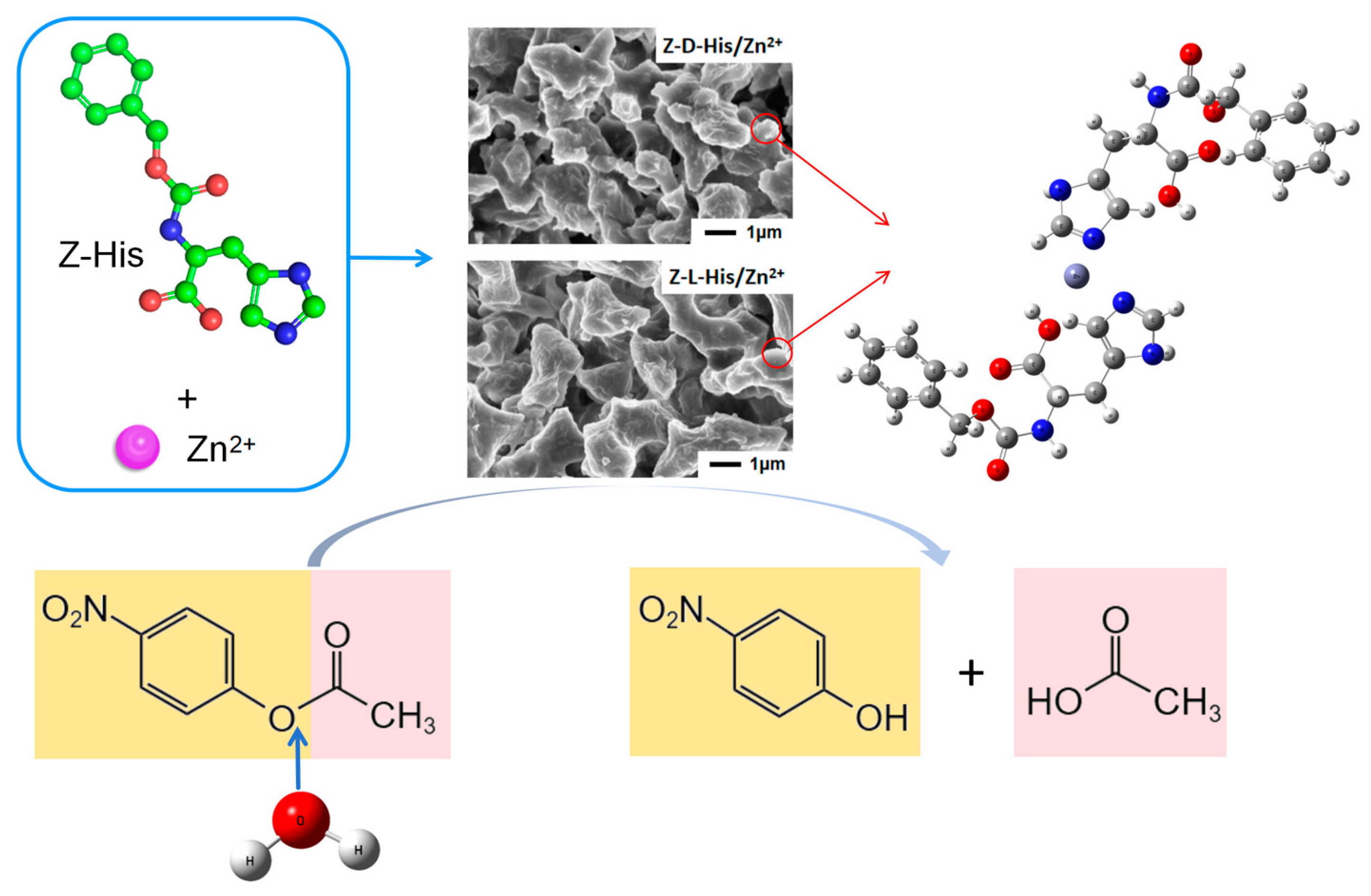
2.1. Characterization
Two supramolecular assemblies, Z-L-His/Zn
2+ and Z-D-His/Zn
2+, were synthesized by self-assembling histidine derivatives of different optical isomers, Z-L-His and Z-D-His, with Zn
2+, and their morphology and structure characterization results are presented in
Figure 2,
Figure 3 and
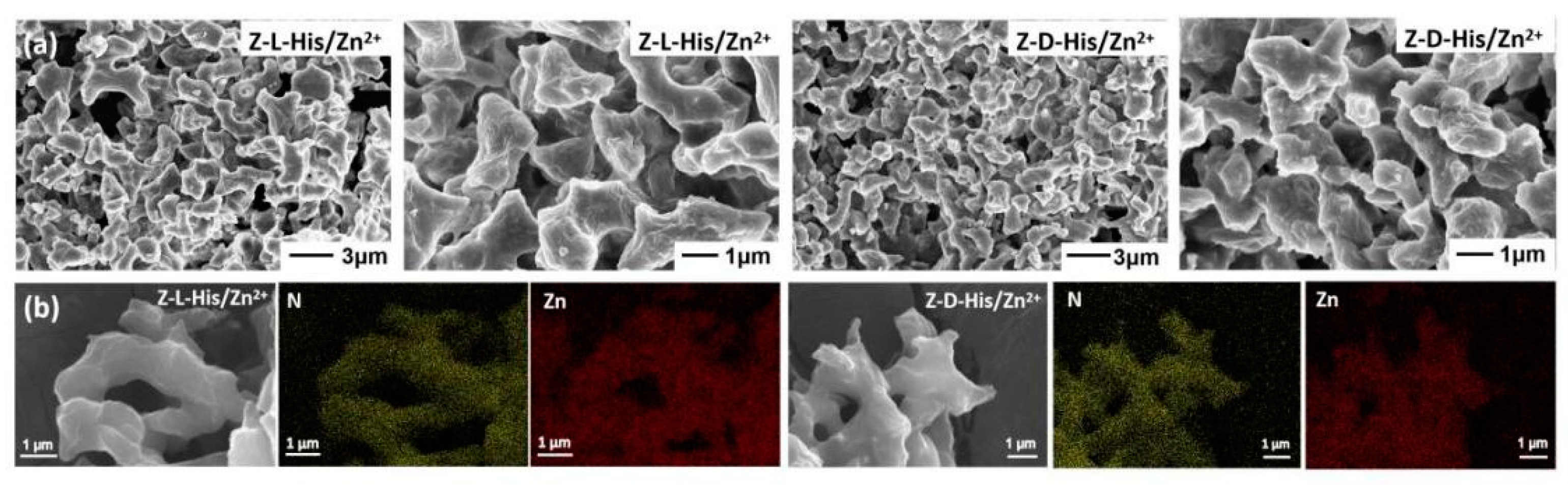
Figure 4. The scanning electron microscope (SEM) images in
Figure 2a show that both Z-L-His/Zn
2+ and Z-D-His/Zn
2+ exhibited block-like structures of varying sizes. The energy-dispersive X-ray spectroscopy (EDS) spectra in
Figure 2b confirmed the presence of Zn and N elements in both assemblies, supporting the successful coordination of Zn
2+ with Z-L-His and Z-D-His. Elemental analysis and inductively coupled plasma optical emission spectrometry (ICP-OES) were employed to determine the ratios of C, H, O, N, and Zn within the assemblies, and the results are summarized in
Table S1. Based on the elemental ratios of N and Zn, the molar ratio of Zn
2+ to Z-L-His or Z-D-His in the assemblies was calculated to be 1:2.
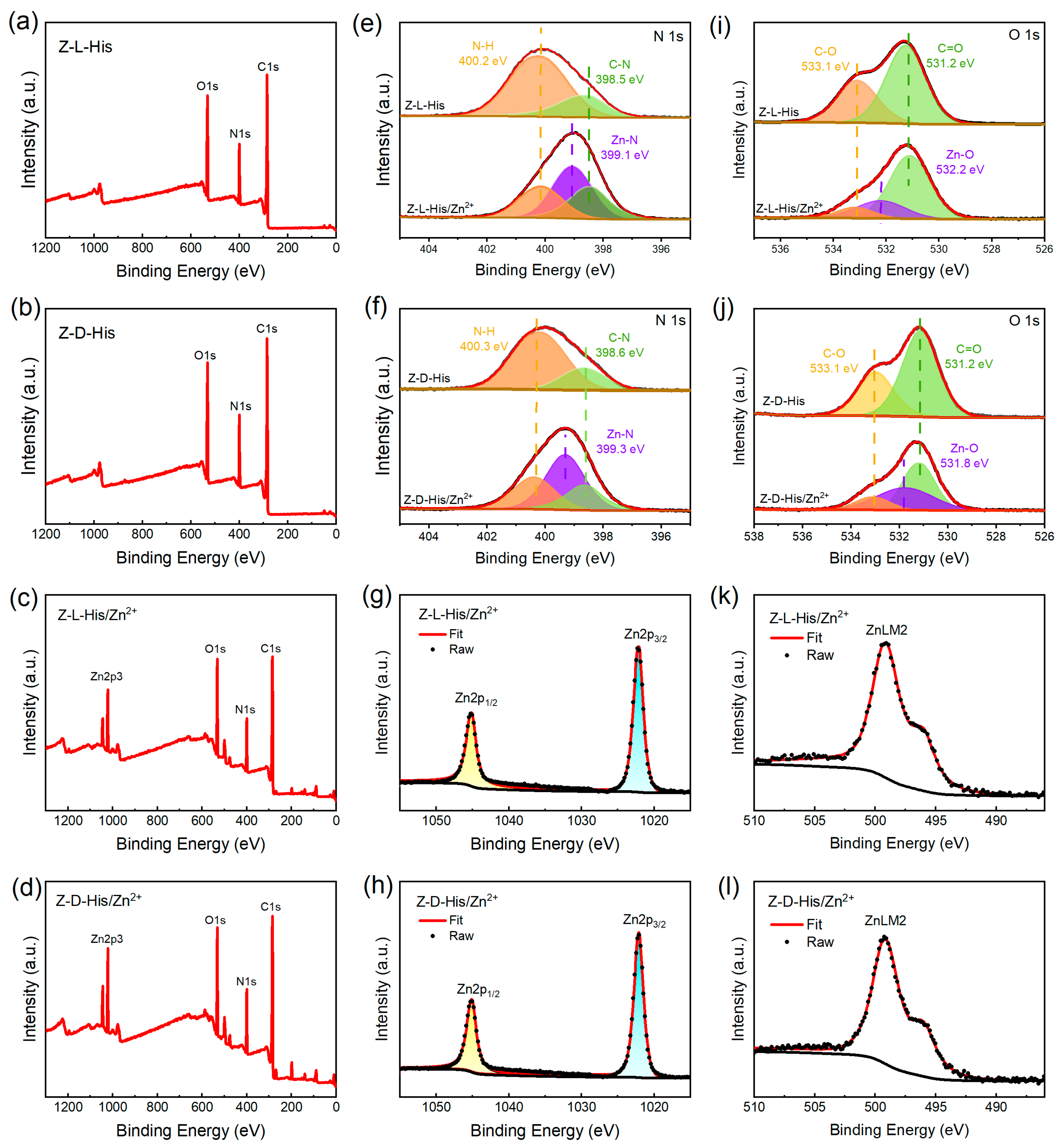
The full-scan X-ray photoelectron spectroscopy (XPS) spectra of Z-L-His, Z-D-His, Z-L-His/Zn
2+, and Z-D-His/Zn
2+ in
Figure 3a–d show a new Zn 2p peak in both assemblies (Z-L-His/Zn
2+ and Z-D-His/Zn
2+) compared to the histidine derivatives (Z-L-His and Z-D-His), indicating the successful coordination of Zn
2+ with the histidine derivatives during self-assembly. The N1s spectra in
Figure 3e,f show peaks at 398.5 eV and 400.2 eV, corresponding to C-N and N-H bonds, respectively [
25]. Additionally, Zn-N peaks are present at 399.1 eV in Z-L-His/Zn
2+ and 399.2 eV in Z-D-His/Zn
2+. Meanwhile, Zn-O peaks appear at 532.2 eV for Z-L-His/Zn
2+ and 531.8 eV for Z-D-His/Zn
2+, as illustrated in
Figure 3i,j, suggesting that Zn
2+ coordinated with both N and O atoms in the histidine derivatives to form the assemblies.
Figure 3g,h,k,l further confirm that Zn
2+ is present in a divalent state within both assemblies.
The X-ray diffractometer (XRD) patterns of Z-L-His, Z-D-His, Z-L-His/Zn
2+, and Z-D-His/Zn
2+ in
Figure 4a,b show that both Z-L-His and Z-D-His exhibit clear crystalline structures, while the two assemblies of Z-L-His/Zn
2+ and Z-D-His/Zn
2+ displayed smoother, broader curves, further confirming that Zn
2+ interacts with the histidine derivatives in the formation of the assemblies. Additionally, the XRD patterns indicate that Z-L-His/Zn
2+ and Z-D-His/Zn
2+ are amorphous in nature [
26]. The Fourier transform infrared (FTIR) spectra in
Figure 4c,d show that for the two histidine derivatives, the absorption peaks at 1540 cm
−1 and 3240 cm
−1 correspond to the N-H deformation and stretching vibrations of the imidazole ring, respectively [
27]. However, these peaks shift to lower wavelengths in the assemblies, indicating coordination of the imidazole nitrogen with Zn
2+. In addition, the two histidine derivatives also exhibited characteristic carboxylic acid peaks at 1410 cm
−1 and 1630 cm
−1, and these characteristic peaks similarly shifted to lower wavelengths for the assemblies, suggesting that their carboxyl oxygen also coordinated with Zn
2+ [
28,
29]. The peak observed at 3400 cm
−1 for the assemblies was attributed to the stretching vibration of O-H or hydrogen bonding [
30], indicating potential hydrogen bonding or the presence of water within Z-L-His/Zn
2+ and Z-D-His/Zn
2+, and the hydrogen bonding or the presence of water may play an important role in the hydrolysis of substrates (
Figure 1). Based on these characterization results, it can be concluded that both assemblies were successfully synthesized, with probable structures as illustrated in
Figure 1, where Zn
2+ coordinates with the nitrogen of the imidazole group and the oxygen of the carboxylic acid group in Z-His, forming a zinc-based hydrolase-like active center. Additionally, hydrogen bonding, hydrophobic interactions, and π-π stacking processes in the self-assembled units further helped the formation of esterase-like mimics.
The pore size structure and specific surface area of the assemblies (Z-L-His/Zn
2+ and Z-D-His/Zn
2+) were characterized using nitrogen adsorption–desorption experiments (
Figure 4e–h). Both isotherms (
Figure 4e,g) are type IV isotherms, indicating the presence of mesopores in the assemblies. Due to the weak nitrogen adsorption capacity of Z-L-His and Z-D-His, their isothermal adsorption–desorption curves were not tested.
Figure 4f,h show the pore size distributions of the two assemblies, and the Brunauer–Emmett–Teller (BET) specific surface areas of Z-L-His/Zn
2+ and Z-D-His/Zn
2+ were 2.82 m
2/g and 2.79 m
2/g, respectively. In addition, their total pore volumes were 7.5 × 10
−3 cm
3/g and 5.8 × 10
−3cm
3/g, while their average pore sizes based on the Barrett–Joyner–Halend (BJH) method were 6.6 nm and 7.6 nm, respectively. The porous structure of Z-L-His/Zn
2+ and Z-D-His/Zn
2+ increases specific surface area, providing more catalytic active sites to substances and finally resulting in favorable catalytic performances.
2.2. Catalytic Activity Analysis of Z-His and Z-His/Zn2+
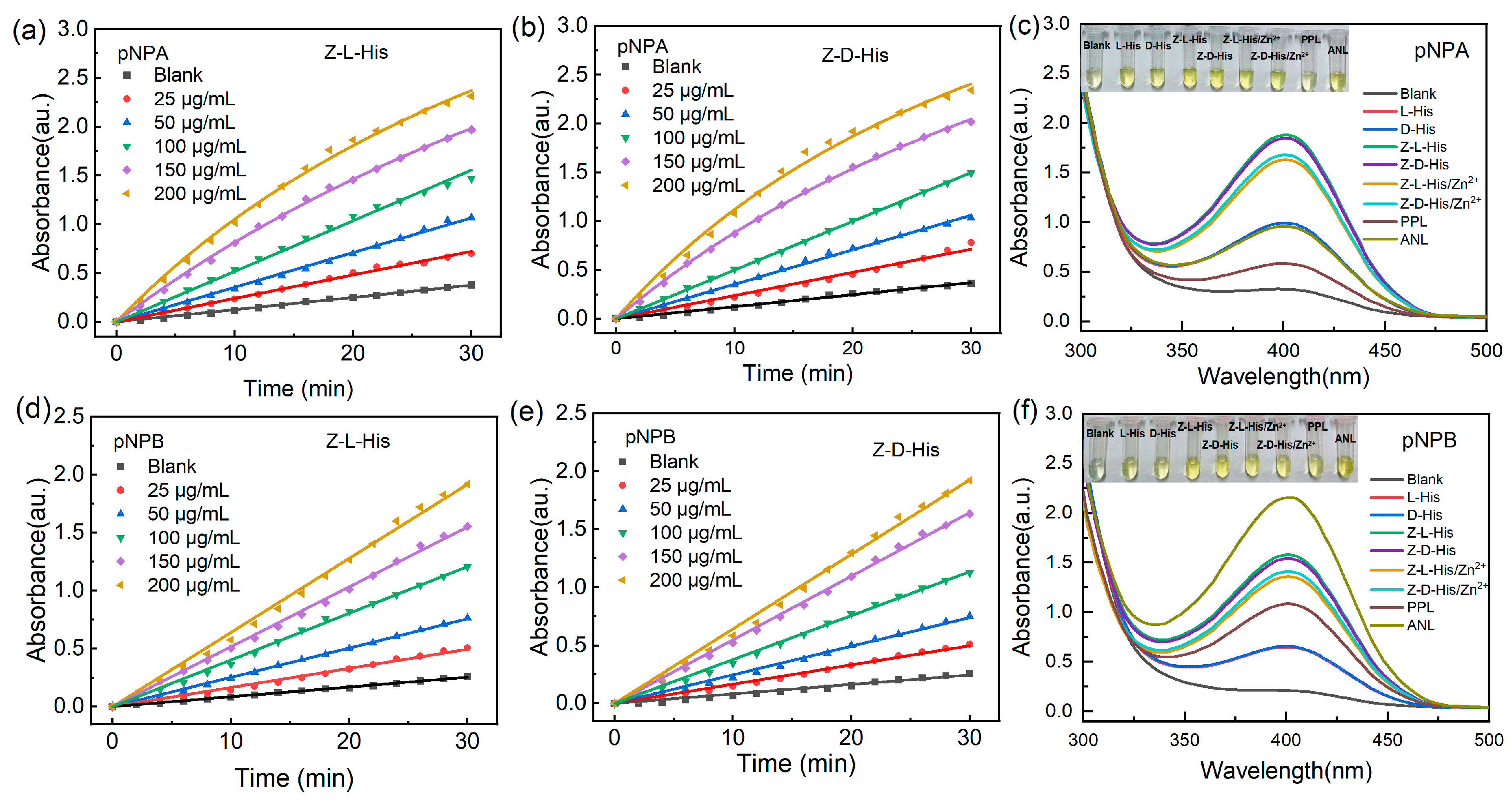
The ester hydrolysis reaction is one of the most common reactions in industrial production. Thus, p-nitrophenyl acetate (pNPA) and 4-Nitrophenyl butyrate (pNPB) were used as model substrates, and the hydrolysis ability of various catalysts was assessed by detecting the production of the colored product p-nitrophenol (pNP) at 405 nm (
Figure 5 and
Figure 6). Firstly, the absorption peaks of the substrates (pNPA and pNPB), the product pNP, and the various catalysts used in the 2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) buffer solution (pH 8) were determined, with the results presented in
Figure 5a. The strong absorption peak of pNP at 405 nm is characteristic of its adsorption, while the tiny adsorption at 405 nm for the substrates pNPA and pNPB was attributed to their spontaneous hydrolysis. In contrast, the catalysts showed no absorption in the wavelength range of 375–500 nm. Thus, the generation of pNP can be monitored by measuring the absorption intensity at 405 nm to determine the reaction process.
Subsequently, the catalytic activities of the assemblies Z-L-His/Zn
2+ and Z-D-His/Zn
2+ were investigated, with their relative activities calculated using the catalytic activity of Z-L-His as 100% (
Figure 5b,c). Due to the self-hydrolysis of pNPA and pNPB in an alkaline environment (pH 8), the blank group exhibited a tiny activity. However, ZnCl
2, a salt of a strong acid and a weak base, lowered the pH of the reaction system, reducing the self-hydrolysis of pNPA and pNPB, and resulting in an activity (Zn
2+ groups in
Figure 5b,c) lower than that of the blank group. For the hydrolysis of both pNPA and pNPB, the two histidine derivatives and two assemblies exhibited similar ester hydrolysis activity. In particular, the assemblies achieved over 80% of the activity of the corresponding histidine derivatives, while the molar ratio of histidine derivatives in the assemblies was 0.9:1, as calculated from the data in
Table S1, This implies the esterase-like activity of the assemblies mainly originates from the histidine derivatives. Based on the structure characterization in
Section 2.1, it was hypothesized that the activity of the assemblies arises from two sources: one is the formation of a zinc active center similar to a metal hydrolase through self-assembly, and the other is the catalytic activity of the imidazole group in histidine derivatives. Therefore, the catalytic efficiency of the imidazole group in the assemblies was reduced due to metal coordination, while the zinc active center in the assemblies partially compensated, finally maintaining the overall activity of the assemblies at a high level. Overall, although the assemblies exhibited slightly reduced activity compared to the histidine derivatives, Z-L-His/Zn
2+ and Z-D-His/Zn
2+ could be recovered by centrifugation or filtration, making them low-cost for industrial applications.
Considering the faster catalysis of the histidine derivatives than their assemblies, the time-dependent absorbance of the system during the hydrolysis of pNPA and pNPB catalyzed at varying concentrations of Z-L-His and Z-D-His were checked to ensure linear kinetics of the 30-min reaction for the subsequent experiments (
Figure 6). Clearly, the absorbance of the reaction system appeared to increase with increasing the concentration of histidine derivatives (
Figure 6a,b,d,e). When using the same substrate (pNPA or pNPB), the increase in absorbance for Z-L-His and Z-D-His followed a similar trend, indicating that both catalysts exhibited comparable catalytic rates. Additionally, the hydrolysis of pNPA occurred more rapidly than that of pNPB, demonstrating a substrate preference for pNPA over pNPB. Furthermore, when the concentrations of Z-L-His or Z-D-His in the reaction system exceeded 100 μg/mL, the absorbance increased nonlinearly within 30 min with pNPA as the substrate. Hence, a catalyst concentration of 100 μg/mL was selected for subsequent studies.
L-His, D-His, and two lipases, lipase from
Aspergillus niger (ANL) and lipase from porcine pancreas (PPL), both of which are widely used in similar studies [
24,
31], were selected for comparison, as shown in
Figure 6c,f. The hydrolysis of pNPA by Z-L-His/Zn
2+ and Z-D-His/Zn
2+ was slightly slower than that of Z-L-His and Z-D-His but significantly faster than that of lipases (ANL and PPL) and histidines (L-His, D-His), as illustrated in
Figure 6c. When pNPB was the substrate, the catalytic activities of Z-L-His/Zn
2+ and Z-D-His/Zn
2+ were slightly lower than that of Z-L-His and Z-D-His but significantly higher than that of PPL and histidines (L-His, D-His), demonstrating robust hydrolysis abilities for the histidine derivatives than their assemblies against both pNPA and pNPB. However, the two natural enzymes displayed distinct substrate preferences: ANL exhibited the strongest hydrolysis activity toward pNPB over the other catalysts in
Figure 6f, which was quite higher than that toward pNPA, while PPL showed a slight preference for catalyzing the hydrolysis of pNPB over pNPA.
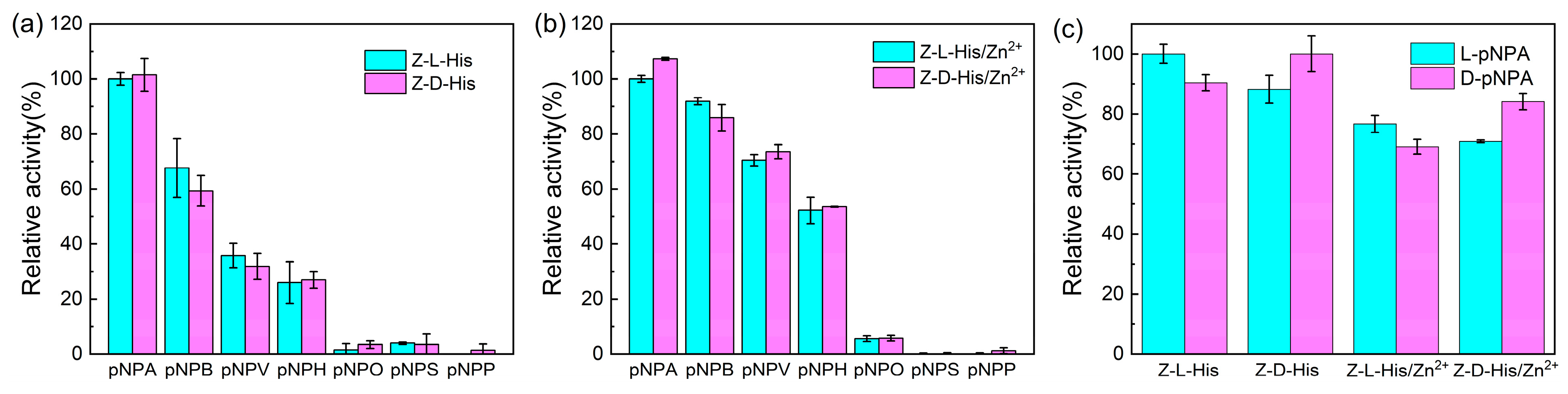
To evaluate substrate universality, carboxylic esters composed of fatty acids of varying lengths [pNPA, pNPB, p-nitrophenyl valerate (pNPV), p-nitrophenyl hexanoate (pNPH), and p-nitrophenyl octanoate (pNPO)], along with the phosphate ester disodium 4-nitrophenyl phosphate hexahydrate (pNPP) and aromatic acid ester p-nitrophenyl salicylate (pNPS), were tested (
Figure 7a,b). The activities were normalized by the hydrolysis activity of pNPA by Z-L-His and Z-L-His/Zn
2+, respectively. It was evident that the optical isomerism of the catalysts did not influence their catalytic activity, as Z-L-His and Z-D-His exhibited similar hydrolysis rates for the tested substrates (
Figure 7a), and Z-L-His/Zn
2+ and Z-D-His/Zn
2+ showed comparable activity (
Figure 7b). Additionally, the hydrolysis activity of each catalyst decreased as the lipid chain length increased, consistent with the findings of Ehud’s group, where the activity of the enzyme mimic of Phe-Zn also declined with longer lipid chains [
16]. Notably, as the lipid chain length increased from pNPA to pNPO, the relative activity of the assemblies decreased more slowly compared to the histidine derivatives, suggesting better substrate universality of Z-L-His and Z-L-His/Zn
2+. Furthermore, none of the four catalysts exhibited significant hydrolysis activity toward esters containing phosphate or aromatic acid groups (pNPP and pNPS), indicating that these catalysts specifically catalyzed the hydrolysis of esters derived from aliphatic acids, rather than those with more complex functional groups.
To further elucidate the impact of the stereoselectivity of catalysts, enantiomers (L- and D-Boc-phenylalanine 4-nitrophenyl ester, L-pNPA, and D-pNPA, respectively) [
17] were used as substrates (
Figure 7c), and the activity was normalized using the hydrolysis activity of L-pNPA by Z-L-His. The catalysts displayed stereoselectivity to the substrates. For example, Z-L-His only catalyzed the hydrolysis of D-pNPA with 90.4% of the activity for L-pNPA (
Figure 7c). Moreover, Z-D-His exhibited 88.2% of the activity of Z-L-His in catalyzing L-pNPA hydrolysis (
Figure 7c), while both catalysts showed similar activities in catalyzing racemic pNPA hydrolysis (
Figure 7a).
In summary, Z-L-His, Z-D-His, Z-L-His/Zn2+, and Z-D-His/Zn2+ could efficiently catalyze the hydrolysis of a series of aliphatic acid esters, and Z-L-His/Zn2+ and Z-D-His/Zn2+ exhibited both enantioselectivity and broad substrate compatibility. Because the catalysis towards the racemic substrates pNPA and pNPB was not influenced by the optical isomerism of catalysts, Z-L-His and Z-L-His/Zn2+ were subsequently used as the subjects of this study to assess the catalytic performance.
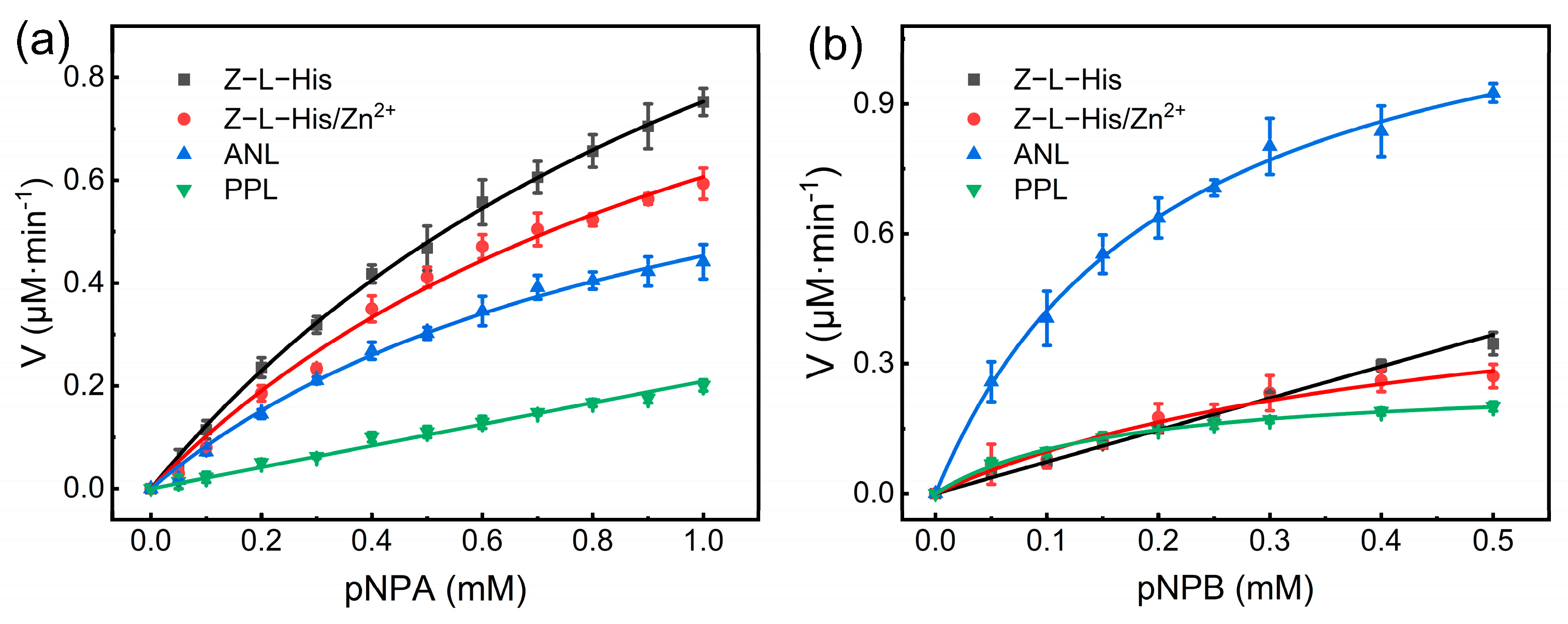
2.3. Kinetic Parameters of Z-L-His and Z-L-His/Zn2+
Kinetic parameters are important for assessing the performance of catalysts, and thus the initial rates of Z-L-His and Z-L-His/Zn
2+ in catalyzing the hydrolysis of the racemic substrates pNPA and pNPB at various substrate concentrations were measured and compared with the two natural enzymes (ANL and PPL) (
Figure 8).
Using pNPA as a substrate, Z-L-His, Z-L-His/Zn
2+, and ANL followed the typical Michaelis–Menten kinetics (
Figure 8a). The kinetic parameters, the maximum initial velocity (
Vmax) and Michaelis–Menten constant (
Km), were determined by fitting the data to the Michaelis–Menten model (
Table S2). However, due to substrate solubility limitations, the rate of PPL-catalyzed product formation increased linearly with substrate concentration within the detected concentration range, and the first-stage reaction kinetics for determining the catalytic efficiency
kcat/
Km was attempted. Similarly, when pNPB was used as the substrate, the
kcat/
Km value for Z-L-His was calculated using first-stage reaction kinetics. The well-fitted experimental data by the Michaelis–Menten model (
Figure 8) for Z-L-His/Zn
2+ in hydrolyzing both pNPA and pNPB confirmed the enzyme-like catalytic characteristics of Z-L-His/Zn
2+. Thus, Z-L-His/Zn
2+ could be regarded as an esterase mimic.
As listed in
Table S2, the
Km value of Z-L-His/Zn
2+ was smaller than that of Z-L-His when pNPA was used as the substrate, indicating that the formation of the assemblies improves the substrate affinity. According to the proposed structure of Z-L-His/Zn
2+ (
Figure 1) and the discussion in the above sections, a zinc active center was formed in Z-L-His/Zn
2+, in comparison to Z-L-His, and it afforded more efficient nucleophilic attack on the substrate [
16], resulting in higher affinity. Furthermore, Z-L-His/Zn
2+ exhibited a high
Vmax value, resulting in a 20% higher
kcat/
Km value than that of the natural enzyme ANL, which is 5.3 times that of the natural enzyme PPL. Moreover, the
kcat/
Km value of Z-L-His was 1.4-fold and 6.3-fold that of ANL and PPL, respectively. When pNPB served as the substrate, ANL exhibited the maximum catalytic efficiency, whereas the catalytic efficiency of Z-L-His/Zn
2+ was similar to that of PPL and 1.6 times higher than that of Z-L-His, suggesting that the formation of the assemblies improved the catalytic ability for the substrate pNPB. The kinetic parameters of other esterase mimics are summarized in
Table S2. Although direct comparisons of catalytic performance are challenging due to differences in reaction systems, the
kcat/
Km values suggest that Z-L-His/Zn
2+ exhibit comparable catalytic efficiency, highlighting their superior catalytic ability and proving their usefulness in mimicking natural esterases.
Overall, the catalytic efficiencies of Z-L-His/Zn2+ for the two substrates were comparable and consistently high, which is even more efficient than the natural enzymes, demonstrating its excellent performance in catalyzing hydrolysis.
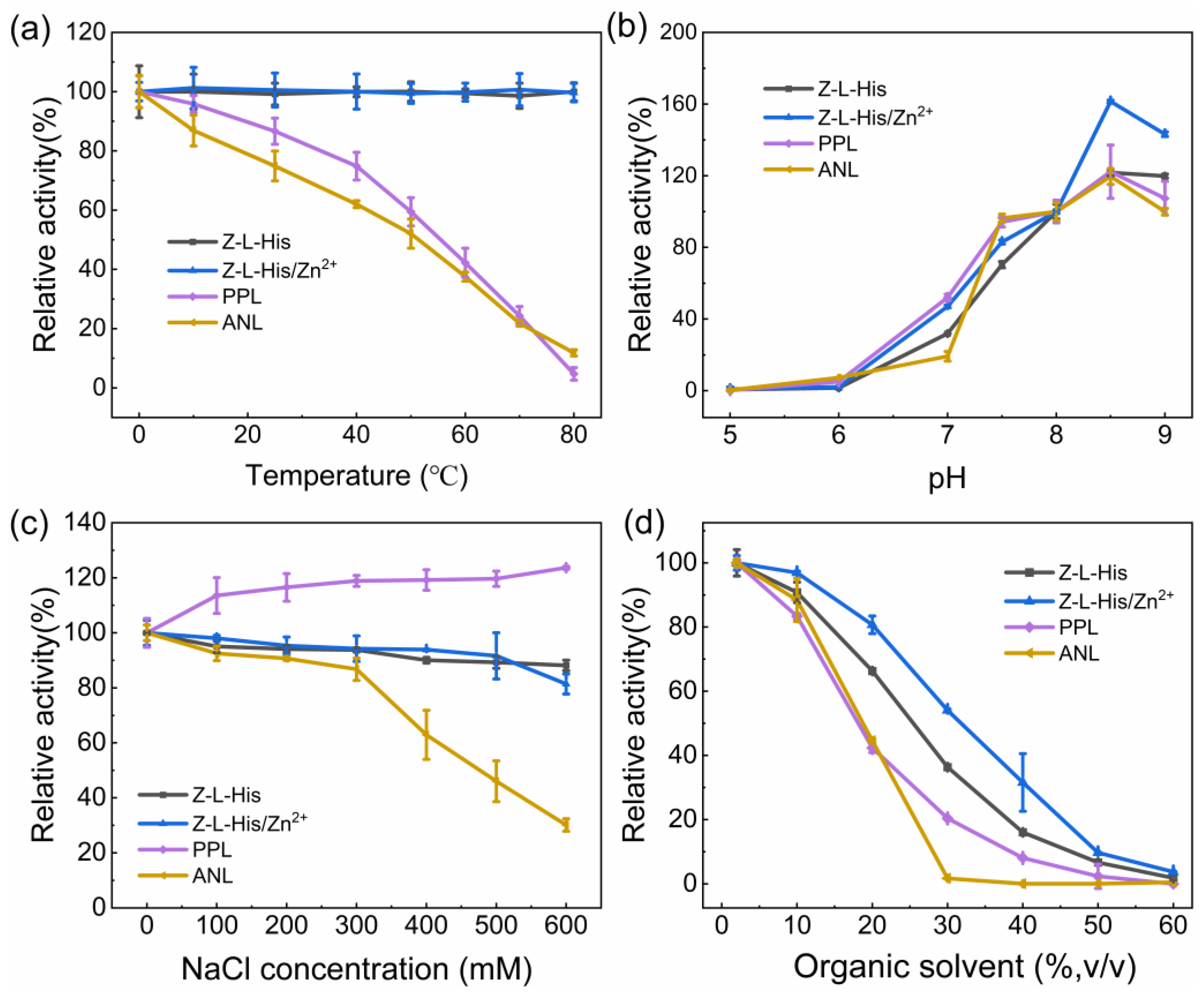
2.4. Catalytic Stability of Z-L-His and Z-L-His/Zn2+
For practical applications of industrial hydrolysis, catalytic stability at various conditions is required. The catalytic activities of Z-L-His and Z-L-His/Zn
2+ after a 30-min incubation at different temperatures and in conditions of varying pH, ionic strength (NaCl concentration), and organic solvent concentration were investigated, and the natural enzymes PPL and ANL were used for comparison (
Figure 9). Because pNPA exhibited more pronounced autohydrolysis than pNPB (
Figure 5b,c) and it aggravated under varying pH conditions, making it hard to control the blank group, pNPB was selected as the substrate for catalytic stability investigation.
Z-L-His and Z-L-His/Zn
2+ displayed excellent thermal stability, maintaining their activities after 30 min of incubation across a temperature range of 0–80 °C (
Figure 9a). In contrast, the natural enzymes PPL and ANL exhibited poor thermal tolerance, with their activities dropping to 4.7% and 11.8%, respectively, after incubation at 80 °C for 30 min. Moreover, compared to other hydrolase mimics [
20], Z-L-His and Z-L-His/Zn
2+ exhibited better thermostability. Due to the activity at pH 8 being set as 100% (
Figure 9b), it is shown that the optimal pH for the catalytic activity of each catalyst is around 8.5, presenting activities larger than 100%. Alkaline conditions enhanced the catalytic reaction, whereas acidic conditions inhibited the catalytic reaction, likely due to the inability to neutralize the hydrolysis product of the aliphatic acid under acidic conditions [
16]. Additionally, Z-L-His/Zn
2+ exhibited significantly higher activity in the pH range of 7–9 than Z-L-His, indicating its advantages under alkaline conditions. For the effect of ionic strength, Z-L-His and Z-L-His/Zn
2+ maintained their catalytic activity even at NaCl concentrations up to 600 mM, while ANL showed poor tolerance to ionic strength, exhibiting a 30.1% residual activity at 600 mM NaCl (
Figure 9c). The high ionic strength tolerance of Z-L-His and Z-L-His/Zn
2+ proved their advantages under complex industrial catalytic environments. The irregular increasing trend with increased ionic strength for PPL might be due to salt-activation, which was similar to porcine pancreas-derived lipases reported by Goswami et al. [
32]. In view of the fact that esters are often poorly soluble, the addition of an organic co-solvent to the hydrolysis system is an effective approach. Therefore, the effect of the organic solvent was explored by adding acetonitrile (ACN) at different concentrations (
Figure 9d). As the organic solvent content increased, the number of water molecules decreased, leading to a gradual reduction in hydrolysis activity. Nevertheless, Z-L-His and Z-L-His/Zn
2+ retained higher activities than the natural enzymes. Specifically, at 20% (
v/
v) ACN, Z-L-His and Z-L-His/Zn
2+ maintained 66.3% and 80.7% activity, respectively, whereas PPL and ANL retained only 42.8% and 44.6% of their initial activity, respectively. Z-L-His/Zn
2+ showed superior organic solvent tolerance over the others, suggesting its strong resistance to organic solvents.
Long-term storage of natural enzymes often results in a decline in enzymatic activity, making it challenging to meet industrial application requirements. In this study, we systematically investigated the long-term storage stability under various incubation conditions of different temperatures, ionic strengths, organic solvent contents, and pH values for 7 d. Samples were collected every 24 h to measure the residual activity, with the activity of the free catalysts on day 0 set as 100% (
Figure 10). Specifically, a 5 mg/mL solution of the natural or mimetic enzyme was incubated and periodically added to the reaction system by a 50-fold dilution, ensuring that the effect of the incubation conditions on the reaction system was negligible.
Figure 10a–c illustrate the changes in the residual activity of the different catalysts during 7 d of incubation at 4, 30, and 60 °C, respectively. Z-L-His and Z-L-His/Zn
2+ exhibited excellent thermostability with no significant loss in activity (residual activity > 95%). In contrast, the residual activity of ANL and PPL gradually decreased over time, and higher incubation temperatures accelerated the loss of activity. After incubation at 60 °C for just 1 d, the residual activity of PPL and ANL dropped to 29.6% and 22.7%, respectively, and the two natural enzymes were completely inactivated in 3 d.
Figure 10a,d,g show the relative activity changes of the catalysts after incubation at pH 7, 8, and 9, where Z-L-His and Z-L-His/Zn
2+ exhibited high tolerance to neutral and alkaline environments, maintaining over 92% activity after 7-d. However, PPL only retained 39.7%, 42.2%, and 32.7% of its original activity after a 7-d incubation at pH 7, 8, and 9, respectively.
Figure 10a,e,h illustrate the effects of ionic strength on the storage stability of the catalysts. Z-L-His and Z-L-His/Zn
2+ exhibited good tolerance to high ionic strength, retaining over 92% activity after a 7-d incubation in 600 mM NaCl. In contrast, the residual activity of ANL reduced to 78.4%, 67.9%, and 59.3%, while that of PPL dropped to 42.2%, 44.0%, and 53.1%, respectively, after a 7-d incubation in systems containing 0, 300, and 600 mM NaCl. It is worth noting, that though PPL showed increased activity at high ionic strength (
Figure 9c), long-term storage in a high ionic strength environment still resulted in a significant decline in enzymatic activity, indicating the poor salt-tolerance of PPL.
Figure 10a,f,i display the changes in the residual activity of different catalysts incubated in a buffer system with ACN contents of 0%, 20%, and 40% (
v/
v), respectively. Z-L-His and Z-L-His/Zn
2+ exhibited good organic solvent tolerance, with unchanged activity in 7 d. However, the residual activity of ANL decreased to 71.7% after 7-d incubation at 40% (
v/
v), indicating that high concentrations of ACN impair ANL stability, a similar phenomenon also observed by Serebryakova et al. [
33]. The residual activity of PPL after a 7 d incubation with 0%, 20%, and 40% (
v/
v) ACN was 42.2%, 62.9%, and 48.3%, respectively, indicating that ACN helped the long-term storage of PPL with higher residual activity [
34]. Nevertheless, this increase in PPL residual activity due to ACN was insufficient to counteract the impairments caused by the overall system and extended incubation times, resulting in a significant loss of activity during long-term storage.
In summary, the above results demonstrated that Z-L-His and Z-L-His/Zn2+ are robust enough for long-term operation under a wide range of temperatures, ionic strengths, organic solvent concentrations, and pH values, highlighting their significant potential for industrial applications.
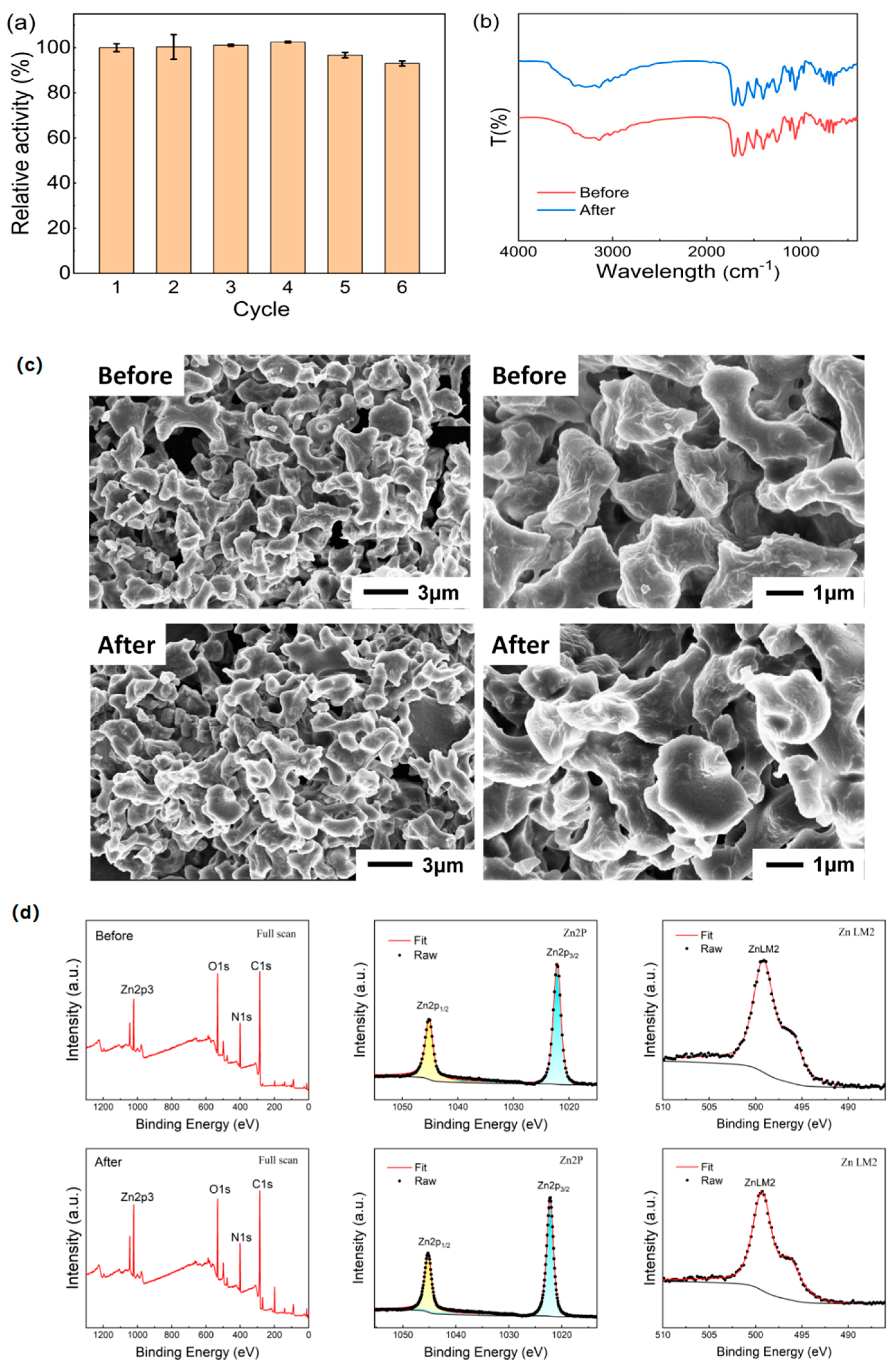
2.5. Reusability of Z-L-His/Zn2+
Beyond operational stability, the reusability of the catalyst is another critical factor in evaluating its industrial application potential. Although both Z-L-His and Z-L-His/Zn
2+ exhibited excellent stability over natural enzymes as discussed in the above sections, the water-soluble feature of histidine derivative Z-L-His made it unable to be recovered from the reaction system after its first use. Thus, the reusability of the esterase mimic Z-L-His/Zn
2+ was evaluated using pNPB as a substrate (
Figure 11).
Remarkably, Z-L-His/Zn
2+ retained 93.0% of its original activity after six cycles of reuse (
Figure 11a). For comparison, the histidine-based nanoenzyme cyclo-HH-ZnI
2 with hydrolase activity only retained 80% of its activity after five cycles [
12]. Additionally, in 2022, a glycine-doped ZIF-8 carbonic anhydrase mimic retained less than 80% of its activity after five cycles. More recently, in 2024, a cobalt-doped carbonic anhydrase mimic with a bimetallic active center (Co50%/ZIF-8) retained only 58.6% of its activity after six cycles using pNPA as a substrate [
35]. These results indicate the superior reusability of Z-L-His/Zn
2+ in this work.
Moreover, FTIR data (
Figure 11b) proved that the chemical bonds and state of Z-L-His/Zn
2+ remained unchanged after recycling six times. SEM images of recycled Z-L-His/Zn
2+ (
Figure 11c) also revealed no significant morphological changes after recycling. Furthermore, the XPS spectral data (
Figure 11d) displayed a consistent elemental composition before and after use, where the atomic ratios of C, N, O, and Zn in Z-L-His/Zn
2+ were 65.78%, 17.44%, 13.43%, and 3.34% before using, and 66.53%, 18.95%, 10.43%, and 4.08% after six cycle, respectively. Thus, the morphology, structural, and elemental integrity of Z-L-His/Zn
2+ before and after six recycles were proved, and the tiny decrease in activity after six cycles is mainly caused by the ordinary mass loss of Z-L-His/Zn
2+ during each recovery. In short, the excellent stability and superior recyclability of Z-L-His/Zn
2+ underscore its significant potential for industrial applications.
2.6. Catalytic Mechanism
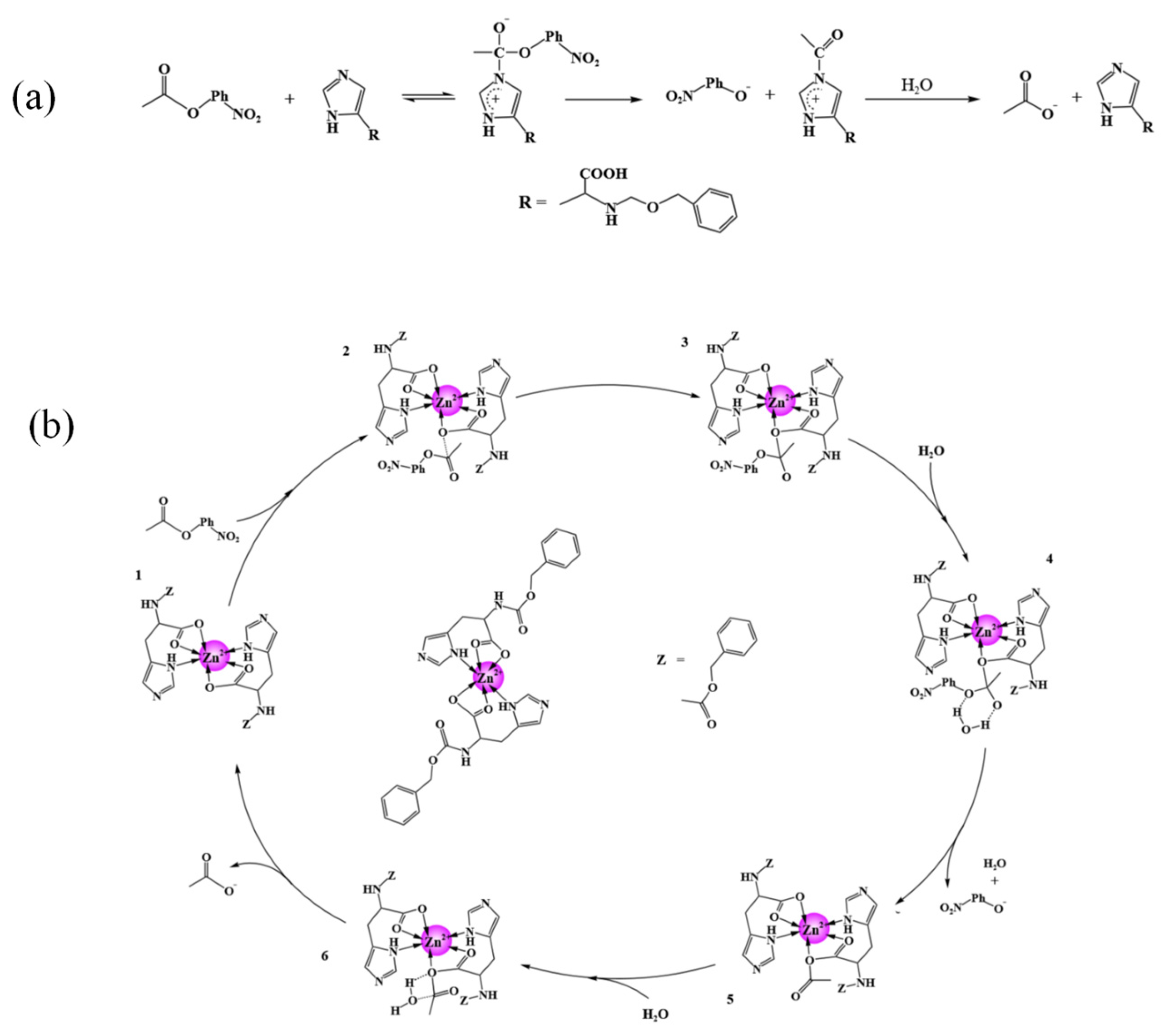
Figure 12 illustrates the possible mechanisms of pNPA hydrolysis catalyzed by the imidazole group of histidine derivatives and the zinc active center within the assemblies, respectively. For the histidine derivatives Z-L-His and Z-D-His, their catalytic activities are from the imidazole group [
16], and the hydrolysis process catalyzed by the imidazole group is shown in
Figure 12a. In brief, the imidazole group works as a nucleophile, attacking the carbonyl carbon of pNPA to form an intermediate. This intermediate then decomposes, releasing pNP and acetate ions in the presence of water molecules, with the imidazole group returning to its original state [
36]. Because the imidazole groups still exist in the assemblies of Z-L-His/Zn
2+ and Z-D-His/Zn
2+, imidazole group-mediated catalysis would also work.
In addition, as detailed in
Section 2.1 and
Section 2.2, the zinc active center is formed in the assemblies, and the structural unit of the Z-His/Zn
2+ can be deduced, where Zn
2+ formes coordination bonds with the nitrogen and carboxyl oxygen atoms of the imidazole groups. In the assemblies, hydrogen bonding, hydrophobic interactions, and π-π stacking between the aromatic rings contribute to facilitating the hydrolysis reaction (
Figure 12b). A possible catalytic mechanism for the zinc active center-mediated catalysis of the pNPA hydrolysis is proposed as follows: In state 1, pNPA binding at the oxygen atoms coordinated with the zinc atom, and generated state 2. Then the oxygen nucleophile attacks the carbonyl carbon of pNPA, leading to the formation of a transition state (state 3). Subsequently, in the presence of water molecules (state 4), one product, pNP, is released from the transition state, forming a new intermediate (state 5). This intermediate is then nucleophilically attacked by a water molecule’s oxygen atom (state 6), and the second product, acetic acid, is finally released, so Z-His/Zn
2+ returns to its initial state (state 1).