Regulation of Ni3S2@NiS Heterostructure Grown on Industrial Nickel Net for Improved Electrocatalytic Hydrogen Evolution
Abstract
1. Introduction
2. Results and Discussion
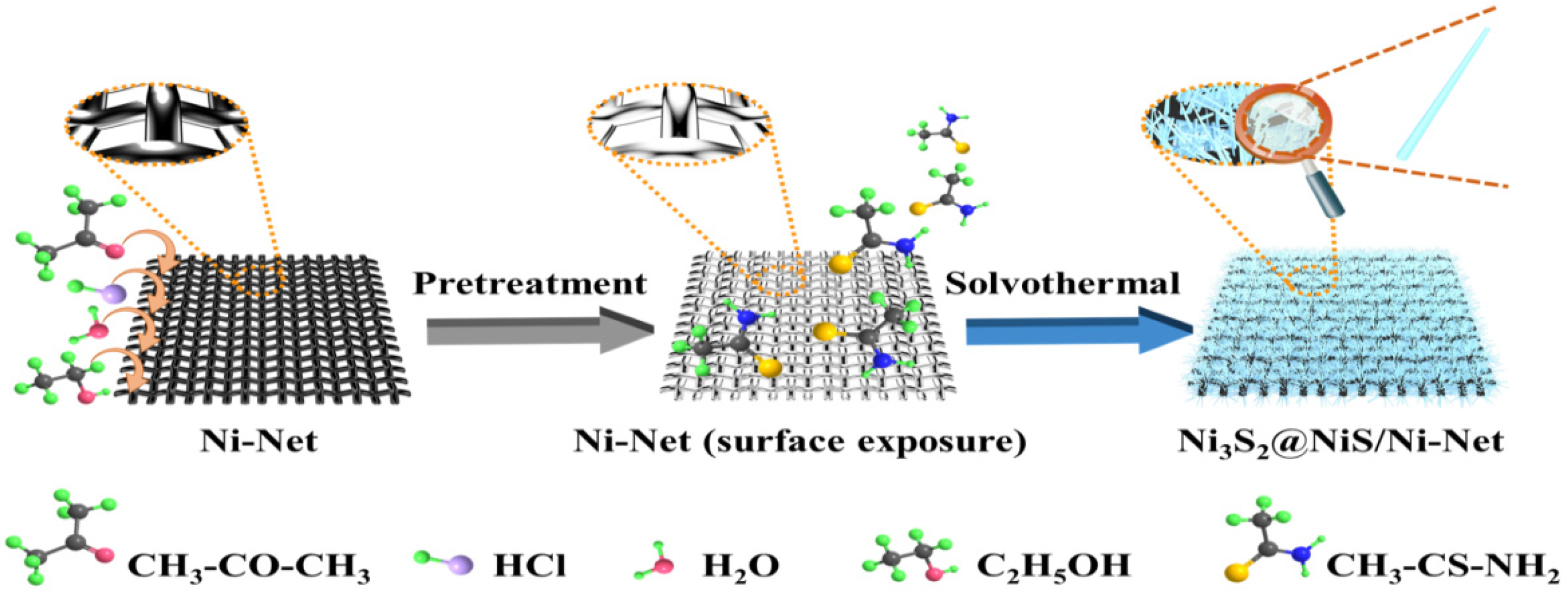
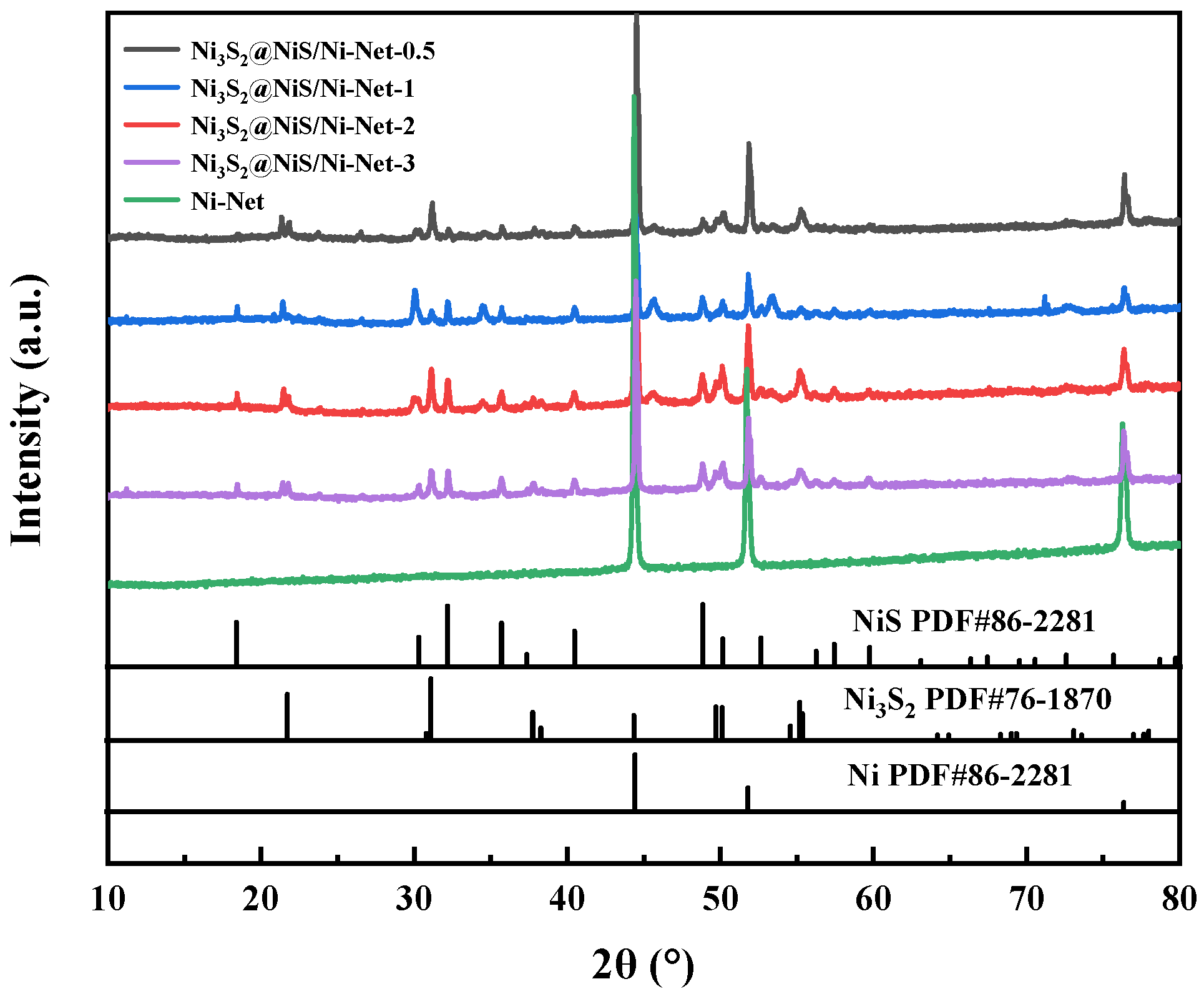
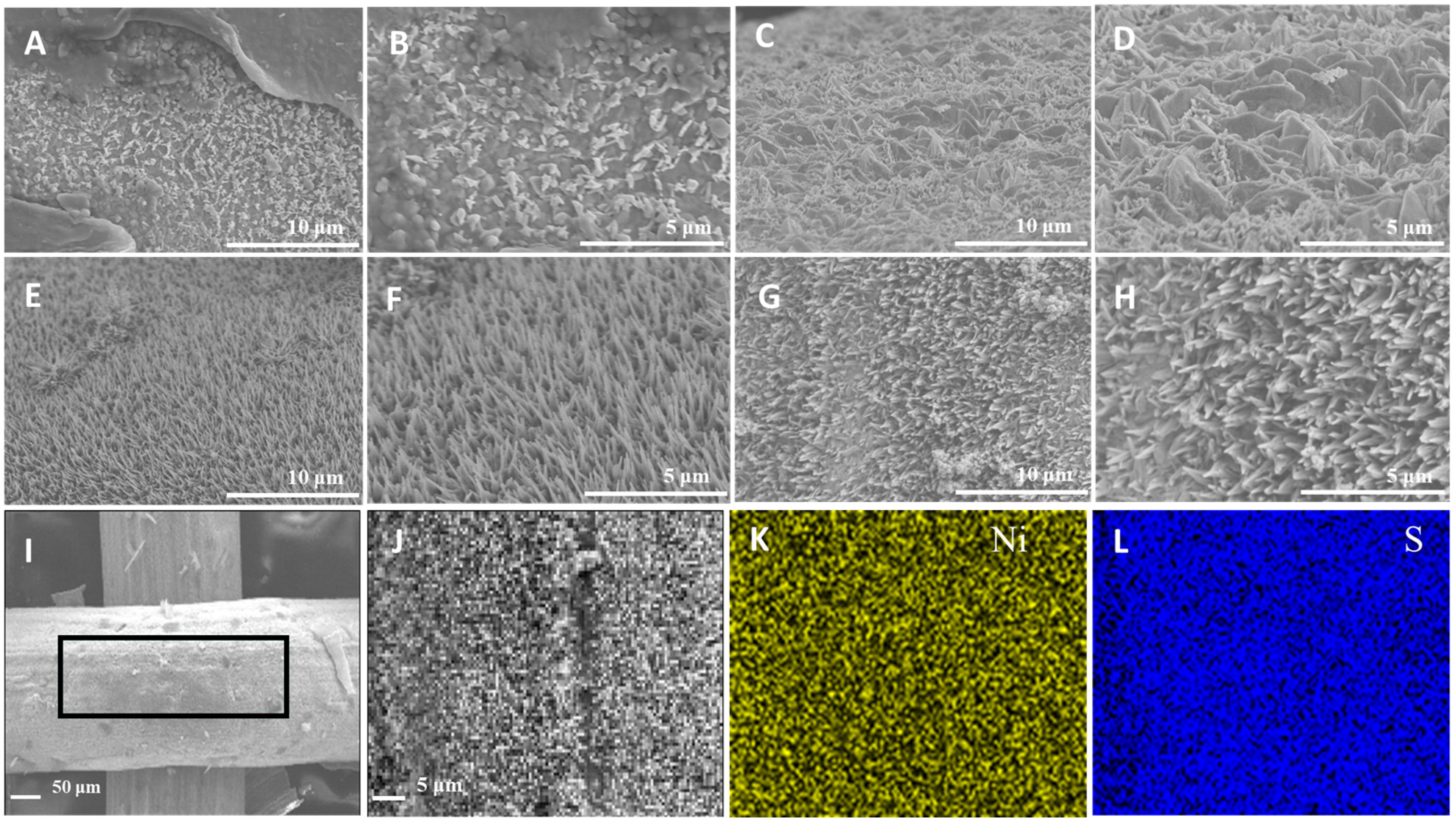
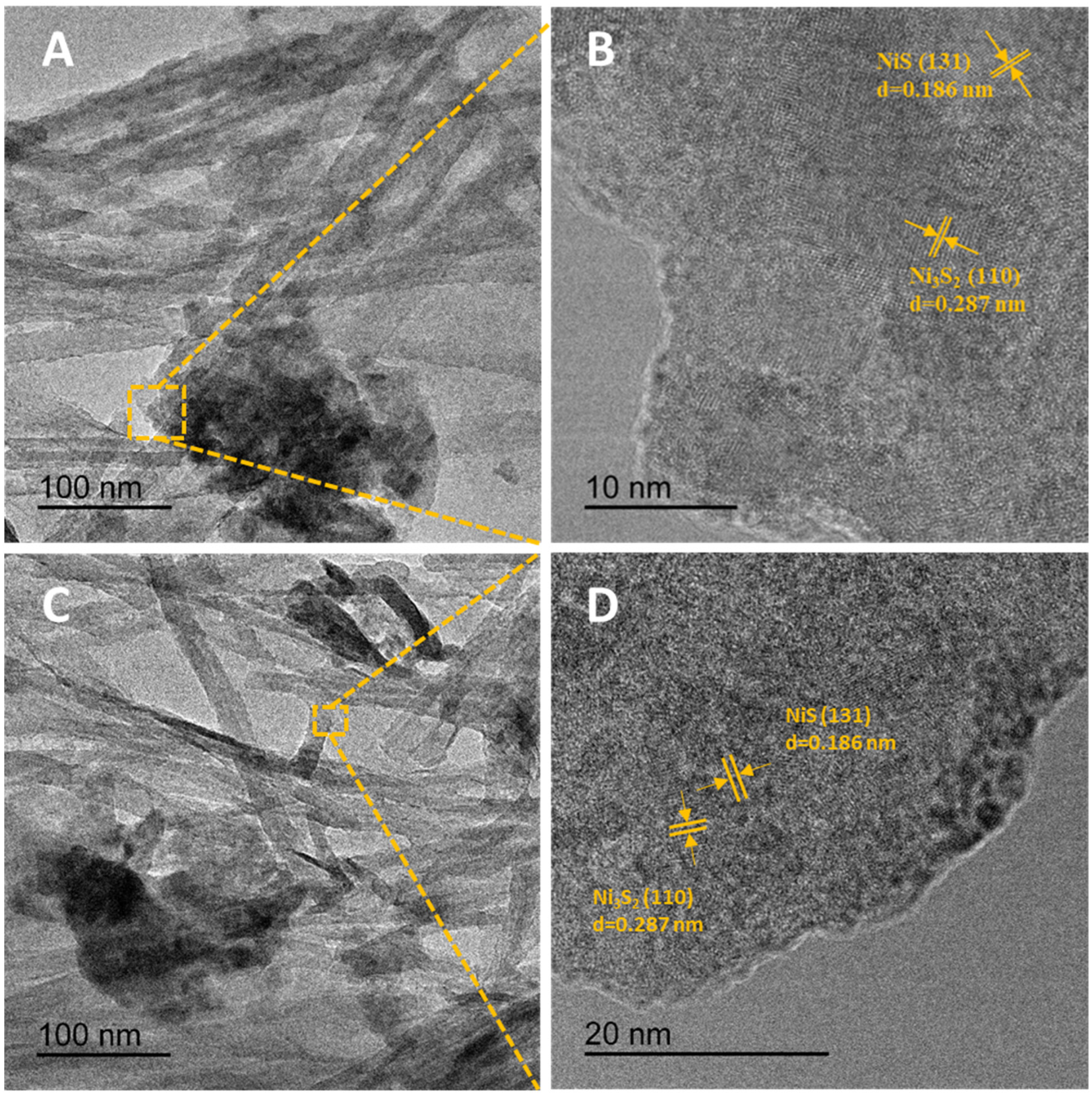
2.1. Synthesis and Microstructural Characterization of Ni3S2@NiS/Ni-Net Materials
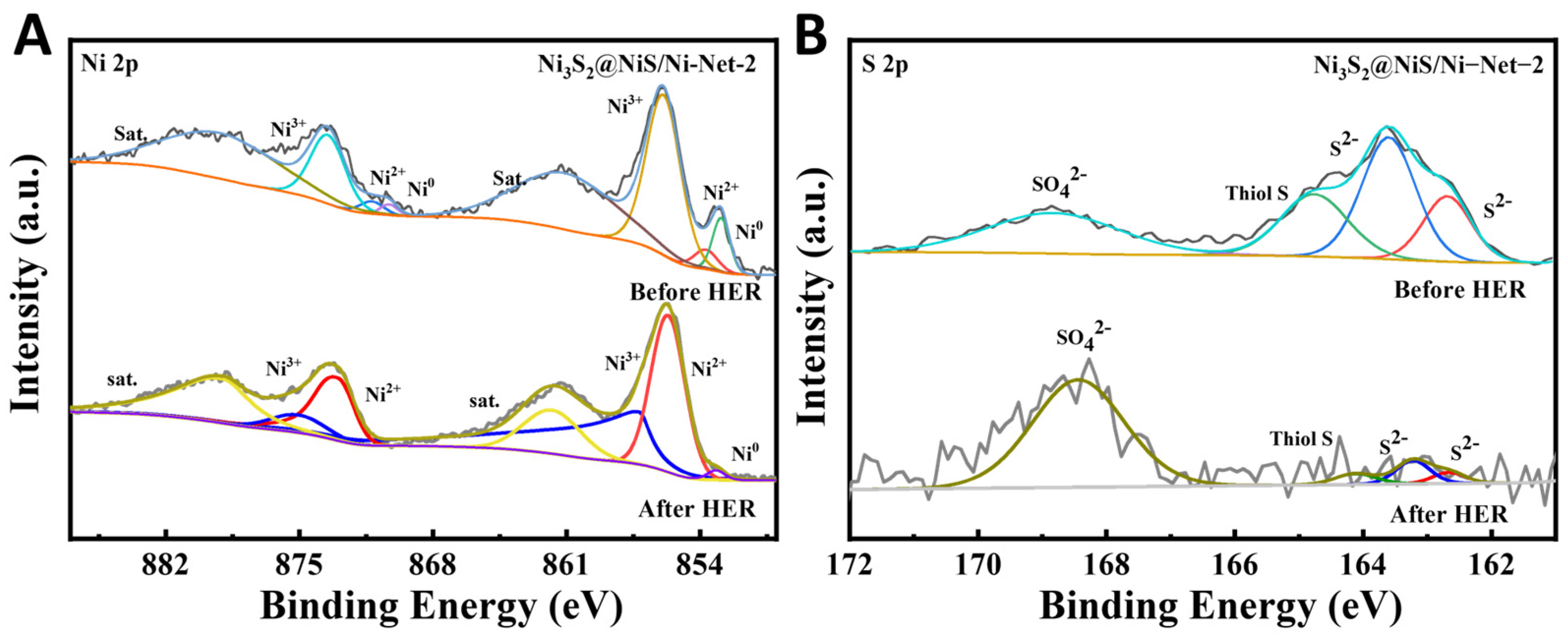
2.2. XPS Analysis of the Resulting Samples
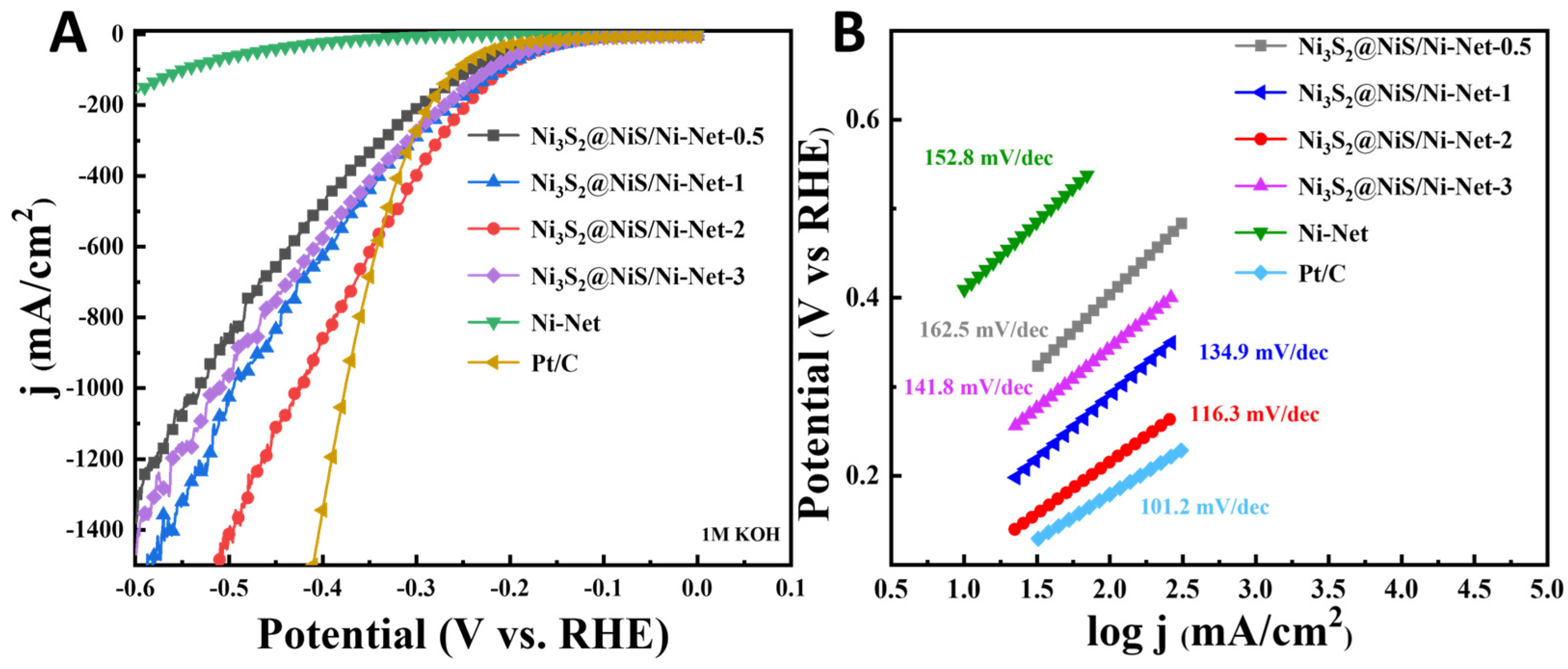
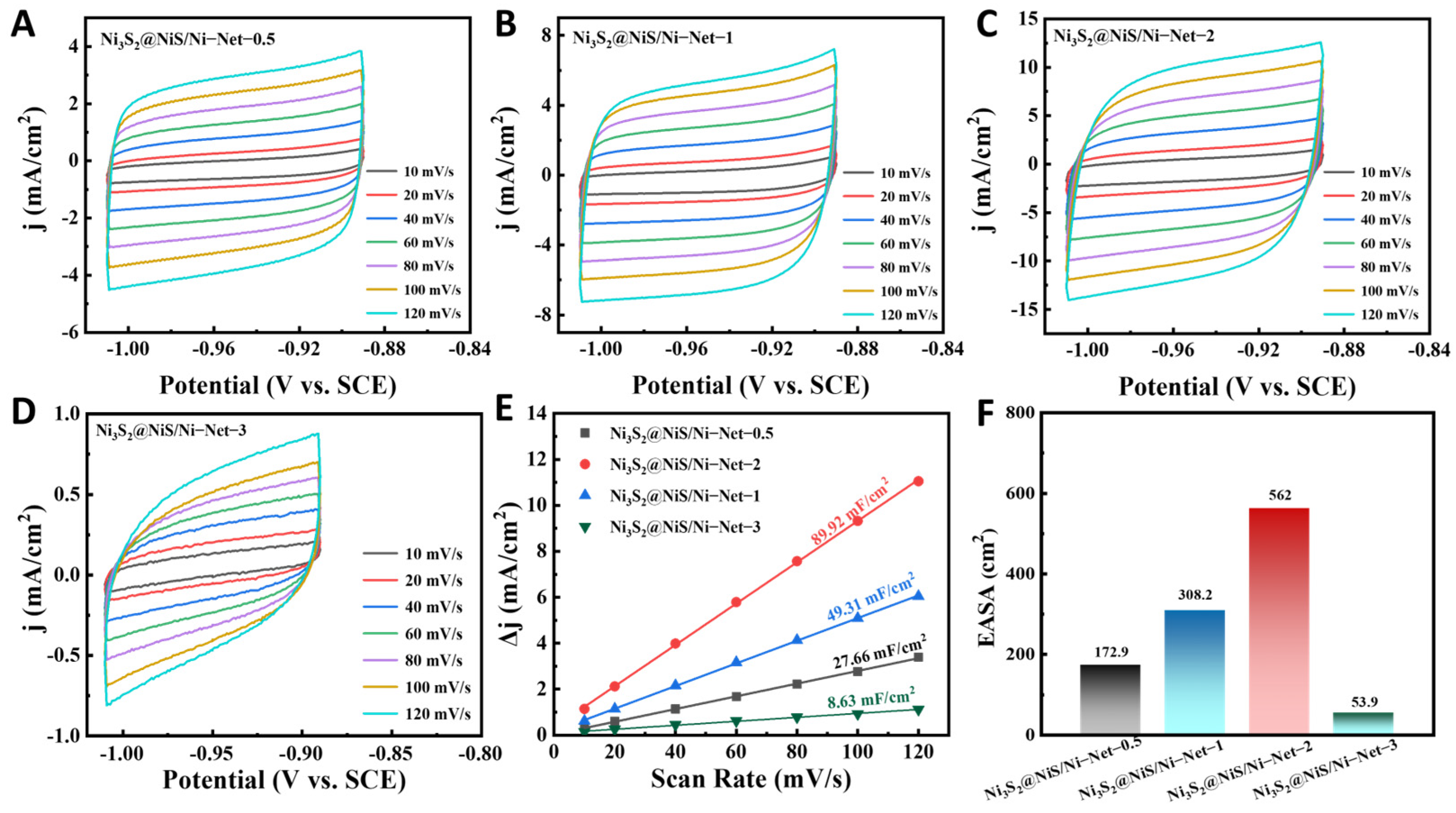
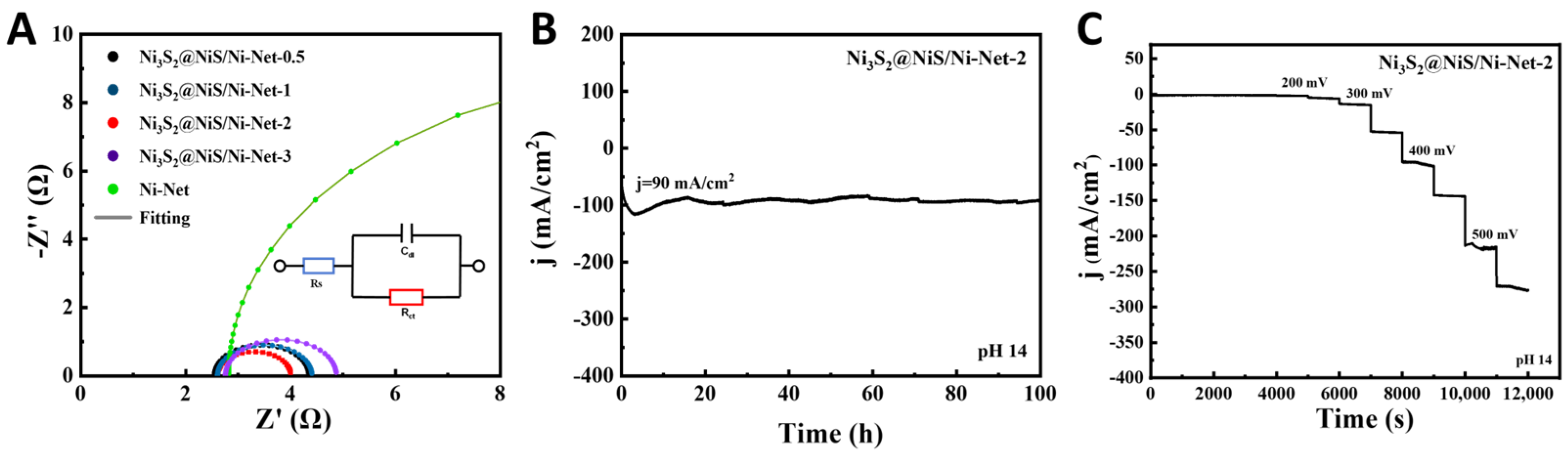
2.3. Electrochemical Evaluation of Ni3S2@NiS/Ni-Net for the HER
3. Materials and Methods
3.1. Preparation of Materials
3.1.1. Chemicals and Reagents
3.1.2. Preparation of Clean Nickel Net
3.1.3. Synthesis of Ni3S2@NiS/Ni-Net
3.2. Material Characterization
3.3. Electrochemical Performance Test Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Marnellos, G.E.; Klassen, T. Welcome to Hydrogen A New International and Interdisciplinary Open Access Journal of Growing Interest in Our Society. Hydrogen 2020, 1, 90–92. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; Ortiz–Ledon, C.; Zhu, J.; Chen, S.; Duan, J. Biomimetic assembly to superplastic metal–organic framework aerogels for hydrogen evolution from seawater electrolysis. Exploration 2021, 1, 20210021. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Chapman, A.; Tsuji, T. Assessing the optimal contributions of renewables and carbon capture and storage toward carbon neutrality by 2050. Sustainability 2023, 15, 13447. [Google Scholar] [CrossRef]
- Yang, H.Y.; Driess, M.; Menezes, P.W. Self-Supported electrocatalysts for practical water electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Doo-Man, C. Fabrication of efficient nanostructured Co3O4-Graphene bifunctional catalysts: Oxygen evolution, hydrogen evolution, and H2O2 sensing. Ceram. Int. 2020, 46, 23479–23498. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Mohaned, M.; Doo-Man, C. Composition dependent electrocatalytic activity of Ni(OH)2-graphene hybrid catalyst deposited by one-step vacuum kinetic spray technique. Mater. Chem. Phys. 2020, 244, 122675. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, K.; Huang, J.; Hui, C.; Li, X.; Feng, L. A review of modulation strategies for improving the catalytic performance of transition metal sulfide self-supported electrodes for the hydrogen evolution reaction. Dalton Trans. 2024, 53, 3959–3969. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, T.; Feng, L.; Cao, L.; Xu, R. One-step Hydrothermal synthesis of MoS2 nanosheets on molybdenum foils for highly efficient hydrogen evolution reaction. J. Shaanxi Univ. Sci. Technol. 2021, 39, 106–111+118. [Google Scholar]
- Sun, T.; Zhong, W.; Zhang, M.; Tang, Y.; Wang, J.; Xu, C.; Hu, L.; Wang, M. A low-cost 2D WO3/Ni3S2 heterojunction for highly stable hydrogen evolution. Mater. Chem. Front. 2021, 5, 8248–8254. [Google Scholar] [CrossRef]
- Feng, J.; Wu, J.; Tong, Y.; Li, G. Efficient hydrogen evolution on cu nanodots-decorated Ni3S2 nanotubes by optimizing Atomic hydrogen adsorption and desorption. J. Am. Chem. Soc. 2018, 140, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Chen, M.; Wu, F.; Tyler, J.; Wang, S.; Wu, Y.; Zhang, Y.; Li, S.; Supriya, L.; Zhang, Z.; et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat. Commun. 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Y.; Cui, Z.; Huang, H.; Cai, D.; Zhang, J.; Zhou, Y.; Xu, M.; Tong, R. Nickel sulfide-based electrocatalysts for overall water splitting. Int. J. Hydrogen Energy. 2023, 48, 27992–28017. [Google Scholar] [CrossRef]
- Haridas, H.; Somapur, S.; Akkera, H.S.; Neella, N.; Kambhala, N. Review on recent advances of nickel sulfide nano electrocatalysts for hydrogen evolution. ChemistrySelect 2024, 9, e202402550. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, X.; Song, L.; Wu, X. Surface Selectivity of Ni3S2 Toward Hydrogen Evolution Reaction: A First-principles Study. Phys. Chem. Chem. Phys. 2020, 22, 25685–25694. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xu, Z.; Pan, H.; Lin, Y.; Yang, Z.; Du, X. Hierarchical Ni3S2 nanosheets coated on Co3O4 nanoneedle arrays on 3D nickel foam as an efficient electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A. 2018, 6, 5098–5106. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Ling, H.; Li, X.; Chan, H.; Yang, L.; Gao, Q. MoS2-Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Feng, L.; Fu, L.; Li, D.; Huang, J. Heterostructured Ni3S2/NiV-LDH nanowires as highly efficient electrocatalyst for overall water splitting reaction. J. Shaanxi Univ. Sci. Technol. 2022, 40, 110–118. [Google Scholar]
- Wang, Y.; He, X.; Chen, X.; Zhang, Y.; Li, F.; Zhou, Y.; Meng, C. Laser synthesis of cobalt-doped Ni3S4-NiS/Ni as high-efficiency supercapacitor electrode and urea oxidation electrocatalyst. Appl. Surf. Sci. 2022, 596, 153600. [Google Scholar] [CrossRef]
- Chen, M.; Su, Q.; Kitiphatpiboon, N.; Zhang, J.; Feng, C.; Li, S.; Zhao, Q.; Abudula, A.; Ma, Y.; Guan, G. Heterojunction Engineering of Ni3S2/NiS Nanowire for Electrochemical Hydrogen Evolution. Fuel 2022, 331, 125794. [Google Scholar] [CrossRef]
- Ye, L.; Du, Y.; Zhao, Y.; Zhao, L. W-doped Ni3S2 nanoparticles modified with NiFeLa hydroxide for hydrogen evolution. ACS Appl. Energy Mater. 2020, 3, 8372–8381. [Google Scholar] [CrossRef]
- Rani, B.J.; Kanjana, P.A.; Ravi, G.; Yuvakkumar, R.; Saravanakumar, B. Superior electrochemical water oxidation of novel NiS@FeS2 nanocomposites. Mater. Sci. Semicond. Process. 2019, 101, 174–182. [Google Scholar] [CrossRef]
- Feng, L.; Li, G.; Liu, Y.; Wu, Y.; Li, G.; Li, H.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef]
- Wang, S.; Geng, Z.; Bi, S.; Wang, Y.; Gao, Z.; Jin, L.; Zhang, C. Recent advances and future prospects on Ni3S2-based electrocatalysts for efficient alkaline water electrolysis. Green Energy Environ. 2024, 9, 659–683. [Google Scholar] [CrossRef]
- Yang, D.; Cao, L.; Feng, L.L.; Huang, J.; Kajiyoshi, K.; Feng, Y.; Liu, Q.; Li, W.; Feng, L.; Hai, G. Formation of hierarchical Ni3S2 nanohorn arrays driven by in-situ generation of VS4 nanocrystals for boosting alkaline water splitting. Appl. Catal. B-Environ. 2019, 257, 117911. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, D.; Meng, T.; Yang, X. Superb hydrogen evolution by a Pt nanoparticle-decorated Ni3S2 microrod array. ACS Appl. Mater. Interfaces 2020, 12, 39163–39169. [Google Scholar] [CrossRef]
- Gao, Y.; He, W.; Cao, D.; Wang, F.; Li, Y.; Hao, Q.; Liu, C.; Liu, H. Mo-doped Ni3S2 nanosheet arrays for overall water splitting. ACS Appl. Nano Mater. 2023, 6, 6066–6075. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, M.Y.; Chai, J.; Tang, Z.; Shao, M.; Kwok, C.T.; Yang, M.; Wang, Z.; Chua, D.; Wang, S.; et al. Facile synthesis of vanadium-doped Ni3S2 nanowire arrays as active electrocatalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 5959–5967. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q. Controllable preparation of N-doped Ni3S2 nanocubes@N-doped graphene-like carbon layers for highly active electrocatalytic overall water splitting. Electrochim. Acta. 2021, 399, 139408. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, Y.; Chu, H.; Baines, R.; Dong, P.; Tang, J.; Yang, Y.; Ajayan, P.M.; Ye, M.; Shen, J. Controlled synthesis of Mo-doped Ni3S2 nano-rods: An efficient and stable electro-catalyst for water splitting. J. Mater. Chem. A 2017, 5, 1595–1602. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, S.; Chen, L.; Li, Y.; Tang, M.; Pei, Q.; Liu, J. Superhydrophilic/superaerophobic amorphous Ni3S2/nimos electrocatalyst for enhanced hydrogen evolution. J. Colloid Interface Sci. 2023, 652, 95–103. [Google Scholar] [CrossRef]
- Feng, L.; Du, Y.; Huang, F.; Cao, L. In situ synthesis of NiAl-LDH nanosheets on nickel foam for highly efficient hydrogen evolution reaction. J. Shaanxi Univ. Sci. Technol. 2020, 38, 123–129. [Google Scholar]
- Zhang, Z.; Dong, Y.; Liu, G.; Li, J.; Sun, H.; Luo, H.; Liu, S. The ultrafine monolayer 1T/2H-Mos2: Preparation, characterization and amazing photocatalytic characteristics. Colloid. Surface A 2020, 589, 124431. [Google Scholar] [CrossRef]
- Lu, X.; Wai, Y.; Bryan, H.; Zhao, C. Electrocatalytic oxygen evolution at surface oxidized multiwall carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhou, M.; Liu, P.; Liu, P.; Yin, H.; Sun, K.; Yang, H.; Al-Mamun, M.; Hu, P.; Wang, H.; et al. Hydrogen spillover-bridged volmer/tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Zhang, X.; Guo, J. Engineering P-doped Ni3S2-NiS hybrid nanorod arrays for efficient overall water electrolysis. J. Alloys Compd. 2021, 862, 158391. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, X.; Guo, X.; Liu, Z.; Asiri, A.M.; Li, B.; Chen, L.; Sun, X. Efficient hydrogen evolution electrocatalysis at alkaline pH by interface engineering of Ni2P-CeO2. Inorg. Chem. 2018, 57, 548–552. [Google Scholar] [CrossRef]
- Yoon, Y.; Yan, B.; Surendranath, Y. Suppressing ion transfer enables versatile measurements of electrochemical surface area for intrinsic activity comparisons. J. Am. Chem. Soc. 2018, 140, 2397–2400. [Google Scholar] [CrossRef]
- Jin, S. Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2017, 2, 1937–1938. [Google Scholar] [CrossRef]
- Ding, Y.; Du, X.; Zhang, X. Controlled synthesis and high performance of Zn–Ni–Co–M (M = O, S, P and Se) nanoneedle arrays as an advanced electrode for overall water splitting. Appl. Surf. Sci. 2021, 548, 148818. [Google Scholar] [CrossRef]
- Sadeghi, E.; Chamani, S.; Erdem, R.; Peighambardoust, N.S.; Aydemir, U. NiMo/CoMoO4 heterostructure with confined oxygen vacancy for active and durable alkaline hydrogen evolution reaction. ACS Appl. Energy Mater. 2023, 6, 7658–7671. [Google Scholar] [CrossRef]
- Ye, S.; Du, Z.; Cai, Z.; Ou, D.; Guo, H.; Liu, Q.; Yang, W.; Shi, Q. Mn-Doped Crystalline Ni3S2/Amorphous MoS2 Core–Shell Nanorods as Bifunctional Electrocatalysts for Highly-Efficient Overall Water Splitting. ACS Appl. Nano Mater. 2024, 7, 3096–3104. [Google Scholar] [CrossRef]
- Li, W.; Sun, Z.; Ge, R.; Li, J.; Li, Y.; Cairney, J.M.; Zheng, R.; Li, Y.; Li, S.; Li, Q.; et al. Nanoarchitectonics of La-Doped Ni3S2/MoS2 Hetetostructural Electrocatalysts for Water Electrolysis. Small Struct. 2023, 4, 2300175. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Y.; Cao, Y.; Yao, F.; He, Z.; Wang, X.; Pan, H.; Wu, M. Amorphous NiWO4 nanoparticles boosting the alkaline hydrogen evolution performance of Ni3S2 electrocatalysts. Appl. Catal. B: Environ. 2020, 274, 119120. [Google Scholar] [CrossRef]
- Huang, J.; Mu, L.; Ou, Y.; Zhao, G.; Huang, J.; Wang, J.; Zhang, B. Bimetallic atom-improved Ni3S2 bifunctional electrocatalysts for efficient hydrogen evolution reaction and overall water splitting performance. CrystEngComm 2024, 26, 4478–4488. [Google Scholar] [CrossRef]
- Qin, X.; Pang, H.; Yu, Z.; Qin, Z.; Jiang, R.; Yao, S.; Huang, J.; Hou, Y.; Fan, B. The cactus bulb-shaped metal-S-C/N nanoparticles supported by highly conductive channels for efficient electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 49, 1070–1084. [Google Scholar] [CrossRef]
- Sun, Y.; Zang, X.; Li, Z.; Zhang, X.; Guo, J. V-doped NiS on carbon fiber cloth for improved electrochemical lithium storage and hydrogen evolution reaction. Solid State Sci. 2024, 154, 107624. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Liu, D.; Li, Y.; Li, X.; Chu, D.; Cao, L.; Huang, J.; Feng, L. Regulation of Ni3S2@NiS Heterostructure Grown on Industrial Nickel Net for Improved Electrocatalytic Hydrogen Evolution. Catalysts 2025, 15, 136. https://doi.org/10.3390/catal15020136
Su Z, Liu D, Li Y, Li X, Chu D, Cao L, Huang J, Feng L. Regulation of Ni3S2@NiS Heterostructure Grown on Industrial Nickel Net for Improved Electrocatalytic Hydrogen Evolution. Catalysts. 2025; 15(2):136. https://doi.org/10.3390/catal15020136
Chicago/Turabian StyleSu, Zihan, Dinghan Liu, Yuhang Li, Xiaoyi Li, Dewei Chu, Liyun Cao, Jianfeng Huang, and Liangliang Feng. 2025. "Regulation of Ni3S2@NiS Heterostructure Grown on Industrial Nickel Net for Improved Electrocatalytic Hydrogen Evolution" Catalysts 15, no. 2: 136. https://doi.org/10.3390/catal15020136
APA StyleSu, Z., Liu, D., Li, Y., Li, X., Chu, D., Cao, L., Huang, J., & Feng, L. (2025). Regulation of Ni3S2@NiS Heterostructure Grown on Industrial Nickel Net for Improved Electrocatalytic Hydrogen Evolution. Catalysts, 15(2), 136. https://doi.org/10.3390/catal15020136








