Electrochemical Synthesis of TiO2 Nanotubes for Photocatalytic Water Splitting: Mechanisms, Challenges, and Improvement Strategies
Abstract
1. Introduction
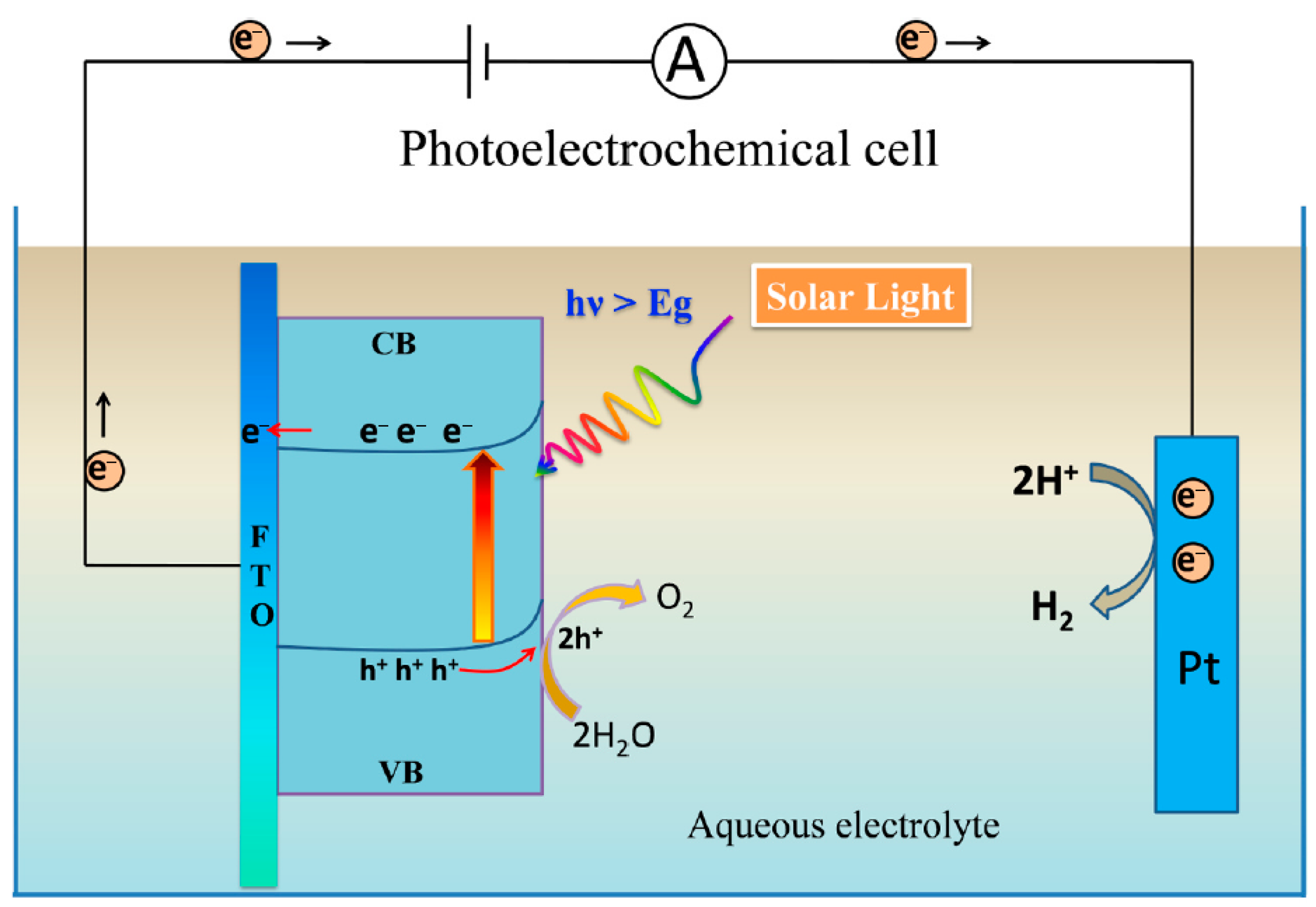
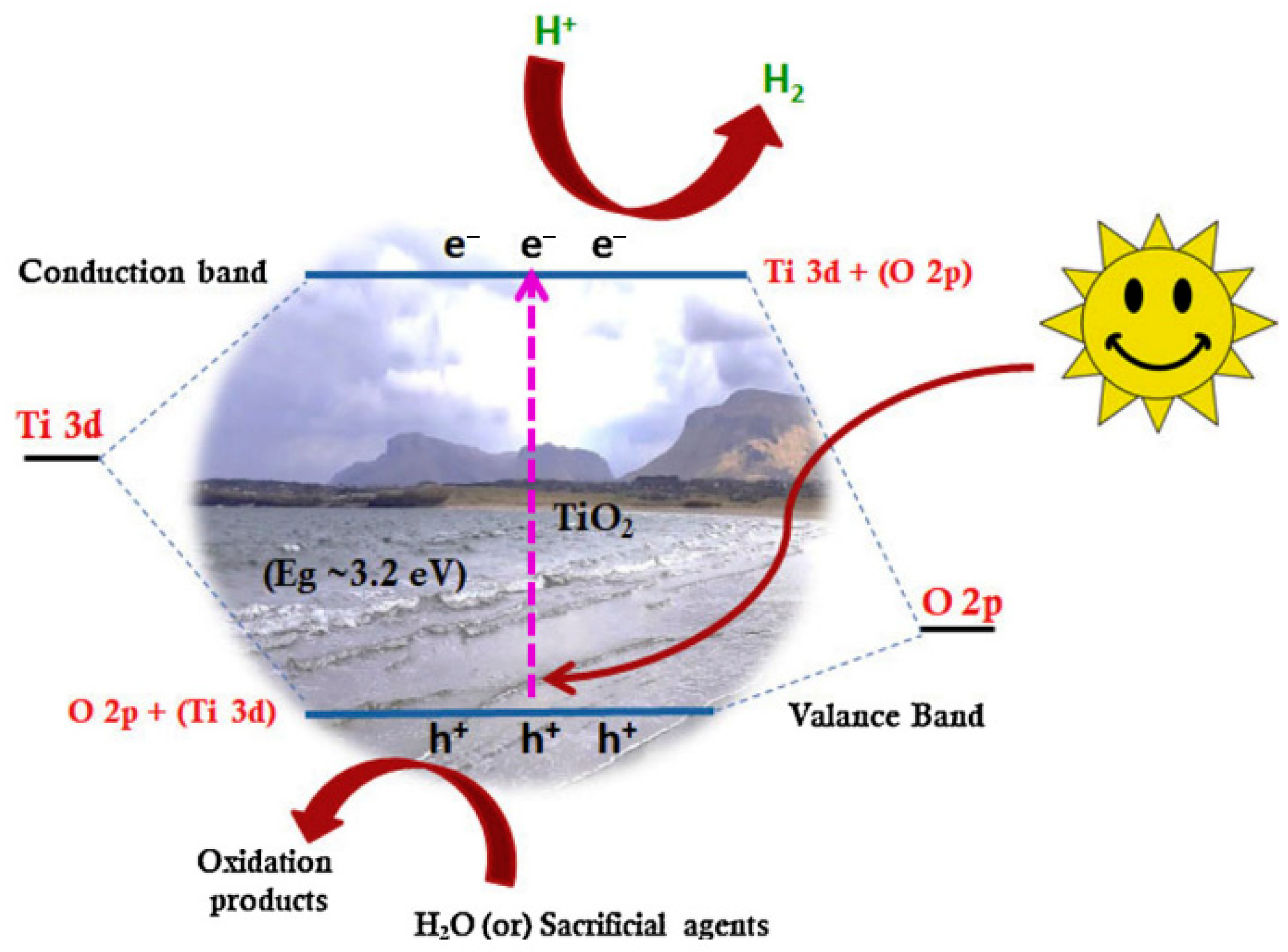
2. Photocatalysis
3. Mechanism of Photocatalysis
- Light Absorption and Electron Excitation
- 2.
- Separation and transport of carriers
- 3.
- Surface reactions (oxidation and reduction)
- -
- Reactions of pores (oxidation): pores (h+) are strongly oxidizing and accept electrons from adsorbed molecules (e.g., water or hydroxide ions). The most important oxidation reaction is the production of the hydroxyl radical (OH.):
- -
- Electron Reactions (Reduction): electrons in the CB can reduce oxygen molecules adsorbed on the TiO2 surface to superoxide radicals :
4. Photocatalytic Evolution of Hydrogen
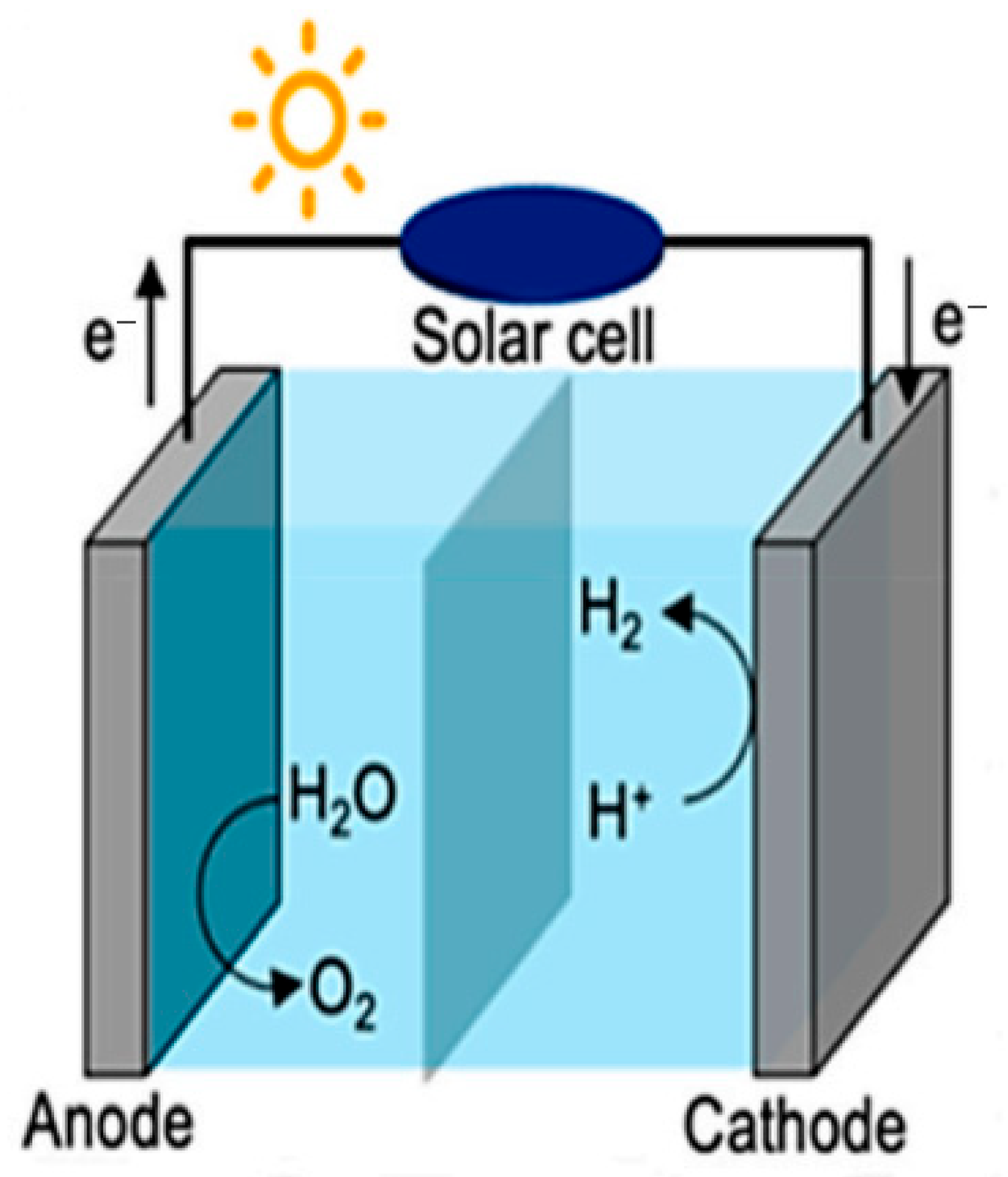
4.1. Photoelectrolysis of Water
4.2. Electroless Photocatalytic Generation of Hydrogen
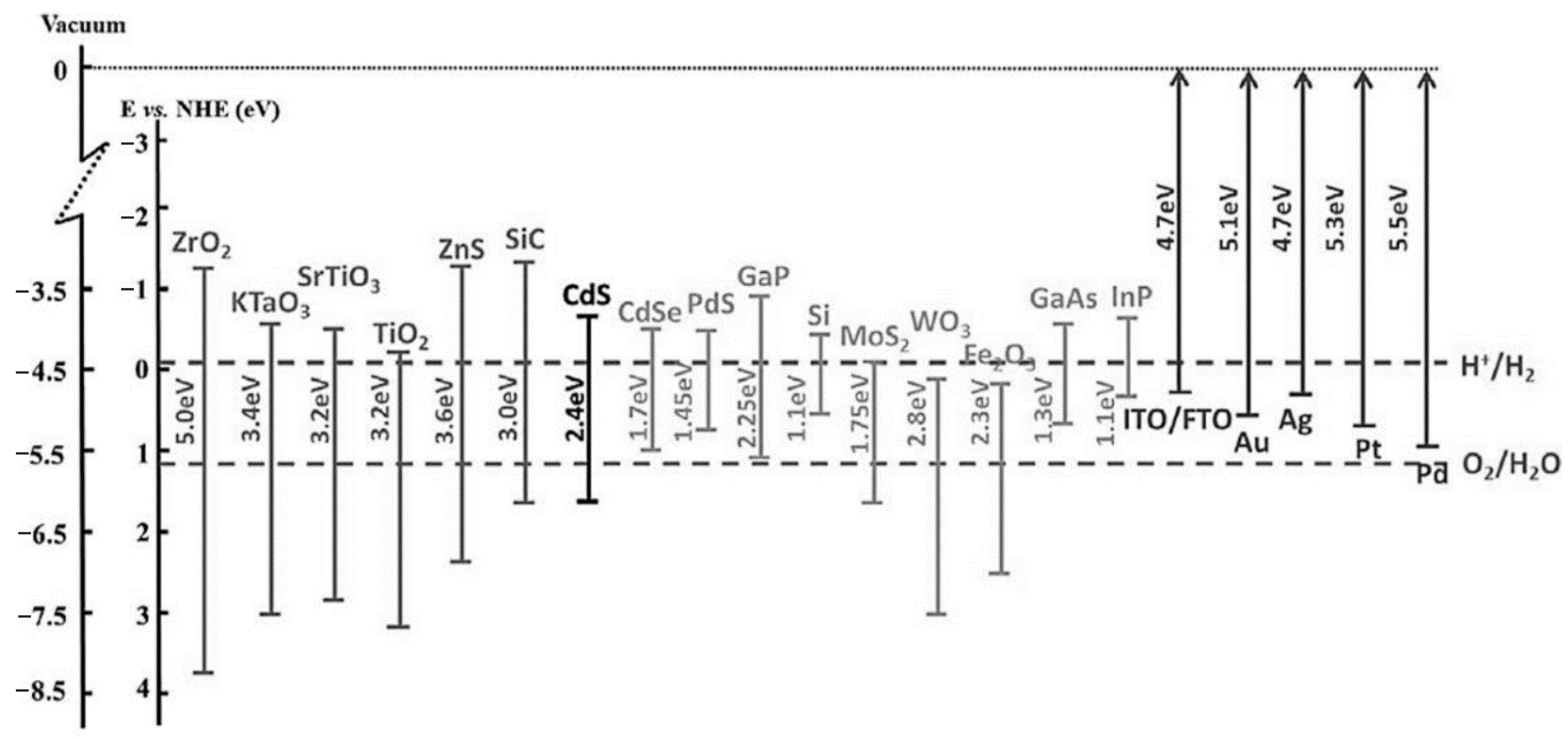
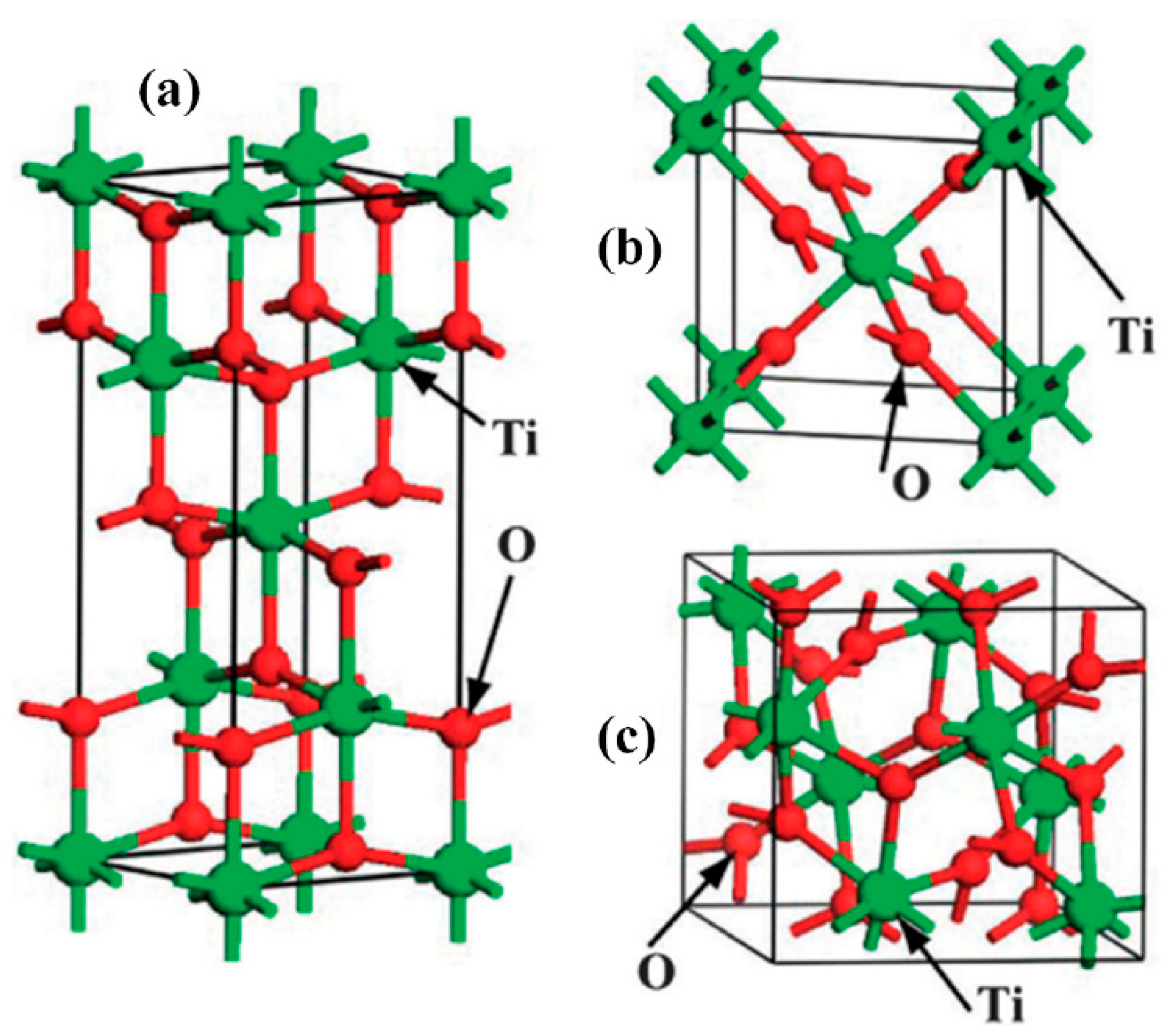
5. Titanium Dioxide (TiO2)
6. Nanostructures
7. Formation Mechanism of TNTs
7.1. Hydrothermal Method
7.2. Using the Template Method
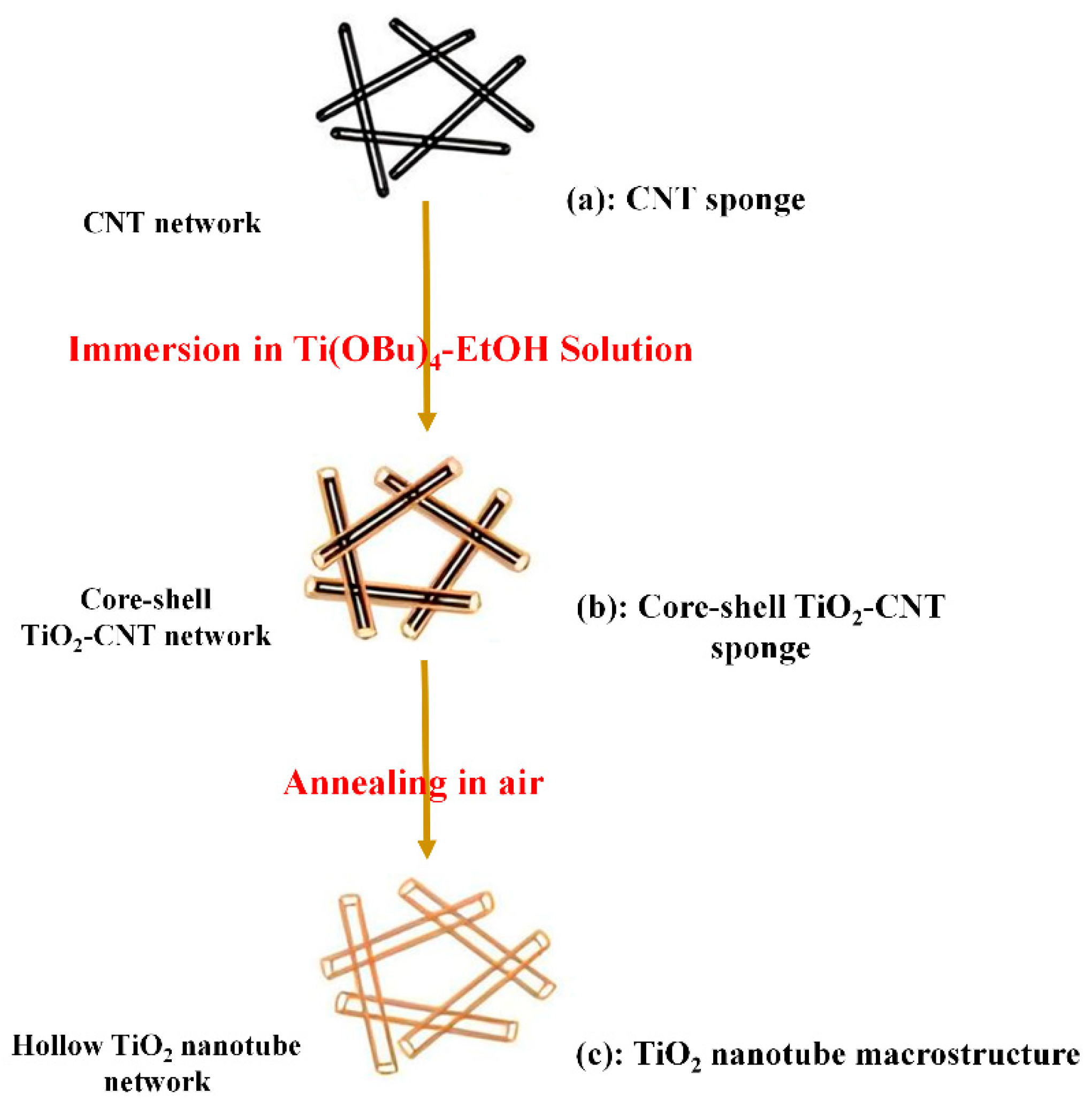
7.3. Atomic Layer Deposition Method
7.4. Sol–Gel Method
7.5. Electrochemical Oxidation Method
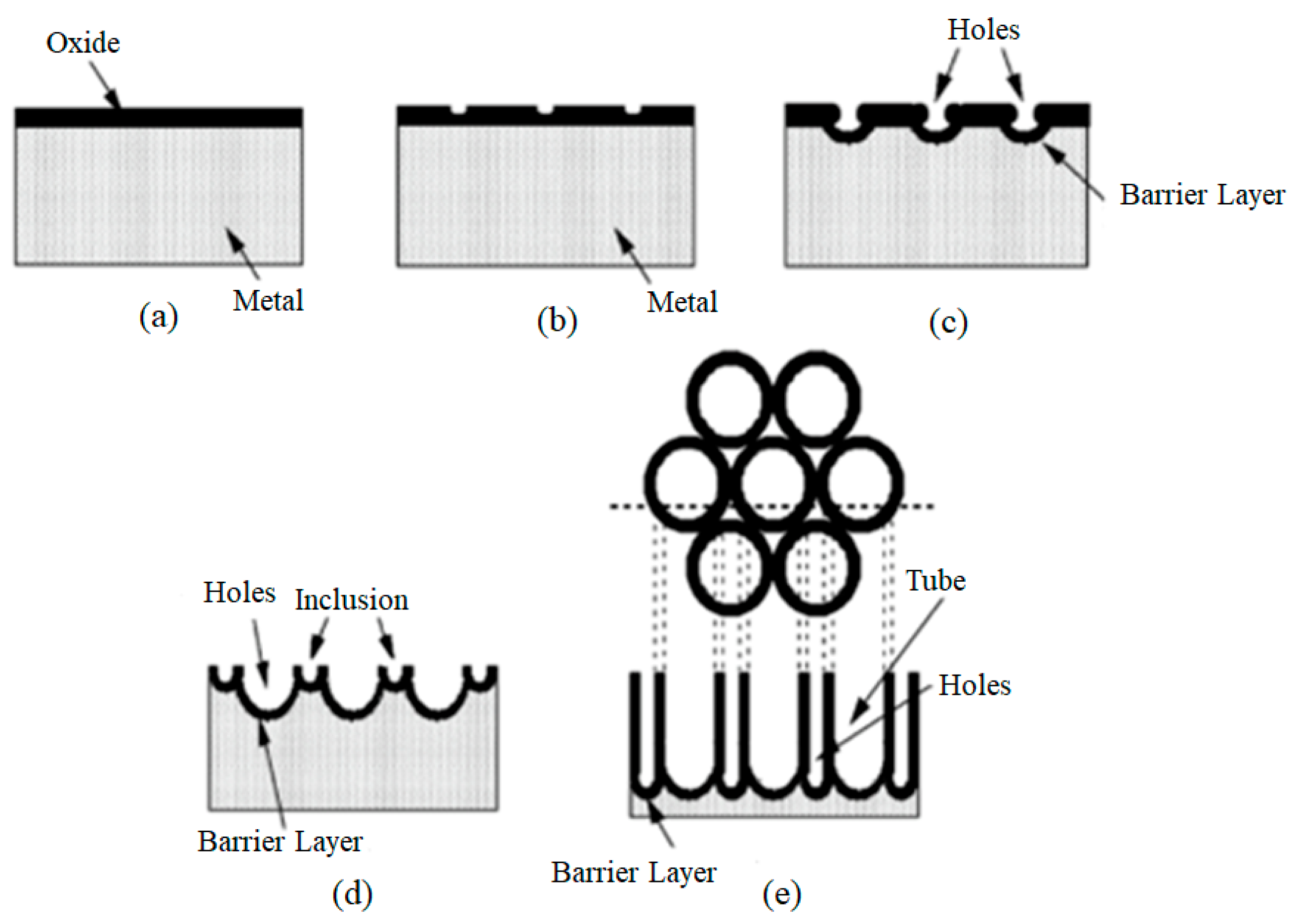
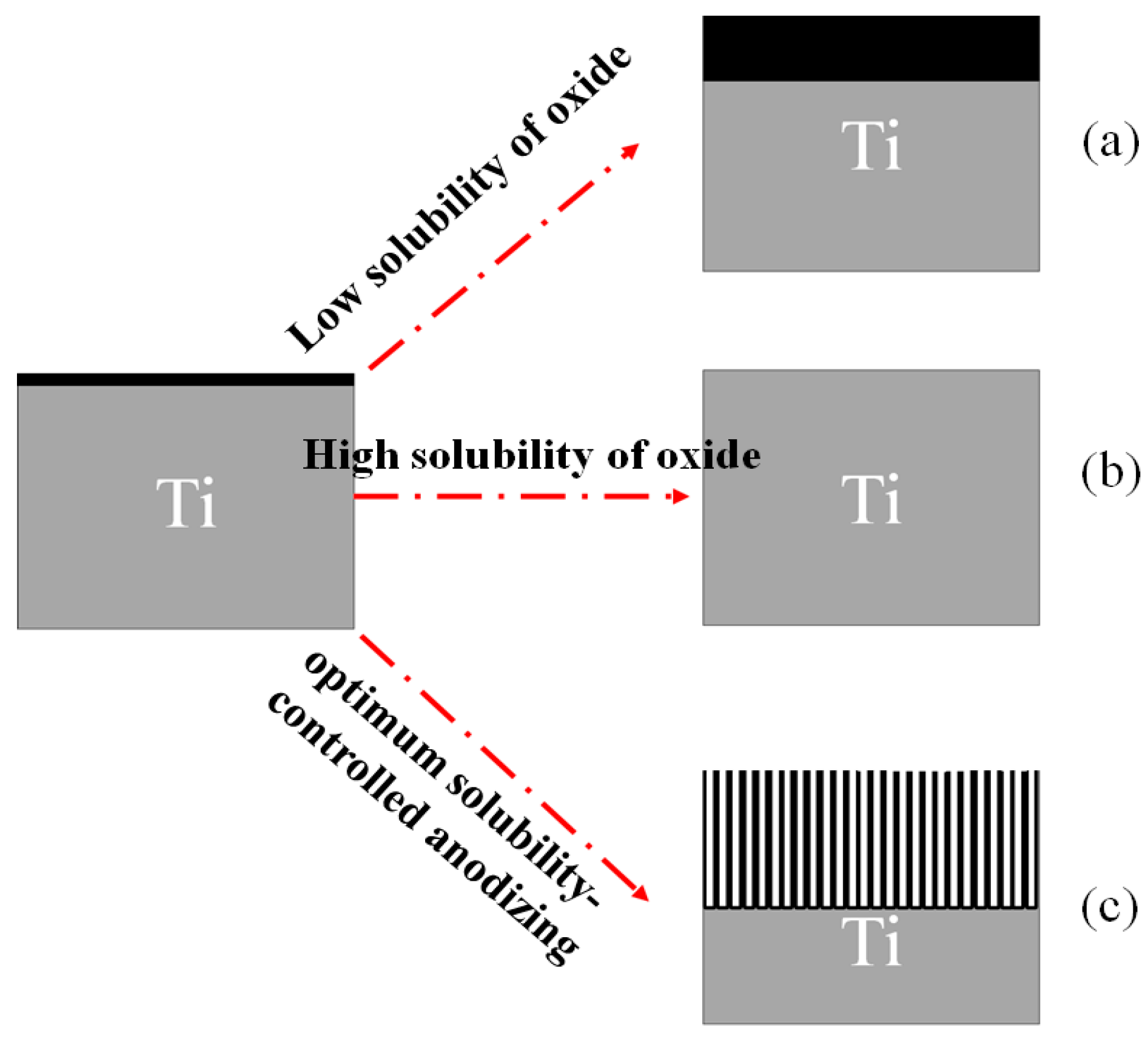
7.5.1. Principles, History and Mechanism
7.5.2. Effects of Parameters in Electrochemical Oxidation Method
Effect of Electrolytes
- Aqueous (acidic) electrolytes—characterized by high water and oxygen content, resulting in greater current densities and faster oxide dissolution.
- Organic or neutral electrolytes—with higher viscosity and lower water content, enabling better control over growth, more uniform structures, and longer tube layers.
Effect of Electrolyte pH
Effect of Applied Voltage
Effect of Electrolyte Temperature
- At 5 °C, the result is a thick, porous oxide layer.
- At 10 °C, the pores are visible but exhibit irregular ordering instead of the characteristic well-defined pattern.
- A significant improvement is seen at 15 °C, where regular pores are achieved, with diameters typically between 45 nm and 50 nm. Interestingly, at this temperature, secondary, smaller pores (two to three) are sometimes observed nucleating inside the primary pores.
- When the temperature is raised further, the resulting pores are highly ordered and exhibit a larger diameter of approximately 84 nm.
Effect of Current Density
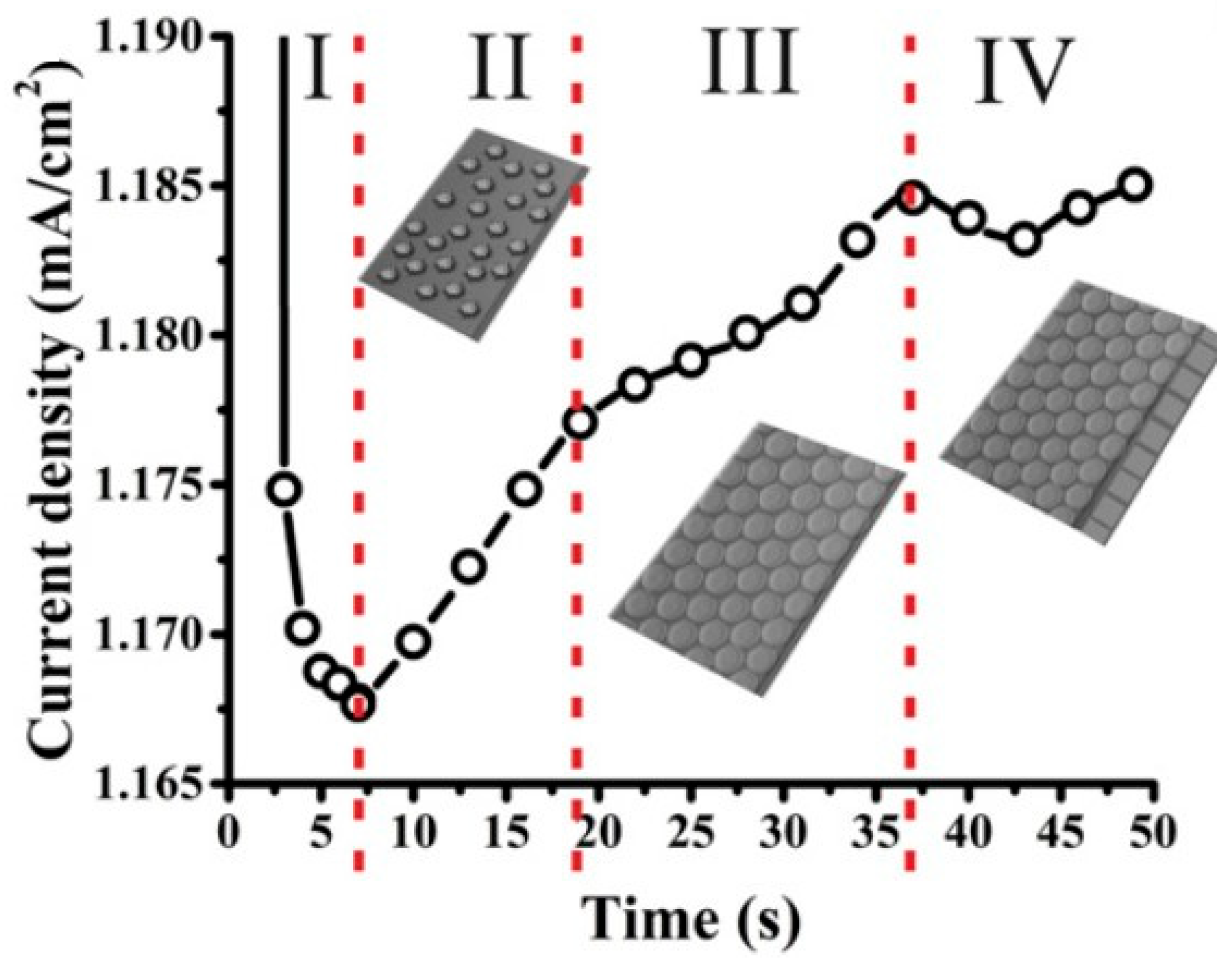
Effect of Anodization Time
Effect of Electrodes
- Stainless Steel (SS): Produced short TNTs with thin (5–10 nm), non-uniform wall thickness, and inconsistent diameters between the top and bottom, leading to conical structures that easily collapse.
- Yielded an aspect ratio similar to carbon, but the top surface re-sembled that from the SS cathode, resulting in bases that were stable but tops prone to collapse. Al-formed TNTs were notably less robust than those from a carbon CE.
- Iron (Fe): Formed well-organized TNTs with high aspect ratios, though these were still less durable than those formed with carbon.
- Carbon (C): Consistently produced TNTs exhibiting the highest aspect ratios, largest diameters, and greatest lengths compared to other tested materials. The quality was comparable to that of a platinum (Pt) cathode, but carbon offers a significant cost advantage.
- Decreasing the inter-electrode distance (e.g., from 4.5 to 0.5 cm) enhances electrolyte conductivity and titanium concentration. This results in TNTs with enlarged pore diameters, thicker walls, and wider inter-tubular spacing. The increased field strength and altered electrolyte properties are believed to drive the self-enlargement and separation of the nanotubes, leading to discrete, well-separated structures.
- Conversely, increasing the separation between electrodes causes the field strength at the anode to drop (due to higher IR), resulting in a significant decrease in pore size and a higher density of TNTs. The overall growth rate of the TNTs also diminishes with greater electrode separation.
8. Applications of TiO2 Nanotubes
8.1. Development of Gas Sensors
8.2. Production of Hydrogen Gas
8.3. Treatment of Water and Industrial Wastewater
8.4. Application as an Electrode
- TNTs provide a high active surface area, which is crucial for catalytic applications.
- They remain stable in both acidic and basic environments.
- TNTs offer a direct charging path compared to powder-based electrodes [143].
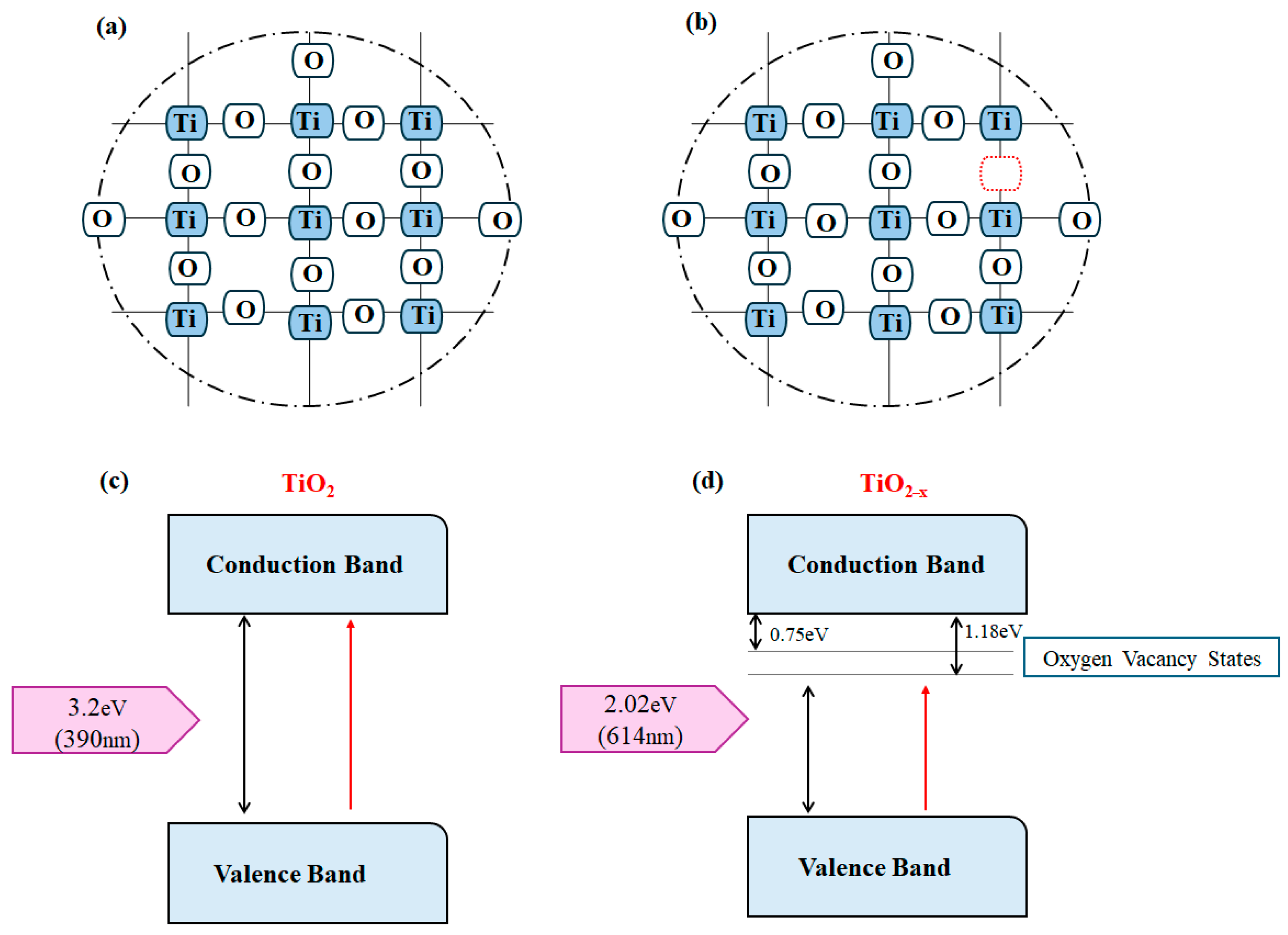
9. Challenges of TiO2 as an Ideal Photocatalyst for Hydrogen Production
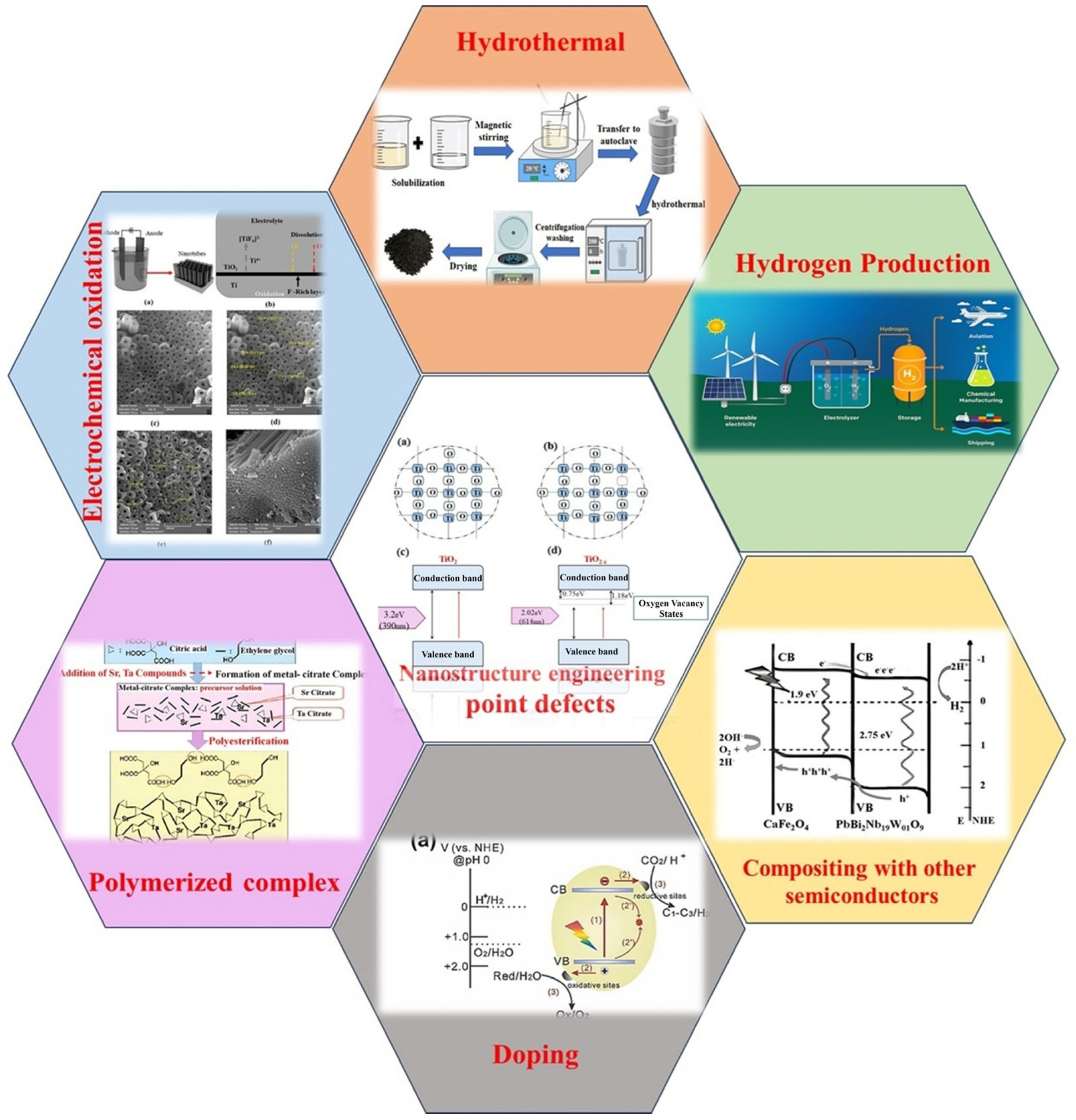
10. Modification of TiO2 Nanotubes for Hydrogen Production
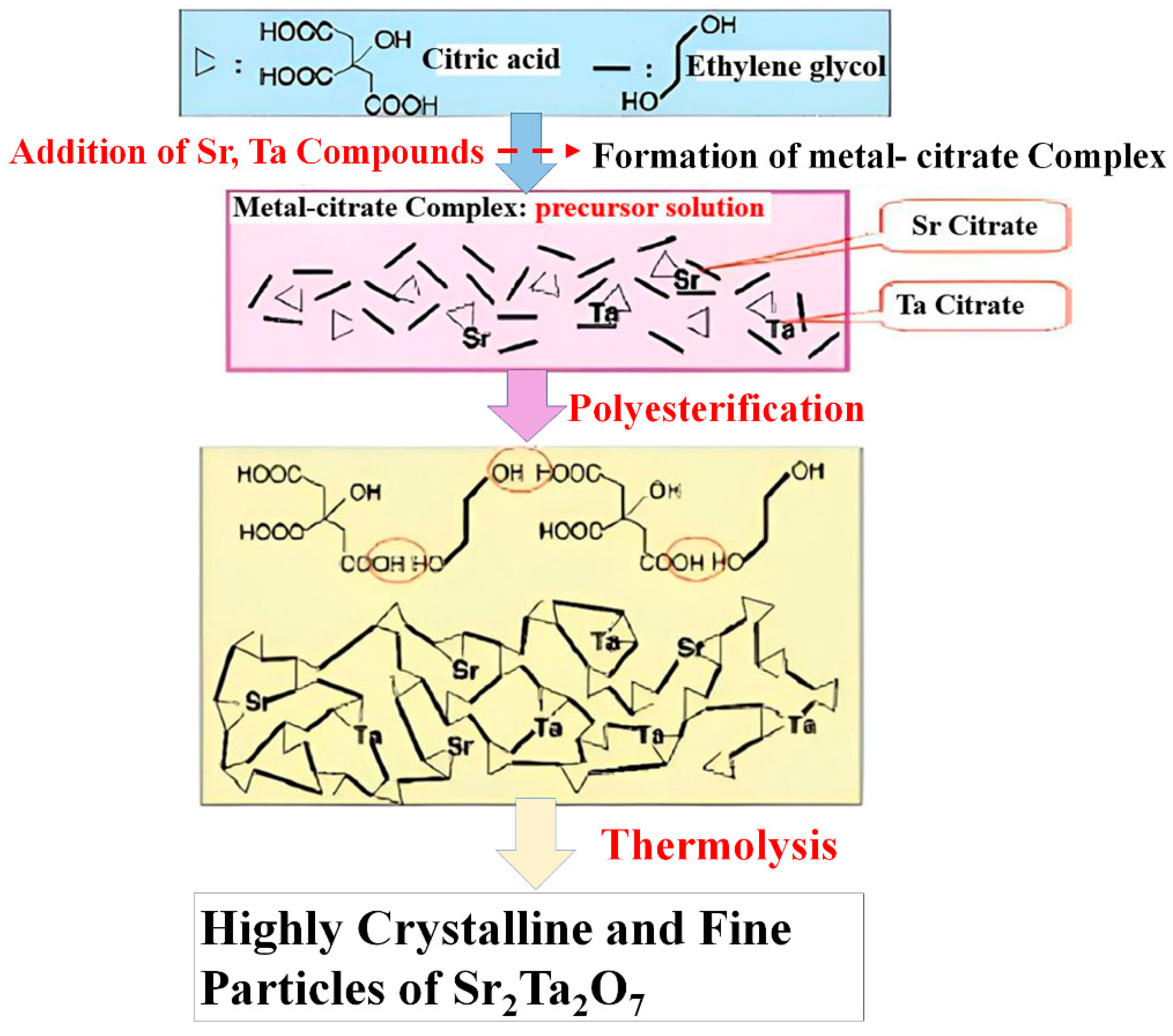
10.1. Polymerized Complex
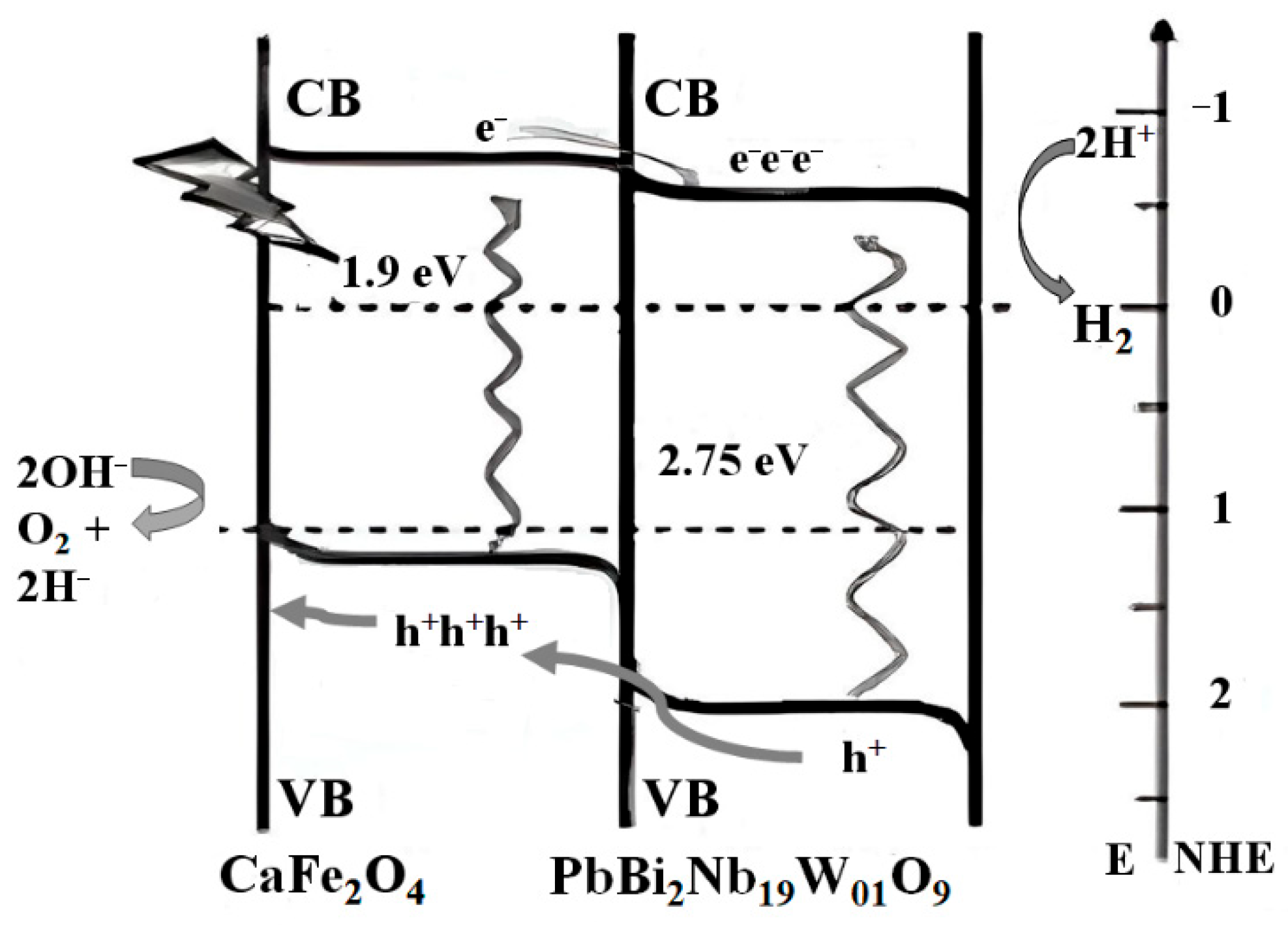
10.2. Compositing Titanium Dioxide with Other Semiconductors
10.3. Surface Modification of TiO2
10.4. Composite Engineering
10.5. Nanostructure Engineering
10.6. Doping
11. Conclusions
- Extension of light-absorption into the visible and solar-spectrum range
- 2.
- Improved charge carrier separation, transport and lifetime
- 3.
- Architectural and morphological optimization for high surface-area and accessibility
- 4.
- Scalable, stable and cost-effective fabrication and integration into devices
- ○
- Developing low-cost, high-throughput anodization or alternative synthesis methods with uniform, reproducible tubes.
- ○
- Ensuring long-term stability under real sunlight, in real water or wastewater conditions (with fouling, ions, pH variation).
- ○
- Integrating TNTs into photoelectrochemical cells, solar reactors or membranes, with minimal auxiliary energy input.
- ○
- Designing systems that minimize noble-metal use, recovery or recycling of catalysts, and enabling modular deployment.
- 5.
- Multi-functional applications beyond hydrogen generation
- ○
- Photocatalytic CO2 reduction to value-added fuels or chemicals via engineered TNT composites.
- ○
- Degradation of emerging contaminants (antibiotics, microplastics), disinfection, self-cleaning surfaces, and coupling photocatalysis with electrocatalysis. For example, TNTs have been applied in antibiotic-resistance-gene degradation in sludge.
- ○
- Coupled systems where solar energy is used simultaneously for pollutant removal and fuel generation, enhancing overall system economics and sustainability.
- 6.
- Mechanistic insight, modeling and smart material design.
- 7.
- Hybrid and tandem systems for full solar spectrum utilization
- 8.
- Environmental & economic assessment, life-cycle and sustainability
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Kashif, M.; Kim, J. Carbon Capture Science & Technology Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar]
- Li, H.; Cui, X. A hydrothermal route for constructing reduced graphene oxide/TiO2 nanocomposites: Enhanced photocatalytic activity for hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 19877–19886. [Google Scholar] [CrossRef]
- Sharma, S.; Pai, M.R.; Kaur, G.; Divya; Satsangi, V.R.; Dass, S.; Shrivastav, R. Efficient hydrogen generation on CuO core/Ag–TiO2 shell nano-hetero-structures by photocatalytic splitting of water. Renew. Energy 2019, 136, 1202–1216. [Google Scholar] [CrossRef]
- Xu, S.; Ng, J.; Zhang, X.; Bai, H.; Sun, D.D. Fabrication and comparison of highly efficient Cu incorporated TiO2 photocatalyst for hydrogen generation from water. Int. J. Hydrogen Energy 2010, 35, 5254–5261. [Google Scholar] [CrossRef]
- Long, L.; Li, J.; Wu, L.; Li, X. Enhanced photocatalytic performance of platinized CdS/TiO2 by optimizing calcination temperature of TiO2 nanotubes. Mater. Sci. Semicond. Process. 2014, 26, 107–111. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sánchez-Sánchez, M.C.; Alvarez-Galvan, M.C.; Del Valle, F.; Fierro, J.L.G. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Liao, C.H.; Huang, C.W.; Wu, J.C.S. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Nosaka, Y. Solar Cells and Photocatalysts. In Comprehensive Nanoscience and Technology; Elsevier: Amsterdam, The Netherlands, 2010; chapter 17; pp. 571–605. [Google Scholar]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Solakidou, M.; Giannakas, A.; Georgiou, Y.; Boukos, N.; Louloudi, M.; Deligiannakis, Y. Efficient photocatalytic water-splitting performance by ternary CdS/Pt-N-TiO2 and CdS/Pt-N, F-TiO2: Interplay between CdS photo corrosion and TiO2-dopping. Appl. Catal. B Environ. 2019, 254, 194–205. [Google Scholar] [CrossRef]
- El Aisnada, A.N.; Miyauchi, M.; Liu, M.; Yamaguchi, A. Recent update on electrochemical CO2 reduction catalyzed by metal sulfide materials. Mater. Rep. Energy 2023, 3, 100190. [Google Scholar] [CrossRef]
- Rayalu, S.S.; Dubey, N.; Labhsetwar, N.K.; Kagne, S.; Devotta, S. UV and visibly active photocatalysts for water splitting reaction. Int. J. Hydrogen Energy 2007, 32, 2776–2783. [Google Scholar] [CrossRef]
- Connelly, K.; Wahab, A.K.; Idriss, H. Photoreaction of Au/TiO2 for hydrogen production from renewables: A review on the synergistic effect between anatase and rutile phases of TiO2. Mater. Renew. Sustain. Energy 2012, 1, 3. [Google Scholar] [CrossRef]
- Wu, J.; Lu, S.; Ge, D.; Zhang, L.; Chen, W.; Gu, H. Photocatalytic properties of Pd/TiO2 nanosheets for hydrogen evolution from water splitting. RSC Adv. 2016, 6, 67502–67508. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, W.-H.; Long, R.; Prezhdo, O.V. Water Splitting with a Single-Atom Cu/TiO2 Photocatalyst: Atomistic Origin of High Efficiency and Proposed Enhancement by Spin Selection. JACS Au 2021, 1, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Galińska, A.; Walendziewski, J. Photocatalytic water splitting over Pt-TiO2 in the presence of sacrificial reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2013, 13, 14–20. [Google Scholar] [CrossRef]
- Choi, J.Y.; Sung, Y.H.; Choi, H.J.; Kim, Y.D.; Huh, D.; Lee, H. Fabrication of Au nanoparticle-decorated TiO2 nanotube arrays for stable photoelectrochemical water splitting by two-step anodization. Ceram. Int. 2017, 43, 14063–14067. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Inoue, Y. Photocatalytic water splitting by RuO2-loaded metal oxides and nitrides with d 0-and d 10-related electronic configurations. Energy Environ. Sci. 2009, 2, 364–386. [Google Scholar] [CrossRef]
- Ikeda, T.; Nomoto, T.; Eda, K.; Mizutani, Y.; Kato, H. Photoinduced dynamics of TiO2 doped with Cr and Sb. J. Phys. Chem. C 2008, 112, 1167–1173. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Dong, J.; Han, J.; Liu, Y.; Nakajima, A.; Matsushita, S.; Wei, S.; Gao, W. Defective black TiO2 synthesized via anodization for visible-light photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.; Keller, N.; Selli, E. Environmental photocatalysis and photochemistry for a sustainable world: A big challenge. Environ. Sci. Pollut. Res. 2017, 24, 12503–12505. [Google Scholar] [CrossRef]
- Prihod, R.V.; Soboleva, N.M. Photocatalysis: Oxidative processes in water treatment. J. Chem. 2013, 2013, 168701. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of Calcination Temperature of SiO2/TiO2 Photocatalysts on UV-VIS and VIS Removal Efficiency of Color Contaminants. Catalysts 2023, 13, 186. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Miseki, Y.; Sayama, K. Photocatalytic Water Splitting for Solar Hydrogen Production Using the Carbonate Effect and the Z-Scheme Reaction. Adv. Energy Mater. 2019, 9, 1801294. [Google Scholar] [CrossRef]
- Kiriakidis, G.; Binas, V. Metal oxide semiconductors as visible light photocatalysts. J. Korean Phys. Soc. 2014, 65, 297–302. [Google Scholar] [CrossRef]
- Martha, S.; Sahoo, P.C.; Parida, K.M. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535–61553. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Mechanism of anodic dissolution reaction of ZnO single crystal electrode under irradiation. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1972, 40, 33–38. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Mahajan, V.K.; Misra, M. Double-side illuminated titania nanotubes for high volume hydrogen generation by water splitting. Nanotechnology 2007, 18, 445705. [Google Scholar] [CrossRef]
- Nozik, A.J. Photoelectrolysis of water using semiconducting TiO2 crystals. Nature 1975, 257, 383–386. [Google Scholar] [CrossRef]
- Zohuri, B. Hydrogen Energy: Challenges and Solutions for a Cleaner Future; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Qureshi, F.; Tahir, M. Photoelectrochemical water splitting with engineering aspects for hydrogen production: Recent advances, strategies and challenges. Int. J. Hydrogen Energy 2024, 69, 760–776. [Google Scholar] [CrossRef]
- Nozik, A.J. Photochemical diodes. Appl. Phys. Lett. 1977, 30, 567–569. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Quiroz, J.; Homsi, M.S.; Camargo, P.H.C. An overview of the photocatalytic H2 evolution by semiconductor-based materials for nonspecialists. J. Braz. Chem. Soc. 2020, 31, 211–229. [Google Scholar] [CrossRef]
- Mekonnen, T.B. An overview on the photocatalytic degradation of organic pollutants in the presence of cerium oxide (CeO2) based nanoparticles: A review. Nanosci. Nanometrol. 2021, 7, 11648. [Google Scholar]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis—Past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Suresh, B.V. Solid State Devices and Technology; Pearson Education India: Chennai, India, 2010. [Google Scholar]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Ünlü, H. A thermodynamic model for determining pressure and temperature effects on the bandgap energies and other properties of some semiconductors. Solid State Electron. 1992, 35, 1343–1352. [Google Scholar] [CrossRef]
- Zhao, Y.; Hoivik, N.; Wang, K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Neamen, D.; Biswas, D. Semiconductor Physics and Devices; McGraw-Hill Higher Education: New York, NY, USA, 2011. [Google Scholar]
- Sze, S.M.; Li, Y.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Photocatalysis; Lecture Notes in Chemistry; Springer: Singapore, 2018. [Google Scholar]
- Wang, X.; Anpo, M.; Fu, X. Current Developments in Photocatalysis and Photocatalytic Materials: New Horizons in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- McGuinness, N.B.; John, H.; Kavitha, M.K.; Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning photocatalytic activity: Materials and applications. In Photocatalysis: Applications; Royal Society of Chemistry: London, UK, 2016; Chapter 8; pp. 204–235. [Google Scholar]
- Hauch, A.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M. Highly efficient high temperature electrolysis. J. Mater. Chem. 2008, 18, 2331–2340. [Google Scholar] [CrossRef]
- Hejazi, S.; Altomare, M.; Schmuki, P. Photo-Electrochemical Solar-to-Fuel Energy Conversion by Hematite-Based Photo-Anodes-The Role of 1D Nanostructuring. Z. Phys. Chem. 2020, 234, 615–631. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Xu, T.; Ji, W.; Zong, X. Recent Advances on Small Band Gap Semiconductor Materials (≤2.1 eV) for Solar Water Splitting. Catalysts 2023, 13, 728. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Álvarez Galván, M.C.; del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L.G. Water splitting on semiconductor catalysts under visiblelight irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Park, K.; Kim, Y.J.; Yoon, T.; David, S.; Song, Y.M. A methodological review on material growth and synthesis of solar-driven water splitting photoelectrochemical cells. RSC Adv. 2019, 9, 30112–30124. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Solar Water Splitting: Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.H.; Li, H.; Huang, D.; Xiao, S.; Qiu, W.; Li, M.; Hu, Y.; Mai, W.; Ji, H.; Yang, S. Enhancing photoelectrochemical water splitting by combining work function tuning and heterojunction engineering. Nat. Commun. 2019, 10, 3687. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Xu, M.; Xu, W.; Liu, X. Construction of g-C3N4-based photoelectrodes towards photoelectrochemical water splitting: A review. J. Alloys Compd. 2023, 969, 172302. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Idriss, H. The elusive photocatalytic water splitting reaction using sunlight on suspended nanoparticles: Is there a way forward? Catal. Sci. Technol. 2020, 10, 304–310. [Google Scholar] [CrossRef]
- Axet, M.R.; Durand, J.; Gouygou, M.; Serp, P. Surface coordination chemistry on graphene and two-dimensional carbon materials for well-defined single atom supported catalysts. Adv. Organomet. Chem. 2019, 71, 53–174. [Google Scholar]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Role of nanoparticles in photocatalysis. J. Nanoparticle Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Singh, A.; Goyal, V.; Singh, J.; Rawat, M. Structural, morphological, optical and photocatalytic properties of green synthesized TiO2 NPs. Curr. Res. Green Sustain. Chem. 2020, 3, 100033. [Google Scholar] [CrossRef]
- Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- Khataee, A.; Mansoori, G.A. Nanostructured Titanium Dioxide Materials: Properties, Preparation and Applications; World Scientific: Singapore, 2011. [Google Scholar]
- Aldosari, O.F.; Hussain, I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. Int. J. Hydrogen Energy 2024, 59, 958–981. [Google Scholar] [CrossRef]
- Kajli, S.K.; Ray, D.; Roy, S.C. Space charge limited conduction in anatase and mixed-phase (anatase/rutile) single TiO2 nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 136, 115030. [Google Scholar] [CrossRef]
- Mohajernia, S. Variation of Grey Titania: Control over Defect Types and Density for Energy Conversion and Energy Storage Application. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2020. [Google Scholar]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Wu, Y.; Andryskova, P.; Zoppellaro, G. Electrochemistry Communications Intrinsic Cu nanoparticle decoration of TiO2 nanotubes: A platform for efficient noble metal free photocatalytic H2 production. Electrochem. Commun. 2018, 98, 82–86. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Yan, K.; Bai, Y.; Yang, S. Unveiling two electron-transport modes in oxygen-deficient TiO2 nanowires and their influence on photoelectrochemical operation. J. Phys. Chem. Lett. 2014, 5, 2890–2896. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for efficient photocatalyst: Fabrication and activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef]
- Sreekantan, S.; Wei, L.C.; Srimala, S.; Wei, L.C. Study on the formation and photocatalytic activity of titanate nanotubes synthesized via hydrothermal method. J. Alloys Compd. 2010, 490, 436–442. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.; Pillai, S.C. Black TiO2 nanomaterials: A review of recent advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Vlachos, C.; Andrews, E.H. Oxide morphology and adhesive bonding on titanium surfaces. J. Mater. Sci. 1984, 19, 3626–3639. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and Physicochemistry of Anodic Oxide Films on Titanium and TA6V Alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Bagher, A.M. Types of Solar Cells and Application. Am. J. Opt. Photonics 2015, 3, 94. [Google Scholar] [CrossRef]
- Nasirpouri, F. Electrodeposition of Nanostructured Materials; Springer International Publishing: Cham, Switzerland, 2017; p. 62. [Google Scholar]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2018, 9, 345–364. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 μm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys. Status Solidi–Rapid Res. Lett. 2007, 1, 65–67. [Google Scholar] [CrossRef]
- Özkan, S. Self-Ordered Arrays of “Spaced” Nanotubes and Their Applications in Energy Conversion; Friedrich-Alexander-Universitaet Erlangen-Nuernberg: Erlangen, Germany, 2017. [Google Scholar]
- Li, Q.; Li, Y.; Zeng, W. Preparation and application of 2D MXene-based gas sensors: A review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Viswanathan, B.; Varadaraju, U.V. Fabrication and characterization of uniform TiO2 nanotube arrays by sol-gel template method. Bull. Mater. Sci. 2006, 29, 705–708. [Google Scholar]
- Hsieh, C.T.; Lai, M.H.; Pan, C. Synthesis and visible-light-derived photocatalysis of titania nanosphere stacking layers prepared by chemical vapor deposition. J. Chem. Technol. Biotechnol. 2010, 85, 1168–1174. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Tamarani, A.; Zainul, R.; Dewata, I. Preparation and characterization of XRD nano Cu-TiO2 using sol-gel method. J. Phys. Conf. Ser. 2019, 1185, 12020. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on TiO2. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Li, H. Templated synthesis of TiO2 nanotube macrostructures and their photocatalytic properties. Nano Res. 2015, 8, 900–906. [Google Scholar] [CrossRef]
- Jung, J.H.; Kobayashi, H.; Van Bommel, K.J.C.; Shinkai, S.; Shimizu, T. Creation of novel helical ribbon and double-layered nanotube TiO2 structures using an organogel template. Chem. Mater. 2002, 14, 1445–1447. [Google Scholar] [CrossRef]
- Roy, P.; Steffen, B.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Tavangar, R.; Namdar-Asl, H.; Esfandiari, N.; Khorashadizade, E. Enhanced photoelectrochemical water splitting via hydrogenated TiO2 nanotubes modified with Cu/CuO species. J. Photochem. Photobiol. A Chem. 2024, 452, 115586. [Google Scholar] [CrossRef]
- Duan, Z.; Huang, Y.; Zhang, D.; Chen, S. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Sci. Rep. 2019, 9, 8008. [Google Scholar] [CrossRef] [PubMed]
- Koohi, F.; Zare, H.R.; Shekari, Z. Decoration of titanium dioxide nanotubes with silver nanoparticles using the photochemical deposition method and their application as an electrocatalyst to determine tinidazole. Anal. Methods 2021, 13, 5343–5350. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Cui, J.; Xu, G.; Xu, G. Ag nanoparticles decorated TiO2 nanotube arrays for ultrasensitive gas sensing. J. Nanosci. Nanotechnol. 2013, 13, 1453–1455. [Google Scholar] [CrossRef]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
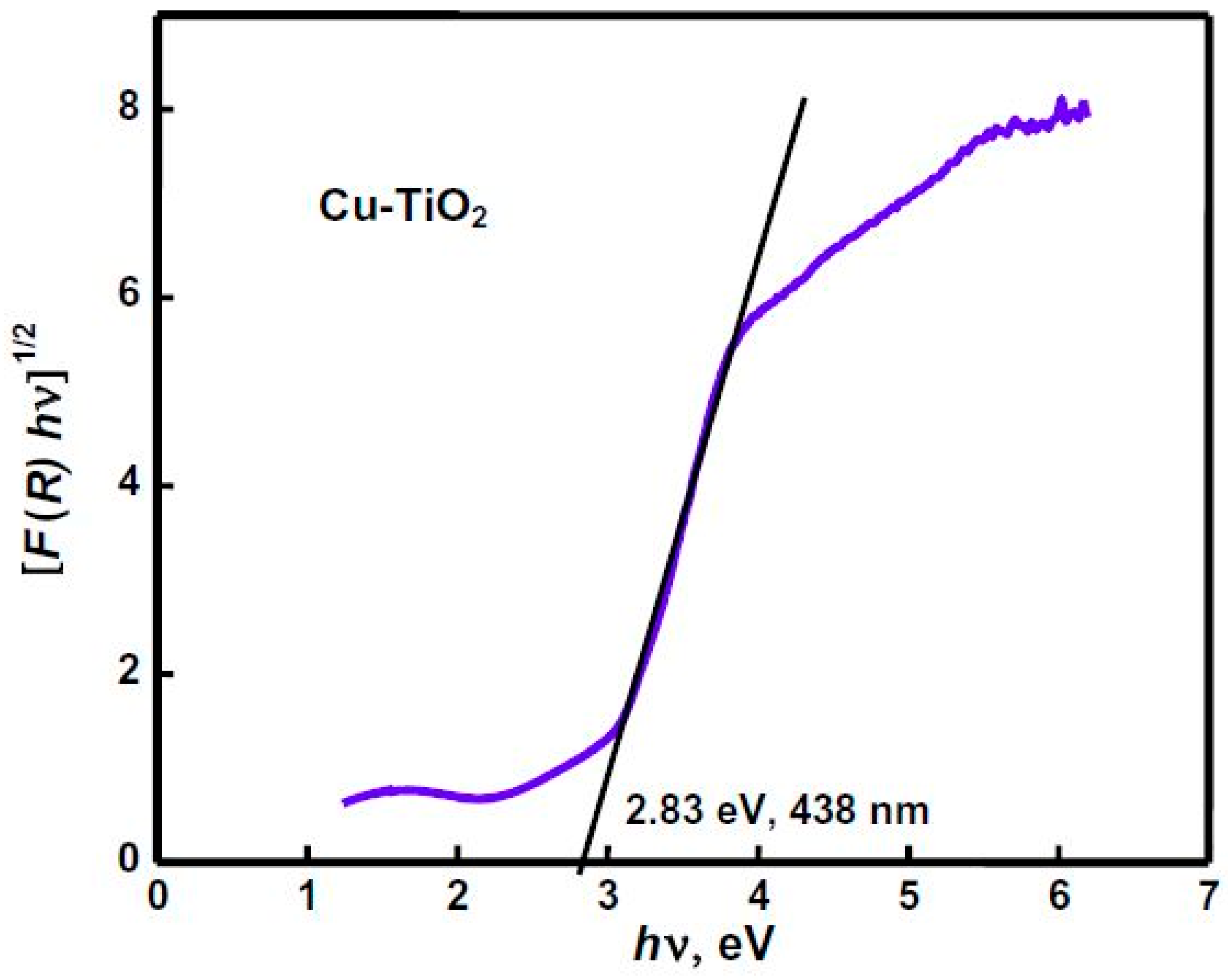
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceram. Int. 2015, 41, 8735–8741. [Google Scholar] [CrossRef]
- Ennaceri, H.; Fischer, K.; Hanus, K.; Chemseddine, A.; Prager, A. Effect of morphology on the photoelectrochemical activity of TiO2 self-organized nanotube arrays. Catalysts 2020, 10, 279. [Google Scholar] [CrossRef]
- Pourandarjani, A.; Nasirpouri, F. The effect of first step anodization time on morphology and photocurrent response of TiO2 nanotube arrays for application in backside illuminated dye-sensitized solar cells. Thin Solid Films 2017, 640, 1–7. [Google Scholar] [CrossRef]
- Bae, S.; Shim, E.; Yoon, J.; Joo, H. Enzymatic hydrogen production by light-sensitized anodized tubular TiO2 photoanode. Sol. Energy Mater. Sol. Cells 2008, 92, 402–409. [Google Scholar] [CrossRef]
- Indira, K.; Ningshen, S.; Mudali, U.K.; Rajendran, N. Effect of anodization parameters on the structural morphology of titanium in fluoride containing electrolytes. Mater. Charact. 2012, 71, 58–65. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Taveira, L.; Schmuki, P. Self-organized porous TiO2 and ZrO2 produced by anodization. Corros. Sci. 2005, 47, 3324–3335. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Li, L. Electrochemical fabrication of well-ordered titania nanotubes in H3PO4/HF electrolytes. Electron. Lett. 2005, 41, 771–772. [Google Scholar] [CrossRef]
- Bae, C.; Yoon, Y.; Yoo, H.; Han, D.; Cho, J.; Lee, B.H. Controlled fabrication of multiwall anatase TiO2 nanotubular architectures. Chem. Mater. 2009, 21, 2574–2576. [Google Scholar] [CrossRef]
- Chen, X.; Schriver, M.; Suen, T.; Mao, S.S. Fabrication of 10 nm diameter TiO2 nanotube arrays by titanium anodization. Thin Solid Films 2007, 515, 8511–8514. [Google Scholar] [CrossRef]
- Ryu, W.H.; Park, C.J.; Kwon, H.S. Synthesis of highly ordered TiO2 nanotube in malonic acid solution by anodization. J. Nanosci. Nanotechnol. 2008, 8, 5467–5470. [Google Scholar] [CrossRef]
- Richter, C.; Panaitescu, E.; Willey, R.; Menon, L. Titania nanotubes prepared by anodization in fluorine-free acids. J. Mater. Res. 2007, 22, 1624–1631. [Google Scholar] [CrossRef]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochimica Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A.; Ong, K.G. Unprecedented ultra-high hydrogen gas sensitivity in undoped titania nanotubes. Nanotechnology 2005, 17, 398. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Tsuchiya, H.; Schmuki, P. Wetting behaviour of layers of TiO2 nanotubes with different diameters. J. Mater. Chem. 2005, 15, 4488–4491. [Google Scholar] [CrossRef]
- Kim, D.; Fujimoto, S.; Schmuki, P.; Tsuchiya, H. Nitrogen doped anodic TiO2 nanotubes grown from nitrogen-containing Ti alloys. Electrochem. Commun. 2008, 10, 910–913. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Balaur, E.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized TiO2 nanotubes prepared in ammonium fluoride containing acetic acid electrolytes. Electrochem. Commun. 2005, 7, 576–580. [Google Scholar] [CrossRef]
- Ghicov, A.; Tsuchiya, H.; Macak, J.M.; Schmuki, P. Titanium oxide nanotubes prepared in phosphate electrolytes. Electrochem. Commun. 2005, 7, 505–509. [Google Scholar] [CrossRef]
- Sun, K.C.; Chen, Y.C.; Kuo, M.Y.; Wang, H.W.; Lu, Y.F.; Chung, J.C. Synthesis and characterization of highly ordered TiO2 nanotube arrays for hydrogen generation via water splitting. Mater. Chem. Phys. 2011, 129, 35–39. [Google Scholar] [CrossRef]
- Joo, S.; Muto, I.; Hara, N. In situ ellipsometric analysis of growth processes of anodic TiO2 nanotube films. J. Electrochem. Soc. 2008, 155, C154. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.; Chen, X. Self-Assembled TiO2 Nanotube Arrays with U-Shaped Profile by Controlling Anodization Temperature. J. Nanomater. 2010, 1, 753253. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Räder, A.S.; Taveira, L.; Schmuki, P. Characterization of electronic properties of TiO2 nanotube films. Corros. Sci. 2007, 49, 203–210. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Han, J.; Ji, L.; Wang, J.; Chen, J.; Wang, Y. Controllable preparation, growth mechanism and the properties research of TiO2 nanotube arrays. Appl. Surf. Sci. 2014, 297, 103–108. [Google Scholar] [CrossRef]
- Regonini, D.; Clemens, F.J. Anodized TiO2 nanotubes: Effect of anodizing time on film length, morphology and photoelectrochemical properties. Mater. Lett. 2015, 142, 97–101. [Google Scholar] [CrossRef]
- Xin, W.; Meng, C.; Jie, W.; Junchao, T.; Yan, S.; Ning, D. Morphology dependence of TiO2 nanotube arrays on anodization variables and buffer medium. J. Semicond. 2010, 31, 063003. [Google Scholar] [CrossRef]
- Sreekantan, S.; Saharudin, K.A.; Wei, L.C. Formation of TiO2 nanotubes via anodization and potential applications for photocatalysts, biomedical materials, and photoelectrochemical cell. IOP Conf. Ser. Mater. Sci. Eng. 2011, 21, 012002. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Effect of cathode material on the morphology and photoelectrochemical properties of vertically oriented TiO2 nanotube arrays. Sol. Energy Mater. Sol. Cells 2008, 92, 1468–1475. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Yam, F.K.; Eid, A.M. Water-splitting properties of bi-phased TiO2 nanotube arrays subjected to high-temperature annealing. Ceram. Int. 2020, 46, 21471–21481. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Yam, F.K.; Eid, A.M.; Nazeer, A.A. Enhanced photoelectrochemical properties of hierarchical black TiO2-x nanolaces for Cr (VI) photocatalytic reduction. Int. J. Hydrogen Energy 2020, 45, 22674–22690. [Google Scholar] [CrossRef]
- Cui, H. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 2, 8612–8616. [Google Scholar] [CrossRef]
- Feng, T.; Yam, F.K. The influence of hydrothermal treatment on TiO2 nanostructure films transformed from titanates and their photoelectrochemical water splitting properties. Surf. Interfaces 2023, 38, 102767. [Google Scholar] [CrossRef]
- Feng, T.; Yam, F.K. Photoelectrochemical properties of anatase TiO2 nanostructures prepared by hydrothermal method: Effect of illumination and external bias on charge transport. Ceram. Int. 2024, 50, 19886–19897. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, R.J. Ball, Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen production using TiO2-based photocatalysts: A comprehensive review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- Raj, C.C.; Prasanth, R. Review—Advent of TiO2 Nanotubes as Supercapacitor Electrode. J. Electrochem. Soc. 2018, 165, 345–358. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Fujihara, K.; Ohno, T.; Matsumura, M. Splitting of water by electrochemical combination of two photocatalytic reactions on TiO2 particles. J. Chem. Soc. Faraday Trans. 1998, 94, 3705–3709. [Google Scholar] [CrossRef]
- Tabata, M. Modified Ta3N5 powder as a photocatalyst for O2 evolution in a two-step water splitting system with an iodate/iodide shuttle redox mediator under visible light. Langmuir 2010, 26, 9161–9165. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; John, B.K.; Abraham, T.; Mathew, B. Metal-Doped Titanium Dioxide for Environmental Remediation”, Hydrogen Evolution and Sensing: A Review. ChemistrySelect 2021, 6, 12742–12751. [Google Scholar] [CrossRef]
- Chen, J.; Dai, S.; Liu, L.; Maitz, M.F.; Liao, Y. Photo-functionalized TiO2 nanotubes decorated with multifunctional Ag nanoparticles for enhanced vascular biocompatibility. Bioact. Mater. 2021, 6, 45–54. [Google Scholar] [CrossRef]
- Esmaeilnejad, A.; Mahmoudi, P.; Zamanian, A.; Mozafari, M. Synthesis of titanium oxide nanotubes and their decoration by MnO nanoparticles for biomedical applications. Ceram. Int. 2019, 45, 19275–19282. [Google Scholar] [CrossRef]
- Abe, H.; Kimura, Y.; Ma, T.; Tadaki, D.; Hirano-Iwata, A. Response characteristics of a highly sensitive gas sensor using a titanium oxide nanotube film decorated with platinum nanoparticles. Sens. Actuators B Chem. 2020, 321, 128525. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Ng, J.; Xu, S.; Zhang, X.; Yang, H.Y.; Sun, D.D. Hybridized nanowires and cubes: A novel architecture of a heterojunctioned TiO2/SrTiO3 thin film for efficient water splitting. Adv. Funct. Mater. 2010, 20, 4287–4294. [Google Scholar] [CrossRef]
- Shareef, M.; Batool, H.; Shehzadi, A.; Aftab, F.; Munir, A.; Manzoor, S.; Gohar, R.S.; Rehman, Z.; Ashiq, M.N.; Nazar, M.F. Structural functionalized TiO2-mediated photocatalysts as dye degradation agents: A systematic review. Mater. Res. Bull. 2025, 195, 113852. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, M.M.; Akhtar, S. Studies on Synthesis, Characterization, and Photocatalytic Activity of TiO2 and Cr-Doped TiO2 for the Degradation of p-Chlorophenol. ACS Omega 2023, 8, 1979–1988. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Pava-Gómez, B.; Vargas-Ramírez, X.; Díaz-Uribe, C. Physicochemical study of adsorption and photodegradation processes of methylene blue on copper-doped TiO2 films. J. Photochem. Photobiol. A Chem. 2018, 360, 13–25. [Google Scholar] [CrossRef]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the Photocatalytic Activity of Copper Doped TiO2 nanoparticles for the Purification and/or Disinfection of Industrial Effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Dopant induced changes in structural and optical properties of Cr3+ doped TiO2 nanoparticles. Mater. Chem. Phys. 2012, 132, 1112–1118. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumble, S.; Gil, A.; Munoz, D. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surfaces Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- Vo, T.L.N.; Dao, T.T.; Duong, A.T.; Bui, V.H.; Nguyen, V.H.; Nguyen, D.L.; Nguyen, H.T. Cuong Nguyen, enhanced photocatalytic degradation of organic dyes using Ce-doped TiO2 thin films. J. Sol-Gel Sci. Technol. 2023, 108, 423–434. [Google Scholar] [CrossRef]
- Jedi-Soltanabadi, Z.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Anodic growth of Nitrogen-doped Titanium dioxide nanotubes by anodization process of elemental Titanium in ethylene glycol-based electrolyte solution with different water contents. Vacuum 2018, 155, 387–390. [Google Scholar] [CrossRef]
- Jennifer, M.M.; Prabha, A.J.; Mahesh, R.; Sasikumar, M.; Ashok, S.; Ganesh, V. Synergistic effects of boron and carbon doping in 1D TiO2 nanotube arrays for enhanced bi-functional electrochemical water splitting application. Inorg. Chem. Commun. 2025, 183, 115711. [Google Scholar] [CrossRef]
- Amaral, R.; Blois, C.; Lunz, J.; Mello, A.; Jardim, P. Physical and optical properties of Ag3PO4 decorated TiO2 based nanostructures. J. Solid State Chem. 2022, 305, 122655. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, T.; Huang, S.; Pu, Y.; Wei, S.; Gao, W. Co-deposition of Ag and Co3O4 on black TiO2-x nanotubes with enhanced photocatalytic activity under visible light irradiation. J. Mater. Sci. 2022, 57, 2455–2466. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Yoon, J. Enhanced photocatalytic activity of TiO2 nanotubes decorated with erbium and reduced graphene oxide. Appl. Surf. Sci. 2021, 565, 150459. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C. Nanostructured Materials: Physicochemical Fundamentals for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Khaliq, N.; Rasheed, M.A.; Cha, G.; Khan, M.; Karim, S.; Schmuki, P. Development of non-enzymatic cholesterol bio-sensor based on TiO2 nanotubes decorated with Cu2O nanoparticles. Sens. Actuators B Chem. 2020, 302, 127200. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ba-Abbad, M.M.; Mohammad, A.W. Novel nanohybrid polysulfone membrane embedded with silver nanoparticles on graphene oxide nanoplates. Chem. Eng. J. 2015, 277, 107. [Google Scholar] [CrossRef]
- Hejazi, S. Utilization of Noble Metal Co-Catalysts on TiO2 for Photocatalytic H2 Production: From Nanoparticle Positioning to Single-Atom Catalysis. Ph.D. Thesis, Friedrich-Alexander-Universitaet Erlangen-Nuernberg, Bavaria, Germany, 2020. [Google Scholar]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, L.; Cai, Q. An electro-catalytic biosensor fabricated with Pt-Au nanoparticle-decorated titania nanotube array. Bioelectrochemistry 2008, 74, 62–65. [Google Scholar] [CrossRef]
- Yu, A.; Xun, H.; Yi, J. Improving hydrogen sensing performance of TiO2 nanotube arrays by zno modification. Front. Mater. 2019, 6, 3389. [Google Scholar] [CrossRef]
- Joo, S.; Muto, I.; Hara, N. Hydrogen gas sensor using Pt-and Pd-added anodic TiO2 nanotube films. J. Electrochem. Soc. 2010, 157, J221–J226. [Google Scholar] [CrossRef]
- Michalicka, J.; Macak, J.M. TiO2 nanotube layers decorated by titania nanoparticles as anodes for Li-ion microbatteries. Mater. Chem. Phys. 2022, 276, 356–363. [Google Scholar]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, N.; Zhang, Y.; Xiao, X. Drug Release Effect on Large-Clearance TiO2 Nanotube Arrays Decorated with P25 Nanoparticles. J. Nanomater. 2021, 2021, 3973784. [Google Scholar] [CrossRef]
- Xie, Y.L.; Ben, C.J.; Guo, L.F. Enhanced Photocatalytic Performance of Anodized TiO2 Nanotube Arrays Decorated with BiVO4 Nanoparticles and Its Application for Rhodamine B Degradation. Int. J. Electrochem. Sci. 2021, 16, 20964. [Google Scholar] [CrossRef]
- Cicmancova, V.; Dvorak, F.; Macak, J.M. One-step decoration of TiO2 nanotubes with Fe3O4 nanoparticles: Synthesis and photocatalytic and magnetic properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar]
- Li, Y.; Si, W.; Pan, Y.; Chen, R.; Tan, H.; Wang, Y. Tuning active sites of carbon nitride using NiO nanoparticles for efficient photocatalytic generation of hydrogen peroxide. Appl. Surf. Sci. 2024, 662, 160111. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, H.J.; Youn, J.I.; Kim, Y.J.; Suh, S.J.; Oh, H.J. Photocatalytic characterization of TiO2 nanotubes with Pd particles synthesized by photoreduction process. Korean J. Met. Mater. 2019, 57, 510–520. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, Z.; Lin, S.; Wu, Z.; Sun, C.Y. Electrochemical synthesis of perovskite LaFeO3 nanoparticle-modified TiO2 nanotube arrays for enhanced visible-light photocatalytic activity. New J. Chem. 2019, 43, 16506–16514. [Google Scholar] [CrossRef]
- Pisarek, M.; Kędzierzawski, P.; Andrzejczuk, M.; Hołdyński, M.; Mikołajczuk-Zychora, A.; Borodziński, A.; Janik-Czachor, M. TiO2 nanotubes with Pt and Pd nanoparticles as catalysts for electro-oxidation of formic acid. Materials 2020, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Pisarek, M.; Krawczyk, M.; Kosiński, A.; Hołdyński, M.; Andrzejczuk, M.; Krajczewski, J.; Lisowski, W. Materials characterization of TiO2 nanotubes decorated by Au nanoparticles for photoelectrochemical applications. RSC Adv. 2021, 11, 38727–38738. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Pan, Y.; Wang, M. Adsorptive-photocatalytic performance and mechanism of Me (Mn, Fe)-N co-doped TiO2/SiO2 in cyanide wastewater. J. Alloys Compd. 2021, 867, 159020. [Google Scholar] [CrossRef]
- Jyothilakshmi, V.P.; Bhabhina, N.M.; Dharsana, M.V.; Swaminathan, S. Wet chemical synthesis of lead sulfide nanoparticles and its application as light harvester in photovoltaic cell. Mater. Today Proc. 2020, 33, 2125–2129. [Google Scholar] [CrossRef]
- Iqbal, T.; Khan, M.A.R.; Batool, S.K.; Shafique, M.; Waheed, A.; Wee, M.M.R.; Iqbal, Q. Synthesis of hematite (α-Fe2O3) nanoparticles by a liquid-phase microplasma-assisted electrochemical process for photocatalytic activity. Phys. Scr. 2023, 98, 045810. [Google Scholar] [CrossRef]
- Sagadevan, S.; Sivasankaran, R.P.; Lett, J.A.; Fatimah, I.; Weldegebrieal, G.K.; Léonard, E.; Soga, T. Evaluation of photocatalytic activity and electrochemical properties of hematite nanoparticles. Symmetry 2023, 15, 1139. [Google Scholar] [CrossRef]
- Fazil, M.; Ahmad, T. Pristine TiO2 and Sr-doped TiO2 nanostructures for enhanced photocatalytic and electrocatalytic water splitting applications. Catalysts 2023, 13, 93. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Tu, L.T.N.; Man, T.V.; Mathesh, M.; Nam, N.D.; Thu, V.T.H. A high-efficiency photoelectrochemistry of Cu2O/TiO2 nanotubes-based composite for hydrogen evolution under sunlight. Compos. Part B Eng. 2019, 174, 106969. [Google Scholar] [CrossRef]
- Li, S.; Ng, Y.H.; Zhu, R.; Lv, S.; Wu, C.; Liu, Y.; Jing, L.; Deng, J.; Dai, H. In situ construction of elemental phosphorus nanorod-modified TiO2 photocatalysts for efficient visible-light-driven H2 generation. Appl. Catal. B Environ. 2021, 297, 120412. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Q.; Zhu, S.; Zhang, X.; Li, Y.; Cui, Y.; Xue, Z.; Gao, S. Hydrothermal construction of flower-like MoS2 on TiO2 NTs for highly efficient environmental remediation and photocatalytic hydrogen evolution. Sep. Purif. Technol. 2021, 265, 118463. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Jiang, Y.; Lan, H.; Guo, W.; Cao, Y. Origin of the improved photocatalytic activity of Cu incorporated TiO2 for hydrogen generation from water. Appl. Surf. Sci. 2016, 382, 170–177. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, P.; Wang, Q.; Wu, Y.; Cao, D.; Qiao, Q.A. Construction of Bi2S3-BiOBr nanosheets on TiO2 NTA as the effective photocatalysts: Pollutant removal, photoelectric conversion and hydrogen generation. J. Colloid Interface Sci. 2021, 585, 459–469. [Google Scholar] [CrossRef] [PubMed]


| Brookite | Rutile | Anatase | |
|---|---|---|---|
| Orthorhombic | Tetragonal | Tetragonal | Unit Cell |
| a = 5.455 | a, b = 4.593 | a, b = 3.784 | Latice Parameter (Å) |
| b = 9.181 | c = 2.959 | c = 9.515 | |
| c = 5.142 | |||
| 1843 | 1840 | 1825 | Melting point (°C) |
| 3.3 | 3 | 3.2 | Gap energy (eV) |
| 4.18 | 4.52 | 3.89 | Density (g/cm3) |
| n-type | n-type | n-type | Semiconductors type |
| 2.58 | 2.94 | 2.56 | Refractive index (ηα) |
| 56.2 | 55.06 | 55.52 | Heat capacity, Cp (J/kg·K) |
| Synthesis Method | Advantages | Disadvantages | Diameter of Nanotubes |
|---|---|---|---|
| Hydrothermal | 1—Simple preparation for industrial-scale applications 2—Ability to manipulate numerous variables to produce nanotubes with diverse characteristics 3—High length-to-diameter ratio and significant cation exchange capacity | 1—Long reaction times 2—High concentrations of NaOH 3—Thermal instability 4—Synthesizing identical nanotubes is challenging. | Inner diameter: 3 to 10 nm Length of TNTs: 50 to 500 nm |
| Electrochemical oxidation | 1—Suitable for study applications 2—Synthesis of regular nanotubes with a very high length-to-diameter ratio 3—Ability to control the diameter, wall thickness, and height of nanotubes 4—Applicable for various uses, including electrochemical, photocatalytic, and self-cleaning applications | 1—Limitations of mass production 2—Difficulty in separating nanotubes from the surface 3—High cost of essential equipment 4—Toxicity of certain materials used, such as fluoride ions | Inner diameter: 0.1 to 2.4 nm Length of TNTs: 20 to 110 nm |
| Using of the mold | 1—Controllable scaling of nanotubes in various formats 2—Suitable for research applications | 1—Need to high-cost materials and their limited shelf life 2—The complex manufacturing process may lead to the destruction of nanotubes during production. | Inner diameter: 0.05 to 200 nm Length of TNTs: 5.2 to 600 nm |
| Preparation Method | Synthesize Parameters | Phase | Morphology | Band Gap Energy | Ref |
|---|---|---|---|---|---|
| Anodizing | at 60 V for 2 h and annealing at 850 °C | Rutile | nanotubes | 3.09 eV | [135] |
| Anodizing | at 60 V for 2 h and annealing at 650 °C | Rutile | nanotubes | 3.39 eV | [136] |
| Anodizing | Two step anodizing at 100 V for 25 min and 100 V for another 5 min | Amorphous | Nanotube | 3.06 eV | [137] |
| Hydrothermal | at 140 °C for 12 h, annealing at 500 °C for 90 min | anatase | nanosheets | 3.22 eV | [138] |
| Hydrothermal | at 210 °C for 6 h, annealing at 500 °C for 90 min | anatase | nanowires | 3.29 eV | [139] |
| Sol–gel | hydrolysis and polymerization of a metal–organic precursor to form a colloidal suspension at 80 °C | A combination of anatase and brookite | Aggregated nanoparticles | 3.31 eV | [140] |
| Sol–gel | Sol–gel at 150 °C | anatase | Aggregated spherical particles | 2.98 eV | [141] |
| Doped Elements | Method | Effect of Doping |
|---|---|---|
| Fe | One-step electrochemical oxidation at 25 °C and 60 V for 6 h | Energy gap: 2.2–3 eV (undoped sample: 3.18 eV) |
| Cu | One-step electrochemical oxidation at room temperature and a voltage of 20 V for 1 h | Energy gap: 2.65 eV (undoped sample: 3.20 eV) Amount of hydrogen produced: 29 µL.cm−2.2 h (undoped sample: 7.6 µL.cm−2.2 h) |
| Cr | One-step electrochemical oxidation at 25 °C and 60 V for 6 h | Energy gap: 2.2–2.82 eV (undoped sample: 3.2 eV) Amount of hydrogen produced: 12–37 µL.cm−2.2 h (undoped sample: 0 µL.cm−2.2 h) |
| Zr | Two-step electrochemical oxidation | Offering higher photocatalytic activities than pure TNTs arrays and being reusable |
| Zn | One-step electrochemical oxidation at 25 °C with 30 V applied for 15 h | Energy gap: 2.2 eV (undoped sample: 3 eV) |
| V | Hydrothermal method at 130 °C for 3 h | Energy gap: 2.91 eV (undoped sample: 3.18 eV) |
| Catalyst | Syntheses Method | Band Gap Energy | Ref | |
|---|---|---|---|---|
| With Dopant | Without Dopant | |||
| Cu doped TiO2 | Sol–gel | 2.34 eV | 3.2 eV | [157] |
| TiO2-Cu2+ 2% | A combination of sol–gel and microwave hydrothermal method | 2.86 eV | 3.1 eV | [158] |
| Fe10 * doped TiO2 | Sol–gel | 2.69 eV | 3.32 eV | [159] |
| Cr doped TiO2 | Sol–gel | 1.8 eV | 3.2 eV | [160] |
| Sol–gel | Nitrogen doped TiO2: 1.6 eV | [161] | ||
| Cr doped TiO2: 1.1 eV | ||||
| Cr/N-TiO2: 1.5 eV | ||||
| Ce doped TiO2 | Sol–gel | 3.21 eV | 3.38 eV | [162] |
| Nitrogen doped TiO2 | Anodizing | 3.2 eV | Less than 3 eV | [163] |
| B and C doped TiO2 | Anodizing | TiO2: 3.01 eV | [164] | |
| Boron Doped TiO2: 2.94 eV | ||||
| Carbon doped TiO2: 2.79 eV | ||||
| Decorated with | Method | Results | Ref |
|---|---|---|---|
| Ag nanoparticles | Electrochemical oxidation of titanium sheets and photochemical deposition of silver nanoparticles on TiO2 nanotubes | As an electrocatalyst for the reduction of tinidazole, creating a surface with long-lasting antibacterial properties to prevent implant-related infection, improving gas sensing properties, particularly for ethanol. | [102,148] |
| Nanoparticles MnO | Thermal decomposition process | Apatite formation in the body-simulating solution, electrochemical oxidation for 20 min at 25 V is desirable as the high heating rate during the synthesis process results in a reduced MnO particle size with a uniform morphology. | [149] |
| Nanoparticles Pt | Atomic layer deposition, electrochemical oxidation | Development of gas sensors to remove bacteria and volatile organic compounds, Sensitivity to hydrogen and carbon monoxide gases is significantly enhanced in decorated titanium nanotubes, achieving complete bacterial inactivation within 60 min. | [150] |
| Nanoparticles Ni | Electrochemical oxidation followed by annealing, and electrochemical oxidation followed by electron deposition | Improving hydrogen sensing performance and ion transfer in lithium batteries Sensitive to hydrogen atmosphere from room temperature to 200 °C. | [172] |
| Nanoparticles Pt/Au | Electrochemical Oxidation followed by electrochemical deposition of gold and platinum particles | Exhibiting high catalytic efficiency in H2O2 oxidation, owing to the high surface area of the nanotube structure, the excellent biocompatibility of TiO2, and the high catalytic activity of the loaded platinum nanoparticles. | [173] |
| Nanoparticles ZnO | Electrochemical oxidation followed by immersion and calcination to decorate the nanotubes with oxide nanoparticles | The significantly improved sensitivity to H2 and excellent performance, mainly attributing to the highly ordered morphology of the produced nanotubes and the formation of heterogeneous bonding at the interface between ZnO and TiO2. | [174] |
| Nanoparticles Nb | Electrochemical oxidation at room temperature with Nb particles | Sensitivity to CO, H2, NO2, ethanol, and acetone gases. Nb greatly improves the conductivity and enhances the gas sensing performance of TiO2 nanotubes. Nb-doped nanotubes exhibit greater electron mobility and require lower activation energy for electron transfer than the undoped state. | [142] |
| Pt, Pd particles in Pt-Ti, Pd-Ti alloy | Electrochemical oxidation | Development of catalytic oxide semiconductors Ti-2%Pt and Ti-2%Pd are suitable for the formation of TiO2 nanotube layers with Pt and Pd, respectively. The increase in hydrogen sensitivity of TiO2 nanotubes with platinum or palladium nanoparticles is due to the acceleration of the chemisorption of hydrogen into the nanotube wall, where it acts as an electron donor. | [166,175] |
| Titania nanoparticles (TiO2) | Electrochemical oxidation | Employed in lithium micro batteries as the anode, with an increased capacitance of nanotubes in the presence of titania particles. | [176] |
| Ag3PO4 nanoparticles | Alkaline hydrothermal followed by in situ deposition of nanoparticles | Development of solar photocatalysts with an enhanced absorption ability in the visible light spectrum. | [165] |
| Ag and Co3O4 nanoparticles | Two-stage electrochemical oxidation | Defect formation in TiO2 nanotube arrays caused by oxygen vacancies and the creation of Ti3+ increases the photodegradation properties of catalyst under visible light irradiation. | [166] |
| Er and reduced graphene oxide | Electrochemical oxidation, followed by either electrochemical deposition or drop casting. | Increased the photocatalytic efficiency and hydrogen evolution rate. | [167] |
| CuO and Cu2O | Hydrothermal | Increased photocatalytic activity, including antibacterial activity due to the better ability of Cu2O to absorb visible light compared to CuO. | [169,177] |
| P25 nanoparticles | hydrothermal oxidation | Studying the release of ibuprofen drug | [178] |
| BiVO4 nanoparticles | Electrochemical oxidation and adsorption | Improvement of photocatalytic activity through increasing visible light absorption Suitable for preparing highly efficient heterogeneous structures for photocatalytic and sensing applications. | [179] |
| Fe3O4 nanoparticles | Electrochemical and hydrothermal oxidation | Development of a magnetically conductive photocatalyst. | [180] |
| NiO nanoparticles | Two-step electrochemical oxidation | Increased photocatalytic activity in water splitting. | [181] |
| Pd particles | Electrochemical oxidation | Enhanced photocatalytic activity for hydrogen production. | [182] |
| LaFeO3 Perovskite Nanoparticles | Electrochemical oxidation followed by electrochemical deposition | Increased photocatalytic activity in the presence of perovskite minerals. | [183] |
| Pt, Pd nanoparticles | Electrochemical oxidation in organic solution followed by using various physical and chemical methods | Reducing the price of anodes employed in fuel cells, having a larger surface area and lower toxicity than bulk electrodes. | [184] |
| Au nanoparticles | Electrochemical oxidation and deposition | Fabrication of flexible sensors for use in lithium batteries Infection control of orthopedic implants. | [185] |
| Mn and Fe | Electrochemical oxidation followed by electrochemical deposition | Improvement of photocatalytic performance in the photocatalytic water splitting. | [186] |
| Lead sulfide nanoparticles | Electrochemical oxidation and immersion | Prevention of metal corrosion by photocathodic method Improved visible light corrosion protection. | [187] |
| Fe2O3 nanoparticles | Environmentally safe liquid-phase micro plasma technique | Expanding the light absorption spectrum from the ultraviolet into the visible range in the photocatalytic processes of hydrogen production via photoelectrochemical decomposition. | [188] |
| Fe2O3 nanoparticles | hydrothermal method | Improved optical, and photocatalytic properties It was reported that 33% of the dye could be decomposed after 2 h of radiation exposure at natural pH without the addition of oxidants. | [189] |
| Cu particles | Adsorption and in situ method using simultaneous electrochemical oxidation in copper nitrate electrolyte | Improved photocatalytic properties in the visible light range; reduction in band gap to enhance the hydrogen production through the photocatalytic process. | [107] |
| Cu/CuO Spices | Electrochemical oxidation and immersion | Improved photocatalytic activity in the visible region. | [100] |
| Sr Particles | Hydrothermal | Enhanced rate of HER. | [190] |
| Catalyst | Synthesis Method | Light Source | The Rate of Hydrogen Production | Ref |
|---|---|---|---|---|
| Cu2O/TiO2 | A combination of hydrothermal and impregnation methods | Sunlight | 48 mmol/g. h | [191] |
| P/TiO2 | Chemical vapor deposition | Visible light | 681 μmol/g. h | [192] |
| MoS2/TiO2 | A combination of anodizing and hydrothermal methods | 300 W Xe-lamp | 143.32 μmol/cm2. h | [193] |
| Cu/TiO2 | Sputtering | 300 W Xe-lamp | 28 μmol/cm2 | [194] |
| Bi2S3-BiOBr/TiO2 | A combination of anodizing and solvothermal method | Xe lamp (CEL-S500) | 17.26 μmol/cm2. h | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namdar-Asl, H.; Shiran-Jang, F.; Fathyunes, L.; Mohtadi-Bonab, M.A.; Pour-Ali, S. Electrochemical Synthesis of TiO2 Nanotubes for Photocatalytic Water Splitting: Mechanisms, Challenges, and Improvement Strategies. Catalysts 2025, 15, 1155. https://doi.org/10.3390/catal15121155
Namdar-Asl H, Shiran-Jang F, Fathyunes L, Mohtadi-Bonab MA, Pour-Ali S. Electrochemical Synthesis of TiO2 Nanotubes for Photocatalytic Water Splitting: Mechanisms, Challenges, and Improvement Strategies. Catalysts. 2025; 15(12):1155. https://doi.org/10.3390/catal15121155
Chicago/Turabian StyleNamdar-Asl, Hamed, Farzaneh Shiran-Jang, Leila Fathyunes, M. A. Mohtadi-Bonab, and Sadegh Pour-Ali. 2025. "Electrochemical Synthesis of TiO2 Nanotubes for Photocatalytic Water Splitting: Mechanisms, Challenges, and Improvement Strategies" Catalysts 15, no. 12: 1155. https://doi.org/10.3390/catal15121155
APA StyleNamdar-Asl, H., Shiran-Jang, F., Fathyunes, L., Mohtadi-Bonab, M. A., & Pour-Ali, S. (2025). Electrochemical Synthesis of TiO2 Nanotubes for Photocatalytic Water Splitting: Mechanisms, Challenges, and Improvement Strategies. Catalysts, 15(12), 1155. https://doi.org/10.3390/catal15121155








