Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications
Abstract
1. Introduction
2. Results
2.1. Optical, Structural, and Morphological Properties of CuO NPs Derived from Durian Husk Extract
2.1.1. UV-Vis Spectroscopy
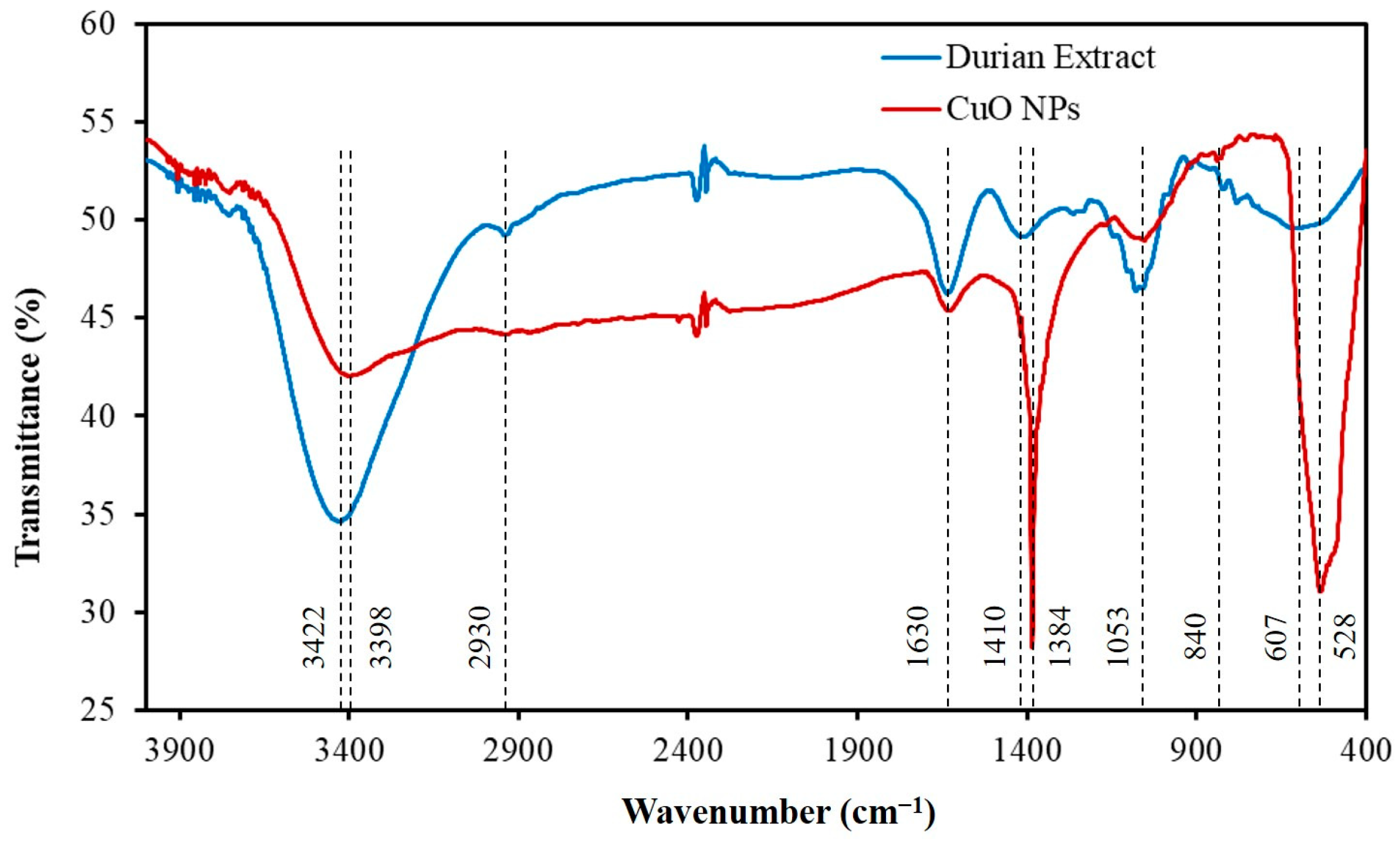
2.1.2. FTIR Spectroscopy
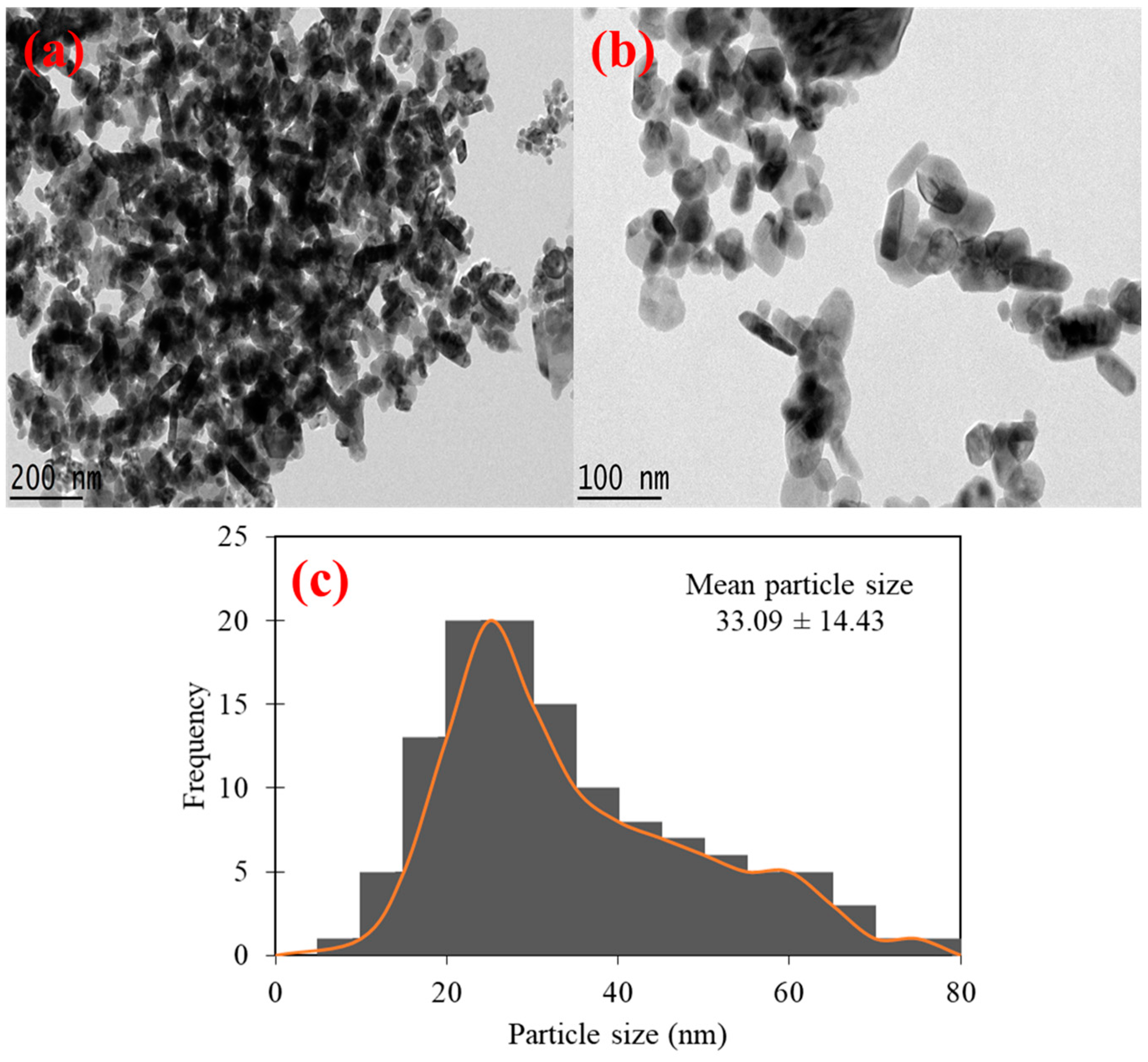
2.1.3. Morphological Analysis by SEM and HR-TEM
2.1.4. XRD Analysis
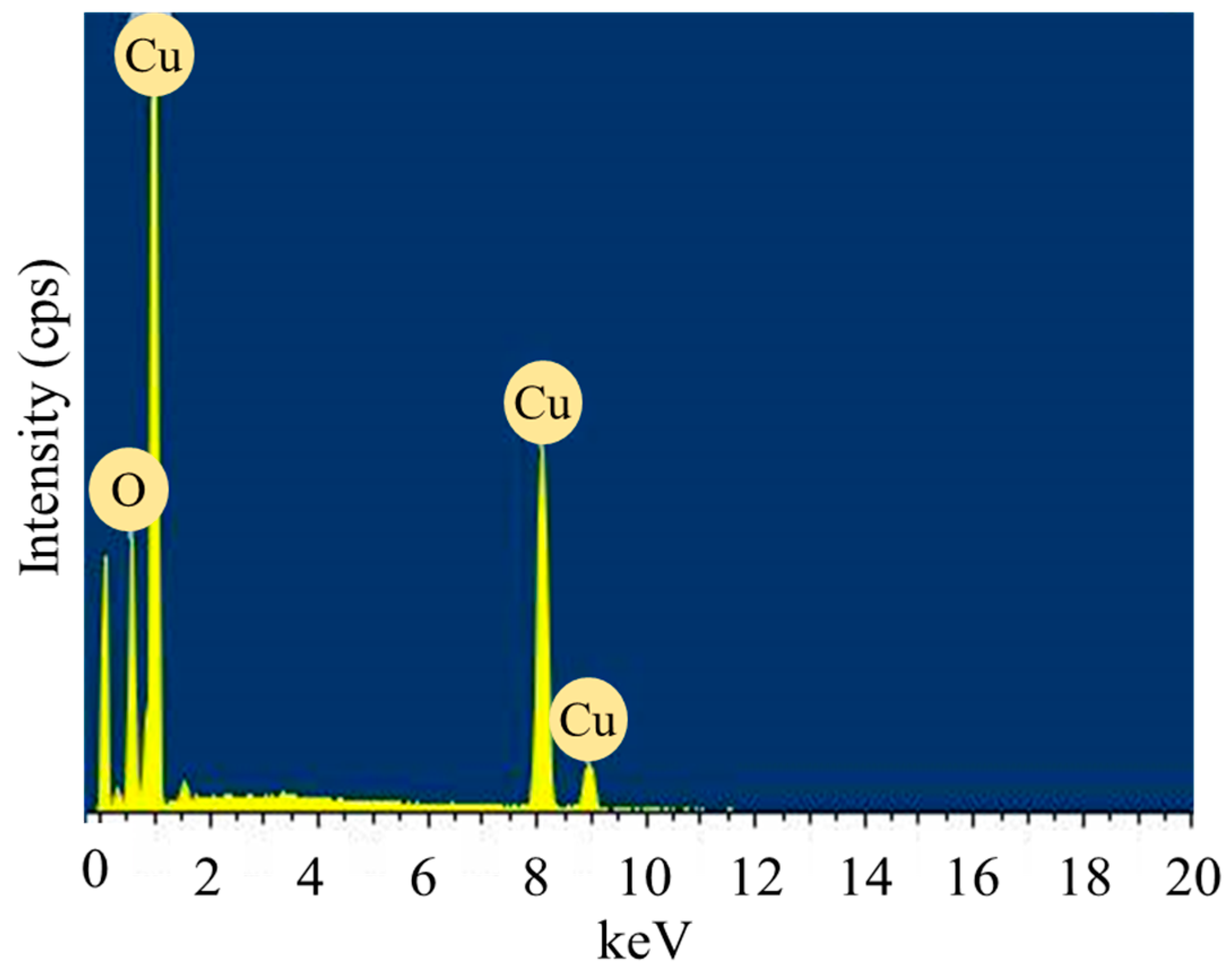
2.1.5. EDX Analysis
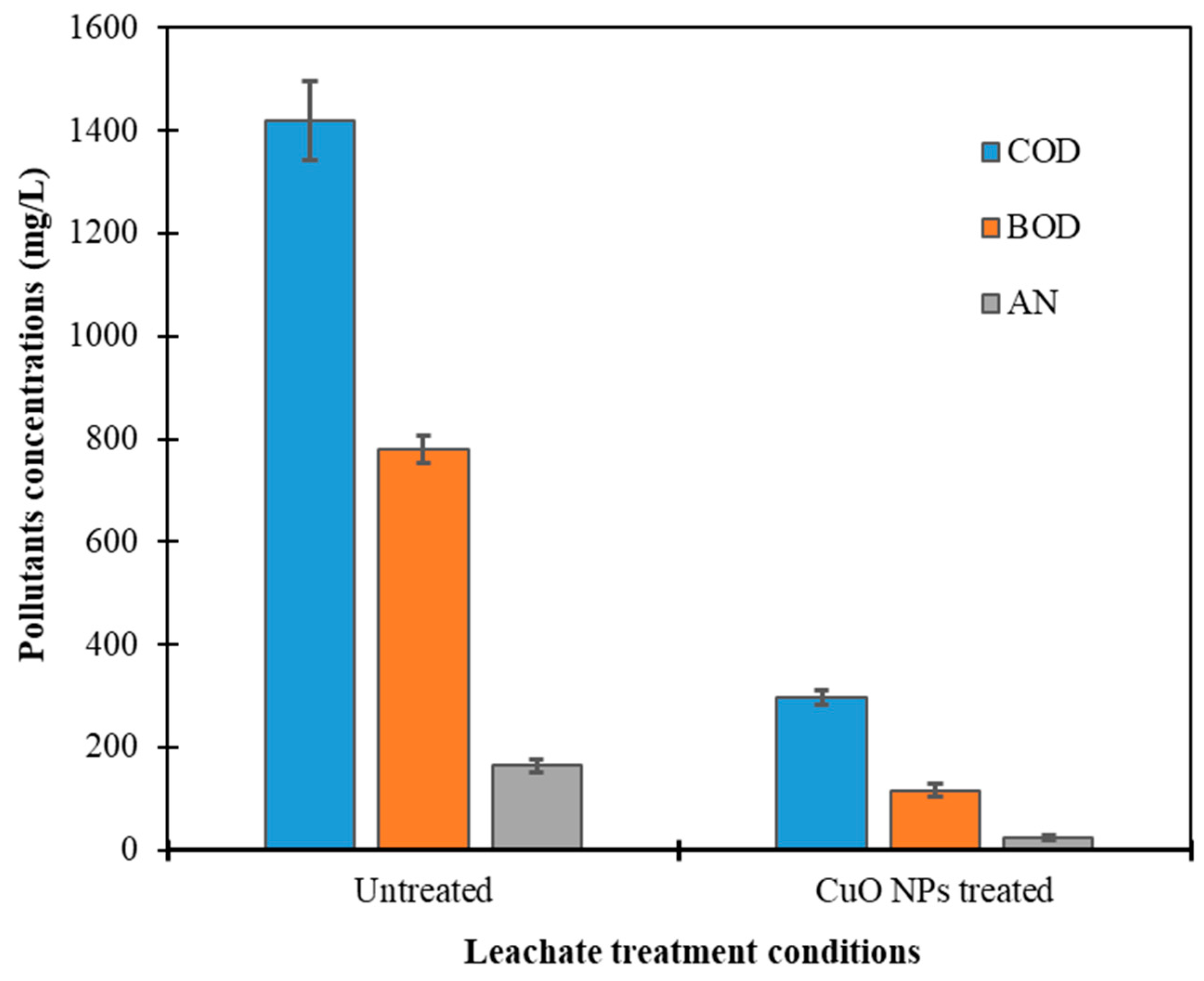
2.2. Photocatalytic Activity of CuO Nanoparticles in Landfill Leachate Treatment
2.2.1. Reduction of Chemical Oxygen Demand (COD)
2.2.2. Removal of Biochemical Oxygen Demand (BOD)
2.2.3. Ammoniacal Nitrogen Removal
3. Discussion
3.1. Optical Properties and Bandgap Analysis
3.2. Structural and Morphological Characteristics
3.3. Photocatalytic Activity in Leachate Treatment
Mechanism of Photocatalytic Activity
3.4. Environmental and Industrial Implications
4. Materials and Methods
4.1. Materials
4.2. Preparation of Durian Husk Extract (DHE)
4.3. Green Synthesis of CuO Nanoparticles
4.4. Characterization of CuO Nanoparticles
4.5. Photocatalytic Treatment of Landfill Leachate
- i = initial value of parameter in mg/L;
- f = value of parameter after photo-degradation in mg/L.
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamaruddin, M.A.; Yusoff, M.S.; Rui, L.M.; Isa, A.M.; Zawawi, M.H.; Alrozi, R. An Overview of Municipal Solid Waste Management and Landfill Leachate Treatment: Malaysia and Asian Perspectives. Environ. Sci. Pollut. Res. 2017, 24, 26988–27020. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2023, 203, 117525. [Google Scholar] [CrossRef]
- Sasidharan, S.; Tey, L.H.; Djearamane, S.; Ab Rashid, N.K.M.; Raajeswari, P.A.; Wong, L.S.; Dhanapal, A.C.T.A. Development of Novel Biofilm Using Musa acuminata (Waste Banana Leaves) Mediated Biogenic Zinc Oxide Nanoparticles Reinforced with Chitosan Blend. J. King Saud Univ. Sci. 2024, 36, 103080. [Google Scholar] [CrossRef]
- Lindamulla, L.; Nanayakkara, N.; Othman, M.; Jinadasa, S.; Herath, G.; Jegatheesan, V. Municipal Solid Waste Landfill Leachate Characteristics and Their Treatment Options in Tropical Countries. Curr. Pollut. Rep. 2022, 8, 273–287. [Google Scholar] [CrossRef]
- Jamrah, A.; AL-Zghoul, T.M.; Al-Qodah, Z. An Extensive Analysis of Combined Processes for Landfill Leachate Treatment. Water 2024, 16, 1640. [Google Scholar] [CrossRef]
- Chan, Y.B.; Aminuzzaman, M.; Tey, L.-H.; Win, Y.F.; Watanabe, A.; Djearamame, S.; Akhtaruzzaman, M. Impact of Diverse Parameters on the Physicochemical Characteristics of Green-Synthesized Zinc Oxide–Copper Oxide Nanocomposites Derived from an Aqueous Extract of Garcinia mangostana L. Leaf. Materials 2023, 16, 5421. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.B.; Aminuzzaman, M.; Rahman, M.K.; Win, Y.F.; Sultana, S.; Cheah, S.Y.; Watanabe, A.; Wong, L.S.; Guha, S.K.; Djearamane, S.; et al. Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study. Green Process. Synth. 2024, 13, 20230251. [Google Scholar] [CrossRef]
- Chan, Y.B.; Aminuzzaman, M.; Win, Y.F.; Djearamane, S.; Wong, L.S.; Guha, S.K.; Almohammadi, H.; Akhtaruzzaman, M.; Tey, L.-H. Garcinia mangostana L. Leaf-Extract-Assisted Green Synthesis of CuO, ZnO and CuO-ZnO Nanomaterials for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Catalysts 2024, 14, 486. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tan, L.X.; Win, Y.F.; Cheah, E.S.G.; Heng, M.H.; Tey, L.H.; Arullappan, S.; Algethami, N.; Alharthi, S.S.; et al. Synthesis, characterization, and preliminary in vitro antibacterial evaluation of ZnO nanoparticles derived from soursop (Annona muricata L.) leaf extract as a green reducing agent. J. Mater. Res. Technol. 2022, 20, 2931–2941. [Google Scholar] [CrossRef]
- Zango, Z.U.; Khoo, K.S.; Garba, A.; Garba, Z.N.; Danmallam, U.N.; Aldaghri, O.; Ibnaouf, K.H.; Ahmad, N.M.; Binzowaimil, A.M.; Lim, J.W.; et al. A review on titanium oxide nanoparticles modified metal-organic frameworks for effective CO2 conversion and efficient wastewater remediation. Environ. Res. 2024, 252, 119024. [Google Scholar] [CrossRef]
- Derakhshani, E.; Asri, M.; Naghizadeh, A. Plant-Based Green Synthesis of Copper Oxide Nanoparticles Using Berberis vulgaris Leaf Extract: An Update on Their Applications in Antibacterial Activity. BioNanoScience 2023, 13, 212–218. [Google Scholar] [CrossRef]
- Phang, Y.K.; Aminuzzaman, M.; Akhtaruzzaman, M.; Muhammad, G.; Ogawa, S.; Watanabe, A.; Tey, L.H. Green Synthesis and Characterization of CuO Nanoparticles Derived from Papaya Peel Extract for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME). Sustainability 2021, 13, 796. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and environmental applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Exploring the chemical composition, emerging applications, potential uses, and health benefits of durian: A review. Food Control 2020, 113, 107189. [Google Scholar]
- Ali, N.A.W.A.; Wong, G.R.; Tan, B.C.; Lum, W.S.; Mazumdar, P. Unleashing the Potential of Durian: Challenges, Opportunities, and the Way Forward. Appl. Fruit Sci. 2025, 67, 1–15. [Google Scholar] [CrossRef]
- Gamay, R.A.J.; Botecario, P.M.N.; Sanchez, P.D.C.; Alvarado, M.C. Durian (Durio zibenthinus) waste: A promising resource for food and diverse applications—A comprehensive review. Food Prod. Process. Nutr. 2024, 6, 27. [Google Scholar] [CrossRef]
- Zhivkova, V. Durian waste valorization–some research tendencies: A review. IOP Conf. Ser. Earth Environ. Sci. 2024, 1420, 12032. [Google Scholar] [CrossRef]
- Masudi, A.; Jusoh, N.W.C.; Bakri, Z.; Jalil, A.A.; Ali, R.R.; Jaafar, N.F. Durian Shell Husk Extract Assisted Synthesis of Copper Oxides Nanoparticles for the Photodegradation of Paracetamol. Malays. J. Anal. Sci. 2019, 23, 818–827. [Google Scholar]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Spongy ball-like copper oxide nanostructure modified by reduced graphene oxide for enhanced photocatalytic hydrogen production. Mater. Res. Bull. 2021, 133, 111026. [Google Scholar] [CrossRef]
- Duong, N.L.; Nguyen, V.M.; Tran, T.A.N.; Phan, T.D.T.; Tran, T.B.Y.; Do, B.L.; Phung Anh, N.; Nguyen, T.A.T.; Ho, T.G.T.; Nguyen, T. Durian shell-mediated simple green synthesis of nanocopper against plant pathogenic fungi. ACS Omega 2023, 8, 10968–10979. [Google Scholar] [CrossRef]
- Al-Balushi, F.; Ibrahim, O.; Rajamohan, N. Sustainable Treatment of Landfill Leachate: A Review on Methods. Int. J. Environ. Sci. Technol. 2024, 21, 1–16. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Hung, Y.T. Sustainable Treatment of Landfill Leachate. Appl. Water Sci. 2015, 5, 113–126. [Google Scholar] [CrossRef]
- Vaidh, S.; Parekh, D.; Patel, D.; Vishwakarma, G.S. Leachate Treatment Potential of Nanomaterial Based Assemblies: A Systematic Review on Recent Development. Water Sci. Technol. 2022, 11, 3285–3300. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Hassan, S.E.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-based copper oxide nanoparticles; biosynthesis, characterization, antibacterial activity, tanning wastewater treatment, and heavy metals sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Rayan, D.A.; Ismail, M.M. Synthesis of Cu and CuO nanoparticles from e-waste and evaluation of their antibacterial and photocatalytic properties. Environ. Sci. Pollut. Res. 2023, 38, 89690–89704. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. Eco-friendly Ocimum tenuiflorum green route synthesis of CuO nanoparticles: Characterizations on photocatalytic and antibacterial activities. J. Environ. Chem. Eng. 2021, 9, 105395. [Google Scholar] [CrossRef]
- Kayalvizhi, S.; Sengottaiyan, A.; Selvankumar, T.; Senthilkumar, B.; Sudhakar, C.; Selvam, K. Eco-friendly cost-effective approach for synthesis of copper oxide nanoparticles for enhanced photocatalytic performance. Optik 2020, 202, 163507. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Majumder, S.; Mourad, A.H.I.; Islam, M.M.; Sakurai, T.; Kang, S.W. Multiplicative rGO/Cu-BDC MOF for 4-nitrophenol reduction and supercapacitor applications. J. Colloid Interface Sci. 2025, 677, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shamin-Shazwana, K.; Shaharia, R.; Amria, C.N.; Zinb, N.H. A Review: Potential Of Durio Zibethinus L. (Durian) Waste. Science 2022, 6, 21–26. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant mediated green synthesis of CuO nanoparticles: Comparison of toxicity of engineered and plant mediated CuO nanoparticles towards Daphnia Magna. Nanometers 2016, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Jayakodi, S.; Shanmugam, V.K. Green synthesis of CuO nanoparticles and its application on toxicology evaluation. Biointerface Res. Appl. Chem 2020, 10, 6343–6353. [Google Scholar]
- Awewomom, J.; Dzeble, F.; Takyi, Y.D.; Ashie, W.B.; Ettey, E.N.; Afua, P.E.; Sackey, L.N.; Opoku, F.; Akoto, O. Addressing global environmental pollution using environmental control techniques: A focus on environmental policy and preventive environmental management. Discov. Environ. 2024, 2, 8. [Google Scholar] [CrossRef]
- Siddique, I. Sustainable Water Management in Urban Areas: Integrating Innovative Technologies and Practices to Address Water Scarcity and Pollution. Pharm. Chem. J. 2021, 8, 172–178. [Google Scholar] [CrossRef]
- Bernstein, S. The United Nations and the Governance of Sustainable Development Goals. In Governing Through Goals: Sustainable Development Goals as Governance Innovation; MIT Press: Cambridge, MA, USA, 2017; pp. 213–239. [Google Scholar]
- Rathi, V.H.; Jeice, A.R. Green-fabrication of CuO nanoparticles using various plant extracts and their multifaceted applications in photocatalytic cationic dye degradation and antimicrobial activities. Biomass Convers. Biorefin. 2024, 14, 18551–18562. [Google Scholar] [CrossRef]
- Papitha, R.; Selvaraj, C.I. Synthesis of CuO-based nanomaterials and its biological studies using Parkia timoriana bark. Biomass Convers. Biorefin. 2024, 1–17. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M. Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry 2021, 7, 145. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z. Fabrication of CuO nanoparticles for structural, optical and dielectric analysis using chemical precipitation method. J. Mater. Sci. Mater. Electron. 2017, 28, 12591–12597. [Google Scholar] [CrossRef]
- Felix, S.; Chakkravarthy, R.B.P.; Grace, A.N. Microwave assisted synthesis of copper oxide and its application in electrochemical sensing. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 12115. [Google Scholar] [CrossRef]
- Vetrimani, A.; Geetha, K.; Jemima, E.A.; Arulnathan, N.; Kim, H.S.; Kathalingam, A. Effect of the green synthesis of CuO plate-like nanoparticles on their photodegradation and antibacterial activities. Phys. Chem. Chem. Phys. 2022, 24, 28923–28933. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.A.; Abebe, B.; Prakash, C.H.; Shantaveerayya, K. A review on green synthesis of Cu and CuO nanomaterials for multifunctional applications. Mater. Sci. Res. India 2018, 15, 279–295. [Google Scholar] [CrossRef]
- Reshma Shaik, H.; Venkatesan, K.; Kadeer, M.D.; Somaiah, P.V. Green Synthesis, Characterization, Biological Activities of CuO Nanoparticles and Their Photocatalytic Degradative Mechanistic Study of Methylene Blue Dye Using Mass Spectroscopy. Russ. J. Gen. Chem. 2024, 94, 220–233. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. Water Reuse 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Prakruthi, R.; Deepakumari, H.N. CuO nanoparticles: Green combustion synthesis, applications to antioxidant, photocatalytic and sensor studies. RSC Adv. 2024, 14, 28703–28715. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ejaz, U.; Vithanage, M.; Bolan, N.; Siddique, K.H. Synthesis, characterization, and advanced sustainable applications of copper oxide nanoparticles: A review. Clean Technol. Environ. Policy 2024, 13, 1–26. [Google Scholar] [CrossRef]
- Alangari, A.; Aboul-Soud, M.A.; Alqahtani, M.S.; Shahid, M.; Syed, R.; Lakshmipathy, R.; Modigunta, J.K.; Jaiswal, H.; Verma, M. Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications. Nanotechnol. Rev. 2024, 13, 20240039. [Google Scholar] [CrossRef]
- Kamala, S.S.; Tey, L.H.; Sim, Y.L. Combined chemical, physical and biological treatment using Chlorella vulgaris sp. on landfill leachate. AIP Conf. Proc. 2018, 2026, 2006. [Google Scholar]



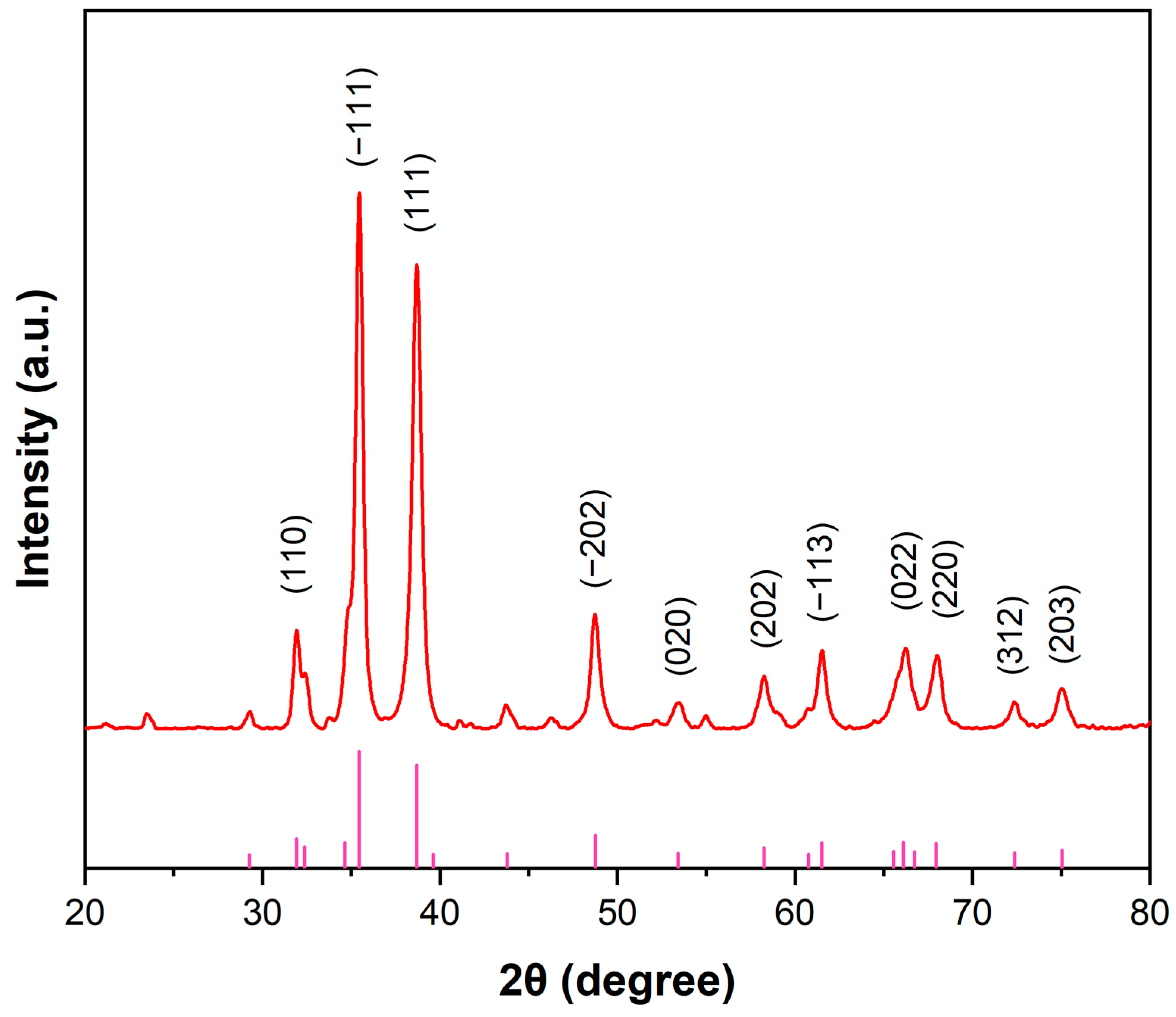

| Peak Position (2θ) | FWHM | β (Rad) | Crystalline Size (nm) |
|---|---|---|---|
| 31.92 | 0.4218 | 0.0074 | 19.59 |
| 35.45 | 0.4859 | 0.0085 | 17.16 |
| 38.69 | 0.6056 | 0.0106 | 13.9 |
| 48.75 | 0.5701 | 0.0100 | 15.3 |
| 53.41 | 0.71418 | 0.0125 | 12.45 |
| 58.26 | 0.6774 | 0.0118 | 13.43 |
| 61.52 | 0.572 | 0.0100 | 16.16 |
| 66.12 | 1.001 | 0.0175 | 9.47 |
| 67.95 | 0.7428 | 0.0130 | 12.9 |
| 72.36 | 0.5962 | 0.0104 | 16.51 |
| 75.06 | 0.7167 | 0.0125 | 13.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-P.; Chan, Y.-B.; Aminuzzaman, M.; Shahinuzzaman, M.; Djearamane, S.; Thiagarajah, K.; Leong, S.-Y.; Wong, L.-S.; Tey, L.-H. Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications. Catalysts 2025, 15, 275. https://doi.org/10.3390/catal15030275
Liang Y-P, Chan Y-B, Aminuzzaman M, Shahinuzzaman M, Djearamane S, Thiagarajah K, Leong S-Y, Wong L-S, Tey L-H. Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications. Catalysts. 2025; 15(3):275. https://doi.org/10.3390/catal15030275
Chicago/Turabian StyleLiang, Yan-Peng, Yu-Bin Chan, Mohammod Aminuzzaman, Mohammad Shahinuzzaman, Sinouvassane Djearamane, Kokila Thiagarajah, Siew-Yoong Leong, Ling-Shing Wong, and Lai-Hock Tey. 2025. "Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications" Catalysts 15, no. 3: 275. https://doi.org/10.3390/catal15030275
APA StyleLiang, Y.-P., Chan, Y.-B., Aminuzzaman, M., Shahinuzzaman, M., Djearamane, S., Thiagarajah, K., Leong, S.-Y., Wong, L.-S., & Tey, L.-H. (2025). Green Synthesis and Characterization of Copper Oxide Nanoparticles from Durian (Durio zibethinus) Husk for Environmental Applications. Catalysts, 15(3), 275. https://doi.org/10.3390/catal15030275






