Hydroxyamide-Functionalized Azolium Anchored on Merrifield Resin for Enantioselective Ir-Catalyzed Reduction of Ketones with Silane
Abstract
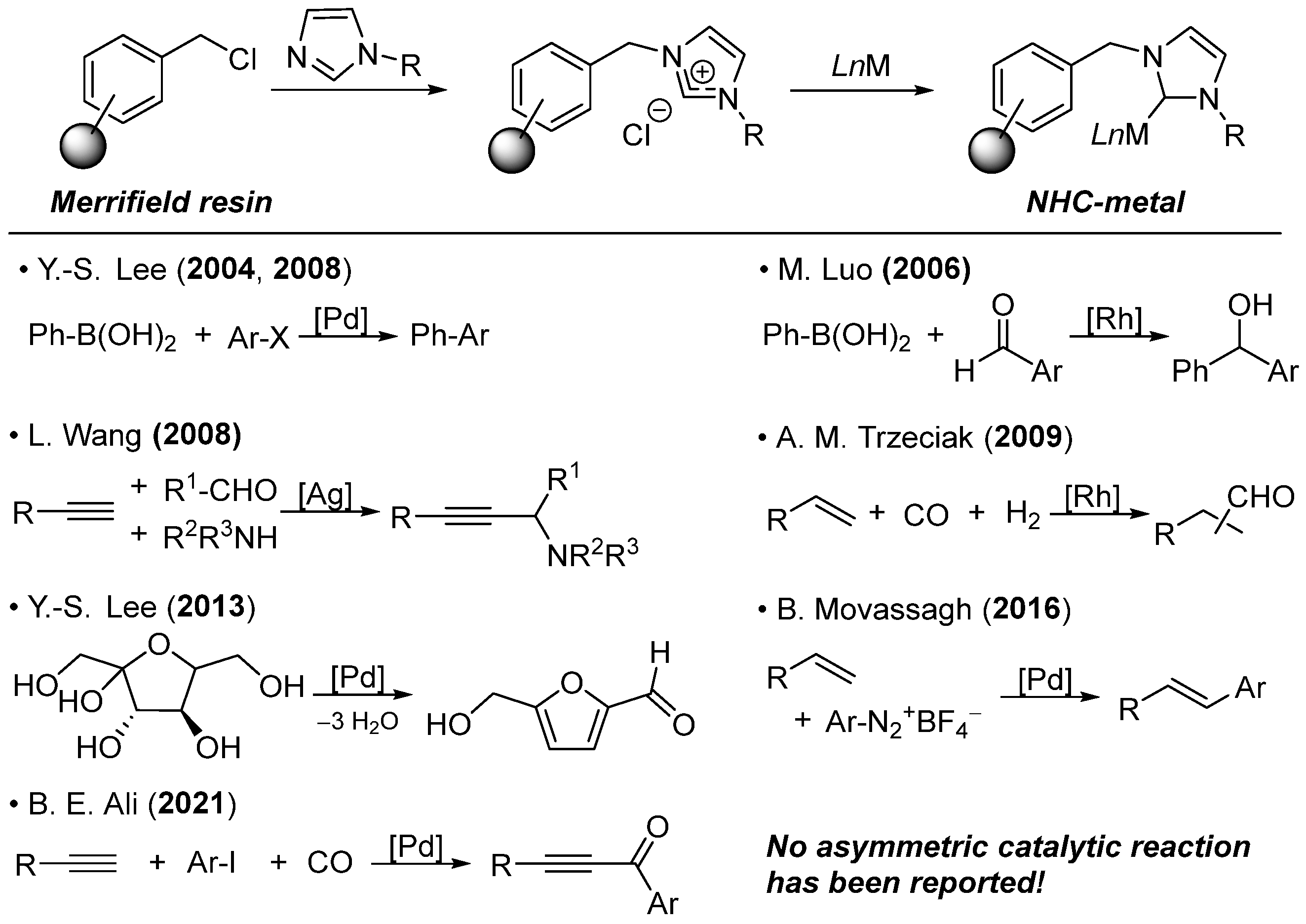
1. Introduction
2. Results and Discussion
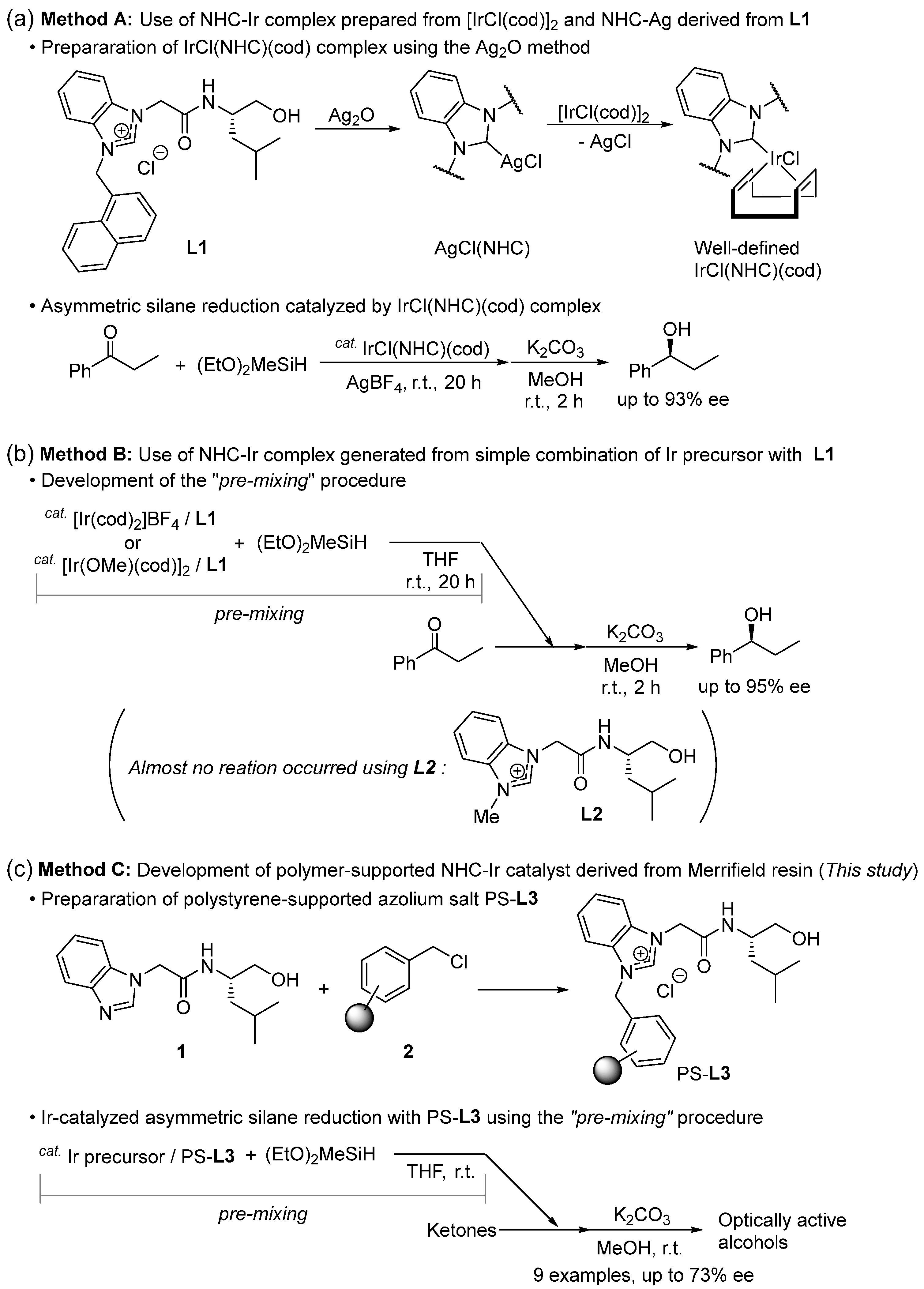
2.1. Preparation of Polymer-Supported Azolium Salt PS-L3 Using Merrifield Resin
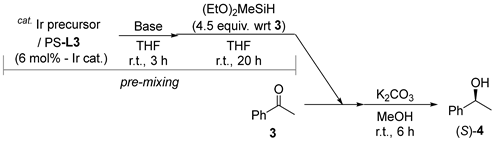
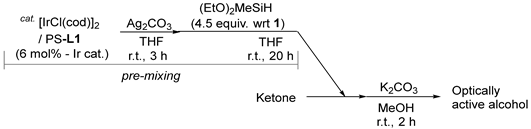
2.2. Ir-Catalyzed Symmetric Silane Reduction of Ketone Using PS-L3
2.3. Reusability of the Polymer-Supported Asymmetric Catalytic System
3. Materials and Methods
3.1. General
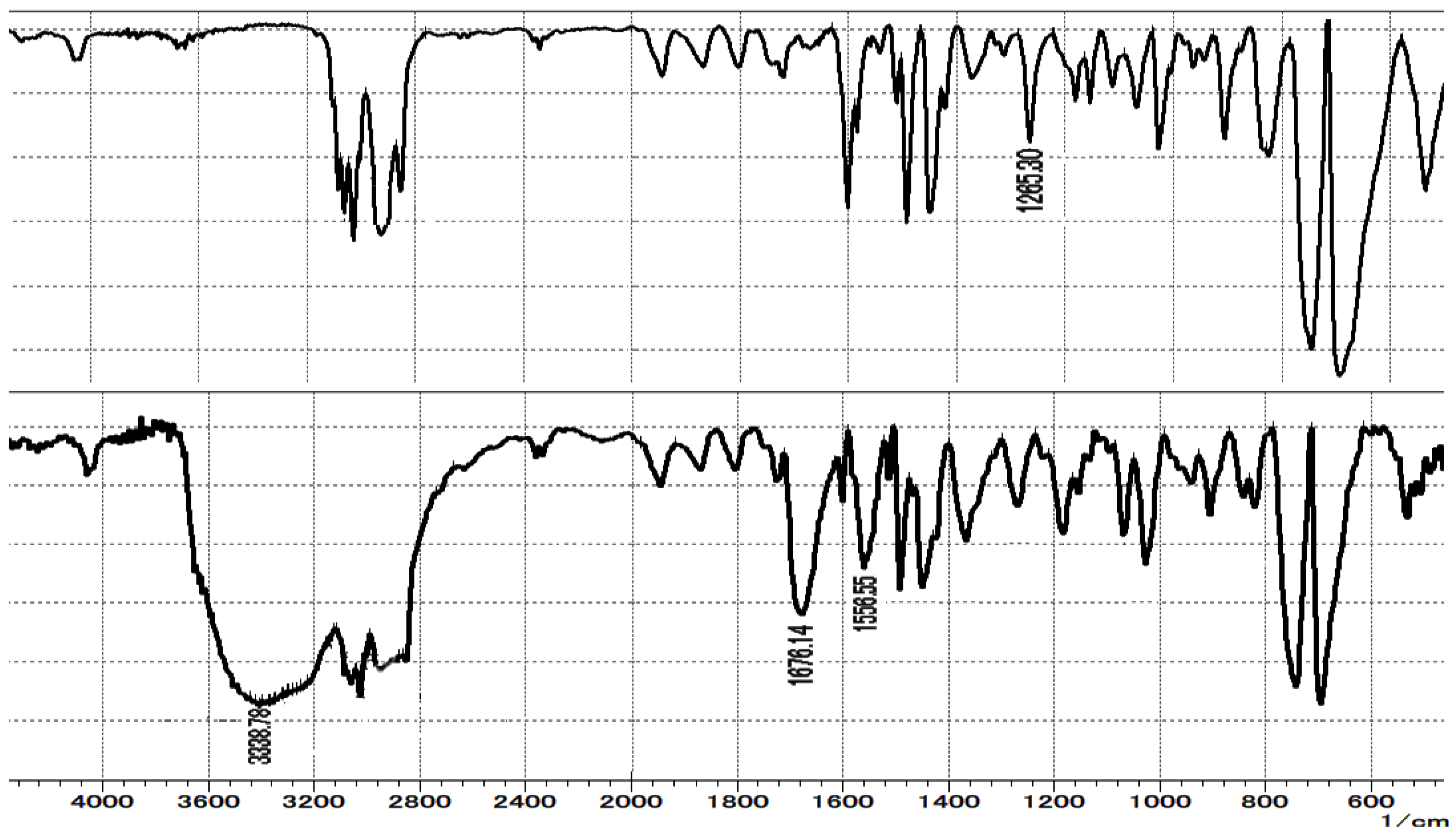
3.2. Procedure for the Preparation of Polymer-Supported Azolium Salt PS-L3 Using Merrifield Resin
3.3. General Procedure for Catalytic Asymmetric Reduction of Ketone with (EtO)2MeSiH
3.4. Procedure for the Catalytic Asymmetric Reduction of 7 with the Recovered Solid-State Resin X
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayaraj, A.; Raveedran, A.V.; Latha, A.T.; Priyadarshini, D.; Swamy, P.C.A. Coordination Versatility of NHC-metal Topologies in Asymmetric Catalysis: Synthetic Insights and Recent Trends. Coord. Chem. Rev. 2023, 478, 214922. [Google Scholar] [CrossRef]
- Neshat, A.; Mastrorilli, P.; Mobarakeh, A.M. Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands. Molecules 2022, 27, 95. [Google Scholar] [CrossRef]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Nahra, F.; Nelson, D.J.; Nolan, S.P. Design Concepts for N-Heterocyclic Carbene Ligands. Trends Chem. 2020, 2, 1096–1111. [Google Scholar] [CrossRef]
- Fliedel, C.; Labande, A.; Manoury, E.; Rinaldo, P. Chiral N-heterocyclic carbene ligands with additional chelating group(s) applied to homogeneous metal-mediated asymmetric catalysis. Coord. Chem. Rev. 2019, 394, 65–103. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Pape, F.; Teichert, J.F. Dealing at Arm’s Length: Catalysis with N-Heterocyclic Carbene Ligands Bearing Anionic Tethers. Eur. J. Org. Chem. 2017, 38, 4206–4229. [Google Scholar] [CrossRef]
- Hopkinson, M.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Wanga, F.; Liua, L.-J.; Wanga, W.; Li, S.; Shi, M. Chiral NHC–metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Perspective on Recycling Strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef]
- Koy, M.; Bellotti, P.; Das, M.; Glorius, F. N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces. Nat. Catal. 2021, 4, 352–363. [Google Scholar] [CrossRef]
- Pentela, N.; Gayathri, V.; Samanta, D. N-heterocyclic carbene bearing thermoresponsive poly(NIPAM) supported palladium (II) complex: Synthetic strategy and application. J. Organomet. Chem. 2020, 913, 121196. [Google Scholar] [CrossRef]
- Ye, R.; Zhukhovitskiy, A.; Kazantsev, R.; Fakra, S.; Wickemeyer, B.; Toste, F.; Somorjai, G. Supported Au Nanoparticles with N-Heterocyclic Carbene Ligands as Active and Stable Heterogeneous Catalysts for Lactonization. J. Am. Chem. Soc. 2018, 140, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Hassan, A.; Chandrasoma, N.; Bogdan, A.; Djuric, S.; Organ, M. Pd-PEPPSI-IPent-SiO2: A Supported Catalyst for Challenging Negishi Coupling Reactions in Flow. Angew. Chem. Int. Ed. 2017, 56, 13347–13350. [Google Scholar] [CrossRef]
- Price, G.; Bogdan, A.; Aguirre, A.; Iwai, T.; Djuric, S.; Organ, M. Continuous flow Negishi cross-couplings employing silica-supported Pd-PEPPSI-IPr precatalyst. Catal. Sci. Technol. 2016, 6, 4733–4742. [Google Scholar] [CrossRef]
- Ernst, J.; Muratsugu, S.; Wang, F.; Tada, M.; Glorius, F. Tunable Heterogeneous Catalysis: N-Heterocyclic Carbenes as Ligands for Supported Heterogeneous Ru/K-Al2O3 Catalysts to Tune Reactivity and Selectivity. J. Am. Chem. Soc. 2016, 138, 10718–10721. [Google Scholar] [CrossRef]
- Fujita, K.; Fujii, A.; Sato, J.; Yasuda, H. Magnetically Recoverable N-Heterocyclic Carbene-Gold(I) Catalyst for Hydroamination of Terminal Alkynes. Synlett 2016, 27, 1941–1944. [Google Scholar] [CrossRef]
- Romanenko, I.; Norsic, S.; Veyre, L.; Sayah, R.; D’Agosto, F.; Raynaud, J.; Boisson, C.; Lacôte, E.; Thieuleux, C. Active and Recyclable Polyethylene-Supported Iridium-(N-Heterocyclic Carbene) Catalyst for Hydrogen/Deuterium Exchange Reactions. Adv. Synth. Catal. 2016, 358, 2317–2323. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Chen, J.; Huang, C.; Tu, T. Robust Iridium Coordination Polymers: Highly Selective, Efficient, and Recyclable Catalysts for Oxidative Conversion of Glycerol to Potassium Lactate with Dihydrogen Liberation. ACS Catal. 2015, 5, 6573–6578. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Zhang, Y.; Li, S.; Qian, H.; Lin, Z. A magnetically separable palladium catalyst containing a bulky N-heterocyclic carbene ligand for the Suzuki-Miyaura reaction. Green Chem. 2015, 17, 413–420. [Google Scholar] [CrossRef]
- Khairnar, B.; Bhanage, B. Amidation of Aryl Halides with Isocyanides Using a Polymer-Supported Palladium-N-Heterocyclic Carbene Complex as an Efficient, Phosphine-Free and Heterogeneous Recyclable Catalyst. Synthesis 2014, 46, 1236–1242. [Google Scholar] [CrossRef]
- Monge-Marcet, A.; Pleixats, R.; Cattoën, X.; Man, M. Catalytic applications of recyclable silica immobilized NHC-ruthenium complexes. Tetrahedron 2013, 69, 341–348. [Google Scholar] [CrossRef]
- Khedkar, M.; Khan, S.; Dhake, K.; Bhanage, B. Carbonylative Cyclization of o-Halobenzoic Acids for Synthesis of N-Substituted Phthalimides Using Polymer-Supported Palladium-N-Heterocyclic Carbene as an Efficient, Heterogeneous, and Reusable Catalyst. Synthesis 2012, 44, 2623–2629. [Google Scholar] [CrossRef]
- Qureshi, Z.; Revankar, S.; Khedkar, M.; Bhanage, B. Aminocarbonylation of aryl iodides with primary and secondary amines in aqueous medium using polymer supported palladium-N-heterocyclic carbene complex as an efficient and heterogeneous recyclable catalyst. Catal. Today 2012, 198, 148–153. [Google Scholar] [CrossRef]
- Bagal, D.; Watile, R.; Khedkar, M.; Dhake, K.; Bhanage, B. PS-Pd-NHC: An efficient and heterogeneous recyclable catalyst for direct reductive amination of carbonyl compounds with primary/secondary amines in aqueous medium. Catal. Sci. Tecnol. 2012, 2, 354–358. [Google Scholar] [CrossRef]
- Ranganath, K.; Schäfer, A.; Glorius, F. Comparison of Superparamagnetic Fe3O4-Supported N-Heterocyclic Carbene-Based Catalysts for Enantioselective Allylation. ChemCatChem 2011, 3, 1889–1891. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Qin, Y.; Chong, Y.; Yang, Q.; Li, G.; Zhang, L.; Li, W. One-pot preparation of magnetic N-heterocyclic carbene-functionalized silica nanoparticles for the Suzuki-Miyaura coupling of aryl chlorides: Improved activity and facile catalyst recovery. Green Chem. 2011, 13, 1352–1361. [Google Scholar] [CrossRef]
- Yu, T.; Li, Y.; Yao, C.; Wu, H.; Liu, Y.; Wu, P. An Efficient and Recyclable Mesostructured Polymer-Supported N-Heterocyclic Carbene-Palladium Catalyst for Sonogashira Reactions. Chin. J. Catal. 2011, 32, 1712–1718. [Google Scholar] [CrossRef]
- Bagal, D.; Qureshi, Z.; Dhake, K.; Khan, S.; Bhanage, B. An efficient and heterogeneous recyclable palladium catalyst for chemoselective conjugate reduction of α,β-unsaturated carbonyls in aqueous medium. Green Chem. 2011, 13, 1490–1494. [Google Scholar] [CrossRef]
- Ranganath, K.; Kloesges, J.; Schäfer, A.; Glorius, F. Asymmetric Nanocatalysis: N-Heterocyclic Carbenes as Chiral Modifiers of Fe3O4/Pd nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7786–7789. [Google Scholar] [CrossRef]
- Worm-Leonhard, K.; Meldal, M. Green Catalysts: Solid-Phase Peptide Carbene Ligands in Aqueous Transition-Metal Catalysis. Eur. J. Org. Chem. 2008, 2008, 5244–5253. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Patra, P.; Ying, J. Synthesis and catalytic applications of mesoporous polymer colloids in olefin hydrosilylation. Adv. Synth. Catal. 2008, 350, 662–666. [Google Scholar] [CrossRef]
- Jun, B.; Lee, Y. Preparation of polymer-supported palladium/N-heterocyclic carbene complex for Suzuki cross-coupling reactions. Tetrahedron Lett. 2004, 45, 1837–1840. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.; Jun, B.; Kang, H.; Park, J.; Lee, Y. Macroporous polystyrene-supported palladium catalyst containing a bulky N-heterocyclic carbene ligand for Suzuki reaction of aryl chlorides. Org. Lett. 2008, 10, 1609–1612. [Google Scholar] [CrossRef]
- Yan, C.; Zeng, X.; Zhang, W.; Luo, M. Polymer-supported N-heterocyclic carbene-rhodium complex catalyst for the addition of arylboronic acids to aldehydes. J. Organomet. Chem. 2006, 691, 3391–3396. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Zhang, Y.; Wang, M. Highly efficient three-component (aldehyde-alkyne-amine) coupling reactions catalyzed by a reusable PS-supported NHC-Ag(I) under solvent-free reaction conditions. Tetrahedron Lett. 2008, 49, 6650–6654. [Google Scholar] [CrossRef]
- Gil, W.; Boczon, K.; Trzeciak, A.; Ziólkowski, J.; Garcia-Verdugo, E.; Luis, S.; Sans, V. Supported N-heterocyclic carbene rhodium complexes as highly selective hydroformylation catalysts. J. Mol. Catal. A Chem. 2009, 309, 131–136. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, S.; Yoon, H.; Kim, J.; Cho, J.; Lee, Y. Polymer-supported N-heterocyclic carbene-iron (III) catalyst and its application to dehydration of fructose into 5-hydroxymethyl-2-furfural. Catal. Commun. 2013, 40, 18–22. [Google Scholar] [CrossRef]
- Mohammadi, E.; Movassagh, B. Synthesis of polystyrene-supported Pd(II)-NHC complex derived from theophylline as an efficient and reusable heterogeneous catalyst for the Heck-Matsuda cross-coupling reaction. J. Mol. Catal. A Chem. 2016, 418, 158–167. [Google Scholar] [CrossRef]
- Mansour, W.; Fettouhi, M.; Saleem, Q.; El Ali, B. Robust alkyl-bridged bis(N-heterocyclic carbene)palladium(II) complexes anchored on Merrifield’s resin as active catalysts for the selective synthesis of flavones and alkynones. Appl. Organomet. Chem. 2021, 35, e6195. [Google Scholar] [CrossRef]
- Yoshida, K.; Horiuchi, S.; Takeichi, T.; Shida, H.; Imamoto, T.; Yanagisawa, A. Bicyclic Imidazoles for Modular Synthesis of Chiral Imidazolium Salts. Org. Lett. 2010, 12, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Shinohara, K.; Nakamura, H.; Teramoto, H.; Sakaguchi, S. Chiral N-heterocyclic carbene iridium catalyst for the enantioselective hydrosilane reduction of ketones. J. Mol. Catal. A Chem. 2016, 421, 138–145. [Google Scholar] [CrossRef]
- Shinohara, K.; Kawabata, S.; Nakamura, H.; Manabe, Y.; Sakaguchi, S. Enantioselective Hydrosilylation of Ketones Catalyzed by a Readily Accessible N-Heterocyclic Carbene-Ir Complex at Room Temperature. Eur. J. Org. Chem. 2014, 2014, 5532–5539. [Google Scholar] [CrossRef]
- Kawabata, S.; Tokura, H.; Chiyojima, H.; Okamoto, M.; Sakaguchi, S. Asymmetric Hydrosilane Reduction of Ketones Catalyzed by an Iridium Complex Bearing a Hydroxyamide-Functionalized NHC Ligand. Adv. Synth. Catal. 2012, 354, 807–812. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Nagao, C.; Ichihara, R.; Matsuo, S. Operationally Simple Enantioselective Silane Reduction of Ketones by the [Ir(OMe)(cod)]2/Azolium Catalytic System. Int. J. Org. Chem. 2024, 14, 1–19. [Google Scholar] [CrossRef]
- Matsuki, T.; Teramoto, H.; Ichihara, R.; Inui, K.; Sakaguchi, S. Asymmetric silane reduction of ketones and β-Keto esters catalyzed by a chiral azolium/iridium system in the presence of a base in methanol at room temperature. Results Chem. 2022, 4, 100364. [Google Scholar] [CrossRef]
- Teramoto, H.; Sakaguchi, S. Enantioselective catalytic hydrosilylation of propiophenone with a simple combination of a cationic iridium complex and a chiral azolium salt. J. Organomet. Chem. 2018, 875, 52–58. [Google Scholar] [CrossRef]
- Riener, K.; Bitzer, M.J.; Pöthig, A.; Raba, A.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. On the Concept of Hemilability: Insights into a Donor-Functionalized Iridium(I) NHC Motif and Its Impact on Reactivity. Inorg. Chem. 2014, 53, 12767–12777. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Jan, M.T.; Ghiviriga, I.; Abboud, K.A.; Veige, A.S. New iridium and rhodium chiral di-N-heterocyclic carbene (NHC) complexes and their application in enantioselective catalysis. Dalton Trans. 2009, 15, 2764–2776. [Google Scholar] [CrossRef]
- Ortega-Lepe, I.; Rossin, A.; Sánchez, P.; Santos, L.L.; Rendón, N.; Álvarez, E.; López-Serrano, J.; Suárez, A. Ammonia-Borane Dehydrogenation Catalyzed by Dual-Mode Proton-Responsive Ir-CNNH Complexes. Inorg. Chem. 2021, 60, 18490–18502. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Entry | Elemental Analysis of PS-L3 | Azolium Content 1 | |
| 1 2 | run 1 | C, 79.94; H, 7.43; N, 3.55 | 0.84 mmol/g |
| run 2 | C, 79.66; H, 7.39; N, 3.62 | 0.86 mmol/g | |
| run 3 | C, 79.86; H, 7.58; N, 3.48 | 0.83 mmol/g | |
| 2 3 | run 1 | C, 85.08; H, 7.23; N, 1.22 | 0.29 mmol/g |
 | ||||||
|---|---|---|---|---|---|---|
| Entry 1 | Ir Precursor | Azolium | Base | Product | Yield [%] 2 | Ee [%] 3 |
| 1 | [IrCl(cod)]2 | PS-L3 | Ag2O | (S)-4 | 68 | 74 |
| 2 | [IrCl(cod)]2 | PS-L3 | none | (S)-4 | 31 | 13 |
| 3 | [IrCl(cod)]2 | PS-L3 | tBuOK 4 | (S)-4 | 23 | 39 |
| 4 | [IrCl(cod)]2 | PS-L3 | AgOAc 4 | (S)-4 | 24 | 44 |
| 5 | [IrCl(cod)]2 | PS-L3 | Ag2CO3 | (S)-4 | 58 | 68 |
| 6 | [IrCl(cod)]2 | PS-L4 5 | Ag2CO3 | (R)-4 | 52 | 75 |
| 7 | [IrOMe(cod)]2 6 | PS-L3 | none | (S)-4 | 49 | 38 |
| 8 | Ir(acac)(cod) 7 | PS-L3 | none | (S)-4 | 26 | 41 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry 1 | Substrate | Product | Yield [%] 2 | Ee [%] | ||
| 1 3,4 | 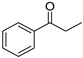 | 5 | 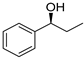 | (S)-6 | 47 | 73 |
| 2 5 |  | 7 | 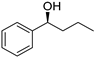 | (S)-8 | 68 | 66 |
| 3 5 | 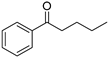 | 9 | 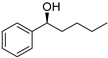 | (S)-10 | 34 | 61 |
| 4 5 | 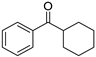 | 11 | 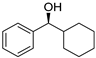 | (S)-12 | 23 | 71 |
| 5 3 | 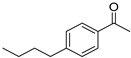 | 13 | 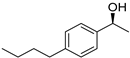 | (S)-14 | 40 | 61 |
| 6 3 |  | 15 | 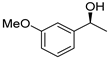 | (S)-16 | 33 | 65 |
| 7 5 | 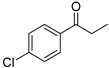 | 17 |  | (S)-18 | 76 | 65 |
| 8 3 | 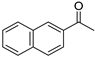 | 19 | 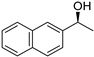 | (S)-20 | 37 | 50 |
| Entry | Substrate | Catalytic System | Product | Yield [%] 1 | Ee [%] 2 | |
|---|---|---|---|---|---|---|
| 1 | 7 | run 1 3,4 | [IrCl(cod)]2 combined with PS-L3 5 | (S)-8 | 68 | 66 |
| 7 | run 2 6 | [IrCl(cod)]2 combined with recovered resin X | (S)-8 | 52 | 77 | |
| 7 | run 2A 7 | recovered resin X | No reaction occurred. | |||
| 2 | 17 | run 1 3,8 | [IrCl(cod)]2 combined with PS-L3 5 | (S)-18 | 76 | 65 |
| 17 | run 2 9 | [IrCl(cod)]2 combined with recovered resin Y 6 | (S)-18 | 80 | 61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, S.; Koyabu, M.; Inui, K. Hydroxyamide-Functionalized Azolium Anchored on Merrifield Resin for Enantioselective Ir-Catalyzed Reduction of Ketones with Silane. Catalysts 2025, 15, 303. https://doi.org/10.3390/catal15040303
Sakaguchi S, Koyabu M, Inui K. Hydroxyamide-Functionalized Azolium Anchored on Merrifield Resin for Enantioselective Ir-Catalyzed Reduction of Ketones with Silane. Catalysts. 2025; 15(4):303. https://doi.org/10.3390/catal15040303
Chicago/Turabian StyleSakaguchi, Satoshi, Masamune Koyabu, and Kazuki Inui. 2025. "Hydroxyamide-Functionalized Azolium Anchored on Merrifield Resin for Enantioselective Ir-Catalyzed Reduction of Ketones with Silane" Catalysts 15, no. 4: 303. https://doi.org/10.3390/catal15040303
APA StyleSakaguchi, S., Koyabu, M., & Inui, K. (2025). Hydroxyamide-Functionalized Azolium Anchored on Merrifield Resin for Enantioselective Ir-Catalyzed Reduction of Ketones with Silane. Catalysts, 15(4), 303. https://doi.org/10.3390/catal15040303







