Pore-Rich Ni-Co Spinel Oxides for Treating Soot Oxidation in Engine Exhausts
Abstract
1. Introduction
2. Results
2.1. Microstructure Properties
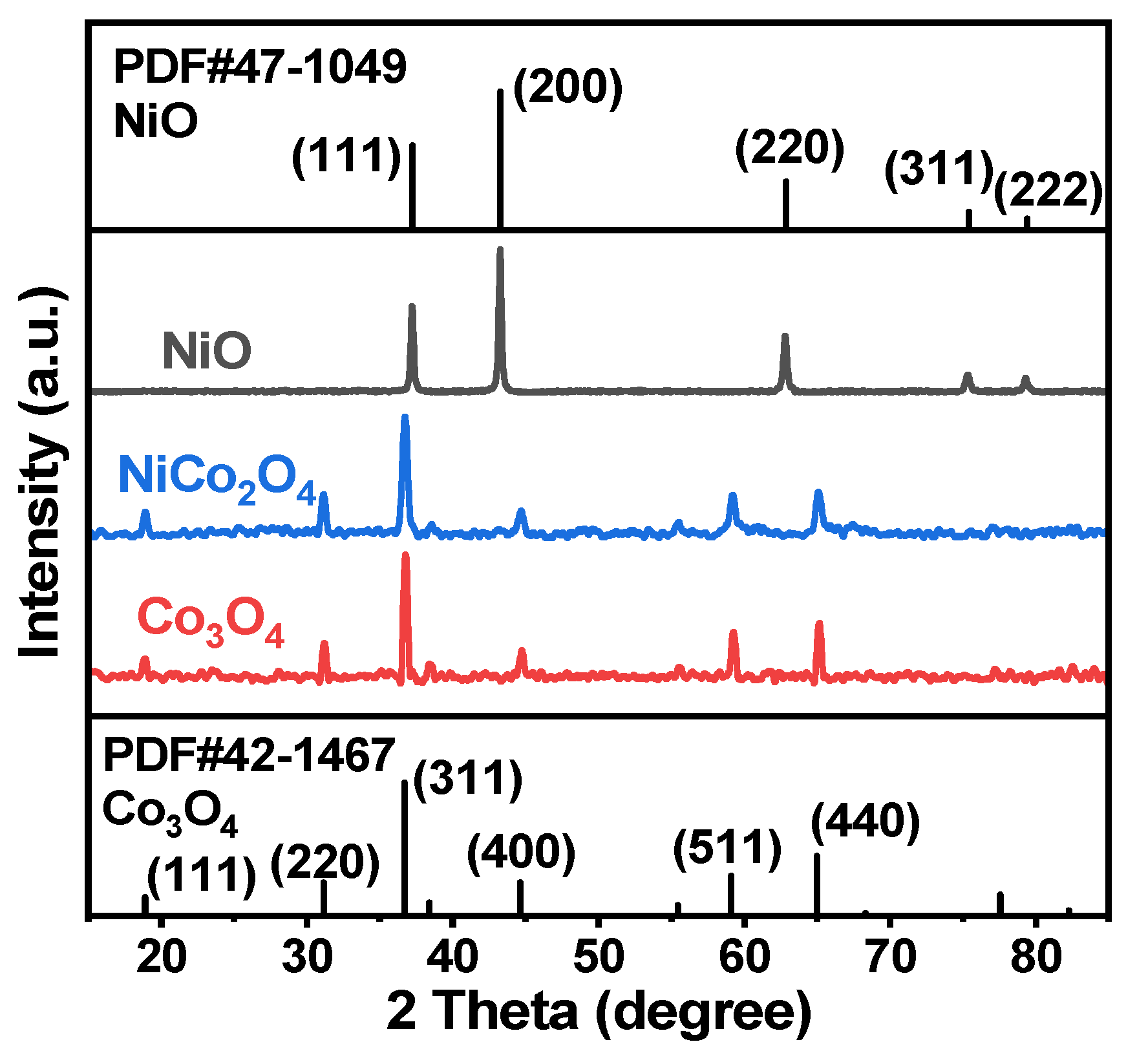
2.1.1. XRD
2.1.2. The N2 Adsorption–Desorption Experiments
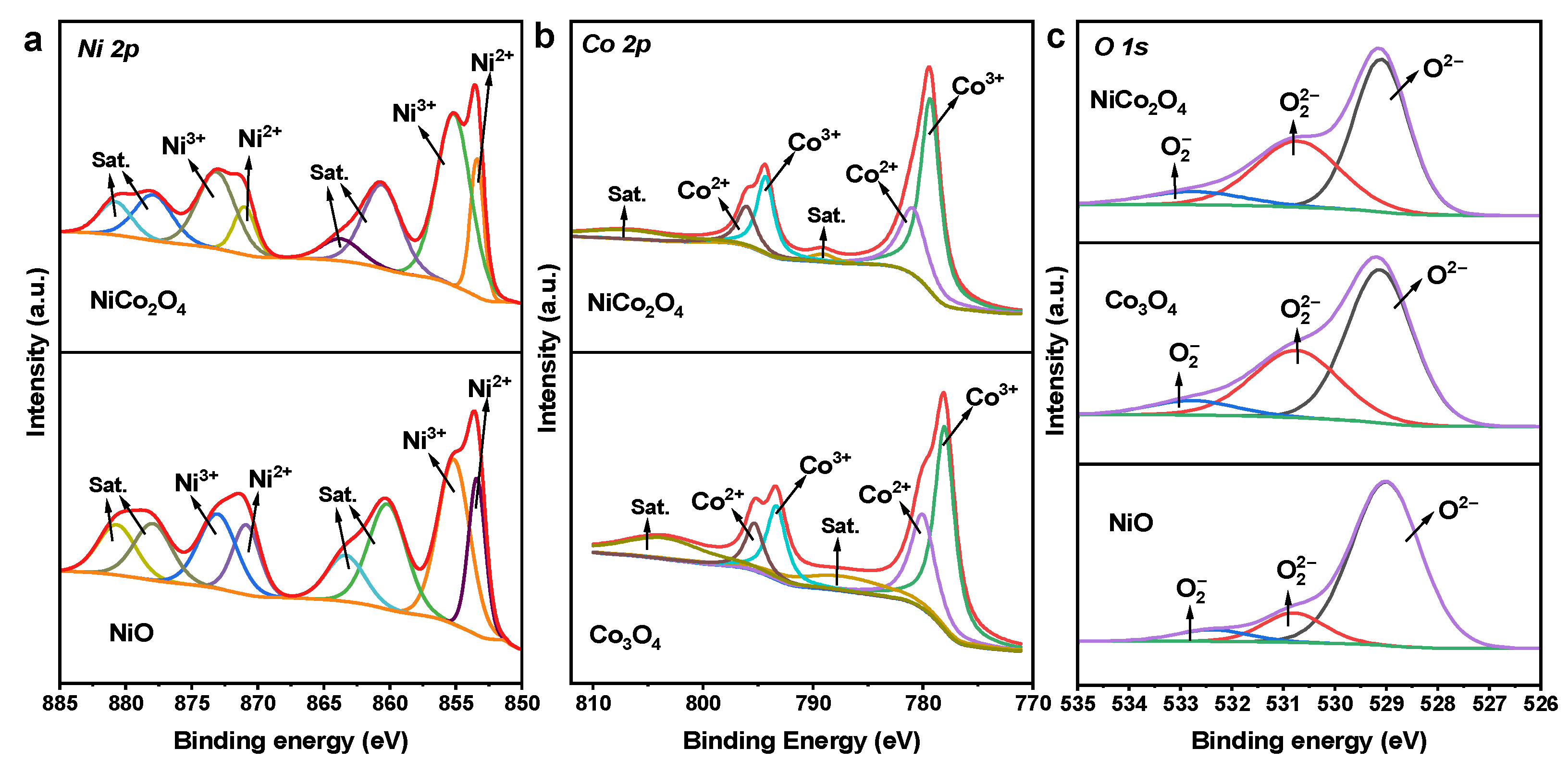
2.1.3. XPS
2.2. Catalytic Performance
2.2.1. Soot-TPO
2.2.2. NO-TPO
2.2.3. H2-TPR
2.3. Intermediates and Mechanisms
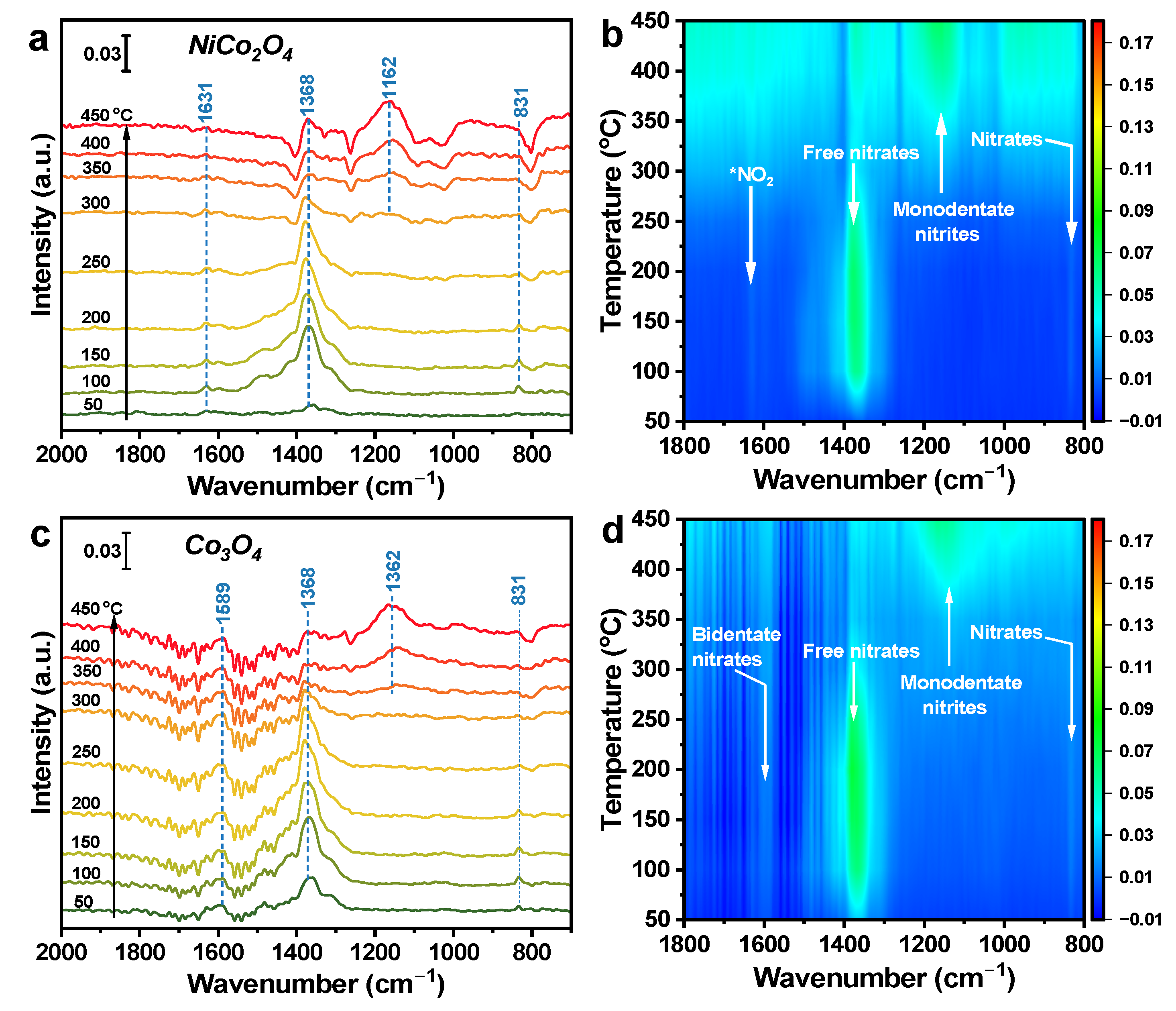
In Situ DRIFTS of NO Oxidation
3. Materials and Methods
3.1. Synthesis of NiO, Co3O4, and NiCo2O4 Catalysts
3.2. Catalysts Characterization
3.3. Evaluation of Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Rauch, S.; Hemond, H.F.; Barbante, C.; Owari, M.; Morrison, G.M.; Peucker-Ehrenbrink, B.; Wass, U. Importance of Automobile Exhaust Catalyst Emissions for the Deposition of Platinum, Palladium, and Rhodium in the Northern Hemisphere. Environ. Sci. Technol. 2005, 39, 8156–8162. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Denisov, S.P.; Danchenko, N.M.; Ismagilov, Z.R. Synergetic effect of Pd addition on catalytic behavior of monolithic platinum–manganese–alumina catalysts for diesel vehicle emission control. Appl. Catal. B Environ. 2016, 185, 322–336. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The recent development of efficient Earth-abundant transition-metal nanocatalysts. Chem. Soc. Rev. 2017, 46, 816–854. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Kaczmarczyk, J.; Zasada, F.; Janas, J.; Indyka, P.; Piskorz, W.; Kotarba, A.; Sojka, Z. Thermodynamic Stability, Redox Properties, and Reactivity of Mn3O4, Fe3O4, and Co3O4 Model Catalysts for N2O Decomposition: Resolving the Origins of Steady Turnover. ACS Catal. 2016, 6, 1235–1246. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, C.; Song, C.; Qu, Z. Spinel-Based Catalysts That Enable Catalytic Oxidation of Volatile Organic Compounds. Environ. Sci. Technol. 2024, 58, 20785–20811. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Ojala, S.; Siffert, S.; Cousin, R. Influence of CO addition on the toluene total oxidation over Co based mixed oxide catalysts. Appl. Catal. B Environ. 2019, 247, 163–172. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Yi, T.; Sun, L.; Zhang, Y.; Hu, X.; Cui, W.; Yang, X. Surface density of synthetically tuned spinel oxides of Co3+ and Ni3+ with enhanced catalytic activity for methane oxidation. Chin. J. Catal. 2018, 39, 1228–1239. [Google Scholar] [CrossRef]
- Gu, D.; Jia, C.-J.; Weidenthaler, C.; Bongard, H.-J.; Spliethoff, B.; Schmidt, W.; Schüth, F. Highly Ordered Mesoporous Cobalt-Containing Oxides: Structure, Catalytic Properties, and Active Sites in Oxidation of Carbon Monoxide. J. Am. Chem. Soc. 2015, 137, 11407–11418. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, H.; Wang, S.; Dai, Y.; Liu, B.; Liu, Y.; Dang, F.; Smith, K.J.; Nie, X.; Hou, S.; et al. Engineering Morphology and Ni Substitution of NixCo3−xO4 Spinel Oxides to Promote Catalytic Combustion of Ethane: Elucidating the Influence of Oxygen Defects. ACS Catal. 2023, 13, 4683–4699. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Li, R.; Zhao, J.; Liu, B.; Wang, D. Atomic Distance Engineering in Metal Catalysts to Regulate Catalytic Performance. Adv. Mater. 2024, 36, 2308653. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, Y.; Zhang, Y.; Zhang, P.; Yu, Q.; Mei, X.; Liu, X.; Zhao, Z.; Liu, J. Synergetic Effect of K Sites and Pt Nanoclusters in an Ordered Hierarchical Porous Pt-KMnOx/Ce0.25Zr0.75O2 Catalyst for Boosting Soot Oxidation. ACS Catal. 2020, 10, 7123–7135. [Google Scholar] [CrossRef]
- Xiong, J.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Xie, Z.; Li, J. Efficiently multifunctional catalysts of 3D ordered meso-macroporous Ce0.3Zr0.7O2-supported PdAu@CeO2 core-shell nanoparticles for soot oxidation: Synergetic effect of Pd-Au-CeO2 ternary components. Appl. Catal. B Environ. 2019, 251, 247–260. [Google Scholar] [CrossRef]
- Cai, P.; Zhao, J.; Zhang, X.; Zhang, T.; Yin, G.; Chen, S.; Dong, C.-L.; Huang, Y.-C.; Sun, Y.; Yang, D.; et al. Synergy between cobalt and nickel on NiCo2O4 nanosheets promotes peroxymonosulfate activation for efficient norfloxacin degradation. Appl. Catal. B Environ. 2022, 306, 121091. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Liu, D.; Sun, H.; Yan, W.; Wang, J.; Dong, M.; Tong, X.; Fan, W. Hollow and porous NiCo2O4 nanospheres for enhanced methanol oxidation reaction and oxygen reduction reaction by oxygen vacancies engineering. Appl. Catal. B Environ. 2021, 291, 120065. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, H.; Xu, L.; Yuan, J.; Gong, J.; Liu, X. Pr0.7Ba0.3Co0.8-xFe0.2NixO3−δ perovskite: High activity and durable cathode for intermediate-to-low-temperature proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 33633–33643. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, Q.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Wu, D.; Li, J. Fabrication of Spinel-Type PdxCo3−xO4 Binary Active Sites on 3D Ordered Meso-macroporous Ce-Zr-O2 with Enhanced Activity for Catalytic Soot Oxidation. ACS Catal. 2018, 8, 7915–7930. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Chen, Y.; Xu, C.; Duan, A.; Jiang, G.; He, H. Highly Active Catalysts of Gold Nanoparticles Supported on Three-Dimensionally Ordered Macroporous LaFeO3 for Soot Oxidation. Angew. Chem. Int. Ed. 2011, 50, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G.; Xu, C.; Gao, J.; He, H.; Wang, X. Three-dimensionally ordered macroporous Ce0.8Zr0.2O2-supported gold nanoparticles: Synthesis with controllable size and super-catalytic performance for soot oxidation. Energy Environ. Sci. 2011, 4, 2959–2970. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Wei, Y.; Xiong, J.; Zhang, P.; Lai, K.; Chi, H.; Liu, X.; Chen, L.; Yu, X.; et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nat. Commun. 2023, 14, 7149. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Huang, J.; Jin, H.; Fang, X.; Liu, W.; Zhang, N.; Wang, H.; Wang, X. Engineering Ni3+ Cations in NiO Lattice at the Atomic Level by Li+ Doping: The Roles of Ni3+ and Oxygen Species for CO Oxidation. ACS Catal. 2018, 8, 8033–8045. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Nie, Z.; Tian, J.; Liu, Y.; Ma, C.; Liu, P.; Ren, Q.; Xiong, J.; Huang, H.; et al. Ni Doping Promotes C–H Bond Activation and Conversion of Key Intermediates for Total Oxidation of Methane over Co3O4 Catalysts. ACS Catal. 2023, 13, 15779–15793. [Google Scholar] [CrossRef]
- Yan, Y.; Ouyang, M.; Xing, Y.; Tian, J.; Song, L.; Chen, P.; Liu, P.; Yang, L.; Song, X.; Fu, M.; et al. Promoted deep oxidation of m-xylene and inhibited the generation of carbon-deposited species by Ce modified Co3O4: The key role of modulating internal electron transport pathway. Appl. Catal. B Environ. Energy 2025, 365, 124864. [Google Scholar] [CrossRef]
- Zhang, P.; Mei, X.; Zhao, X.; Xiong, J.; Li, Y.; Zhao, Z.; Wei, Y. Boosting Catalytic Purification of Soot Particles over Double Perovskite-Type La2–xKxNiCoO6 Catalysts with an Ordered Macroporous Structure. Environ. Sci. Technol. 2021, 55, 11245–11254. [Google Scholar] [CrossRef]
- Zhang, Q.-N.; Zhang, Y.; Cai, C.; Guo, Y.-C.; Reid, J.P.; Zhang, Y.-H. In Situ Observation on the Dynamic Process of Evaporation and Crystallization of Sodium Nitrate Droplets on a ZnSe Substrate by FTIR-ATR. J. Phys. Chem. A 2014, 118, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yu, Q.; Ji, Z.; Lv, Y.; Cao, Y.; Tang, C.; Gao, F.; Dong, L.; Chen, Y. A comparative study of different doped metal cations on the reduction, adsorption and activity of CuO/Ce0.67M0.33O2 (M = Zr4+, Sn4+, Ti4+) catalysts for NO+CO reaction. Appl. Catal. B Environ. 2013, 130–131, 293–304. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Ma, C.; Qin, Y. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO. Appl. Catal. B Environ. 2017, 201, 636–651. [Google Scholar] [CrossRef]
- Kang, L.; Han, L.; He, J.; Li, H.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. Improved NOx Reduction in the Presence of SO2 by Using Fe2O3-Promoted Halloysite-Supported CeO2–WO3 Catalysts. Environ. Sci. Technol. 2019, 53, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wasalathanthri, N.D.; SantaMaria, T.M.; Kriz, D.A.; Dissanayake, S.L.; Kuo, C.-H.; Biswas, S.; Suib, S.L. Mesoporous manganese oxides for NO2 assisted catalytic soot oxidation. Appl. Catal. B Environ. 2017, 201, 543–551. [Google Scholar] [CrossRef]
- Wang, H.; Peng, Y.; Zhou, B.; Yuan, J.; Wang, R.; Si, W.; Li, J. Formation mechanisms of N2O and NH3 on Pd/ZrO2 and Pd/Al2O3 for NO reduction. Chem. Eng. J. 2023, 475, 145379. [Google Scholar] [CrossRef]





| Catalyst | Specific Surface Areas (m2/g) | Average Pore Volumes (cm3/kg) | Average Mesopore Sizes (nm) |
|---|---|---|---|
| NiO | 14.9 | 74.0 | 8.3 |
| Co3O4 | 10.5 | 65.8 | 8.5 |
| NiCo2O4 | 21.7 | 122.3 | 12.2 |
| Catalyst | Ni 2p | Co 2p | O 1s | |||||
|---|---|---|---|---|---|---|---|---|
| Ni3+ | Ni2+ | Co3+ | Co2+ | O2− | O22− | O2− | O22− + O2− | |
| NiO | 64.0 | 36.0 | - | - | 81.1 | 12.9 | 6.0 | 18.9 |
| Co3O4 | - | - | 75.9 | 24.1 | 58.9 | 34.0 | 7.1 | 41.1 |
| NiCo2O4 | 75.4 | 24.6 | 79.8 | 20.2 | 54.9 | 36.6 | 8.5 | 35.1 |
| Catalyst | T10 (°C) | T50 (°C) | T90 (°C) | SCO2m (%) * |
|---|---|---|---|---|
| Without catalysts | 485 | 599 | 651 | 22.83 |
| NiO | 403 | 464 | 496 | 99.69 |
| Co3O4 | 316 | 369 | 407 | 99.80 |
| NiCo2O4 | 316 | 356 | 388 | 99.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Chen, K.; Li, Y.; Ma, Y.; Cui, B.; Xiong, J.; Wei, Y. Pore-Rich Ni-Co Spinel Oxides for Treating Soot Oxidation in Engine Exhausts. Catalysts 2025, 15, 267. https://doi.org/10.3390/catal15030267
Xu L, Chen K, Li Y, Ma Y, Cui B, Xiong J, Wei Y. Pore-Rich Ni-Co Spinel Oxides for Treating Soot Oxidation in Engine Exhausts. Catalysts. 2025; 15(3):267. https://doi.org/10.3390/catal15030267
Chicago/Turabian StyleXu, Linsheng, Kaixuan Chen, Yuanfeng Li, Yaxiao Ma, Baolong Cui, Jing Xiong, and Yuechang Wei. 2025. "Pore-Rich Ni-Co Spinel Oxides for Treating Soot Oxidation in Engine Exhausts" Catalysts 15, no. 3: 267. https://doi.org/10.3390/catal15030267
APA StyleXu, L., Chen, K., Li, Y., Ma, Y., Cui, B., Xiong, J., & Wei, Y. (2025). Pore-Rich Ni-Co Spinel Oxides for Treating Soot Oxidation in Engine Exhausts. Catalysts, 15(3), 267. https://doi.org/10.3390/catal15030267







