A Unique Dual-Shell Structure with Highly Active Ni@SiC/CNT/CNF Microwave Catalysts
Abstract
1. Introduction
2. Results
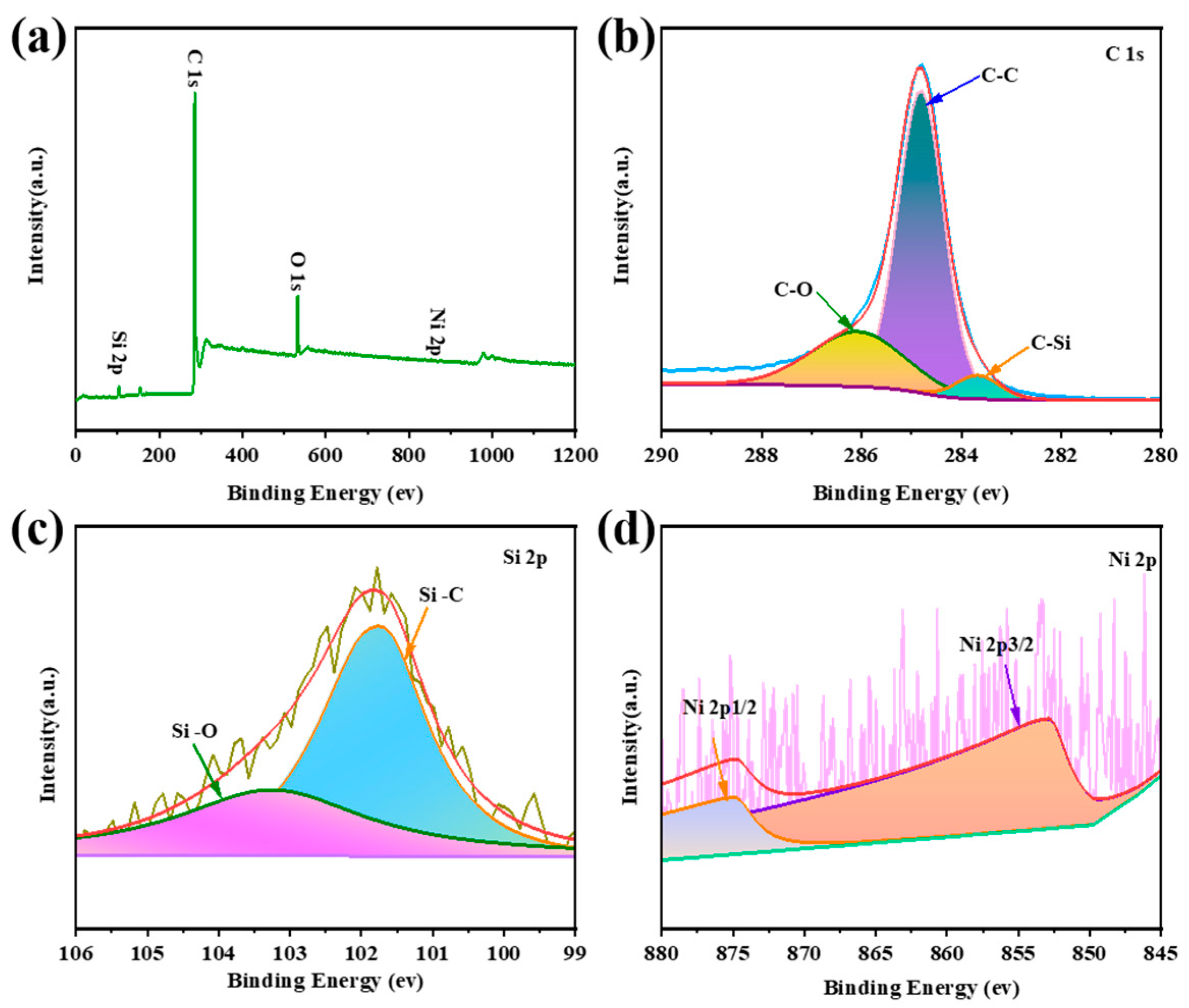
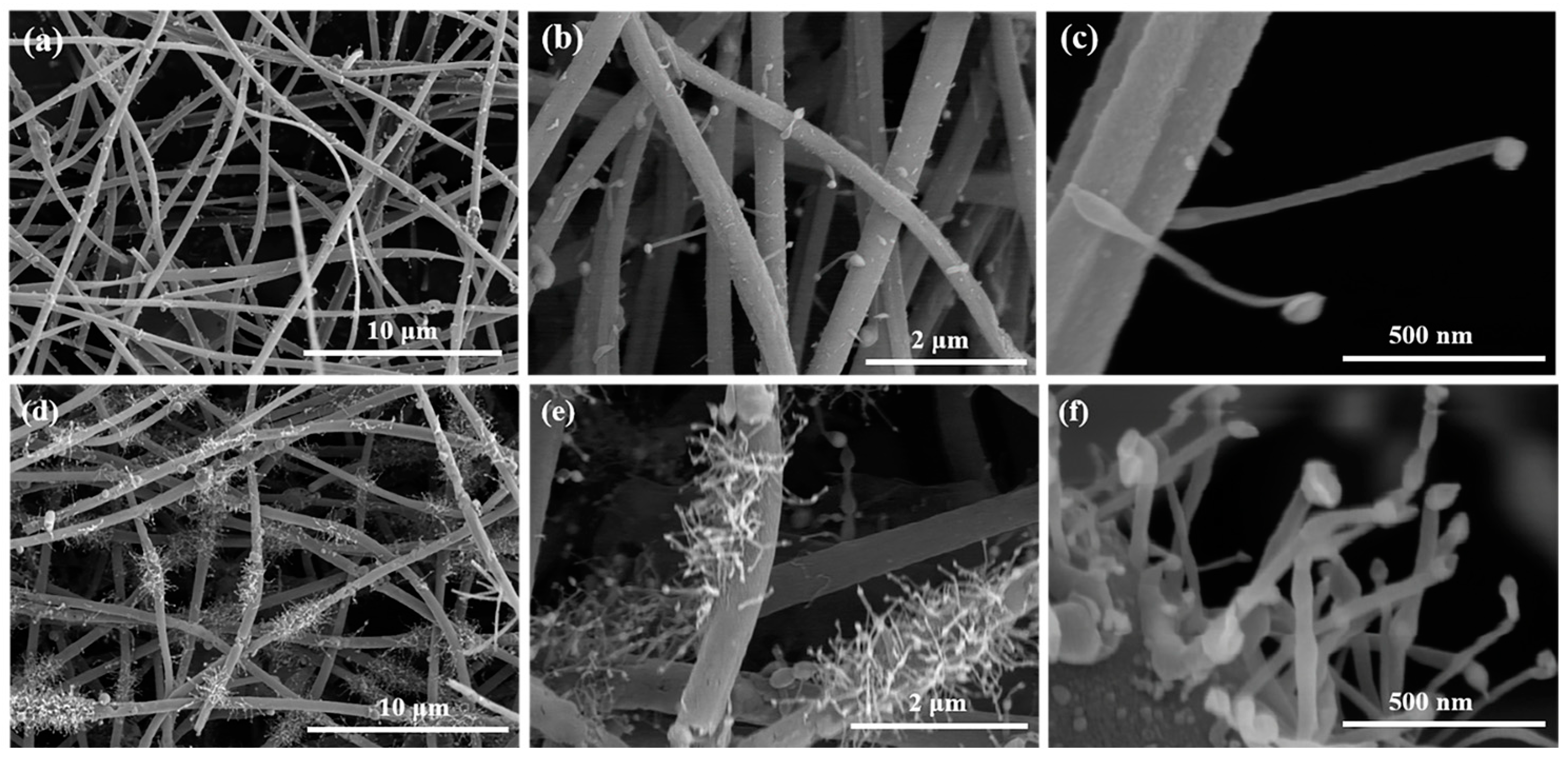
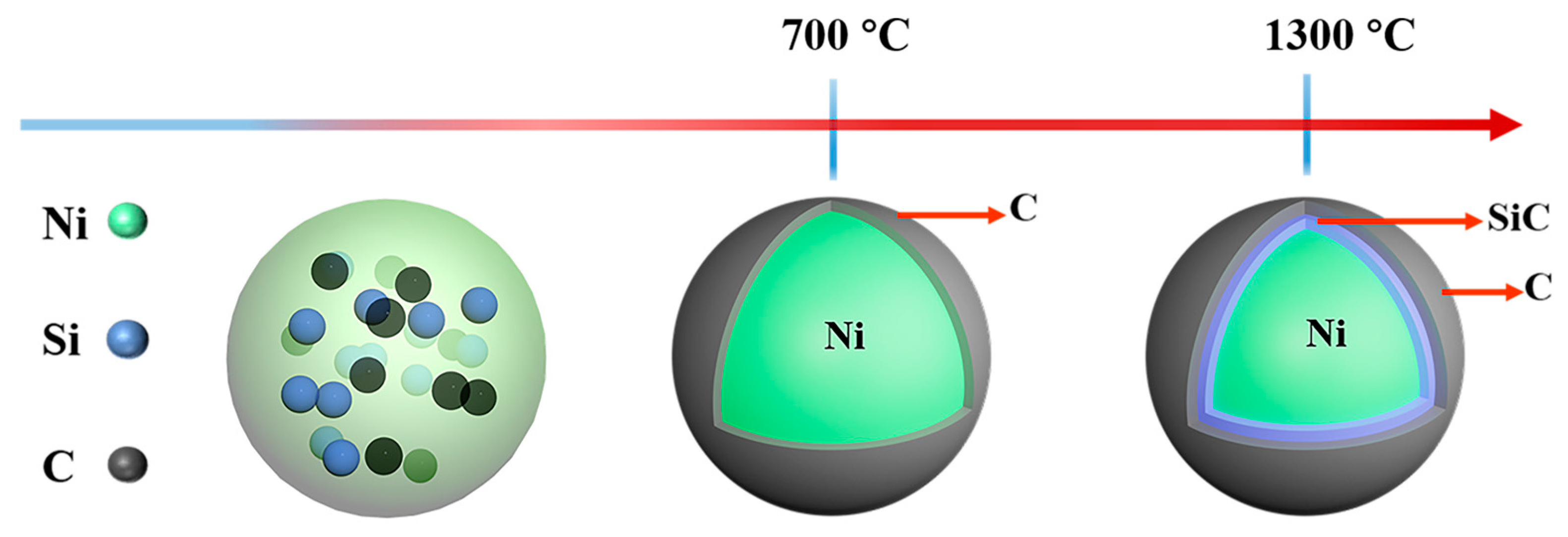
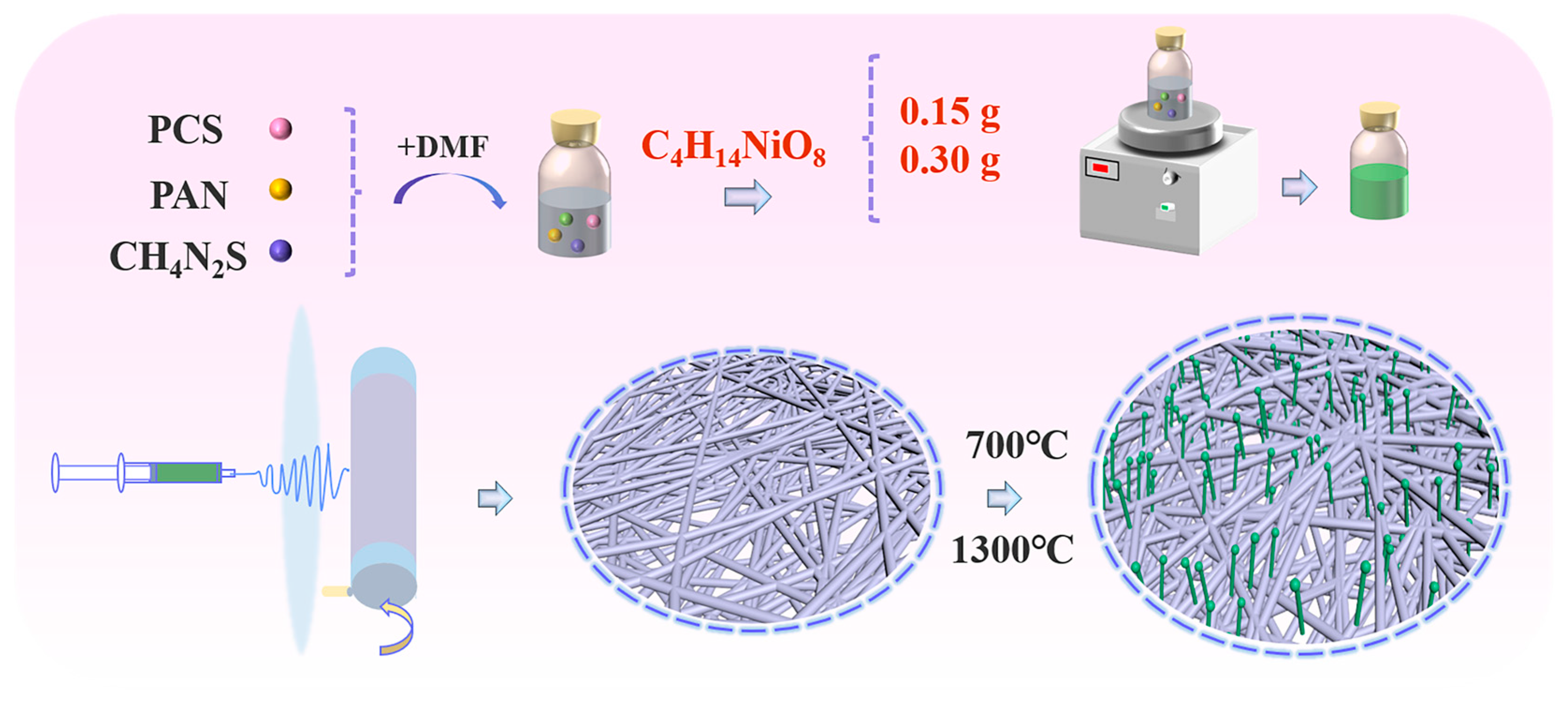
2.1. Microscopic Morphology and Structural Composition of Ni@SiC/CNT/CNF Composites
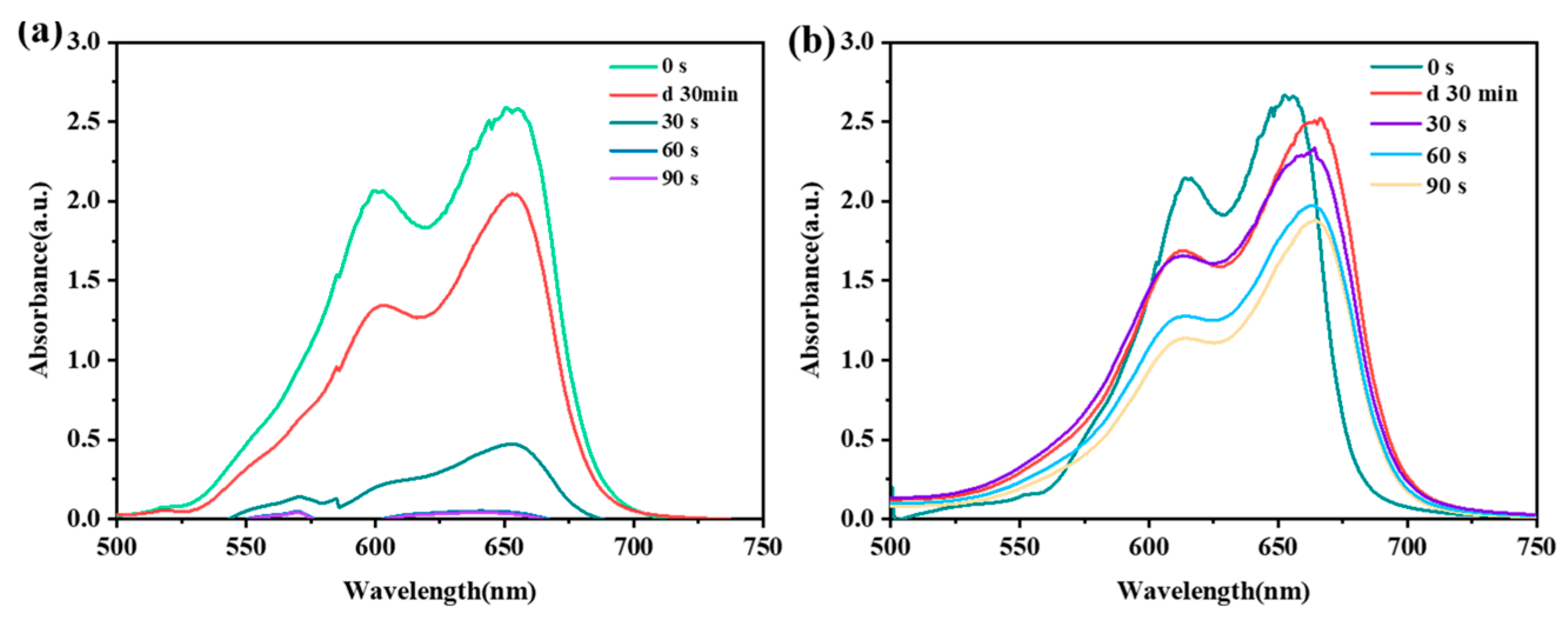
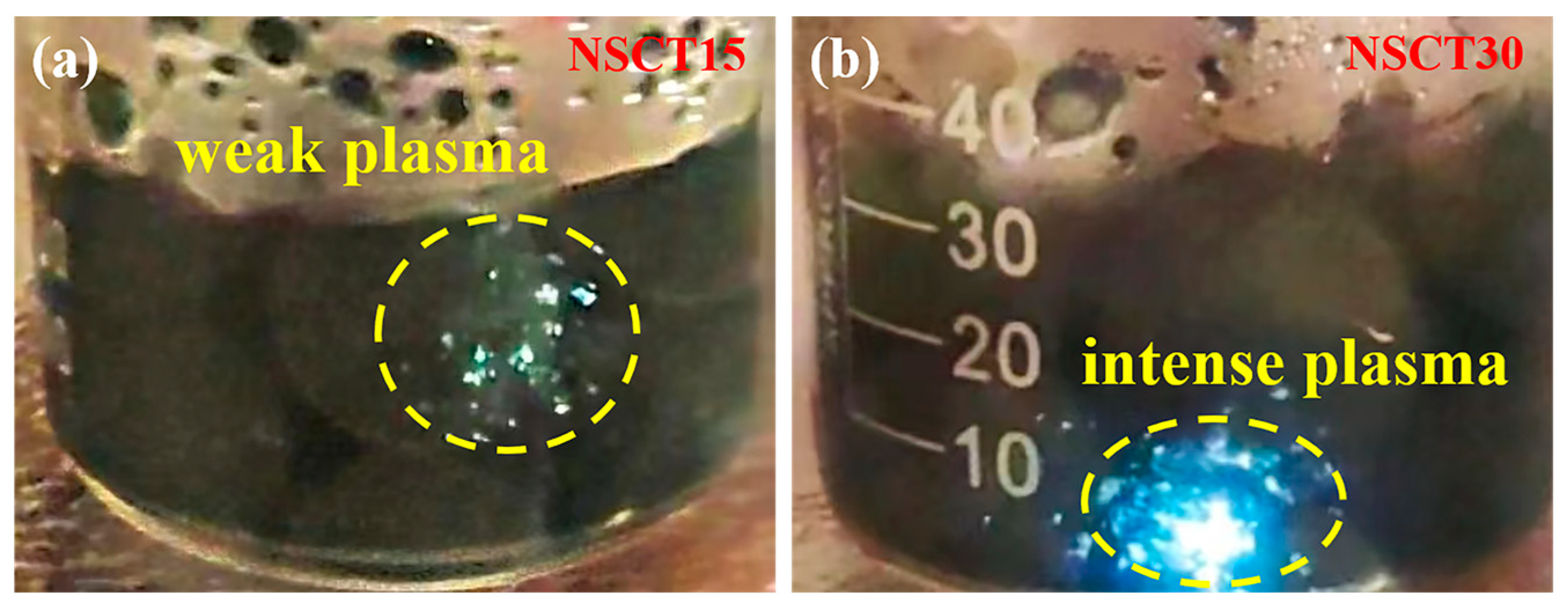
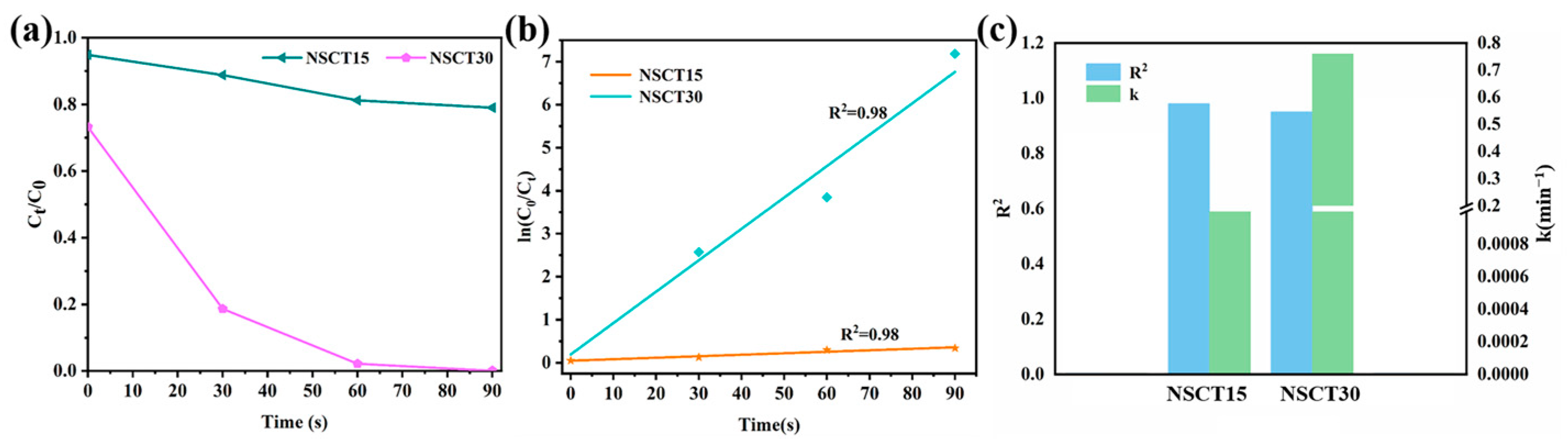
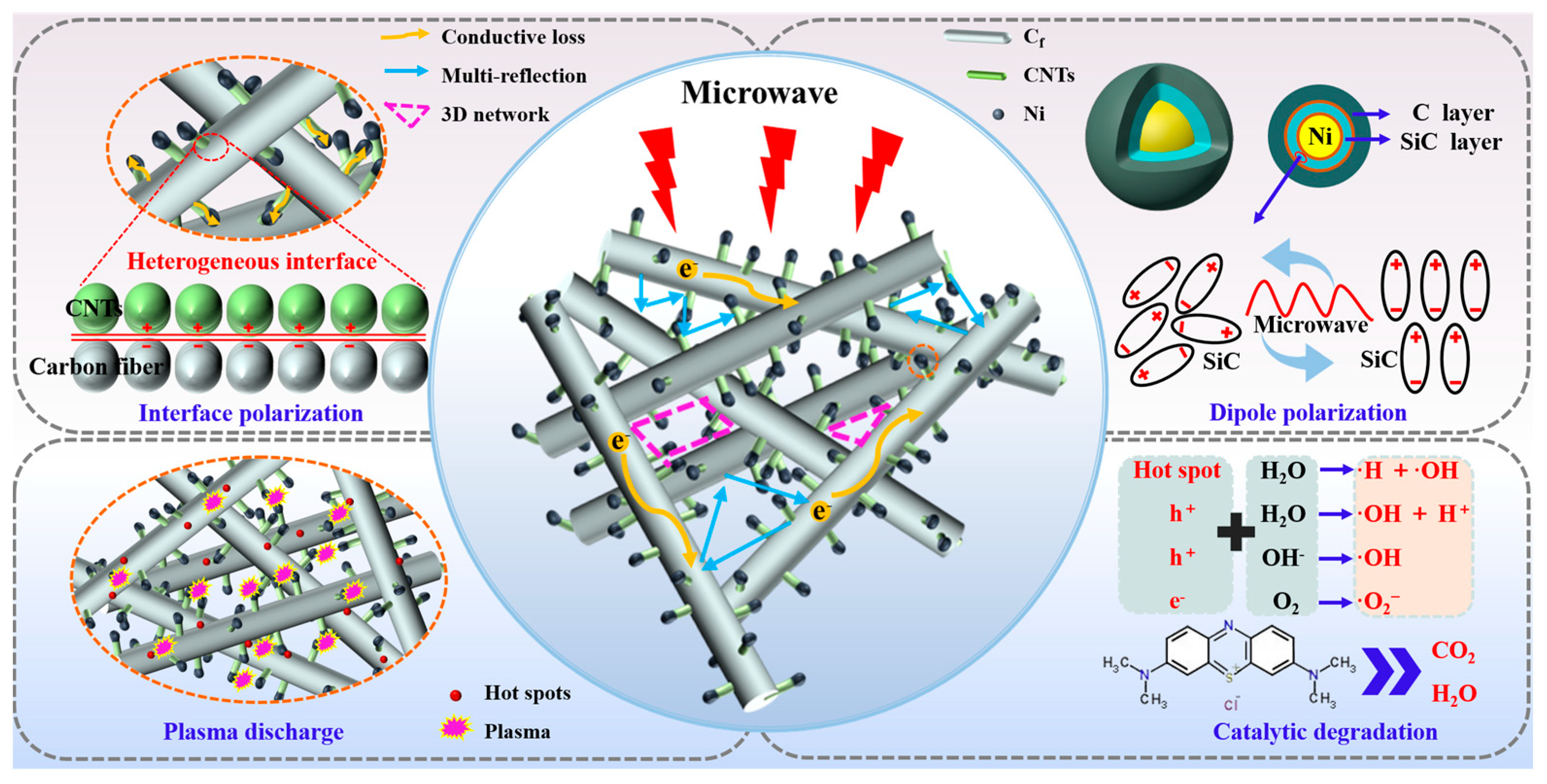
2.2. Degradation of Methylene Blue Wastewater Dye
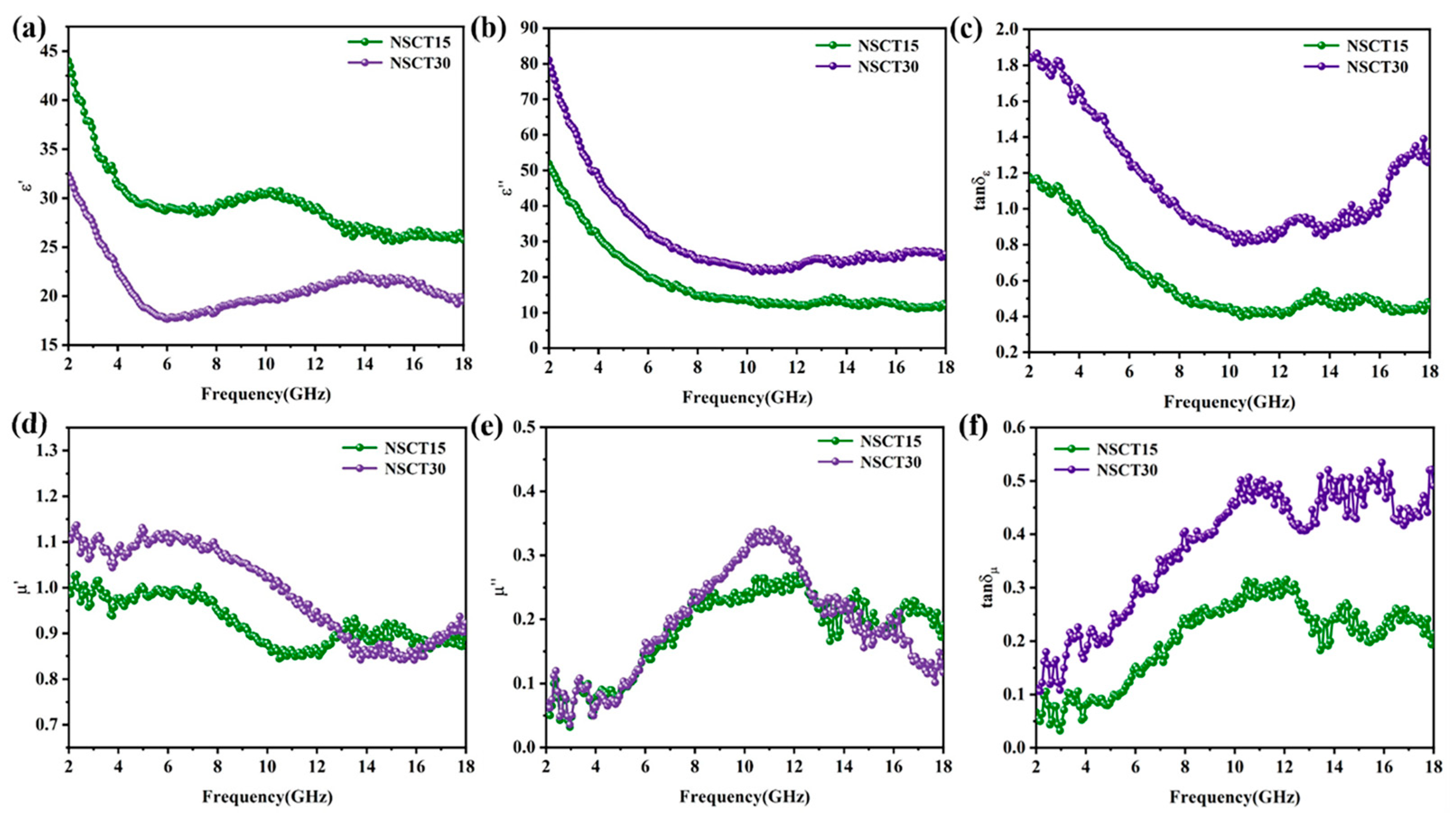
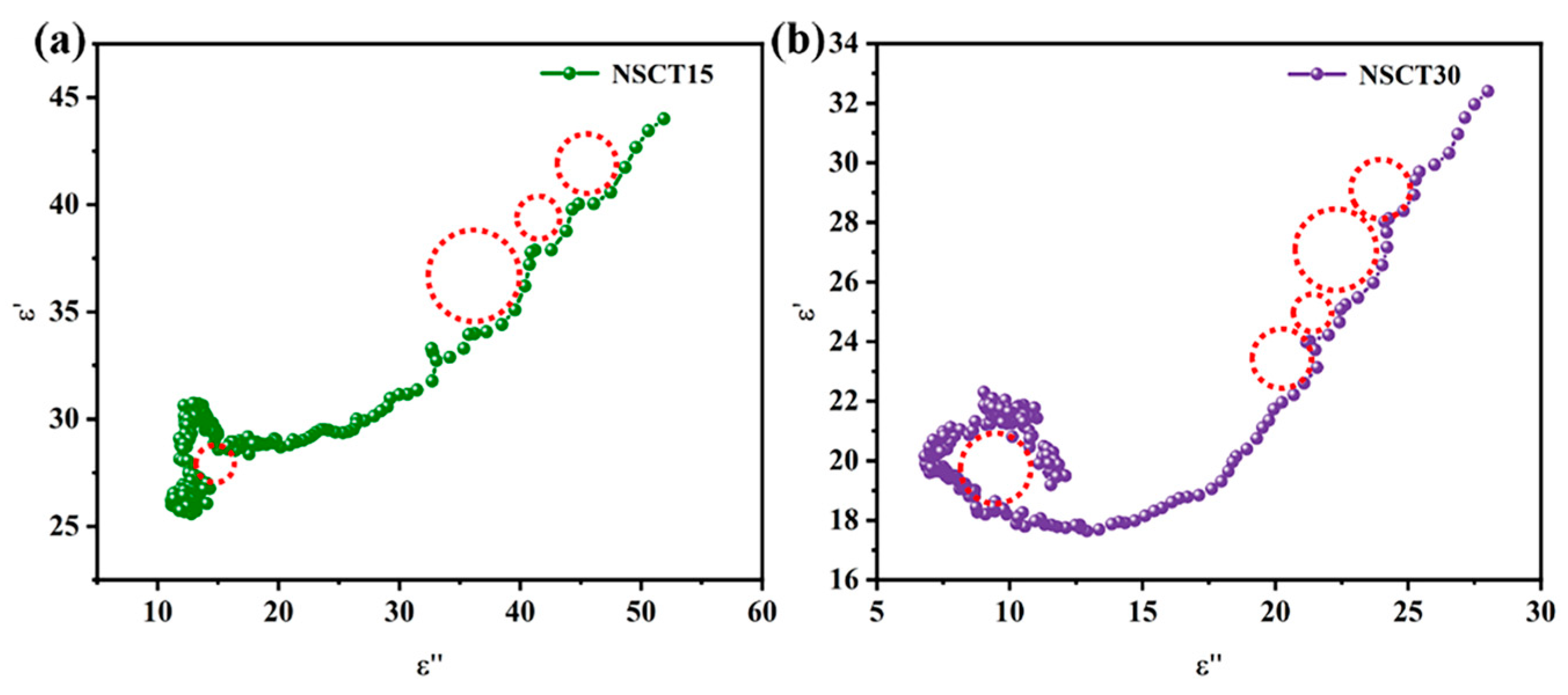
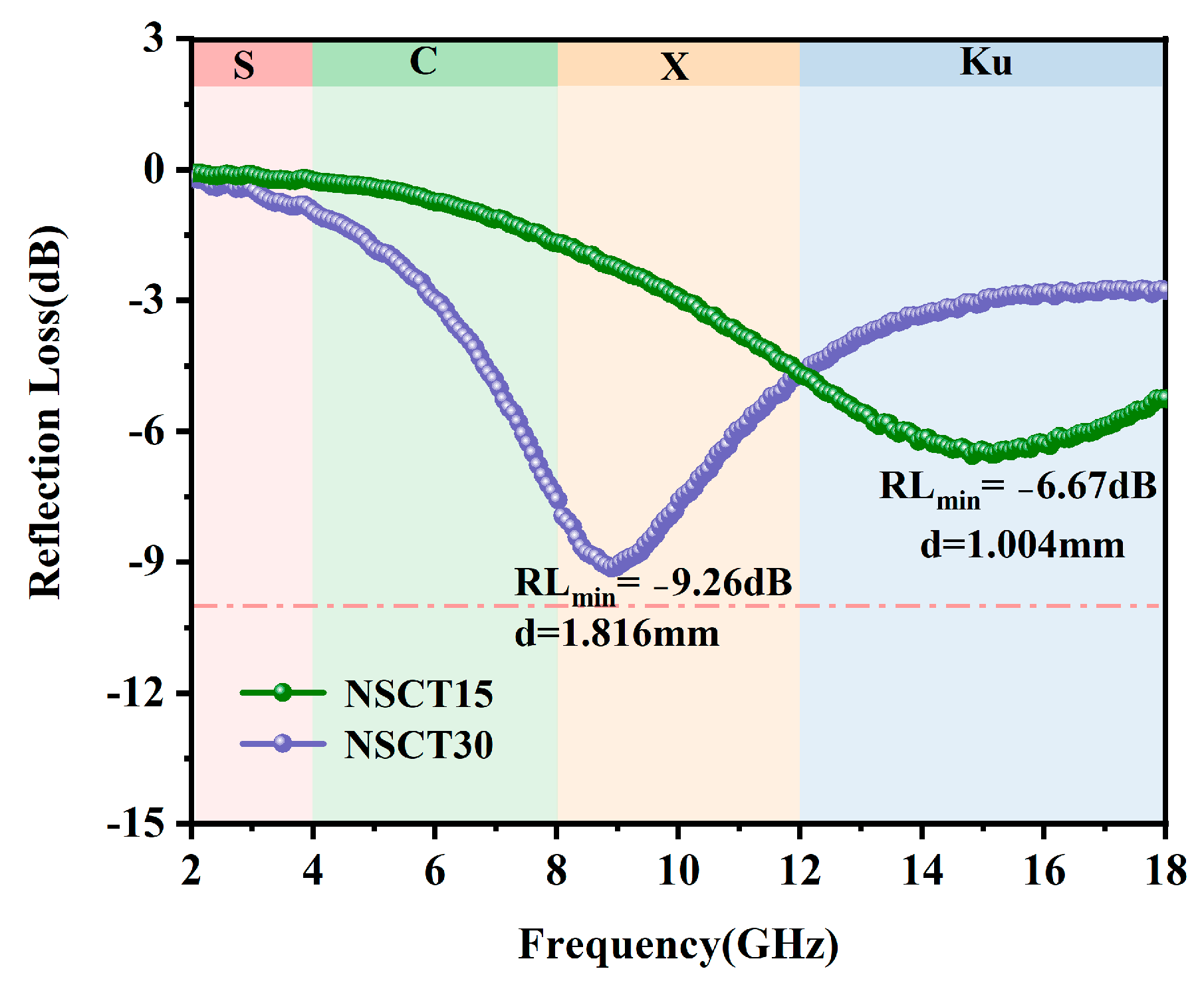
2.3. Electromagnetic Wave Absorption Properties of NSCT Composite Materials
3. Materials and Methods
3.1. Preparation of Ni@SiC/CNT/CNF Composites
3.2. Characterization
3.3. Microwave Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Issabayeva, G.; Hang, S.Y.; Wong, M.C.; Aroua, M.K. A review on the adsorption of phenols from wastewater onto diverse groups of adsorbents. Rev. Chem. Eng. 2018, 34, 855–873. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, M.; Wang, Y.; Ma, Q.; Yin, Q. Construction of a novel magnetic levitation iron–carbon micro-electrolysis treatment system for dye wastewater and its anti-passivation strategy. Environ. Sci. Water Res. Technol. 2023, 9, 2076–2088. [Google Scholar] [CrossRef]
- Liang, C.; Niu, H.-Y.; Guo, H.; Niu, C.-G.; Yang, Y.-Y.; Liu, H.-Y.; Tang, W.-W.; Feng, H.-P. Efficient photocatalytic nitrogen fixation to ammonia over bismuth monoxide quantum dots-modified defective ultrathin graphitic carbon nitride. Chem. Eng. J. 2021, 406, 126868. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Liu, L.; Hao, X.; Cai, K.; Xiong, J.; Hong, W.; Tao, J. New optimization approach for amphoteric/magnetic ramie biosorbent in dyestuff adsorption. Biochem. Eng. J. 2022, 181, 108379. [Google Scholar] [CrossRef]
- Han, Y.; Li, H.; Liu, M.; Sang, Y.; Liang, C.; Chen, J. Purification treatment of dyes wastewater with a novel micro-electrolysis reactor. Sep. Purif. Technol. 2016, 170, 241–247. [Google Scholar] [CrossRef]
- Meng, Q.-B.; He, Z.-W.; Yang, W.; Li, W.-T.; Tang, C.-C.; Zhou, A.-J.; Ren, Y.-X.; Liu, W.; Li, Z.; Wang, A. Roles and fates of antibiotics in anaerobic digestion of waste activated sludge: Insights to pro- and re-duction of antibiotic resistance genes. Chem. Eng. J. 2024, 500, 156633. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Zhou, J.; Ni, B. Anaerobic membrane bioreactors for antibiotic wastewater treatment: Performance and membrane fouling issues. Bioresour. Technol. 2018, 267, 714–724. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, Y.; Gao, Y.; Tian, Z.; Yang, M. Anaerobic treatment of antibiotic production wastewater pretreated with enhanced hydrolysis: Simultaneous reduction of COD and ARGs. Water Res. 2017, 110, 211–217. [Google Scholar] [CrossRef]
- Nabi, G.; Ali, M.; Khan, S.; Kumar, S. The crisis of water shortage and pollution in Pakistan: Risk to public health, biodiversity, and ecosystem. Environ. Sci. Pollut. Res. Int. 2019, 26, 10443–10445. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Pang, C.; Li, X.; Gao, X. The Advances in the Special Microwave Effects of the Heterogeneous Catalytic Reactions. Front. Chem. 2020, 8, 355. [Google Scholar] [CrossRef]
- Pérez-Martínez, B.T.; Aboudzadeh, M.A.; Schubert, U.S.; Leiza, J.R.; Tomovska, R. Microwave irradiation versus conventional heating assisted free-radical copolymerization in solution. Chem. Eng. J. 2020, 399, 125761. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, J.; Ou, Y.; Luo, Y.; You, Z. Microwave selective effect: A new approach towards oxygen inhibition removal for highly-effective NO decomposition by microwave catalysis over BaMn(x)Mg(1−x)O3 mixed oxides at low temperature under excess oxygen. Chem. Commun. (Camb.) 2015, 51, 4073–4076. [Google Scholar] [CrossRef]
- Strekalova, A.A.; Shesterkina, A.A.; Kustov, A.L.; Kustov, L.M. Recent Studies on the Application of Microwave-Assisted Method for the Preparation of Heterogeneous Catalysts and Catalytic Hydrogenation Processes. Int. J. Mol. Sci. 2023, 24, 8272. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, W.; Chen, J.; Gan, Z.; Wang, X.; Zhou, J. Development of core–shell structured Mo2C@BN as novel microwave catalysts for highly effective direct decomposition of H2S into H2 and S at low temperature. Catal. Sci. Technol. 2020, 10, 6769–6779. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zhu, J.; Wang, X.; Zhou, J. Highly effective direct decomposition of H2S by microwave catalysis on core-shell Mo2N-MoC@SiO2 microwave catalyst. Appl. Catal. B Environ. 2020, 268, 118454. [Google Scholar] [CrossRef]
- Xu, W.; Luo, M.; Peng, R.; Xiang, M.; Hu, X.; Lan, L.; Zhou, J. Highly effective microwave catalytic direct decomposition of H2S into H2 and S over MeS-based (Me = Ni, Co) microwave catalysts. Energy Convers. Manag. 2017, 149, 219–227. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Chen, J.; Zhou, J.; Xu, W. Highly effective direct conversion of H2S into COx-free H2 and S at low temperature over novel MoxC@ZrO2 microwave catalysts. Int. J. Hydrogen Energy 2024, 87, 515–525. [Google Scholar] [CrossRef]
- Hou, S.; Gao, Y.; Song, P.; Zhao, J.; Gong, B.; Zhu, X.; Liu, Q. Synthesis of Pd-modified Fe3O4 loaded on montmorillonite catalyst for photocatalytic degradation of tetracycline. Inorg. Chem. Commun. 2024, 159, 111745. [Google Scholar] [CrossRef]
- Lv, G.; Xing, X.; Liao, L.; An, P.; Yin, H.; Mei, L.; Li, Z. Synthesis of birnessite with adjustable electron spin magnetic moments for the degradation of tetracycline under microwave induction. Chem. Eng. J. 2017, 326, 329–338. [Google Scholar] [CrossRef]
- Cai, B.; Feng, J.F.; Peng, Q.Y.; Zhao, H.F.; Miao, Y.C.; Pan, H. Super-fast degradation of high concentration methyl orange over bifunctional catalyst Fe/Fe(3)C@C with microwave irradiation. J. Hazard. Mater. 2020, 392, 122279. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, M.A.; Lee, H.H.; Chae, W.-S.; Cho, M.; Jang, J.S. Energy-efficient photoelectrochemical water splitting and degradation of organic dyes over microwave-assisted WO3 nanosheets/W foil with rapid charge transport. Sol. Energy Mater. Sol. Cells 2022, 246, 111939. [Google Scholar] [CrossRef]
- Asati, H.; Mondal, R.; Tripathi, K.M. Ultra-fast microwave catalytic degradation of multiple dyes by waste derived carbon nano onions. Mater. Today Sustain. 2024, 26, 100724. [Google Scholar] [CrossRef]
- Vieira, Y.; Ceretta, M.B.; Foletto, E.L.; Wolski, E.A.; Silvestri, S. Application of a novel rGO-CuFeS2 composite catalyst conjugated to microwave irradiation for ultra-fast real textile wastewater treatment. J. Water Process Eng. 2020, 36, 101397. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Tian, L.; Lv, G.; Wu, L.; Bian, L.; Liu, M.; Liao, L. Role of holes in microwave-induced catalyst Co3O4 for efficient high-concentration tetracycline degradation. Appl. Surf. Sci. 2023, 621, 156801. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, L.; Ye, F.; Li, Z.; Xu, Z. Synthesis and characterization of carbon-poor SiC nanowires via vapor-liquid-solid growth mechanism. Ceram. Int. 2019, 45, 6440–6446. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Zhu, G.; Wang, Z.; Lian, G.; Xiong, X.; You, W.; Che, R. Hollow porous FeCo/Cu/CNTs composite microspheres with excellent microwave absorption performance. Nano Res. 2024, 17, 9857–9864. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yue, K.; Li, A.; Qian, L. Multi-scale structural nitrogen-doped rGO@CNTs composites with ultra-low loading towards microwave absorption. Appl. Surf. Sci. 2021, 538, 147943. [Google Scholar] [CrossRef]
- Wang, Y.; Man, Q.; Zhu, S.; Lei, Z.; Tan, G.; Guan, H. Fe/Co decorated C@CNTs derived from bimetallic metal-organic-framework for microwave absorption with wide bandwidth. J. Alloys Compd. 2023, 967, 171645. [Google Scholar] [CrossRef]
- Ouyang, H.; Liu, J.; Li, C.; Bao, L.; Shen, T.; Li, Y. Synthesis of Ni@SiC/CNFs Composite and Its Microwave-Induced Catalytic Activity. C 2024, 10, 72. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, K.; Hao, N.; Wang, J.; Liu, X.; Xu, L.; Li, Y. Study on microwave absorption performance of nickel cobalt bimetallic nanosheet arrays @ hydrophilic carbon cloth composite with core-sheath structure. J. Alloys Compd. 2024, 970, 172594. [Google Scholar] [CrossRef]
- Bi, S.; Song, Y.-Z.; Hou, G.-L.; Li, H.; Liu, Z.-H.; Hou, Z.-L.; Zhang, J. Sandwich nanoarchitectonics of heterogenous CB/CNTs honeycomb composite for impedance matching design and microwave absorption. J. Alloys Compd. 2023, 943, 169154. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Xie, N.; Ye, L.; Chen, J.; Chen, J. Electromagnetic and absorbing properties of the composites based on iron, cobalt, B and rare earth Nd. J. Mater. Sci. Mater. Electron. 2018, 30, 401–405. [Google Scholar] [CrossRef]
- Liang, X.; Wang, C.; Yao, Z.; Zhang, Y.; Liu, S.; Liu, J.; Yu, M. A facile synthesis of Fe/C composite derived from Fe-metal organic frameworks: Electromagnetic wave absorption with thin thickness. J. Alloys Compd. 2022, 922, 166299. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Lu, H.; Guo, W.; He, X.; Yuan, Y. Heterogeneous rod-like Ni@C composites toward strong and stable microwave absorption performance. Carbon 2021, 181, 358–369. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.; Sun, J.; Ma, X.; Song, Z.; Zhao, X.; Mao, Y. Direct calorimetry study of metal discharge heating effects induced by microwave irradiation. Appl. Therm. Eng. 2017, 125, 386–393. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, P.; Ding, Q.; Li, Y.; Yue, L.; Zhang, H.; Wang, W. Magnetic plasmons in plasmonic nanostructures: An overview. J. Appl. Phys. 2023, 133, 030902. [Google Scholar] [CrossRef]
- Kim, J.; Son, S.; Choe, M.; Lee, Z. In situ TEM investigation of nickel catalytic graphitization. Mater. Today Adv. 2024, 22, 100494. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.; Jia, Z.; Zhang, X.; Liu, X.; Wang, K.; Xu, B.; Wu, G. Dielectric behavior of Fe(3)N@C composites with green synthesis and their remarkable electromagnetic wave absorption performance. J. Colloid Interface Sci. 2021, 582, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, S.; Tong, H.; Liu, Y.; Wu, A.; Hao, H. The capacitive loss of microwave energy in Ni@SiC@C core/bi-shell nanoparticles. Chem. Eng. J. 2022, 434, 134655. [Google Scholar] [CrossRef]
- Chang, C.-B.; Tsai, C.-Y.; Chen, K.-T.; Tuan, H.-Y. Solution-Grown Phosphorus-Hyperdoped Silicon Nanowires/Carbon Nanotube Bilayer Fabric as a High-Performance Lithium-Ion Battery Anode. ACS Appl. Energy Mater. 2021, 4, 3160–3168. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Choi, W.C.; Chae, S.; Lee, J.; Kim, B.-S.; Park, M.; Kim, H.Y. Highly flexible, erosion resistant and nitrogen doped hollow SiC fibrous mats for high temperature thermal insulators. J. Mater. Chem. A 2017, 5, 2664–2672. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lei, Y.; Wu, N.; Gou, Y.; Han, C.; Fang, D. Hierarchically porous SiC ultrathin fibers mat with enhanced mass transport, amphipathic property and high-temperature erosion resistance. J. Mater. Chem. A 2014, 2, 20873–20881. [Google Scholar] [CrossRef]
- Huo, Y.; Tan, Y.; Zhao, K.; Lu, Z.; Zhong, L.; Tang, Y. Enhanced electromagnetic wave absorption properties of Ni magnetic coating-functionalized SiC/C nanofibers synthesized by electrospinning and magnetron sputtering technology. Chem. Phys. Lett. 2021, 763, 138230. [Google Scholar] [CrossRef]
- Li, D.; Liao, H.; Kikuchi, H.; Liu, T. Microporous Co@C Nanoparticles Prepared by Dealloying CoAl@C Precursors: Achieving Strong Wideband Microwave Absorption via Controlling Carbon Shell Thickness. ACS Appl. Mater. Interfaces 2017, 9, 44704–44714. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fan, S.; Li, X.; Qu, X.; Tian, Y.; Zhang, X.; Zhang, Z.; Dong, X.; Cao, T. Enhanced dielectric and conductivity properties of carbon-coated SiC nanocomposites in the terahertz frequency range. Nanotechnology 2021, 32, 265705. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, P.; Deng, D.; Yu, L.; Yang, F.; Bao, X. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar] [CrossRef]
- Cui, X.; Ren, P.; Deng, D.; Deng, J.; Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 2016, 9, 123–129. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Gu, W.; Zhou, M.; Tang, S.; Cao, J.; Zou, Z.; Ji, G. Research progress on nanostructure design and composition regulation of carbon spheres for the microwave absorption. Carbon 2022, 189, 617–633. [Google Scholar] [CrossRef]
- Cai, Z.; Su, L.; Wang, H.; Xie, Q.; Gao, H.; Niu, M.; Lu, D. Hierarchically assembled carbon microtube@SiC nanowire/Ni nanoparticle aerogel for highly efficient electromagnetic wave absorption and multifunction. Carbon 2022, 191, 227–235. [Google Scholar] [CrossRef]
- Quan, B.; Gu, W.; Sheng, J.; Lv, X.; Mao, Y.; Liu, L.; Huang, X.; Tian, Z.; Ji, G. From intrinsic dielectric loss to geometry patterns: Dual-principles strategy for ultrabroad band microwave absorption. Nano Res. 2020, 14, 1495–1501. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Hu, H.; Xiu, Z.; Huang, X.; Yue, J.; Wang, Y. Enhancement of the microwave absorption properties of PyC-SiCf/SiC composites by electrophoretic deposition of SiC nanowires on SiC fibers. Ceram. Int. 2020, 46, 9303–9310. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Z.; Guo, D.; Shi, B.; Zhou, N.; Xu, B. Low-density, large-sized SiCnw/SiOC composite aerogels with excellent thermal insulation, microwave absorption, and thermostability. Ceram. Int. 2024, 50, 33315–33324. [Google Scholar] [CrossRef]
- Yin, S.; Jiang, Y.; Su, K.; Fang, X.; Wang, Y.; Li, Q.; Yang, J. Preparation, mechanical, dielectric and microwave absorption properties of hierarchical porous SiCnw-Si3N4 composite ceramics. J. Eur. Ceram. Soc. 2022, 42, 3820–3830. [Google Scholar] [CrossRef]
- Ouyang, H.; You, X.; Yang, Y.; Ren, M.; Zhang, Q.; Deng, R.; Zhang, X.; Yang, J.; Dong, S. Hierarchical porous SiCnws/SiC composites with one-dimensional oriented assemblies for high-temperature broadband wave absorption. J. Mater. Sci. Technol. 2025, 214, 1–10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, Y.; Wu, H.; Zhang, D.; Liu, J.; Ouyang, H. A Unique Dual-Shell Structure with Highly Active Ni@SiC/CNT/CNF Microwave Catalysts. Catalysts 2025, 15, 132. https://doi.org/10.3390/catal15020132
Liu X, Zhang Y, Wu H, Zhang D, Liu J, Ouyang H. A Unique Dual-Shell Structure with Highly Active Ni@SiC/CNT/CNF Microwave Catalysts. Catalysts. 2025; 15(2):132. https://doi.org/10.3390/catal15020132
Chicago/Turabian StyleLiu, Xizong, Yulei Zhang, Heng Wu, Dongsheng Zhang, Jiaqi Liu, and Haibo Ouyang. 2025. "A Unique Dual-Shell Structure with Highly Active Ni@SiC/CNT/CNF Microwave Catalysts" Catalysts 15, no. 2: 132. https://doi.org/10.3390/catal15020132
APA StyleLiu, X., Zhang, Y., Wu, H., Zhang, D., Liu, J., & Ouyang, H. (2025). A Unique Dual-Shell Structure with Highly Active Ni@SiC/CNT/CNF Microwave Catalysts. Catalysts, 15(2), 132. https://doi.org/10.3390/catal15020132






