Sulfur-Doped CoFe/NF Catalysts for High-Efficiency Electrochemical Urea Oxidation and Hydrogen Production: Structure Optimization and Performance Enhancement
Abstract
1. Introduction
2. Results and Discussion
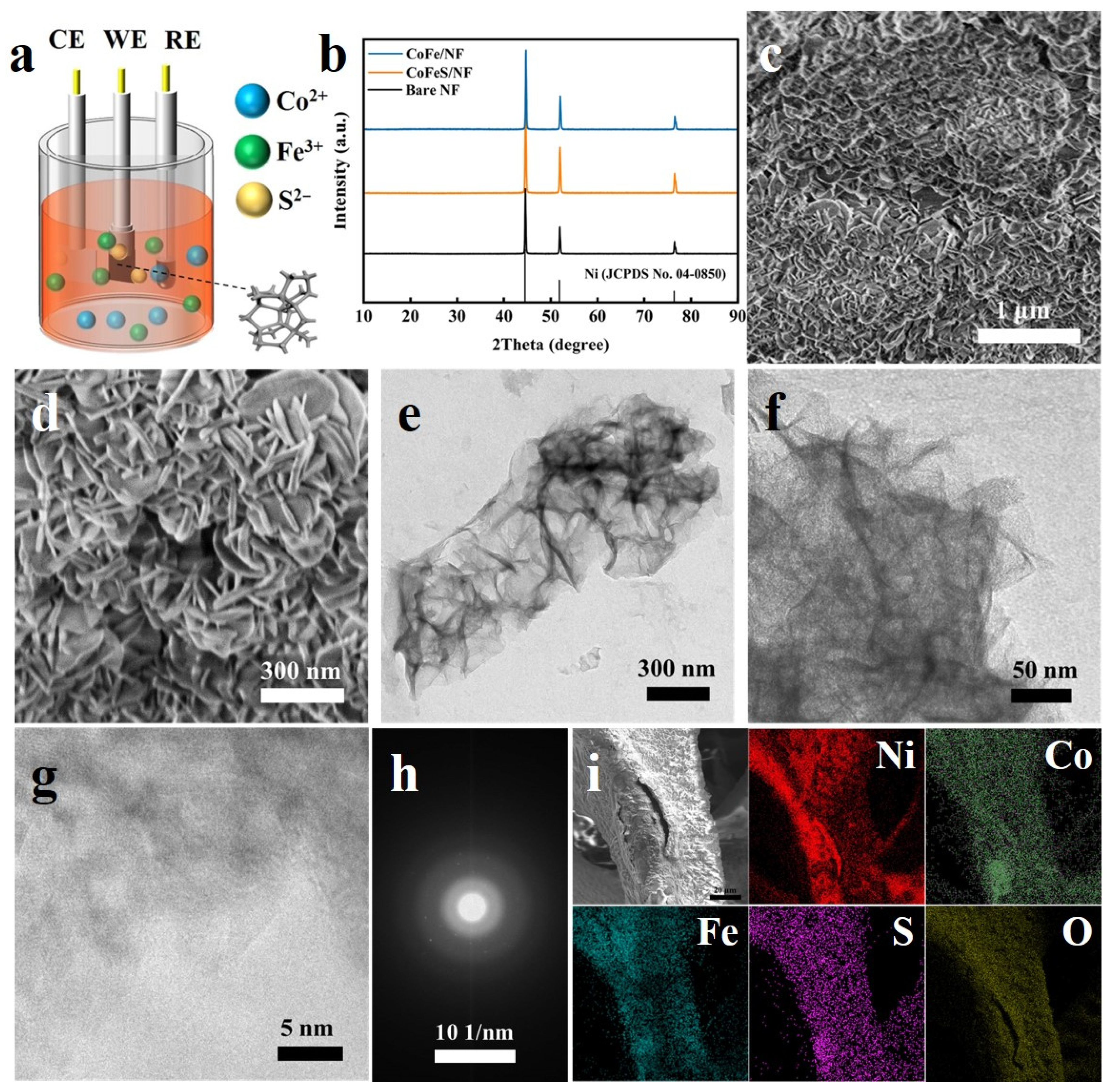
2.1. Characterization of Samples
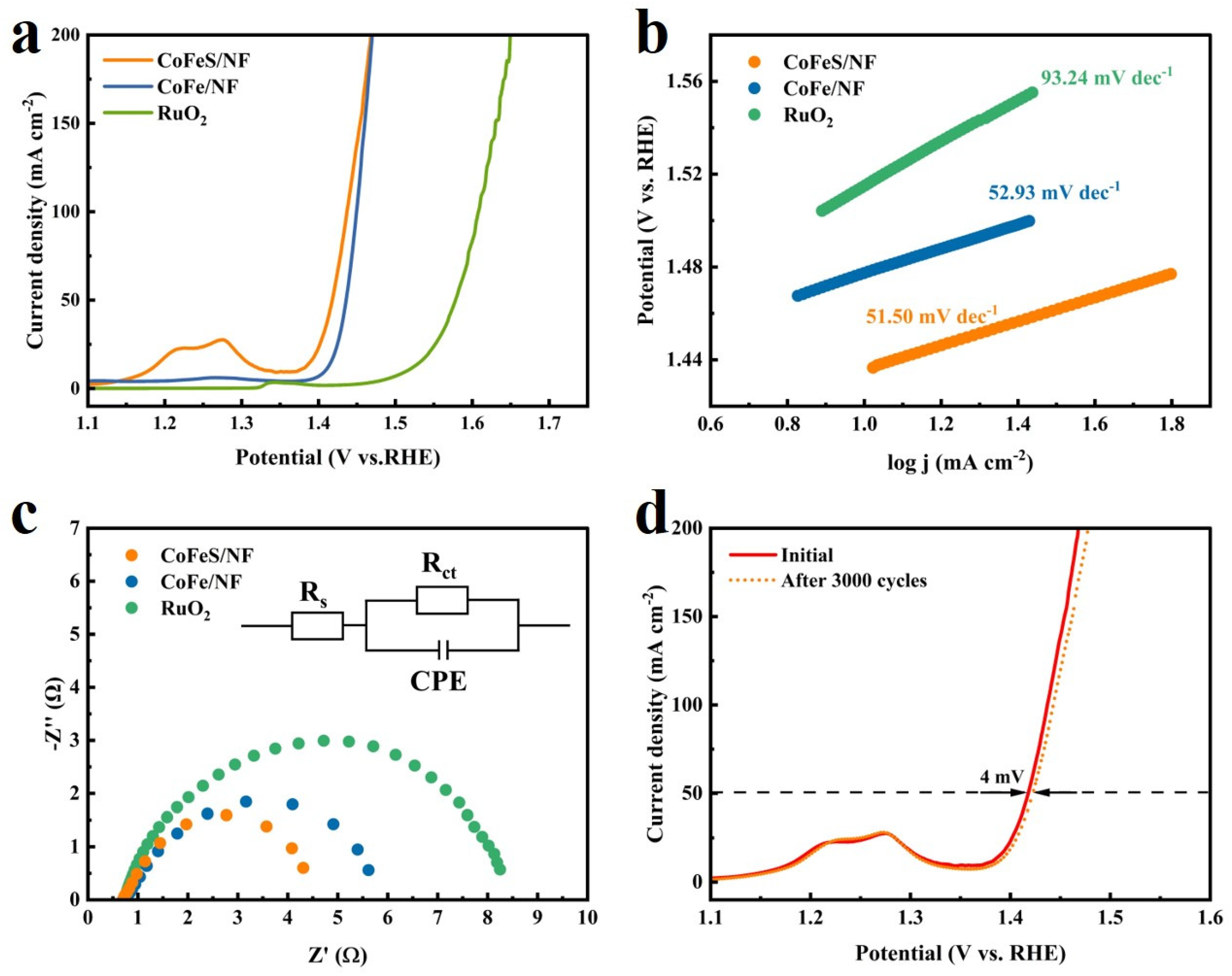
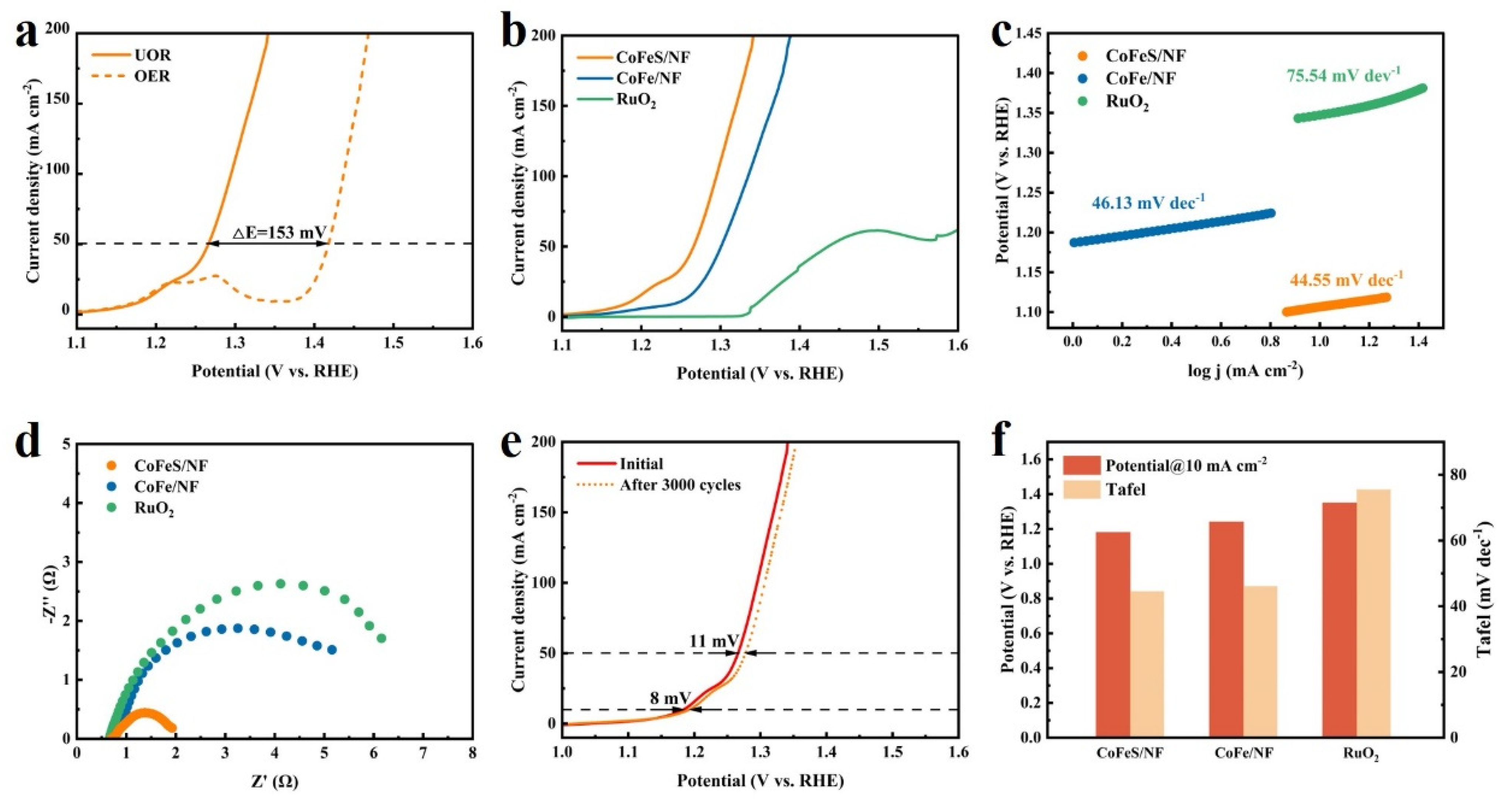
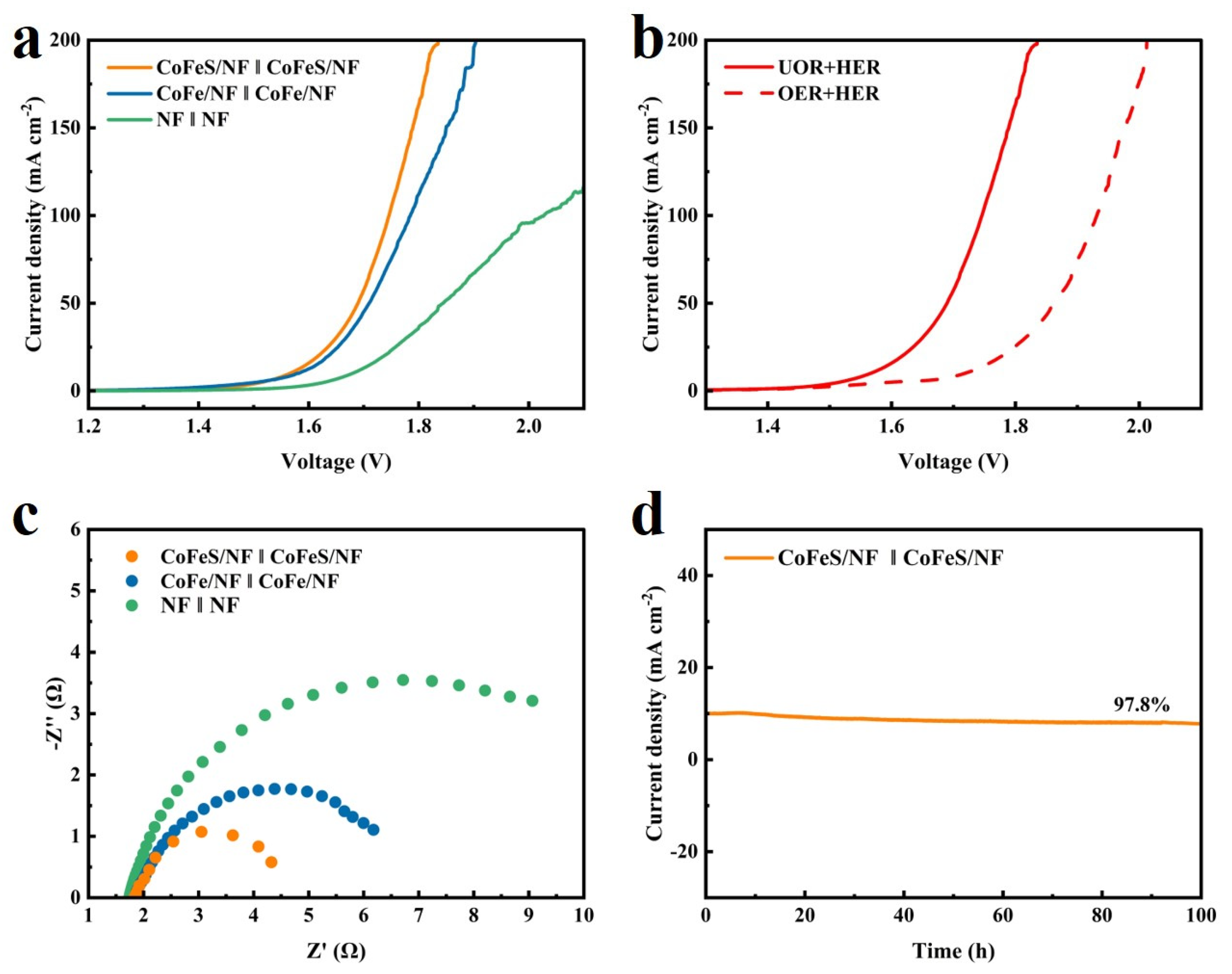
2.2. Electrochemical Testing of Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of CoFe/NF and CoFeS/NF
3.3. Preparation of Pt/C/NF and RuO2/NF
3.4. Characterization of Materials
3.5. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akadiri, A.C.; Saint Akadiri, S.; Gungor, H. The role of natural gas consumption in Saudi Arabia’s output and its implication for trade and environmental quality. Energy Policy 2019, 129, 230–238. [Google Scholar] [CrossRef]
- Child, M.; Koskinen, O.; Linnanen, L.; Breyer, C. Sustainability guardrails for energy scenarios of the global energy transition. Renew. Sustain. Energy Rev. 2018, 91, 321–334. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Merge, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, L.J.; Sun, W.P.; Chen, Y.; Yu, H.; Li, K.K.; Jia, B.H.; Zhang, L.; Ma, T.Y. Electrocatalytic Water Splitting: From Harsh and Mild Conditions to Natural Seawater. Small 2022, 18, 2105830. [Google Scholar] [CrossRef]
- Shi, L.N.; Cui, L.T.; Ji, Y.R.; Xie, Y.; Zhu, Y.R.; Yi, T.F. Towards high-performance electrocatalysts: Activity optimization strategy of 2D MXenes-based nanomaterials for water-splitting. Coord. Chem. Rev. 2022, 469, 214668. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. Energy 2023, 325, 122388. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Ma, X.; Shen, Y.; Deng, Q.; Zhang, W.; Zhao, Y. Hydrogen Production from Urea Sewage on NiFe-Based Porous Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, J.; Meng, G.; Wu, T.; Chen, C.; Tian, H.; Chen, Y.; Kong, F.; Chang, Z.; Cui, X.; et al. Active site recovery and N-N bond breakage during hydrazine oxidation boosting the electrochemical hydrogen production. Nat. Commun. 2023, 14, 1997. [Google Scholar] [CrossRef]
- Qiao, L.L.; Zhu, A.Q.; Liu, D.; Feng, J.X.; Chen, Y.Y.; Chen, M.P.; Zhou, P.F.; Yin, L.H.; Wu, R.C.; Ng, K.W.; et al. Crystalline phosphides/amorphous oxides composite for energy-saving hydrogen production assisted by efficient urea oxidation reaction. Chem. Eng. J. 2022, 454, 140380. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Dai, X.F.; Wang, Y.P.; Ji, X.Y.; Ma, Z.; Liu, S.Q. The polyoxometalates mediated preparation of phosphate-modified NiMoO4-x with abundant O-vacancies for H2 production via urea electrolysis. J. Colloid Interface Sci. 2022, 629, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Shrestha, N.K.; Inamdar, A.I.; Bathula, C.; Jung, J.; Hussain, S.; Nazir, G.; Kaseem, M.; Im, H.; Kim, H. Bimetallic Cu/Fe MOF-Based Nanosheet Film via Binder-Free Drop-Casting Route: A Highly Efficient Urea-Electrolysis Catalyst. Nanomaterials 2022, 12, 1916. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, M.S.; Zhu, J.; Yang, S.D.; Chen, L.; Yang, X.L.; Wang, P.; Li, K.; Xue, F.N.; Lu, Y.; et al. New strategy to synthesize oxygen vacancy-rich CoFe nanoneedles for overall water splitting and urea electrolysis. Chem. Eng. J. 2021, 432, 134275. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Wu, D.; Chen, L.; Chen, S.; Wan, H.; Chen, G.; Zhang, N.; Liu, X.H.; Ma, R.Z. Collaborative optimization of thermodynamic and kinetic for Ni-based hydroxides in electrocatalytic urea oxidation reaction. Appl. Catal. B Environ. 2023, 340, 123214. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, M.Z.; Wu, S.L.; Huang, B.L.; Lee, C.S.; Zhang, W.J. Oxygen-Incorporated NiMoP Nanotube Arrays as Efficient Bifunctional Electrocatalysts For Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte. Adv. Funct. Mater. 2021, 31, 2104951. [Google Scholar] [CrossRef]
- Huang, C.J.; Xu, H.M.; Shuai, T.Y.; Zhan, Q.N.; Zhang, Z.J.; Li, G.R. Modulation Strategies for the Preparation of High-Performance Catalysts for Urea Oxidation Reaction and Their Applications. Small 2023, 19, 2301130. [Google Scholar] [CrossRef]
- Diao, Y.X.; Liu, Y.S.; Hu, G.X.; Zhao, Y.Y.; Qian, Y.H.; Wang, H.D.; Shi, Y.; Li, Z. NiFe nanosheets as urea oxidation reaction electrocatalysts for urea removal and energy-saving hydrogen production. Biosens. Bioelectron. 2022, 211, 114380. [Google Scholar] [CrossRef]
- Ge, J.H.; Kuang, J.E.; Xiao, Y.H.; Guan, M.H.; Yang, C.Z. Recent development of nickel-based catalysts and in situ characterization techniques for mechanism understanding of the urea oxidation reaction. Surf. Interfaces 2023, 41, 103230. [Google Scholar] [CrossRef]
- Huang, C.J.; Zhan, Q.N.; Xu, H.M.; Zhu, H.R.; Shuai, T.Y.; Li, G.R. Fe-Doped Ni2P/NiSe2 Composite Catalysts for Urea Oxidation Reaction (UOR) for Energy-Saving Hydrogen Production by UOR-Assisted Water Splitting. Inorg. Chem. 2024, 63, 8925–8937. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, H.M.; Tian, Y.F.; Yan, Y.; Xue, Q.; He, T.; Liu, H.F.; Wang, C.D.; Chen, Y.; Xia, B.Y. Anodic hydrazine oxidation assists energy-efficient hydrogen evolution over a bifunctional cobalt perselenide nanosheet electrode. Angew. Chem. Int. Ed. 2018, 57, 7649–7653. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhao, L.Y.; Li, J.X.; Tian, X.L.; Wu, X.; Feng, L.G. High valence state of Ni and Mo synergism in NiS2-MoS2 hetero-nanorods catalyst with layered surface structure for urea electrocatalysis. J. Energy Chem. 2021, 66, 483–492. [Google Scholar] [CrossRef]
- Liang, Y.H.; Liu, Q.; Asiri, A.M.; Sun, X.P. Enhanced electrooxidation of urea using NiMoO4·xH2O nanosheet arrays on Ni foam as anode. Electrochim. Acta 2014, 153, 456–460. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Lang, C.C.; Gao, M.R.; Chen, Y.; Fu, Q.Q.; Duan, Y.; Yu, S.H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Zhuo, X.Y.; Jiang, W.J.; Yu, T.Q.; Qian, G.F.; Chen, J.L.; Yang, H.F.; Yin, S.B. Crystalline-Amorphous Ni3S2-NiMoO4 Heterostructure for Durable Urea Electrolysis-Assisted Hydrogen Production at High Current Density. ACS Appl. Mater. Interfaces 2022, 14, 46481–46490. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, Y.M.; Qian, G.F.; Yu, T.Q.; Wang, Z.L.; Luo, L.; Shen, F.; Yin, S.B. In Situ Growth of Volcano-like FeIr Alloy on Nickel Foam as Efficient Bifunctional Catalyst for Overall Water Splitting at High Current Density. Chem. Eng. J. 2021, 421, 129892. [Google Scholar] [CrossRef]
- Wang, Z.J.; Guo, P.; Liu, M.; Guo, C.; Liu, H.J.; Wei, S.X.; Zhang, J.; Lu, X.Q. Rational Design of Metallic NiTex (x = 1 or 2) as Bifunctional Electrocatalysts for Efficient Urea Conversion. ACS Appl. Energy Mater. 2019, 2, 3363–3372. [Google Scholar] [CrossRef]
- Zhu, X.J.; Dou, X.Y.; Dai, J.; An, X.D.; Guo, Y.Q.; Zhang, L.D.; Tao, S.; Zhao, J.Y.; Chu, W.S.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 12465–12469. [Google Scholar] [CrossRef]
- Ren, H.N.; Yu, L.X.; Yang, L.P.; Huang, Z.H.; Kang, F.Y.; Lv, R.T. Efficient electrocatalytic overall water splitting and structural evolution of cobalt iron selenide by one-step electrodeposition. J. Energy Chem. 2021, 60, 194–201. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. Tailoring the bifunctional electrocatalytic activity of electrodeposited molybdenum sulfide/iron oxide heterostructure to achieve excellent overall water splitting. Chem. Eng. J. 2021, 417, 129333. [Google Scholar] [CrossRef]
- Liu, S.S.; Ma, L.J.; Li, J.S. Facile preparation of amorphous NiFe hydroxide by corrosion engineering for electrocatalytic water and urea oxidation. J. Alloys Compd. 2022, 936, 168271. [Google Scholar] [CrossRef]
- Song, Y.L.; Huang, J.L.; Tang, C.L.; Wang, T.; Liu, Y.S.; He, X.S.; Xie, C.P.; Chen, G.; Deng, C.F.; He, Z.B. Improved Urea Oxidation Performance via Interface Electron Redistributions of the NiFe(OH)x/MnO2/NF p-p Heterojunction. Small 2024, 20, 2403612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, C.Q.; Kuai, C.G.; Sokaras, D.; Zheng, X.L.; Sainio, S.; Lin, F.; Dong, C.K.; Nordlund, D.; Du, X.W. Unveiling the critical role of the Mn dopant in a NiFe(OH)2 catalyst for water oxidation. J. Mater. Chem. A 2020, 8, 17471–17476. [Google Scholar] [CrossRef]
- Li, Y.L.; Jia, B.M.; Chen, B.Y.; Liu, Q.L.; Cai, M.K.; Xue, Z.Q.; Fan, Y.N.; Wang, H.P.; Su, C.Y.; Li, G.Q. MOF-derived Mn doped porous CoP nanosheets as efficient and stable bifunctional electrocatalysts for water splitting. Dalton Trans. 2018, 47, 14679–14685. [Google Scholar] [CrossRef]
- Luo, M.; Liu, S.Q.; Zhu, W.W.; Ye, G.Y.; Wang, J.; He, Z. An electrodeposited MoS2-MoO3-x/Ni3S2 heterostructure electrocatalyst for efficient alkaline hydrogen evolution. Chem. Eng. J. 2021, 428, 131055. [Google Scholar] [CrossRef]
- Qin, H.Y.; Ye, Y.K.; Li, J.H.; Jia, W.Q.; Zheng, S.Y.; Cao, X.J.; Lin, G.L.; Jiao, L.F. Synergistic engineering of doping and vacancy in Ni(OH)2 to boost urea electrooxidation. Adv. Funct. Mater. 2022, 33, 2209698. [Google Scholar] [CrossRef]
- Yi, X.; He, X.; Yin, F.; Chen, B.; Li, G.; Yin, H. Amorphous Ni-Fe-Se hollow nanospheres electrodeposited on nickel foam as a highly active and bifunctional catalyst for alkaline water splitting. Dalton Trans. 2020, 49, 6764–6775. [Google Scholar] [CrossRef]
- Li, S.R.; Zhang, Y.P.; Yu, X.; Wang, Z.L.; Zhang, G.F.; Zhao, Z.Y.; Yan, Z.Y.; Xiao, X.C. One-step electrodeposition synthesis of amorphous NiCoFe(OH)x/NF as an efficient catalyst for urea-assisted overall water splitting. Electrochim. Acta 2023, 463, 142803. [Google Scholar] [CrossRef]
- Yao, L.C.; Zhang, H.M.; Humayun, M.; Fu, Y.J.; Xu, X.F.; Feng, C.D.; Wang, C.D. Constructing Nanoporous Crystalline/Amorphous NiFe2O4/NiO Electrocatalyst for High Efficiency OER/UOR. J. Alloys Compd. 2022, 936, 168206. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, S.R.; Zhang, G.F.; Yu, X.; Shi, Y.; Zhang, Y.P.; Xiao, X.C. Facile synthesis of FeCoW oxides: Effects of amorphous structure, electronic configuration and catalytic sites on water oxidation. J. Alloys Compd. 2022, 933, 167787. [Google Scholar] [CrossRef]
- Mosallaei, H.; Hadadzadeh, H.; Ensafi, A.A.; Mousaabadi, K.Z.; Weil, M.; Foelske, A.; Sauer, M. Evaluation of HER and OER electrocatalytic activity over RuO2–Fe2O3 nanocomposite deposited on HrGO nanosheets. Int. J. Hydrogen Energy 2022, 48, 1813–1830. [Google Scholar] [CrossRef]
- Raja, A.; Son, N.; Swaminathan, M.; Kang, M. Electrochemical behavior of heteroatom doped on reduced graphene oxide with RuO2 for HER, OER, and supercapacitor applications. J. Taiwan Inst. Chem. Eng. 2022, 138, 104471. [Google Scholar] [CrossRef]
- He, W.; Zhang, R.; Liu, H.; Hao, Q.; Li, Y.; Zheng, X.; Liu, C.; Zhang, J.; Xin, H. Atomically Dispersed Silver Atoms Embedded in NiCo Layer Double Hydroxide Boost Oxygen Evolution Reaction. Small 2023, 19, 2301610. [Google Scholar] [CrossRef]
- Ren, L.P.; Yang, D.; Li, J.Q.; Li, H.S.; Yang, J.H. Nitrogen doped carbon fiber supported nickel phosphide for efficient electrocatalytic overall urea splitting. Appl. Surf. Sci. 2023, 624, 157173. [Google Scholar] [CrossRef]
- Xie, H.; Feng, Y.F.; He, X.Y.; Zhu, Y.; Li, Z.Y.; Liu, H.H.; Zeng, S.Y.; Qian, Q.Z.; Zhang, G.Q. Construction of Nitrogen-Doped Biphasic Transition-Metal Sulfide Nanosheet Electrode for Energy-Efficient Hydrogen Production via Urea Electrolysis. Small 2022, 19, 2207425. [Google Scholar] [CrossRef]
- Shamloofard, M.; Shahrokhian, S. Morphology Modulation and Phase Transformation of Manganese-Cobalt Carbonate Hydroxide Caused by Fluoride Doping and Its Effect on Boosting the Overall Water Electrolysis. Inorg. Chem. 2023, 62, 1178–1191. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.; Lu, S.; Dong, B.; Gao, W.; Chi, J.; Liu, Z.; Chai, Y.; Liu, C. Controlling electrodeposited ultrathin amorphous Fe hydroxides film on V-doped nickel sulfide nanowires as efficient electrocatalyst for water oxidation. J. Power Sources 2017, 363, 44–53. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3-δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Li, Q.; Chen, B.; Huang, L.; Zhu, S.; Qian, Y.; Wu, D.; Luo, S.; Xie, A. S-doped Ni(Fe)OOH bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2023, 51, 1392–1406. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, L.P.; Lin, H.P.; Liu, Y.X.; Ye, J.Y.; Wen, Y.Z.; Chen, A.; Wang, L.; Ni, F.L.; Zhou, Z.Y.; et al. A Lattice-Oxygen-Involved Reaction Pathway to Boost Urea Oxidation. Angew. Chem. Int. Ed. 2019, 58, 16820–16825. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Q.; Xu, Q.L.; Chen, J.L.; Qian, G.F.; Zhuo, X.Y.; Yang, H.F.; Yin, S.B. Boosting urea-assisted water splitting by constructing sphere-flower-like NiSe2-NiMoO4 heterostructure. Chem. Eng. J. 2022, 449, 137791. [Google Scholar] [CrossRef]
- Xu, H.; Liao, Y.; Gao, Z.F.; Qing, Y.; Wu, Y.Q.; Xia, L.Y. A branch-like Mo-doped Ni3S2 nanoforest as a high-efficiency and durable catalyst for overall urea electrolysis. J. Mater. Chem. A 2021, 9, 3418–3426. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Zakharov, V.; Kuznetsova, I.; Aslanov, L.; Kustov, L. Triazine derivatives as metal-free electrocatalysts: Do three nitrogen atoms mimic a metal? Sustain. Energy Fuels 2024, 9, 1464–1479. [Google Scholar] [CrossRef]
- Li, J.N.; Li, J.L.; Liu, T.; Chen, L.; Li, Y.F.; Wang, H.L.; Chen, X.R.; Gong, M.; Liu, Z.P.; Yang, X.J. Deciphering and suppressing over-oxidized nitrogen in nickel-catalyzed urea electrolysis. Angew. Chem. Int. Ed. 2021, 60, 26656–26662. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhang, H.Y.; Miao, J.H.; Hu, F.X.; Wang, L.; Tang, Y.J.; Qiao, M.; Guo, C.X. Strategies for designing more efficient electrocatalysts towards the urea oxidation reaction. J. Mater. Chem. A 2022, 10, 3296–3313. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Kordek, K.; Yin, H.J.; Rutkowski, P.; Zhao, H.J. Cobalt-based composite films on electrochemically activated carbon cloth as high performance overall water splitting electrodes. Int. J. Hydrogen Energy 2018, 44, 23–33. [Google Scholar] [CrossRef]
- Lai, Y.Q.; Li, Y.; Jiang, L.X.; Xu, W.; Lv, X.J.; Li, J.; Liu, Y.X. Electrochemical behaviors of co-deposited Pb/Pb-MnO2 composite anode in sulfuric acid solution—Tafel and EIS investigations. J. Electroanal. Chem. 2012, 671, 16–23. [Google Scholar] [CrossRef]
- Wu, J.; Yu, Z.J.; Zhang, Y.Y.; Niu, S.Q.; Zhao, J.Y.; Li, S.W.; Xu, P. Understanding the effect of second metal on CoM (M = Ni, Cu, Zn) metal-organic frameworks for electrocatalytic oxygen evolution reaction. Small 2021, 17, 2105150. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; He, W.; Yin, D.D.; Xie, Y.R.; Zhang, H.L.; Ma, Q.L.; Yu, W.S.; Yang, Y.; Dong, X.T. Achieving Efficient Urea Electrolysis by Spatial Confinement Effect and Heterostructure. Chem. Eng. J. 2023, 462, 142254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yao, L.; Wang, Z.; Xu, Z.; Xiao, X. Sulfur-Doped CoFe/NF Catalysts for High-Efficiency Electrochemical Urea Oxidation and Hydrogen Production: Structure Optimization and Performance Enhancement. Catalysts 2025, 15, 285. https://doi.org/10.3390/catal15030285
Li S, Yao L, Wang Z, Xu Z, Xiao X. Sulfur-Doped CoFe/NF Catalysts for High-Efficiency Electrochemical Urea Oxidation and Hydrogen Production: Structure Optimization and Performance Enhancement. Catalysts. 2025; 15(3):285. https://doi.org/10.3390/catal15030285
Chicago/Turabian StyleLi, Sirong, Lang Yao, Zhenlong Wang, Zhonghe Xu, and Xuechun Xiao. 2025. "Sulfur-Doped CoFe/NF Catalysts for High-Efficiency Electrochemical Urea Oxidation and Hydrogen Production: Structure Optimization and Performance Enhancement" Catalysts 15, no. 3: 285. https://doi.org/10.3390/catal15030285
APA StyleLi, S., Yao, L., Wang, Z., Xu, Z., & Xiao, X. (2025). Sulfur-Doped CoFe/NF Catalysts for High-Efficiency Electrochemical Urea Oxidation and Hydrogen Production: Structure Optimization and Performance Enhancement. Catalysts, 15(3), 285. https://doi.org/10.3390/catal15030285





