From Cellulose to Highly Aromatic Hydrochar: Catalytic Carbonization and Catalytic Aromatization Mechanism of Lanthanide (III) Ions
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of the Basic Properties of Samples
2.1.1. The Fuel Quality and Chemical Composition of the Hydrochar
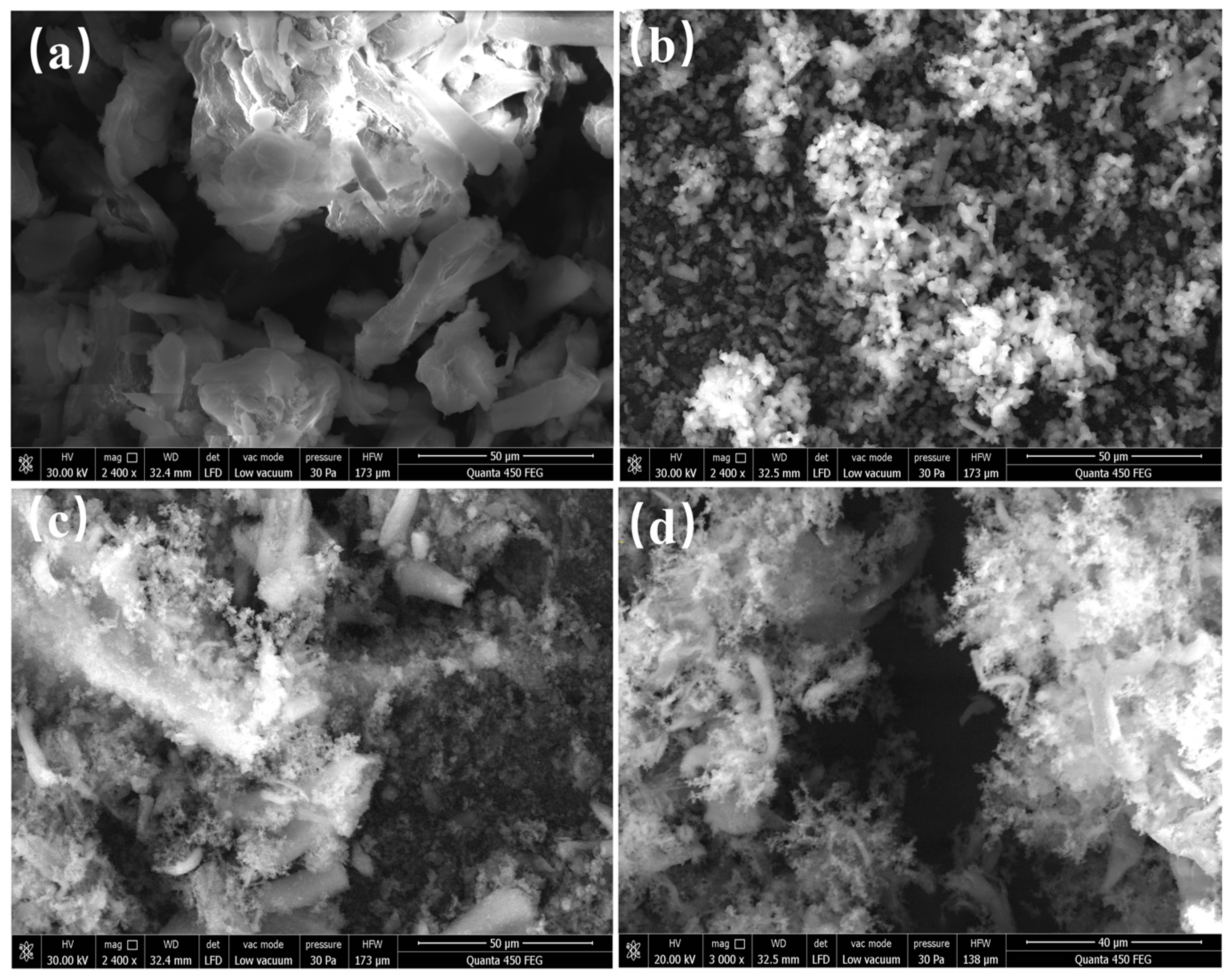
2.1.2. The Evolution of the Microscopic Morphology of Hydrochar
2.2. The Variations in Chemical Structure
2.2.1. Evolution of the Functional Groups from Cellulose to Hydrochar
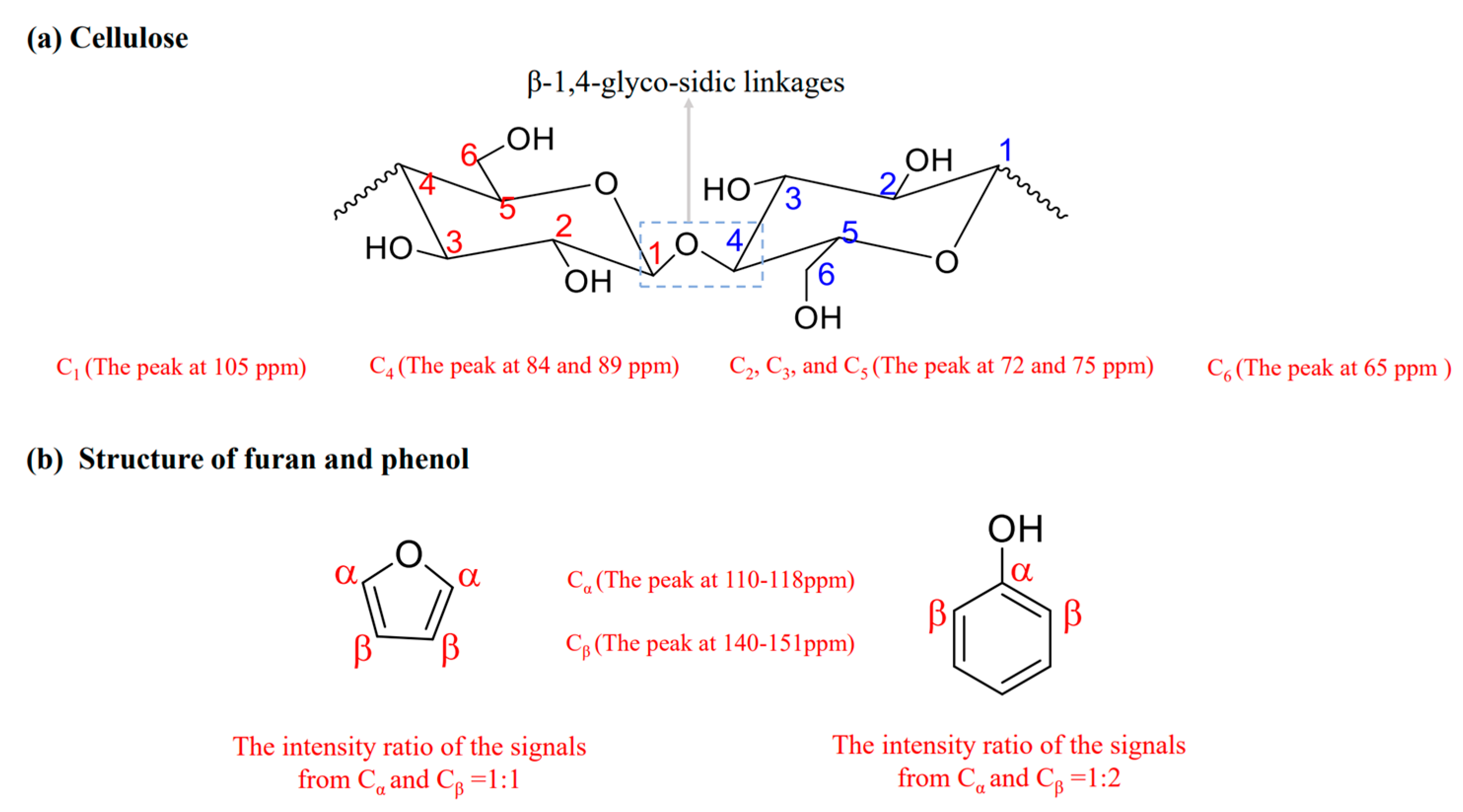
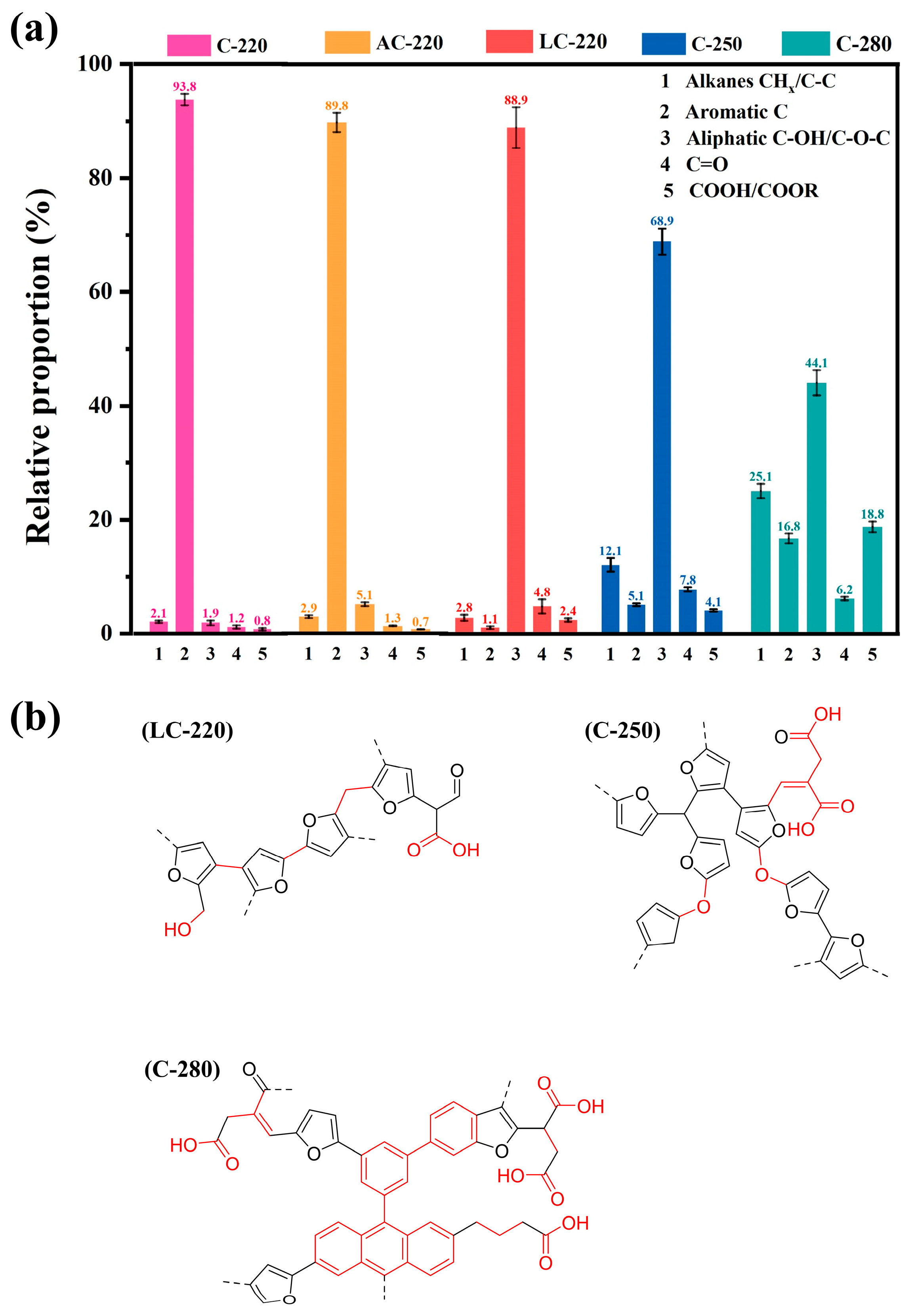
2.2.2. Evolution of the C-Containing Functional Groups and Chemical Structure of Hydrochar
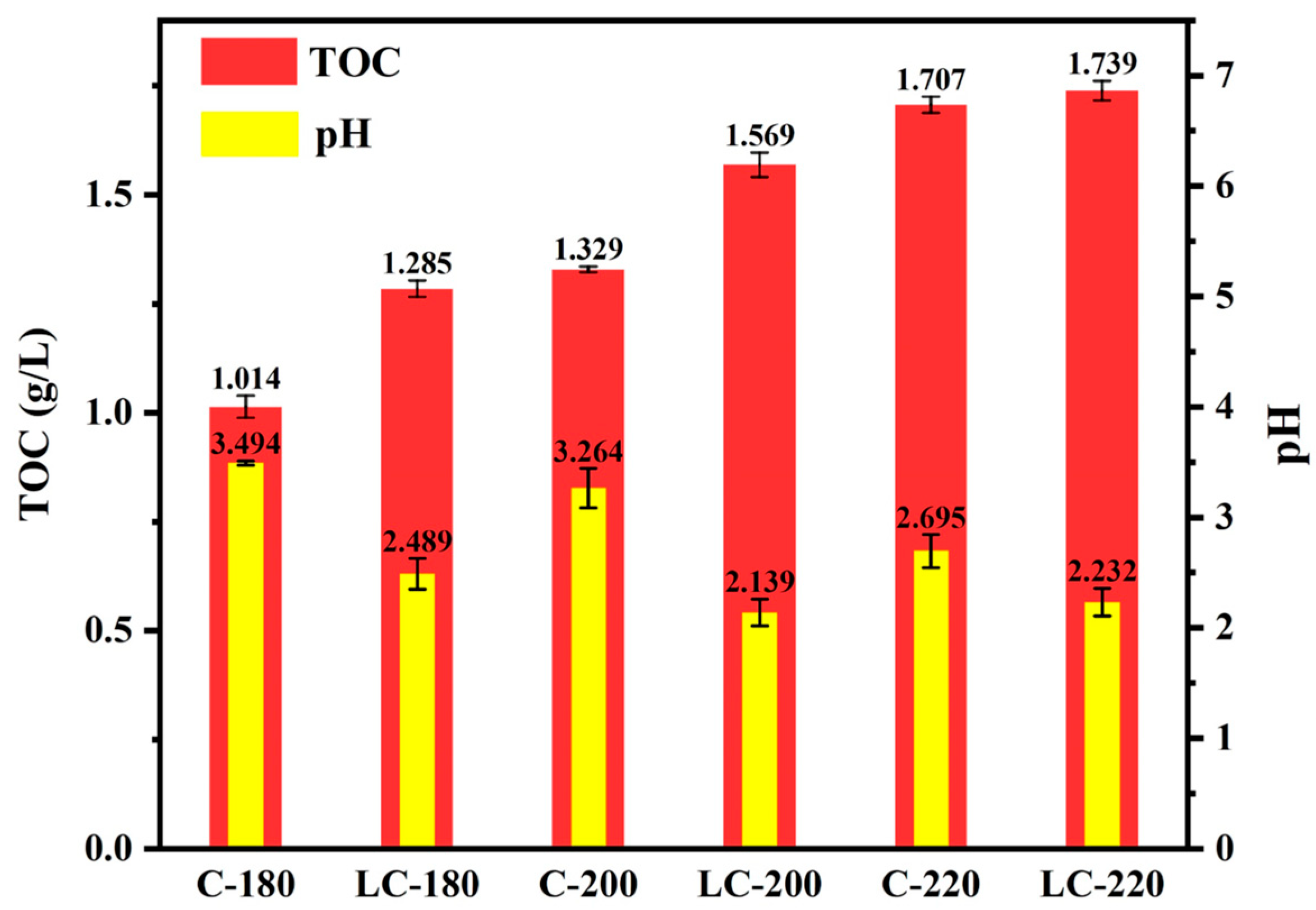
2.3. Distribution of pH and TOC of Liquid-Phase Products
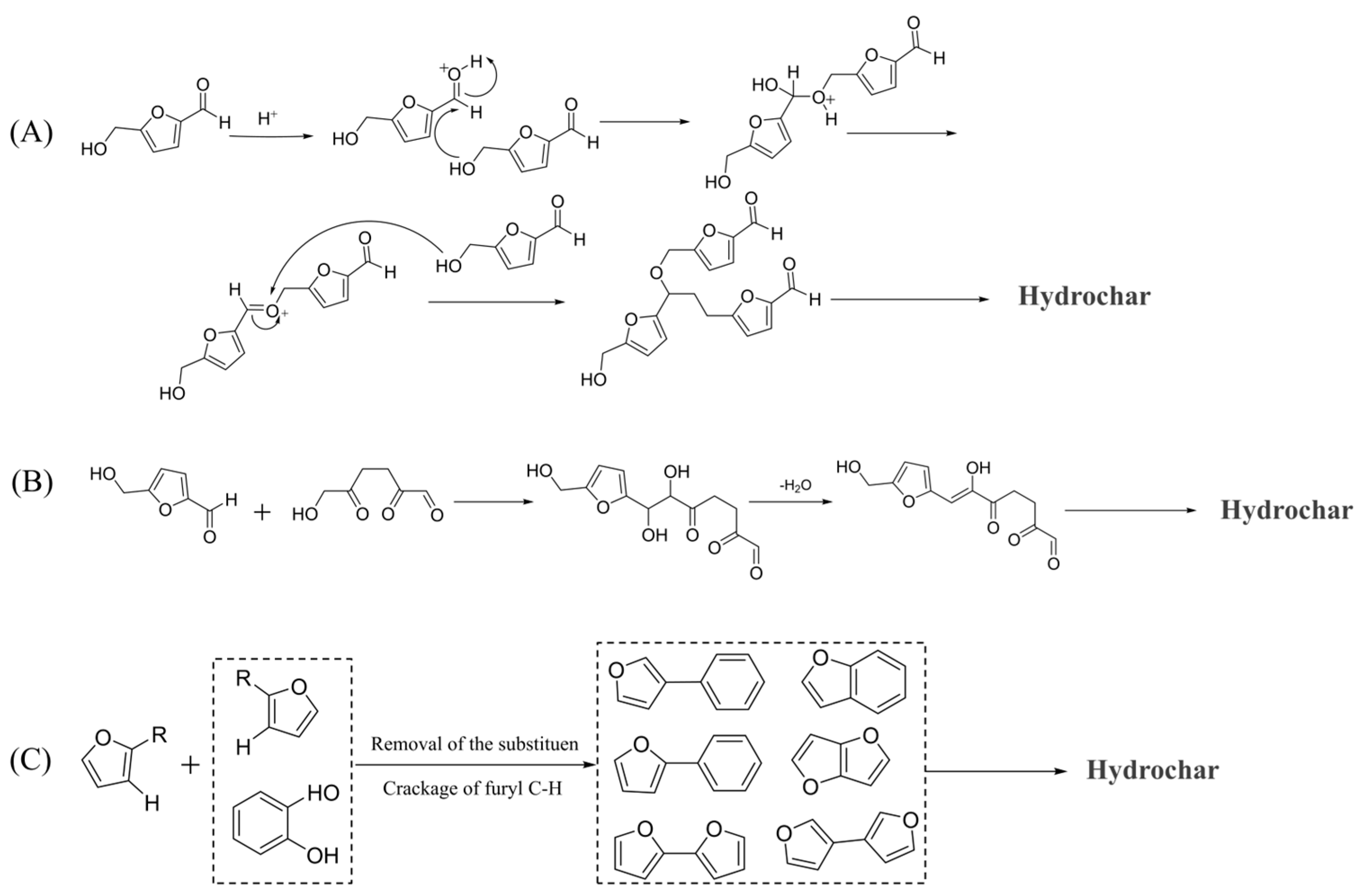
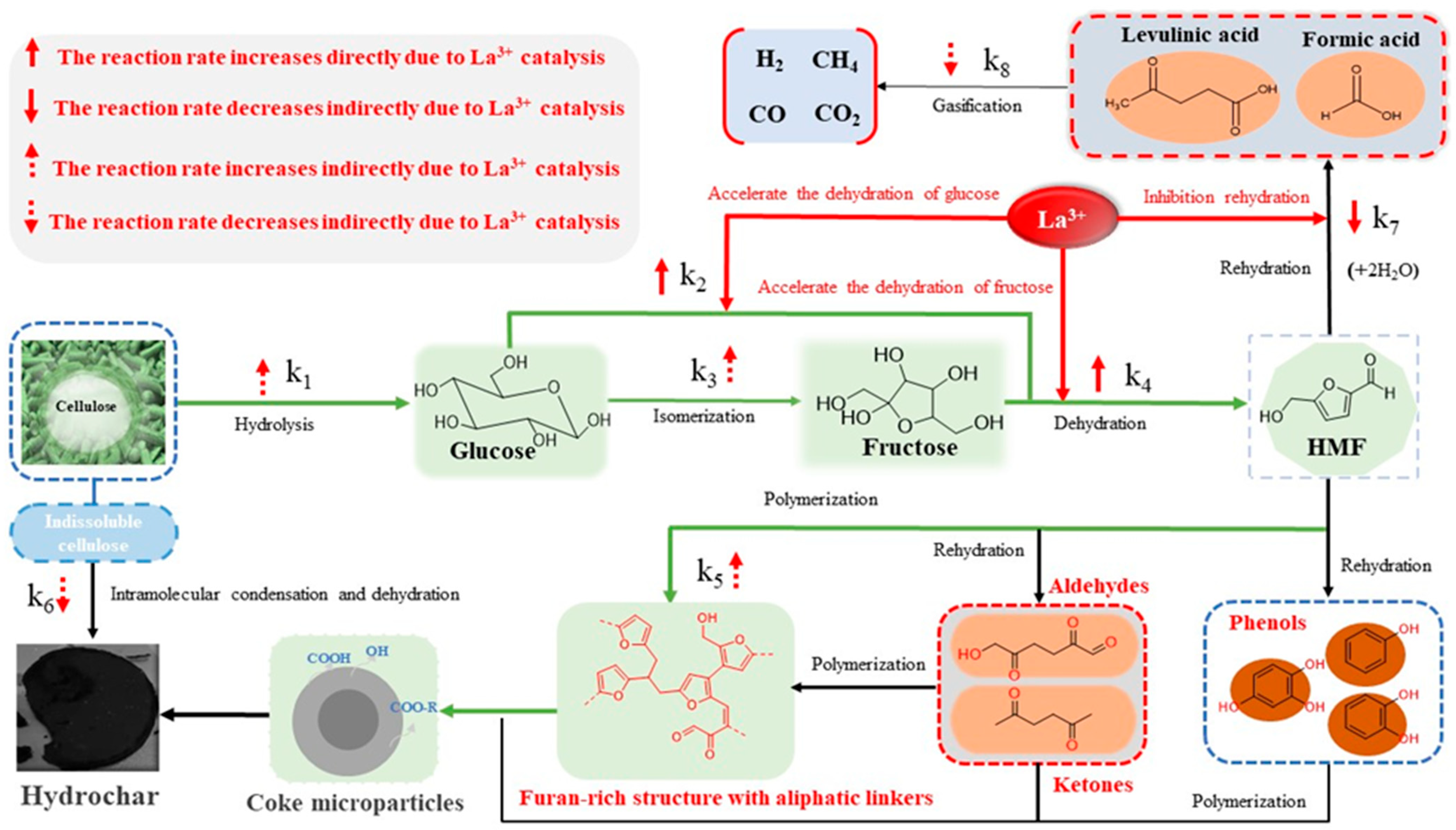
2.4. Catalytic Carbonization Mechanism
3. Materials and Methods
3.1. Materials
3.2. Hydrothermal Carbonization Experiment
3.3. Characterization of the Hydrochars and Aqueous Products
4. Conclusions
- Compared with the uncatalyzed HTC process and the traditional acetic acid catalytic process, lanthanide (III) ions showed superior catalytic effects. At 220 °C, it could facilitate the formation of coke microparticles, and as a result, the fuel properties of hydrochar were similar to those of hydrochar catalyzed by acetic acid at 220 °C. The HHV of hydrochar catalyzed by lanthanide (III) ions at 220 °C was comparable to that of the HHV of hydrochar formed at 250 °C.
- The effective acceleration of the dehydration reaction of glucose to HMF and the inhibition of the rehydration reaction of HMF to LA also indirectly accelerated the hydrolysis of cellulose and the formation of coke microparticles, accelerating the process from cellulose to highly aromatized hydrochar.
- Lanthanide (III) ions could effectively promote the conversion of carbohydrates into furanic units in the HTC process of cellulose. Therefore, it is promising to investigate the potential of using LaCl3 as a catalyst for the aromatization of natural lignocellulosic biomass.
- Although at 220 °C, lanthanide (III) ions could convert cellulose into furan-rich hydrochar, it was unable to accelerate the further conversion to arene-rich hydrochar structures. Therefore, it is necessary to further develop a catalyst to promote the transformation of the furanyl structure into the phenyl structure in the future, increasing the aromatization and graphitization degree of hydrochar.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Böttger, J.; Eckhard, T.; Pflieger, C.; Senneca, O.; Muhler, M.; Cerciello, F. Green coal substitutes for boilers through hydrothermal carbonization of biomass: Pyrolysis and combustion behavior. Fuel 2023, 344, 128025. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Du, Y.-H.; Aleem, R.D.; Shan, Y.-Q.; Cao, C.-Q.; Duan, P.-G.; Jia, D.; Ramzan, N. Preparation of carbon materials for supercapacitors in energy storage by direct hydrothermal carbonization of cellulose and pyrazine. J. Energy Storage 2024, 76, 109825. [Google Scholar] [CrossRef]
- Huier, J.; Fang, D.; Yiping, L.; Zhijie, X.; Yichao, C.; Pan, Z.; Xiaofeng, L.; Dong, L. Hydrothermal carbonization of corn straw in biogas slurry. J. Clean. Prod. 2022, 353, 131682. [Google Scholar]
- Assis, E.I.; Gidudu, B.; Chirwa, E.M. Hydrothermal carbonisation of paper sludge: Effect of process conditions on hydrochar fuel characteristics and energy recycling efficiency. J. Clean. Prod. 2022, 373, 133775. [Google Scholar] [CrossRef]
- Bardhan, M.; Novera, T.M.; Tabassum, M.; Islam, M.A.; Islam, M.A.; Hameed, B.H. Co-hydrothermal carbonization of different feedstocks to hydrochar as potential energy for the future world: A review. J. Clean. Prod. 2021, 298, 126734. [Google Scholar] [CrossRef]
- Krishna, Y.; Roshan, P.; Sheeja, J. Enhanced defluoridation in household filter using binary metal hydrochar composite. J. Clean. Prod. 2022, 370, 133525. [Google Scholar]
- Lozano Pérez, A.S.; Romero Mahecha, V.; Guerrero Fajardo, C.A. Comparative Analysis of Optimal Reaction Conditions for Hydrothermal Carbonization and Liquid Hot-Water Processes in the Valorization of Peapods and Coffee Cherry Waste into Platform Chemicals. ChemEngineering 2024, 8, 98. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Z.; Peng, P.; Tan, S.; Zhao, Z.; Yin, Q.; Wang, C. Influence mechanism of aqueous organic components on the hydrochar formation reaction during the biomass hydrothermal carbonization wastewater recycling. Fuel 2022, 326, 125033. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Leng, S.; Leng, L.; Chen, L.; Chen, J.; Chen, J.; Zhou, W. The effect of aqueous phase recirculation on hydrothermal liquefaction/carbonization of biomass: A review. Bioresour. Technol. 2020, 318, 124081. [Google Scholar] [CrossRef] [PubMed]
- Greta, S.; Attila, K.; Jozsef, T.A.; Peter, M.; Pieter, B.; Daniel, F. Catalytic hydrothermal carbonization of microalgae biomass for low-carbon emission power generation: The environmental impacts of hydrochar co-firing. Fuel 2021, 300, 120927. [Google Scholar]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valori. 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Seri, K.-I.; Inoue, Y.; Ishida, H. Catalytic Activity of Lanthanide(III) Ions for the Dehydration of Hexose to 5-Hydroxymethyl-2-furaldehyde in Water. Bull. Chem. Soc. Jpn. 2001, 74, 1145–1150. [Google Scholar] [CrossRef]
- Zhang, S.; Sheng, K.; Yan, W.; Liu, J.; Shuang, E.; Yang, M.; Zhang, X. Bamboo derived hydrochar microspheres fabricated by acid-assisted hydrothermal carbonization. Chemosphere 2021, 263, 128093. [Google Scholar] [CrossRef]
- Jideani, T.; Chukwuchendo, E.; Khotseng, L. An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review. Catalysts 2023, 13, 1425. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Okpala, C.O.R.; Igwegbe, C.A.; Białowiec, A. Catalyst-Enhancing Hydrothermal Carbonization of Biomass for Hydrochar and Liquid Fuel Production—A Review. Materials 2024, 17, 2579. [Google Scholar] [CrossRef]
- Gao, L.; Chen, Q.; Wang, Y.; Che, D.; Sun, B.; Guo, S. Density Functional Theory Study on Na+ and K+ Catalysis in the Transformation of Glucose to Fructose and HMF in Hydrothermal Environments. Molecules 2024, 29, 4849. [Google Scholar] [CrossRef]
- Lim, S.R.; Kim, G.H.; Um, B.H. Hydrothermal Carbonization and Torrefaction of Kenaf, Rice Husk, Corncob, and Wood Chip: Characteristics and Differences of Hydrochar and Torrefied Char. BioEnergy Res. 2024, 17, 1816–1831. [Google Scholar] [CrossRef]
- Wang, R.; Jin, Q.; Ye, X.; Lei, H.; Jia, J.; Zhao, Z. Effect of process wastewater recycling on the chemical evolution and formation mechanism of hydrochar from herbaceous biomass during hydrothermal carbonization. J. Clean. Prod. 2020, 277, 123281. [Google Scholar] [CrossRef]
- Geng, M.; Tao, Y.; Zhang, Z.; Dai, Y.; Huang, J.; Liu, F.; Na, H.; Zhu, J. Formation Mechanisms of Hydroxyl-Rich Carbon Layers on Carbon Nanotube Surfaces for Promoting the Hydrolysis of Cellulose to Sugar. ACS Appl. Nano Mater. 2023, 6, 588–597. [Google Scholar] [CrossRef]
- Wang, R.; Jia, J.; Jin, Q.; Chen, H.; Liu, H.; Yin, Q.; Zhao, Z. Forming mechanism of coke microparticles from polymerization of aqueous organics during hydrothermal carbonization process of biomass. Carbon 2022, 192, 50–60. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Ribeiro, F.C.P.; Santos, J.L.; Araujo, R.O.; Santos, V.O.; Chaar, J.S.; Tenório, J.A.S.; de Souza, L.K.C. Sustainable catalysts for esterification: Sulfonated carbon spheres from biomass waste using hydrothermal carbonization. Renew. Energy 2024, 220, 119653. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W. REE recovery and hydrochar production from a hyperaccumulator by microwave-assisted hydrothermal carbonization. Miner. Eng. 2024, 208, 108595. [Google Scholar] [CrossRef]
- Vojtová, I.; Leinweber, P.; Weidlich, T. Small Molecules Effective for Conversion of Lignocellulosic Biomass to Furfural and Its Derivatives. Catalysts 2024, 14, 791. [Google Scholar] [CrossRef]
- Xing, X.; Liu, W.; Xu, S.; Hao, J. H-Beta Zeolite as Catalyst for the Conversion of Carbohydrates into 5-Hydroxymethylfurfural: The Role of Calcination Temperature. Catalysts 2024, 14, 248. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, B.; Wu, S.; Xu, X.; Liu, B.; Wang, Q.; Zhang, X.; Zhang, J. From sewage sludge and lignocellulose to hydrochar by co-hydrothermal carbonization: Mechanism and combustion characteristics. Energy 2024, 305, 132414. [Google Scholar] [CrossRef]
- Liu, Z. A review on the emerging conversion technology of cellulose, starch, lignin, protein and other organics from vegetable-fruit-based waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, S.; Zhang, Y.S.; Cheng, H.; Yang, X.; Li, Q.; Zhang, Y.; Zhou, H. Ni transformation and hydrochar properties during hydrothermal carbonization of cellulose. Fuel 2025, 382, 133772. [Google Scholar] [CrossRef]
- Gromov, N.V.; Ogorodnikova, O.L.; Medvedeva, T.B.; Panchenko, V.N.; Yakovleva, I.S.; Isupova, L.A.; Timofeeva, M.N.; Taran, O.P.; Aymonier, C.; Parmon, V.N. Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions. Catalysts 2023, 13, 1298. [Google Scholar] [CrossRef]
- Arifin; Puripat, M.; Yokogawa, D.; Parasuk, V.; Irle, S. Glucose transformation to 5-hydroxymethylfurfural in acidic ionic liquid: A quantum mechanical study. J. Comput. Chem. 2016, 37, 327–335. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, H.T.; Yang, L.; Zhang, W.; Hu, Y.; Guo, Y.; Yang, L.; Leng, S.; Chen, J.; Chen, J. Co-hydrothermal carbonization of cellulose, hemicellulose, and protein with aqueous phase recirculation: Insight into the reaction mechanisms on hydrochar formation. Energy 2022, 251, 123965. [Google Scholar] [CrossRef]
- Maxime, D.; Geert, H.; Anne, R.; Elsa, W.H.; Jacques, F. Hydrothermal liquefaction of blackcurrant pomace and model molecules: Understanding of reaction mechanisms. Sustain. Energy Fuels 2017, 1, 555–582. [Google Scholar]
- Ma, R.; Fakudze, S.; Liu, S.; Wei, Y.; Chen, J.; Han, J.; Wang, C.; Wang, J. Surfactants/citric acid catalyzed hydrothermal carbonization of pomelo peel for solid fuels: Conversion mechanism and combustion performance. Fuel 2023, 342, 127762. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Wang, X.; Zhang, X.; Chen, H. Generalized two-dimensional correlation infrared spectroscopy to reveal the mechanisms of lignocellulosic biomass pyrolysis. Proc. Combust. Inst. 2019, 37, 3013–3021. [Google Scholar] [CrossRef]
- Zhong, J.; Zhu, W.; Sun, J.; Mu, B.; Wang, X.; Xue, Z.; Cao, J. Hydrothermal carbonization of coking sludge: Formation mechanism and fuel characteristic of hydrochar. Chemosphere 2024, 346, 140504. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, C.; Jin, C.; Zhu, Y.; Huang, J.; Na, H.; Zhu, J. In situ bifunctional solid acids bearing B–OH and –COOH groups for efficient hydrolysis of cellulose to sugar in a pure aqueous phase. Green Chem. 2024, 26, 948–959. [Google Scholar] [CrossRef]
- Longo, A.; Alves, O.; Sen, A.U.; Nobre, C.; Brito, P.; Gonçalves, M. Dry and Hydrothermal Co-Carbonization of Mixed Refuse-Derived Fuel (RDF) for Solid Fuel Production. Reactions 2024, 5, 77–97. [Google Scholar] [CrossRef]
- GB/T 30732-2014; Proximate Analysis of Coal by Instrumental Method. Standards Press of China: Beijing, China, 2014.
- GB/T 213-2008; Determination of Calorific Value of Coal. Standards Press of China: Beijing, China, 2008.
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

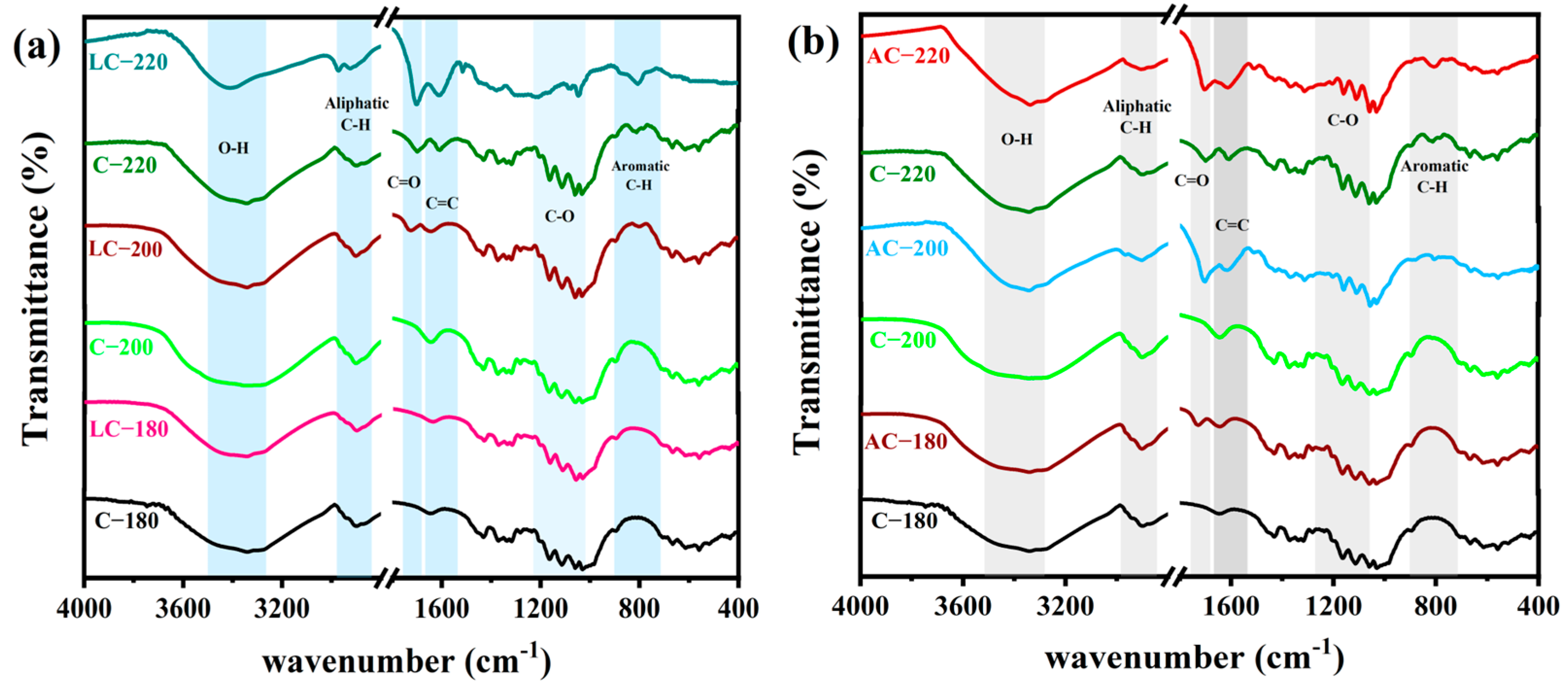
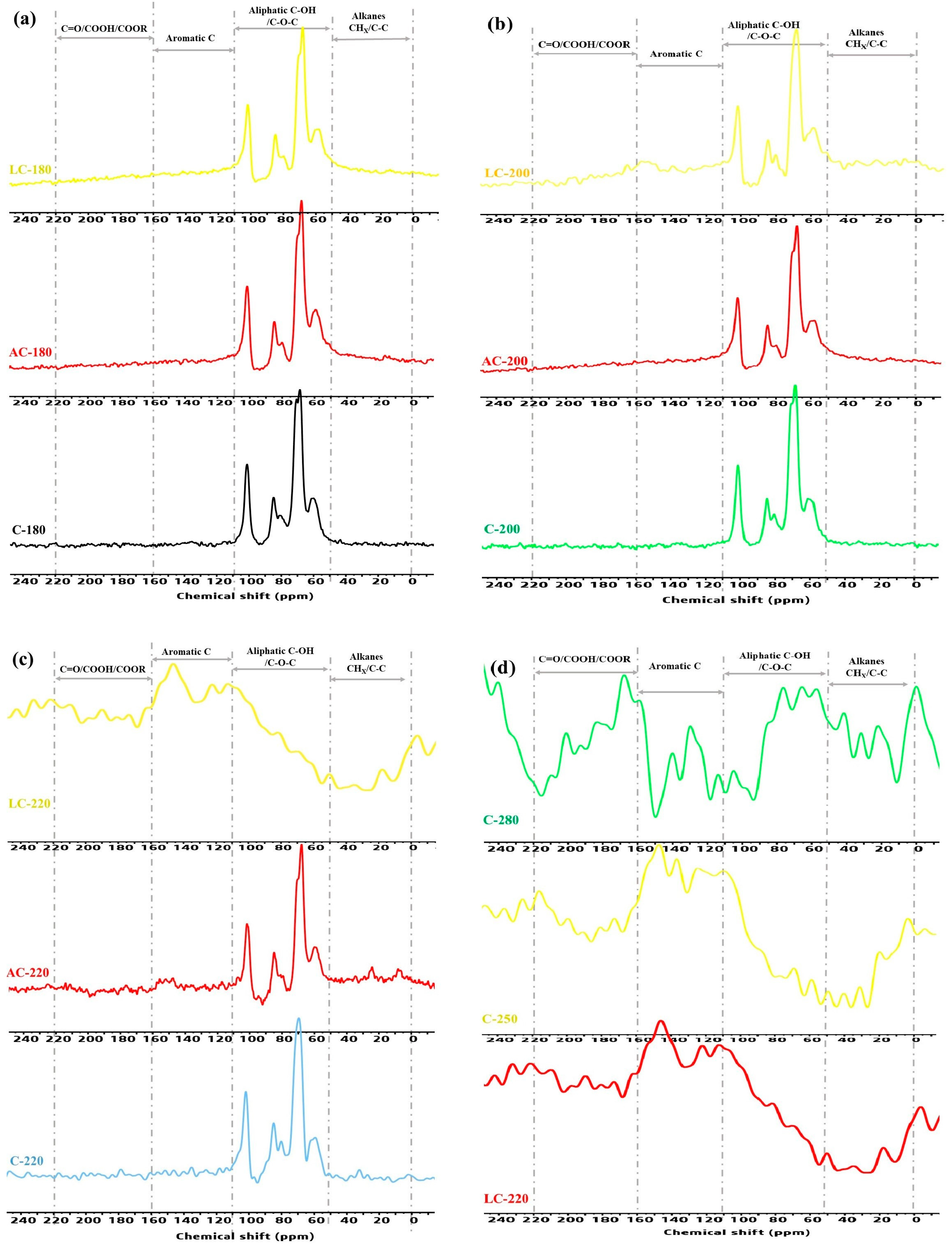
| Wavenumber (cm−1) | Functional Groups | Description |
|---|---|---|
| 3350 | O-H stretching | Alcohol, phenol, or carboxylic acid |
| 2923, 2848 | C-H stretching | Aliphatic |
| 1732, 1700 | C=O stretching | Carbonyl, ester, or carboxyl |
| 1602, 1513 | C=C stretching | Aromatic structure or olefins |
| 1050–1249 | C-O stretching | Alcohol, aliphatic ether, or carboxylic acid |
| 815 | C-H stretching | Aromatic |
| δ (ppm) | Functional Groups | Chemical Formula |
|---|---|---|
| 185–220 | Ketone | C=O |
| 160–185 | Acid or ester | COOH/COOR |
| 140–151 | Cα phenol or linked furan | C=C-OH or C=C-O |
| 142 | Cα free furan | C=CH-O |
| 125–129 | sp2-hybridized aromatic C | C-C=C-C |
| 110–118 | Cβ phenol or linked furan | C=C-OH or C-C=C-O |
| 90–110 | Anomeric C | C-O-C |
| 50–90 | O-alkyl C (alcohol, ether, or aliphatic) | C-OH, C-O-C |
| 40 | Methine or quaternary carbon | -C-H<, >C< |
| 30 | Methylene | -CH2- |
| 15 | Methyl | -CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Chi, M.; Xu, X.; Bai, L.; Wu, J.; Guo, Y. From Cellulose to Highly Aromatic Hydrochar: Catalytic Carbonization and Catalytic Aromatization Mechanism of Lanthanide (III) Ions. Catalysts 2025, 15, 245. https://doi.org/10.3390/catal15030245
Han S, Chi M, Xu X, Bai L, Wu J, Guo Y. From Cellulose to Highly Aromatic Hydrochar: Catalytic Carbonization and Catalytic Aromatization Mechanism of Lanthanide (III) Ions. Catalysts. 2025; 15(3):245. https://doi.org/10.3390/catal15030245
Chicago/Turabian StyleHan, Shuaijie, Mingshu Chi, Xiuling Xu, Li Bai, Junquan Wu, and Yizhuo Guo. 2025. "From Cellulose to Highly Aromatic Hydrochar: Catalytic Carbonization and Catalytic Aromatization Mechanism of Lanthanide (III) Ions" Catalysts 15, no. 3: 245. https://doi.org/10.3390/catal15030245
APA StyleHan, S., Chi, M., Xu, X., Bai, L., Wu, J., & Guo, Y. (2025). From Cellulose to Highly Aromatic Hydrochar: Catalytic Carbonization and Catalytic Aromatization Mechanism of Lanthanide (III) Ions. Catalysts, 15(3), 245. https://doi.org/10.3390/catal15030245






