Novel Ni/SBA-15 Catalyst Pellets for Tar Catalytic Cracking in a Dried Sewage Sludge Pyrolysis Pilot Plant
Abstract
1. Introduction
2. Results and Discussion
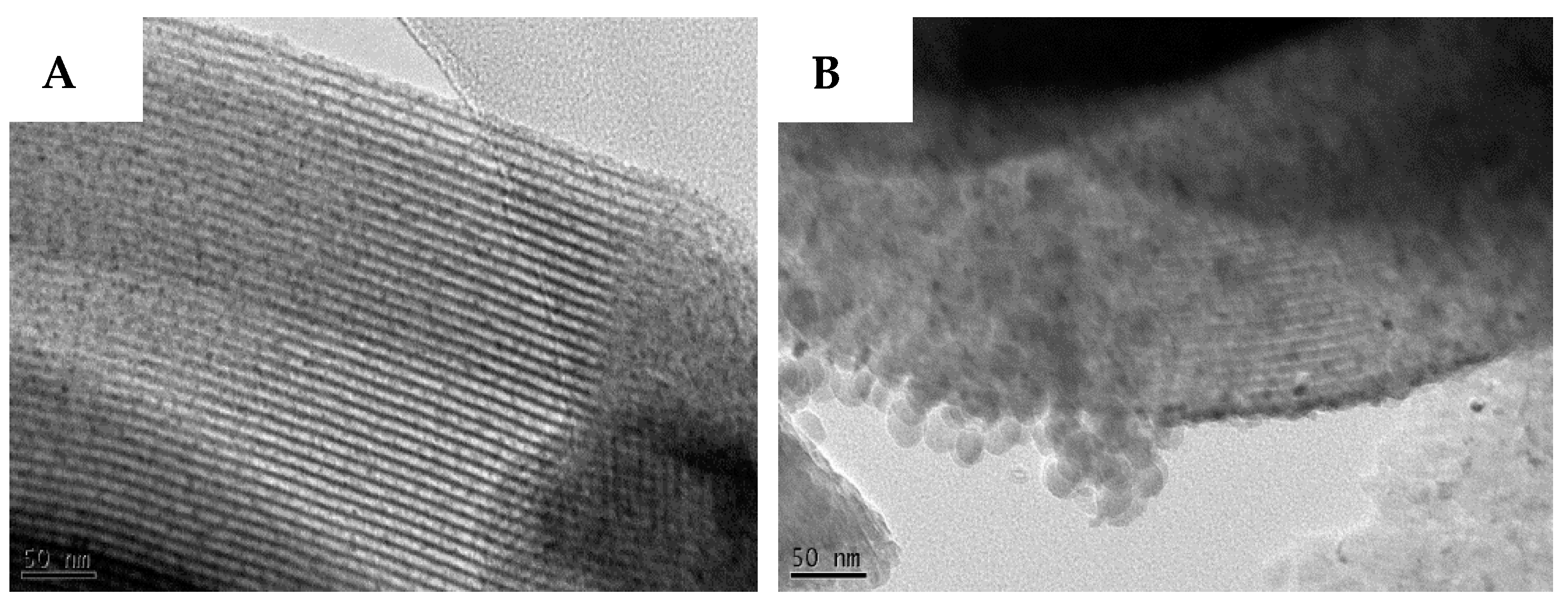
2.1. Catalyst Characterisation
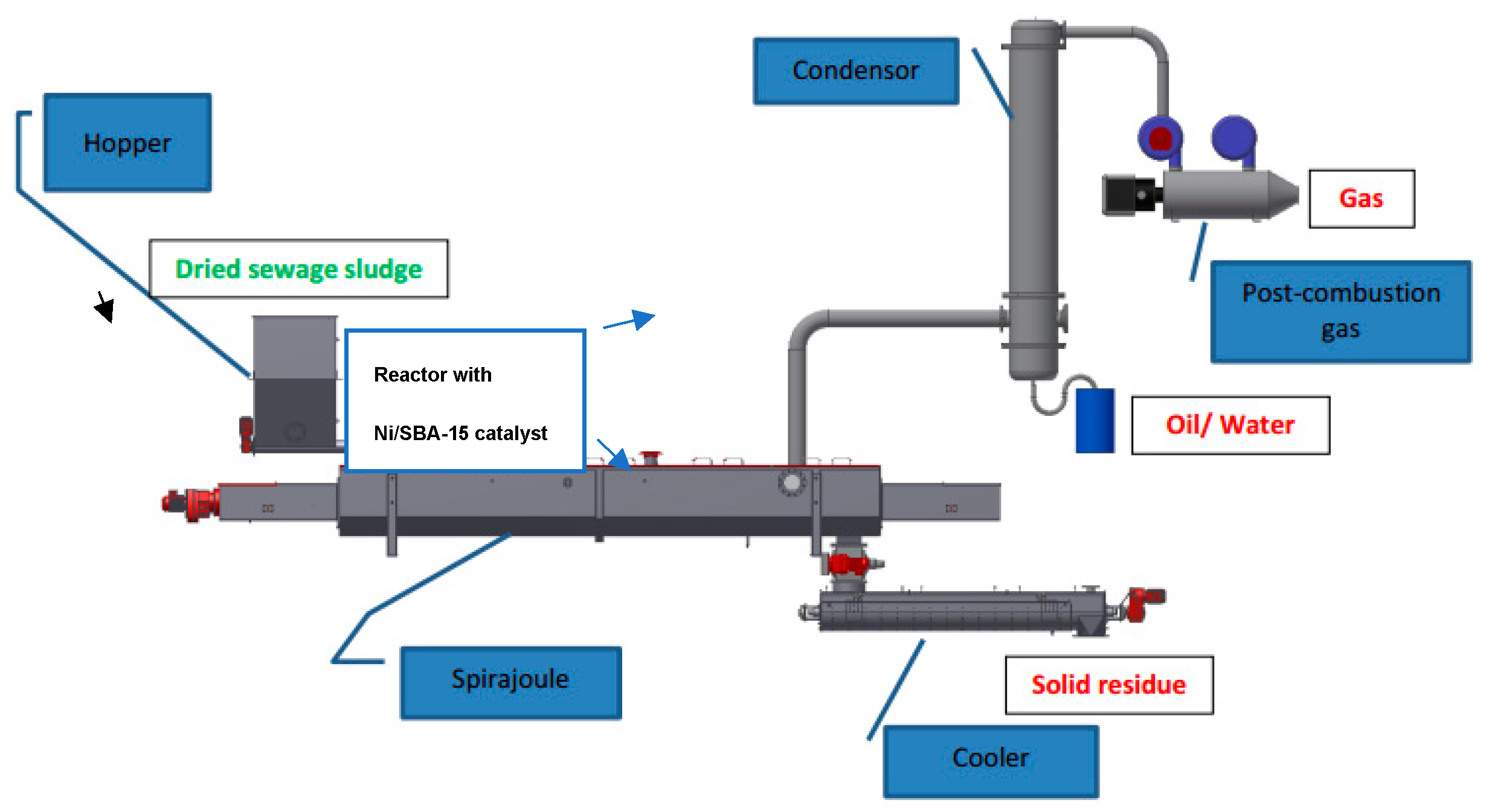
2.2. Pilot Plant Results and Discussion
2.2.1. Characterisation of Pyrolysis Liquid Fraction Without Catalytic Cracking
2.2.2. Pyrolysis Gas Composition
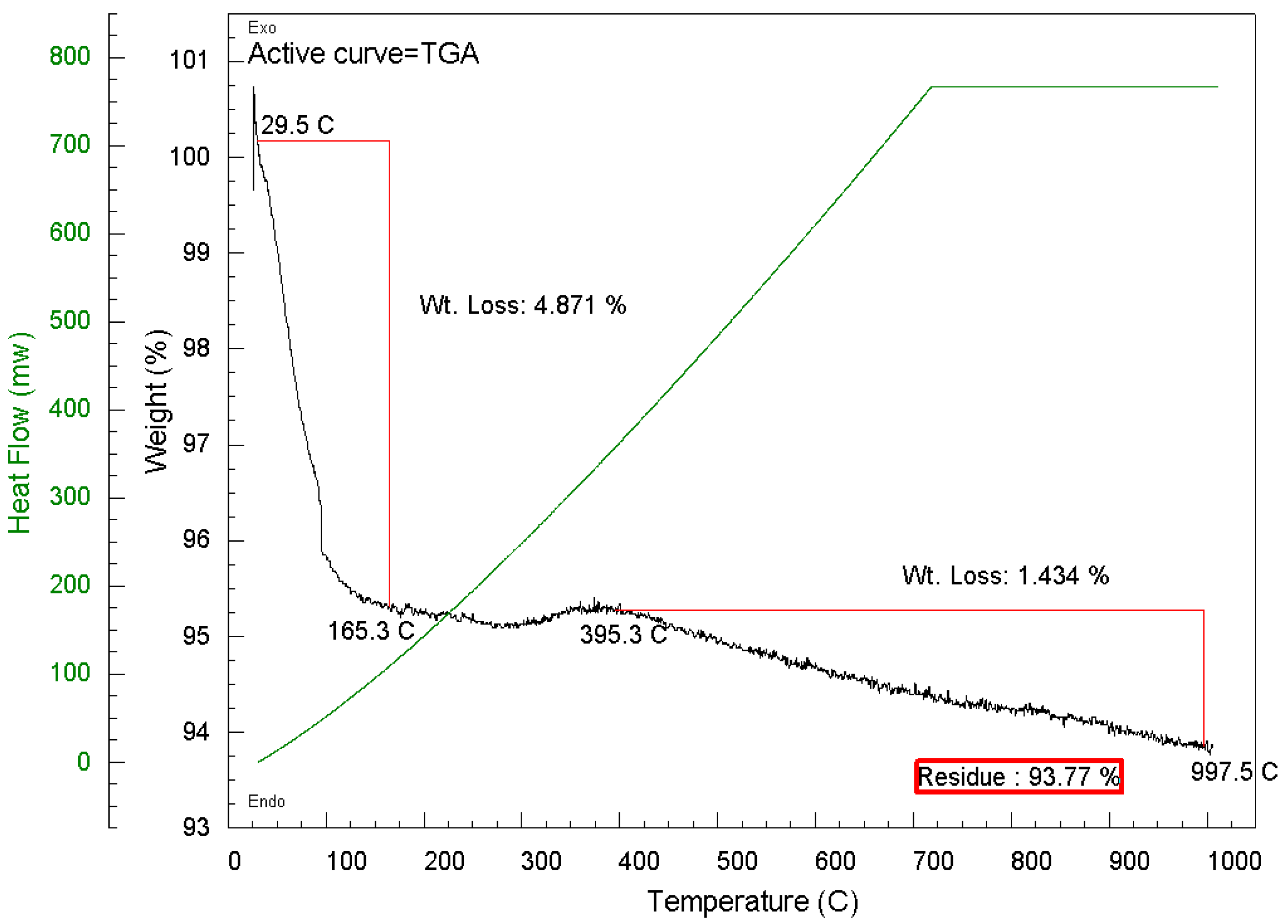
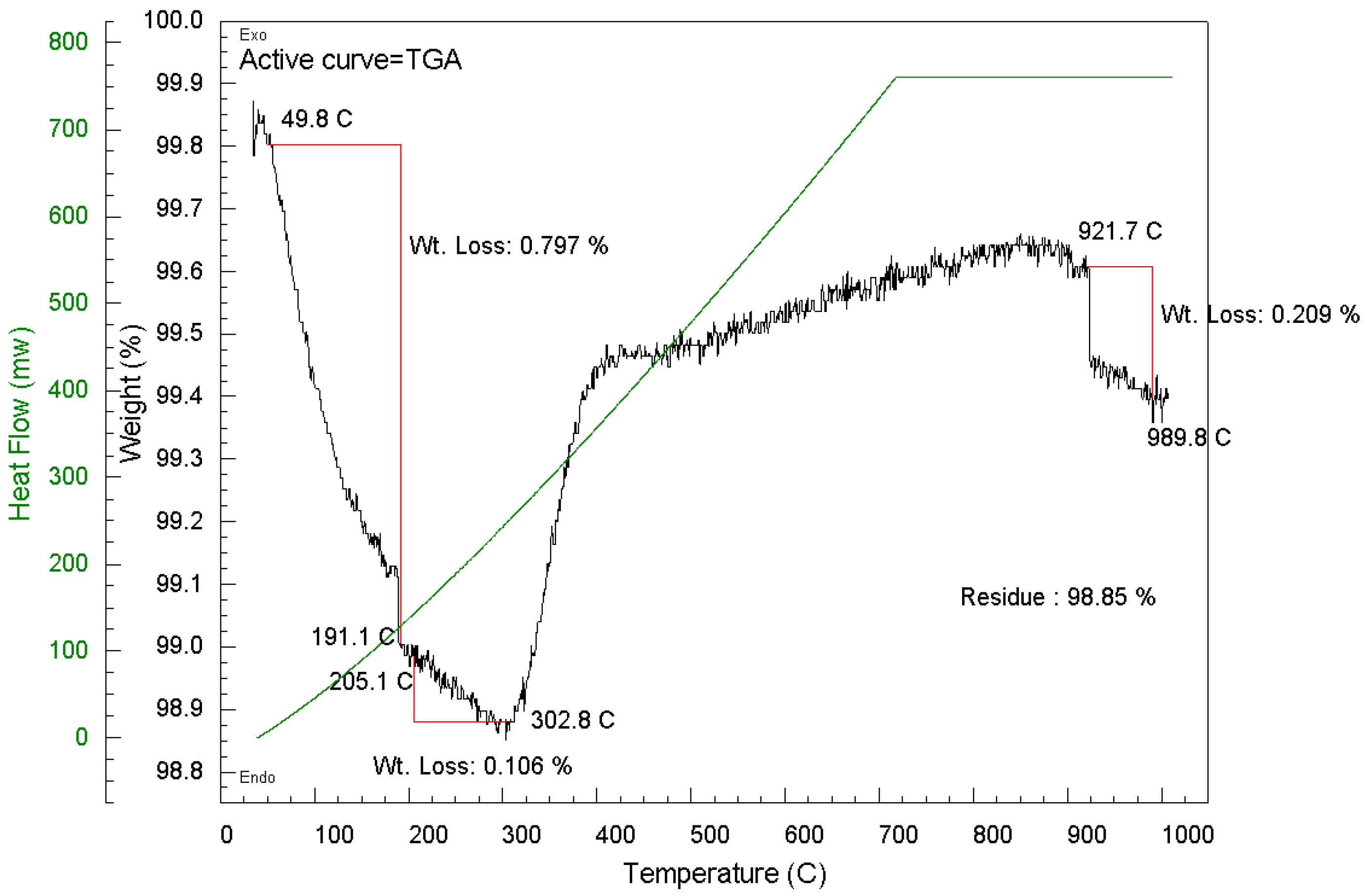
2.2.3. Thermogravimetric Analysis (TGA) of Spent Pellet Catalyst
2.3. Catalytic Activity Evaluation
2.3.1. Catalytic Activity Evaluation via Temperature-Programmed Reaction (TPRx)
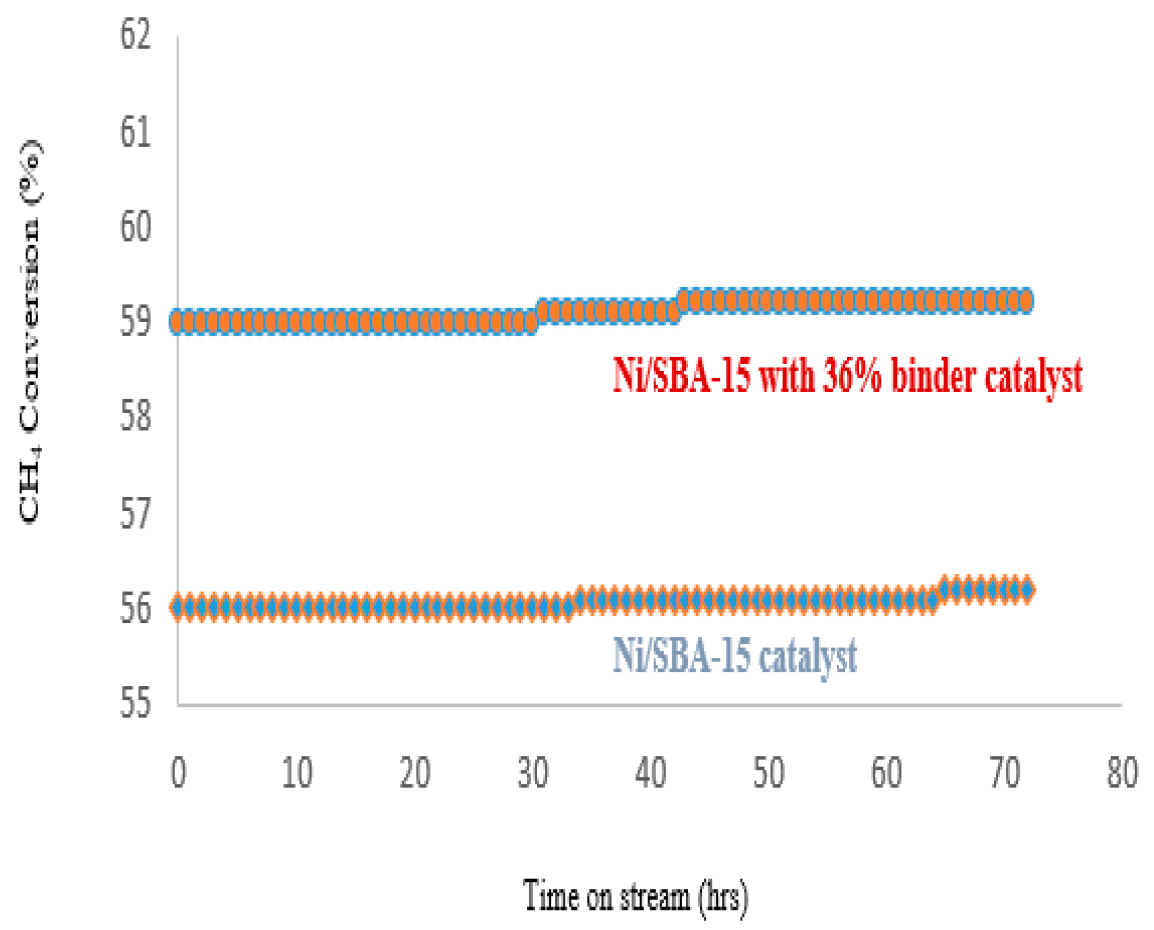
2.3.2. Catalyst Life-Time Test on Stream
3. Experimental Procedure
3.1. Preparation of Ni/SBA-15 Powder Catalysts
3.2. Preparation of Ni/SBA-15 Catalyst Pellets
3.3. Characterisation of Prepared Catalyst Pellets
3.4. Pilot Plant Test Procedure
3.5. Analysis of Liquid and Gas Pyrolysis Fractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission of European Communities. Council Directive 91/271/EEC of 21 March 1991 Concerning Urban Waste-Water Treatment (Amended by the 98/15 EC of 27 February 1998), Official Journal of the European Communities, 30.5.91, No L 135/40. Available online: https://www.legislation.gov.uk/eudr/1998/15/contents (accessed on 28 January 2025).
- Williams, G.; Aitkenhead, N. Lessons from Loscoe: The uncontrolled migration of landfill gas. Q. J. Eng. Geol. Hydrogeol. 1991, 24, 191–207. [Google Scholar] [CrossRef]
- Boeckx, P.; Cleemput, O.V.; Villaralvo, I. Methane emission from a landfill and the methane oxidising capacity of its covering soil. Soil Biol. Biochem. 1996, 28, 1397–1405. [Google Scholar] [CrossRef]
- Mosher, B.W.; Czepiel, P.M.; Harriss, R.C.; Shorter, J.H.; Kolb, C.E.; McManus, J.B.; Allwine, E.; Lamb, B.K. Methane Emissions at Nine Landfill Sites in the Northeastern United States. Environ. Sci. Technol. 1999, 33, 2088–2094. [Google Scholar] [CrossRef]
- Al-Dabbas, M.D.A.F. Reduction of methane emissions and utilization of municipal waste for energy in Amman. Renew. Energy 1998, 14, 427–434. [Google Scholar] [CrossRef]
- Zamorano, M.; Pérez, J.I.P.; Pavés, I.A.; Ridao, Á.R. Study of the energy potential of the biogas produced by an urban waste landfill in Southern Spain. Renew. Sustain. Energy Rev. 2007, 11, 909–922. [Google Scholar] [CrossRef]
- Lundin, M.; Olofsson, M.; Pettersson, G.J.; Zetterlund, H. Environmental and economic assessment of sewage sludge handling options. Resour. Conserv. Recycl. 2004, 41, 255–278. [Google Scholar] [CrossRef]
- Harakeh, S.E.; Mahmood, N.; Zhang, Y. Sewage Sludge Process Improvement Investigation for London, Ontario in: Green Fuels and Chemicals; University of Western Ontario, Department of Chemical and Biochemical Engineering: London, UK, 2011. [Google Scholar]
- Maschio, G.; Koufopanos, C.; Lucchesi, A. Pyrolysis, a promising route for biomass utilization. Bioresour. Technol. 1992, 42, 219–231. [Google Scholar] [CrossRef]
- Bridgwater, A.V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1995, 74, 631–653. [Google Scholar] [CrossRef]
- Higman, C.; Van der Burgt, M. Gasification; Gulf Professional Publishing: Houston, TX, USA, 2011. [Google Scholar]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-B.; Saman, W.R.G.; Navarro, R.R.; Wu, D.-Y.; Zhang, D.-L.; Matsumura, M.; Kong, H.-N. Removal of PCDD/Fs and PCBs from sediment by oxygen free pyrolysis. J. Environ. Sci. 2006, 18, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M.B. Study of the pyrolysis liquids obtained from different sewage sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Domínguez, A.; Fernández, Y.; Fidalgo, B.; Pis, J.J.; Menéndez, J.A. Bio-syngas production with low concentrations of CO2 and CH4 from microwave-induced pyrolysis of wet and dried sewage sludge. Chemosphere 2008, 70, 397–403. [Google Scholar] [CrossRef]
- Karayildirim, T.; Yanik, J.; Yuksel, M.; Bockhorn, H. Characterisation of products from pyrolysis of waste sludges. Fuel 2006, 85, 1498–1508. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Conesa, J.A.; Llavador, F. Analysis of the pyrolysis and combustion of different sewage sludges by TG. J. Anal. Appl. Pyrolysis 2001, 58–59, 927–941. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Martínez, O.; Gómez, X.; Morán, A. Pyrolysis of mixtures of sewage sludge and manure: A comparison of the results obtained in the laboratory (semi-pilot) and in a pilot plant. Waste Manag. 2007, 27, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, F. Introduction to pyrolysis of biomass. J. Anal. Appl. Pyrolysis 1982, 3, 283–305. [Google Scholar] [CrossRef]
- Inguanzo, M.; Domı, A.; Menéndez, J.A.; Blanco, C.G.; Pis, J.J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Bridgwater, A. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Ábrego, J.; Arauzo, J.; Sánchez, J.L.; Gonzalo, A.; Cordero, T.; Rodríguez-Mirasol, J. Structural changes of sewage sludge char during fixed-bed pyrolysis. Ind. Eng. Chem. Res. 2009, 48, 3211–3221. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Park, Y.-K.; Yim, J.-H.; Jeon, J.-K.; Park, J.; Ryu, C.; Kim, S.-S. Clean bio-oil production from fast pyrolysis of sewage sludge: Effects of reaction conditions and metal oxide catalysts. Bioresour. Technol. 2010, 101, S83–S85. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, J.; Scott, D.S.; Westerberg, I.B. Flash pyrolysis of sewage sludge. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 265–270. [Google Scholar] [CrossRef]
- Mercader, F.D.M.; Groeneveld, M.J.; Kersten, S.R.A.; Venderbosch, R.H.; Hogendoorn, J.A. Pyrolysis oil upgrading by high pressure thermal treatment. Fuel 2010, 89, 2829–2837. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bakhshi, N.N. Catalytic upgrading of pyrolysis oil. Energy Fuels 1993, 7, 306–314. [Google Scholar] [CrossRef]
- Doshi, V.A.; Vuthaluru, H.B.; Bastow, T. Investigations into the control of odour and viscosity of biomass oil derived from pyrolysis of sewage sludge. Fuel Process. Technol. 2005, 86, 885–897. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Grassi, G. Biomass Pyrolysis Liquids Upgrading and Utilization; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Gas cleaning for IC engine applications from fixed bed biomass gasification. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Stevens, C.; Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Xu, C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Roddy, D.J.M.-W. Biomass Gasification and Pyrolysis. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 133–153. [Google Scholar]
- Cao, Y.; Wang, Y.; Riley, J.T.; Pan, W.P. A novel biomass air gasification process for producing tar-free higher heating value fuel gas. Fuel Process. Technol. 2006, 87, 343–353. [Google Scholar] [CrossRef]
- Antonopoulos, I.-S.; Karagiannidis, A.; Elefsiniotis, L.; Perkoulidis, G.; Gkouletsos, A. Development of an innovative 3-stage steady-bed gasifier for municipal solid waste and biomass. Fuel Process. Technol. 2011, 92, 2389–2396. [Google Scholar] [CrossRef]
- Zhang, S.; Asadullah, M.; Dong, L.; Tay, H.-L.; Li, C.-Z. An advanced biomass gasification technology with integrated catalytic hot gas cleaning. Part II: Tar reforming using char as a catalyst or as a catalyst support. Fuel 2013, 112, 646–653. [Google Scholar] [CrossRef]
- Dou, B.; Gao, J.; Sha, X.; Baek, S.W. Catalytic cracking of tar component from high-temperature fuel gas. Appl. Therm. Eng. 2003, 23, 2229–2239. [Google Scholar] [CrossRef]
- Delgado, J.; Aznar, M.P.; Corella, J. Calcined Dolomite, Magnesite, and Calcite for Cleaning Hot Gas from a Fluidized Bed Biomass Gasifier with Steam: Life and Usefulness. Ind. Eng. Chem. Res. 1996, 35, 3637–3643. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Wang, T.; Chang, J.; Lv, P.; Zhu, J. Novel catalyst for cracking of biomass tar. Energy Fuels 2005, 19, 22–27. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Cao, W.T.J.-P.; He, Z.-M.; Jiang, W.; Wang, Z.-H. Recent progress of catalysts for reforming of biomass tar/tar models at low temperature—A short review. ChemCatChem 2023, 15, e202300581. [Google Scholar] [CrossRef]
- Mitchell, S.; Michels, N.-L.; Pérez-Ramírez, J. From powder to technical body: The undervalued science of catalysy scale up. Chem. Soc. Rev. 2013, 42, 6094–6112. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.-Y.; Wah, H.H.A.; Zeng, G.; Li, Y.; Yang, Y. Carbon dioxide reforming of methane over nickel-grafted SBA-15 and MCM-41 catalysts. Catal. Today 2009, 148, 243–250. [Google Scholar] [CrossRef]
- Iro, E.; Ariga-Miwa, H.; Sasaki, T.; Asakura, K.; Olea, M. Elimination of Indoor Volatile Organic Compounds on Au/SBA-15 Catalysts: Insights into the Nature, Size, and Dispersion of the Active Sites and Reaction Mechanism. Catalysts 2022, 12, 1365. [Google Scholar] [CrossRef]
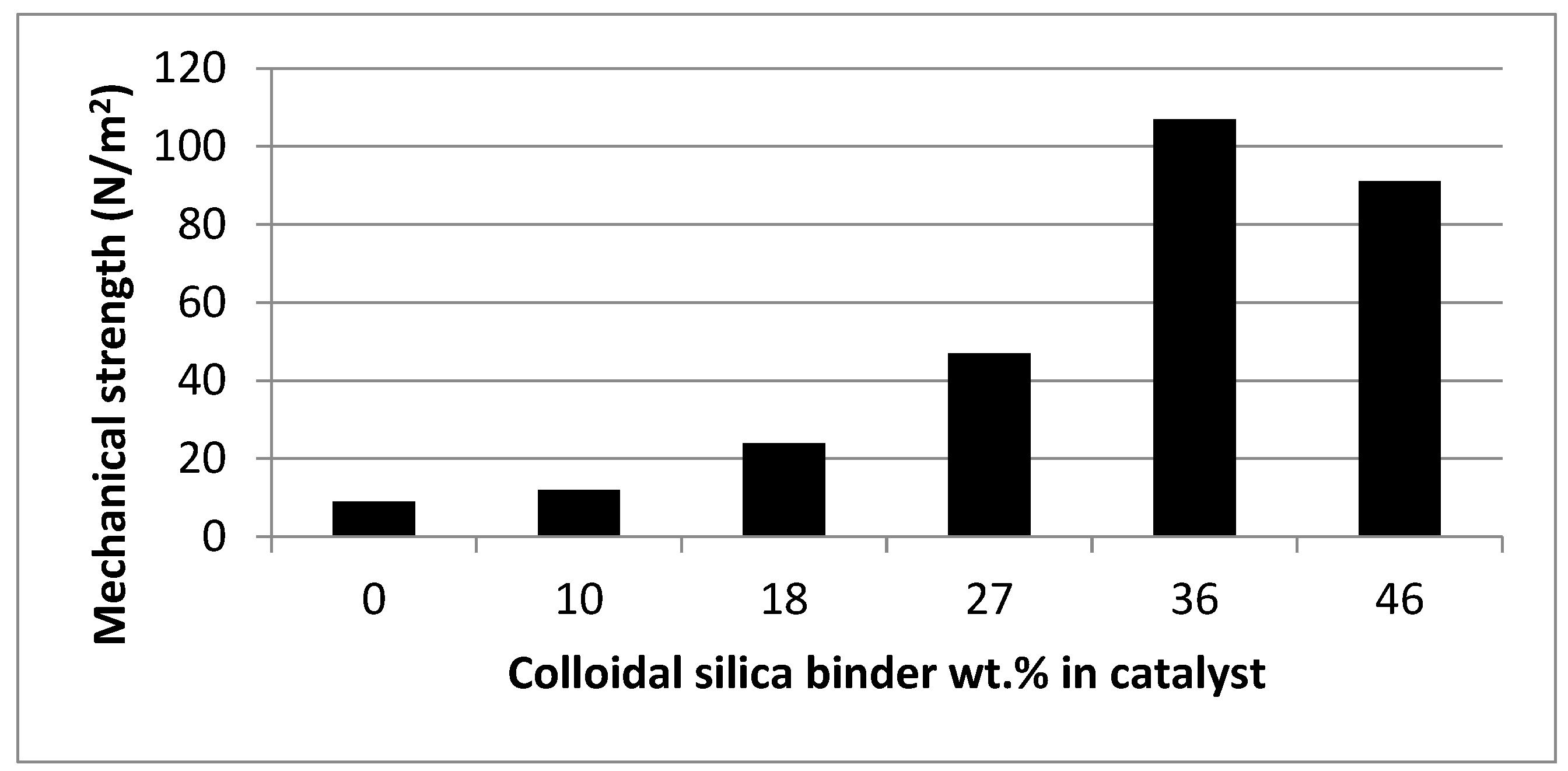


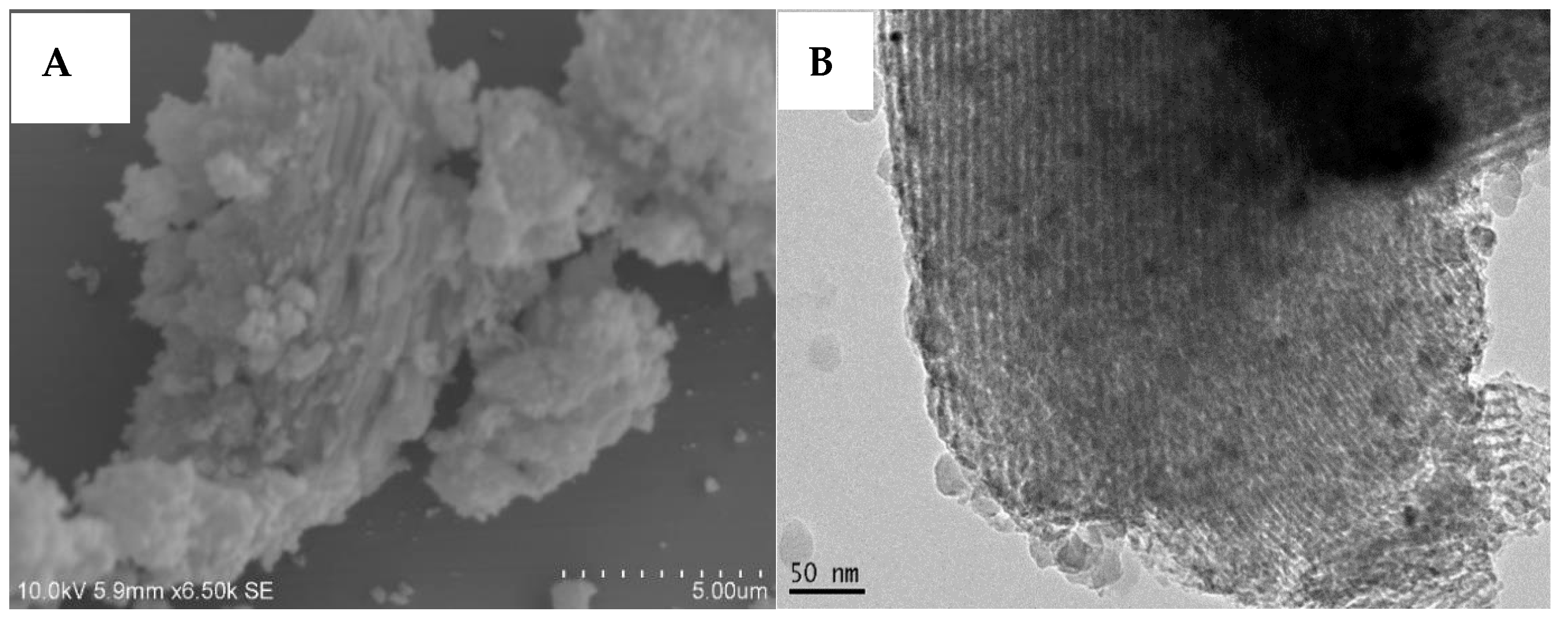
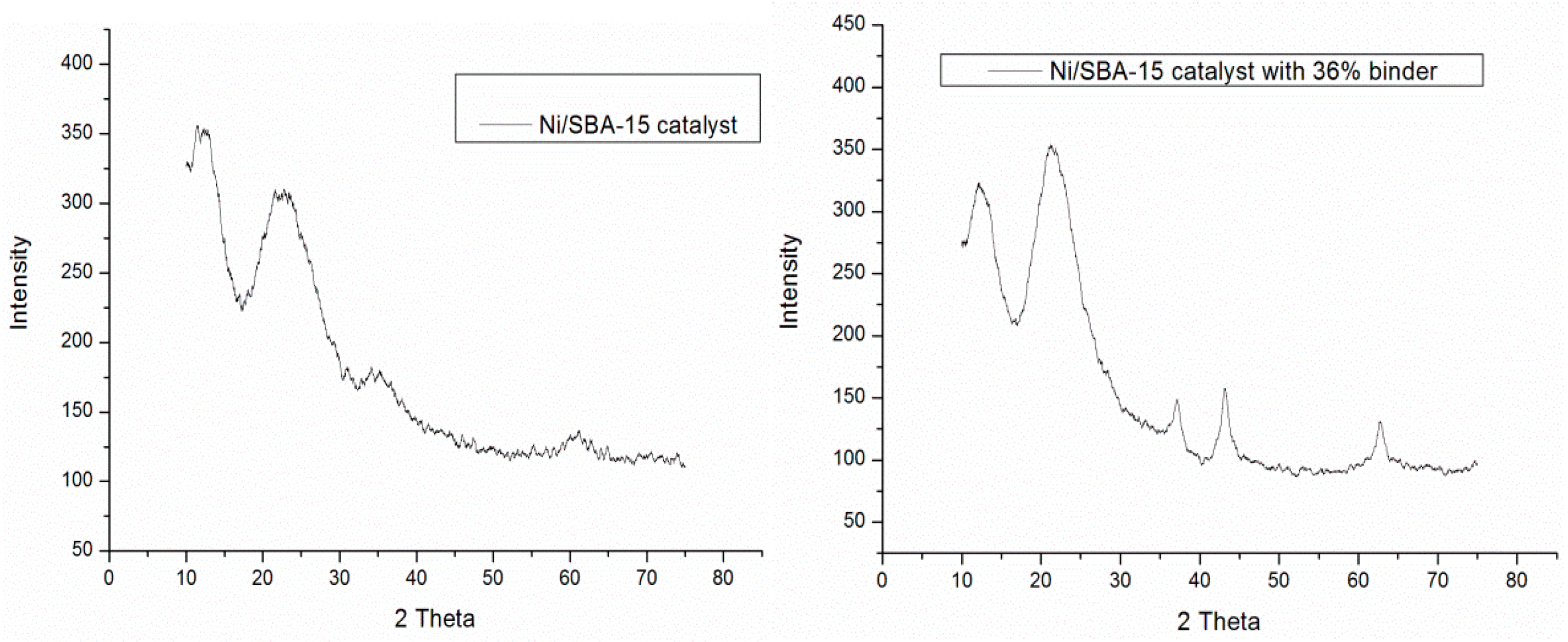
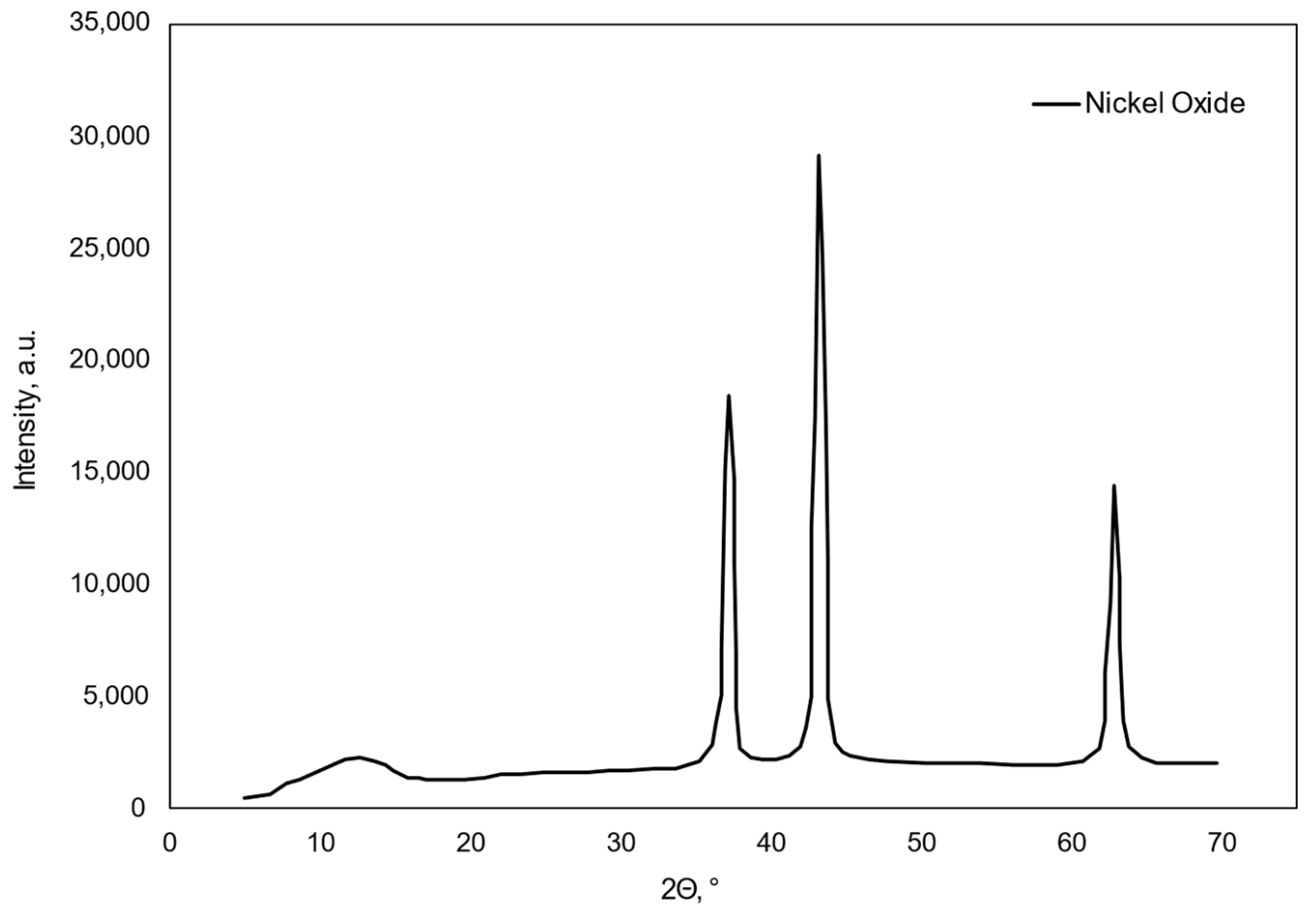
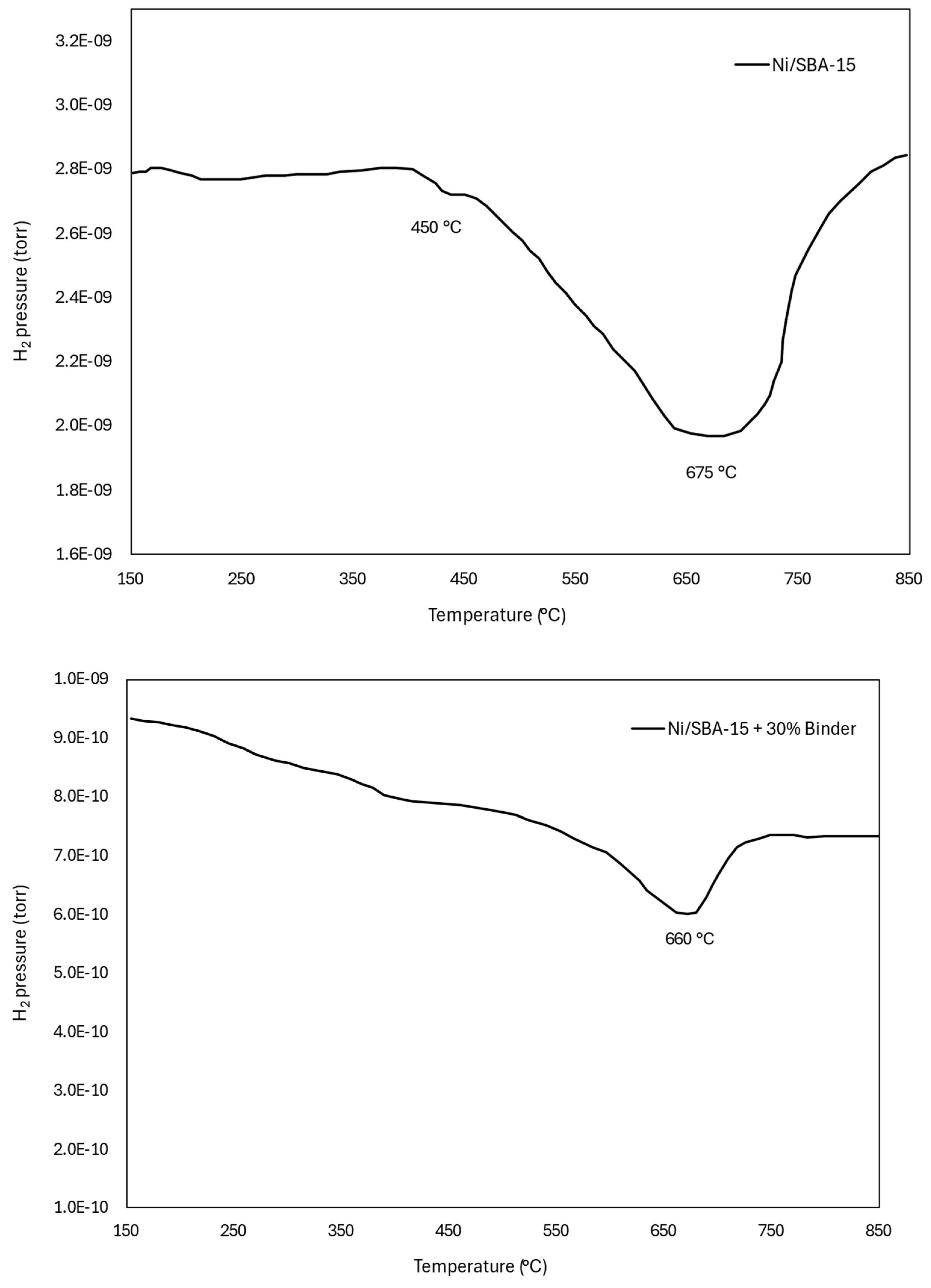
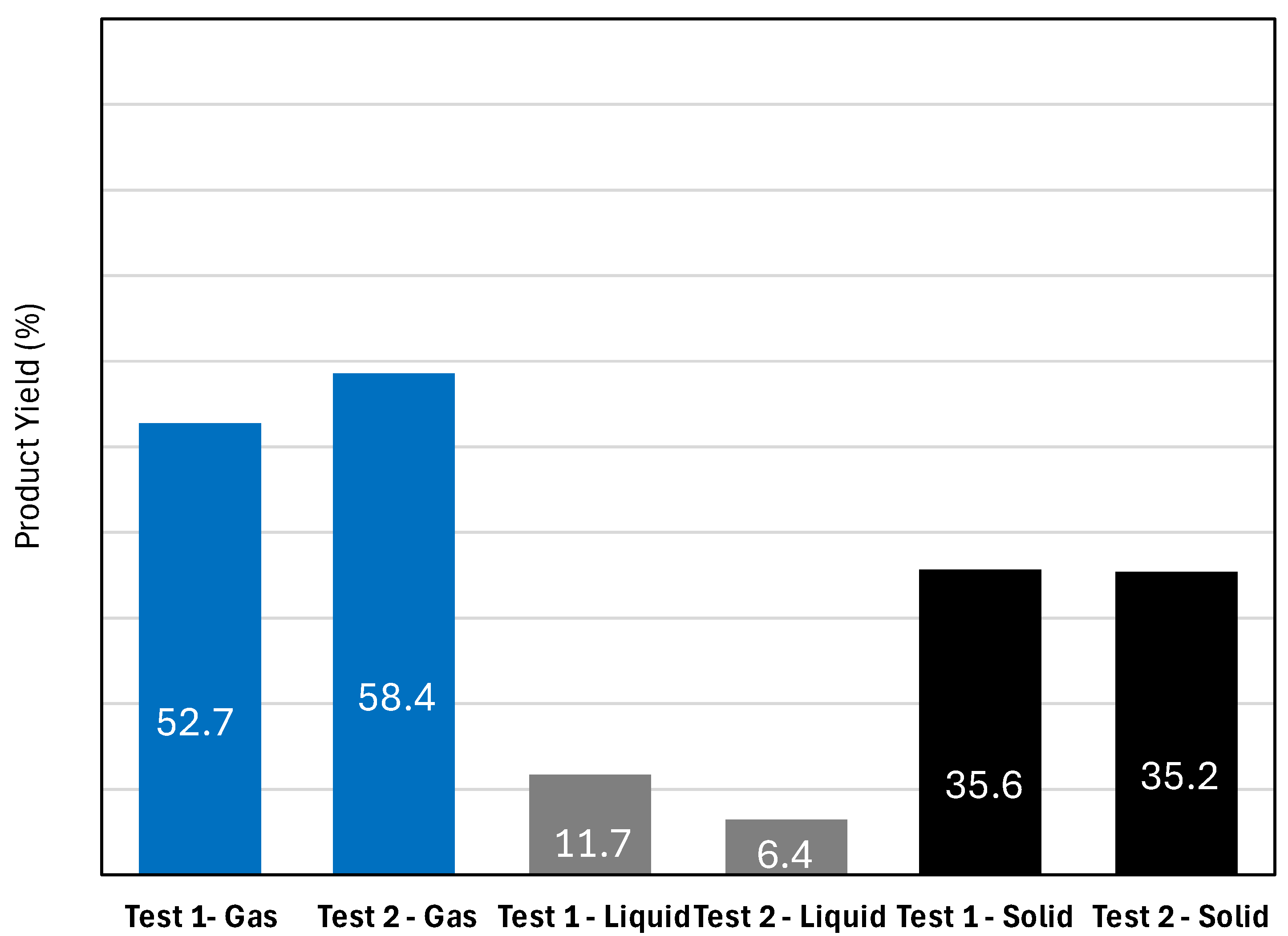

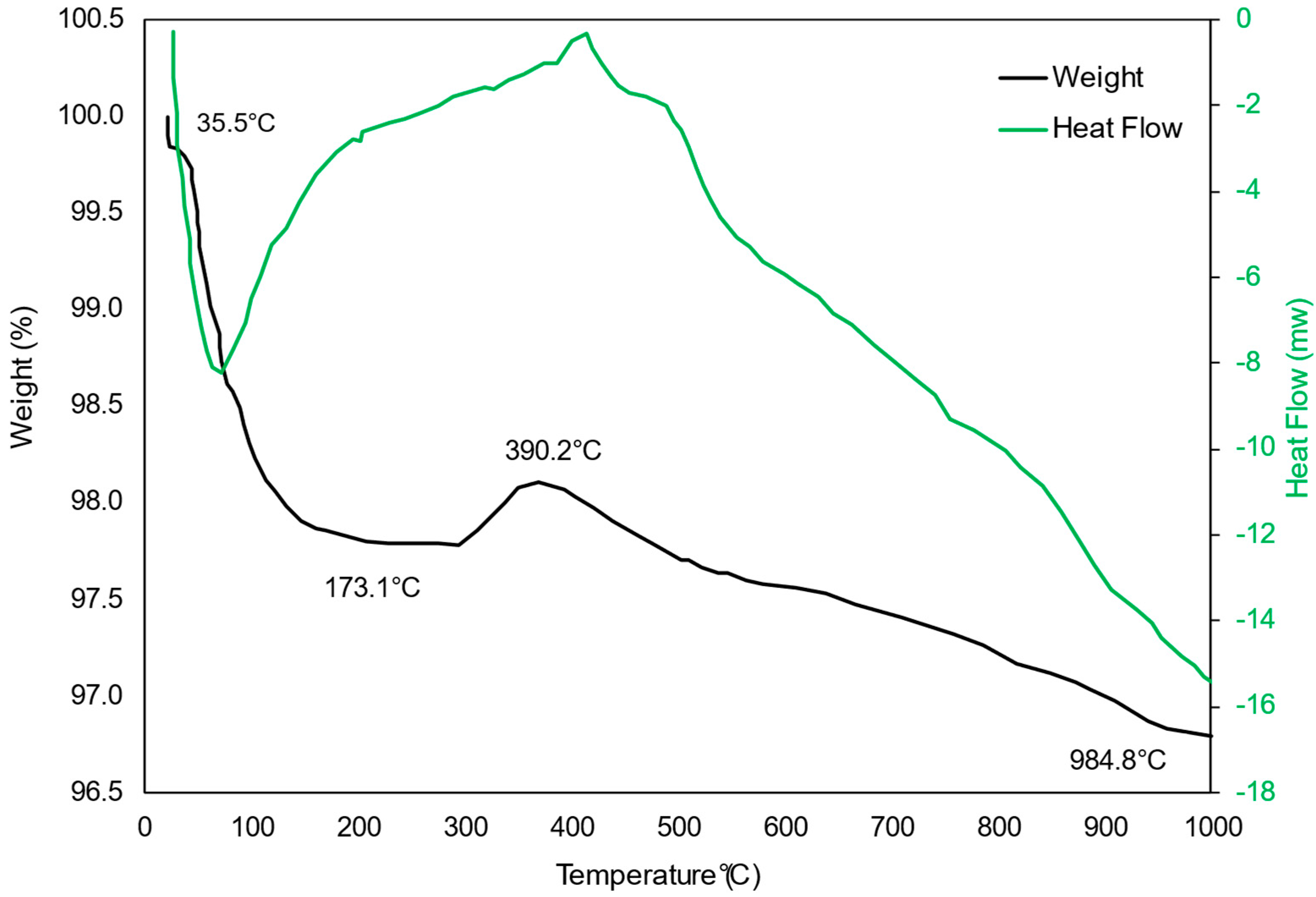
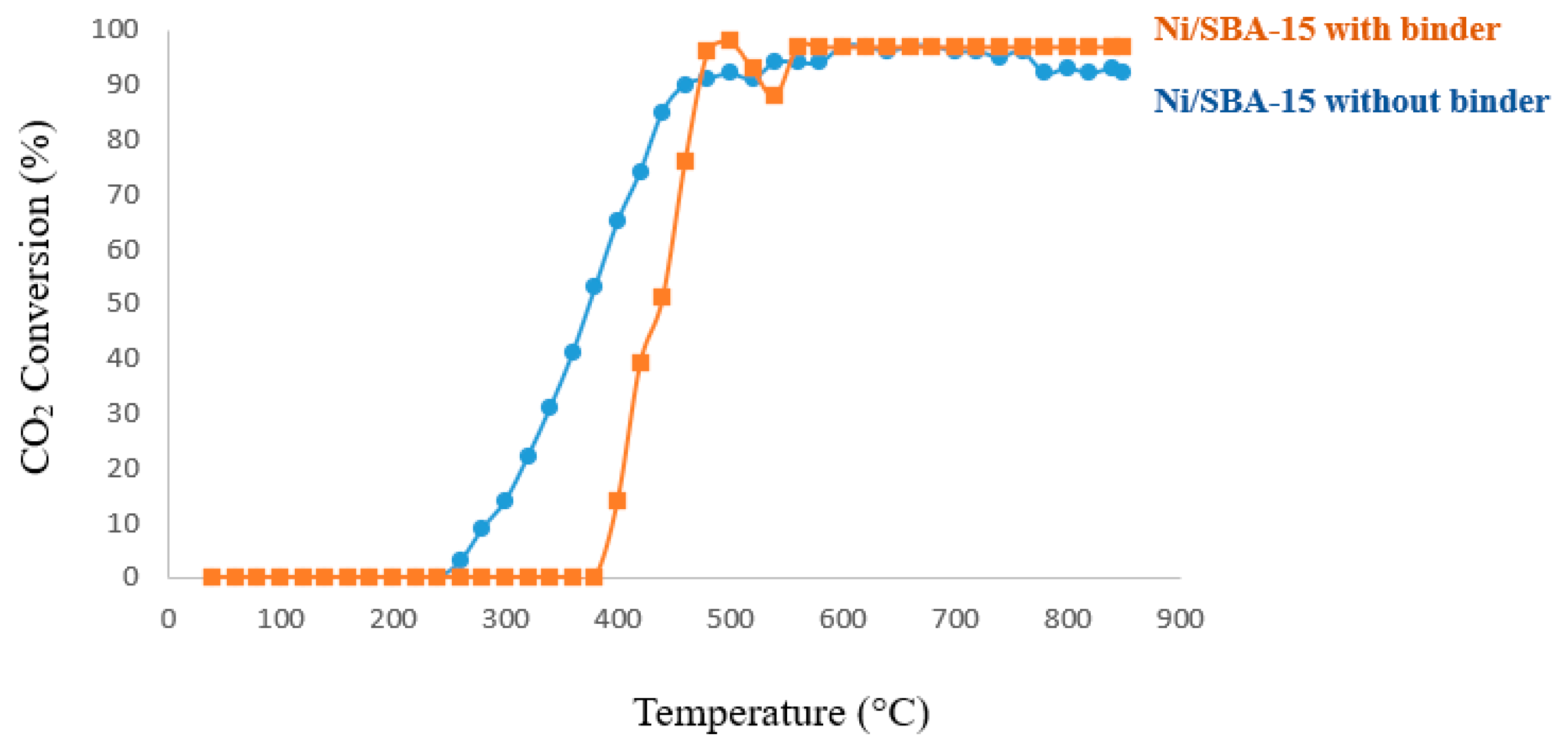


| Catalyst | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm2/g) | Ni Loading (wt.%) | Ni Size (nm) |
|---|---|---|---|---|---|
| Ni/SBA-15 | 539 | 5.3 | 0.65 | 5.7 | 1–2 |
| Ni/SBA-15 + 36% binder | 4.0 | 7 |
| Liquid Density (g/cm3) | pH of Liquid | Water Content (%) | Tar Content (%) | Nitrogen Content (µg/mL)/wt. ppm | Sulphur Content (µg/mL)/wt. ppm |
|---|---|---|---|---|---|
| 1.0347 | 9 | 55 | 45 | 4803/4642 | 704/680 |
| Tar Compounds | Amount (%) |
|---|---|
| Light aromatics excluding benzene | 22.2 |
| Pyridine | 20.2 |
| Dimethylpyrazine | 9.2 |
| Acids | 9.1 |
| Aliphatic | 7.4 |
| Phenol | 3.6 |
| 4-Pyridiamine | 3.0 |
| Others | 25.3 |
| No. | Ni/SBA-15 Catalyst (g) | Methyl Cellulose (g) | Colloidal Silica + H2O Suspension (g) | Amount of Colloidal Silica in Ni/SBA-15 Catalyst (%) |
|---|---|---|---|---|
| 1 | 5 | - | - | - |
| 2 | 6 | 5 | 10 | 46 |
| 3 | 7 | 5 | 8 | 36 |
| 4 | 8 | 5 | 6 | 27 |
| 5 | 9 | 5 | 4 | 18 |
| 6 | 10 | 5 | 2 | 10 |
| Pyrolysis at 800 °C | Sewage Sludge | Plant Operating Parameters | |||
|---|---|---|---|---|---|
| Test Runs | Moisture Content (%) | Density | Residence Time | Feed Flow Rate (kg/h) | Duration (min) |
| Test 1 without catalyst | 7.8 | 0.7 | 20 | 3.7 | 85 |
| Test 2 with Ni/SBA-15 catalyst pellets | 7.8 | 0.7 | 20 | 3.3 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iro, E.; Hajimirzaee, S.; Sasaki, T.; Olea, M. Novel Ni/SBA-15 Catalyst Pellets for Tar Catalytic Cracking in a Dried Sewage Sludge Pyrolysis Pilot Plant. Catalysts 2025, 15, 142. https://doi.org/10.3390/catal15020142
Iro E, Hajimirzaee S, Sasaki T, Olea M. Novel Ni/SBA-15 Catalyst Pellets for Tar Catalytic Cracking in a Dried Sewage Sludge Pyrolysis Pilot Plant. Catalysts. 2025; 15(2):142. https://doi.org/10.3390/catal15020142
Chicago/Turabian StyleIro, Emmanuel, Saeed Hajimirzaee, Takehiko Sasaki, and Maria Olea. 2025. "Novel Ni/SBA-15 Catalyst Pellets for Tar Catalytic Cracking in a Dried Sewage Sludge Pyrolysis Pilot Plant" Catalysts 15, no. 2: 142. https://doi.org/10.3390/catal15020142
APA StyleIro, E., Hajimirzaee, S., Sasaki, T., & Olea, M. (2025). Novel Ni/SBA-15 Catalyst Pellets for Tar Catalytic Cracking in a Dried Sewage Sludge Pyrolysis Pilot Plant. Catalysts, 15(2), 142. https://doi.org/10.3390/catal15020142







