Bi4Ti3O12-V/Ag Composite with Oxygen Vacancies and Schottky Barrier with Photothermal Effect for Boosting Nizatidine Degradation
Abstract
1. Introduction
2. Results and Discussion
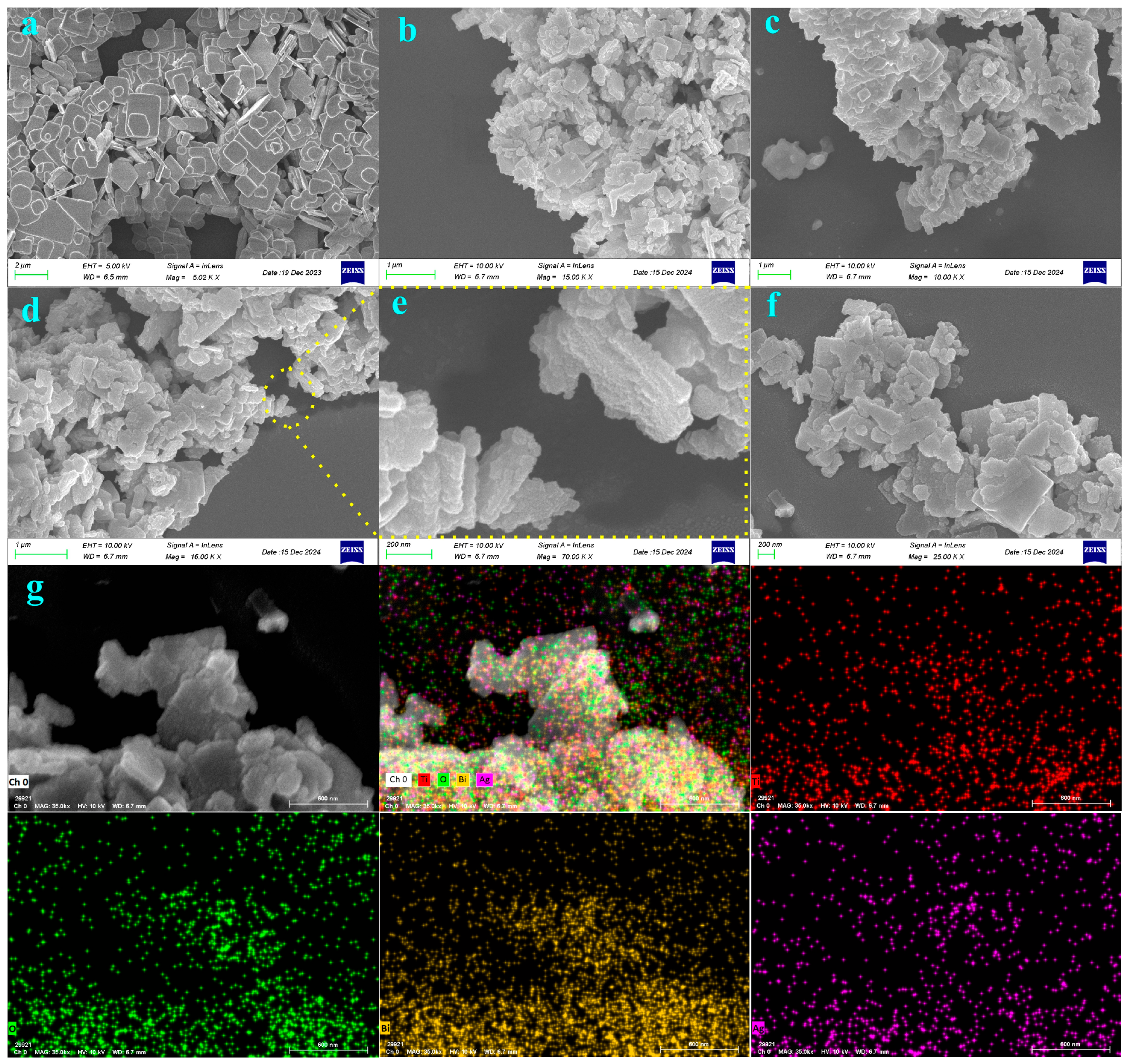
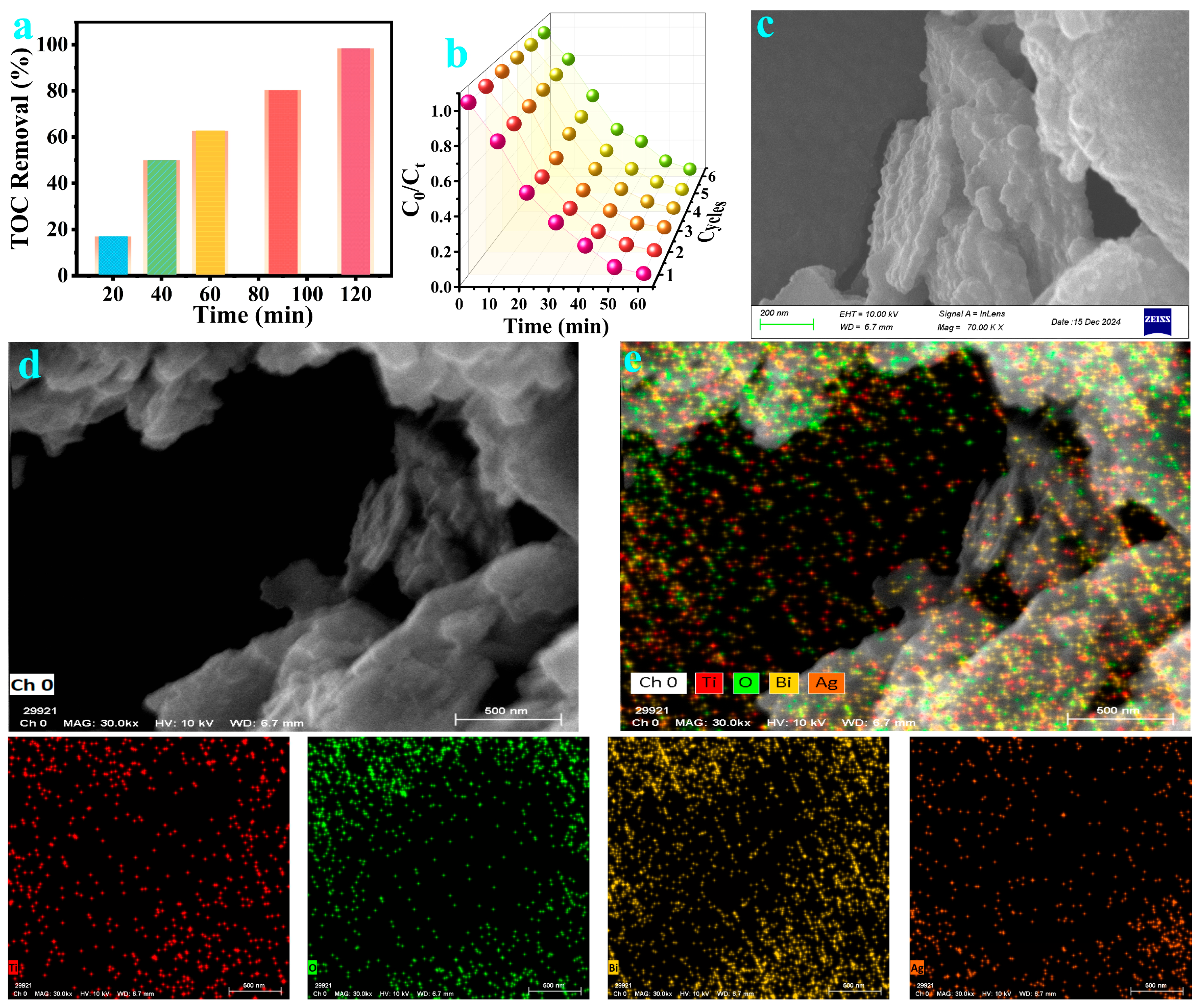
2.1. Structure and Morphology of BTO-V/Ag Composite Catalyst
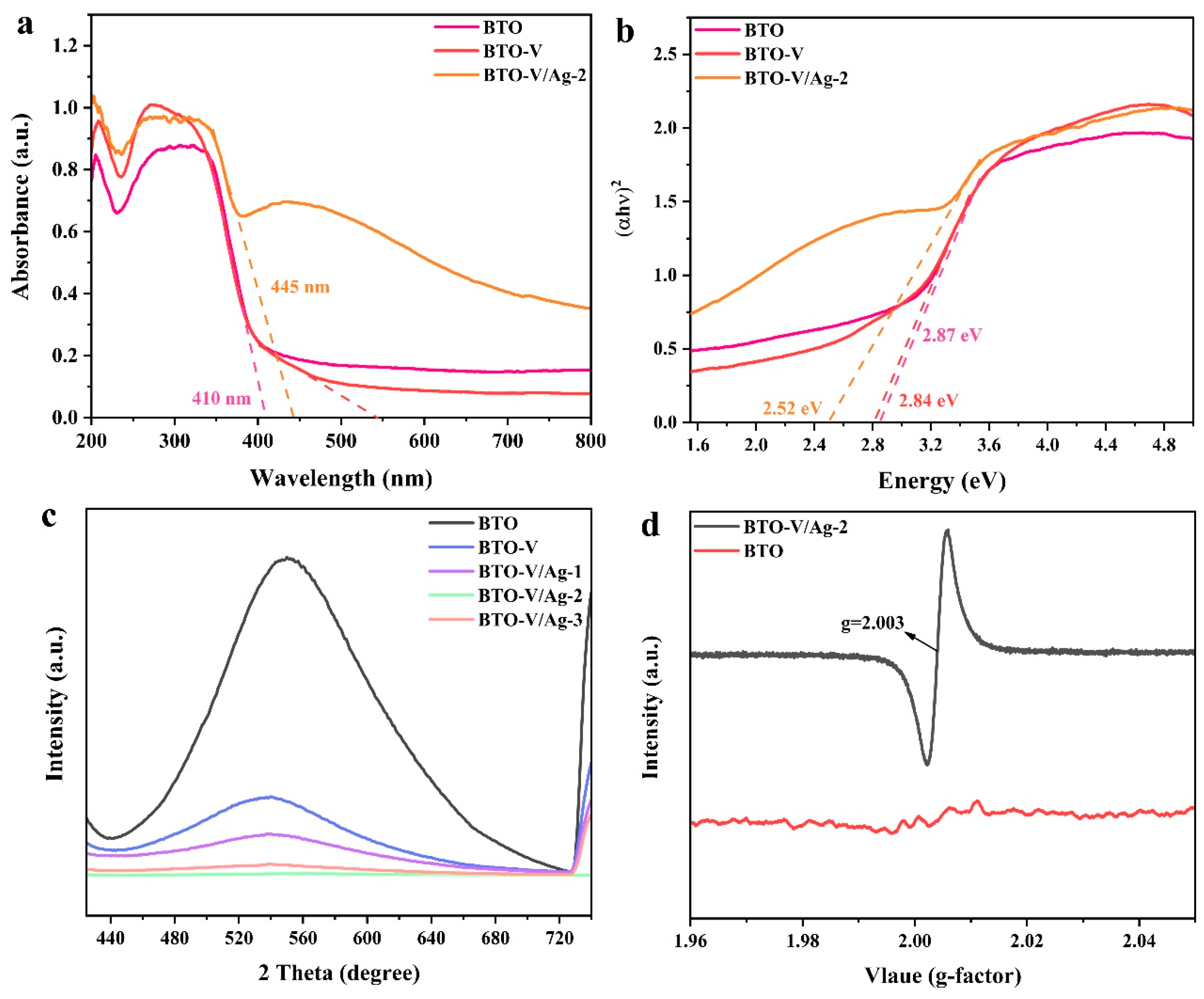
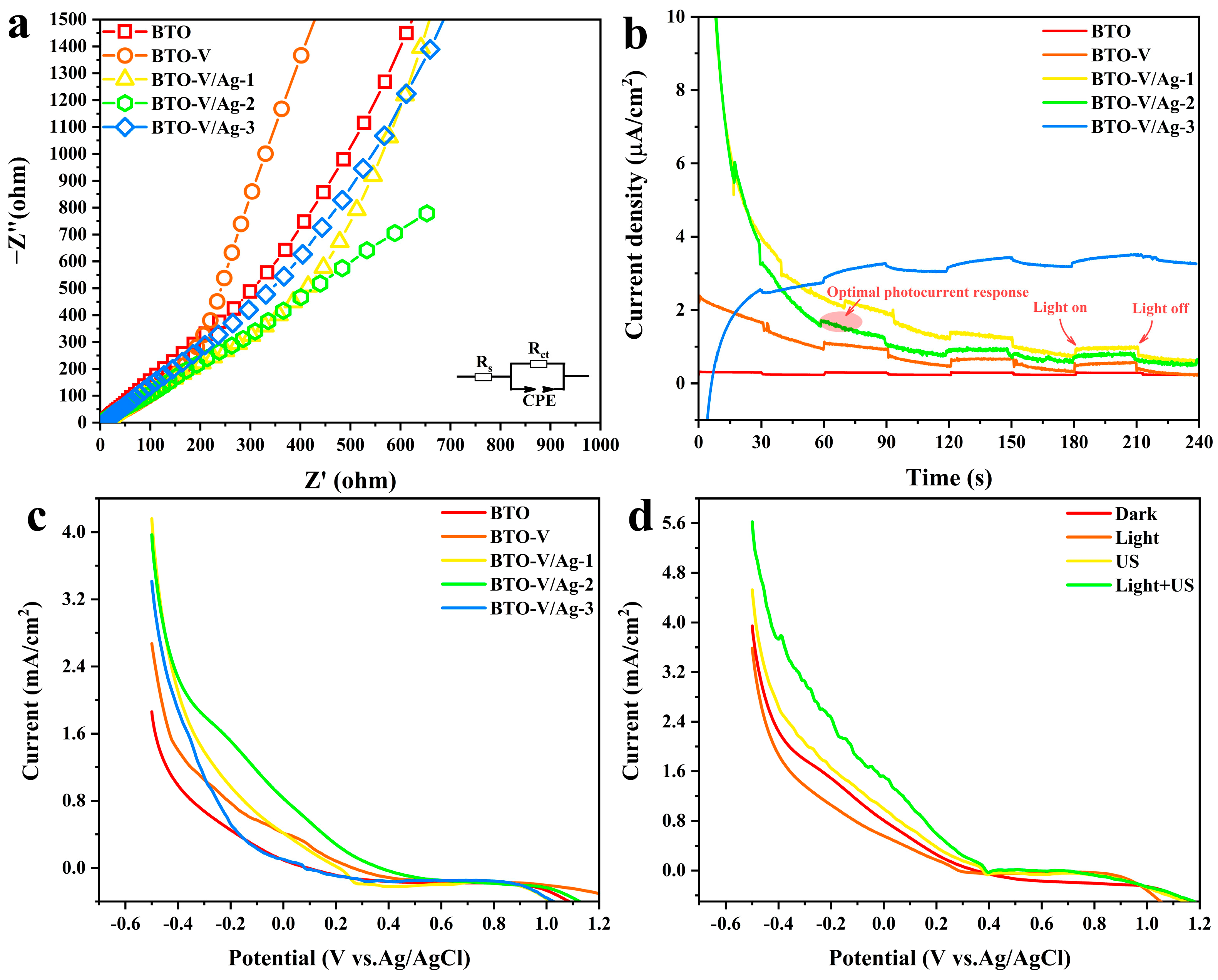
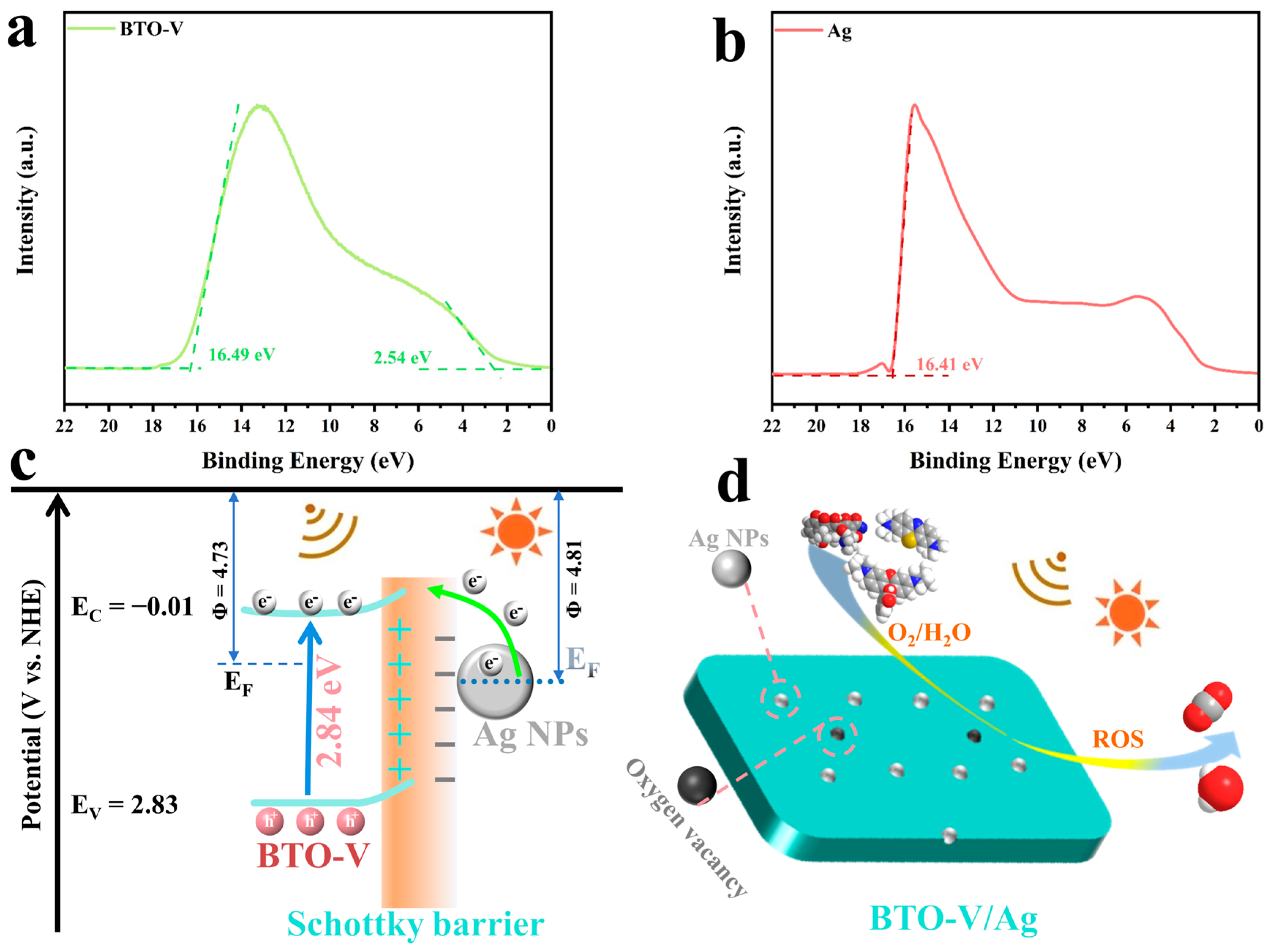
2.2. Photogenerated Charge Separation and Piezo-Photocatalytic Synergy Properties
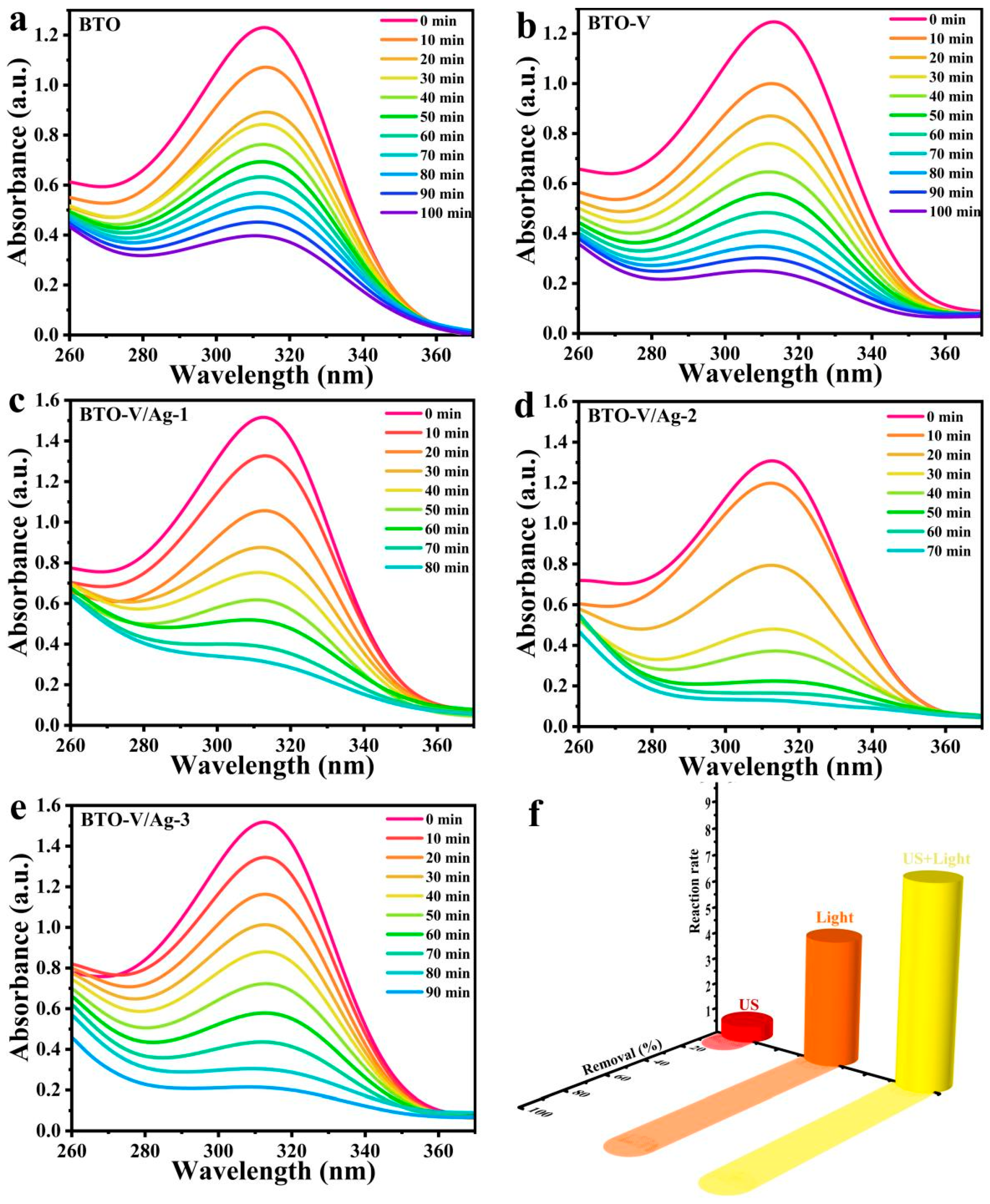
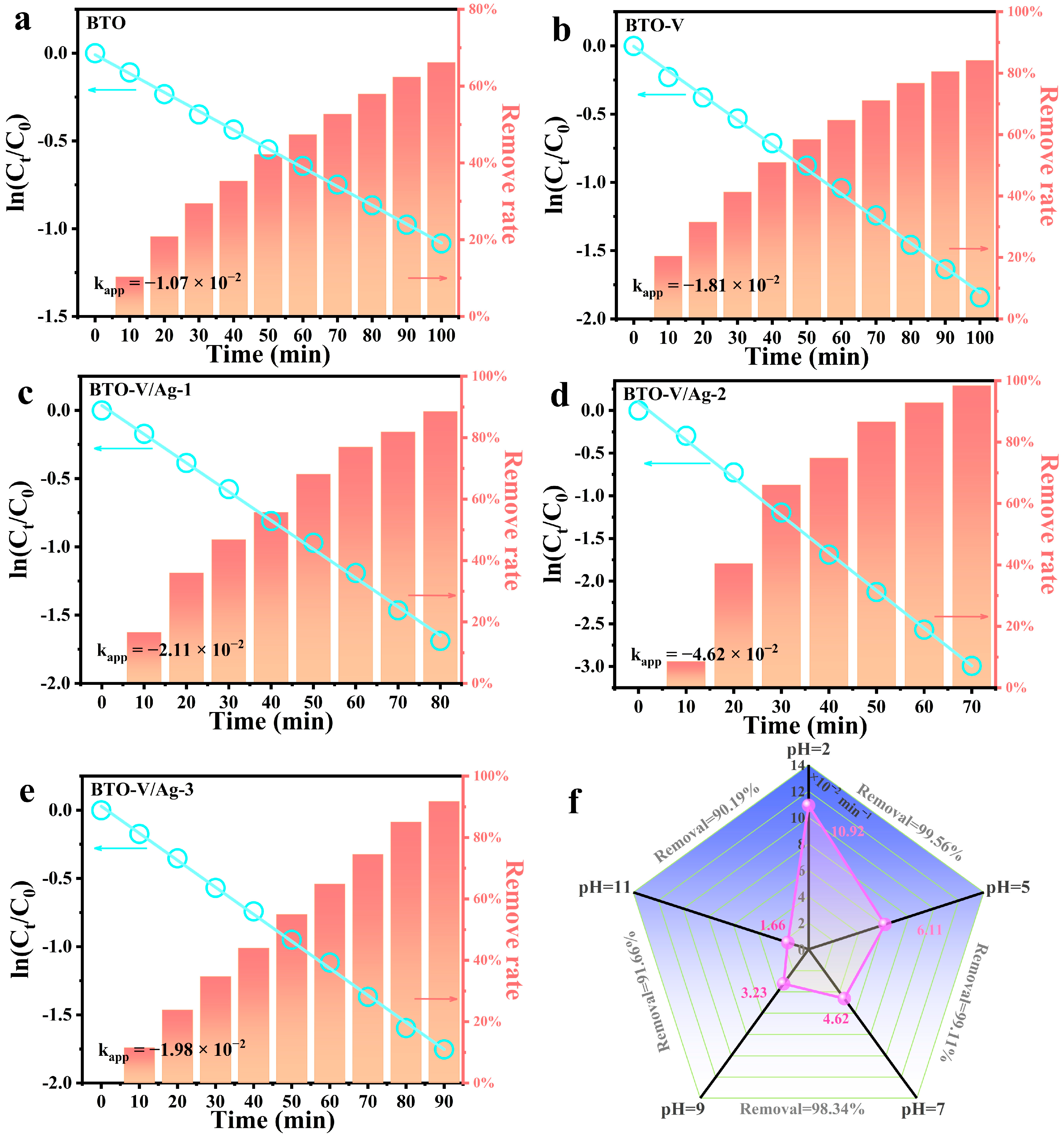
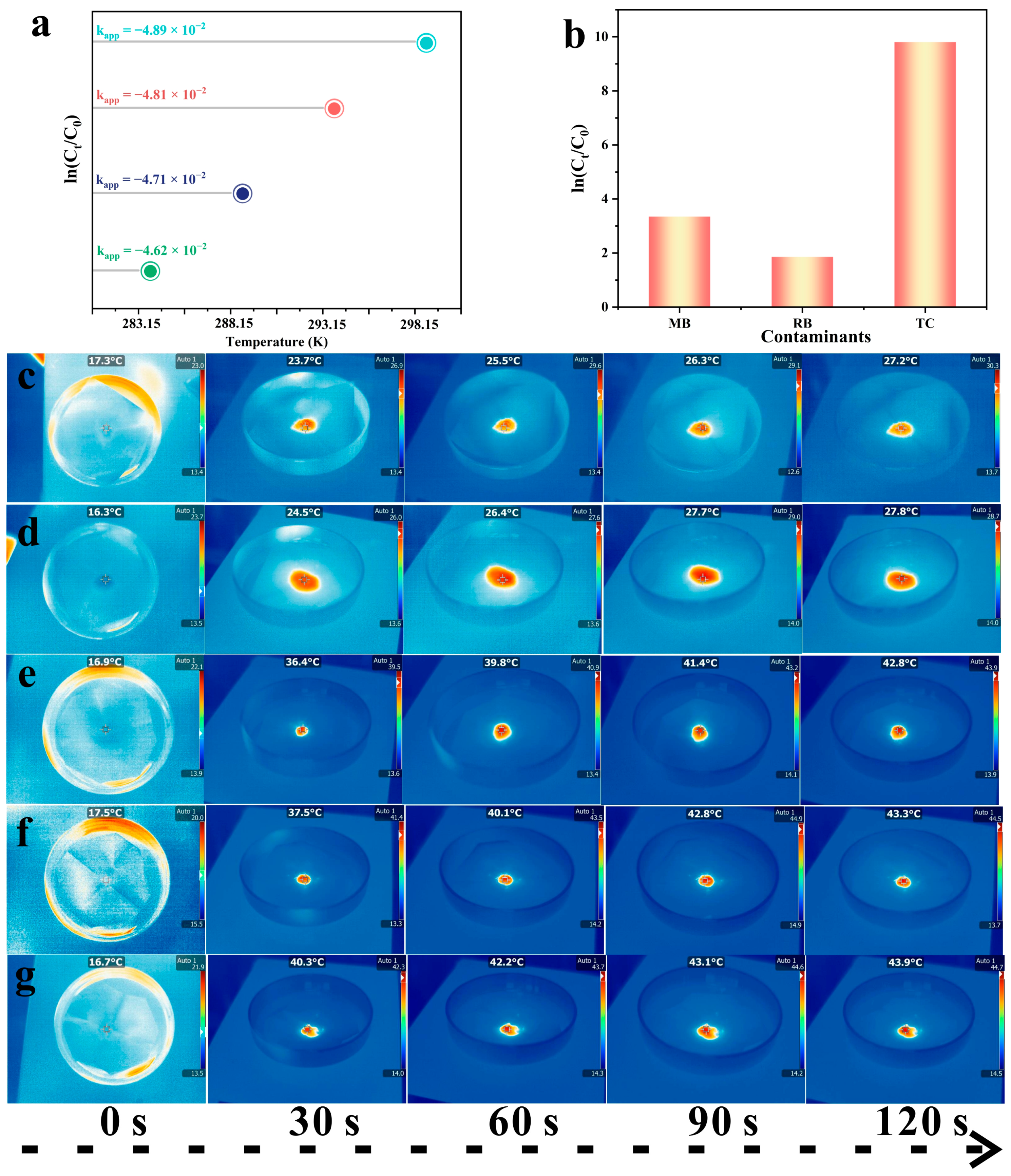
2.3. Piezo-Photocatalytic Activity of BTO-V/Ag Schottky Heterojunction
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Piezo-Photocatalytic Performance Assays
3.4. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Imgharn, A.; Sun, T.; Nicolle, J.; Naciri, Y.; Hsini, A.; Albourine, A.; Ania, C. A simple approach to prepare a C3N4/MoO3 heterojunction with improved photocatalytic performance for the degradation of methylparaben. Catalysts 2024, 14, 170. [Google Scholar] [CrossRef]
- Reincke, M.; Arlt, W.; Damdimopoulou, P.; Köhrle, J.; Bertherat, J. Endocrine disrupting chemicals are a threat to hormone health: A commentary on behalf of the ESE. Nat. Rev. Endocrinol. 2024, 20, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.; Bartosova, Z.; Völker, J.; Wagner, M. Migration of endocrine and metabolism disrupting chemicals from plastic food packaging. Environ. Int. 2024, 189, 108791. [Google Scholar] [CrossRef] [PubMed]
- Devendrapandi, G.; Liu, X.; Balu, R.; Ayyamperumal, R.; Arasu, M.V.; Lavanya, M.; Reddy, V.R.M.; Kim, W.K.; Karthika, P. Innovative remediation strategies for persistent organic pollutants in soil and water: A comprehensive review. Environ. Res. 2024, 249, 118404. [Google Scholar] [CrossRef]
- Yan, X.; Xia, Y.; Ti, C.; Shan, J.; Wu, Y.; Yan, X. Thirty years of experience in water pollution control in Taihu Lake: A review. Sci. Total Environ. 2024, 914, 169821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Cao, X.; Li, J.; Hou, X. Synthesis of MOF on MOF photocatalysts using PCN-134 as seed through epitaxial growth strategy towards nizatidine degradation. Chem. Eng. J. 2023, 465, 143000. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhang, Z. Nanoconfined catalytic water purification within CoFeCu LDH-assembled membrane nanochannels. Appl. Catal. B Environ. Energy 2024, 357, 124290. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, J.; Zeng, Z.; Gao, Y.; Zhang, Z.; Han, B.; Ma, J.; Jiang, J. Oxidation of amine-based pharmaceuticals with unactivated peroxymonosulfate: Kinetics, mechanisms, and elimination efficiency of NDMA formation. J. Hazard. Mater. 2024, 463, 132961. [Google Scholar] [CrossRef]
- You, S.C.; Seo, S.I.; Falconer, T.; Yanover, C.; Duarte-Salles, T.; Seager, S.; Posada, J.D.; Shah, N.H.; Nguyen, P.-A.; Kim, Y. Ranitidine use and incident cancer in a multinational cohort. JAMA Netw. Open 2023, 6, e2333495. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water pollutants: Sources and impact on the environment and human health. Sens. Water Pollut. Monit. Role Mater. 2020, 12, 43–62. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-C.; Chuang, Y.-H.; Liou, S.Y.H.; Li, Q.; Hou, C.-H. In situ engineering of highly conductive TiO2/carbon heterostructure fibers for enhanced electrocatalytic degradation of water pollutants. J. Hazard. Mater. 2022, 429, 128328. [Google Scholar] [CrossRef]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Weng, Z.; Lin, Y.; Han, B.; Zhang, X.; Guo, Q.; Luo, Y.; Ou, X.; Zhou, Y.; Jiang, J. Donor-acceptor engineered g-C3N4 enabling peroxymonosulfate photocatalytic conversion to 1O2 with nearly 100% selectivity. J. Hazard. Mater. 2023, 448, 130869. [Google Scholar] [CrossRef] [PubMed]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants–A review. Appl. Surface Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Bai, Z.; Xie, Y.; Wei, X.; Zhou, L.; Bao, J.; Tang, R.; Wen, Y.; Zhao, Z.; Deng, J.; Zhao, Z. Intrinsic metal ion dual-regulated fabrication of multifunctional nano-interfacial BiFeVO4/Fe-MOF for high photo-Fenton-like synergistic catalytic oxytetracycline removal. Appl. Catal. B Environ. Energy 2025, 361, 124634. [Google Scholar] [CrossRef]
- Chee, S.S.; Seo, D.; Kim, H.; Jang, H.; Lee, S.; Moon, S.P.; Lee, K.H.; Kim, S.W.; Choi, H.; Ham, M.H. Lowering the Schottky barrier height by graphene/Ag electrodes for high-mobility MoS2 field-effect transistors. Adv. Mater. 2019, 31, 1804422. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Pham, M.-T.; Nguyen, H.Q.; Cao, T.M.; Van Pham, V. Boosting visible-light-driven photocatalysis of nitrogen oxide degradation by Mott–Schottky Pd/TiO2 heterojunctions. Sep. Purif. Technol. 2025, 354, 129012. [Google Scholar] [CrossRef]
- Jia, P.; Han, Z.; Chen, J.; Liu, J.; Wang, L.; Zhang, X.; Guo, Y.; Zhou, J. Pt@WS2 Mott–Schottky heterojunction boosts light-driven active ion transport for enhanced ionic power harvesting. ACS Nano 2024, 31, 233–239. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.R.; Hernandez-Rodríguez, Y.; Casas-Espínola, J.; Cigarroa-Mayorga, O. Nanostructural modulation of Schottky barrier in Au/α-MoO3 heterojunction via Au nanoparticle size control. Appl. Surface Sci. 2024, 670, 160624. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.; Liu, J.; Wang, K.; Chen, X.; Liu, H. Narrow bandgap schottky heterojunction sonosensitizer with high electron–hole separation boosted sonodynamic therapy in bladder cancer. Adv. Mater. 2024, 36, 2401252. [Google Scholar] [CrossRef] [PubMed]
- Ghodselahi, T.; Neishaboorynejad, T.; Arsalani, S. Fabrication LSPR sensor chip of Ag NPs and their biosensor application based on interparticle coupling. Appl. Surface Sci. 2015, 343, 194–201. [Google Scholar] [CrossRef]
- Lu, X.; Ma, Z.; Chang, Y.; Wang, S.; Li, X.; Xu, D.; Bao, J.; Liu, Y. Mott-Schottky construction boosted plasmon thermal and electronic effects on the Ag/CoV-LDH nanohybrids for highly-efficient water oxidation. Adv. Mater. 2024, 26, 2313057. [Google Scholar] [CrossRef]
- Wen, Y.; Che, H.; Tang, C.; Liu, B.; Ao, Y. A Schottky heterojunction with spatially separated active sites for piezo-photocatalytic dual-channel hydrogen peroxide generation. Nano Energy 2024, 128, 109837. [Google Scholar] [CrossRef]
- Jia, P.; Li, J.; Huang, H. Piezocatalysts and piezo-photocatalysts: From material design to diverse applications. Adv. Funct. Mater. 2024, 34, 2407309. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.T.; Yuan, Z.Y. Enabling internal electric fields to enhance energy and environmental catalysis. Adv. Energy Mater. 2023, 13, 2203720. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Wang, Y.; Xiong, Y.; Yang, P.; Qin, J.; Xiong, X.; Lei, Y. Designing a built-in electric field for efficient energy electrocatalysis. ACS Nano 2022, 16, 19959–19979. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Liu, J.; Yan, K.; Zhu, K. Recent advancements in the use of novel piezoelectric materials for piezocatalytic and piezo-photocatalytic applications. Appl. Catal. B Environ. Energy 2024, 341, 123335. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Chen, X.-Z.; Sanchis-Gual, R.; Veciana, A.; Franco, C.; Kim, D.; Surin, I.; Pérez-Ramírez, J.; Mattera, M. Tuning oxygen vacancies in Bi4Ti3O12 nanosheets to boost piezo-photocatalytic activity. Nano Energy 2023, 108, 108202. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, X.; Wang, F. Investigation of the physical characteristics of Bi4Ti3O12, Bi2Ti4O11, Bi12TiO20, Bi2Ti2O7 ternary semiconductors. Mat. Sci. Semicon. Proc. 2024, 181, 108658. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumar, R.; Raizada, P.; Khan, A.A.P.; Ahamad, T.; Van Le, Q.; Nguyen, V.-H.; Thakur, S.; Singh, P.; Sudhaik, A. Advances in bismuth titanate (Bi12TiO20)-based photocatalysts for environmental remediation: Fundamentals and practical applications. J. Water Proc. Eng. 2024, 59, 104974. [Google Scholar] [CrossRef]
- Man, R.; Dan, Y.; Fan, J.; Wang, C.; Li, Z.; Zhou, T.; Lu, X.; Zhao, M. Enhanced piezoelectricity in Al2O3 (0001) single crystal substrate sintered Bi12TiO20-BaTiO3 composite ceramics. New J. Chem. 2024, 12, 33–39. [Google Scholar] [CrossRef]
- Nesa Soheli, S.; Lu, Z.; Sun, D.; Shyha, I. Lead-free NaNbO3-based ceramics for electrostatic energy storage capacitors. Ceramics 2024, 7, 712–734. [Google Scholar] [CrossRef]
- Gumiel, C.; Colado, M.; Calatayud, D.G.; Barea, R.; Villegas, M.; Jardiel, T. Microstructure modulation of a Bi4Ti3O12 thin film system by combining the effect of a simple processing methodology with a co-doping strategy involving Nd3+ and Nb5+. Boletín Soc. Española Cerámica Vidr. 2024, 63, 425–433. [Google Scholar] [CrossRef]
- Murthy, R.K.; Shivanna, M.; Ahamed, N.N.; Bhoomika, V.; Ravikumar, C.; Murthy, H.A. Photocatalytic and electrochemical sensor study of combustion synthesized bismuth oxide (Bi2O3) nanoparticles using lemon and urea fuels. Mater. Sci. Eng. B 2024, 307, 117487. [Google Scholar] [CrossRef]
- Wang, C.; Chen, F.; Hu, C.; Ma, T.; Zhang, Y.; Huang, H. Efficient piezocatalytic H2O2 production of atomic-level thickness Bi4Ti3O12 nanosheets with surface oxygen vacancy. Chem. Eng. J. 2022, 431, 133930. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Kim, D.; Franco, C.; Terzopoulou, A.; Veciana, A.; Puigmartí-Luis, J.; Chen, X.Z.; Nelson, B.J.; Pané, S. Enhanced piezocatalytic performance of BaTiO3 nanosheets with highly exposed {001} facets. Adv. Funct. Mater. 2022, 32, 2202180. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Y.; Yi, S.; Yan, S.; Ouyang, C.; Deng, F.; Li, C.; Liao, G.; Li, Q. Facile synthesis of pure silicon zeolite-confined silver nanoparticles and their catalytic activity for the reduction of 4-nitrophenol and methylene blue. Sep. Purif. Technol. 2023, 307, 122727. [Google Scholar] [CrossRef]
- Hu, X.; Li, K.; Wu, X.; Wang, N.; Li, X.; Li, Q.; Li, L.; Lv, K. Dramatic promotion of visible-light photoreactivity of TiO2 hollow microspheres towards NO oxidation by introduction of oxygen vacancy. Appl. Catal. B Environ. Energy 2019, 256, 117860. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Lu, Y.; Wang, T.; Huo, P.; Yan, Y. Increasing visible-light absorption for photocatalysis with black 2D Bi4Ti3O12 nanosheets. Adv. Powder Technol. 2019, 30, 1043–1050. [Google Scholar] [CrossRef]
- Yang, L.; Tian, B.; Xie, Y.; Dong, S.; Yang, M.; Gai, S.; Lin, J. Oxygen-vacancy-rich piezoelectric BiO2−x nanosheets for augmented piezocatalytic, sonothermal, and enzymatic therapies. Adv. Mater. 2023, 35, 2300648. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.; Soleimani-Javid, Z.; Arshid, E.; Amir, S.; Civalek, Ö. Vibration analysis of graphene nanoplatelets’ reinforced composite plates integrated by piezo-electromagnetic patches on the piezo-electromagnetic media. Wave. Random Complex Media 2024, 34, 2394–2424. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, L.; Yan, W.; Wang, H.; Mao, W.; Cui, Y.; Li, Y.; Zhu, X. Synergizing the internal electric field and ferroelectric polarization of the BiFeO3/ZnIn2S4 Z-scheme heterojunction for photocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 434–446. [Google Scholar] [CrossRef]
- Kang, X.; Dong, G.; Dong, T. Oxygen vacancy defect engineering of heterophase junction TiO2: Interfacial/surface oxygen vacancies coadjust the photocatalytic ROS production. ACS Appl. Energy Mater. 2022, 6, 1025–1036. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, K.; Liu, Z.; Tao, R.; Sun, Z.; Zhang, H.; An, G. In situ controllable loading of ultrafine noble metal particles on titania. J. Am. Chem. Soc. 2009, 131, 6648–6649. [Google Scholar] [CrossRef]
- Ding, J.; Guo, D.; Wang, N.; Wang, H.F.; Yang, X.; Shen, K.; Chen, L.; Li, Y. Defect engineered metal–organic framework with accelerated structural transformation for efficient oxygen evolution reaction. Angew. Chem. Int. Edit. 2023, 62, e202311909. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, C.; Zhang, X.; Guo, R.; Zheng, Y.; Chu, H.; Fu, H.; Wang, P.; Wang, C.-C. Piezo-photocatalytic synergetic for H2O2 generation via dual-pathway over Z-scheme ZIF-L/g-C3N4 heterojunction. Nano Energy 2024, 128, 109795. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric nanogenerator: A foundation of the energy for the new era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Cui, W.-G.; Gao, F.; Na, G.; Wang, X.; Li, Z.; Yang, Y.; Niu, Z.; Qu, Y.; Wang, D.; Pan, H. Insights into the pH effect on hydrogen electrocatalysis. Chem. Soc. Rev. 2024, 53, 10253–10311. [Google Scholar] [CrossRef]
- Alkaim, A.; Aljeboree, A.; Alrazaq, N.; Baqir, S.; Hussein, F.; Lilo, A. Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem. 2014, 26, 8445. [Google Scholar] [CrossRef]
- Liu, S.; Deng, F.; Guo, Y.; Ouyang, C.; Yi, S.; Li, C.; Liao, G.; Li, Q. Silver nanocatalysts supported by multiple melanin carriers with a photothermal effect for reduction of methylene blue and 4-nitrophenol. ACS Appl. Nano Mater. 2023, 7, 889–903. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar]
- Ouyang, C.; Liu, S.; Guo, Y.; Yi, S.; Li, Q. Silver nanoparticles decorated polydopamine-coated pure silica zeolite as an efficient nanocatalyst for organic pollutants removal. Appl. Surface Sci. 2024, 652, 159281. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Sun, Q.; Hou, X.; Wang, T. Enhanced photocatalytic degradation of sulfathiazole via dual-ligand Zr-MOFs: A porphyrin and pyrene-based energy transfer. Sep. Purif. Technol. 2025, 354, 128727. [Google Scholar]
- Hattali, O.A.; Marzouqi, F.; Al Mamari, S.; Kuvarega, A.T.; Selvaraj, R. CdO nanoplates for photocatalytic degradation of Levofloxacin and Nizatidine under natural solar light irradiation. Inorg. Chem. Commun. 2022, 146, 110071. [Google Scholar]
- Zhao, Y.; Cao, X.; Zhang, Y.; Li, J.; Chen, P.; Hou, X. Novel edge-epitaxial MOF on MOF for efficient nizatidine removal. J. Taiwan Inst. Chem. Eng. 2024, 163, 105643. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Hu, C.; Gong, Y.; Guo, Y.; Cheng, Z.; Yuan, M.; Liao, Z.; Xiao, X.; Xu, Z.; Du, J.; et al. Bi4Ti3O12-V/Ag Composite with Oxygen Vacancies and Schottky Barrier with Photothermal Effect for Boosting Nizatidine Degradation. Catalysts 2025, 15, 117. https://doi.org/10.3390/catal15020117
Liu S, Hu C, Gong Y, Guo Y, Cheng Z, Yuan M, Liao Z, Xiao X, Xu Z, Du J, et al. Bi4Ti3O12-V/Ag Composite with Oxygen Vacancies and Schottky Barrier with Photothermal Effect for Boosting Nizatidine Degradation. Catalysts. 2025; 15(2):117. https://doi.org/10.3390/catal15020117
Chicago/Turabian StyleLiu, Sheng, Chen Hu, Ying Gong, Yujuan Guo, Zhenping Cheng, Mengyi Yuan, Zixiang Liao, Xuewen Xiao, Zushun Xu, Jun Du, and et al. 2025. "Bi4Ti3O12-V/Ag Composite with Oxygen Vacancies and Schottky Barrier with Photothermal Effect for Boosting Nizatidine Degradation" Catalysts 15, no. 2: 117. https://doi.org/10.3390/catal15020117
APA StyleLiu, S., Hu, C., Gong, Y., Guo, Y., Cheng, Z., Yuan, M., Liao, Z., Xiao, X., Xu, Z., Du, J., Shen, P., & Li, Q. (2025). Bi4Ti3O12-V/Ag Composite with Oxygen Vacancies and Schottky Barrier with Photothermal Effect for Boosting Nizatidine Degradation. Catalysts, 15(2), 117. https://doi.org/10.3390/catal15020117








