Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Property and Morphology
2.2. Pore Structure Property
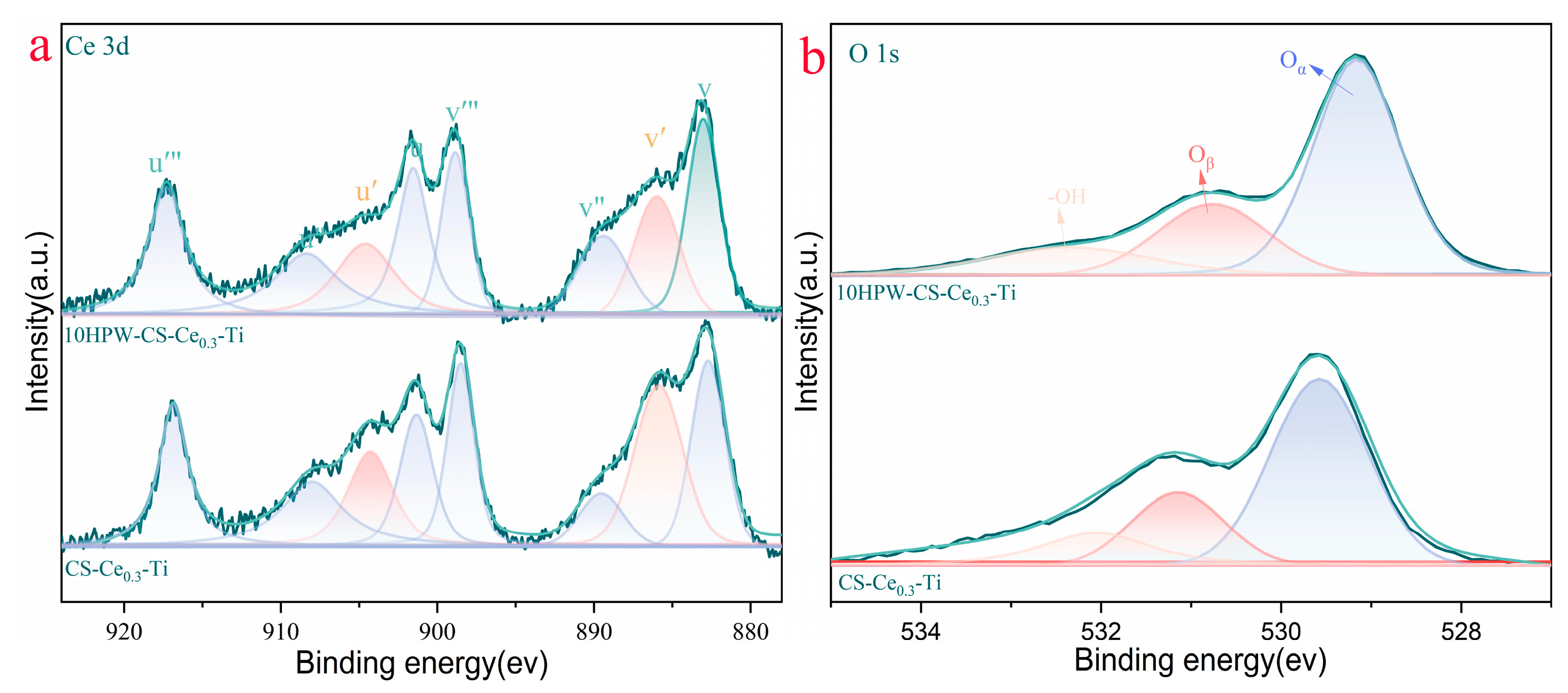
2.3. Surface Chemistry Analysis
2.4. Redox and Acid Sites Properties Analysis
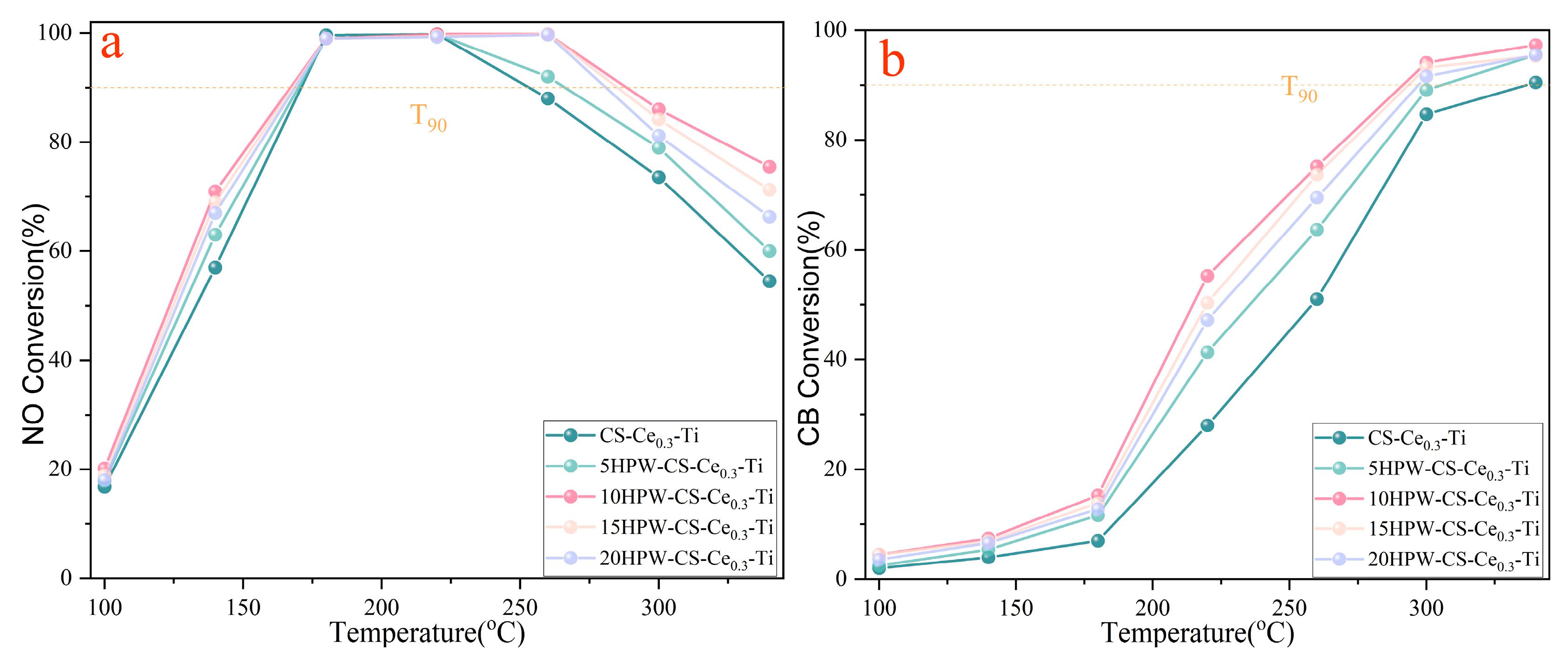
2.5. Catalyst Performance
2.6. Promotional Effects of Phosphotungstic Acid and Chitosan
3. Experimental Procedure
3.1. Catalyst Preparation
3.2. Characterization of Catalyst
3.3. Catalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gan, L.; Li, K.; Xiong, S.; Zhang, Y.; Chen, J.; Peng, Y.; Li, J. MnOx-CeO2 catalysts for effective NOx reduction in the presence of chlorobenzene. Catal. Commun. 2018, 117, 1–4. [Google Scholar] [CrossRef]
- Chu, P.; Zhang, L.; Wang, Z.; Wei, L.; Liu, Y.; Dai, H.; Duan, E.; Deng, J. Synergistic catalytic elimination of NOx and VOCs: State of the art and open challenges. Surf. Interfaces 2024, 51, 104718. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, J.; Zhang, R.; Ma, M.; Li, X.; Gong, P. Recent advances in different catalysts for synergistic removal of NOx and VOCs: A minor review. Environ. Chem. Eng. 2023, 12, 111764. [Google Scholar] [CrossRef]
- Hussain, B.; Chen, J.S.; Huang, S.W.; Tsai, I.S.; Rathod, J.; Hsu, B.M. Underpinning the ecological response of mixed chlorinated volatile organic compounds (CVOCs) associated with contaminated and bioremediated groundwaters: A potential nexus of microbial community structure and function for strategizing efficient bioremediation. Environ. Pollut. 2023, 334, 122215. [Google Scholar]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel. 2023, 331, 125885. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.; Hu, H.; Yang, H.; He, F.; Fu, Z. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Jia, Y.; Wang, Z.; Jiang, M.; Shen, K.; Zhao, H.; Guo, Y.; Guo, Y.; Wang, L.; et al. Significant improvement of catalytic performance for chlorinated volatile organic compound oxidation over RuOx supported on acid-etched Co3O4. Environ. Sci. Technol. 2021, 55, 10734–10743. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Gong, B.; Shao, S.; Tu, C.; Pan, J.; Wang, Y.; Dai, Q.; Guo, Y.; Wang, X. Catalytic combustion of CVOCs over MoOx/CeO2 catalysts. Appl. Catal. B-Environ. 2022, 310, 121240. [Google Scholar] [CrossRef]
- Xie, B.; Wang, Z.; Zhang, X.; Ding, M.; Li, M.; Guo, X.; Dai, Q.; Wang, L.; Zhan, W.; Guo, Y.; et al. Morphology effect of cerium dioxide on the catalytic performance of Ru/CeO2 catalyst for the oxidation of different CVOCs. Sep. Purif. Technol. 2024, 345, 127428. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayu, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakrishna, S. Catalytic oxidation of chlorinated volatile organic compounds (CVOCs) over CeO2-based catalysts: A review. Appl. Catal. B Environ. 2019, 253, 117855. [Google Scholar] [CrossRef]
- Kang, D.; Bian, Y.; Shi, Q.; Wang, J.; Yuan, P.; Shen, B. A review of synergistic catalytic removal of nitrogen oxides and chlorobenzene from waste incinerators. Catalysts 2022, 12, 1360. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, Y.; Bi, F.; Sun, P.; Weng, X.; Wu, Z. Synergistic elimination of NOx and chloroaromatics on a commercial V2O5-WO3/TiO2 catalyst: Byproduct analyses and the SO2 effect. Environ. Sci. Technol. 2019, 53, 12657–12667. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Peng, Y.; Zhao, X.; Liu, H.; Gao, C.; Si, W.; Li, J. Roles of Ru on the V2O5–WO3/TiO2 Catalyst for the Simultaneous Purification of NOx and Chlorobenzene: A Dechlorination Promoter and a Redox Inductor. ACS Catal. 2022, 12, 11505–11517. [Google Scholar] [CrossRef]
- Lin, B.; Guo, Z.; Li, J.; Xiao, G.; Ye, D.; Hu, Y. V-Cu bimetallic oxide supported catalysts for synergistic removal of toluene and NOx from coal-fired flue gas: The crucial role of support. Chem. Eng. J. 2023, 458, 141443. [Google Scholar] [CrossRef]
- Yin, R.; Chen, J.; Mi, J.; Liu, H.; Yan, T.; Shan, L.; Lang, J.; Li, J. Breaking the activity–selectivity trade-off for simultaneous catalytic elimination of nitric oxide and chlorobenzene via FeVO4–Fe2O3 interfacial charge transfer. ACS Catal. 2022, 12, 3797–3806. [Google Scholar] [CrossRef]
- Kang, D.; Shi, Q.; Zhang, C.; Zhao, P.; Lyu, H.; Yang, M.; Bian, Y.; Shen, B. Synergistic removal of NOx and CB by Co-MnOx catalysts in a low-temperature window. Chem. Eng. J. 2023, 476, 146369. [Google Scholar] [CrossRef]
- Gong, P.; Cao, R.; Yu, Y.; Zhang, J. Promotion effect of SO42−/Fe2O3 modified MnOx catalysts for simultaneous control of NO and CVOCs. Surf. Interfaces 2022, 33, 102253. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Wang, W.; Lan, K.; Zhao, D. Ordered mesoporous crystalline frameworks toward promising energy applications. Adv. Mater. 2024, 36, 2311460. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous molecular sieve-based materials for catalytic oxidation of VOC: A review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X.J. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(χ)-CeO2 at low temperatures. Appl. Catal. B-Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.F.; Hamoudi, S.; Belkacemi, K. Synthesis of gold catalysts supported on mesoporous silica materials: Recent developments. Catalysts 2011, 1, 97–154. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tadé, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B-Environ. 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Gu, Q.; Lei, Y.; Huang, Q.; Chen, M. A superior Mn5CeTi5Ox catalysts for synergistic catalytic removal of chlorobenzene and NOx: Performance enhancement and mechanism studies. Mol. Catal. 2024, 569, 114634. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Z.; Ye, C.; Qi, Z.; Chen, J.; Huang, Z.; Qiu, T. Encapsulation of HPW and preparation of composites rich in Zr-defects by manual grinding: Synergistic catalysis for efficient oxidative desulfurization at room temperature. Chem. Eng. J. 2023, 451, 138906. [Google Scholar] [CrossRef]
- Lan, T.; Yalavarthi, R.; Shen, Y.; Gao, M.; Wang, F.; Hu, Q.; Hu, P.; Beladi-Mousavi, M.; Chen, X.; Hu, X.; et al. Polyoxometalates-Mediated Selectivity in Pt Single-Atoms on Ceria for Environmental Catalysis. Angew. Chem. Int. Edit. 2024, 64, e202415786. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Zhang, Q.; Wang, H.; Zhang, X.; Chen, L.; Ma, L.; Liu, J. The efficient promoting hydrodeoxygenation of bioderived furans over Pd/HPW-SiO2 by phosphotungstic acid. Fuel Process. Technol. 2024, 258, 108095. [Google Scholar] [CrossRef]
- Wu, J.; Jin, S.; Wei, X.; Gu, F.; Han, Q.; Lan, Y.; Qian, C.; Li, J.; Wang, X.; Zhang, R.; et al. Enhanced sulfur resistance of H3PW12O40-modified Fe2O3 catalyst for NH3-SCR: Synergistic effect of surface acidity and oxidation ability. Chem. Eng. J. 2021, 412, 128712. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Jiang, M.; Chen, J.; Jia, H. Polyoxometallate functionalizing CeO2 via redox-etching precipitation to synergistically catalyze oxidation of gaseous chlorinated pollutants: From lab to practice. Appl. Catal. B-Environ. 2020, 278, 119263. [Google Scholar] [CrossRef]
- Xia, C.; Jin, X.; Parandoust, A.; Sheibani, R.; Khorsandi, Z.; Montazeri, N.; Wu, Y.; Van Le, Q. Chitosan-supported metal nanocatalysts for the reduction of nitroaromatics. Int. J. Biol. Macromol. 2023, 239, 124135. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of chitosan aerogels: Three-dimensional pore control for tailored applications. Angew. Chem. Int. Edit. 2021, 60, 9828–9851. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Nezafat, Z.; Pakzad, K.; Ahmadpoor, F. Synthesis of magnetic chitosan supported metformin-Cu (II) complex as a recyclable catalyst for N-arylation of primary sulfonamides. J. Organomet. Chem. 2021, 948, 121915. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.; Zhang, G. A general strategy for constructing transition metal Oxide/CeO2 heterostructure with oxygen vacancies toward hydrogen evolution reaction and oxygen evolution reaction. Power Sources 2021, 512, 230514. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, K.; Yu, Z.; Su, Y.; Han, R.; Liu, Q. Oxygen vacancies in a catalyst for VOCs oxidation: Synthesis, characterization, and catalytic effects. J. Mater. Chem. A 2022, 10, 14171–14186. [Google Scholar] [CrossRef]
- Liu, H.; Fu, H.; Liu, Y.; Chen, X.; Yu, K.; Wang, L. Synthesis, characterization and utilization of oxygen vacancy contained metal oxide semiconductors for energy and environmental catalysis. Chemosphere 2021, 272, 129534. [Google Scholar] [CrossRef]
- Bratan, V.; Vasile, A.; Chesler, P.; Hornoiu, C. Insights into the Redox and Structural Properties of CoOx and MnOx: Fundamental Factors Affecting the Catalytic Performance in the Oxidation Process of VOCs. Catalysts 2022, 12, 1134. [Google Scholar] [CrossRef]
- Gan, L.; Ye, P.; Tian, X.; Mi, J.; Xing, J.; Xue, Q.; Wu, Q.; Chen, J.; Li, J. Simultaneous removal of NOx and VOCs by Si doping CeO2 based catalysts: An acidity and redox properties balance strategy. Fuel 2024, 366, 131396. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Liu, X.; He, F.; Li, F. Novel heterogeneous denitrification catalyst over a wide temperature range: Synergy between CeO2, ZrO2 and TiO2. Chem. Eng. J. 2019, 356, 598–608. [Google Scholar] [CrossRef]
- Kim, S.; Sa, Y.J. Ce4+/Ce3+ Redox-Promoted Electron Transfer for Efficient Neutral H2O2 Electrosynthesis from Two-Electron Oxygen Reduction. ACS Catal. 2024, 14, 6842–6855. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Han, D.; He, F.; Li, F.X.; Qi, K. Effects of surface physicochemical properties on NH3-SCR activity of MnO2 catalysts with different crystal structures. Chin. J. Catal. 2017, 38, 1925–1934. [Google Scholar] [CrossRef]
- Geng, Y.; Xiong, S.; Li, B.; Liao, Y.; Xiao, X.; Yang, S. H3PW12O40 grafted on CeO2: A high-performance catalyst for the selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 856–866. [Google Scholar] [CrossRef]
- Su, Y.; Fu, K.; Pang, C.; Zheng, Y.; Song, C.; Ji, N.; Ma, D.; Lu, X.; Liu, C.; Han, R.; et al. Recent advances of chlorinated volatile organic compounds’ oxidation catalyzed by multiple catalysts: Reasonable adjustment of acidity and redox properties. Environ. Sci. Technol. 2022, 56, 9854–9871. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Dong, F.; Yao, J.; Tang, Z.; Zhang, J. Pt nanoparticles confined in the ordered mesoporous CeO2 as a highly efficient catalyst for the elimination of VOCs. J. Catal. 2022, 412, 42–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Wang, M.; Li, Y.; Tong, Y.; Yang, P.; Zhu, Y. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B-Environ. 2021, 282, 119542. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, R.; Wu, J.; Tang, Z. Exploring the Influence of CeTiOx Morphology on Wet Catalytic Oxidation Performance of Phenol over the Ru-Based Catalyst. Ind. Eng. Chem. Res. 2024, 63, 17014–17024. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Li, B.; Yang, Y.; Ma, S.; Wang, B.; Huang, Z. Synergistic Promotion Effect of Acidity and Redox Capacity in the Simultaneous Removal of CB and NOx in NH3-SCR Unit. Fuel 2023, 342, 127838. [Google Scholar] [CrossRef]
- Kang, D.; Shi, Q.; Zhang, C.; Zhao, P.; Lyu, H.; Huang, A.; Shen, B. Modulation of Acidic and Redox Properties of Mn-Based Catalysts by Co Doping: Application to the Synergistic Removal of NOx and Chlorinated Organics. Sep. Purif. Technol. 2024, 339, 126695. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, J.; Wu, X.; Liu, B.; Mei, J.; Yang, S. Exceptional Performance of Chlorobenzene Oxidation on Antimony-Loaded Commercial Selective Catalytic Reduction Catalyst as a Co-Benefit of Nitrogen Oxides Reduction: Notable Enhancement of Chlorobenzene Oxidation Due to Antimony Loading. J. Colloid Interface Sci. 2025, 680, 274–285. [Google Scholar] [CrossRef]
- Wang, F.; Chen, A.; Lan, T.; Chen, X.; Wang, M.; Hu, X.; Wang, P.; Cheng, D.; Zhang, D. Synergistic Catalytic Removal of NOx and Chlorinated Organics through the Cooperation of Different Active Sites. J. Hazard. Mater. 2024, 468, 133722. [Google Scholar] [CrossRef]
- Xu, M.; Liu, T.; Gao, X.; Jin, Q.; Yang, J.; Zhu, C.; Wang, S.; Xu, H. Simultaneous Catalytic Removal of NO and Chlorobenzene over MnCeSmSnOx: Catalytic Performance and Removal Mechanism. Chem. Phys. Impact 2023, 6, 100228. [Google Scholar] [CrossRef]





| Catalysts | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| CS-Ce0.3-Ti | 217 | 0.43 | 8.5 |
| 5HPW-CS-Ce0.3-Ti | 210 | 0.41 | 8.3 |
| 10HPW-CS-Ce0.3-Ti | 204 | 0.39 | 8.3 |
| 15HPW-CS-Ce0.3-Ti | 178 | 0.35 | 7.9 |
| 20HPW-CS-Ce0.3-Ti | 157 | 0.33 | 7.6 |
| Samples | Ce4+/(Ce3+ + Ce4+) (%) | Oα/(Oα + Oβ) (%) |
|---|---|---|
| CS-Ce0.3-Ti | 72.6 | 21.4 |
| 10HPW-CS-Ce0.3-Ti | 78.3 | 25.5 |
| Catalysts | NO Conversion (°C) | CB Conversion (°C) | Space Velocity (h−1) |
|---|---|---|---|
| MnOx/TiO2 | 90% at 120 | 80% at 330 | 80,000 [47] |
| MnCoOx | 90% at 104 | 90% at 181 | 60,000 [48] |
| MnCeTiOx | 80% at 250 | 96% at 300 | 60,000 [49] |
| CoSmMn2O5 | 90% at 160 | 90% at 270 | 50,000 [50] |
| MnCeSmSnOx | 90% at 120 | 90% at 238 | 24,000 [51] |
| This study | 90% at 167 | 90% at 291 | 60,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Zhang, R.; Shen, Y.; Zhou, X.; Zhai, Y.; Han, Y.; Wang, D.; Zhang, L.; Song, X.; Fang, D.; et al. Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx. Catalysts 2025, 15, 119. https://doi.org/10.3390/catal15020119
Ma M, Zhang R, Shen Y, Zhou X, Zhai Y, Han Y, Wang D, Zhang L, Song X, Fang D, et al. Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx. Catalysts. 2025; 15(2):119. https://doi.org/10.3390/catal15020119
Chicago/Turabian StyleMa, Mingyang, Ruhan Zhang, Yanan Shen, Xin Zhou, Yumeng Zhai, Yumeng Han, Dan Wang, Longjin Zhang, Xinru Song, De Fang, and et al. 2025. "Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx" Catalysts 15, no. 2: 119. https://doi.org/10.3390/catal15020119
APA StyleMa, M., Zhang, R., Shen, Y., Zhou, X., Zhai, Y., Han, Y., Wang, D., Zhang, L., Song, X., Fang, D., & Gong, P. (2025). Mesoporous Ce-Ti Catalysts Modified by Phosphotungstic Acid and Chitosan for the Synergistic Catalysis of CVOCs and NOx. Catalysts, 15(2), 119. https://doi.org/10.3390/catal15020119








