Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation
Abstract
1. Introduction
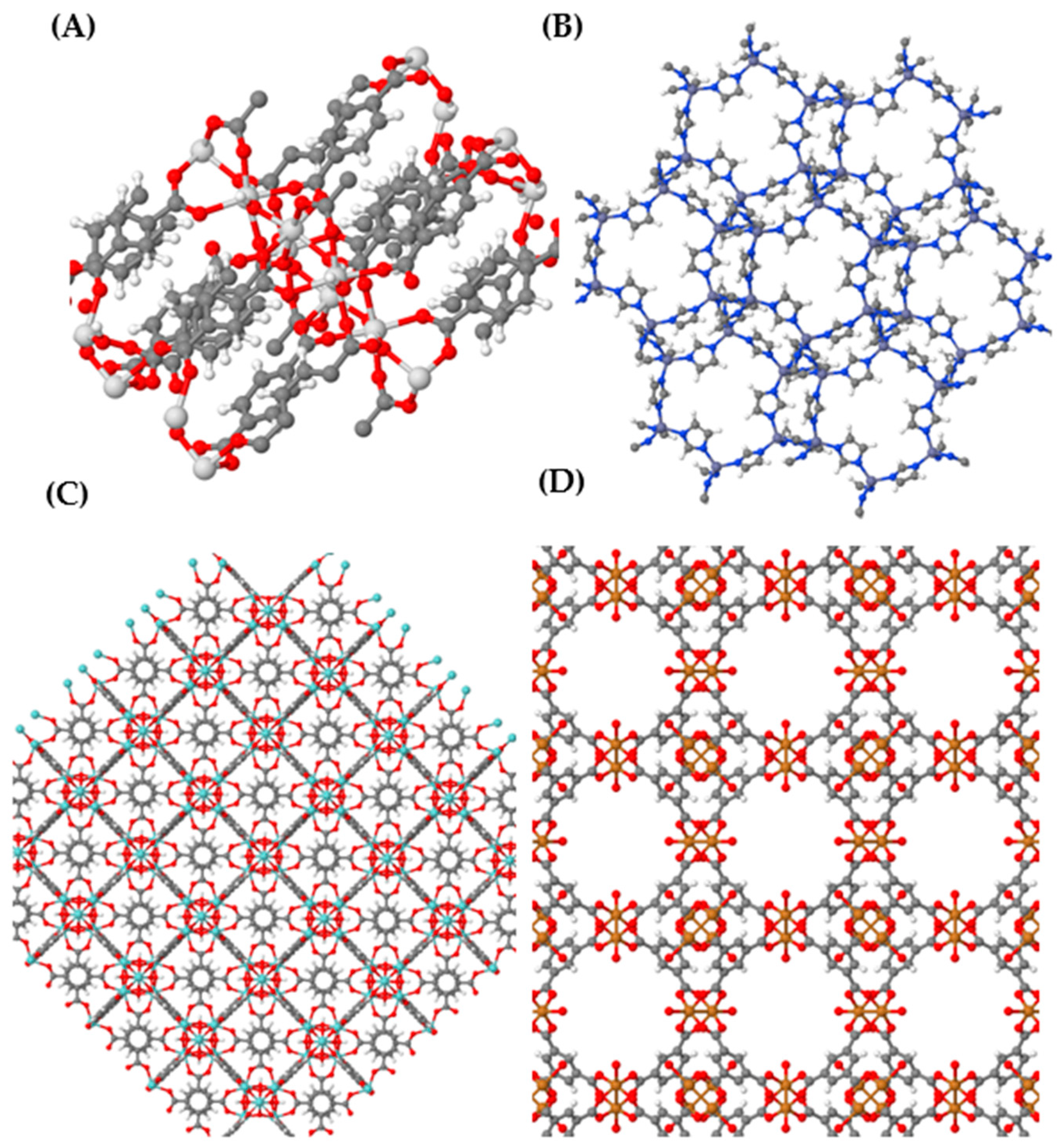
2. MOFs: Fundamentals, Properties and Synthesis
2.1. Fundamentals and Properties
| MOF | Ligand Linker | Metal Clusters | Synthesis Method | Temperature (°C) | Time (h) | Solvent | Reference |
|---|---|---|---|---|---|---|---|
| MIL53 | H2BDC | Zn, Fe | Solvothermal | 90–150 | 15 | DMF *1/EtOH *2 | [44] |
| H2BDC | Fe | Sonochemical | 70 | 2 | DMF *1 | [45] | |
| MIL101 | NH2-BDC | Fe | Hydrothermal | 110–150 | 24 | H2O | [46] |
| MIL125 | H2BDC | Ti | Solvothermal | 150–220 | 12 | DMF *1/EtOH *2 | [47] |
| ZIF-8 | 2-methylimidazole | Zn | Solvothermal | 100–150 | 6 | DMF *1/MeOH *3 | [48,49] |
| 2-methylimidazole | Zn | Microwave | 100–150 | 4 | DMF *1/MeOH *3 | [50] | |
| HKUST | BTC | Cu | Solvothermal | 150 | 24 | DMF *1/EtOH *2 | [51] |
| BTC | Cu, Ru | Mechanochemical | 25 | 0.33 | - | [52] | |
| UiO-66 | H2BDC | Zr | Solvothermal | 120–220 | 12 | DMF *1 | [53] |
| UiO-67 | BPDC | Zr | Solvothermal | 120–220 | 48 | DMF *1 | [54] |
| MOF-5 | H2BDC | Zn | Electrochemical | 25 | 2 | DMF *1/EtOH *2 | [55] |
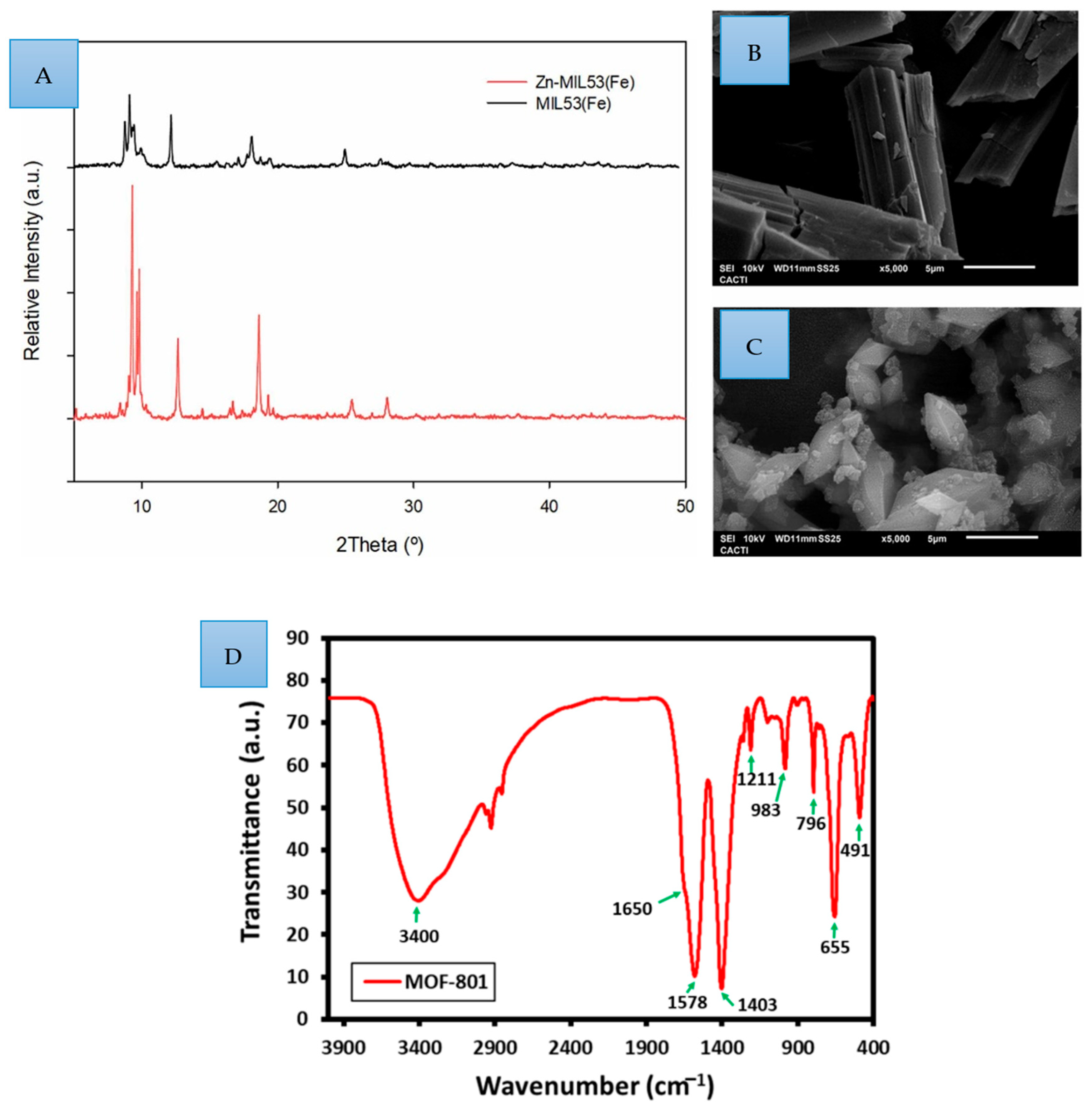
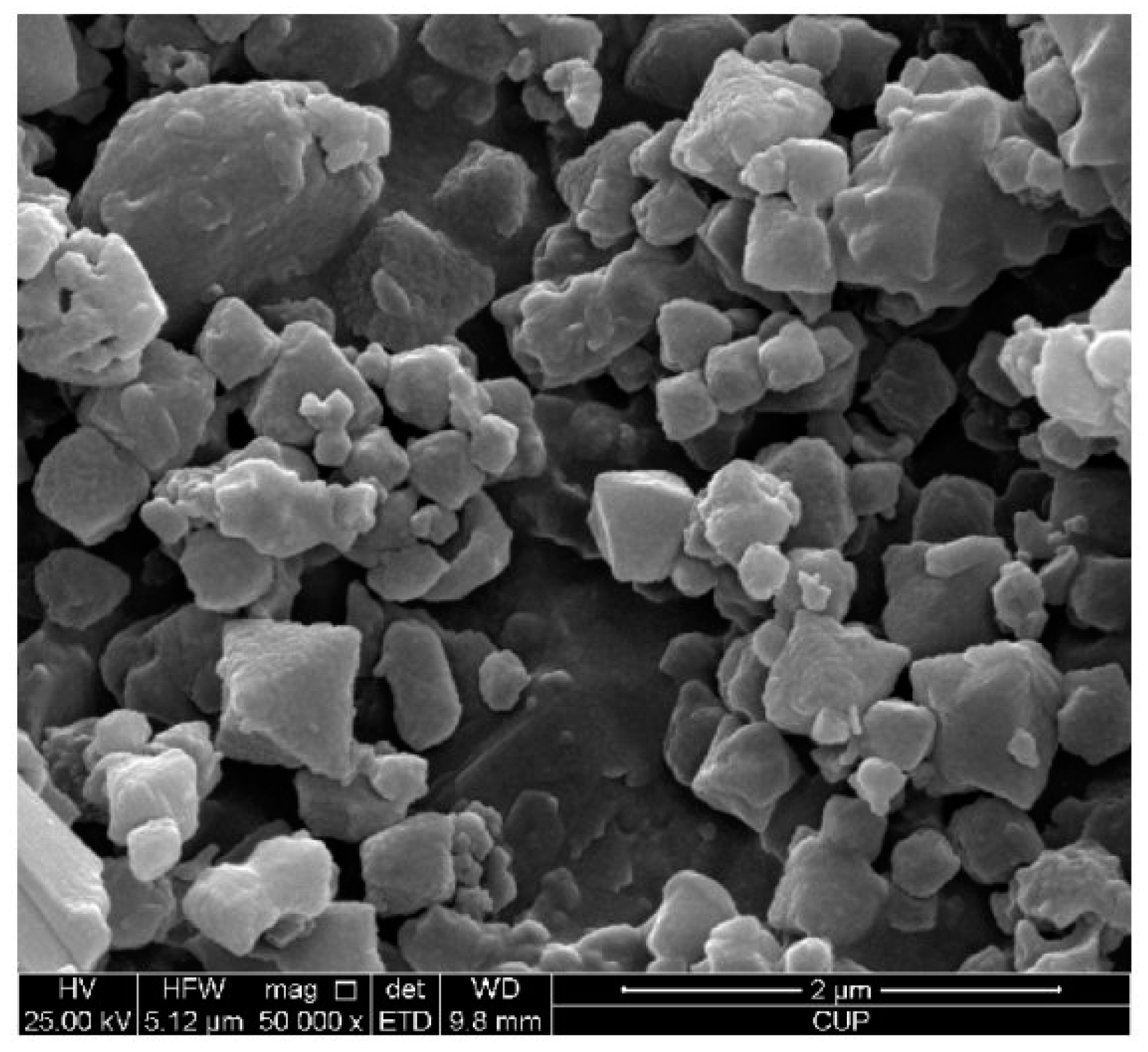
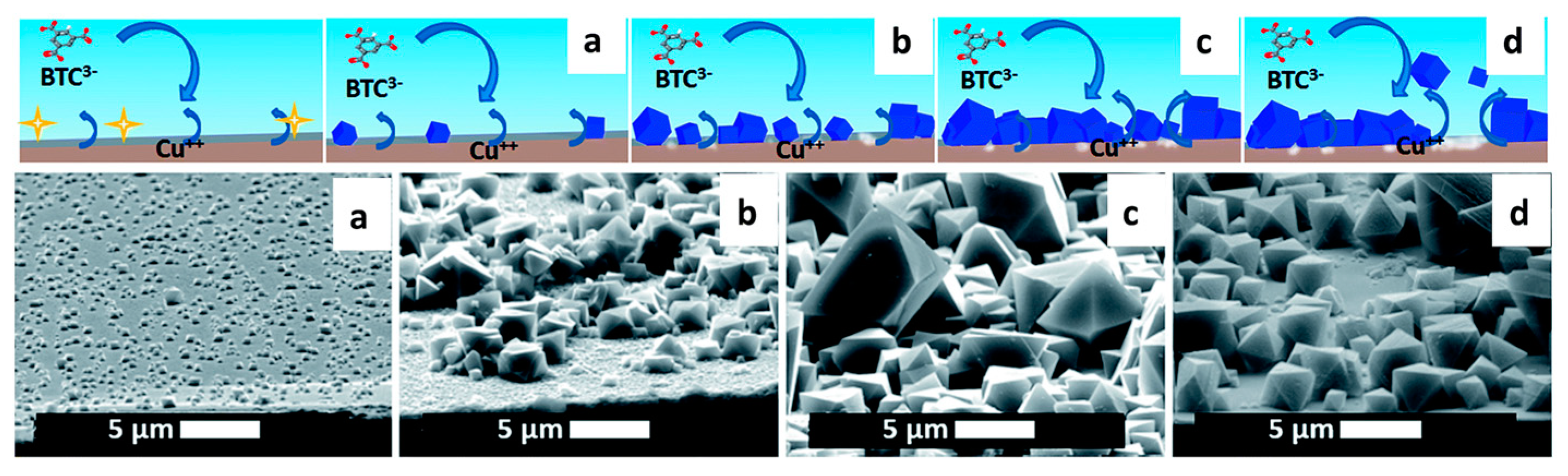
2.2. Synthesis
3. MOFs for Wastewater Treatment
3.1. MOFs as Adsorbents for Pollutants
| MOF MOF-Derived | Dosage (g/L) | Efficiency (%) | Pollutant | Time (min) | Reference |
|---|---|---|---|---|---|
| HKUST-1-derived porous carbon | 0.33 | 80 | Tetracycline | 360 | [126] |
| Ni8BDP6 | 0.73 | 97 | Atenolol | 60 | [124] |
| MOF-525 | 0.20 | 97 | Sulfamethoxazole | 360 | [122] |
| MOF-545 | 0.20 | 95 | Sulfamethoxazole | 360 | [122] |
| MIL101(Fe) | 0.40 | 32 11 22 | Methylene Blue Malachite Green Rhodamine B | 25 25 25 | [123] |
| MIL53(Fe) | 0.43 | 35 | Rhodamine B | 90 | [44] |
| Zn-MIL53(Fe) | 0.43 | 48 | Rhodamine B | 90 | [44] |
| ZIF-8 | 1.10 | 80 | Ibuprofen | 360 | [50] |
| ZIF-67 | 0.50 | 96 | Ibuprofen | 240 | [133] |
| ZIF-67/PVDF | 1.50 | 99 | Congo Red | 60 | [134] |
| ZIF-8/PVDF | 0.40 | 60 | Ibuprofen | 60 | [131] |
| ZIF-8/PVDF | 1.20 | 94 | Paper | 540 | [135] |
| Ni-UiO-66 | 0.20 | 90 | Tetracycline | 500 | [125] |
| UiO-66-biochar composite | 0.40 | 35 | Sulfamethoxazole | 600 | [130] |
| UiO-67/Biochar | 1.00 | 89 | B3+ | 160 | [136] |
| UiO-66/GO | 0.50 | 94 | Tetracycline | 600 | [137] |
| NH2-MIL53(Al)/PAN | 0.20 | 95 | Co2+ | 360 | [132] |
| TiO2-MIL101(Fe) | 0.50 | 88 | Fluoxetine | 120 | [138] |
| MIL101(Fe)/GO | 0.05 | 82 | As5+ | 300 | [127] |
3.2. Enhancements Strategies for MOF Adsorption
3.3. MOF as Catalyst for AOP of Organic Pollutants
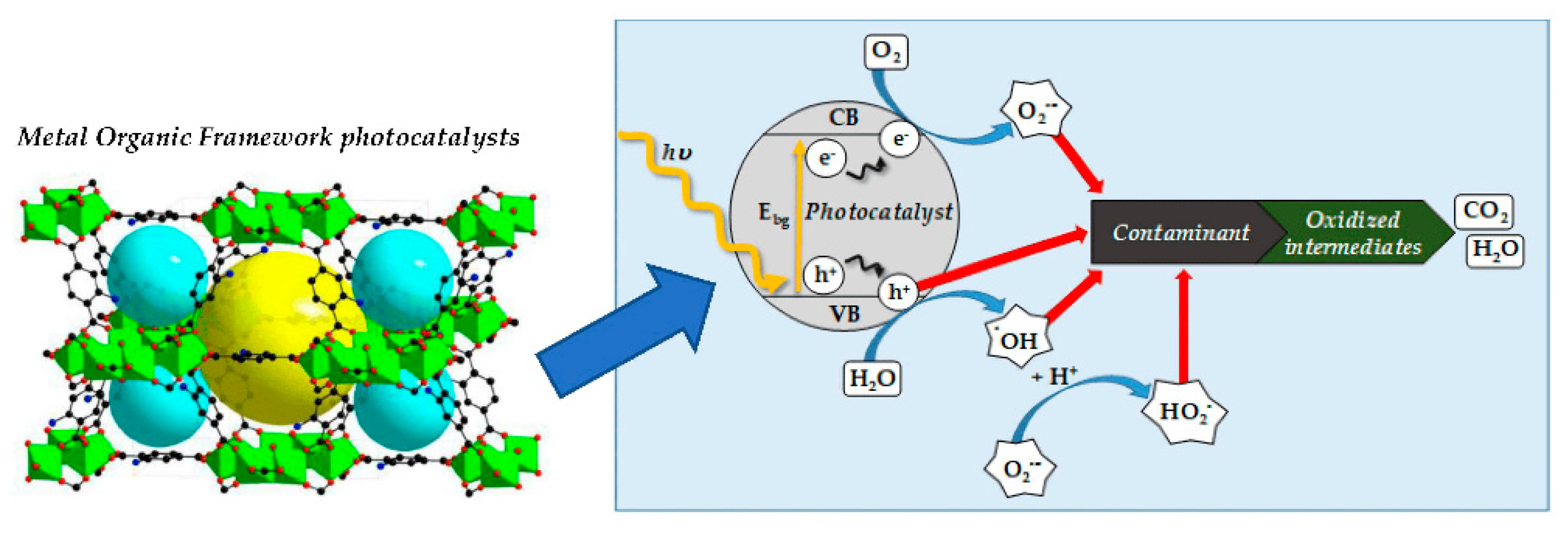
3.3.1. Photocatalytic Processes
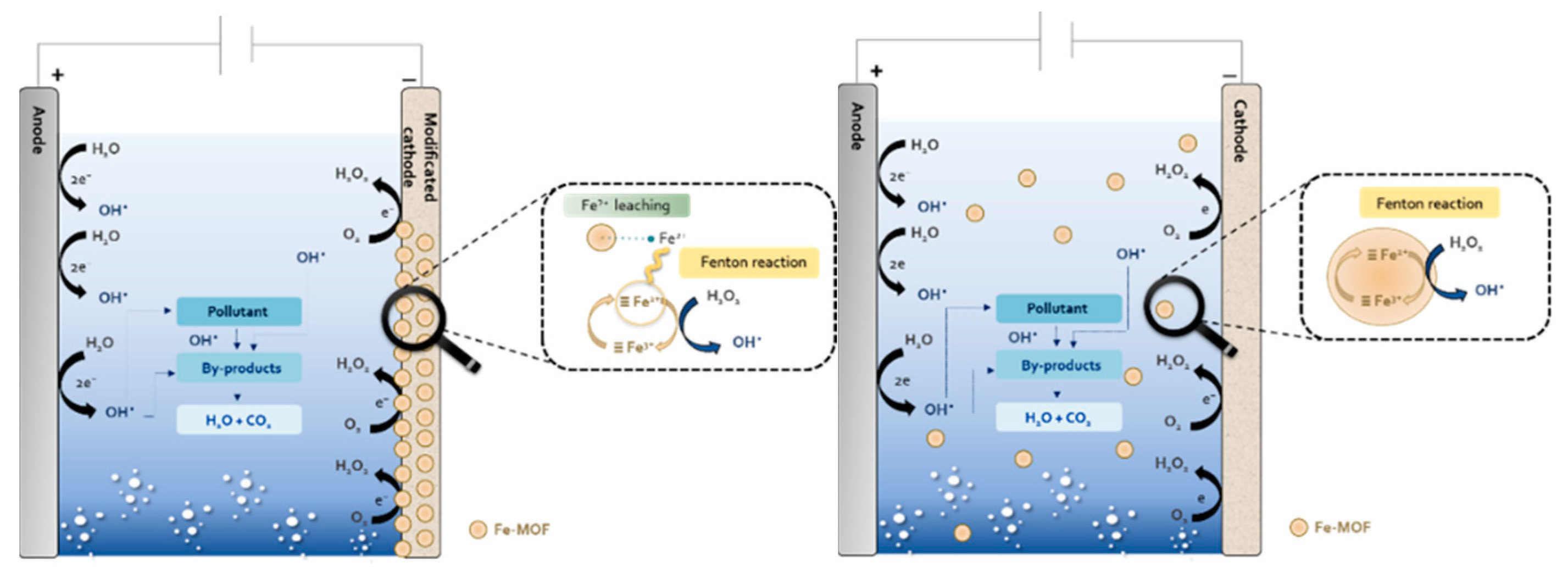
3.3.2. Fenton and Fenton-Based Processes
| MOF MOF-Derived | Process | Dosage (g/L) | Efficiency (%) | Pollutant | Time (min) | Reference |
|---|---|---|---|---|---|---|
| Fe-BTC | Electro-Fenton | 0.75 | 100 | Tetracycline | 40 | [190] |
| Zn-MIL53(Fe) | Electro-Fenton | 0.43 | 100/100 | Sulfamethoxazole/Fluoxetine | 90 | [43] |
| HKUST-1@C/Graphene | Electro-Fenton | 0.25 | 100 | Rhodamine B | 150 | [186] |
| MIL53(Fe) | Fenton | 0.50 0.50 0.50 | 100 100 97 | Methylene Blue Phenol Rhodamine B | 20 20 30 | [28] |
| Co-MIL53(Fe) | Fenton | 0.13 | 90 | Oxytetracycline | 90 | [61] |
| FeCu-MOF | Fenton-like (PMS) | 1.19 | 81 | Sulfamethoxazole | 90 | [164] |
| Ni-MOF | Fenton-Like (PS) | 0.2 | 15 | Tetracycline | 45 | [198] |
| MnFeO-ZIF-8 | Fenton-like (PMS) | 0.5 | 100 | Bisphenol A | 15 | [199] |
| HKUST-1 | Fenton-like (PMS) | 0.66 | 100 | Antipyrine | 300 | [200] |
| Zn-MIL53(Fe) | Fenton-like (PMS) | 0.43 | 85/100 | Sulfamethoxazole/Fluoxetine | 240 | [44] |
| Mn-MIL53(Fe) | Fenton-like (PMS) | 0.2 | 90 | Tetracycline | 60 | [201] |
| Ni-MIL101(Fe) | Fenton-like (PS) | 0.2 | 96 | Tetracycline | 30 | [198] |
| MIL101(Fe) | Fenton-like (PS) | 0.2 | 31 | Tetracycline | 45 | [198] |
| MIL101(Fe) | Fenton-like (PMS) | 0.4 | 72 | Methylene Blue | 25 | [123] |
| Cu-MIL101(Fe) | Fenton-like (PS) photocatalysis | 0.3/0.05 | 91 | Tetracycline | 120 | [202] |
| MIL101(Fe) | Fenton-like (PS) photocatalysis | 0.3/0.05 | 52 | Tetracycline | 120 | [202] |
| UiO-66(Zr)/MIL125(Ti)@GN | Photocatalysis | 0.1 | 99 | Ofloxacin | 50 | [170] |
| AgWO-ZIF-8 | Photocatalysis | 0.1 | 98 | Methylene Blue | 120 | [169] |
| MIL101(Fe)/TiO2 | Adsorption-Photocatalysis | 0.5 | 92 | Fluoxetine | 20 | [138] |
| HF-UiO-67 | Photocatalysis-Ultrasound | 0.6 | 91 | Rhodamine B | 1 | [183] |
| OH-UiO-66 | Photo-Fenton | 8 | 95 | Sulfamethoxazole | 120 | [23] |
| Ag-ZIF-8 | Photo-Fenton | 0.2 | 68 | Methylene Blue | 90 | [203] |
| UiO-66 | Photo-Fenton | 0.1 | 95 | Tetracycline | 120 | [204] |
3.4. Enhancing Catalyst Performance of MOFs
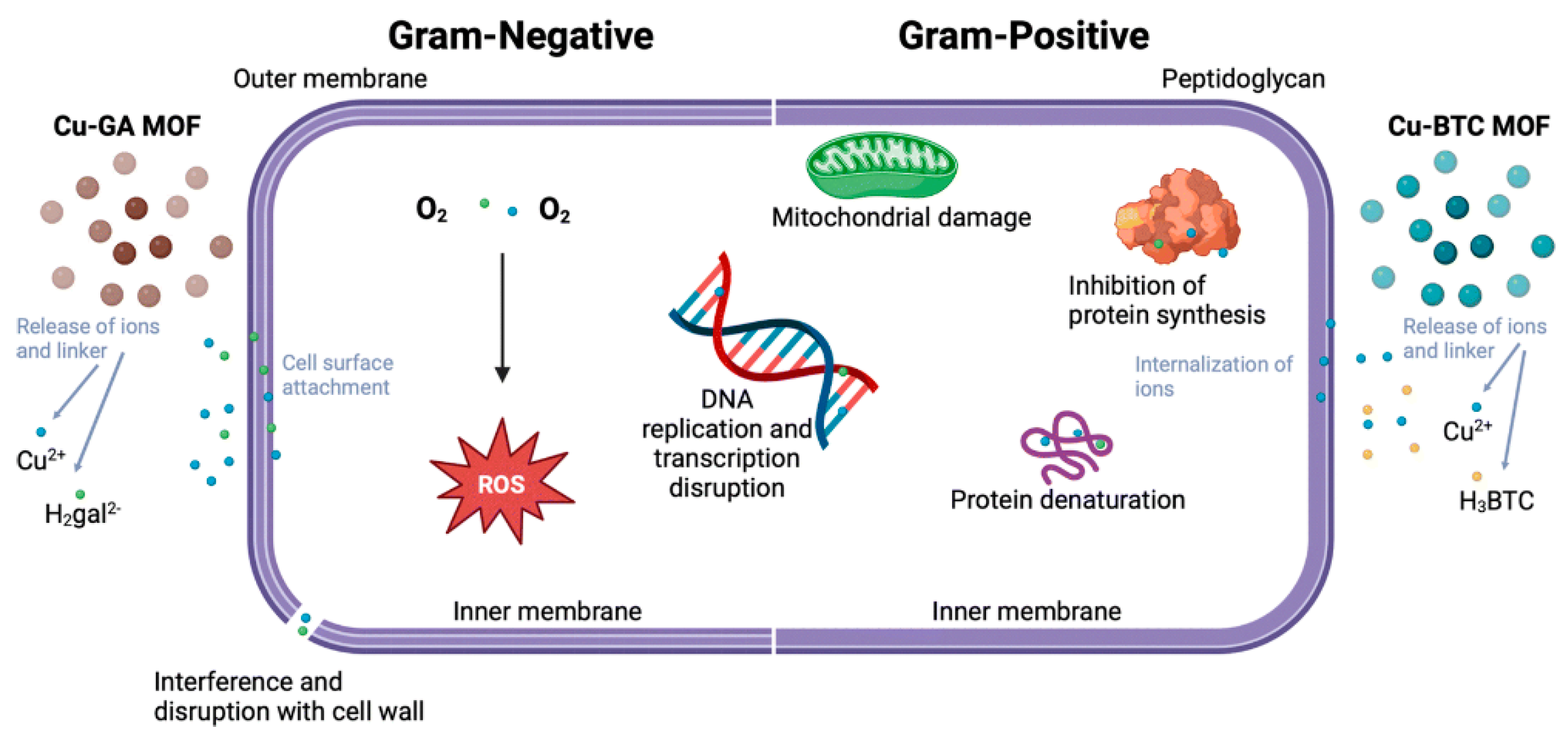
3.5. Antibacterial and Antimicrobial Properties of MOFs
3.6. Influence of Real Water Matrices on the Performance of MOFs in AOPs and Antimicrobial Applications
| MOF MOF-Derived | Process | Dosage (g/L) | Efficiency (%) | Pathogen | Time (min) | Reference |
|---|---|---|---|---|---|---|
| CuO-MOF2/MMT | Agar-diffusion | - | Inhibition | E. coli, B. subtilis | 24 h | [232] |
| ZnO-MIL53(Fe) | Agar-diffusion | - | Inhibition | E. coli | 24 h | [208] |
| CuO-MIL53(Fe) | Agar-diffusion | - | Inhibition | E. coli | 24 h | [208] |
| CuBTC | Agar-diffusion | - | Inhibition | E. coli, Lactobacillus | 90 | [217] |
| ZIF-8 | Agar-diffusion | - | Inhibition | E. coli | 24 h | [210] |
| SH-MOF(UiO-66)@Cotton | Membrane | 1 | >90 | S. aureus | 60 | [218] |
| SrTiO/CuFeO-MIL101(Co) | Agar-diffusion | - | Inhibition | S. aureus, Candida albicans, P. aeruginosa | 24 h | [220] |
| MIL53(Fe) | Photo-electro-Fenton | 0.68 | >85 | Pseudomonae, Enterobacteriae | 60 | [221] |
| MIL100(Fe) | Fenton | 0.1 | >99 | S. aureusP. aeruginosa | 24 h | [222] |
| NiCoFe-MOF | Fenton | 0.1 | 100/100 | E. coli, S. aureus | 120 | [27] |
| HKUST-1 | Fenton-Like (PMS) | 0.66 | 100 | E. coli | 5 | [200] |
| PAN-HKUST-1 | Fenton-Like (PMS) | 0.61 | 100 | E. coli | 30 | [224] |
| CuFe-MOF | Fenton-Like (PMS) | 0.25 | 100 | E. coli | 60 | [223] |
| Zn-MIL53(Fe) | Electro-Fenton | 0.43 | 100 | E. coli, P. aeruginosa, L. crispatus | 5 | [43] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, B.; Tyagi, R.D. Microbiology of Hospital Wastewater. In Current Developments in Biotechnology and Bioengineering: Environmental and Health Impact of Hospital Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–148. [Google Scholar] [CrossRef]
- Kumari, A.; Maurya, N.S.; Tiwari, B. Hospital Wastewater Treatment Scenario around the Globe. In Current Developments in Biotechnology and Bioengineering Environmental and Health Impact of Hospital Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–570. [Google Scholar] [CrossRef]
- Gatou, M.A.; Vagena, I.A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environ and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, W.; Hossain, M.S.A.; You, J.; Kim, J. Recent Progress of Functional Metal–Organic Framework Materials for Water Treatment Using Sulfate Radicals. Env. Res. 2022, 211, 112956. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of Various Dimensional Metal Organic Frameworks (MOFs) and Their Hybrid Composites for Emerging Applications—A Review. Chemosphere 2022, 298, 134184. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Yang, T.; Sridhar, D.; Algadi, H.; Bin Xu, B.; El-Bahy, Z.M.; Li, H.; Ma, Y.; Li, T.; et al. An Overview of Metal-Organic Frameworks and Their Magnetic Composites for the Removal of Pollutants. Sep. Purif. Technol. 2023, 320, 124144. [Google Scholar] [CrossRef]
- Marghade, D.; Shelare, S.; Prakash, C.; Soudagar, M.E.M.; Yunus Khan, T.M.; Kalam, M.A. Innovations in Metal-Organic Frameworks (MOFs): Pioneering Adsorption Approaches for Persistent Organic Pollutant (POP) Removal. Env. Res. 2024, 258, 119404. [Google Scholar] [CrossRef]
- Bradai, M.; Ait Radi, M.; Zeggai, F.Z.; Karkachi, N.; Meghabar, R. In-situ Synthesis of Highly Potent Antibacterial Copper-Based MOFs/Sodium Alginate Composite Beads. ChemSelect 2024, 9, e202304855. [Google Scholar] [CrossRef]
- Sivaprakash, B.; Rajamohan, N.; Singaramohan, D.; Ramkumar, V.; Elakiya, B.T. Techniques for Remediation of Pharmaceutical Pollutants Using Metal Organic Framework—Review on Toxicology, Applications, and Mechanism. Chemosphere 2022, 308, 136417. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Yu, Y.; Yu, Z.; Lai, L.; Liu, F.; Wei, L.; Chen, Y. Bifunctional Catalysts for Heterogeneous Electro-Fenton Processes: A Review. Env. Chem. Lett. 2022, 20, 3837–3859. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Su, R.; Lv, G.; Wang, Z.; Gao, B.; Zhou, W. Enhanced Degradation of Bisphenol F in a Porphyrin-MOF Based Visible-Light System under High Salinity Conditions. Chem. Eng. J. 2022, 428, 132106. [Google Scholar] [CrossRef]
- Hubab, M.; Al-Ghouti, M.A. Recent Advances and Potential Applications for Metal-Organic Framework (MOFs) and MOFs-Derived Materials: Characterizations and Antimicrobial Activities. Biotech. Rep. 2024, 42, e00837. [Google Scholar] [CrossRef]
- Kumar, A.; Kataria, R. MOFs as Versatile Scaffolds to Explore Environ Contaminants Based on Their Luminescence Bustle. Sci. Total Environ. 2024, 926, 172129. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–Organic Frameworks (MOFs) for the Efficient Removal of Contaminants from Water: Underlying Mechanisms, Recent Advances, Challenges, and Future Prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent Advances in Application of Metal-Organic Frameworks (MOFs) as Adsorbent and Catalyst in Removal of Persistent Organic Pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef]
- Malekian, M.; Fahimi, H.; Niri, N.M.; Khaleghi, S. Development of Novel Chimeric Endolysin Conjugated with Chitosan-Zn-Metal–Organic Framework Nanocomposites with Antibacterial Activity. Appl. Biochem. Biotechnol. 2024, 196, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, M.A.; Abbas, Q.; Mouselly, M.; Alawadhi, H.; Olabi, A.G. High-Performance Effective Metal–Organic Frameworks for Electrochem Applications. J. Sci: Adv. Mater. Devices 2022, 7, 100465. [Google Scholar] [CrossRef]
- Kong, X.J.; Li, J.R. An Overview of Metal–Organic Frameworks for Green Chem Eng. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Gupta, N.K.; Bae, J.; Kim, S.; Kim, K.S. Fabrication of Zn-MOF/ZnO Nanocomposites for Room Temperature H2S Removal: Adsorption, Regeneration, and Mechanism. Chemosphere 2021, 274, 129789. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zhang, Y.; Lv, X.-X.; Cui, M.-S.; Cui, K.-P.; Dai, Z.-L.; Wang, B.; Weerasooriya, R.; Chen, X. Efficient Degradation of Sulfamethoxazole by Diatomite-Supported Hydroxyl-Modified UIO-66 Photocatalyst after Calcination. Nanomaterials 2023, 13, 3116. [Google Scholar] [CrossRef]
- Salari Joo, H.; Behzadi Tayemeh, M.; Abaei, H.; Johari, S.A. On How Zeolitic Imidazolate Framework-8 Reduces Silver Ion Release and Affects Cytotoxicity and Antimicrobial Properties of AgNPs@ZIF8 Nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131411. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Tan, Y.; Jiang, C.; Wang, H.; Zhang, S. Preparation of Millimeter-Scale MIL-53(Fe)@polyethersulfone Balls to Optimize Photo-Fenton Process. Chem. Eng. J. 2022, 441, 135881. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal- and Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (PMS) Activation, with Focus on Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Pu, H.; Ouyang, Q.; Zhou, X.; Sun, D.-W. Multifunctional Trimetal-Organic Frameworks with Enhanced Fenton-like Catalytic Activity for Inhibiting Bacteria. J. Food Meas. Charact. 2024, 18, 5130–5144. [Google Scholar] [CrossRef]
- Dinh Du, P.; Ngoc Hoai, P. Synthesis of MIL-53(Fe) Metal-Organic Framework Material and Its Application as a Catalyst for Fenton-Type Oxidation of Organic Pollutants. Adv. Mater. Sci. Eng. 2021, 2021, 5540344. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Y.; Rushlow, J.; Huo, J.; Liu, Z.; Hsu, Y.C.; Yin, R.; Wang, M.; Liang, R.; Wang, K.Y.; et al. Development of the Design and Synthesis of Metal–Organic Frameworks (MOFs)—From Large Scale Attempts, Functional Oriented Modifications, to Artificial Intelligence (AI) Predictions. Chem. Soc. Rev. 2025, 54, 367–395. [Google Scholar] [CrossRef]
- Jin, C.; Shi, S.; Liao, S.; Liu, S.; Xia, S.; Luo, Y.; Wang, S.; Wang, H.; Chen, C. Post-Synthetic Ligand Exchange by Mechanochem: Toward Green, Efficient, and Large-Scale Preparation of Functional Metal-Organic Frameworks. Chem. Mater. 2023, 35, 4489–4497. [Google Scholar] [CrossRef]
- Gupta, M.; Chatterjee, N.; De, D.; Saha, R.; Chattaraj, P.K.; Oliver, C.L.; Bharadwaj, P.K. Metal-Organic Frameworks of Cu(II) Constructed from Functionalized Ligands for High Capacity H2 and CO2 Gas Adsorption and Catalytic Studies. Inorg. Chem. 2020, 59, 1810–1822. [Google Scholar] [CrossRef]
- Sun, X.; Shi, L.; Hu, H.; Huang, H.; Ma, T.; Sun, X.; Shi, L.; Hu, H.; Ma, T.; Huang, H.W. Ligand Functionalization in Zirconium-Based Metal-Organic Frameworks for Enhanced Carbon Dioxide Fixation. Adv. Sustain. Syst. 2020, 4, 2000098. [Google Scholar] [CrossRef]
- Kim, D.; Ha, H.; Kim, Y.; Son, Y.; Choi, J.; Park, M.H.; Kim, Y.; Yoon, M.; Kim, H.; Kim, D.; et al. Experimental, Structural, and Computational Investigation of Mixed Metal-Organic Frameworks from Regioisomeric Ligands for Porosity Control. Cryst. Growth Des. 2020, 20, 5338–5345. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Son, Y.; Kim, J.Y.; Cha, S.; Kwak, D.; Lee, J.; Kwak, J.; Yoon, M.; Kim, M. Uncoordinated Tetrazole Ligands in Metal–Organic Frameworks for Proton-Conductivity Studies. Bull. Korean Chem. Soc. 2022, 43, 912–917. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Wang, W.; Burger, S.; Wang, Z.; Wang, Y.; Wöll, C.; Cokoja, M.; Fischer, R.A. Defect Eng of Copper Paddlewheel-Based Metal-Organic Frameworks of Type NOTT-100: Implementing Truncated Linkers and Its Effect on Catalytic Properties. ACS Appl. Mater. Interfaces 2020, 12, 37993–38002. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Gao, D.; Cheng, P.; Ma, J.G.; Song, D. Impact of Ligands on the Properties of Lanthanide Metal-Organic Frameworks. Z. Anorg. Allg. Chem. 2023, 649, e202200379. [Google Scholar] [CrossRef]
- Wen, Y.; Shi, D.; Duan, Z. Two Copper (II) Complexes Based on Different Copper Salts, 1,3-Benzenedicarboxylic Acid and 1,4-Di(Imidazolidin-1-Yl) Benzene and Their Fluorescence Recognition to Nitrobenzene Derivatives. J. Solid. State Chem. 2020, 287, 121334. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Jiao, S.; Song, X.; Li, S.; Liu, K.; Wang, L. Coordination Polymers Driven by 2,5-Dibromoterephthalic Acid and Chelating Co-Ligands: Syntheses, Structures and Luminescent Properties. J. Solid. State Chem. 2020, 292, 121721. [Google Scholar] [CrossRef]
- Łuczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology Control through the Synthesis of Metal-Organic Frameworks. Adv. Colloid. Interface Sci. 2023, 314, 102864. [Google Scholar] [CrossRef]
- Omkaramurthy, B.M.; Krishnamurthy, G.; Prasad, N.L. Two New Zn (II) Bdc Metal-Organic Frameworks Based on Benzene 1, 4-Dicarboxylic Acid: Synthesis, Crystal Structures, Luminescent Properties and Electrochem Studies. Mater. Today Proc. 2020, 22, 2179–2190. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, R.; Shu, T.; Wang, Y.; Li, L. Synthesis of Co-Doped Fe Metal–Organic Framework MIL-101(Fe,Co) and Efficient Degradation of Organic Dyes in Water. Sep. Purif. Technol. 2023, 304, 122300. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y.; Cheng, J.; Chen, Y. Enhanced Performance of Sulfamethoxazole Degradation Using Achromobacter Sp. JL9 with In-Situ Generated Biogenic Manganese Oxides. Bioresour. Technol. 2021, 333, 125089. [Google Scholar] [CrossRef]
- Terrón, D.; Holgado-Vázquez, J.P.; Rosales, E.; Sanromán, M.A.; Pazos, M. Zn-MIL53(Fe) as an Electro-Fenton Catalyst: Application in Organic Pollutant Degradation and Pathogen Inactivation. Sep. Purif. Technol. 2024, 360, 130881. [Google Scholar] [CrossRef]
- Terrón, D.; Holgado-Vázquez, J.P.; Giráldez, A.; Rosales, E.; Sanromán, M.A.; Pazos, M. Application of Novel Zn-MIL53(Fe) for Removal of Micropollutants Using an Activated Peroxymonosulphate System. J. Env. Chem. Eng. 2024, 12, 113403. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, Y.; Kwak, S.Y. Facile Sonochem Synthesis of Flexible Fe-Based Metal-Organic Frameworks and Their Efficient Removal of Organic Contaminants from Aqueous Solutions. ACS Omega 2022, 7, 23213–23222. [Google Scholar] [CrossRef]
- Lv, Y.; Xue, J.; Chen, Z.; Qu, J.; Huang, K.; Wang, M.; Sun, W. Development of Hydrothermal Carbonaceous Carbon/NH2-MIL-101(Fe) Composite Photocatalyst with in-Situ Production and Activation of H2O2 Capabilities for Effective Sterilization. Chem. Eng. J. 2024, 498, 155263. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, Y.; Yang, Y.; Wang, H.; Ma, J.; Wang, D.; Gao, N.; Li, L.; Liu, W.; Wang, H. Synthesis Optimization of Metal-Organic Frameworks MIL-125 and Its Adsorption Separation on C8 Aromatics Measured by Pulse Test and Simulation Calculation. J. Solid. State Chem. 2021, 296, 121956. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Li, F.; Wu, Y.; Xi, H. Ultrafast Room-Temperature Synthesis of Hierarchically Porous Metal–Organic Frameworks with High Space–Time Yields. CrystEngComm 2020, 22, 2675–2680. [Google Scholar] [CrossRef]
- Xia, W.; Lau, S.K.; Yong, W.F. Comparative Life Cycle Assessment on Zeolitic Imidazolate Framework-8 (ZIF-8) Production for CO2 Capture. J. Clean. Prod. 2022, 370, 133354. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, H.; Le, P.H.; Nguyen, D.T.; Nguyen, T.T.; Van Nguyen, C.; Vo, D.V.; Nguyen, T.D. Microwave-Assisted Solvothermal Fabrication of Hybrid Zeolitic–Imidazolate Framework (ZIF-8) for Optimizing Dyes Adsorption Efficiency Using Response Surface Methodology. J. Env. Chem. Eng. 2020, 8, 104189. [Google Scholar] [CrossRef]
- Morales, E.M.C.; Méndez-Rojas, M.A.; Torres-Martínez, L.M.; Garay-Rodríguez, L.F.; López, I.; Uflyand, I.E.; Kharisov, B.I. Ultrafast Synthesis of HKUST-1 Nanoparticles by Solvothermal Method: Properties and Possible Applications. Polyhedron 2021, 210, 115517. [Google Scholar] [CrossRef]
- Sondermann, L.; Smith, Q.; Strothmann, T.; Vollrath, A.; Beglau, T.H.; Janiak, C. Christoph Janiak Mechanochem Synthesis and Application of Mixed-Metal Copper–Ruthenium HKUST-1 Metal–Organic Frameworks in the Electrocatalytic Oxygen Evolution Reaction. RSC Mechanochem. 2024, 1, 296–307. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, F.; Huelsenbeck, L.; Smith, N. Comparison between Conventional Solvothermal and Aqueous Solution-Based Production of UiO-66-NH2: Life Cycle Assessment, Techno-Economic Assessment, and Implications for CO2 Capture and Storage. J. Env. Chem. Eng. 2021, 9, 105159. [Google Scholar] [CrossRef]
- Su, X.; Xu, T.; Ye, R.; Guo, C.; Wabaidur, S.M.; Chen, D.L.; Aftab, S.; Zhong, Y.; Hu, Y. One-Pot Solvothermal Synthesis of In-Doped Amino-Functionalized UiO-66 Zr-MOFs with Enhanced Ligand-to-Metal Charge Transfer for Efficient Visible-Light-Driven CO2 Reduction. J. Colloid. Interface Sci. 2023, 646, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Liu, X.; Song, X.L.; Yang, T.L.; Liang, Z.H.; Fan, C.M. In Situ Electrochem Synthesis of MOF-5 and Its Application in Improving Photocatalytic Activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- Mahdipoor, H.R.; Halladj, R.; Ganji Babakhani, E.; Amjad-Iranagh, S.; Sadeghzadeh Ahari, J. Synthesis, Characterization, and CO2 Adsorption Properties of Metal Organic Framework Fe-BDC. RSC Adv. 2021, 11, 5192–5203. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jia, Y.; Tang, Q.; Mao, D.; Jiang, L.; Kang, J.; Li, J.; Wang, J. Binary Metal Organic Frameworks (UIO-66 and ZIF-67) for Adsorptive Removal of Sb. J. Nanoparticle Res. 2022, 24, 158. [Google Scholar] [CrossRef]
- Yeskendir, B.; Dacquin, J.P.; Lorgouilloux, Y.; Courtois, C.; Royer, S.; Dhainaut, J. From Metal–Organic Framework Powders to Shaped Solids: Recent Devs and Challenges. Mater. Adv. 2021, 2, 7139–7186. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Xu, M.; Liu, S.; Yao, J. Fast Adsorption of Methyl Blue on Zeolitic Imidazolate Framework-8 and Its Adsorption Mechanism. RSC Adv. 2016, 6, 109608–109612. [Google Scholar] [CrossRef]
- Aryee, A.A.; Liu, Y.; Han, R.; Qu, L. Bimetallic Adsorbents for Wastewater Treatment: A Review. Environ. Chem. Lett. 2023, 21, 1811–1835. [Google Scholar] [CrossRef]
- Rajeendre Katugampalage, T.; Waribam, P.; Opaprakasit, P.; Kaewsaneha, C.; Hsu, S.H.; Chooaksorn, W.; Jong, C.A.; Sooksaen, P.; Ratanatawanate, C.; Sreearunothai, P. Bimetallic Fe:Co Metal–Organic Framework (MOF) with Unsaturated Metal Sites for Efficient Fenton-like Catalytic Degradation of Oxytetracycline (OTC) Antibiotics. Chem. Eng. J. 2024, 479, 147592. [Google Scholar] [CrossRef]
- Luo, J.; Luo, X.; Gan, Y.; Xu, X.; Xu, B.; Liu, Z.; Ding, C.; Cui, Y.; Sun, C. Advantages of Bimetallic Organic Frameworks in the Adsorption, Catalysis and Detection for Water Contaminants. Nanomaterials 2023, 13, 2194. [Google Scholar] [CrossRef]
- Du, X.; Zhou, M. Strategies to Enhance Catalytic Performance of Metal–Organic Frameworks in Sulfate Radical-Based Advanced Oxidation Processes for Organic Pollutants Removal. Chem. Eng. J. 2021, 403, 126346. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, T.D.; Bach, L.G.; Hoang, T.; Bui, Q.T.P.; Tran, L.D.; Nguyen, C.V.; Vo, D.V.N.; Do, S.T. Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp. Catalysts 2018, 8, 487. [Google Scholar] [CrossRef]
- Shaik, M.R.; Adil, S.F.; Alothman, Z.A.; Alduhaish, O.M. Fumarate Based Metal–Organic Framework: An Effective Catalyst for the Transesterification of Used Vegetable Oil. Crystals 2022, 12, 151. [Google Scholar] [CrossRef]
- Ji, F.; Jiao, D.; Wang, H.; Cao, Q.; Guo, J.; Cao, X.; Li, Y.; Gao, Y. Study on Adsorption Performance of Fe-Modified ZIF-67 Bimetallic Organic Framework for Toluene. E3S Web Conf. 2023, 406, 02045. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Li, Z.; Goyal, N.; Du, T.; He, J.; Li, G.K. Shaping of Metal-Organic Frameworks: A Review. Energy Fuels 2022, 36, 2927–2944. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Z.; Wei, N.; Lin, X.; Hu, C. Microfluidic Synthesis of Defective and Hierarchical Pore Zr Metal-Organic Framework Materials and CO2 Adsorption Performance Study. Cryst. Growth Des. 2024, 24, 9084–9096. [Google Scholar] [CrossRef]
- Jiang, M.; Liao, J.; Liu, C.; Liu, J.; Chen, P.; Zhou, J.; Du, Z.; Liu, Y.; Luo, Y.; Liu, Y.; et al. Metal-Organic Frameworks/Metal Nanoparticles as Smart Nanosensing Interfaces for Electrochem Sensors Applications: A Mini-Review. Front. Bioeng. Biotechnol. 2023, 11, 1251713. [Google Scholar] [CrossRef]
- Al Amery, N.; Abid, H.R.; Al-Saadi, S.; Wang, S.; Liu, S. Facile Directions for Synthesis, Modification and Activation of MOFs. Mater. Today Chem. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Phan, P.T.; Hong, J.; Tran, N.; Le, T.H. The Properties of Microwave-Assisted Synthesis of Metal–Organic Frameworks and Their Applications. Nanomaterials 2023, 13, 352. [Google Scholar] [CrossRef]
- Iram, G.; Ateeq-Ur-Rehman; Iqbal, M.A.; Zafar, A.; Majeed, A.; Hayat, S.; Nawaz, M. Advanced Synthetic Routes of Metal Organic Frameworks and Their Diverse Applications. Rev. Inorg. Chem. 2024, 44, 449–470. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T.; Maleki, A.; Tarlani, A.; Soroush, M.R. Synthesis and Characterization of Ultrapure HKUST-1 MOFs as Reusable Heterogeneous Catalysts for the Green Synthesis of Tetrazole Derivatives. ChemSelect 2020, 5, 3164–3172. [Google Scholar] [CrossRef]
- Liu, K. Metal-Organic Frameworks Synthetic Approaches and Applications in Energy Industry. E3S Web Conf. 2024, 553, 02009. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, C.A. Metal–Organic Framework Gels for Adsorption and Catalytic Detoxification of Chem Warfare Agents: A Review. Gels 2023, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wei, T. Electrochem Synthesis Methods of Metal-Organic Frameworks and Their Environ Analysis Applications: A Review. ChemElectroChem 2022, 9, e202200196. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent Advances in Metal-Organic Frameworks: Synthesis, Application and Toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Gao, A.; Cao, Y.; Svec, F.; Tan, T.; Lv, Y. Layer-by-Layer Assembly of Metal–Organic Frameworks in Macroporous Polymer Monolith and Their Use for Enzyme Immobilization. Macromol. Rapid Commun. 2016, 37, 551–557. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Zhao, A.; Tan, K.; Cui, Y. High-Alumina Fly Ash as Sustainable Aluminum Sources for the in Situ Preparation of Al-Based Eco-MOFs. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128421. [Google Scholar] [CrossRef]
- Burgaz, E.; Erciyes, A.; Andac, M.; Andac, O. Synthesis and Characterization of Nano-Sized Metal Organic Framework-5 (MOF-5) by Using Consecutive Combination of Ultrasound and Microwave Irradiation Methods. Inorganica Chim. Acta 2019, 485, 118–124. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Dong, S.; Miao, X.; Zhang, M.; Sun, K.; Zhang, Y.; Cao, Z.; Sun, J. Wool-Ball-like BiOBr@ZnFe-MOF Composites for Degradation Organic Pollutant under Visible-Light: Synthesis, Performance, Characterization and Mechanism. Opt. Mater. 2022, 131, 112580. [Google Scholar] [CrossRef]
- Liu, S.; Huo, Q.; Chen, R.; Chen, P.; Li, Y.; Han, Y. Synthesis and Characterization of an Iron Nitride Constructed by a Novel Template of Metal Organic Framework. J. Spectrosc. 2015, 2015, 362103. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, T.D.; Van Nguyen, T. Microwave-Assisted Solvothermal Synthesis and Photocatalytic Activity of Bismuth(III) Based Metal–Organic Framework. Top. Catal. 2020, 63, 1109–1120. [Google Scholar] [CrossRef]
- Chongdar, S.; Ghosh, A.; Bal, R.; Bhaumik, A. Microwave-Assisted Synthesis of ZIF-9@xGO Composites as Cooperative Electrocatalysts for Electro-Oxidation of Benzyl Alcohols Coupled with H2 Production. J. Mater. Chem. A Mater. 2023, 12, 233–246. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, T.; Zeng, Y.; Zheng, M.-Y.; Zhang, F.-Z.; Wang, Y.-L.; Lin, Z.-J.; Lin, R.-G. Hierarchical Micro- and Mesoporous Zeolitic Imidazolate Framework-8-Based Enzyme Hybrid for Combination Antimicrobial by Lysozyme and Lactoferrin. Inorg. Chem. 2024, 63, 12377–12384. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Gao, J.; Deng, J.; Zheng, L.; Liu, M.; Wei, P.; Wang, Y.; Li, Y. A Novel ZIF-8 and ZIF-67 Co-Modified TiO2 Nanospheres for Highly Efficient Degradation of Methylene Blue under Simulated Sunlight. J. Mater. Sci. Mater. Electron. 2023, 34, 2291. [Google Scholar] [CrossRef]
- Pouramini, Z.; Mousavi, S.M.; Babapoor, A.; Hashemi, S.A.; Lai, C.W.; Mazaheri, Y.; Chiang, W.H. Effect of Metal Atom in Zeolitic Imidazolate Frameworks (ZIF-8 & 67) for Removal of Dyes and Antibiotics from Wastewater: A Review. Catalysts 2023, 13, 155. [Google Scholar] [CrossRef]
- Lo Presti, F.; Pellegrino, A.L.; Consoli, N.; Malandrino, G. Green Ultrasound-Assisted Synthesis of Rare-Earth-Based MOFs. Molecules 2023, 28, 6088. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kim, J.; Kim, S.N.; Ahn, W.S. High Yield 1-L Scale Synthesis of ZIF-8 via a Sonochem Route. Microporous Mesoporous Mater. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Shamspur, T.; Mostafavi, A.; Sargazi, G. A Novel Ultrasonic Reverse Micelle-Assisted Electrospun Efficient Route for Eu-MOF and Eu-MOF/CA Composite Nanofibers: A High Performance Photocatalytic Treatment for Removal of BG Pollutant. Env. Sci. Pollut. Res. 2021, 28, 4317–4328. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, C.; Thummavichai, K.; Feng, C.; Li, Z.; Liu, S.; Zhang, S.; Wang, N.; Zhu, Y. MOF-Derived Biochar Composites for Enhanced High Performance Photocatalytic Degradation of Tetracycline Hydrochloride. RSC Adv. 2022, 12, 31900–31910. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochem Deposition of Metal–Organic Framework Films and Their Applications. J. Mater. Chem. A Mater. 2020, 8, 7569–7587. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.R.C.; Li, M.; Stappers, L.; Dinca, M.; Denayer, J.F.M.; Binnemans, K.; De Vos, D.E.; Fransaer, J. On the Electrochem Deposition of Metal–Organic Frameworks. J. Mater. Chem. A Mater. 2016, 4, 3914–3925. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Afkhami, A.; Madrakian, T.; Ahmadi, M. Electrochem Synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 177–195. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zeng, X.; Li, M.; Wang, M.Q.; Su, T.Y.; Tian, X.C.; Tang, J. Rapid Electrochem Synthesis of HKUST-1 on Indium Tin Oxide. RSC Adv. 2017, 7, 9316–9320. [Google Scholar] [CrossRef]
- Gómez-López, P.; Murat, M.; Hidalgo-Herrador, J.M.; Carrillo-Carrión, C.; Balu, A.M.; Luque, R.; Rodríguez-Padrón, D. Mechanochem Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions. Catalysts 2021, 11, 526. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochem Synthesis of MOF-303 and Its CO2 Adsorption at Ambient Conditions. Molecules 2024, 29, 2698. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Ding, W.H. Mechanochemly Synthesized Zeolitic Imidazolate Framework-8 as Sorbent for Dispersive Solid-Phase Extraction of Benzophenone-Type Ultraviolet Filters in Aqueous Samples. J. Chromatogr. A 2022, 1681, 463443. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, H.; Fu, X.C.; Deng, N.M. Continuous, Solvent-Free, and Mechanochem Synthesis of UIO-66 (Mn) by Gas-Solid Two-Phase Flow and Mechanism Research. J. Solid. State Chem. 2024, 338, 124863. [Google Scholar] [CrossRef]
- El-Sayed, E.S.M.; Yuan, D. Waste to MOFs: Sustainable Linker, Metal, and Solvent Sources for Value-Added MOF Synthesis and Applications. Green. Chem. 2020, 22, 4082–4104. [Google Scholar] [CrossRef]
- Marino, P.; Donnarumma, P.R.; Bicalho, H.A.; Quezada-Novoa, V.; Titi, H.M.; Howarth, A.J. A Step toward Change: A Green Alternative for the Synthesis of Metal-Organic Frameworks. ACS Sustain. Chem. Eng. 2021, 9, 16356–16362. [Google Scholar] [CrossRef]
- Rajendran, H.K.; Deen, M.A.; Ray, J.P.; Singh, A.; Narayanasamy, S. Harnessing the Chem Functionality of Metal–Organic Frameworks Toward Removal of Aqueous Pollutants. Langmuir 2024, 40, 3963–3983. [Google Scholar] [CrossRef]
- Naseer, M.N.; Jaafar, J.; Junoh, H.; Zaidi, A.A.; Kumar, M.; Alqahtany, A.; Jamil, R.; Alyami, S.H.; Aldossary, N.A. Metal-Organic Frameworks for Wastewater Decontamination: Discovering Intellectual Structure and Research Trends. Materials 2022, 15, 5053. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Rangasamy, G. Removal of Toxic Pollutants From Industrial Effluent: Sustainable Approach and Recent Advances in Metal Organic Framework. Ind. Eng. Chem. Res. 2022, 61, 15754–15765. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Pramono, E.; Belva, J.; Yusariarta Parmita, A.W.; Patah, A.; Eddy, D.R.; Aimon, A.H.; Ramelan, A. Recent Improvement Strategies on Metal-Organic Frameworks as Adsorbent, Catalyst, and Membrane for Wastewater Treatment. Molecules 2021, 26, 5261. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, Y.; He, P.; Wang, Y.; Wei, G. Recent Advances in Metal–Organic Framework (MOF)-Based Composites for Organic Effluent Remediation. Materials 2024, 17, 2660. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Bahi, A.; Fernández, R.; Alaee, P.; Wu, S.; Wuttke, S.; Ko, F.; Arjmand, M. Metal-Organic Frameworks and Electrospinning: A Happy Marriage for Wastewater Treatment. Adv. Funct. Mater. 2022, 32, 2207723. [Google Scholar] [CrossRef]
- Evangelou, D.A.; Pournara, A.D.; Tziasiou, C.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Robust Al MOF With Selective As(V) Sorption and Efficient Luminescence Sensing Properties Toward Cr(VI). Inorg. Chem. 2022, 61, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Modified-Mof-808-Loaded Polyacrylonitrile Membrane for Highly Efficient, Simultaneous Emulsion Separation and Heavy Metal Ion Removal. ACS Appl. Mater. Interfaces 2020, 12, 39227–39235. [Google Scholar] [CrossRef]
- Le, T.; Chen, X.; Dong, H.; Tarpeh, W.A.; Perea-Cachero, A.; Coronas, J.; Martin, S.M.; Mohammad, M.; Razmjou, A.; Esfahani, A.R.; et al. An Evolving Insight Into Metal Organic Framework-Functionalized Membranes for Water and Wastewater Treatment and Resource Recovery. Ind. Eng. Chem. Res. 2021, 60, 6869–6907. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, J.; Lü, F.; Li, Y.; He, Y.; Wang, Y.; Li, L. Controllable Preparation of Cu-Mof-Coated Carboxyl Filter Paper for Simultaneous Removal of Organic Dye and Metal Ions. Ind. Eng. Chem. Res. 2021, 60, 7311–7319. [Google Scholar] [CrossRef]
- Roy, N.; Das, C.; Paul, M.; Im, J.; Biswas, G. Adsorptive Elimination of a Cationic Dye and a Hg (II)-Containing Antiseptic From Simulated Wastewater Using a Metal Organic Framework. Molecules 2024, 29, 886. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Osella, S.; Webb, S.M.; Yang, X.; Goddard, W.A.; Hoffmann, M.R. Modular Functionalization of Metal-Organic Frameworks for Nitrogen Recovery From Fresh Urine**. Angew. Chem. 2023, 62, e202309258. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Ghaffarkhah, A.; Molavi, H.; Dutta, S.; Lu, Y.; Wuttke, S.; Kamkar, M.; Rojas, O.J.; Arjmand, M. COF and MOF Hybrids: Advanced Materials for Wastewater Treatment. Adv. Funct. Mater. 2023, 34, 2305527. [Google Scholar] [CrossRef]
- Kumar, P.; Abbas, Z.; Kumar, P.; Das, D.; Mobin, S.M. Highlights in Interface of Wastewater Treatment by Utilizing Metal Organic Frameworks: Purification and Adsorption Kinetics. Langmuir 2024, 40, 5040–5059. [Google Scholar] [CrossRef]
- Guo, Z.; Jiao, W.; Du, P.; Yang, H.; Liu, X.; Chen, D.; Xing, H. Modified Metal–Organic Frameworks for Sonophotocatalytic Elimination of Wastewater Pollutants. Appl. Organomet. Chem. 2024, 38, e7523. [Google Scholar] [CrossRef]
- Badea, S.; Niculescu, V. Recent Progress in the Removal of Legacy and Emerging Organic Contaminants From Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes. Materials 2022, 15, 3850. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.; Serio, M. Di Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 581487. [Google Scholar] [CrossRef]
- Ji, Y.; An, C.; Liu, T. Photocatalytic Activity of Two MOFs Materials for Degradation of Dye Wastewater; IOS Press: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Ihsanullah, I. Applications of MOFs as Adsorbents in Water Purification: Progress, Challenges and Outlook. Curr. Opin. Env. Sci. Health 2022, 26, 100335. [Google Scholar] [CrossRef]
- Opinion of the European Economic and Social Committee on the Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (COM(2022) 541 Final—2022/0345 (COD)). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52022PC0541 (accessed on 24 February 2025).
- Yu, K.; Ahmed, I.; Won, D.I.; Lee, W.I.; Ahn, W.S. Highly Efficient Adsorptive Removal of Sulfamethoxazole from Aqueous Solutions by Porphyrinic MOF-525 and MOF-545. Chemosphere 2020, 250, 126133. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Fan, L.; Wang, Y.; Li, L. Degradation of Organic Dyes by Peroxymonosulfate Activated with Water-Stable Iron-Based Metal Organic Frameworks. J. Colloid. Interface Sci. 2021, 589, 298–307. [Google Scholar] [CrossRef]
- Rojas, S.; Navarro, J.A.R.; Horcajada, P. Metal-Organic Frameworks for the Removal of the Emerging Contaminant Atenolol under Real Conditions. Dalton Trans. 2021, 50, 2493–2500. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Hu, X.; Tan, W.; Li, J.; Su, D.; Wang, H.; Yang, M. A Study on the Performance of a Novel Adsorbent UiO-66 Modified by a Nickel on Removing Tetracycline in Wastewater. Chemosphere 2023, 338, 139399. [Google Scholar] [CrossRef]
- Pan, J.; Bai, X.; Li, Y.; Yang, B.; Yang, P.; Yu, F.; Ma, J. HKUST-1 Derived Carbon Adsorbents for Tetracycline Removal with Excellent Adsorption Performance. Env. Res. 2022, 205, 112425. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Li, W.; Wang, Y.; Zhang, T.C.; Wei, Y.; Yuan, S. Zr-Doped MIL-101(Fe)/Graphene Oxide Nanocomposites: An Efficient and Water-Stable MOF-Based Adsorbent for As(V) Adsorption in Aqueous Solution. Sep. Purif. Technol. 2024, 339, 126681. [Google Scholar] [CrossRef]
- Ibrahim, Q.; Gharbia, S. Adsorption Performance of G-C3N4/Graphene, and MIL-101(Fe)/Graphene for the Removal of Pharmaceutical Contaminants: A Molecular Dynamics Simulation Study. Sci. Rep. 2024, 14, 27109. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Zhang, C.; Zhang, X.; Zhang, R. Covalent Integration of Fe-Based Metal–Organic Framework and Triazine-Containing Covalent Organic Framework for Enhanced Adsorptive Removal of Antibiotics. J. Clean. Prod. 2024, 434, 140259. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Ma, S.; Xing, X.; Zhou, L.; Liu, Z.; Zhang, C. Adsorption Removal of Sulfamethoxazole from Water Using UiO-66 and UiO-66-BC Composites. Particuology 2022, 62, 71–78. [Google Scholar] [CrossRef]
- Hassan, A.; Hasan, S.W.; Van der Bruggen, B.; Al-Zuhair, S. Efficient Ibuprofen Removal Using Enzymatic Activated ZIF-8-PVDF Membranes. Clean. Eng. Technol. 2024, 23, 100824. [Google Scholar] [CrossRef]
- Peng, Y.; Pan, T.; Chen, C.; Zhang, Y.; Yuan, G.; Liu, D.; Pu, X.; Xiong, W. In Situ Synthesis of NH2-MIL-53-Al/PAN Nanofibers for Removal Co(II) through an Electrospinning Process. Langmuir 2024, 40, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Qamer Abbas, M.; Javeria, H.; Shuhuan, C.; Khan, J.; Nazir, A.; Ibrahim, S.; Du, Z. High-Performance Novel ZIF-67 and ZIF-67@MWNTs Composite Adsorbents for Efficient Removal of Pharmaceutical Contaminant from Water: Exceptional Capacity and Excellent Reusability. Sep. Purif. Technol. 2025, 355, 129645. [Google Scholar] [CrossRef]
- Sharma, U.; Pandey, R.; Basu, S.; Saravanan, P. ZIF-67 Blended PVDF Membrane for Improved Congo Red Removal and Antifouling Properties: A Correlation Establishment between Morphological Features and Ultra-Filtration Parameters. Chemosphere 2023, 320, 138075. [Google Scholar] [CrossRef]
- Sonawane, A.V.; Murthy, Z.V.P. Synthesis and Characterization of ZIF-8-Based PVDF Mixed Matrix Membranes and Application to Treat Pulp and Paper Industry Wastewater Using a Membrane Bioreactor. Env. Sci. 2022, 8, 881–896. [Google Scholar] [CrossRef]
- Ghaedi, S.; Rajabi, H.; Hadi Mosleh, M.; Babakhani, P.; Sedighi, M. UiO-67 Metal-Organic Framework Loaded on Hardwood Biochar for Sustainable Management of Environ Boron Contaminations. J. Env. Chem. Eng. 2024, 12, 114511. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.; Zhu, M.; Zheng, Y.Z.; Tao, X. Highly Effective PH-Universal Removal of Tetracycline Hydrochloride Antibiotics by UiO-66-(COOH)2/GO Metal–Organic Framework Composites. J. Solid. State Chem. 2020, 284, 121200. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M.; Vatanpour, V. Adsorption and Photocatalytic Degradation of Fluoxetine Using TiO2-Supported-Clinoptilolite, NaX and MIL-101 (Fe) Metal Organic Framework. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2154–2171. [Google Scholar] [CrossRef]
- Kaur, H.; Devi, N.; Siwal, S.S.; Alsanie, W.F.; Thakur, M.K.; Thakur, V.K. Metal-Organic Framework-Based Materials for Wastewater Treatment: Superior Adsorbent Materials for the Removal of Hazardous Pollutants. ACS Omega 2023, 8, 9004–9030. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y. Research on Improved MOF Materials Modified by Functional Groups for Purification of Water. Molecules 2023, 28, 2141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qileng, A.; Li, J.; Cao, Y.; Liu, W.; Liu, Y. Grafting a Porous Metal-Organic Framework [NH2-MIL-101(Fe)] with AgCl Nanoparticles for the Efficient Removal of Congo Red. ACS Omega 2023, 8, 4639–4648. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.K.; Woo, H.C.; Jhung, S.H. Removal of Particulate Matters with Isostructural Zr-Based Metal-Organic Frameworks Coated on Cotton: Effect of Porosity of Coated MOFs on Removal. ACS Appl. Mater. Interfaces 2020, 12, 34423–34431. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Y.; Zhang, H.; Zhuang, Z.; Yu, Y. Hierarchically Porous S-Scheme CdS/UiO-66 Photocatalyst for Efficient 4-Nitroaniline Reduction. Chin. J. Catal. 2021, 42, 78–86. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Bandosz, T.J. Activated Carbon versus Metal-Organic Frameworks: A Review of Their PFAS Adsorption Performance. J. Hazard. Mater. 2022, 425, 127810. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sert, E.; Atalay, F.S. Synthesis, Characterization of a Metal Organic Framework: MIL-53 (Fe) and Adsorption Mechanisms of Methyl Red onto MIL-53 (Fe). J. Taiwan. Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Quang, T.T.; Truong, N.X.; Minh, T.H.; Tue, N.N.; Ly, G.T.P. Enhanced Photocatalytic Degradation of MB Under Visible Light Using the Modified MIL-53(Fe). Top. Catal. 2020, 63, 1227–1239. [Google Scholar] [CrossRef]
- Jeong, C.; Ansari, M.Z.; Hakeem Anwer, A.; Kim, S.H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A Review on Metal-Organic Frameworks for the Removal of Hazardous Environ Contaminants. Sep. Purif. Technol. 2023, 305, 122416. [Google Scholar] [CrossRef]
- Singh, S.; Sivaram, N.; Nath, B.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Metal Organic Frameworks for Wastewater Treatment, Renewable Energy and Circular Economy Contributions. npj Clean Water 2024, 7, 124. [Google Scholar] [CrossRef]
- Chang, J.; Bian, Y.; Wang, Y. MOFs-Coupled Fiber Membranes: A Versatile Platform for Water Purification. Sep. Purif. Technol. 2025, 357, 130059. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochem. Angew. Chem. 2010, 122, 9834–9837. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of Organic Pollutants from Aqueous Solution Using Metal Organic Frameworks (MOFs)-Based Adsorbents: A Review. Chemosphere 2021, 284, 131393. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhu, J.; Zhuo, S.; Chen, J.; Huang, W.; Cheng, H.; Li, L.; Tang, T.; Feng, J. Magnetic/Zeolitic Imidazolate Framework-67 Nanocomposite for Magnetic Solid-Phase Extraction of Five Flavonoid Components from Chinese Herb Dicranopteris Pedata. Molecules 2023, 28, 702. [Google Scholar] [CrossRef]
- Beamish-Cook, J.; Shankland, K.; Murray, C.A.; Vaqueiro, P. Insights into the Mechanochem Synthesis of MOF-74. Cryst. Growth Des. 2021, 21, 3047–3055. [Google Scholar] [CrossRef]
- Chen, Y.; Idrees, K.B.; Mian, M.R.; Son, F.A.; Zhang, C.; Wang, X.; Farha, O.K. Reticular Design of Precise Linker Installation into a Zirconium Metal-Organic Framework to Reinforce Hydrolytic Stability. J. Am. Chem. Soc. 2023, 145, 3055–3063. [Google Scholar] [CrossRef]
- Zhang, J.; An, B.; Li, Z.; Cao, Y.; Dai, Y.; Wang, W.; Zeng, L.; Lin, W.; Wang, C. Neighboring Zn-Zr Sites in a Metal-Organic Framework for CO2Hydrogenation. J. Am. Chem. Soc. 2021, 143, 8829–8837. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Xia, K.; Huang, C.; Liu, L.; Wang, J. Bioinspired Neuron-like Adsorptive Networks for Heavy Metal Capture and Tunable Electrochemly Mediated Recovery. ACS Appl. Mater. Interfaces 2021, 13, 45077–45088. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; de Fernandez-de Luis, R.; Salazar, H.; Gonçalves, B.F.; King, S.; Almásy, L.; Kriechbaum, M.; Laza, J.M.; Vilas-Vilela, J.L.; Martins, P.M.; et al. On The Multiscale Structure and Morphology of PVDF-HFP@MOF Membranes in The Scope of Water Remediation Applications. Adv. Mater. Interfaces 2023, 10, 2300424. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Pb(II) Adsorption from Aqueous Solution by an Aluminum-Based Metal Organic Framework–Graphene Oxide Nanocomposite. Mater. Adv. 2021, 2, 3051–3059. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Moghanlo, S.; Kazemi, M.S.; Nazari, S.; Ghadiri, S.K.; Saleh, H.N.; Sillanpää, M. Comparative Removal of Hazardous Cationic Dyes by MOF-5 and Modified Graphene Oxide. Sci. Rep. 2022, 12, 15314. [Google Scholar] [CrossRef]
- Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ye, Z. Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks. Nanomaterials 2020, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, K.; Zhang, S.; Chen, Y.; Ning, L. Removal of Endocrine-Disrupting Chems from Environment Using A Robust Platform Based on Metal-Organic Framework Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 3646–3651. [Google Scholar] [CrossRef]
- Capsoni, D.; Guerra, G.; Puscalau, C.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Sturini, M. Zinc Based Metal-Organic Frameworks as Ofloxacin Adsorbents in Polluted Waters: ZIF-8 vs. Zn3(BTC)2. Int. J. Env. Res. Public. Health 2021, 18, 1433. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanromán, A. One-Pot Synthesis of Bimetallic Fe–Cu Metal–Organic Frameworks Composite for the Elimination of Organic Pollutants via Peroxymonosulphate Activation. Env. Sci. Pollut. Res. 2023, 40, 3963–3983. [Google Scholar] [CrossRef]
- Ramalingam, G.; Pachaiappan, R.; Kumar, P.S.; Dharani, S.; Rajendran, S.; Vo, D.-V.N.; Hoang, T.K.A. Hybrid Metal Organic Frameworks as an Exotic Material for the Photocatalytic Degradation of Pollutants Present in Wastewater: A Review. Chemosphere 2022, 288, 132448. [Google Scholar] [CrossRef]
- Xiao, K.; Shu, B.; Lv, K.; Huang, P.; Chang, Q.; Wu, L.; Wang, S.; Cao, L. Recent Progress of MIL MOF Materials in Degradation of Organic Pollutants by Fenton Reaction. Catalysts 2023, 13, 734. [Google Scholar] [CrossRef]
- Li, Y.; Yao, B.; Chen, Y.; Zhou, Y.; Duan, X. Metal-Organic Frameworks (MOFs) as Efficient Catalysts for Electro-Fenton (EF) Reactions: Current Progress and Prospects. Chem. Eng. J. 2023, 463, 142287. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Jiang, L.; Yuan, X.; Yu, H.; Wang, H.; Zhang, J.; Xia, Q. Recent Advances in Titanium Metal–Organic Frameworks and Their Derived Materials: Features, Fabrication, and Photocatalytic Applications. Chem. Eng. J. 2020, 395, 125080. [Google Scholar] [CrossRef]
- Mohammed, R.O.; Amooey, A.A.; Ammar, S.H.; Salman, M.D. Enhanced Photocatalytic Degradation Activity of ZIF-8 Doped with Ag2WO4 Photocatalyst. J. Taiwan. Inst. Chem. Eng. 2023, 151, 105141. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Alamgholiloo, H.; Noroozi Pesyan, N.; Zolfigol, M.A. A MOF-on-MOF Strategy to Construct Double Z-Scheme Heterojunction for High-Performance Photocatalytic Degradation. Appl. Catal. B 2023, 321, 122082. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- He, X.; Ding, Y.; Huang, Z.; Liu, M.; Chi, M.; Wu, Z.; Segre, C.U.; Song, C.; Wang, X.; Guo, X. Eng a Self-Grown TiO2/Ti-MOF Heterojunction with Selectively Anchored High-Density Pt Single-Atomic Cocatalysts for Efficient Visible-Light-Driven Hydrogen Evolution. Angew. Chem. 2023, 135, e202217439. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, M.; Liu, Y.; Wang, J.; Zhang, G.; Li, L.; Du, L.; Wang, G.; Yang, S.; Wang, X. Single Atoms Meet Metal–Organic Frameworks: Collaborative Efforts for Efficient Photocatalysis. Energy Env. Sci. 2022, 15, 3722–3749. [Google Scholar] [CrossRef]
- Abdel Aziz, Y.S.; Sanad, M.M.S.; Abdelhameed, R.M.; Zaki, A.H. In-Situ Construction of Zr-Based Metal-Organic Framework Core-Shell Heterostructure for Photocatalytic Degradation of Organic Pollutants. Front. Chem. 2023, 10, 1102920. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent Progress in 2D Metal-Organic Framework Photocatalysts: Synthesis, Photocatalytic Mechanism and Applications. J. Phys. Energy 2021, 3, 032010. [Google Scholar] [CrossRef]
- Qi, X.C.; Lang, F.; Li, C.; Liu, M.W.; Wang, Y.F.; Pang, J. Synergistic Effects of MOFs and Noble Metals in Photocatalytic Reactions: Mechanisms and Applications. Chempluschem 2024, 89, e202400158. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Yang, Z.; Huang, Z.; Jia, Q.; Zhu, Y.; Xia, Y. Recent Advances in Metal–Organic Frameworks Derived Nanocomposites for Photocatalytic Applications in Energy and Environment. Adv. Sci. 2021, 8, 2100625. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.S.; Hu, H.; Wang, J.; Lu, L.; Muddassir, M.; Srivastava, D.; Chauhan, R.; Wu, Y.; Wang, X.; Kumar, A. New 5,5-(1,4-Phenylenebis(Methyleneoxy)Diisophthalic Acid Appended Zn(II) and Cd(II) MOFs as Potent Photocatalysts for Nitrophenols. Molecules 2023, 28, 7180. [Google Scholar] [CrossRef]
- Mu, C.; Jian, S.; Zhang, M. Metal-Organic Frameworks (MOFs) and Metal-Organic Cages (MOCs) for Photocatalytic Hydrogen Production. Chem.—A Eur. J. 2024, 30, e202401264. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Zhang, W.; Zhou, M.; Jiang, H.L. Heteroatom-Doped Ag25 Nanoclusters Encapsulated in Metal–Organic Frameworks for Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2024, 63, e202401443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Reji, R.; Drummer, M.C.; He, H.; Niklas, J.; Weingartz, N.P.; Bolotin, I.L.; Chen, L.X.; Poluektov, O.G.; Zapol, P.; et al. Facile Optical Gap Tuning in Nanographene Metal-Organic Frameworks. ACS Appl. Opt. Mater. 2023, 1, 1643–1650. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, C.; Gao, Y.; Tao, X.; Ding, C.; Fan, F.; Jiang, H.-L. Charge Separation by Creating Band Bending in Metal–Organic Frameworks for Improved Photocatalytic Hydrogen Evolution. Angew. Chem. 2022, 134, e202204108. [Google Scholar] [CrossRef]
- Wang, S.; Teng, X.; Liu, C.; Zhu, H.; Cheng, H.; Yan, J.; Wang, L.; Liang, Z.; Ouyang, J. Ultrafast Piezo-Photocatalytic Degradation of Dye Pollutants Using UiO-66-NH2(Hf) Metal-Organic Framework-Based Nanoparticles. Opt. Mater. 2024, 147, 114758. [Google Scholar] [CrossRef]
- Peng, L.; Gong, X.; Wang, X.; Yang, Z.; Liu, Y. In Situ Growth of ZIF-67 on a Nickel Foam as a Three-Dimensional Heterogeneous Catalyst for Peroxymonosulfate Activation. RSC Adv. 2018, 8, 26377–26382. [Google Scholar] [CrossRef]
- Lu, S.; You, S.; Hu, J.; Li, X.; Li, L. Magnetic MnFe2O4/ZIF-67 Nanocomposites with High Activation of Peroxymonosulfate for the Degradation of Tetracycline Hydrochloride in Wastewater. RSC Adv. 2024, 14, 7528–7539. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Fang, X.; Miao, W.; Chen, X.; Sun, J.; Ni, B.J.; Mao, S. Heterogeneous Electro-Fenton Catalysis with HKUST-1-Derived Cu@C Decorated in 3D Graphene Network. Chemosphere 2020, 243, 125423. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanromán, M.A.; Rosales, E. Heterogeneous Electro-Fenton System Using Fe-MOF as Catalyst and Electrocatalyst for Degradation of Pharmaceuticals. Chemosphere 2023, 340, 139942. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Moreno, G.A.; Ayala-Durán, S.C.; Barbero, B.P.; Narda, G.E.; Bernini, M.C.; Pupo Nogueira, R.F. Photo-Fenton Degradation of Sulfamethoxazole Using MIL-53(Fe) under UVA LED Irradiation and Natural Sunlight. J. Env. Chem. Eng. 2022, 10, 107678. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Li, X.; Deng, S.; Zhao, S.; Zhang, W. Enhanced Degradation of Sulfamethoxazole (SULFAMETHOXAZOLE) in Toilet Wastewater by Photo-Fenton Reactive Membrane Filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef]
- Fisher, T.M.; dos Santos, A.J.; Garcia-Segura, S. Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline. Catalysts 2024, 14, 314. [Google Scholar] [CrossRef]
- Qiu, P.; Liao, X.; Jiang, Y.; Yao, Y.; Shi, L.; Lu, S.; Li, Z. Unraveling the Photocatalytic Electron Transfer Mechanism in a Ti-MOF/g-C3N4 Heterojunction for High-Efficient Coupling Performance of Primary Amines. New J. Chem. 2022, 46, 20711–20722. [Google Scholar] [CrossRef]
- Kinik, F.P.; Ortega-Guerrero, A.; Ebrahim, F.M.; Ireland, C.P.; Kadioglu, O.; Mace, A.; Asgari, M.; Smit, B. Toward Optimal Photocatalytic Hydrogen Generation from Water Using Pyrene-Based Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 57118–57131. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Tan, Y.; Xu, B.; Cai, J.; Zhang, Y.; Wang, Q.; Wu, Q.; Yang, B.; Huang, J. Recent Advances in Metal-Organic Framework (MOF)-Based Photocatalysts: Design Strategies and Applications in Heavy Metal Control. Molecules 2023, 28, 6681. [Google Scholar] [CrossRef]
- Karmakar, S.; Barman, S.; Rahimi, F.A.; Biswas, S.; Nath, S.; Maji, T.K. Developing Post-Modified Ce-MOF as a Photocatalyst: A Detail Mechanistic Insight into CO2 Reduction toward Selective C2 Product Formation. Energy Env. Sci. 2023, 16, 2187–2198. [Google Scholar] [CrossRef]
- Li, X.; Zhou, T.; Liao, S.; Shi, W.; Shi, J.Y. Regulating the Electronic Band Structure of the Ti-Based Metal-Organic Framework toward Boosting Light-Driven Hydrogen Evolution. ACS Appl. Mater. Interfaces 2024, 16, 67771–67777. [Google Scholar] [CrossRef]
- Fang, X.; Choi, J.Y.; Stodolka, M.; Pham, H.T.B.; Park, J. Advancing Electrically Conductive Metal-Organic Frameworks for Photocatalytic Energy Conversion. Acc. Chem. Res. 2024, 57, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.; Gao, H.; Wang, J.; Li, A.; Zhang, X. Fine-Tuning the Metal Oxo Cluster Composition and Phase Structure of Ni/Ti Bimetallic MOFs for Efficient CO2 Reduction. J. Phys. Chem. C 2021, 125, 9200–9209. [Google Scholar] [CrossRef]
- Wang, D.; Suo, M.; Lai, S.; Deng, L.; Liu, J.; Yang, J.; Chen, S.; Wu, M.F.; Zou, J.P. Photoinduced Acceleration of Fe3+/Fe2+ Cycle in Heterogeneous FeNi-MOFs to Boost Peroxodisulfate Activation for Organic Pollutant Degradation. Appl. Catal. B 2023, 321, 122054. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, D.; Chen, X.; Zhang, D. Facilitated Activation of Peroxymonosulfate by Loading ZIF-8 on Fe3O4-MnO2 for Deep Mineralization of Bisphenol A. ACS ES T Water 2021, 1, 417–429. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanroman, A. Peroxymonosulphate Activation by Basolite® F-300 for Escherichia Coli Disinfection and Antipyrine Degradation. Int. J. Env. Res. Public. Health 2022, 19, 6852. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Zhu, J. One-Step Synthesis of Mn-Doped MIL-53(Fe) for Synergistically Enhanced Generation of Sulfate Radicals towards Tetracycline Degradation. J. Colloid. Interface Sci. 2020, 580, 470–479. [Google Scholar] [CrossRef]
- Ma, L.; Xu, J.; Liu, Y.; An, Y.; Pan, Z.; Yang, B.; Li, L.; Hu, T.; Lai, B. Improved Degradation of Tetracycline by Cu-Doped MIL-101(Fe) in a Coupled Photocatalytic and Persulfate Oxidation System: Efficiency, Mechanism, and Degradation Pathway. Sep. Purif. Technol. 2023, 305, 122450. [Google Scholar] [CrossRef]
- Fan, G.; Luo, J.; Guo, L.; Lin, R.; Zheng, X.; Snyder, S.A. Doping Ag/AgCl in Zeolitic Imidazolate Framework-8 (ZIF-8) to Enhance the Performance of Photodegradation of Methylene Blue. Chemosphere 2018, 209, 44–52. [Google Scholar] [CrossRef]
- Jia, X.; Wang, F.; Xu, X.; Liu, C.; Zhang, L.; Jiao, S.; Zhu, G.; Wang, X.; Yu, G. Highly Efficient Photocatalytic Degradation of Tetracycline by Modifying UiO-66 via Different Regulation Strategies. ACS Omega 2023, 8, 27375–27385. [Google Scholar] [CrossRef]
- Zhao, B.; Han, J.; Liu, B.; Zhang, S.L.; Guan, B. Hierarchical Metal–Organic Framework Nanoarchitectures for Catalysis. Chem. Synth. 2024, 4, 41. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and Photocatalysis by Metal Organic Frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–Organic Frameworks as Multifunctional Solid Catalysts. Trends Chem. 2020, 2, 454–466. [Google Scholar] [CrossRef]
- Arshadi Edlo, A.; Akhbari, K. Modulated Antibacterial Activity in ZnO@MIL-53(Fe) and CuO@MIL-53(Fe) Nanocomposites Prepared by Simple Thermal Treatment Process. Appl. Organomet. Chem. 2024, 38, e7326. [Google Scholar] [CrossRef]
- Qi, Y.; Ren, S.; Che, Y.; Ye, J.; Ning, G. Research Progress of Metal-Organic Frameworks Based Antibacterial Materials. Acta Chim. Sin. 2020, 78, 613–624. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; Toson, E.A.; Shoueir, K.R.; Aljohani, H.A.; Abo-Ser, M.M. Metal–Organic Frameworks as Efficient Materials for Drug Delivery: Synthesis, Characterization, Antioxidant, Anticancer, Antibacterial and Molecular Docking Investigation. Appl. Organomet. Chem. 2020, 34, e5905. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, F.; Yu, P.; Zhou, Y.; Luo, R.; Liu, M.; Ritz, U. Metal-Organic Framework-Based Advanced Therapeutic Tools for Antimicrobial Applications. Acta Biomater. 2024, 175, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud. Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Sun, H.Q.; Lu, X.M.; Gao, P.J. The Exploration of the Antibacterial Mechanism of Fe3+ against Bacteria. Braz. J. Microbiol. 2011, 42, 410–414. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorozyński, P. Metal-Organic Frameworks: Mechanisms of Antibacterial Action and Potential Applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, F.; Wang, D. MOFs and MOF-Derived Materials for Antibacterial Application. J. Funct. Biomater. 2022, 13, 215. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial Applications of Metal–Organic Frameworks and Their Composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef]
- Elmehrath, S.; Ahsan, K.; Munawar, N.; Alzamly, A.; Nguyen, H.L.; Greish, Y. Antibacterial Efficacy of Copper-Based Metal-Organic Frameworks against Escherichia Coli and Lactobacillus. RSC Adv. 2024, 14, 15821–15831. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rana, A.; Patel, A.; Manna, D.; Biswas, S. Superhydrophobic Nanosized Metal-Organic Framework Composites for the Targeted Removal of Hydrophobic Pharmaceuticals with Outstanding Bacterial Anti-Adhesion Properties. Environ. Sci. Nano 2024, 11, 1233–1244. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, M.; Wu, D.; Shen, M.; Liu, D.; Ding, T. Synthesis of UiO-66 Loaded-Caffeic Acid and Study of Its Antibacterial Mechanism. Food Chem. 2023, 402, 134248. [Google Scholar] [CrossRef]
- Janani, B.J.; Syed, A.; Majeed, N.A.; Shleghm, M.R.; abdulkhudur ali azlze Alkhafaij, M.; Bahair, H.; Abdulwahab, H.M.H.; Elgorban, A.M.; AL-Shwaiman, H.A.; Wong, L.S. Synergistic Effect of SrTiO3/CuFe2O4/MIL-101(Co) as a MOF Composite under Gamma-Rays for Antimicrobial Potential versus Bacteria and Pathogenic Fungi. Colloids Surf. B Biointerfaces 2024, 241, 114015. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pachón, D.; Echeverry-Gallego, R.A.; Serna-Galvis, E.A.; Villarreal, J.M.; Botero-Coy, A.M.; Hernández, F.; Torres-Palma, R.A.; Moncayo-Lasso, A. Treatment of Wastewater Effluents from Bogotá—Colombia by the Photo-Electro-Fenton Process: Elimination of Bacteria and Pharmaceutical. Sci. Total Environ. 2021, 772, 144890. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Mukherjee, M.; Bhattacharya, P.; Neogi, S.; De, S. Mechanistic Insights into the Antibacterial Property of MIL-100 (Fe) Metal-Organic Framework. J. Env. Chem. Eng. 2024, 12, 113088. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Lomba-Fernández, B.; Pazos, M.; Rosales, E.; Sanromán, A. Peroxymonosulfate Activation by Different Synthesized CuFe-MOFs: Application for Dye, Drugs, and Pathogen Removal. Catalysts 2023, 13, 820. [Google Scholar] [CrossRef]
- Giráldez, A.; Fdez-Sanromán, A.; Terrón, D.; Sanromán, M.A.; Pazos, M. Nanostructured Copper-Organic Frameworks for the Generation of Sulphate Radicals: Application in Wastewater Disinfection. Env. Sci. Pollut. Res. Int. 2023, 1–5. [Google Scholar] [CrossRef]
- Ghaedi, S.; Rajabi, H.; Hadi Mosleh, M.; Sedighi, M. MOF Biochar Composites for Environ Protection and Pollution Control. Bioresour. Technol. 2025, 418, 131982. [Google Scholar] [CrossRef]
- Yang, F.; Du, M.; Yin, K.; Qiu, Z.; Zhao, J.; Liu, C.; Zhang, G.; Gao, Y.; Pang, H.; Yang, F.Y.; et al. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Tang, D.Y.; Wang, X.H.; Yan, L.L.; Deng, L.L.; Zhao, M.Q.; Deng, E.N.; Zhou, Q.H. Molecularly Imprinted MOF/PAN Hybrid Nanofibrous Membranes for Selective Bisphenol A Adsorption and Antibacterial Fouling in Water Treatment. Sep. Purif. Technol. 2024, 328, 124984. [Google Scholar] [CrossRef]
- Negro, C.; Pérez-Cejuela, H.M.; Simó-Alfonso, E.F.; Iqbal, W.; Herrero-Martínez, J.M.; Armentano, D.; Ferrando-Soria, J.; Pardo, E. (Multivariate)-Metal−Organic Framework for Highly Efficient Antibiotic Capture from Aquatic Environ Matrices. ACS Appl. Mater. Interfaces 2023, 15, 3069–3076. [Google Scholar] [CrossRef]
- Ali, I.; Wan, P.; Raza, S.; Peng, C.; Tan, X.; Sun, H.; Li, J. Development of Novel MOF-Mixed Matrix Three-Dimensional Membrane Capsules for Eradicating Potentially Toxic Metals from Water and Real Electroplating Wastewater. Env. Res. 2022, 215, 113945. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.R.; Wu, H.; Yu, C.X.; Ding, J.; Zhou, Y.L.; Liu, L.L. Fabrication of 2D MOF Nanosheets Decorated with SO42− for Highly Efficient and Selective Hg2+ Capture from Wastewater. Sep. Purif. Technol. 2025, 360, 131048. [Google Scholar] [CrossRef]
- Gao, S.; Liu, W.; Wang, M.; Fu, D.; Zhao, Z.; Liu, X. In Situ Self-Grown Synthesis of c-MOF@NiO Heterostructure Anchored to c-MOF/RGA Particle Electrode: Promoting Sustained and Efficient Degradation of Phenol in Coking Wastewater. Appl. Catal. B 2025, 365, 124911. [Google Scholar] [CrossRef]
- Jafarnia, S.; Sohrabnezhad, S.; Foulady-Dehaghi, R. Triplet Cu2O/MOF-2/MMT Nanocomposite: Antibacterial and Photocatalyst Agent. J. Mol. Struct. 2023, 1289, 135870. [Google Scholar] [CrossRef]

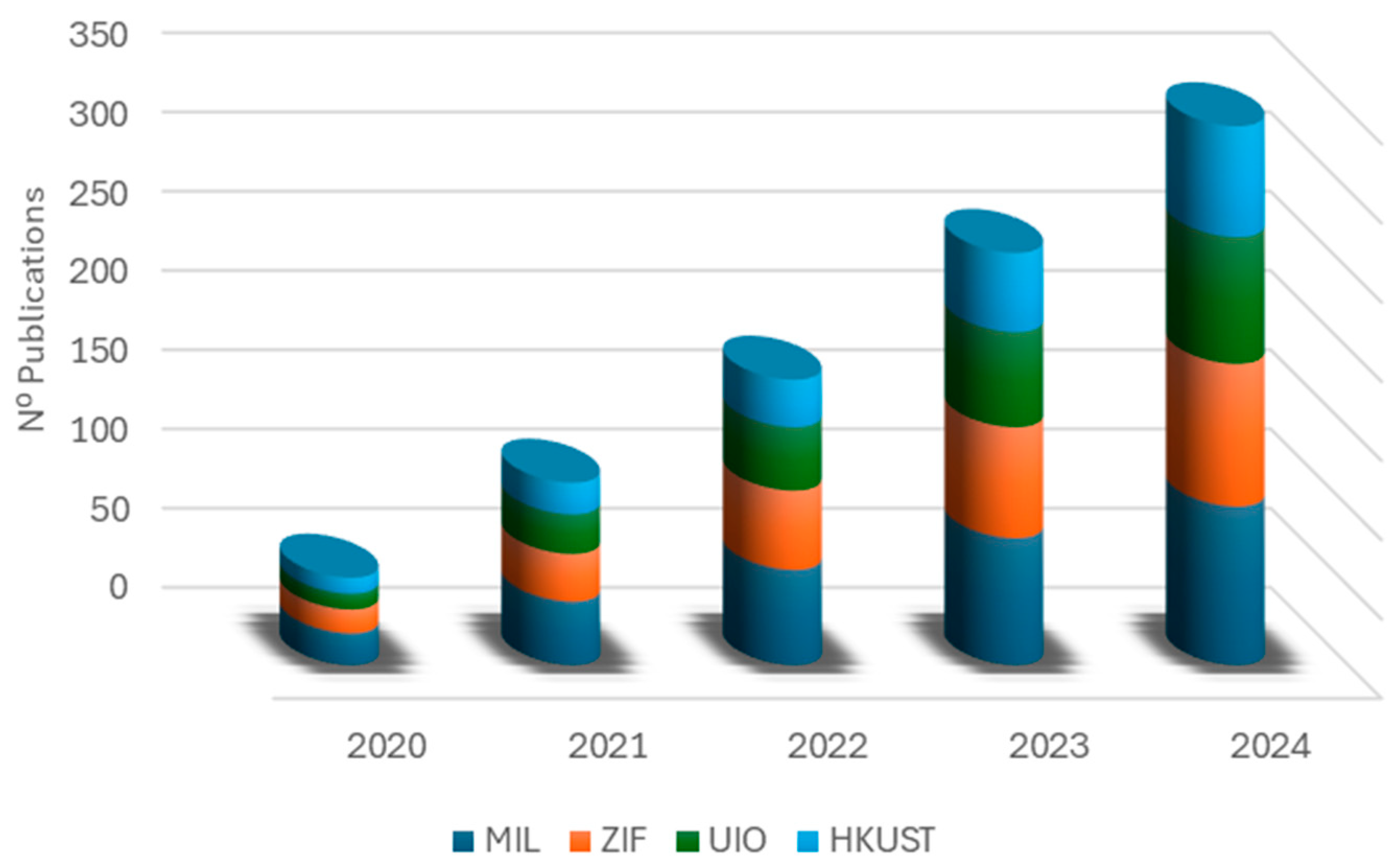
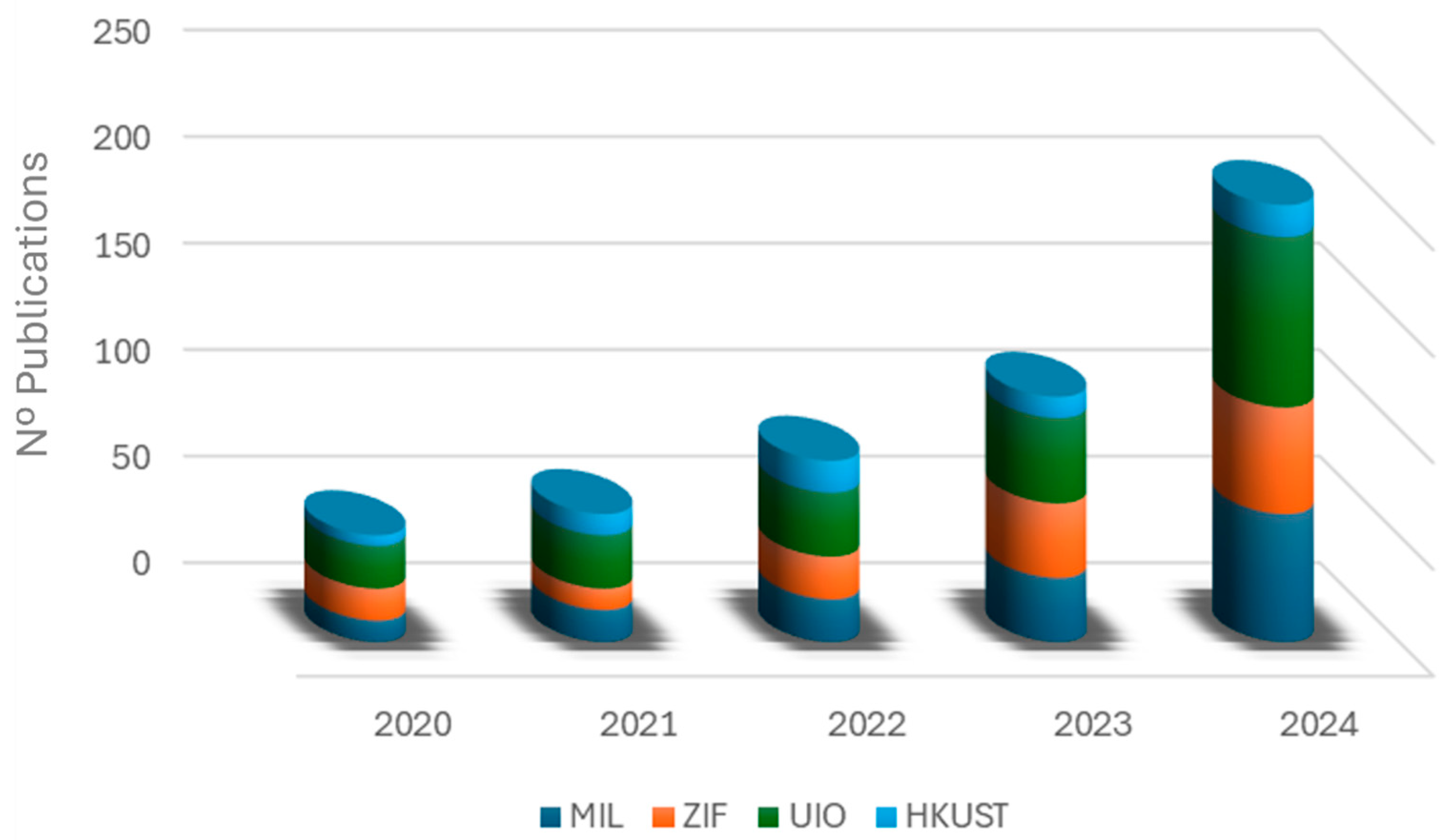
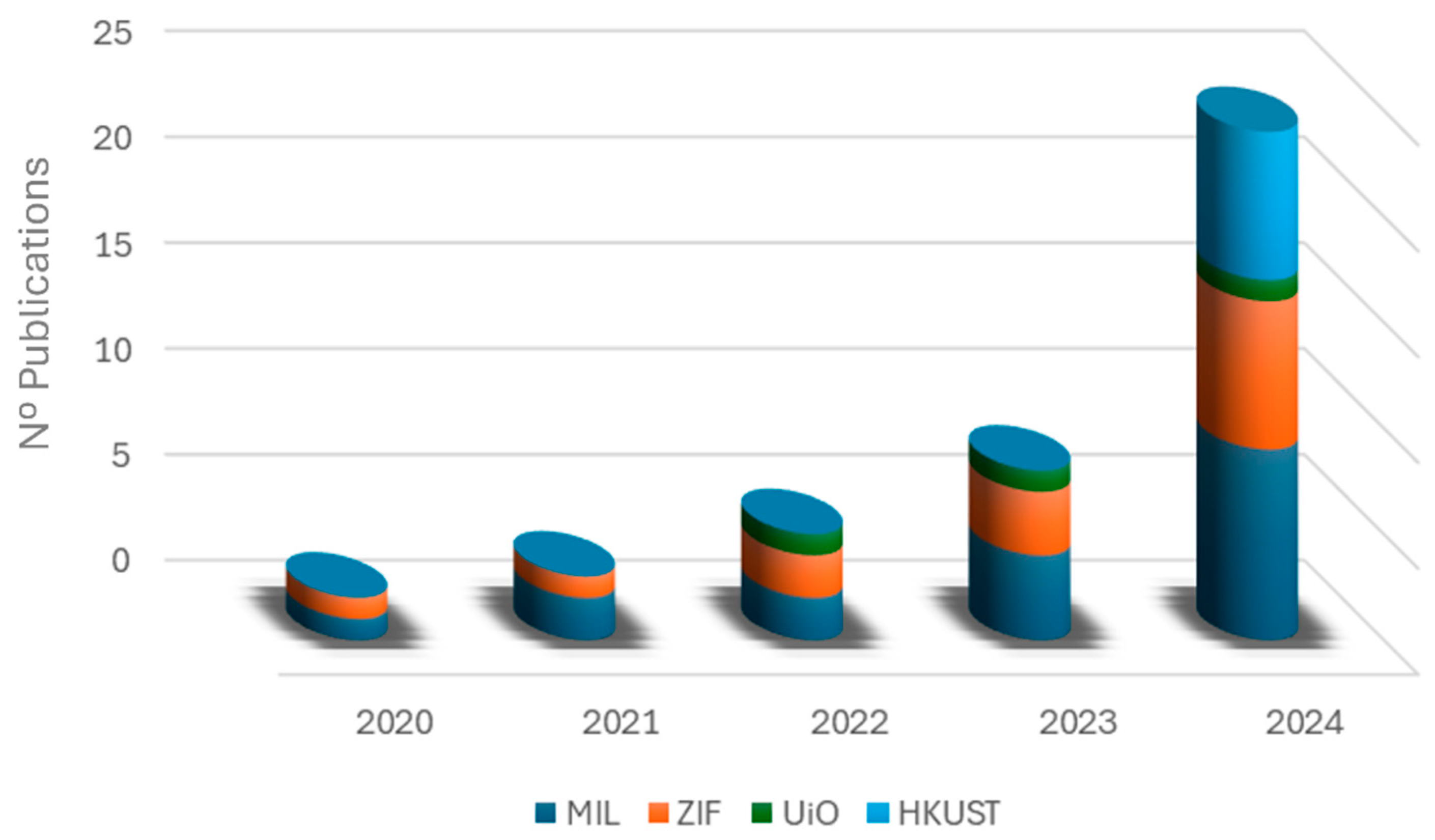

| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Solvothermal | High crystallinity and purity Tunable morphology Scalable | Long reaction times High temperatures and organic solvents |
| Sonochemical | Fast synthesis Energy efficient Nanosized | Possible structural defects Limited scalability |
| Hydrothermal | Environmentally friendly Good crystallinity Scalable | High temperatures Long reaction times |
| Microwave-Assisted | Rapid synthesis Uniform heating for better crystallinity | Limited morphology Potential side reactions Scarce solvents for synthesis Limited scalability |
| Mechanochemical | Environmentally friendly Fast and cost-effective Scalable | Limited control over morphology |
| Electrochemical | Precise control over crystal growth Low temperatures | Require conductive substrates Limited scalability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrón, D.; Sanromán, A.; Pazos, M. Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation. Catalysts 2025, 15, 244. https://doi.org/10.3390/catal15030244
Terrón D, Sanromán A, Pazos M. Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation. Catalysts. 2025; 15(3):244. https://doi.org/10.3390/catal15030244
Chicago/Turabian StyleTerrón, Daniel, Angeles Sanromán, and Marta Pazos. 2025. "Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation" Catalysts 15, no. 3: 244. https://doi.org/10.3390/catal15030244
APA StyleTerrón, D., Sanromán, A., & Pazos, M. (2025). Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation. Catalysts, 15(3), 244. https://doi.org/10.3390/catal15030244








