Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acid Anhydrides
Abstract
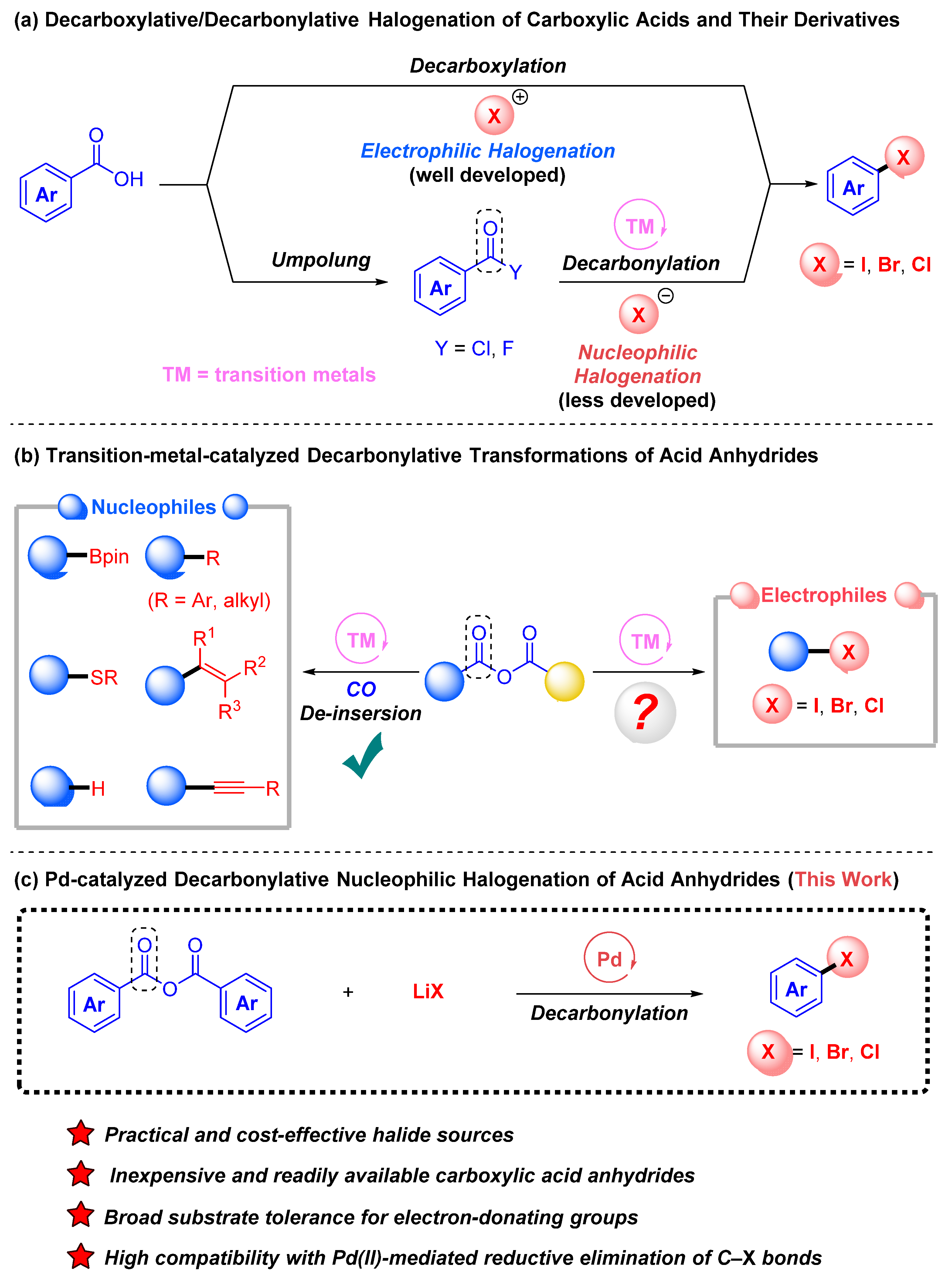
1. Introduction
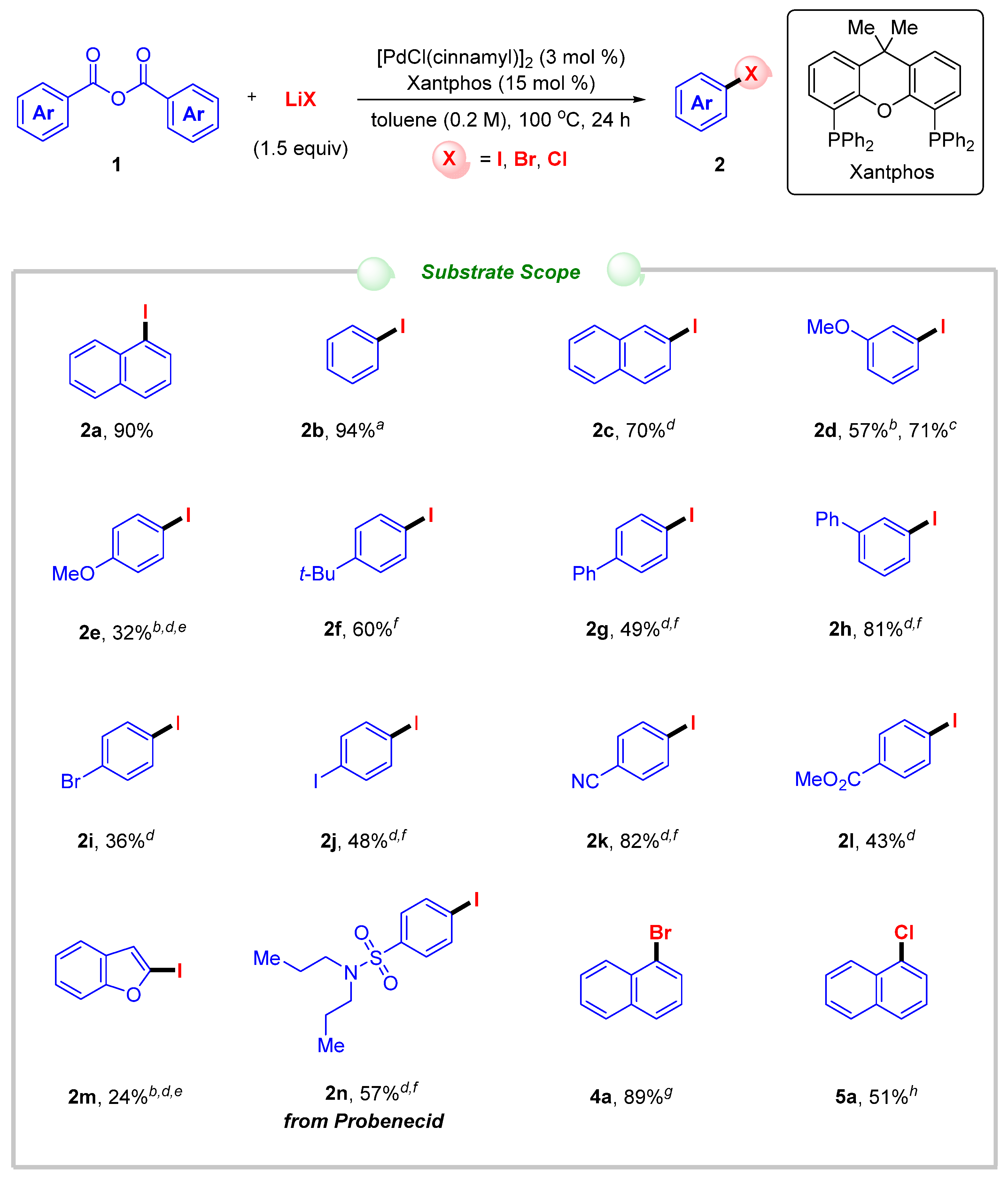
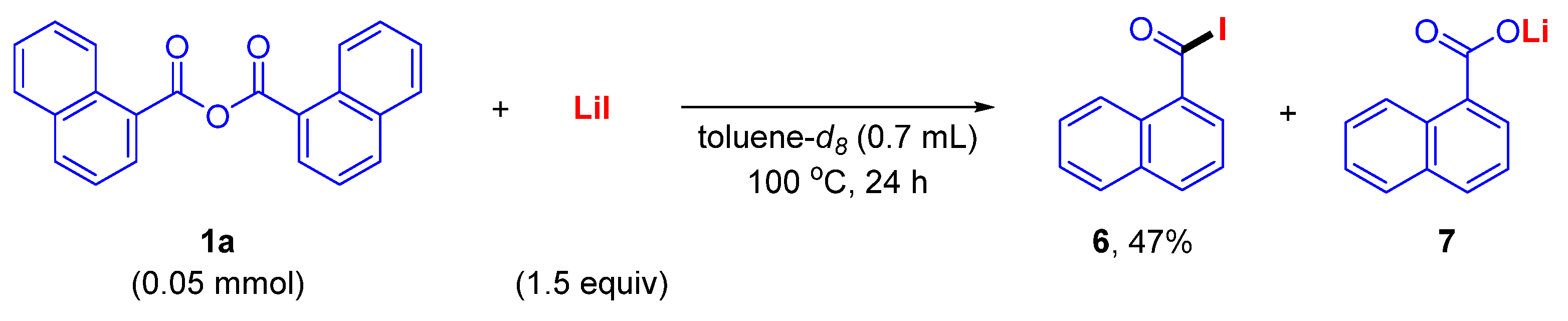
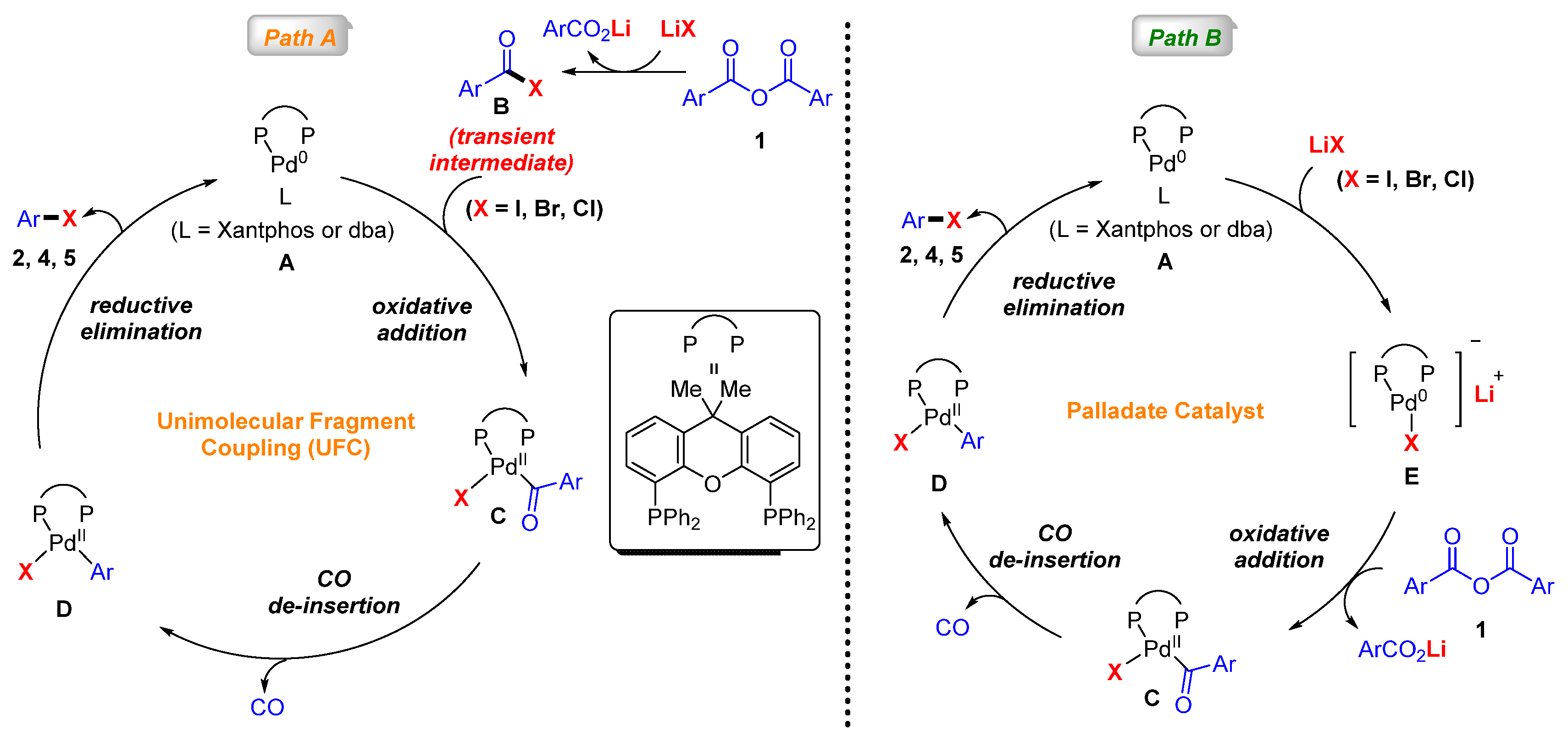
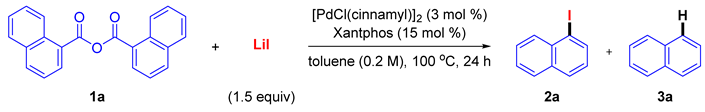
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Experimental Procedure
3.2.1. Representative Procedure for Decarbonylative Iodination of Acid Anhydrides 1 (Procedure D)
3.2.2. Representative Procedure for Decarbonylative Iodination of Acid Anhydrides 1 (Procedure E) (Open System)
3.2.3. Representative Procedure for Decarbonylative Bromination of 1-Naphthoic Anhydrides 1a (Procedure F)
3.2.4. Representative Procedure for Decarbonylative Chlorination of 1-Naphthoic Anhydrides 1a (Procedure G)
3.3. Characterization Data of Products
- 1-Iodonaphthalene (2a) [117]. Prepared according to procedure D as a yellow oil. Rf = 0.60 (hexane). The isolated yield was 90% (45.9 mg) from 1a. 1H NMR (600 MHz, CDCl3): δ 8.14–8.10 (m, 2H), 7.85 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 7.8 Hz, 1H), 7.60 (t, J = 7.6 Hz, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.8 Hz, 1H); 13C{1H} NMR (151 MHz, CDCl3): δ 137.5, 134.5, 134.2, 132.2, 129.1, 128.7, 127.8, 127.0, 126.8, 99.7.
- 2-Iodonaphthalene (2c) [117]. Prepared according to procedure D as a white solid. Rf = 0.60 (hexane). The isolated yield was 70% (35.4 mg) from 1c. 1H NMR (400 MHz, CDCl3): δ 8.24–8.24 (m, 1H), 7.81–7.78 (m, 1H), 7.73–7.71 (m, 2H), 7.58 (d, J = 8.6 Hz, 1H), 7.50 (dt, J = 6.3, 3.5 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3): δ 136.8, 135.1, 134.5, 132.2, 129.6, 128.0, 126.9, 126.8, 126.6, 91.6.
- 1-Iodo-3-methoxybenzene (2d) [117]. Prepared according to procedure E as a colorless oil. Rf = 0.40 (DCM/hexane = 1/10). The isolated yield was 57% (26.8 mg) from 1d. 1H NMR (600 MHz, CDCl3): δ 7.28 (ddt, J = 7.8, 1.8, 0.9 Hz, 1H), 7.25 (dt, J = 2.5, 1.2 Hz, 1H), 7.00 (t, J = 8.1 Hz, 1H), 6.87 (dd, J = 8.4, 2.5 Hz, 1H), 3.78 (s, 3H); 13C{1H} NMR (151 MHz, CDCl3): δ 160.3, 130.9, 130.0, 123.1, 113.9, 94.5, 55.5.
- 1-Iodo-4-methoxybenzene (2e) [117]. Prepared according to procedure E as a colorless oil. Rf = 0.40 (DCM/hexane = 1/10). The isolated yield was 32% (15.2 mg) from 1e. 1H NMR (600 MHz, CDCl3): δ 7.57–7.54 (m, 2H), 6.70–6.67 (m, 2H), 3.78 (s, 3H); 13C{1H} NMR (151 MHz, CDCl3): 159.6, 138.3, 116.5, 82.8, 55.4.
- 1-(tert-butyl)-4-iodobenzene (2f) [117]. Prepared according to procedure D as a white solid. Rf = 0.80 (hexane). The isolated yield was 60% (31.1 mg) from 1f. 1H NMR (400 MHz, CDCl3): δ 7.64–7.60 (m, 2H), 7.17–7.13 (m, 2H), 1.30 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3): δ 151.0, 137.2, 127.7, 90.8, 34.7, 31.3.
- 4-Iodo-1,1′-biphenyl (2g) [117]. Prepared according to procedure D as a white solid. Rf = 0.55 (hexane). The isolated yield was 49% (27.6 mg) from 1g. 1H NMR (600 MHz, CDCl3): δ 7.78–7.76 (m, 2H), 7.57–7.55 (m, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.39–7.36 (m, 1H), 7.35–7.33 (m, 2H); 13C{1H} NMR (151 MHz, CDCl3): δ 140.9, 140.2, 138.0, 129.1, 129.0, 127.8, 127.0, 93.2.
- 3-Iodo-1,1′-biphenyl (2h) [117]. Prepared according to procedure D as a colorless oil. Rf = 0.55 (hexane). The isolated yield was 81% (45.1 mg) from 1h. 1H NMR (600 MHz, CDCl3): δ 7.95 (t, J = 1.7 Hz, 1H), 7.68 (ddd, J = 7.9, 1.8, 1.0 Hz, 1H), 7.56–7.54 (m, 3H), 7.46–7.43 (m, 2H), 7.39–7.36 (m, 1H), 7.18 (t, J = 7.8 Hz, 1H); 13C{1H} NMR (151 MHz, CDCl3): δ 143.6, 139.8, 136.32, 136.28, 130.5, 129.0, 128.0, 127.2, 126.5, 94.9.
- 1-Bromo-4-iodobenzene (2i) [117]. Prepared according to procedure D as a white solid. Rf = 0.80 (hexane). The isolated yield was 36% (20.3 mg) from 1i. 1H NMR (600 MHz, CDCl3): δ 7.56–7.53 (m, 2H), 7.24–7.22 (m, 2H); 13C{1H} NMR (151 MHz, CDCl3): δ 139.2, 133.6, 122.3, 92.2.
- 1,4-Diiodobenzene (2j) [117]. Prepared according to procedure D as a white solid. Rf = 0.80 (hexane). The isolated yield was 48% (31.1 mg) from 1j. 1H NMR (600 MHz, CDCl3): δ 7.41 (s, 4H); 13C{1H} NMR (151 MHz, CDCl3): δ 139.5, 93.5.
- 4-Iodobenzonitrile (2k) [117]. Prepared according to procedure D as a white solid. Rf = 0.30 (DCM/hexane = 1/1). The isolated yield was 82% (37.3 mg) from 1k. 1H NMR (600 MHz, CDCl3): δ 7.86–7.83 (m, 2H), 7.37–7.35 (m, 2H); 13C{1H} NMR (151 MHz, CDCl3): δ 138.6, 133.3, 118.3, 111.9, 100.4.
- Methyl 4-Iodobenzoate (2l) [117]. Prepared according to procedure D as a white solid. Rf = 0.40 (DCM/hexane = 1/1). The isolated yield was 43% (22.6 mg) from 1l. 1H NMR (600 MHz, CDCl3): δ 7.81–7.79 (m, 2H), 7.75–7.73 (m, 2H), 3.91 (s, 3H); 13C{1H} NMR (151 MHz, CDCl3): δ 166.7, 137.9, 131.2, 129.7, 100.9, 52.4.
- 2-Iodobenzofuran (2m) [117]. Prepared according to procedure E as a colorless oil. Rf = 0.60 (hexane). The isolated yield was 24% (11.8 mg) from 1m. 1H NMR (600 MHz, CDCl3): δ 7.52–7.50 (m, 1H), 7.48–7.46 (m, 1H), 7.24–7.19 (m, 2H), 6.96 (s, 1H); 13C{1H} NMR (151 MHz, CDCl3): δ 158.3, 129.3, 124.4, 123.3, 119.8, 117.4, 111.0, 96.0.
- 4-Iodo-N,N-dipropylbenzenesulfonamide (2n) [117]. Prepared according to procedure D as a white solid. Rf = 0.40 (DCM/hexane = 2/1). The isolated yield was 57% (41.8 mg) from 1n. 1H NMR (400 MHz, CDCl3): δ 7.86–7.82 (m, 2H), 7.52–7.49 (m, 2H), 3.07–3.04 (m, 4H), 1.54 (dq, J = 14.9, 7.4 Hz, 4H), 0.86 (t, J = 7.4 Hz, 6H); 13C{1H} NMR (101 MHz, CDCl3): δ 140.0, 138.3, 128.6, 99.5, 50.1, 22.1, 11.3.
- 1-Bromonaphthalene (4a) [117]. Prepared according to procedure F as a colorless oil. Rf = 0.60 (hexane). The isolated yield was 89% (36.7 mg) from 1a. 1H NMR (400 MHz, CDCl3): δ 8.28 (d, J = 8.4 Hz, 1H), 7.87–7.80 (m, 3H), 7.62 (ddd, J = 8.5, 6.9, 1.5 Hz, 1H), 7.56 (td, J = 7.5, 6.8, 1.4 Hz, 1H), 7.34 (t, J = 7.8 Hz, 1H); 13C{1H} NMR (151 MHz, CDCl3): δ 134.7, 132.1, 130.0, 128.4, 128.0, 127.4, 127.2, 126.8, 126.3, 122.9.
- 1-Chloronaphthalene (5a) [117]. Prepared according to procedure G as a colorless oil. Rf = 0.55 (hexane). The isolated yield was 51% (16.5 mg) from 1a. 1H NMR (400 MHz, CDCl3): δ 8.33–8.30 (m, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.2 Hz, 1H), 7.65–7.55 (m, 3H), 7.42–7.38 (m, 1H); 13C{1H} NMR (101 MHz, CDCl3): δ 134.7, 132.0, 130.9, 128.3, 127.3, 127.2, 126.8, 126.3, 125.8, 124.5.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandes, M.Z.; Cavalcanti, S.M.T.; Moreira, D.R.M.; de Azevedo, W.F., Jr.; Leite, A.C.L. Halogen Atoms in the Modern Medicinal Chemistry: Hints for the Drug Design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Tang, M.L.; Bao, Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2011, 23, 446–455. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally Occurring Organohalogen Compounds. Acc. Chem. Res. 1998, 31, 141–152. [Google Scholar] [CrossRef]
- Gribble, G.W. Structure and Biosynthesis of Halogenated Alkaloids. In Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; Fattorusso, E., Tagilialatela-Scafati, O., Eds.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Jeschke, P. The Unique Role of Halogen Substituents in the Design of Modern Crop Protection Compounds. In Modern Methods in Crop Protection Research; Jeschke, P., Krämer, W., Schirmer, U., Witschel, M., Eds.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Beale, T.M.; Chudzinski, M.G.; Sarwar, M.G.; Taylor, M.S. Halogen bonding in solution: Thermodynamics and applications. Chem. Soc. Rev. 2013, 42, 1667–1680. [Google Scholar] [CrossRef]
- Stille, J.K.; Lau, K.S.Y. Mechanisms of Oxidative Addition of Organic Halides to Group 8 Transition-Metal Complexes. Acc. Chem. Res. 1977, 10, 434–442. [Google Scholar] [CrossRef]
- Labinger, J.A. Tutorial on Oxidative Addition. Organometallics 2015, 34, 4784–4795. [Google Scholar] [CrossRef]
- Rio, J.; Liang, H.; Perrin, M.-E.L.; Perego, L.A.; Grimaud, L.; Payard, P.-A. We Already Know Everything about Oxidative Addition to Pd(0): Do We? ACS Catal. 2023, 13, 11399–11421. [Google Scholar] [CrossRef]
- Kvasovs, N.; Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 2021, 50, 2244–2259. [Google Scholar] [CrossRef]
- Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Visible Light Mediated Photoredox Catalytic Arylation Reactions. Acc. Chem. Res. 2016, 49, 1566–1577. [Google Scholar] [CrossRef]
- Pan, Q.; Ping, Y.; Kong, W. Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings. Acc. Chem. Res. 2023, 56, 515–535. [Google Scholar] [CrossRef]
- Li, Y.; Hari, D.P.; Vita, M.V.; Waser, J. Cyclic Hypervalent Iodine Reagents for Atom-Transfer Reactions: Beyond Trifluoromethylation. Angew. Chem. Int. Ed. 2016, 55, 4436–4454, Angew. Chem. 2016, 128, 4512–4531. [Google Scholar] [CrossRef]
- Brand, J.P.; Waser, J. Electrophilic alkynylation: The dark side of acetylene chemistry. Chem. Soc. Rev. 2012, 41, 4165–4179. [Google Scholar] [CrossRef]
- Charpentier, J.; Früh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650–682. [Google Scholar] [CrossRef]
- Wang, X.; Studer, A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 2017, 50, 1712–1724. [Google Scholar] [CrossRef]
- Cui, B.; Jia, S.; Tokunaga, E.; Shibata, N. Defluorosilylation of fluoroarenes and fluoroalkanes. Nat. Commun. 2018, 9, 4393. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, L.J.; Rodríguez, N.; Gooßen, K. Carboxylic Acids as Substrates in Homogeneous Catalysis. Angew. Chem. Int. Ed. 2008, 47, 3100–3120, Angew. Chem. 2008, 120, 3144–3164. [Google Scholar] [CrossRef]
- Dzik, W.I.; Lange, P.P.; Gooßen, L.J. Carboxylates as sources of carbon nucleophiles and electrophiles: Comparison of decarboxylative and decarbonylative pathways. Chem. Sci. 2012, 3, 2671–2678. [Google Scholar] [CrossRef]
- Borodine, A. Ueber Bromvaleriansäure Und Brombutersäure. Liebigs Ann. 1861, 119, 121–123. [Google Scholar] [CrossRef]
- Hunsdiecker, H.; Hunsdiecker, C. Über den Abbau der Salze aliphatischer Säuren durch Brom. Ber. Dtsch. Chem. Ges. B 1942, 75, 291–297. [Google Scholar] [CrossRef]
- Hunsdiecker, H.; Hunsdiecker, C.; Vogt, E. Method of Manufacturing Organic Chlorine and Bromine derivatives. U.S. Patent 2176181, 17 October 1939. [Google Scholar]
- Varenikov, A.; Shapiro, E.; Gandelman, M. Decarboxylative Halogenation of Organic Compounds. Chem. Rev. 2021, 121, 412–484. [Google Scholar] [CrossRef] [PubMed]
- Cornella, J.; Rosillo-Lopez, M.; Larrosa, I. A Novel Mode of Reactivity for Gold(I): The Decarboxylative Activation of (Hetero) Aromatic Carboxylic Acids. Adv. Synth. Catal. 2011, 353, 1359–1366. [Google Scholar] [CrossRef]
- Cristol, S.; Firth, W., Jr. A Convenient Synthesis of Alkyl Halides from Carboxylic Acids. J. Org. Chem. 1961, 26, 280. [Google Scholar] [CrossRef]
- Zhan, K.; Li, Y. Microwave-Assisted Silver-Catalyzed Protodecarboxylation and Decarboxylative Iodination of Aromatic Carboxylic Acids. Catalysts 2017, 7, 314. [Google Scholar] [CrossRef]
- Hazarika, D.; Phukan, P. TsNBr2 promoted decarboxylative bromination of α,β-unsaturated carboxylic acids. Tetrahedron Lett. 2018, 59, 4593–4596. [Google Scholar] [CrossRef]
- Jayaraman, A.; Cho, E.; Kim, J.; Lee, S. Decarboxylative Tribromination for the Selective Synthesis of Tribromomethyl Ketone and Tribromovinyl Derivatives. Adv. Synth. Catal. 2018, 360, 3978–3989. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, X.; Zhang, H.; Yamamoto, Y.; Bao, M. Transition-metal-free decarboxylative halogenation of 2-picolinic acids with dihalomethane under oxygen conditions. Green Chem. 2019, 21, 5565–5570. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Deng, G.J.; Gong, H. Simple, Efficient and Controllable Synthesis of Iodo/Di-Iodoarenes via Ipso iododecarboxylation/Consecutive Iodination Strategy. Sci. Rep. 2017, 7, 40430. [Google Scholar]
- Fu, Z.; Li, Z.; Song, Y.; Yang, R.; Liu, Y.; Cai, H. Decarboxylative Halogenation and Cyanation of Electron-Deficient Aryl Carboxylic Acids via Cu Mediator as Well as Electron-Rich Ones through Pd Catalyst under Aerobic Conditions. J. Org. Chem. 2016, 81, 2794–2803. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, H.; Jin, Y.; Ou, L.; Fu, H. Visible-Light-Induced Decarboxylative Iodination of Aromatic Carboxylic Acids. Synlett 2018, 29, 1572–1577. [Google Scholar]
- Patra, T.; Mukherjee, S.; Ma, J.; Strieth-Kalthoff, F.; Glorius, F. Visible-Light-Photosensitized Aryl and Alkyl Decarboxylative Functionalization Reactions. Angew. Chem. Int. Ed. 2019, 58, 10514–10520, Angew. Chem. 2019, 131, 10624–10630. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, Y.; Jiang, L.; Li, Z.; Guo, S.; Cai, H. Cu-catalyzed decarboxylative iodination of aryl carboxylic acids with NaI: A practical entry to aryl iodides under aerobic conditions. Tetrahedron Lett. 2018, 59, 4458–4461. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Liu, Z.-Q. Transition-Metal-Free Hunsdiecker Reaction of Electron-Rich Arene-carboxylic Acids and Aryl Aldehydes in Water. Synlett 2014, 25, 2508–2512. [Google Scholar] [CrossRef]
- Perry, G.J.P.; Quibell, J.M.; Panigrahi, A.; Larrosa, I. Transition-Metal-Free Decarboxylative Iodination: New Routes for Decarboxylative Oxidative Cross-Couplings. J. Am. Chem. Soc. 2017, 139, 11527–11536. [Google Scholar] [CrossRef]
- Quibell, J.M.; Perry, G.J.P.; Cannas, D.M.; Larrosa, I. Transition-metal-free decarboxylative bromination of aromatic carboxylic acids. Chem. Sci. 2018, 9, 3860–3865. [Google Scholar] [CrossRef]
- Chen, C.; Tong, X. Synthesis of organic halides via palladium(0) catalysis. Org. Chem. Front. 2014, 1, 439–446. [Google Scholar] [CrossRef]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern Transition-Metal-Catalyzed Carbon–Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- Jones, D.J.; Lautens, M.; McGlacken, G.P. The emergence of Pd-mediated reversible oxidative addition in cross coupling, carbohalogenation and carbonylation reactions. Nat. Catal. 2019, 2, 843–851. [Google Scholar] [CrossRef]
- Echavarren, A.M.; Stille, J.K. Palladium-Catalyzed Coupling of Aryl Triflates with Organostannanes. J. Am. Chem. Soc. 1987, 109, 5478–5486. [Google Scholar] [CrossRef]
- Roy, A.H.; Hartwig, J.F. Reductive Elimination of Aryl Halides from Palladium(II). J. Am. Chem. Soc. 2001, 123, 1232–1233. [Google Scholar] [CrossRef]
- Roy, A.H.; Hartwig, J.F. Directly Observed Reductive Elimination of Aryl Halides from Monomeric Arylpalladium(II) Halide Complexes. J. Am. Chem. Soc. 2003, 125, 13944–13945. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.H.; Hartwig, J.F. Reductive Elimination of Aryl Halides upon Addition of Hindered Alkylphosphines to Dimeric Arylpalladium(II) Halide Complexes. Organometallics 2004, 23, 1533–1541. [Google Scholar] [CrossRef]
- Shen, X.; Hyde, A.M.; Buchwald, S.L. Palladium-Catalyzed Conversion of Aryl and Vinyl Triflates to Bromides and Chlorides. J. Am. Chem. Soc. 2010, 132, 14076–14078. [Google Scholar] [CrossRef]
- Pan, J.; Wang, X.; Zhang, Y.; Buchwald, S.L. An Improved Palladium-Catalyzed Conversion of Aryl and Vinyl Triflates to Bromides and Chlorides. Org. Lett. 2011, 13, 4974–4976. [Google Scholar] [CrossRef]
- Sather, A.C.; Buchwald, S.L. The Evolution of Pd0/PdII-Catalyzed Aromatic Fluorination. Acc. Chem. Res. 2016, 49, 2146–2157. [Google Scholar] [CrossRef]
- Newman, S.G.; Lautens, M. The Role of Reversible Oxidative Addition in Selective Palladium(0)-Catalyzed Intramolecular Cross-Couplings of Polyhalogenated Substrates: Synthesis of Brominated Indoles. J. Am. Chem. Soc. 2010, 132, 11416–11417. [Google Scholar] [CrossRef]
- Newman, S.G.; Lautens, M. Palladium-Catalyzed Carboiodination of Alkenes: Carbon–Carbon Bond Formation with Retention of Reactive Functionality. J. Am. Chem. Soc. 2011, 133, 1778–1780. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qiu, D.; Tong, X. Palladium-Catalyzed Cycloisomerizations of (Z)-1-Iodo-1,6-dienes: Iodine Atom Transfer and Mechanistic Insight to Alkyl Iodide Reductive Elimination. J. Am. Chem. Soc. 2011, 133, 6187–6193. [Google Scholar] [CrossRef]
- Chen, C.; Hou, L.; Cheng, M.; Su, J.; Tong, X. Palladium(0)-Catalyzed Iminohalogenation of Alkenes: Synthesis of 2-Halomethyl Dihydropyrroles and Mechanistic Insights into the Alkyl Halide Bond Formation. Angew. Chem. Int. Ed. 2015, 54, 3092–3096, Angew. Chem. 2015, 127, 3135–3139. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Dong, M.; Yang, N.; Wang, J.; Zhang, Y.; Liu, K.; Tong, X. Pd(0)-Catalyzed Asymmetric Carbohalogenation: H-Bonding-Driven C(sp3)–Halogen Reductive Elimination under Mild Conditions. J. Am. Chem. Soc. 2021, 143, 1924–1931. [Google Scholar] [CrossRef]
- Lee, Y.H.; Morandi, B. Palladium-Catalyzed Intermolecular Aryliodination of Internal Alkynes. Angew. Chem. Int. Ed. 2019, 58, 6444–6448, Angew. Chem. 2019, 131, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Boehm, P.; Kehl, N.; Morandi, B. Rhodium-Catalyzed Anti-Markovnikov Transfer Hydroiodination of Terminal Alkynes. Angew. Chem. Int. Ed. 2023, 62, e202214071, Angew. Chem. 2023, 135, e202214071. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wei, J.; Geng, W.; Zhang, W.-X.; Xi, Z. Transfer of Aryl Halide to Alkyl Halide: Reductive Elimination of Alkylhalide from Alkylpalladium Halides Containing syn-β-Hydrogen Atoms. Angew. Chem. Int. Ed. 2014, 53, 14533–14537, Angew. Chem. 2014, 126, 14761–14765. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Rueping, M. Transition-Metal-Catalyzed Decarbonylative Coupling Reactions: Concepts, Classifications, and Applications. Chem. Eur. J. 2018, 24, 7794–7809. [Google Scholar] [CrossRef]
- Zhao, Q.; Szostak, M. Redox-Neutral Decarbonylative Cross-Couplings Coming of Age. ChemSusChem 2019, 12, 2983–2987. [Google Scholar] [CrossRef]
- Lu, H.; Yu, T.-Y.; Xu, P.-F.; Wei, H. Selective Decarbonylation via Transition-Metal-Catalyzed Carbon–Carbon Bond Cleavage. Chem. Rev. 2021, 121, 365–411. [Google Scholar] [CrossRef]
- Lalloo, N.; Brigham, C.E.; Sanford, M.S. Mechanism-Driven Development of Group 10 Metal-Catalyzed Decarbonylative Coupling Reactions. Acc. Chem. Res. 2022, 55, 3430–3444. [Google Scholar] [CrossRef]
- Keaveney, S.T.; Schoenebeck, F. Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides. Angew. Chem. Int. Ed. 2018, 57, 4073–4077, Angew. Chem. 2018, 130, 4137–4141. [Google Scholar] [CrossRef]
- Okuda, Y.; Xu, J.; Ishida, T.; Wang, C.-A.; Nishihara, Y. Nickel-Catalyzed Decarbonylative Alkylation of Aroyl Fluorides Assisted by Lewis-Acidic Organoboranes. ACS Omega 2018, 3, 13129–13140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Nishihara, Y. Nickel-catalysed decarbonylative borylation of aroyl fluorides. Chem. Commun. 2018, 54, 13969–13972. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Liu, L.; Asanuma, Y.; Nishihara, Y. Nickel-Catalyzed Decarbonylative Stannylation of Acyl Fluorides under Ligand-Free Conditions. Molecules 2019, 24, 1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Nishihara, Y. Nickel/copper-cocatalyzed decarbonylative silylation of acyl fluorides. Chem. Commun. 2019, 55, 10507–10510. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, Q.; Wang, Z.; Nishihara, Y. Palladium-Catalyzed Decarbonylative Alkylation of Acyl Fluorides. Org. Lett. 2020, 22, 2350–2353. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, L.; Nishihara, Y. Palladium/copper-cocatalyzed decarbonylative alkynylation of acyl fluorides with alkynylsilanes: Synthesis of unsymmetrical diarylethynes. Chem. Commun. 2020, 56, 7977–7980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, L.; You, J.; Nishihara, Y. Nickel-Catalyzed Decarbonylative Alkynylation of Acyl Fluorides with Terminal Alkynes under Copper-Free Conditions. Synlett 2021, 32, 1560–1564. [Google Scholar]
- You, J.; Chen, Q.; Nishihara, Y. Nickel-Catalyzed Decarbonylative Thioetherification of Acyl Fluorides via C–F Bond Activation. Synthesis 2021, 53, 3045–3050. [Google Scholar]
- Chen, Q.; Li, Z.; Nishihara, Y. Palladium/Copper-Cocatalyzed Arylsilylation of Internal Alkynes with Acyl Fluorides and Silylboranes: Synthesis of Tetrasubstituted Alkenylsilanes by Three-Component Coupling Reaction. Org. Lett. 2022, 24, 385–389. [Google Scholar] [CrossRef]
- Chen, Q.; You, J.; Tian, T.; Li, Z.; Kashihara, M.; Mori, H.; Nishihara, Y. Nickel-Catalyzed Decarbonylative Reductive Alkylation of Aroyl Fluorides with Alkyl Bromides. Org. Lett. 2022, 24, 9259–9263. [Google Scholar] [CrossRef]
- Tian, T.; Chen, Q.; Li, Z.; Nishihara, Y. Recent Advances in C–F Bond Activation of Acyl Fluorides Directed toward Catalytic Transformation by Transition Metals, N-Heterocyclic Carbenes, or Phosphines. Synthesis 2022, 54, 3667–3697. [Google Scholar]
- Malapit, C.A.; Bour, J.R.; Brigham, C.E.; Sanford, M.S. Base-free nickel-catalysed decarbonylative Suzuki–Miyaura coupling of acid fluorides. Nature 2018, 563, 100–104. [Google Scholar] [CrossRef]
- Malapit, C.A.; Bour, J.R.; Laursen, S.R.; Sanford, M.S. Mechanism and Scope of Nickel-Catalyzed Decarbonylative Borylation of Carboxylic Acid Fluorides. J. Am. Chem. Soc. 2019, 141, 17322–17330. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, N.; Malapit, C.A.; Taimoory, S.M.; Brigham, C.E.; Sanford, M.S. Decarbonylative Fluoroalkylation at Palladium(II): From Fundamental Organometallic Studies to Catalysis. J. Am. Chem. Soc. 2021, 143, 18617–18625. [Google Scholar] [CrossRef] [PubMed]
- Fehér, P.P.; Stirling, A. Theoretical Study on the Formation of Ni(PR3)(Aryl)F Complexes Observed in Ni-Catalyzed Decarbonylative C–C Coupling of Acyl Fluorides. Organometallics 2020, 39, 2774–2783. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Sakurai, Y.; Hattori, H.; Sakai, N. Palladium-Catalyzed Reductive Conversion of Acyl Fluorides via Ligand-Controlled Decarbonylation. Org. Lett. 2018, 20, 4204–4208. [Google Scholar] [CrossRef]
- Sakurai, S.; Yoshida, T.; Tobisu, M. Iridium-catalyzed Decarbonylative Coupling of Acyl Fluorides with Arenes and Heteroarenes via C–H Activation. Chem. Lett. 2019, 48, 94–97. [Google Scholar] [CrossRef]
- Kayumov, M.; Zhao, J.-N.; Mirzaakhmedov, S.; Wang, D.-Y.; Zhang, A. Synthesis of Arylstannanes via Palladium-Catalyzed Decarbonylative Coupling of Aroyl Fluorides. Adv. Synth. Catal. 2020, 362, 776–781. [Google Scholar] [CrossRef]
- Sakurai, S.; Tobisu, M. Palladium-catalyzed Decarbonylative Cyanation of Acyl Fluorides and Chlorides. Chem. Lett. 2021, 50, 151–153. [Google Scholar] [CrossRef]
- He, B.; Liu, X.; Li, H.; Zhang, X.; Ren, Y.; Su, W. Rh-Catalyzed General Method for Directed C–H Functionalization via Decarbonylation of in-Situ-Generated Acid Fluorides from Carboxylic Acids. Org. Lett. 2021, 23, 4191–4196. [Google Scholar] [CrossRef]
- Blanchard, N.; Bizet, V. Acid Fluorides in Transition-Metal Catalysis: A Good Balance between Stability and Reactivity. Angew. Chem. Int. Ed. 2019, 58, 6814–6817, Angew. Chem. 2019, 131, 6886–6889. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Sakai, N. Acyl Fluorides in Late-Transition-Metal Catalysis. Angew. Chem. Int. Ed. 2020, 59, 574–594, Angew. Chem. 2020, 132, 584–605. [Google Scholar] [CrossRef]
- Blum, J.; Lipshes, Z. Catalytic Conversion of Benzoic Anhydrides into Fluorenones. J. Org. Chem. 1969, 34, 3076–3080. [Google Scholar] [CrossRef]
- Zhang, X.; Jordan, F.; Szostak, M. Transition-metal-catalyzed decarbonylation of carboxylic acids to olefins: Exploiting acyl C–O activation for the production of high value products. Org. Chem. Front. 2018, 5, 2515–2521. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Rodríguez, N. A mild and efficient protocol for the conversion of carboxylic acids to olefins by a catalytic decarbonylative elimination reaction. Chem. Commun. 2004, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Kajita, Y.; Kurahashi, T.; Matsubara, S. Nickel-Catalyzed Decarbonylative Addition of Anhydrides to Alkynes. J. Am. Chem. Soc. 2008, 130, 17226–17227. [Google Scholar] [CrossRef]
- Matsuda, T.; Suzuki, K. Rhodium(III)-catalysed decarbonylative coupling of maleic anhydrides with alkynes. RSC Adv. 2014, 4, 37138–37141. [Google Scholar] [CrossRef]
- Prakash, R.; Shekarrao, K.; Gogoi, S.; Boruah, R.C. Ruthenium-catalyzed decarbonylative addition reaction of anhydrides with alkynes: A facile synthesis of isocoumarins and α-pyrones. Chem. Commun. 2015, 51, 9972–9974. [Google Scholar] [CrossRef]
- Jin, W.; Yu, Z.; He, W.; Ye, W.; Xiao, W.-J. Efficient Rh(I)-Catalyzed Direct Arylation and Alkenylation of Arene C–H Bonds via Decarbonylation of Benzoic and Cinnamic Anhydrides. Org. Lett. 2009, 11, 1317–1320. [Google Scholar] [CrossRef]
- Maetani, S.; Fukuyama, T.; Ryu, I. Rhodium-Catalyzed Decarbonylative C–H Arylation of 2-Aryloxybenzoic Acids Leading to Dibenzofuran Derivatives. Org. Lett. 2013, 15, 2754–2757. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, P.; Wang, D.; Wang, M.; Yuan, Y.; Shi, Z. PIII-Chelation-Assisted Indole C7-Arylation, Olefination, Methylation, and Acylation with Carboxylic Acids/Anhydrides by Rhodium Catalysis. Angew. Chem. Int. Ed. 2019, 58, 1504–1508, Angew. Chem. 2019, 131, 1518–1522. [Google Scholar] [CrossRef]
- Han, X.; Yuan, Y.; Shi, Z. Rhodium-Catalyzed Selective C–H Trideuteromethylation of Indole at C7 Position Using Acetic-d6 Anhydride. J. Org. Chem. 2019, 84, 12764–12772. [Google Scholar] [CrossRef]
- Liu, C.; Ji, C.-L.; Zhou, T.; Hong, X.; Szostak, M. Bimetallic Cooperative Catalysis for Decarbonylative Heteroarylation of Carboxylic Acids via C-O/C-H Coupling. Angew. Chem. Int. Ed. 2021, 60, 10690–10699, Angew. Chem. 2021, 133, 10785–10794. [Google Scholar] [CrossRef] [PubMed]
- Stephan, M.S.; Teunissen, A.J.J.M.; Verzijl, G.K.M.; de Vries, J.G. Heck Reactions without Salt Formation: Aromatic Carboxylic Anhydrides as Arylating Agents. Angew. Chem. Int. Ed. 1998, 37, 662–664, Angew. Chem. 1998, 110, 688–690. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Paetzold, J.; Winkel, L. Pd-Catalyzed Decarbonylative Heck Olefination of Aromatic Carboxylic Acids Activated in situ with Di-tert-butyl Dicarbonate. Synlett 2002, 10, 1721–1723. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Bercot, E.A.; Rovis, T. Decarbonylative Cross-Coupling of Cyclic Anhydrides: Introducing Stereochemistry at an sp3 Carbon in the Cross-Coupling Event. J. Am. Chem. Soc. 2003, 125, 10498–10499. [Google Scholar] [CrossRef] [PubMed]
- Gooßen, L.J.; Paetzold, J. New Synthesis of Biaryls via Rh-Catalyzed Decarbonylative Suzuki-Coupling of Carboxylic Anhydrides with Arylboroxines. Adv. Synth. Catal. 2004, 346, 1665–1668. [Google Scholar] [CrossRef]
- Liu, C.; Ji, C.-L.; Qin, Z.-X.; Hong, X.; Szostak, M. Synthesis of Biaryls via Decarbonylative Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling of Carboxylic Acids. iScience 2019, 19, 749–759. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Huang, T.; Tang, Z.; Li, C.; Li, W.; Zhang, T.; Li, Z.; Chen, T. Palladium-Catalyzed Decarbonylative Sonogashira Coupling of Terminal Alkynes with Carboxylic Acids. Org. Lett. 2021, 23, 3304–3309. [Google Scholar] [CrossRef]
- Liu, C.; Szostak, M. Decarbonylative Sonogashira Cross-Coupling of Carboxylic Acids. Org. Lett. 2021, 23, 4726–4730. [Google Scholar] [CrossRef]
- Bie, F.; Liu, X.; Szostak, M.; Liu, C. Decarbonylative Alkynylation of Aryl Anhydrides via Palladium Catalysis. J. Org. Chem. 2023, 88, 4442–4451. [Google Scholar] [CrossRef]
- Liu, C.; Ji, C.-L.; Hong, X.; Szostak, M. Palladium-Catalyzed Decarbonylative Borylation of Carboxylic Acids: Tuning Reaction Selectivity by Computation. Angew. Chem. Int. Ed. 2018, 57, 16721–16726. [Google Scholar] [CrossRef]
- Zhang, W.; Bie, F.; Ma, J.; Zhou, F.; Szostak, M.; Liu, C. Palladium-Catalyzed Decarbonylative Borylation of Aryl Anhydrides. J. Org. Chem. 2021, 86, 17445–17452. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, R.; Cui, Y.; Liu, C. Decarbonylative borylation of aryl anhydrides via rhodium catalysis. Org. Biomol. Chem. 2024, 22, 1693–1698. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Tao, S.-W.; Liu, R.-Q.; Zhu, Y.-M. Forging C–S Bonds through Nickel-Catalyzed Aryl Anhydrides with Thiophenols: Decarbonylation or Decarbonylation Accompanied by Decarboxylation. J. Org. Chem. 2019, 84, 11891–11901. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Cao, H.; Wang, G.; Xing, F.; Szostak, M.; Liu, C. Predominant intermolecular decarbonylative thioetherification of carboxylic acids using nickel precatalysts. Org. Chem. Front. 2023, 10, 4275–4281. [Google Scholar] [CrossRef]
- Liu, C.; Qin, Z.-X.; Ji, C.-L.; Hong, X.; Szostak, M. Highly-chemoselective step-down reduction of carboxylic acids to aromatic hydrocarbons via palladium catalysis. Chem. Sci. 2019, 10, 5736–5742. [Google Scholar] [CrossRef]
- Komiya, S.; Yamamoto, A.; Yamamoto, T. Preparation of Acyl(Carboxylato)Nickel and -Palladium Complexes M(COR)(OCOR’)L2 (M = Ni, Pd) And Reversible Reductive Elimination of Carboxylic Anhydrides, RCOOCOR’. Chem. Lett. 1981, 10, 193–196. [Google Scholar] [CrossRef]
- Sano, K.; Yamamoto, T.; Yamamoto, A. Preparation of new Pt- and Ni-containing Cyclic Esters and Their Reactivities. Chem. Lett. 1983, 12, 115–118. [Google Scholar] [CrossRef]
- Sano, K.; Yamamoto, T.; Yamamoto, A. Preparation of Ni- or Pt-Containing Cyclic Esters by Oxidative Addition of Cyclic Carboxylic Anhydrides and Their Properties. Bull. Chem. Soc. Jpn. 1984, 57, 2741–2747. [Google Scholar] [CrossRef]
- Nagayama, K.; Kawataka, F.; Sakamoto, M.; Shimizu, I.; Yamamoto, A. Preparation and Reactivities of Acyl(carboxylato)palladium Complexes. Chem. Lett. 1995, 24, 367–368. [Google Scholar] [CrossRef]
- Miller, J.A.; Nelson, J.A. Oxidative Addition of Carboxylic Acid Anhydrides to Rhodium(I) Phosphine Complexes To Produce Novel Rhodium(III) Acyl Derivatives. Organometallics 1991, 10, 2958–2961. [Google Scholar] [CrossRef]
- Lee, Y.H.; Morandi, B. Metathesis-active ligands enable a catalytic functional group metathesis between aroyl chlorides and aryl iodides. Nat. Chem. 2018, 10, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Boehm, P.; Martini, T.; Lee, Y.H.; Cacherat, B.; Morandi, B. Palladium-Catalyzed Decarbonylative Iodination of Aryl Carboxylic Acids Enabled by Ligand-Assisted Halide Exchange. Angew. Chem. Int. Ed. 2021, 60, 17211–17217, Angew. Chem. 2021, 133, 17348–17355. [Google Scholar] [CrossRef] [PubMed]
- De La Higuera Macias, M.; Arndtsen, B.A. Functional Group Transposition: A Palladium-Catalyzed Metathesis of Ar–X σ-Bonds and Acid Chloride Synthesis. J. Am. Chem. Soc. 2018, 140, 10140–10144. [Google Scholar] [CrossRef]
- Tian, T.; Kashihara, M.; Yan, W.; Nishihara, Y. Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acyl Fluorides and Chlorides: Synthesis of Aryl Halides via Reductive Elimination of the C–X (X = I, Br, and Cl) Bond and Mechanistic Implications. ACS Catal. 2024, 14, 11905–11917. [Google Scholar] [CrossRef]
- Watson, D.A.; Su, M.; Teverovskiy, G.; Zhang, Y.; García-Fortanet, J.; Kinzel, T.; Buchwald, S.L. Formation of ArF from LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides. Science 2009, 325, 1661–1664. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A.; Suarez, A. Intimate Mechanism of Oxidative Addition to Zerovalent Palladium Complexes in the Presence of Halide Ions and Its Relevance to the Mechanism of Palladium-Catalyzed Nucleophilic Substitutions. J. Am. Chem. Soc. 1993, 115, 9531–9541. [Google Scholar] [CrossRef]
- Miloserdov, F.M.; McMullin, C.L.; Belmonte, M.M.; Benet-Buchholz, J.; Bakhmutov, V.I.; Macgregor, S.A.; Grushin, V.V. The Challenge of Palladium-Catalyzed Aromatic Azidocarbonylation: From Mechanistic and Catalyst Deactivation Studies to a Highly Efficient Process. Organometallics 2014, 33, 736–752. [Google Scholar] [CrossRef]
- Miloserdov, F.M.; Grushin, V.V. Palladium-Catalyzed Aromatic Azidocarbonylation. Angew. Chem. Int. Ed. 2012, 51, 3668–3672, Angew. Chem. 2012, 124, 3728–3732. [Google Scholar] [CrossRef]
- Malapit, C.A.; Ichiishi, N.; Sanford, M.S. Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides. Org. Lett. 2017, 19, 4142–4145. [Google Scholar] [CrossRef]
- Liao, W.-J.; Lin, S.-Y.; Kuo, Y.-S.; Liang, C.-F. Site-Selective Acylation of Phenols Mediated by a Thioacid Surrogate through Sodium Thiosulfate Catalysis. Org. Lett. 2022, 24, 4207–4211. [Google Scholar] [CrossRef]
- Daskiewicz, J.-B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J.M.; Johnson, I.T.; Ndjoko, K.; Hostettmann, K.; et al. Effects of Flavonoids on Cell Proliferation and Caspase Activation in a Human Colonic Cell Line HT29: An SAR Study. J. Med. Chem. 2005, 48, 2790–2804. [Google Scholar] [CrossRef] [PubMed]
- Dhimitruka, I.; SantaLucia, J., Jr. Investigation of the Yamaguchi Esterification Mechanism. Synthesis of a Lux-S Enzyme Inhibitor Using an Improved Esterification Method. Org. Lett. 2006, 8, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, X.; Xu, C.; Pan, Y.; Zhang, G.; Xu, L.; Li, H.; Shi, Z. Rhodium-Catalyzed Decarbonylative Direct C2-Arylation of Indoles with Aryl Carboxylic Acids. ChemCatChem 2014, 6, 3069–3074. [Google Scholar] [CrossRef]
- Bashir, I.A.; Lee, S. Base-Mediated Synthesis of Anhydrides from Activated Amides. J. Org. Chem. 2023, 88, 6159–6167. [Google Scholar] [CrossRef] [PubMed]
- Khatun, N.; Santra, S.K.; Banerjee, A.; Patel, B.K. Nano CuO Catalyzed Cross Dehydrogenative Coupling (CDC) of Aldehydes to Anhydrides. Eur. J. Org. Chem. 2015, 2015, 1309–1313. [Google Scholar] [CrossRef]
- Gaspa, S.; Amura, I.; Porcheddu, A.; De Luca, L. Anhydrides from aldehydes or alcohols via oxidative cross-coupling. New J. Chem. 2017, 41, 931–939. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Majhi, P.K.; Goodfellow, A.S.; Tak, R.K.; Cordes, D.B.; McKay, A.P.; Kasten, K.; Bühl, M.; Smith, A.D. Synthesis of Tetra-Substituted 3-Hydroxyphthalide Esters by Isothiourea-Catalysed Acylative Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2024, 63, e202402909, Angew. Chem. 2024, 136, e202402909. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Entry | Deviations from the Standard Conditions | 2a (%) a | 3a (%) a |
| 1 | None | 97 | <1 |
| 2 | DPEphos instead of Xantphos | 34 | <1 |
| 3 | dtbpf instead of Xantphos | 37 | <1 |
| 4 | P(t-Bu)3·HBF4 instead of Xantphos | <1 | <1 |
| 5 | BrettPhos instead of Xantphos | 5 | <1 |
| 6 | Pd2(dba)3 instead of [PdCl(cinnamyl)]2 | 68 | <1 |
| 7 | PdCl2 instead of [PdCl(cinnamyl)]2 | <1 | <1 |
| 8 | Ni(cod)2 instead of [PdCl(cinnamyl)]2 | <1 | <1 |
| 9 | NaI instead of LiI | <5 | <1 |
| 10 | THF instead of toluene | 30 | <1 |
| 11 | 80 °C instead of 100 °C | 55 | <1 |
| 12 | 1-naphthoic pivalic anhydride instead of 1a | 25 | <1 |
| 13 | w/o [PdCl(cinnamyl)]2 or Xantphos | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Uei, S.; Yan, W.; Nishihara, Y. Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acid Anhydrides. Catalysts 2025, 15, 191. https://doi.org/10.3390/catal15020191
Tian T, Uei S, Yan W, Nishihara Y. Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acid Anhydrides. Catalysts. 2025; 15(2):191. https://doi.org/10.3390/catal15020191
Chicago/Turabian StyleTian, Tian, Shuhei Uei, Weidan Yan, and Yasushi Nishihara. 2025. "Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acid Anhydrides" Catalysts 15, no. 2: 191. https://doi.org/10.3390/catal15020191
APA StyleTian, T., Uei, S., Yan, W., & Nishihara, Y. (2025). Palladium-Catalyzed Decarbonylative Nucleophilic Halogenation of Acid Anhydrides. Catalysts, 15(2), 191. https://doi.org/10.3390/catal15020191








