In Situ Synthesis of Hierarchical Carbon-Encapsulated Pd Nanoparticles as an Efficient Semi-Hydrogenation Catalyst
Abstract
1. Introduction
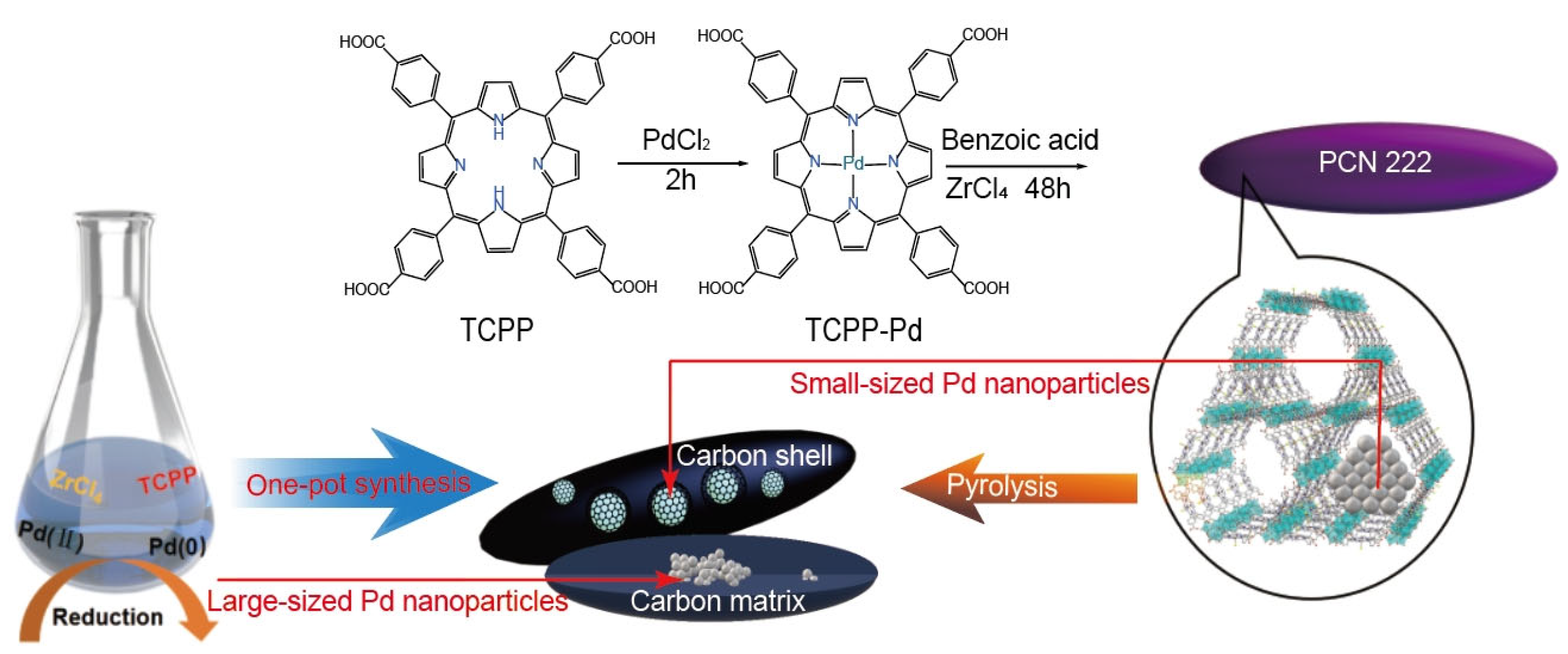
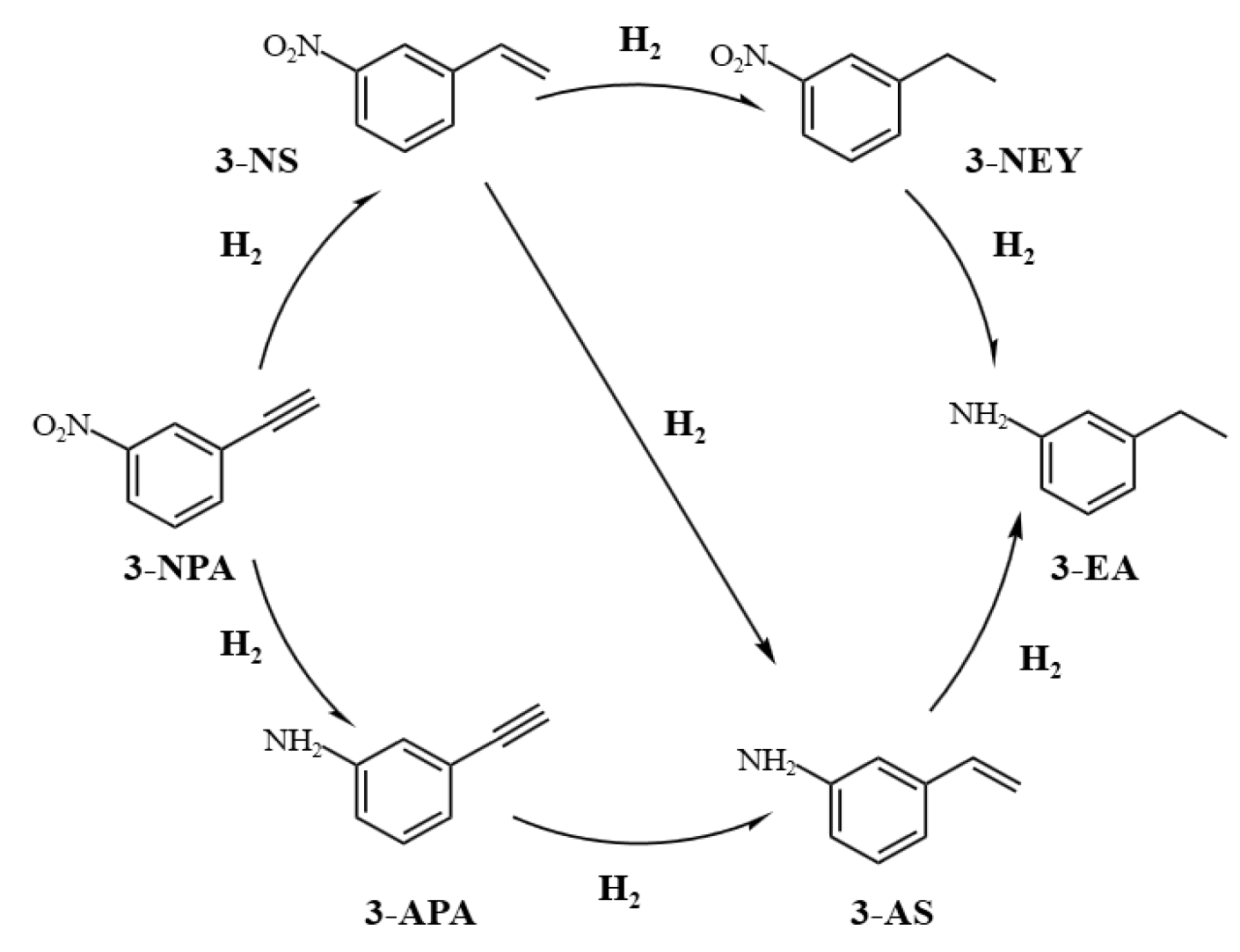
2. Results and Discussion
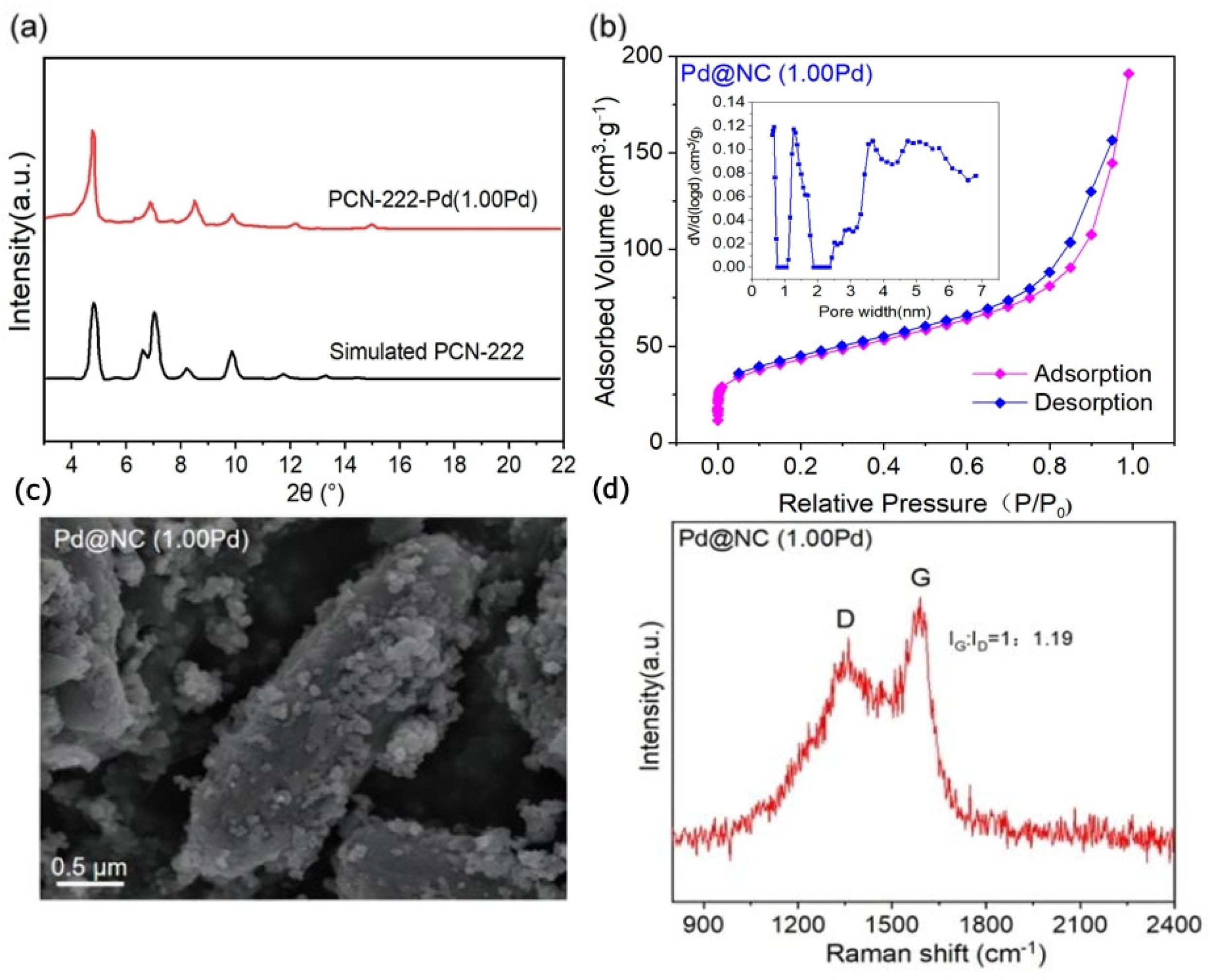
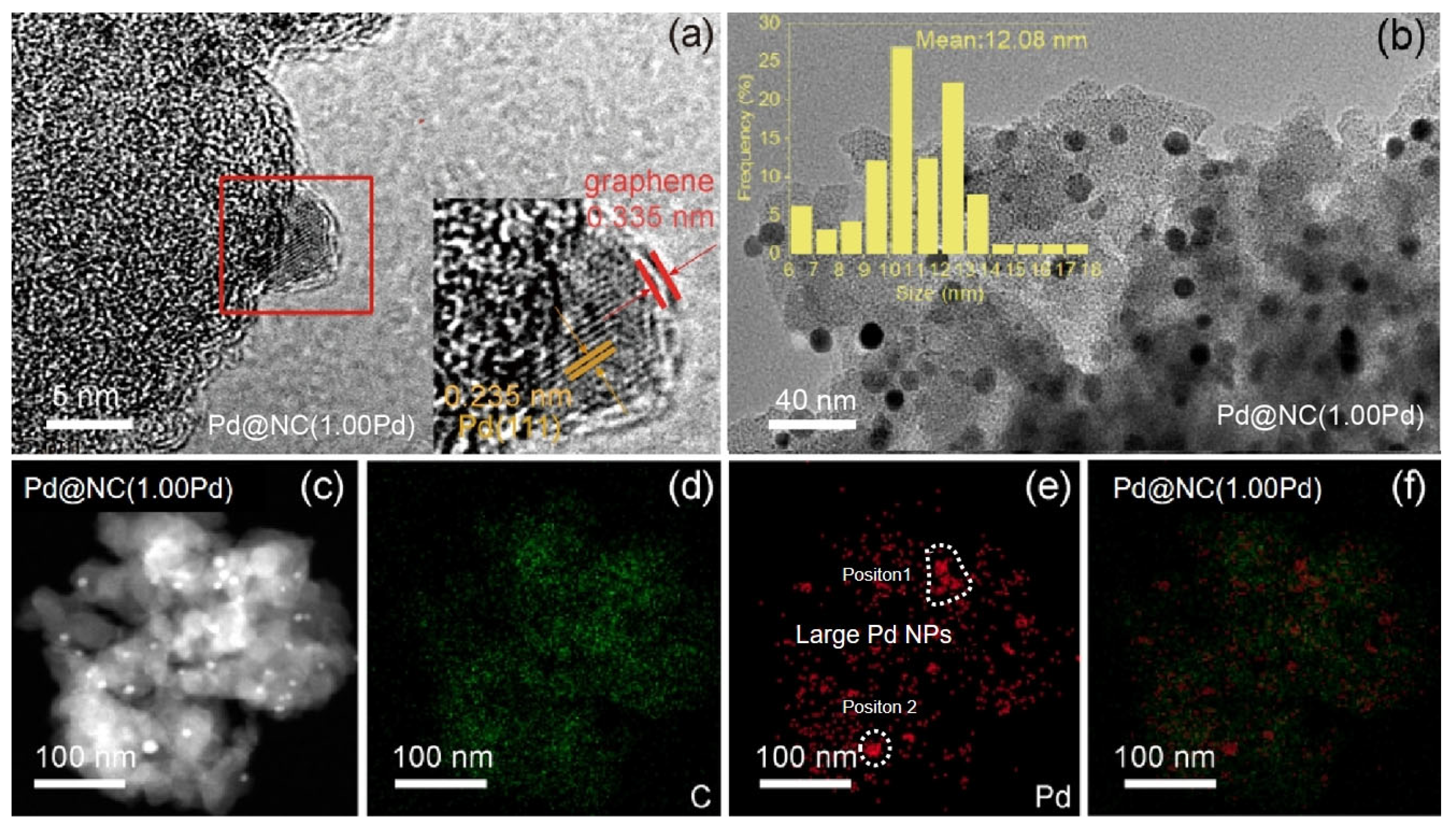
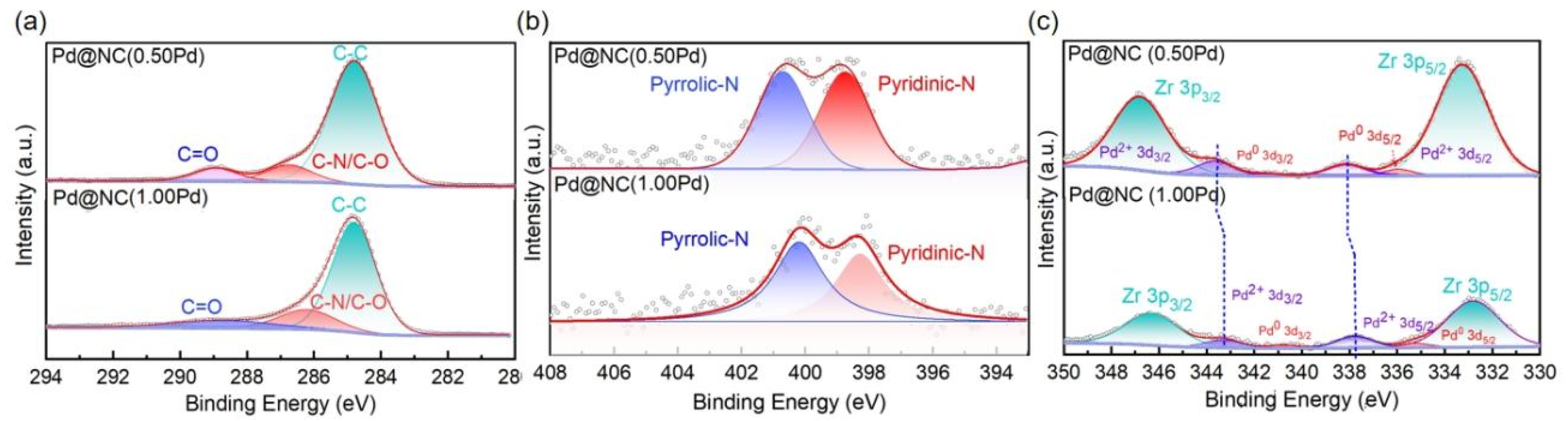
Characterization
3. Experimental Section
3.1. Materials and Chemicals
3.2. Solvothermal “One-Pot” Synthesis of PCN-222-Pd
3.3. In Situ Synthesis of Pd@NC (xPd) Catalysts
3.4. Characterization Method
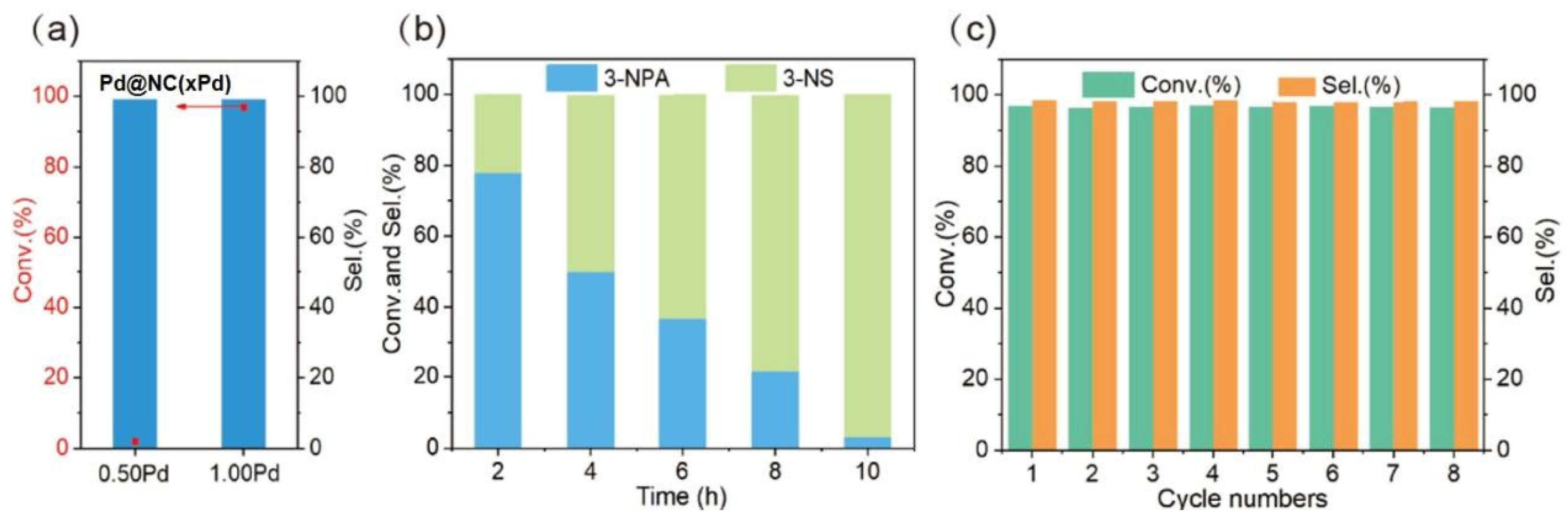
3.5. Catalytic Evaluation Test
4. Mechanism Explanation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Ma, S.; Liu, Y.; Wang, L.; Yuan, D.; Shao, W.-P.; Zhang, L.; Yang, F.; Lin, T.; Ding, H.; et al. Homolytic H2 dissociation for enhanced hydrogenation catalysis on oxides. Nat. Commun. 2024, 15, 540. [Google Scholar] [PubMed]
- Prins, R. Hydrogen spillover. Facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [PubMed]
- Molina, D.L.; Muir, M.; Abdel-Rahman, M.K.; Trenary, M. The influence of palladium on the hydrogenation of acetylene on Ag (111). J. Chem. Phys. 2021, 154, 184701. [Google Scholar] [CrossRef]
- Backhouse, T.; Earley, J.H.; Mugo, J.N.; Goodlet, G.; Jones, G.; Seljamäe-Green, R.; Erden, T.E.; Forster, M.; Gómez, P.; Jackson, S.W. Sn-doped PdAg/Al2O3 catalysts: Efficient catalysts for the selective hydrogenation of acetylene to ethylene under industrial conditions. J. Catal. 2024, 436, 115600. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Baeva, G.N.; Smirnova, N.S.; Vaulina, A.E.; Mel’nikov, D.P.; Stakheev, A.Y. Properties of PdAg/Al2O3 Egg-Shell Single-Atom Catalysts in Front-End Hydrogenation of Acetylene. Pet. Chem. 2024, 64, 1159–1168. [Google Scholar] [CrossRef]
- Markov, P.V.; Baeva, G.N.; Smirnova, N.S.; Vaulina, A.E.; Melnikov, D.P.; Mashkovsky, I.S. Effect of isolated palladium atoms on the performance of PdAg/Al2O3 catalysts for the selective hydrogenation of acetylene. Russ. Chem. Bull. 2024, 73, 1189–1196. [Google Scholar]
- Rajaram, J.; Narula, A.P.S.; Chawla, H.P.S.; Dev, S. Semihydrogenation of acetylenes: Modified Lindlar catalyst. Tetrahedron 1983, 39, 2315–2322. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, B.; Fan, M.; Li, D.; Zhang, R. C2H2 semi-hydrogenation on the metal M (M = Cu, Ag, Au) alloyed single-atom Pd catalysts: Effects of Pd coordination number and environment on the catalytic performance. Chem. Eng. Sci. 2021, 243, 116786. [Google Scholar] [CrossRef]
- Xue, F.; Li, Q.; Ji, W.; Lv, M.; Xu, H.; Zeng, J.; Li, T.; Ren, Y.; Zhou, L.; Chen, X.; et al. Highly efficient semi-hydrogenation in strained ultrathin PdCu shell and the atomic deciphering for the unlocking of activity-selectivity. Chem. Sci. 2024, 15, 11837–11846. [Google Scholar]
- Liu, Y.; He, Y.; Zhou, D.; Feng, J.; Li, D. Catalytic performance of Pd-promoted Cu hydrotalcite-derived catalysts in partial hydrogenation of acetylene: Effect of Pd-Cu alloy formation. Catal. Sci. Technol. 2016, 6, 3027–3037. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Schild, D.; Wang, D.; Kübel, C.; Behrens, S. Pd-In intermetallic nanoparticles with high catalytic selectivity for liquid-phase semi-hydrogenation of diphenylacetylene. Nanoscale 2022, 14, 17661–17669. [Google Scholar] [PubMed]
- Wowsnick, G. Surface Dynamics of Pd2Ga and Its Reactivity in the Liquid Phase Hydrogenation of Phenylacetylene. Ph.D. Thesis, Technische Universität, Berlin, Germany, 2013. [Google Scholar]
- Cao, X.; Tong, R.; Tang, S.; Jang, B.W.-L.; Mirjalili, A.; Li, J.; Guo, X.; Zhang, J.; Hu, J.; Meng, X. Design of PdZn bimetal MOF nanosheets and MOF-derived Pd3.9Zn6.1/CNS catalyst for selective hydrogenation of acetylene under simulated front-end conditions. Molecules 2022, 27, 5736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd-Zn-Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. Acs Catal. 2016, 6, 1054–1061. [Google Scholar]
- Chen, H.; Yang, B.; Zhang, Y.; Che, C.; Zhang, F.; Han, W.; Wen, H.; Wang, A.; Zhang, T. The Geometric and Electronic Effects of Ceria on Promoting PdZn catalyst for Enhanced Acetylene Semi-Hydrogenation. ChemCatChem 2024, 16, e202400566. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Ren, P.; Xiao, D.; Wen, X.; Wang, N.; et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 2018, 140, 13142–13146. [Google Scholar] [CrossRef]
- Huang, F.; Peng, M.; Chen, Y.; Cai, X.; Qin, X.; Wang, N.; Xiao, D.; Jin, L.; Wang, G.; Wen, X.D.; et al. Low-temperature acetylene semi-hydrogenation over the Pd1-Cu1 dual-atom catalyst. J. Am. Chem. Soc. 2022, 144, 18485–18493. [Google Scholar]
- Wang, S.; Zhao, Z.; Chang, X.; Zhao, J.; Tian, H.; Yang, C.; Li, M.; Fu, Q.; Mu, R.; Gong, J. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes. Angew. Chem. Int. Ed. 2019, 58, 7668–7672. [Google Scholar]
- Tang, J.; Liu, P.; Liu, X.; Chen, L.; Wen, H.; Zhou, Y.; Wang, J. In situ encapsulation of Pt nanoparticles within pure silica TON zeolites for space-confined selective hydrogenation. ACS Appl. Mater. Interfaces 2020, 12, 11522–11532. [Google Scholar]
- Liu, H.; Li, J.; Liang, X.; Ren, H.; Yin, H.; Wang, L.; Yang, D.; Wang, D.; Li, Y. Encapsulation of Pd Single-Atom Sites in Zeolite for Highly Efficient Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2024, 146, 24033–24041. [Google Scholar]
- Chai, Y.; Wu, G.; Liu, X.; Ren, Y.; Dai, W.; Wang, C.; Xie, Z.; Guan, N.; Li, L. Acetylene-selective hydrogenation catalyzed by cationic nickel confined in zeolite. J. Am. Chem. Soc. 2019, 141, 9920–9927. [Google Scholar] [CrossRef]
- Bai, R.; He, G.; Li, L.; Zhang, T.; Li, J.; Wang, X.; Wang, X.; Zou, Y.; Mei, D.; Corma, A.; et al. Encapsulation of palladium carbide subnanometric species in zeolite boosts highly selective semihydrogenation of alkynes. Angew. Chem. Int. Ed. 2023, 62, e202313101. [Google Scholar]
- Liu, Y.; Wang, B.; Fu, Q.; Liu, W.; Wang, Y.; Gu, L.; Wang, D.; Li, Y. Polyoxometalate-based metal–organic framework as molecular sieve for highly selective semi-hydrogenation of acetylene on isolated single Pd atom sites. Angew. Chem. Int. Ed. 2021, 60, 22522–22528. [Google Scholar]
- Li, Z.; Hu, M.; Liu, J.; Wang, W.; Li, Y.; Fan, W.; Gong, Y.; Yao, J.; Wang, P.; He, M.; et al. Mesoporous silica stabilized MOF nanoreactor for highly selective semi-hydrogenation of phenylacetylene via synergistic effect of Pd and Ru single site. Nano Res. 2021, 15, 1983–1992. [Google Scholar]
- Shen, C.; Ji, Y.; Wang, P.; Bai, S.; Wang, M.; Li, Y.; Huang, X.; Shao, Q. Interface confinement in metal nanosheet for high-efficiency semi-hydrogenation of alkynes. ACS Catal. 2021, 11, 5231–5239. [Google Scholar]
- Zhang, R.P.; He, B.; Yang, R.P.; Zhang, Y.X.; Li, W.C.; Zhu, L.H.; Wang, S.J.; Wang, D.Q.; Liu, X.; Chen, L.; et al. Nanoengineered Design of inside-Heating Hot Nanoreactor Surrounded by Cool Environment for Selective Hydrogenations. Adv. Mater. 2023, 35, 2302793. [Google Scholar]
- Cai, J.; Chen, Y.; Song, H.; Hou, L.; Li, Z. MOF derived C/Co@ C with a “one-way-valve”-like graphitic carbon layer for selective semi-hydrogenation of aromatic alkynes. Carbon 2020, 160, 64–70. [Google Scholar]
- Liu, J.; Sun, J.; Singh, T.; Lin, S.; Ma, L. Facile synthesis of N-doped graphene encapsulated Ni@ N/C catalyst and its catalysis for highly selective semi-hydrogenation of alkynes. Green Chem. Eng. 2022, 3, 395–404. [Google Scholar]
- Zhao, L.; Qin, X.; Zhang, X.; Cai, X.; Huang, F.; Jia, Z.; Diao, J.; Xiao, D.; Jiang, Z.; Lu, R.; et al. A magnetically separable Pd single-atom catalyst for efficient selective hydrogenation of phenylacetylene. Adv. Mater. 2022, 34, 2110455. [Google Scholar]
- Chen, B.; Zheng, Z.; Hu, C.; Zengcai, Z.; Liu, Z.; Lu, M.; Meng, Q.; Wang, T. High-content graphitized N-doped carbon encapsulated Cu catalyst in aqueous phase reforming of methanol for efficient hydrogen production. Fuel 2024, 371, 131888. [Google Scholar]
- Xia, L.; Li, D.; Long, J.; Huang, F.; Yang, L.; Guo, Y.; Jia, Z.; Xiao, J.; Liu, H. N-doped graphene confined Pt nanoparticles for efficient semi-hydrogenation of phenylacetylene. Carbon 2019, 145, 47–52. [Google Scholar]
- Liu, Y.; Fu, F.; McCue, A.; Jones, W.; Rao, D.; Feng, J.; He, Y.; Li, D. Adsorbate-induced structural evolution of Pd catalyst for selective hydrogenation of acetylene. ACS Catal. 2020, 10, 15048–15059. [Google Scholar]
- Shi, X.; Lin, Y.; Huang, L.; Sun, Z.; Yang, Y.; Zhou, X.; Vovk, E.; Liu, X.; Huang, X.; Sun, M.; et al. Copper catalysts in semihydrogenation of acetylene: From single atoms to nanoparticles. ACS Catal. 2020, 10, 3495–3504. [Google Scholar]
- Zhong, Y.; Liao, P.; Kang, J.; Liu, Q.; Wang, S.; Li, S.; Liu, X.; Li, G. Locking effect in metal@ MOF with superior stability for highly chemoselective catalysis. J. Am. Chem. Soc. 2023, 145, 4659–4666. [Google Scholar]
- Liu, J.; Zhang, A.; Liu, M.; Hu, S.; Ding, F.; Song, C.; Guo, X. Fe-MOF-derived highly active catalysts for carbon dioxide hydrogenation to valuable hydrocarbons. J. CO2 Util. 2017, 21, 100–107. [Google Scholar]
- Nakatsuka, K.; Yoshii, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Controlled Pyrolysis of Ni-MOF-74 as a Promising Precursor for the Creation of Highly Active Ni Nanocatalysts in Size-Selective Hydrogenation. Chem.—Eur. J. 2018, 24, 898–905. [Google Scholar] [PubMed]
- Zhao, M.X.; Yang, N.; Li, Z.X.; Xie, H.S. MOFs Derived Catalysts Prepared by Pyrolysis for Hydrogenation of Bio-Based Furfural: A Mini-Review. ChemistrySelect 2020, 5, 13681–13689. [Google Scholar]
- Hu, A.; Lu, X.; Cai, D.; Pan, H.; Jing, R.; Xia, Q.; Zhou, D.; Xia, Y. Selective hydrogenation of nitroarenes over MOF-derived Co@ CN catalysts at mild conditions. Mol. Catal. 2019, 472, 27–36. [Google Scholar]
- Zhou, H.; Yang, T.; Kou, Z.; Shen, L.; Zhao, Y.; Wang, Z.; Wang, X.; Yang, Z.; Du, J.; Xu, J.; et al. Negative pressure pyrolysis induced highly accessible single sites dispersed on 3D graphene frameworks for enhanced oxygen reduction. Angew. Chem. Int. Ed. 2020, 132, 20645–20649. [Google Scholar]
- Zhang, W.; Wang, Y.; Ding, K.; Li, H.; Sun, Z.; Hu, Z.; Sun, H. A semi-encapsulated PdRh alloy heterojunction for the selective catalytic hydrogenation of nitrophenylacetylene to nitrostyrene. Dalton. Trans. 2022, 51, 14639–14645. [Google Scholar]
- Yu, L.; Deng, D.; Bao, X. Chain mail for catalysts. Angew. Chem. Int. Ed. 2020, 59, 15294–15297. [Google Scholar]
- Guo, K.; Bao, L.; Yu, Z.; Lu, X. Carbon encapsulated nanoparticles: Materials science and energy applications. Chem. Soc. Rev. 2024, 53, 11100–11164. [Google Scholar] [PubMed]
- Yuan, H.; Hong, M.; Huang, X.; Qiu, W.; Dong, F.; Zhou, Y.; Chen, Y.; Gao, J.; Yang, S. Graphene Chainmail Shelled Dilute Ni–Cu Alloy for Selective and Robust Aqueous Phase Catalytic Hydrogenation. Adv. Sci. 2024, 11, 2304349. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Wang, C.; Zhai, X.; Zhang, T.; Wang, Y.; Hu, X. Direct Growth of Poly-Glutamic Acid Film on Peroxidase Mimicking PCN-222(Mn) for Constructing a Novel Sensitive Nonenzymatic Electrochemical Hydrogen Peroxide Biosensor. ACS Sustain. Chem. Eng. 2020, 8, 13226–13235. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Zhang, W.; Wang, Y.; Chen, X.; Ai, Y.; Hu, Z.; Sun, H.-B. In Situ Synthesis of Hierarchical Carbon-Encapsulated Pd Nanoparticles as an Efficient Semi-Hydrogenation Catalyst. Catalysts 2025, 15, 295. https://doi.org/10.3390/catal15030295
Kong W, Zhang W, Wang Y, Chen X, Ai Y, Hu Z, Sun H-B. In Situ Synthesis of Hierarchical Carbon-Encapsulated Pd Nanoparticles as an Efficient Semi-Hydrogenation Catalyst. Catalysts. 2025; 15(3):295. https://doi.org/10.3390/catal15030295
Chicago/Turabian StyleKong, Weijie, Wenhui Zhang, Yiming Wang, Xin Chen, Yongjian Ai, Zenan Hu, and Hong-Bin Sun. 2025. "In Situ Synthesis of Hierarchical Carbon-Encapsulated Pd Nanoparticles as an Efficient Semi-Hydrogenation Catalyst" Catalysts 15, no. 3: 295. https://doi.org/10.3390/catal15030295
APA StyleKong, W., Zhang, W., Wang, Y., Chen, X., Ai, Y., Hu, Z., & Sun, H.-B. (2025). In Situ Synthesis of Hierarchical Carbon-Encapsulated Pd Nanoparticles as an Efficient Semi-Hydrogenation Catalyst. Catalysts, 15(3), 295. https://doi.org/10.3390/catal15030295





