Scalable and Green Engineering of MoOx with Abundant Oxygen Vacancies for Efficient and Recyclable Aerobic Oxidative Desulfurization of Fuels
Abstract
1. Introduction
2. Results and Discussion
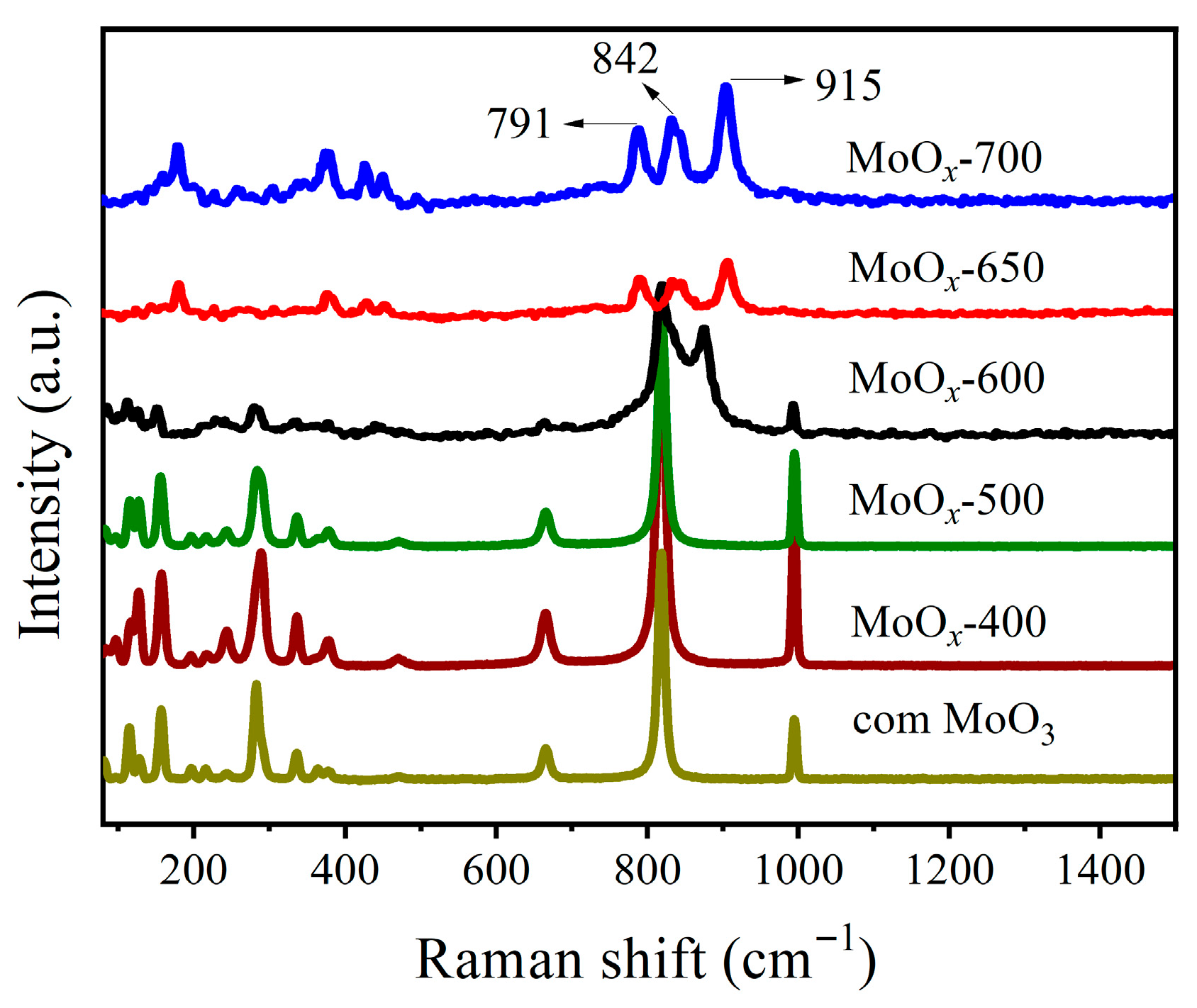
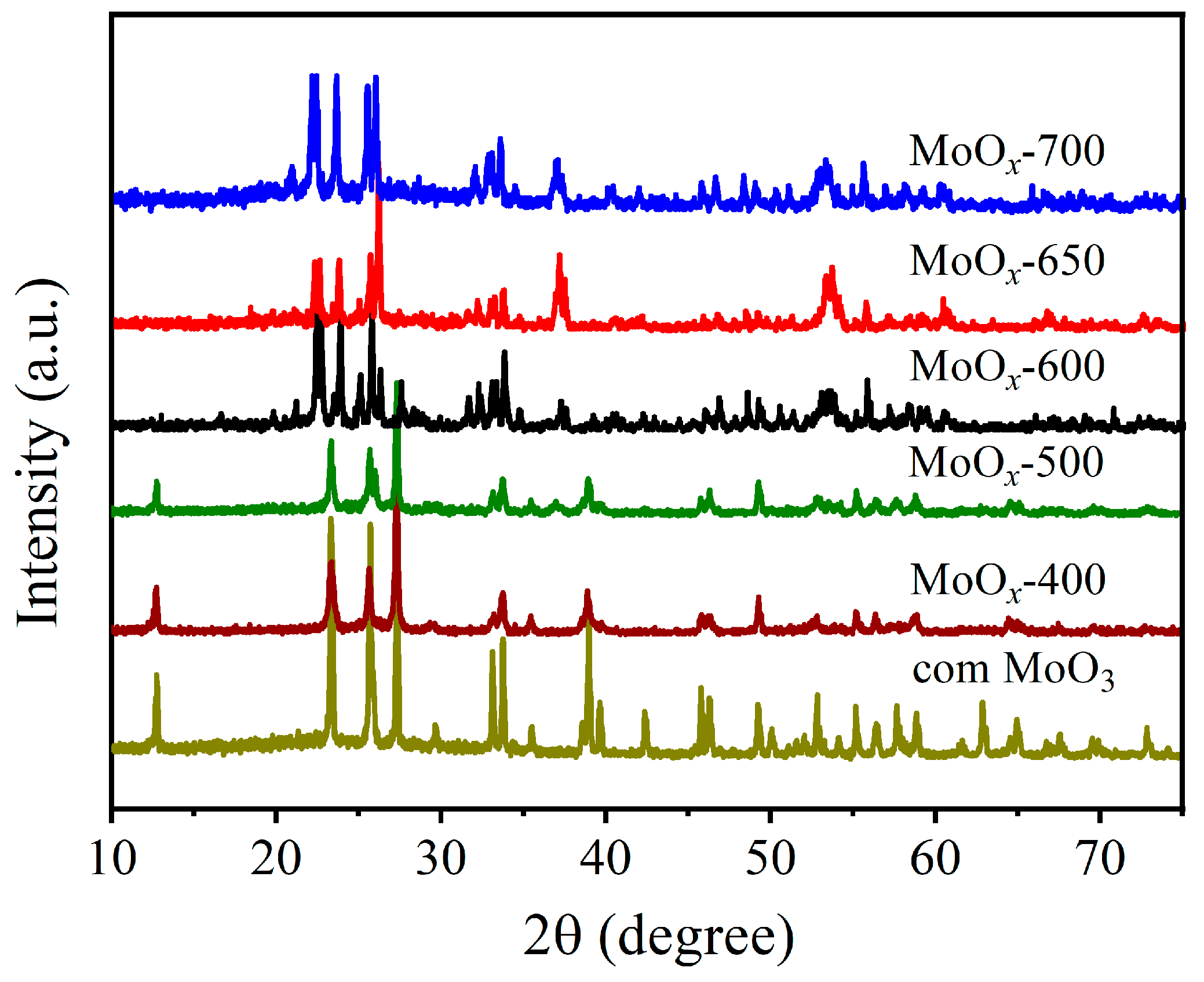
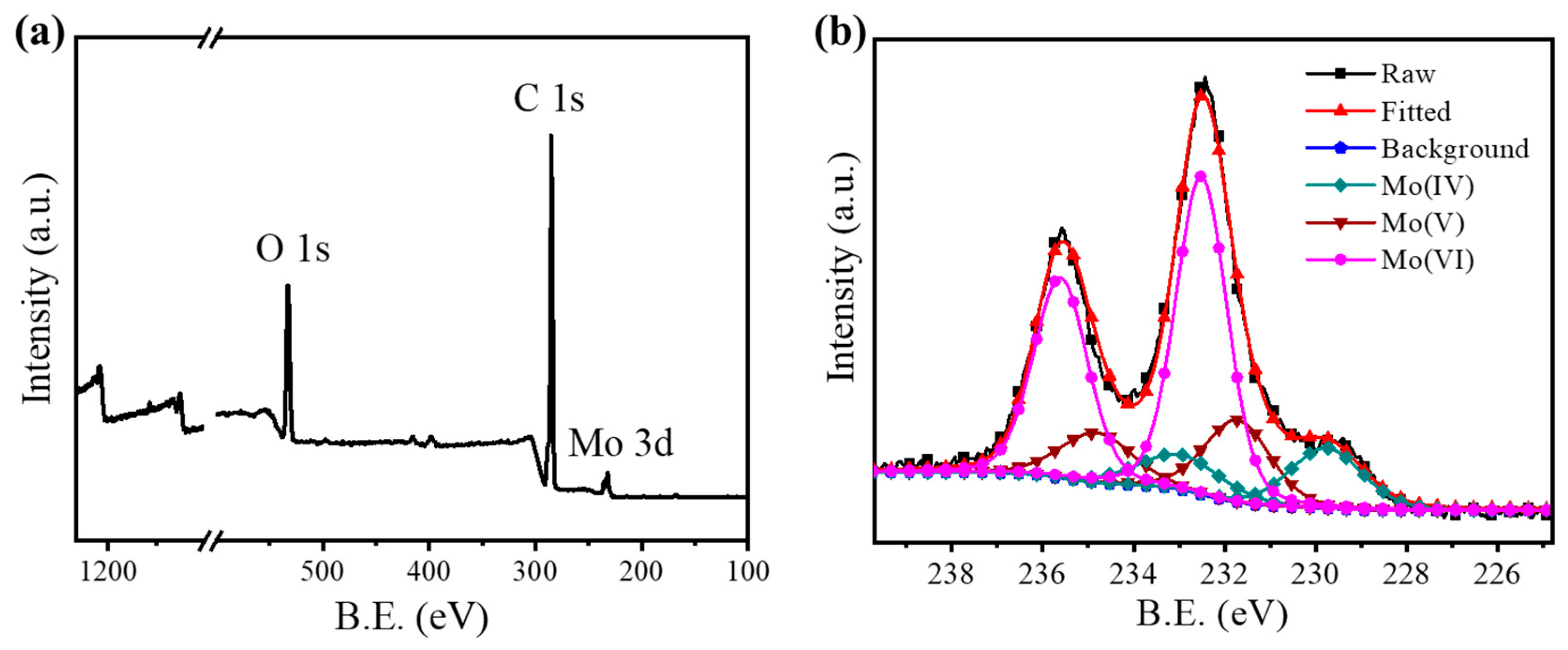
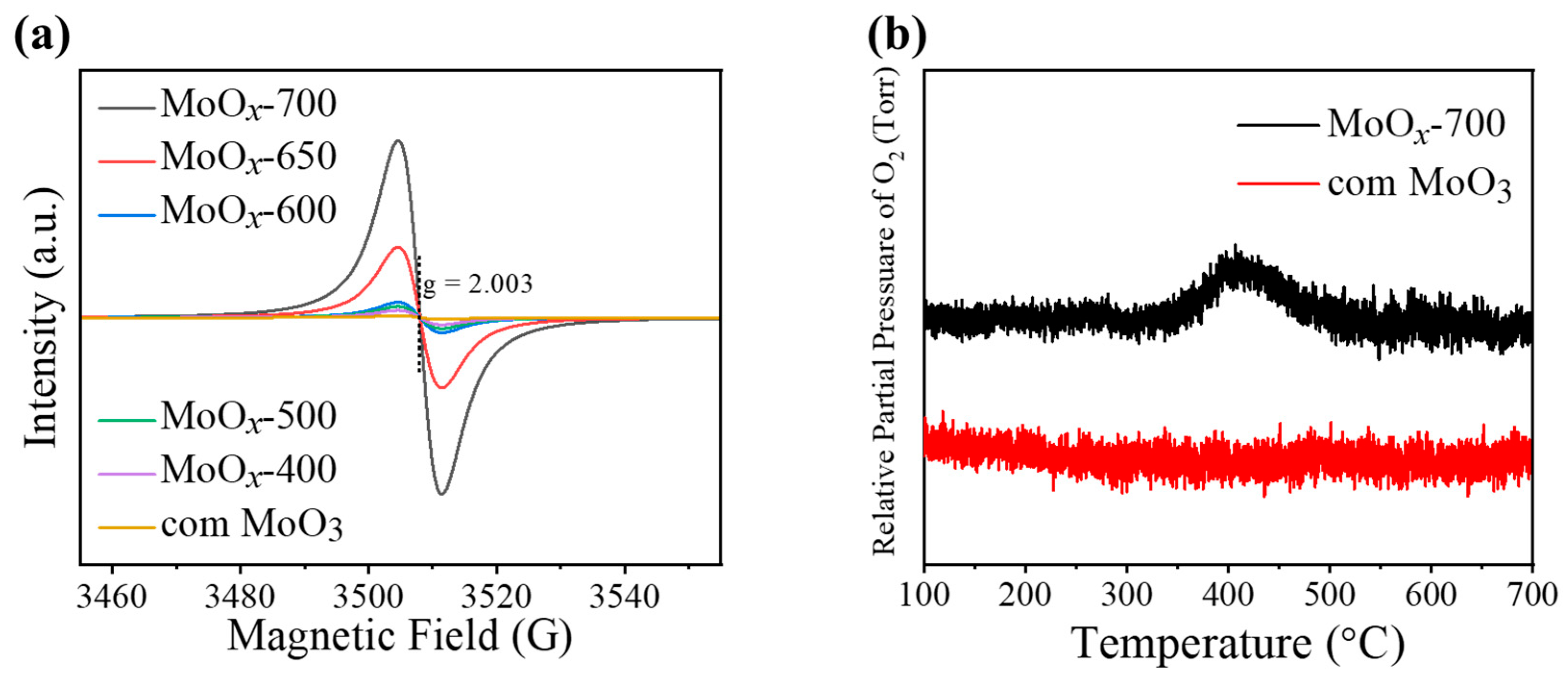
2.1. Characterizations
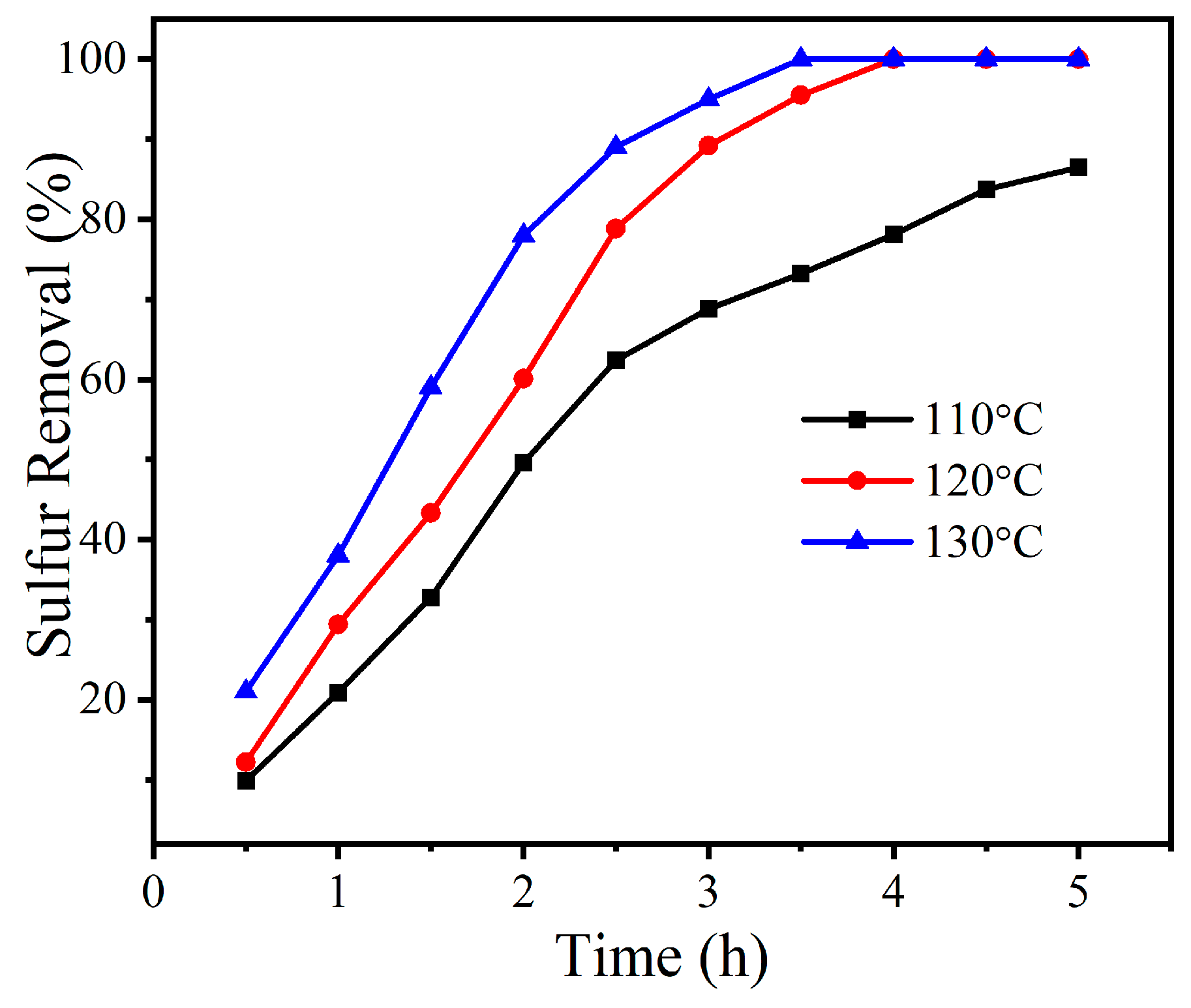
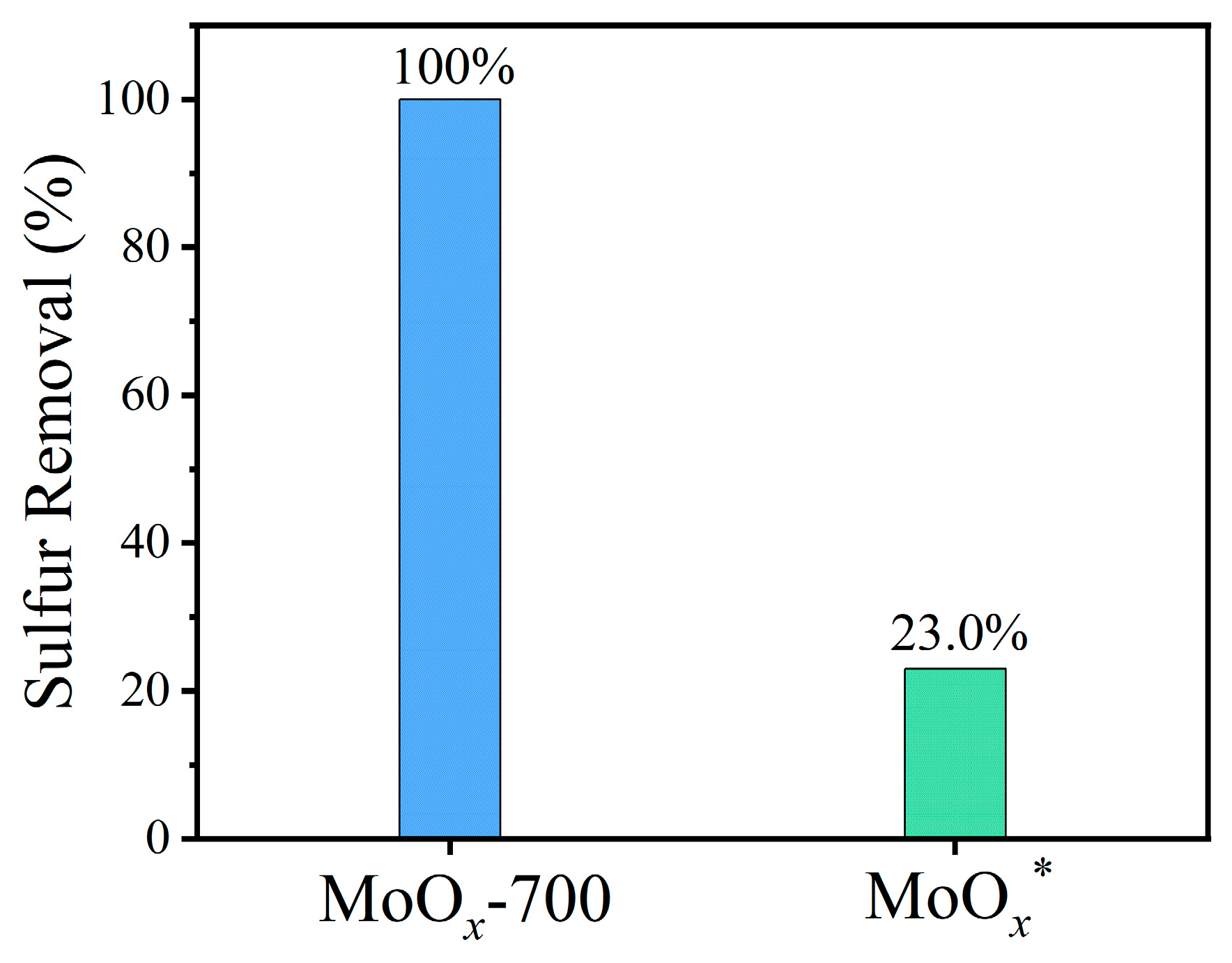
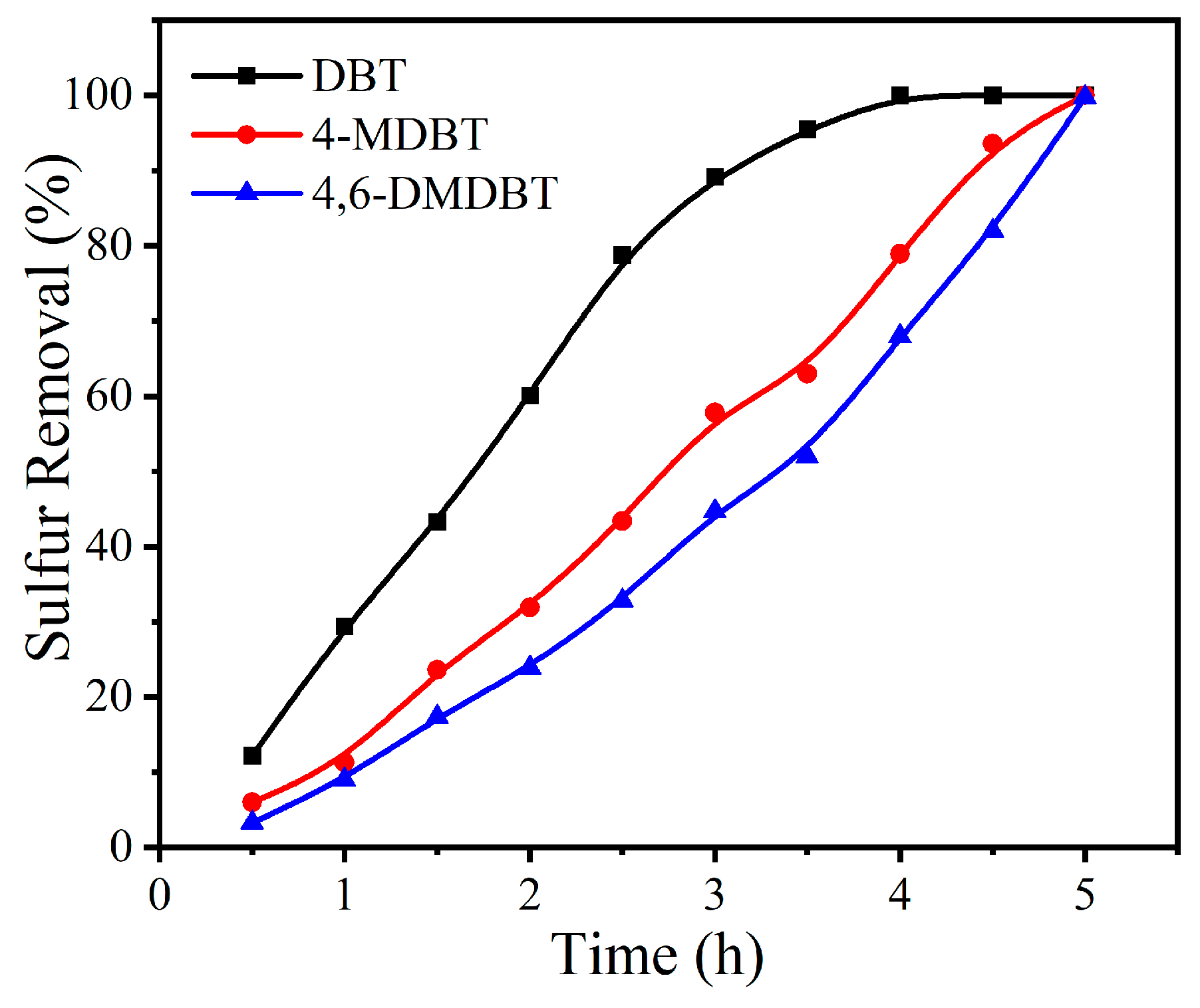
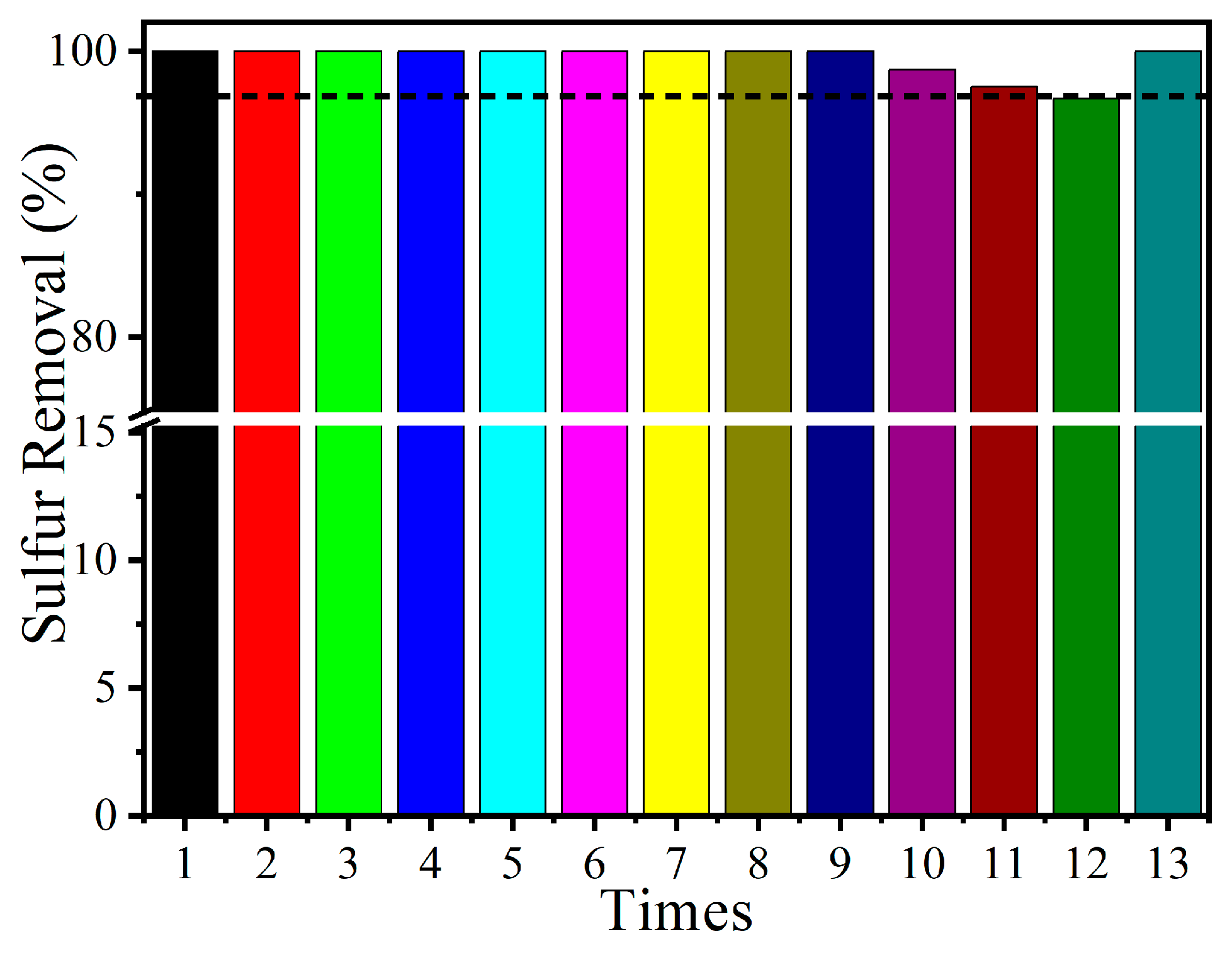
2.2. Sulfur Removal with Different Catalysts
3. Materials and Methods
3.1. Reagents
3.2. The Synthesis of MoOx-M Catalyst
3.3. Reaction Conditions for ESR Analyses
3.4. The Aerobic Oxidative Desulfurization Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-W.; Lee, T.-H. A comparative study of combustion characteristics for the evaluation of the feasibility of crude bioethanol as a substitute for marine fuel oil. J. Mar. Sci. Eng. 2025, 13, 433. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Zhou, Y.; Shi, X. Research the synergistic carbon reduction effects of sulfur dioxide emissions trading policy. J. Clean. Prod. 2024, 447, 141483. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Zou, Y.; Ding, J.; Li, H.; Zhang, M.; Li, H.; Zhu, W. Effects of attractive electrostatic interactions on sulfur dioxide capture by functionalized deep eutectic solvents with abundant negative sites. Chem. Eng. J. 2025, 591, 165030. [Google Scholar] [CrossRef]
- Salazar, N.; Rangarajan, S.; Rodríguez-Fernández, J.; Mavrikakis, M.; Lauritsen, J.V. Site-dependent reactivity of MoS2 nanoparticles in hydrodesulfurization of thiophene. Nat. Commun. 2020, 11, 4369. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, Y.; Zhang, W.; Liang, S.; You, F.; Cheng, H.; Zheng, P.; Wu, P.; Liu, J. Regulating the electron affinity of NiMo/Al2O3 to enhance ultra-deep hydrodesulfurization of diesel. Appl. Catal. B-Environ. Energy 2025, 378, 125565. [Google Scholar] [CrossRef]
- Majid, M.F.; Zaid, H.F.M.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J. Mol. Liq. 2020, 306, 112870. [Google Scholar] [CrossRef]
- Omar, R.A.; Bhaduri, B.; Verma, N. Graphitic carbon nitride-immobilized bacterial endospores: Combined adsorptive-and bio-desulfurization of liquid fuels. Mater. Lett. 2024, 361, 136117. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Lateef, S.A. Review of adsorptive desulfurization process: Overview of the non-carbonaceous materials, mechanism and synthesis strategies. Fuel 2021, 294, 120273. [Google Scholar] [CrossRef]
- Haruna, A.; Merican, Z.M.A.; Musa, S.G. Recent advances in catalytic oxidative desulfurization of fuel oil—A review. J. Ind. Eng. Chem. 2022, 112, 20–36. [Google Scholar] [CrossRef]
- Wang, C.; Ding, J.; Wu, H.; Zhang, J.; Xu, J.; Zhang, Y.; Ma, M.; Zhang, M.; Li, H. The Facile Construction of Defect-Engineered and Surface-Modified UiO-66 MOFs for Promising Oxidative Desulfurization Performance. Nanomaterials 2025, 15, 931. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.S.; Hamasalih, L.O.; Hama Aziz, K.H.; Omer, K.M.; Shafiq, I. Oxidative desulfurization of real high-sulfur diesel using dicarboxylic acid/H2O2 system. Processes 2022, 10, 2327. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A. Optimization of oxidative desulfurization of Malaysian Euro II diesel fuel utilizing tert-butyl hydroperoxide–dimethylformamide system. Fuel 2015, 161, 26–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Yang, H.; Dong, Y.; Liu, Y.; Yang, L.; Wei, D.; Wang, W.; Bai, L.; Chen, H. Efficient aerobic oxidative desulfurization over Co–Mo–O bimetallic oxide catalysts. Catal. Sci. Technol. 2019, 9, 2915–2922. [Google Scholar] [CrossRef]
- Guo, J.; Tian, X.; Liu, H.; Wang, J. Mo–V/g-C3N4 with strong electron donating capacity and abundant oxygen vacancies for low-temperature aerobic oxidative desulfurization. Chem. Commun. 2025, 61, 11461–11464. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, W.; Chen, H.; Zhu, L.; Luo, J.; Yang, W.; Chen, G.; Chen, Z.; Zhu, W.; Li, H. Pt nanoparticles encapsulated on V2O5 nanosheets carriers as efficient catalysts for promoted aerobic oxidative desulfurization performance. Chin. J. Catal. 2021, 42, 557–562. [Google Scholar] [CrossRef]
- Gómez-Paricio, A.; Santiago-Portillo, A.; Navalón, S.; Concepción, P.; Alvaro, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Chen, Z.; Yin, S.; Wu, P.; Xun, S.; Jiang, W.; Zhang, M.; Li, H. Light irradiation induced aerobic oxidative deep-desulfurization of fuel in ionic liquid. RSC Adv. 2015, 5, 99927–99934. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Zhang, X.; Qiu, Y.; Zhu, Q.; Xun, S.; Yang, W.; Li, H.; Chen, Z.; Zhu, W. Atomic-layered α-V2O5 nanosheets obtained via fast gas-driven exfoliation for superior aerobic oxidative desulfurization. Energy Fuels 2020, 34, 2612–2616. [Google Scholar] [CrossRef]
- Asadi, F.; Allahyari, S.; Rahemi, N.; Hussain, M. Ultrasound-assisted aerobic desulfurization of fuel oil using plasma-treated MoO3-boron nitride catalysts. J. Environ. Chem. Eng. 2025, 13, 115248. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y. Engineering cobalt oxide with coexisting cobalt defects and oxygen vacancies for enhanced catalytic oxidation of toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Simlandy, A.K.; Bhattacharyya, B.; Pandey, A.; Mukherjee, S. Picosecond electron transfer from quantum dots enables a general and efficient aerobic oxidation of boronic acids. ACS Catal. 2018, 8, 5206–5211. [Google Scholar] [CrossRef]
- Bai, H.; Yi, W.; Liu, J.; Lv, Q.; Zhang, Q.; Ma, Q.; Yang, H.; Xi, G. Large-scale synthesis of ultrathin tungsten oxide nanowire networks: An efficient catalyst for aerobic oxidation of toluene to benzaldehyde under visible light. Nanoscale 2016, 8, 13545–13551. [Google Scholar] [CrossRef] [PubMed]
- Sádaba, I.; Granados, M.L.; Riisager, A.; Taarning, E. Deactivation of solid catalysts in liquid media: The case of leaching of active sites in biomass conversion reactions. Green Chem. 2015, 17, 4133–4145. [Google Scholar] [CrossRef]
- Niu, K.; Liu, Q.; Liu, C.; Yu, Z.; Zheng, Y.; Su, Y.; Zhao, Y.; Liu, B.; Cui, S.; Zang, G. Unraveling the role of oxygen vacancies in metal oxides: Recent progress and perspectives in NH3-SCR for NOx removal. Chem. Eng. J. 2024, 487, 150714. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Cen, W.; Cheng, Y. Birnessite as a highly efficient catalyst for low-temperature NH3-SCR: The vital role of surface oxygen vacancies. Ind. Eng. Chem. Res. 2020, 59, 14606–14615. [Google Scholar] [CrossRef]
- Malcolmson, S.J.; Meek, S.J.; Sattely, E.S.; Schrock, R.R.; Hoveyda, A.H. Highly efficient molybdenum-based catalysts for enantioselective alkene metathesis. Nature 2008, 456, 933–937. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Yamashita, H. Development of defective molybdenum oxides for photocatalysis, thermal catalysis, and photothermal catalysis. Chem. Commun. 2022, 58, 8466–8479. [Google Scholar] [CrossRef]
- Hu, W.; Xie, L.; Gu, C.; Zheng, W.; Tu, Y.; Yu, H.; Huang, B.; Wang, L. The nature of active sites of molybdenum sulfide-based catalysts for hydrogen evolution reaction. Coord. Chem. Rev. 2024, 506, 215715. [Google Scholar] [CrossRef]
- Cai, G.; Chin, Y.-H.C. Catalytic Consequences of Protons in Methanol Oxidative Dehydrogenation on Molybdenum-Based Polyoxometalate Clusters. ACS Catal. 2024, 14, 6674–6686. [Google Scholar] [CrossRef]
- Yang, F.; Lu, S.; Feng, Y.; Fu, L.; Feng, L. Insights into the confinement effect of NiMo catalysts toward alkaline hydrogen oxidation. ACS Catal. 2024, 14, 2324–2332. [Google Scholar] [CrossRef]
- Xu, L.; An, X.; She, J.; Li, H.; Zhu, L.; Zhu, W.; Li, H.; Jiang, W. Molybdenum-based metal–organic frameworks as highly efficient and stable catalysts for fast oxidative desulfurization of fuel oil. Sep. Purif. Technol. 2023, 326, 124699. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Bhattacharjee, J.; Datta, P.; Roy, S. A comprehensive review of the oxidation states of molybdenum oxides and their diverse applications. Sustain. Chem. Environ. 2024, 7, 100125. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum oxides–from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Lima, C.D.; Moura, J.V.; Pinheiro, G.S.; Araujo, J.F.; Gusmão, S.B.; Viana, B.C.; Freire, P.T.; Luz-Lima, C. Co-doped α-MoO3 hierarchical microrods: Synthesis, structure and phonon properties. Ceram. Int. 2021, 47, 27778–27788. [Google Scholar] [CrossRef]
- Payen, E.; Grimblot, J.; Kasztelan, S. Study of oxidic and reduced alumina-supported molybdate and heptamolybdate species by in situ laser Raman spectroscopy. J. Phys. Chem. 1987, 91, 6642–6648. [Google Scholar] [CrossRef]
- Dieterle, M.; Mestl, G. Raman spectroscopy of molybdenum oxides Part II. Resonance Raman spectroscopic characterization of the molybdenum oxides Mo4O11 and MoO2. Phys. Chem. Chem. Phys. 2002, 4, 822–826. [Google Scholar] [CrossRef]
- Pavoni, E.; Modreanu, M.G.; Mohebbi, E.; Mencarelli, D.; Stipa, P.; Laudadio, E.; Pierantoni, L. First-principles calculation of MoO2 and MoO3 electronic and optical properties compared with experimental data. Nanomaterials 2023, 13, 1319. [Google Scholar] [CrossRef]
- Wang, S.; Lu, S.; Yang, X.; Liu, X. Pseudocapacitive MoOx anode material with super-high rate and ultra-long cycle properties for aqueous zinc ion batteries. J. Electroanal. Chem. 2021, 882, 115033. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Su, J.; Zhao, X.; Pan, X. Inhibition of phase transition from δ-MnO2 to α-MnO2 by Mo-doping and the application of Mo-doped MnO2 in aqueous zinc-ion batteries. Phys. Chem. Chem. Phys. 2023, 25, 30663–30669. [Google Scholar] [CrossRef]
- Lima, C.D.; de Carvalho, T.C.; Mendoza, C.D.; da Costa, M.E.M.; Pinheiro, G.d.S.; Luz-Lima, C.; Silva, B.G.; Sommer, R.L.; Araujo, J.F. Magnetic transition in MoO3: Influence of Mo5+/Mo6+ ratios on paramagnetic to diamagnetic behavior. Solid State Sci. 2025, 162, 107866. [Google Scholar] [CrossRef]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.M.; Lu, Z.H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar] [CrossRef]
- Chen, H.; Liang, C.; Xun, S.; Yu, Z.; Wu, C.; He, M.; Li, H.; Zhu, W. Oxygen vacancy regulation strategy in V-Nb mixed oxides catalyst for enhanced aerobic oxidative desulfurization performance. J. Colloid Interface Sci. 2023, 641, 289–298. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, R.; Huo, Y.; Li, H.; Wang, L. Formation, detection, and function of oxygen vacancy in metal oxides for solar energy conversion. Adv. Funct. Mater. 2022, 32, 2109503. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Yang, L.; He, G.; Zhang, C.; Zhang, R.; He, H. Oxygen vacancies induced by transition metal doping in γ-MnO2 for highly efficient ozone decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.-M.; Yang, X.-F.; Jiao, M.-G.; Zhou, Z.; Zhang, M.-H.; Wang, D.-H.; Bu, X.-H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B-Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Astle, M.A.; Rance, G.A.; Loughlin, H.J.; Peters, T.D.; Khlobystov, A.N. Molybdenum dioxide in carbon nanoreactors as a catalytic nanosponge for the efficient desulfurization of liquid fuels. Adv. Funct. Mater. 2019, 29, 1808092. [Google Scholar] [CrossRef]
- Wang, C.; Miao, Q.; Huang, X.; Li, J.; Duan, Y.; Yan, L.; Jiang, Y.; Lu, S. Fabrication of various morphological forms of a g-C3N4-supported MoO3 catalyst for the oxidative desulfurization of dibenzothiophene. New J. Chem. 2020, 44, 18745–18755. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Yang, Q.; Wang, W.-H.; Bao, M. Improving the stability of subnano-MoO3/meso-SiO2 catalyst through amino-functionalization. Funct. Mater. Lett. 2018, 11, 1850003. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, X.; Bai, J.; Yang, Z.; Chen, H.; Yang, L.; Liang, Y.; Bai, L.; Yang, H. Co, N-codoped MoOx nanoclusters on graphene derived from polyoxometalate for highly efficient aerobic oxidation desulfurization of diesel. J. Catal. 2023, 428, 115186. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Bai, J.; Yang, H.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Liang, Y.; Chen, H. MoOx nanoclusters decorated on spinel-type transition metal oxide porous nanosheets for aerobic oxidative desulfurization of fuels. Fuel 2023, 334, 126753. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, Y.; Shao, S.; Liang, S.; You, F.; Wu, H.; Huang, Y.; Wu, P.; Zhu, W.; Liu, J. Regulating the metal-support interaction of MoO3/LaTiOx to enhance ultra-deep aerobic oxidative desulfurization of diesel. Chem. Eng. J. 2025, 519, 165375. [Google Scholar] [CrossRef]
- Hu, C.; Xun, S.; Yang, B.; Zhu, L.; He, M.; Hua, M.; Li, H.; Zhu, W. Heterostructure MoO3/g-C3N4 efficient enhances oxidative desulfurization: Rational designing for the simultaneously formation of MoO3 nanoparticle and few layers g-C3N4. Sep. Purif. Technol. 2024, 340, 126789. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J. Mesoporous MoO3–x material as an efficient electrocatalyst for hydrogen evolution reactions. Adv. Energy Mater. 2016, 6, 1600528. [Google Scholar] [CrossRef]
- Xun, S.; Wu, C.; Tang, L.; Yuan, M.; Chen, H.; He, M.; Zhu, W.; Li, H. One-pot in-situ synthesis of coralloid supported VO2 catalyst for intensified aerobic oxidative desulfurization. Chin. J. Chem. Eng. 2023, 56, 136–140. [Google Scholar] [CrossRef]
- Sahraei, S. Assessment of reaction parameters in the oxidative desulfurization reaction. Energy Fuels 2023, 37, 15373–15393. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Ishaq, M.; Yang, W.; Hussain, M. Recent breakthroughs in deep aerobic oxidative desulfurization of petroleum refinery products. J. Clean Prod. 2021, 294, 125731. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Yesire, Y.; Zhang, Y.; Ding, J.; Fan, Y.; Zhang, M.; Wang, C.; Li, H. Amphiphilic Catalysts Comprising Phosphomolybdic Acid Fastened on MIL-101 (Cr): Enabling Efficient Oxidative Desulfurization under Solvent-Free and Moderate Reaction Conditions. Energy Fuels 2024, 38, 8553–8563. [Google Scholar] [CrossRef]
- Wu, H.; Bai, S.; Ding, J.; Zhang, J.; Zhang, Y.; Zou, Y.; Wang, C.; Chen, Z.; Li, H.; Zhu, W. Enhanced oxidative desulfurization activity over NH2-MIL-125 (HAc) through facet modulation by acetic acid and surface modification by amino group. Appl. Surf. Sci. 2024, 653, 159315. [Google Scholar] [CrossRef]
- Ren, Z.; Sheng, J.; Yuan, Q.; Su, Y.; Zhu, L.; Dai, C.; Zhao, H. Cross-Linked Polyvinylimidazole Complexed with Heteropolyacid Clusters for Deep Oxidative Desulfurization. Molecules 2024, 29, 4238. [Google Scholar] [CrossRef]
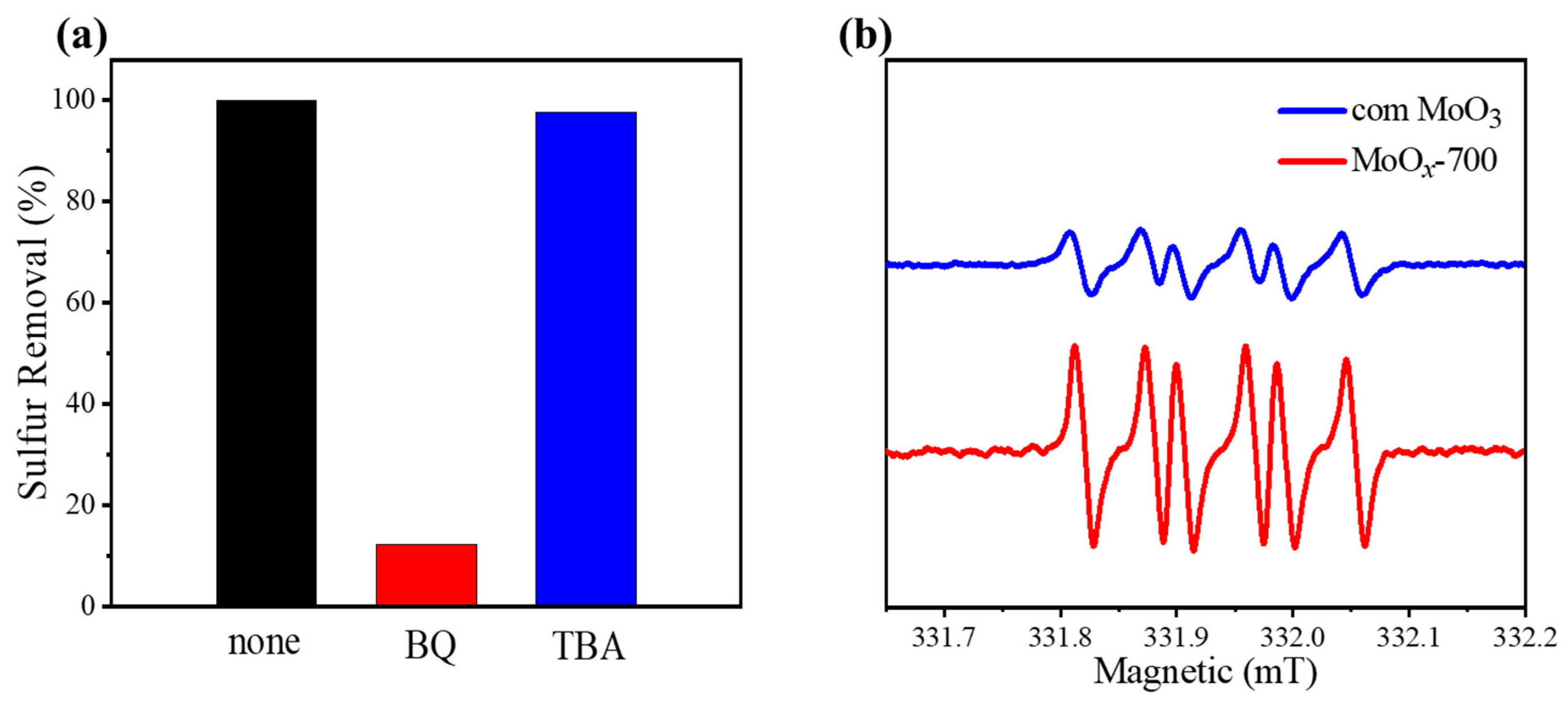
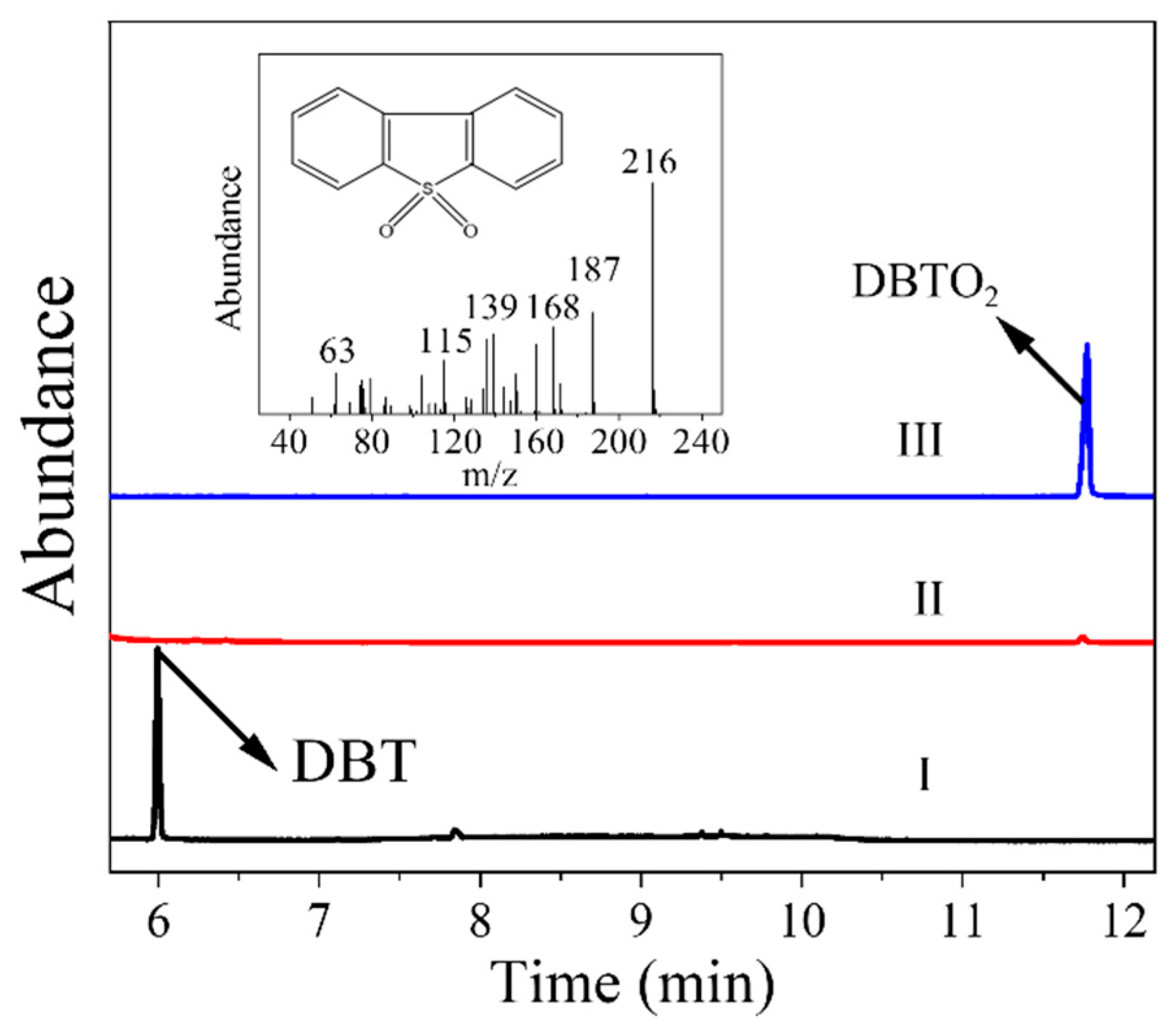
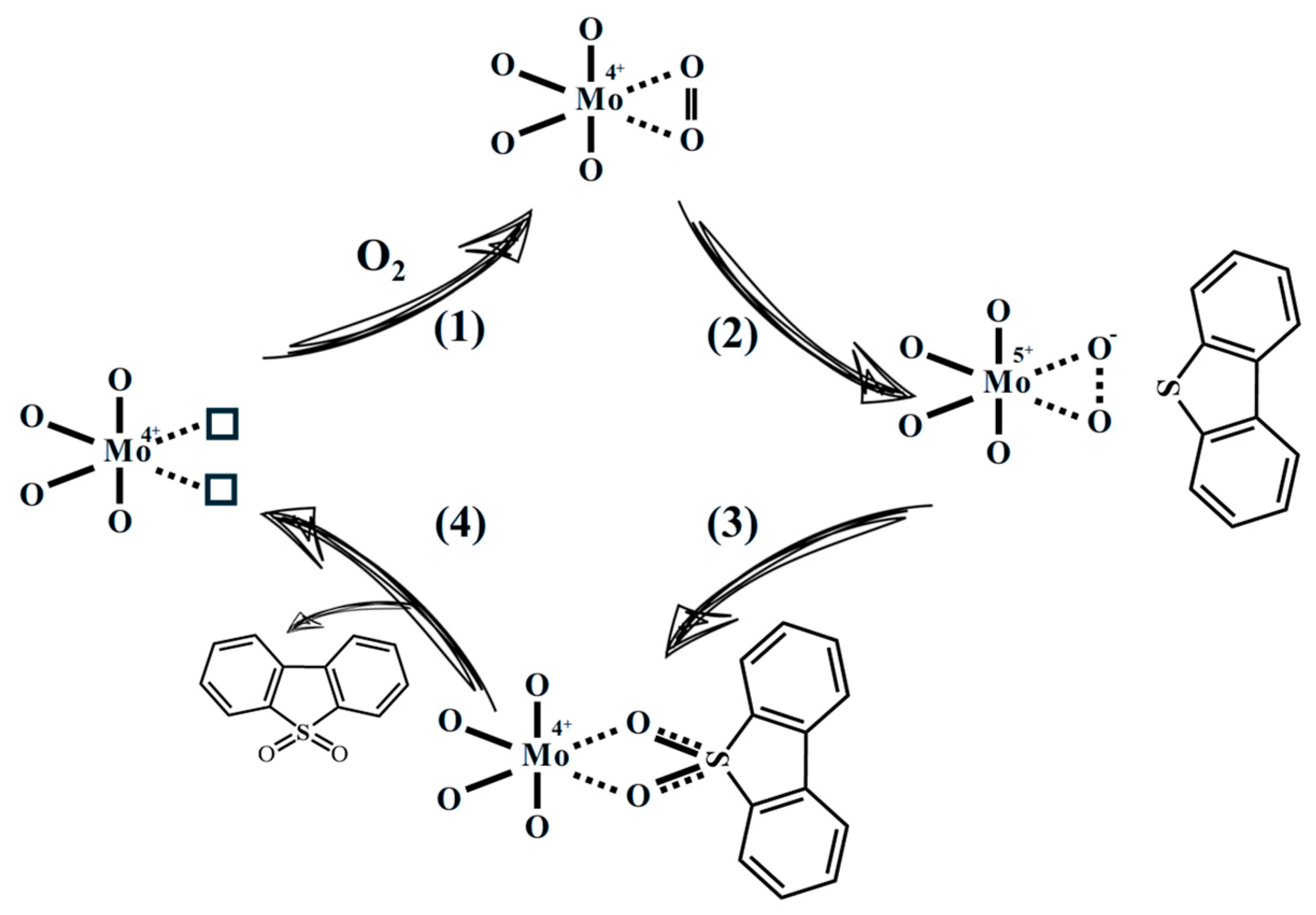
| Entry | Catalyst | Sulfur Removal (%) |
|---|---|---|
| 1 | MoOx-400 | 16.5 |
| 2 | MoOx-500 | 18.7 |
| 3 | MoOx-600 | 20.3 |
| 4 | MoOx-650 | 66.4 |
| 5 | MoOx-700 | 100.0 |
| 6 | com MoO3 | 8.9 |
| Catalyst | Temperature | Oxidant | DBT Removal | Recycling | References |
|---|---|---|---|---|---|
| MoO2/g-C3N4 | 80 °C | TBHP | 100% | — | [46] |
| MoO2@GNF | 60 °C | TBHP | 98.8% | 5 | [47] |
| MoO3/TCN | 60 °C | H2O2 | 100% | 6 | [48] |
| MoO3/SiO2 | 70 °C | H2O2 | 98% | 5 | [49] |
| Co,N-MoOx | 120 °C | Air | 100% | 7 | [50] |
| MoOx/CuCo2O4 | 120 °C | Air | 100% | 6 | [51] |
| MoO3/LaTiOx | 130 °C | Pure O2 | 100% | 16 | [52] |
| MoO3/g-C3N4 | 130 °C | Air | 100% | 6 | [53] |
| MoOx-700 | 120 °C | Air | 100% | 12 | This work |
| Catalysts | Content of Different Forms of Mo (%) | ||
|---|---|---|---|
| Mo(IV) | Mo(V) | Mo(VI) | |
| Fresh catalyst | 35 | 35 | 30 |
| Used catalyst | 33 | 35 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ma, M.; Zhang, Y.; Zhang, Y.; Chen, J.; Li, J.; Lu, Y.; Yao, X.; Zhang, M. Scalable and Green Engineering of MoOx with Abundant Oxygen Vacancies for Efficient and Recyclable Aerobic Oxidative Desulfurization of Fuels. Catalysts 2025, 15, 1146. https://doi.org/10.3390/catal15121146
Wang C, Ma M, Zhang Y, Zhang Y, Chen J, Li J, Lu Y, Yao X, Zhang M. Scalable and Green Engineering of MoOx with Abundant Oxygen Vacancies for Efficient and Recyclable Aerobic Oxidative Desulfurization of Fuels. Catalysts. 2025; 15(12):1146. https://doi.org/10.3390/catal15121146
Chicago/Turabian StyleWang, Chao, Mindan Ma, Ying Zhang, Yijin Zhang, Jiayi Chen, Junjian Li, Yao Lu, Xiaoyu Yao, and Ming Zhang. 2025. "Scalable and Green Engineering of MoOx with Abundant Oxygen Vacancies for Efficient and Recyclable Aerobic Oxidative Desulfurization of Fuels" Catalysts 15, no. 12: 1146. https://doi.org/10.3390/catal15121146
APA StyleWang, C., Ma, M., Zhang, Y., Zhang, Y., Chen, J., Li, J., Lu, Y., Yao, X., & Zhang, M. (2025). Scalable and Green Engineering of MoOx with Abundant Oxygen Vacancies for Efficient and Recyclable Aerobic Oxidative Desulfurization of Fuels. Catalysts, 15(12), 1146. https://doi.org/10.3390/catal15121146






