Investigating the Photocatalytic Properties of Reduced Graphene Oxide-Coated Zirconium Dioxide and Their Impact on Structural and Morphological Features
Abstract
1. Introduction
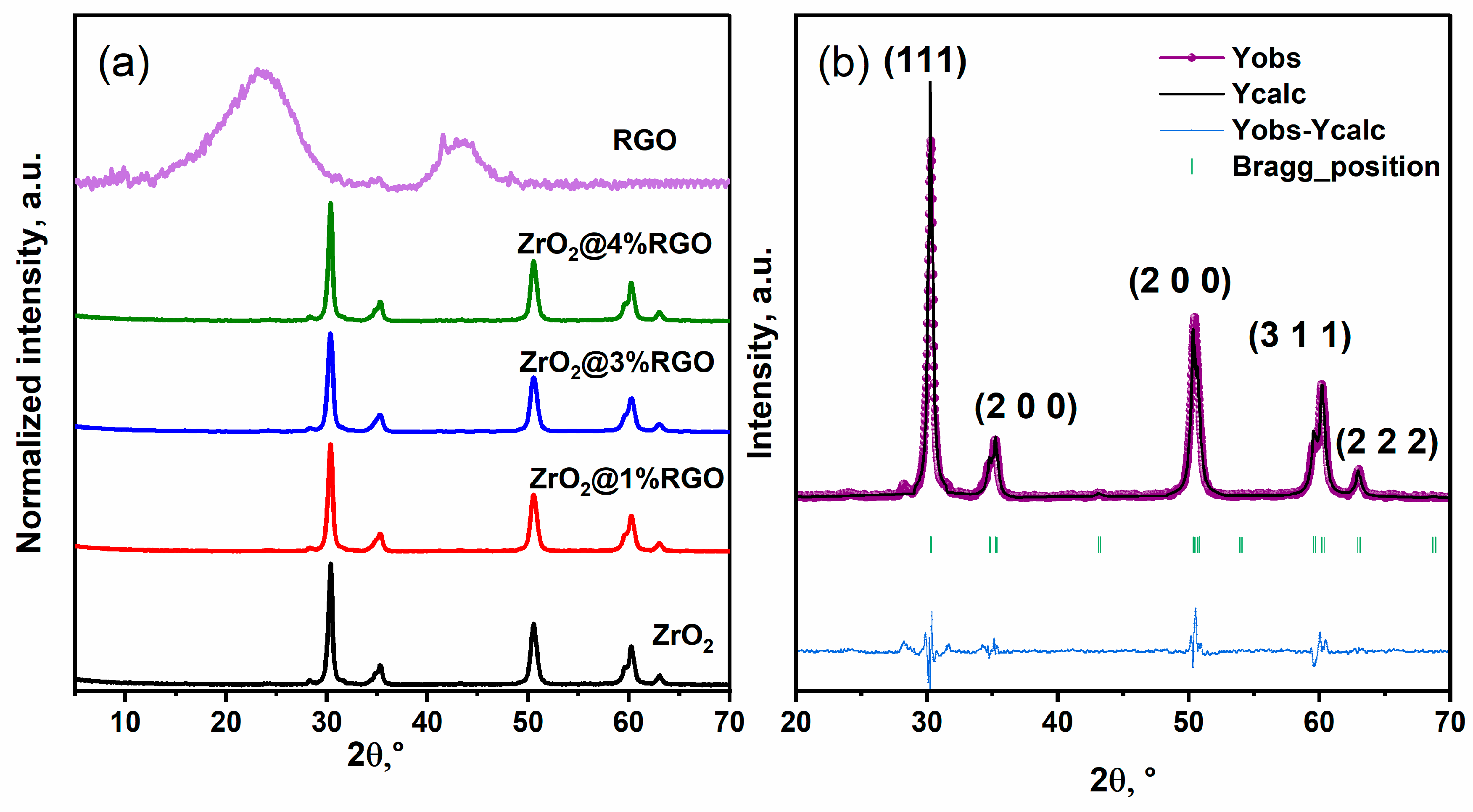
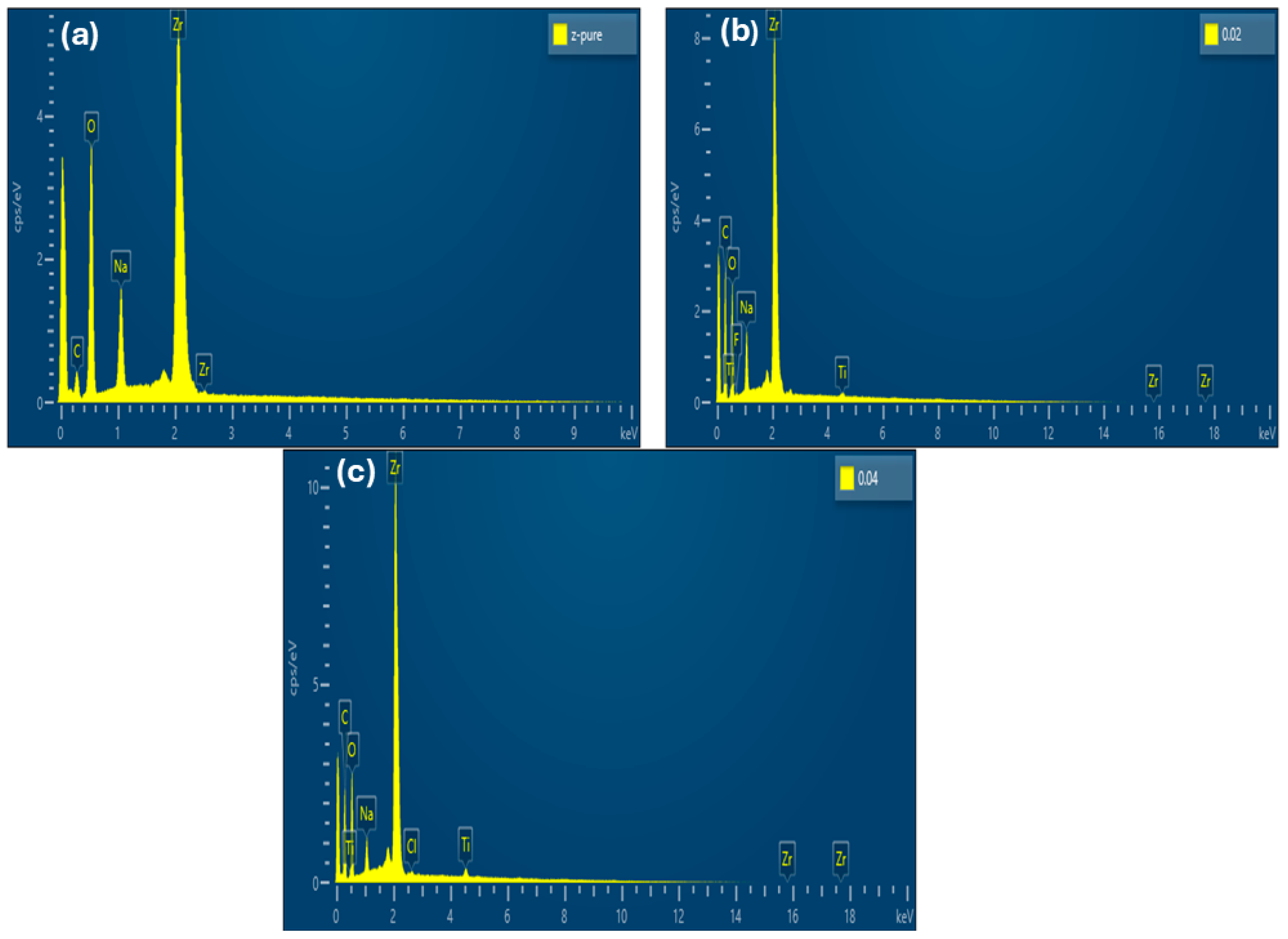
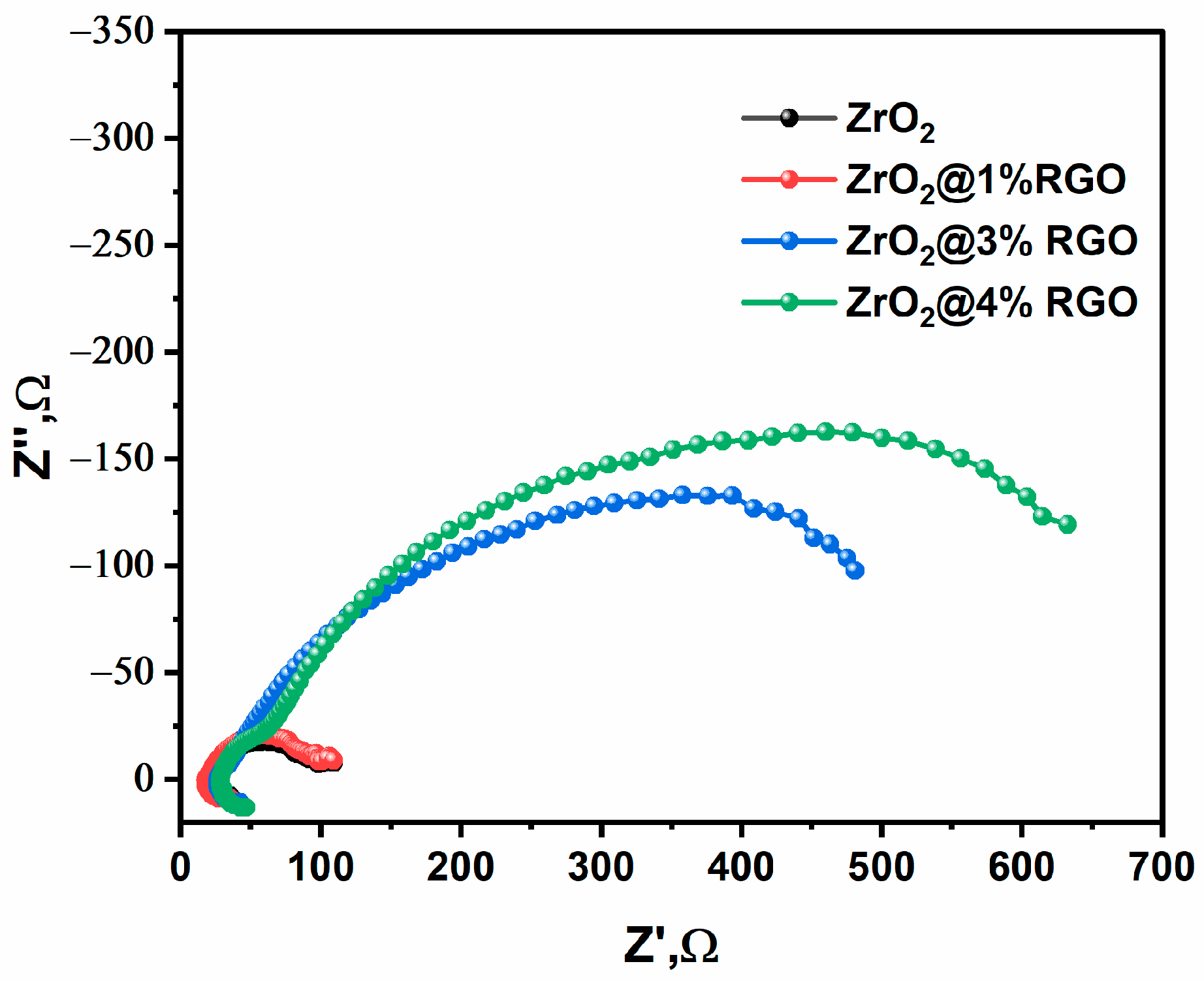
2. Result and Discussion
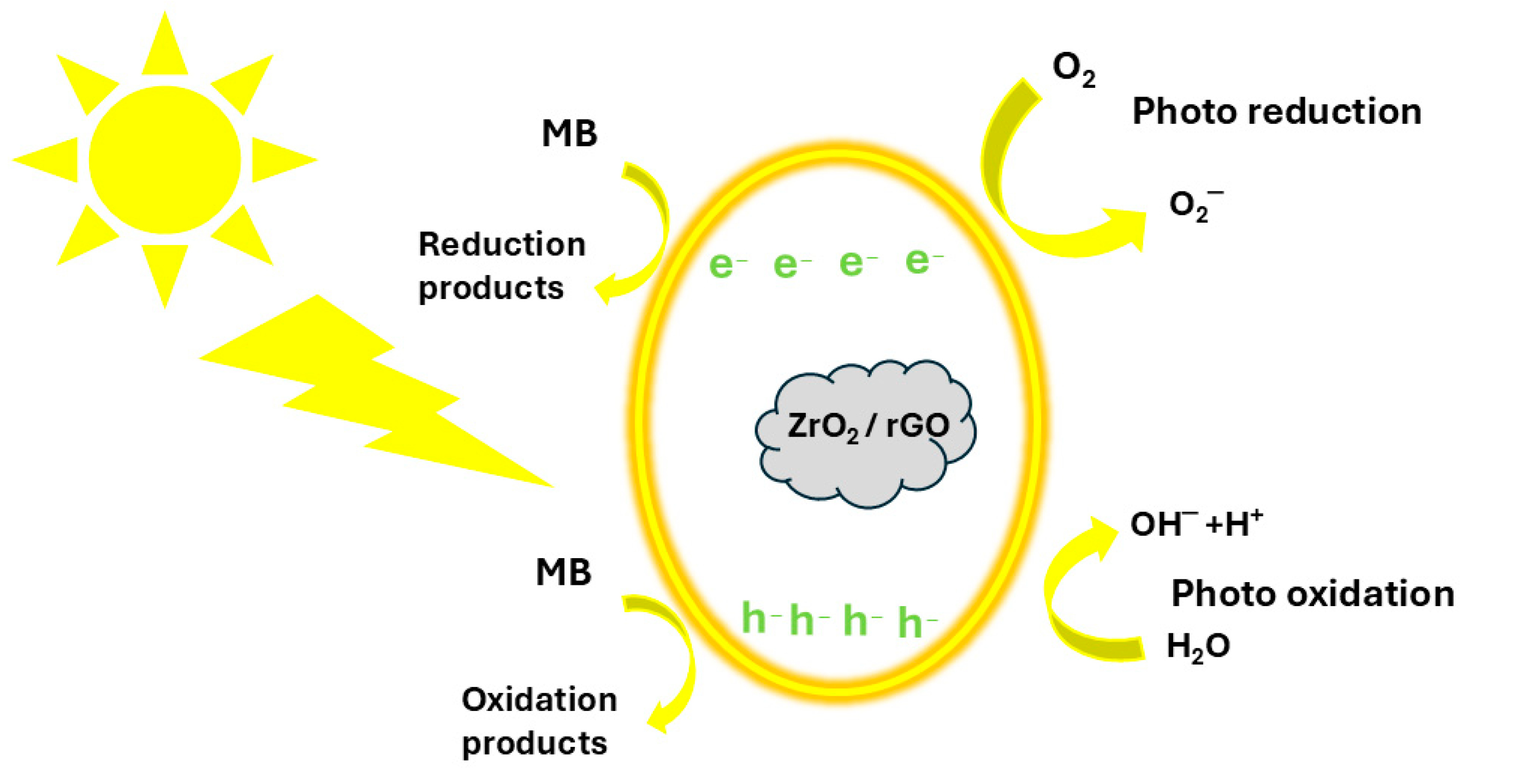
Photocatalytic Performance
3. Experimental Work
3.1. Preparation Technique of ZrO2
3.1.1. Reduced Graphene Preparation from PET Water Bottle Waste
3.1.2. Synthesis ZrO2@%RGO
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Wakawa, Y.M.; Li, J.; Akbour, R.A.; Yap, P.-S.; Lau, S.Y.; Jeevanandam, J. Recent developments in the utilization of modified graphene oxide to adsorb dyes from water: A review. J. Ind. Eng. Chem. 2023, 117, 21–37. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Almohammedi, A.; Alraddadi, S.; Taha, S.A.; Saad, M.; Sharaf, I.; Ismail, Y.A.M. Comparison study between as-synthesized ZnO and ZnO derived from ZiF-8 metalorganic framework in removing methylene blue. Mod. Phys. Lett. B 2024, 38, 2450221. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Rawindran, H.; Alam, M.M.; Leong, W.H.; Sahrin, N.T.; Ng, H.-S.; Chan, Y.J.; Abdelfattah, E.A.; Lim, J.W.; Aliyu, U.S.; et al. Mitigating persistent organic pollutants from marine plastics through enhanced recycling: A review. Environ. Res. 2023, 240, 117533. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Al-omoush, M.; Solayman, M.; Saad, H.M.H.; Khabiri, G.; Saad, M.; Alsulaim, G.M.; Soldatov, A.V.; Ismail, Y.A.M.; Gomaa, H. A heterostructural MoS2QDs@UiO-66 nanocomposite for the highly efficient photocatalytic degradation of methylene blue under visible light and simulated sunlight. RSC Adv. 2023, 13, 34598–34609. [Google Scholar] [CrossRef]
- Sakfali, J.; Chaabene, S.B.; Akkari, R.; Dappozze, F.; Berhault, G.; Guillard, C.; Zina, M.S. High photocatalytic activity of aerogel tetragonal and monoclinic ZrO2 samples. J. Photochem. Photobiol. A Chem. 2022, 430, 113970. [Google Scholar] [CrossRef]
- Simon, S.M.; George, G.; Sajna, M.S.; Prakashan, V.P.; Jose, T.A.; Vasudevan, P.; Saritha, A.C.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Recent advancements in multifunctional applications of sol-gel derived polymer incorporated TiO2-ZrO2 composite coatings: A comprehensive review. Appl. Surf. Sci. Adv. 2021, 6, 100173. [Google Scholar] [CrossRef]
- Rafieerad, A.; Amiri, A.; Sequiera, G.L.; Yan, W.; Chen, Y.; Polycarpou, A.A.; Dhingra, S. Development of fluorine-free tantalum carbide MXene hybrid structure as a biocompatible material for supercapacitor electrodes. Adv. Funct. Mater. 2021, 31, 2100015. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Radhika, D.; Nesaraj, A.S.; Sadasivuni, K.K.; Reddy, K.R.; Kasai, D.; Raghu, A.V. Photocatalytic, antibacterial and electrochemical properties of novel rare earth metal oxides-based nanohybrids. Mater. Sci. Energy Technol. 2020, 3, 853–861. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Wang, J.; Ren, D.; Li, B.-Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef]
- Da, Y.; Li, X.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Advanced characterization techniques for identifying the key active sites of gas-involved electrocatalysts. Adv. Funct. Mater. 2020, 30, 2001704. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. An overview of recent developments on semiconductor catalyst synthesis and modification used in photocatalytic reaction. Mater. Today Proc. 2020, 31, A151–A157. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liu, M.; Luo, H.; Deng, L.; Huang, L.; Wei, S.; Zhou, C.; Xu, Y. Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring. Nanomaterials 2019, 9, 906. [Google Scholar] [CrossRef]
- Sharaf, I.M.; Laifi, J.; Alraddadi, S.; Saad, M.; Koubesy, M.S.I.; Elewa, N.N.; Almohiy, H.; Ismail, Y.M.; Soldatov, A.; Aboraia, A.M. Unraveling the effect of Cu doping on the structural and morphological properties and photocatalytic activity of ZrO2. Heliyon 2024, 10, e23848. [Google Scholar] [CrossRef]
- Mishra, K.; Devi, N.; Siwal, S.S.; Gupta, V.K.; Thakur, V.K. Hybrid semiconductor photocatalyst nanomaterials for energy and environmental applications: Fundamentals, designing, and prospects. Adv. Sustain. Syst. 2023, 7, 2300095. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zhou, Q.; Liang, X.; Liu, D. Facile synthesis of novel gully-like double-sized mesoporous structural Sr-doped ZrO2–TiO2 composites with improved photocatalytic efficiency. J. Solid State Chem. 2019, 269, 375–385. [Google Scholar] [CrossRef]
- Wang, Z.; Nayak, P.K.; Caraveo-Frescas, J.A.; Alshareef, H.N. Recent developments in p-Type oxide semiconductor materials and devices. Adv. Mater. 2016, 28, 3831–3892. [Google Scholar] [CrossRef] [PubMed]
- Selvam, N.C.S.; Manikandan, A.; Kennedy, L.J.; Vijaya, J.J. Comparative investigation of zirconium oxide (ZrO2) nano and microstructures for structural, optical and photocatalytic properties. J. Colloid Interface Sci. 2013, 389, 91–98. [Google Scholar] [CrossRef]
- Gatti, T.; Lamberti, F.; Mazzaro, R.; Kriegel, I.; Schlettwein, D.; Enrichi, F.; Lago, N.; Di Maria, E.; Meneghesso, G.; Vomiero, A. Opportunities from doping of non-critical metal oxides in last generation light-conversion devices. Adv. Energy Mater. 2021, 11, 2101041. [Google Scholar] [CrossRef]
- Zhang, Y.; Jie, W.; Chen, P.; Liu, W.; Hao, J. Ferroelectric and piezoelectric effects on the optical process in advanced materials and devices. Adv. Mater. 2018, 30, 1707007. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis-and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-based nanomaterials: Recent developments in synthesis and applications. Nanoscale Adv. 2022, 4, 4210–4236. [Google Scholar] [CrossRef]
- Batool, S.S.; Saleem, R.; Khan, R.R.M.; Saeed, Z.; Pervaiz, M.; Summer, M. Enhancing photocatalytic performance of zirconia-based nanoparticles: A comprehensive review of factors, doping strategies, and mechanisms. Mater. Sci. Semicond. Process. 2024, 178, 108419. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Porcu, S.; Secci, F.; Ricci, P.C. Advances in hybrid composites for photocatalytic applications: A review. Molecules 2022, 27, 6828. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Gurushantha, K.; Anantharaju, K.S.; Renuka, L.; Sharma, S.C.; Nagaswarupa, H.P.; Prashantha, S.C.; Vidya, Y.S.; Nagabhushana, H. New green synthesized reduced graphene oxide–ZrO2 composite as high performance photocatalyst under sunlight. RSC Adv. 2017, 7, 12690–12703. [Google Scholar] [CrossRef]
- Li, Z.; Li, K.; Du, P.; Mehmandoust, M.; Karimi, F.; Erk, N. Carbon-based photocatalysts for hydrogen production: A review. Chemosphere 2022, 308, 135998. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Elhakeem, A.; Kaytbay, S.; Ahmed, A. A comprehensive review on the thermal, electrical, and mechanical properties of graphene-based multi-functional epoxy composites. Adv. Compos. Hybrid Mater. 2022, 5, 547–605. [Google Scholar] [CrossRef]
- Sambyal, S.; Sharma, R.; Mandyal, P.; Balou, S.; Gholami, P.; Fang, B.; Shandilya, P.; Priye, A. Advancement in two-dimensional carbonaceous nanomaterials for photocatalytic water detoxification and energy conversion. J. Environ. Chem. Eng. 2023, 11, 109517. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Yu, J.; Zhu, Y.; Lin, K.; Wang, B.; Cai, R.; Ekren, D.; Lewis, D.; Kinloch, I.A. Enhancing the thermoelectric properties of TiO2-based ceramics through addition of carbon black and graphene oxide. Carbon 2024, 216, 118509. [Google Scholar] [CrossRef]
- Purohit, S.; Yadav, K.L.; Satapathi, S. Metal halide perovskite heterojunction for photocatalytic hydrogen generation: Progress and future opportunities. Adv. Mater. Interfaces 2022, 9, 2200058. [Google Scholar] [CrossRef]
- Singh, B.R.; Shoeb, M.; Khan, W.; Naqvi, A.H. Synthesis of graphene/zirconium oxide nanocomposite photocatalyst for the removal of rhodamineB dye from aqueous environment. J. Alloys Compd. 2015, 651, 598–607. [Google Scholar] [CrossRef]
- Sikdar, S.; Banu, A.; Ali, S.; Barman, S.; Kalar, P.L.; Das, R. Micro-structural Analysis and Photocatalytic Properties of Green Synthesized t-ZrO2 Nanoparticles. ChemistrySelect 2022, 7, e202103953. [Google Scholar] [CrossRef]
- Shinde, H.M.; Bhosale, T.T.; Gavade, N.L.; Babar, S.B.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of ZrO2 nanoparticles from Ficus benghalensis leaf extract for photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14055–14064. [Google Scholar] [CrossRef]
- Dhandapani, C.; Narayanasamy, R.; Karthick, S.N.; Hemalatha, K.V.; Selvam, S.; Hemalatha, P.; Kumar, M.S.; Kirupha, S.D.; Kim, H.J. Drastic photocatalytic degradation of methylene blue dye by neodymium doped zirconium oxide as photocatalyst under visible light irradiation. Optik 2016, 127, 10288–10296. [Google Scholar] [CrossRef]
- Aljawrneh, B.; Ocak, Y.S.; Albiss, B.A.; Dwiri, A.; Tawalbeh, M.; Al-Othman, A. ZrO2 nanoparticles for effective dye degradation in wastewater: Synthesis, characterization, and photocatalytic performance under sunlight. J. Alloys Compd. 2024, 1008, 176522. [Google Scholar] [CrossRef]








| Catalyst | Synthesis Method | Dye | Irradiation Source | Efficiency, % | References |
|---|---|---|---|---|---|
| t-ZrO2 | Green method | MB | UV light | 68 | [38] |
| ZrO2 | Biogenic | MO | UV light | 69 | [39] |
| Nd-ZrO2 | Polymer assisted | MB | Visible light | 68 | [40] |
| ZrO2 NPs | Hydrothermal | MB | Sunlight | 80 | [41] |
| ZrO2 | Sol–gel combustion | MB | Sunlight | 65 | Our work |
| ZrO2@4%RGO | Sol–gel combustion | MB | Sunlight | 82 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farghly, N.; Abu El-Oyoun, M.; Abousehly, A.; Alkallas, F.H.; Trabelsi, A.B.G.; Shaaban, E.R.; Aboraia, A.M. Investigating the Photocatalytic Properties of Reduced Graphene Oxide-Coated Zirconium Dioxide and Their Impact on Structural and Morphological Features. Catalysts 2025, 15, 289. https://doi.org/10.3390/catal15030289
Farghly N, Abu El-Oyoun M, Abousehly A, Alkallas FH, Trabelsi ABG, Shaaban ER, Aboraia AM. Investigating the Photocatalytic Properties of Reduced Graphene Oxide-Coated Zirconium Dioxide and Their Impact on Structural and Morphological Features. Catalysts. 2025; 15(3):289. https://doi.org/10.3390/catal15030289
Chicago/Turabian StyleFarghly, Norhan, M. Abu El-Oyoun, A. Abousehly, Fatemah H. Alkallas, Amira Ben Gouider Trabelsi, E. R. Shaaban, and Abdelaziz Mohamed Aboraia. 2025. "Investigating the Photocatalytic Properties of Reduced Graphene Oxide-Coated Zirconium Dioxide and Their Impact on Structural and Morphological Features" Catalysts 15, no. 3: 289. https://doi.org/10.3390/catal15030289
APA StyleFarghly, N., Abu El-Oyoun, M., Abousehly, A., Alkallas, F. H., Trabelsi, A. B. G., Shaaban, E. R., & Aboraia, A. M. (2025). Investigating the Photocatalytic Properties of Reduced Graphene Oxide-Coated Zirconium Dioxide and Their Impact on Structural and Morphological Features. Catalysts, 15(3), 289. https://doi.org/10.3390/catal15030289










