Single-Atom Catalysts for Electrochemical Nitrate Reduction to Ammonia: Rational Design, Mechanistic Insights, and System Perspectives
Abstract
1. Introduction
2. Brief Overview for eNO3RR
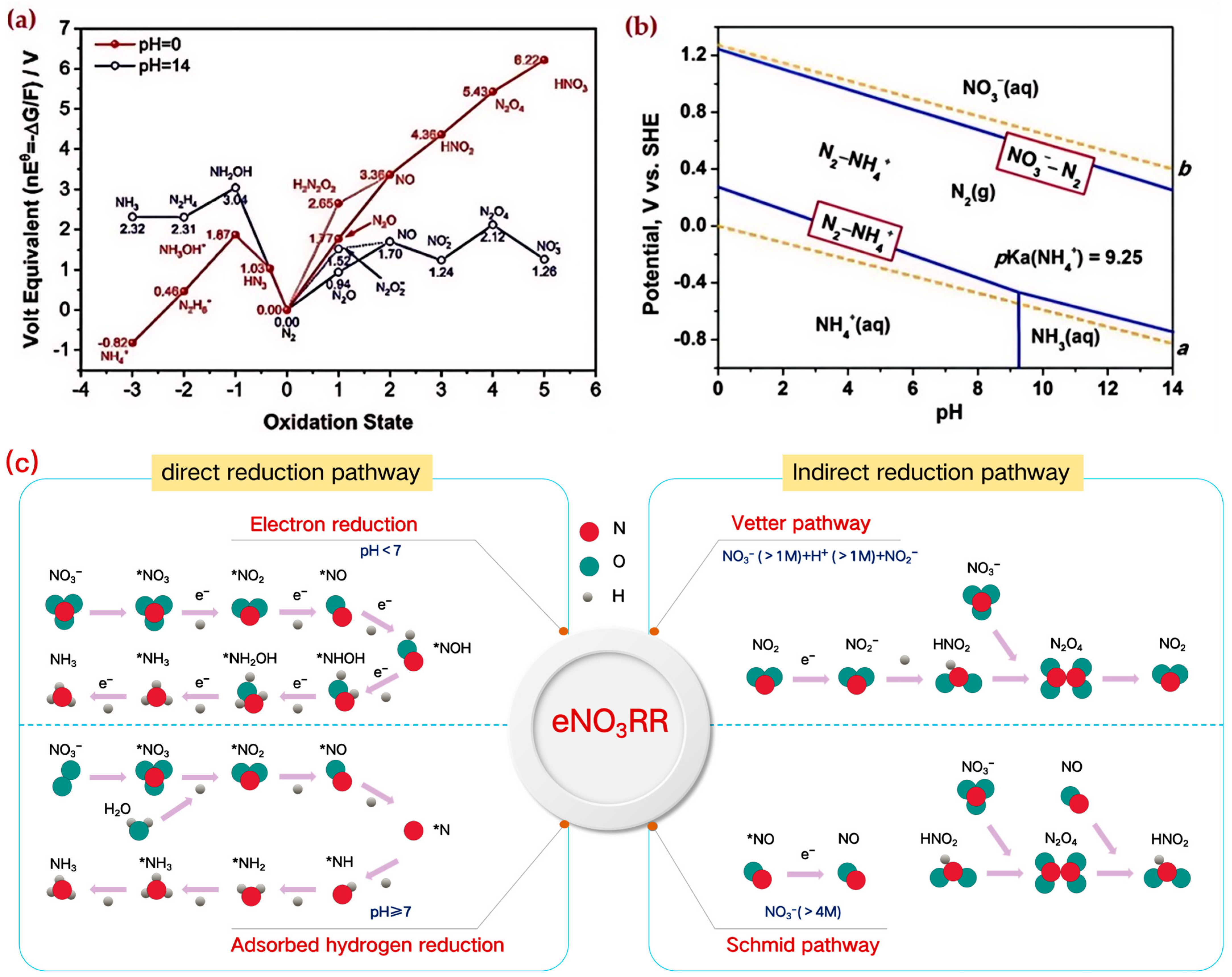
2.1. Mechanisms of Electrocatalytic Nitrate to Ammonia
2.2. In Situ/Operando Characterizations
2.2.1. Operando FTIR Spectroscopy
2.2.2. Operando XAS
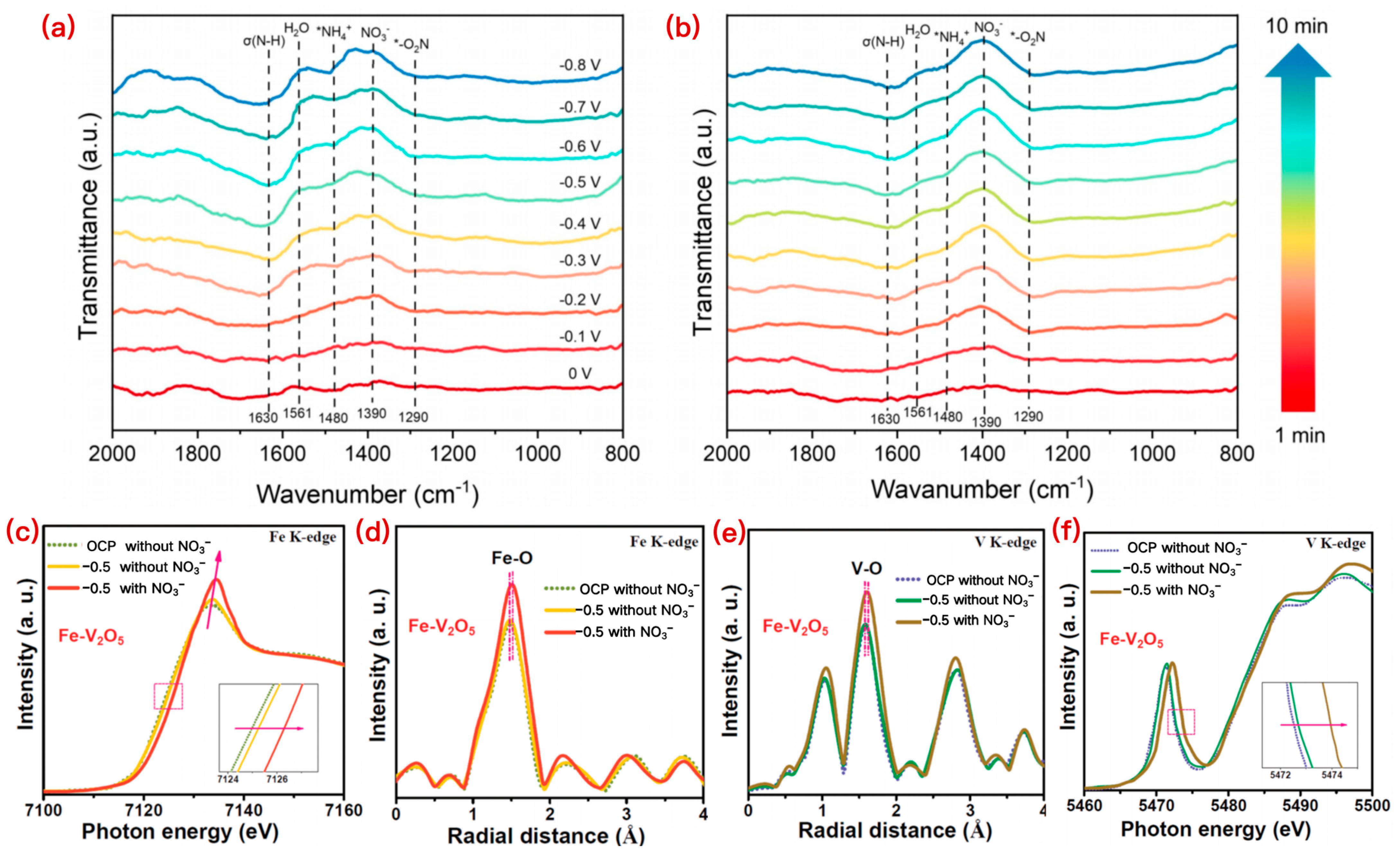
2.2.3. Operando Raman Spectroscopy
2.2.4. Differential Electrochemical Mass Spectrometry (DEMS)
2.2.5. Electron Paramagnetic Resonance (EPR)

2.3. Theoretical Computations
2.3.1. Pre-Screening of Structural Stability and Active Sites
2.3.2. Reaction Pathway Analysis and Identification of the Potential-Determining Step

2.3.3. Deciphering Electronic Structure Modulation and Structure-Activity Relationships
3. Research Progress of SACs for eNO3RR
3.1. Non-Noble Metal-Based SACs
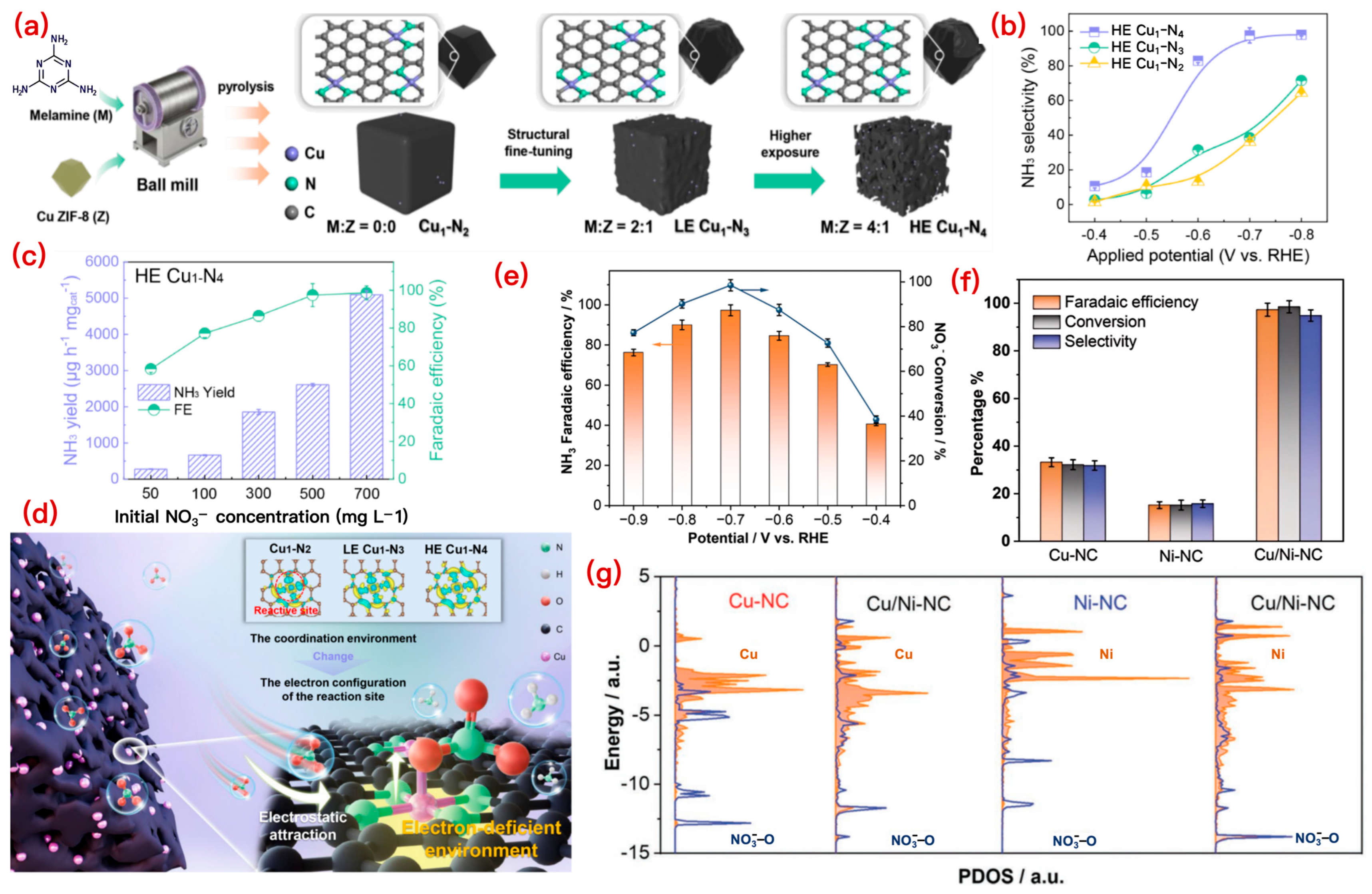
3.1.1. Cu-Based SACs
3.1.2. Fe-Based SACs
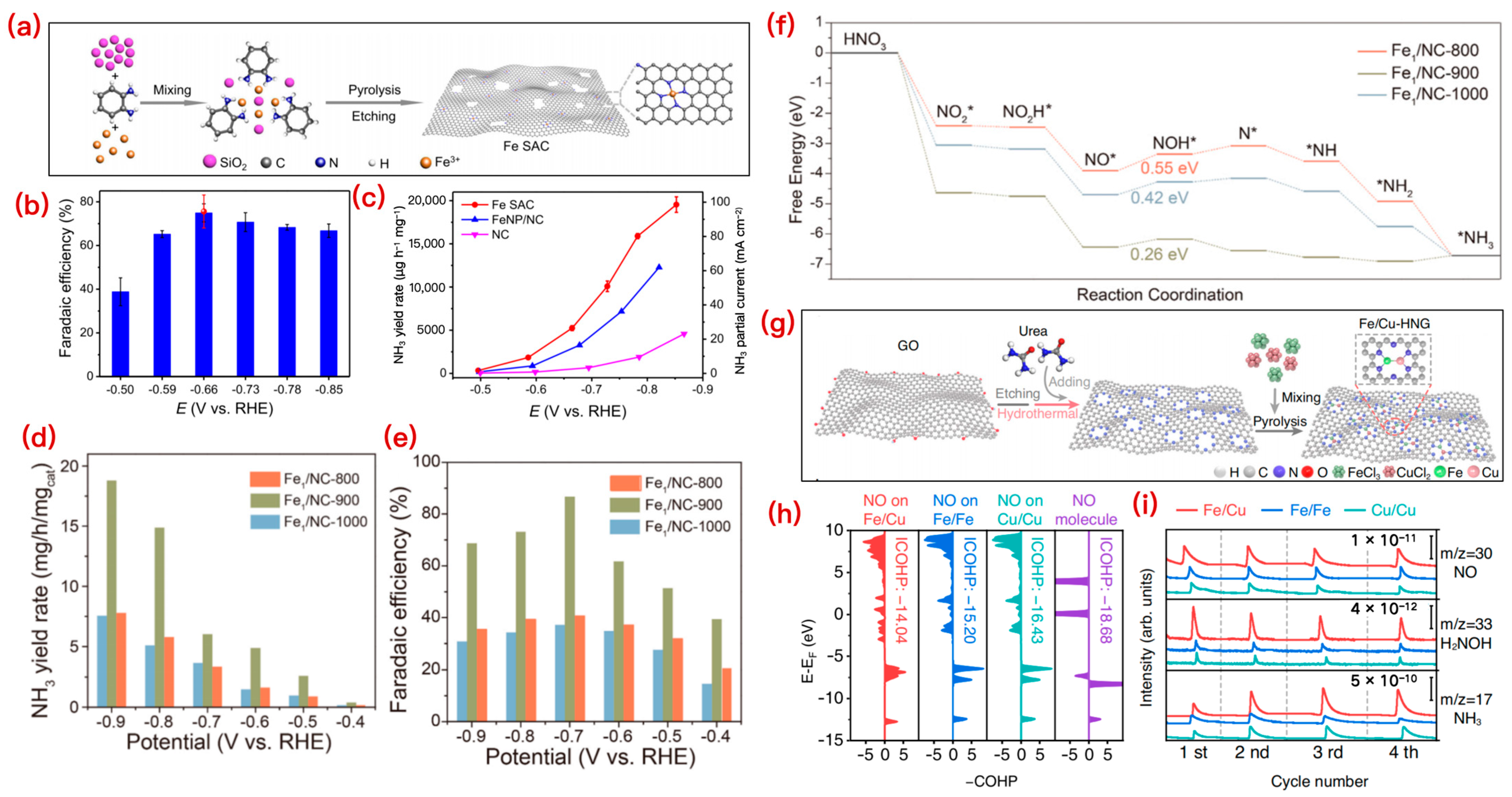
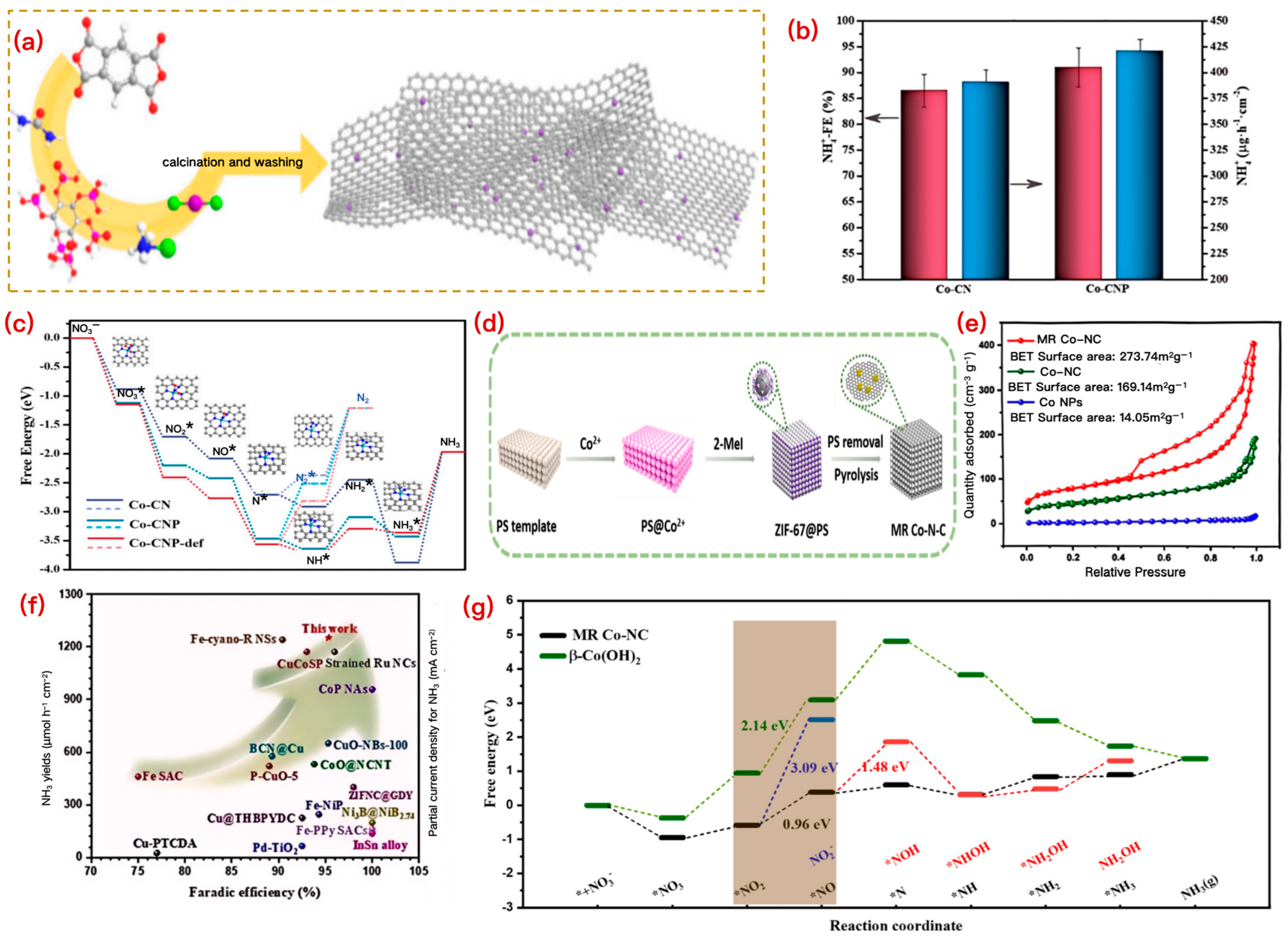
3.1.3. Co-Based Catalysts
3.2. Criteria for Selection of Catalysts
3.3. Strategies for Enhancing SACs Performance
3.3.1. Support Engineering
- (1)
- Defect Engineering: Vacancy Anchoring and Electronic Structure Modulation
- (2)
- Heteroatom Doping: Charge Redistribution and Reaction Pathway Optimization
3.3.2. Coordination Environment Optimization
- (1)
- Coordinating Atom Modulation
- (2)
- Coordination Number Modulation
3.3.3. Dual Single-Atom and Alloying Strategies
- (1)
- Dual Single-Atom Catalysts: Synergistic Catalysis
- (2)
- SAA Strategy: Host Regulation and HER Suppression
3.4. Interface Engineering
4. Effect of Microenvironment on eNO3RR to NH3
4.1. Electrolyte pH
4.2. Electrolyte Composition and Interfacial Ion Behavior
4.3. Potential Regulation and Interfacial Charge Dynamics
5. Conclusions and Outlook
- (1)
- From Atomic Precision to Macroscopic Synthesis: Bridging the Materials Gap
- (2)
- Decoding the Dynamic Interface: Integrating Multi-modal Operando Insights
- (3)
- System Integration and Techno-Economic Viability: The Path to Industrialization.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, W.; Wang, J.; Ni, G.; Liu, K.; Liu, C.; Chen, S.; Wang, Q.; Chen, Y.; Luo, T.; Wang, X.; et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2. Nat. Commun. 2024, 15, 1264. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-driven dinitrogen reduction catalyzed by a CdS: Nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef]
- Wei, M.; Huang, Y.; Wei, Y.; Chen, S.; Zhang, Z.; Ge, Z.; Chen, J.; Saleem, F.; Liu, W. Emerging Trends in Two-Dimensional Nanomaterials for Electrocatalytic Nitrate-to-Ammonia Conversion. ACS Appl. Mater. Interfaces 2025, 17, 27671–27696. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening Ammonia toward the Solar Ammonia Refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, K.; Liang, Z.; Zou, R.; Xu, Q. Electrochemical nitrogen fixation and utilization: Theories, advanced catalyst materials and system design. Chem. Soc. Rev. 2019, 48, 5658–5716. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Liu, F.; Pei, Z.; Shi, J.; Wang, Z.-J.; He, D.; Chen, Y. Homogeneous, Heterogeneous, and Biological Catalysts for Electrochemical N2 Reduction toward NH3 Under Ambient Conditions. ACS Catal. 2019, 9, 5245–5267. [Google Scholar] [CrossRef]
- Mahmood, S.; Wang, H.; Chen, F.; Zhong, Y.; Hu, Y. Recent progress and prospects of electrolytes for electrocatalytic nitrogen reduction toward ammonia. Chin. Chem. Lett. 2024, 35, 108550. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Tugaoen, H.O.N.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Peramaiah, K.; Huang, K.-W. Rethinking nitrate reduction: Redirecting electrochemical efforts from ammonia to nitrogen for realistic environmental impacts. Energy Environ. Sci. 2024, 17, 2682–2685. [Google Scholar] [CrossRef]
- Jiang, Y.; Kong, D.; Huang, L.; Wu, S.; Xu, P.; Ye, L.; Zhou, X.; Qian, J.; Tang, H.; Ge, Y.; et al. Refining the active phases of silver/nickle-based catalysts achieves a highly-selective reduction of nitrate to ammonium at low overpotential. Appl. Catal. B Environ. Energy 2024, 356, 124224. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zheng, L.; Yan, X.; Xie, J.; Yu, Z.; Zhang, S.; Jiang, F.; Chen, H. The synergistic catalysis effect on electrochemical nitrate reduction at the dual-function active sites of the heterostructure. Energy Environ. Sci. 2024, 17, 4582–4593. [Google Scholar] [CrossRef]
- Meng, S.; Ling, Y.; Yang, M.; Zhao, X.; Osman, A.I.; Al-Muhtaseb, A.H.; Rooney, D.W.; Yap, P.-S. Recent research progress of electrocatalytic reduction technology for nitrate wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 109418. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, X.; Liu, X.; Kuang, X.; Wang, H.; Zhang, C.; Wei, Q.; Wu, D. Zn single atom on N-doped carbon: Highly active and selective catalyst for electrochemical reduction of nitrate to ammonia. Chem. Eng. J. 2023, 452, 139533. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Zhou, J.; Liu, F.; Hao, F.; Fan, Z. Electrochemical Nitrate Reduction: Ammonia Synthesis and the Beyond. Adv. Mater. 2024, 36, 2304021. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Liu, Y.; Matanovic, I.; Rüscher, M.; Huang, Y.; Ly, A.; Guo, S.; Zang, W.; Yan, X.; Martini, A.; et al. Elucidating electrochemical nitrate and nitrite reduction over atomically-dispersed transition metal sites. Nat. Commun. 2023, 14, 4554. [Google Scholar] [CrossRef] [PubMed]
- van Langevelde, P.H.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Nitrate Reduction for Sustainable Ammonia Production. Joule 2021, 5, 290–294. [Google Scholar] [CrossRef]
- Mou, T.; Wang, Y.; Deák, P.; Li, H.; Long, J.; Fu, X.; Zhang, B.; Frauenheim, T.; Xiao, J. Predictive Theoretical Model for the Selective Electroreduction of Nitrate to Ammonia. J. Phys. Chem. Lett. 2022, 13, 9919–9927. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, M.; Yu, Y.; Zhang, B. Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 2021, 50, 6720–6733. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Fang, Y.; Wen, L.; Tu, B.; Huang, Y. Recent advances of ammonia synthesis under ambient conditions over metal-organic framework based electrocatalysts. Appl. Catal. B Environ. 2024, 340, 123161. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Sun, M.; Chen, J.; Zhou, J.; Hao, F.; Liu, F.; Lu, P.; Meng, X.; Guo, L.; et al. Regulating the Electrochemical Nitrate Reduction Performance with Controllable Distribution of Unconventional Phase Copper on Alloy Nanostructures. Adv. Mater. 2024, 36, 2407889. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fan, X.; Shan, B.; Qi, L.; Quan, X.; Liu, Y. Insights into lattice oxygen and strains of oxide-derived copper for ammonia electrosynthesis from nitrate. Nature Communications. 2025, 16, 3479. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Chen, J.; Zhang, W.-X.; Yang, J. Electrocatalytic reduction of nitrate–A step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 2022, 51, 2710–2758. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qiao, L.; Peng, S.; Bai, H.; Liu, C.; Ip, W.F.; Lo, K.H.; Liu, H.; Ng, K.W.; Wang, S.; et al. Recent Advances in Electrocatalysts for Efficient Nitrate Reduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2303480. [Google Scholar] [CrossRef]
- Ding, T.; Wang, M.; Wu, F.; Song, B.; Lu, K.; Zhang, H. Recent Advances in Electrocatalytic Nitrate Reduction: Strategies To Promote Ammonia Synthesis. ACS Appl. Energy Mater. 2024, 7, 11475–11496. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Cao, X.; Chen, M.; Liu, Y.; Zhou, Y.; Huang, M.; Xia, L.; Wang, Y.; Li, T.; et al. Unveiling Cutting-Edge Developments in Electrocatalytic Nitrate-to-Ammonia Conversion. Adv. Mater. 2024, 36, 2312746. [Google Scholar] [CrossRef] [PubMed]
- de Vooys, A.C.A.; Beltramo, G.L.; van Riet, B.; van Veen, J.A.R.; Koper, M.T.M. Mechanisms of electrochemical reduction and oxidation of nitric oxide. Electrochim. Acta 2004, 49, 1307–1314. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Zhang, J.; Deng, B.; Cao, Z.; Wang, Z.; Wei, G.; Zhang, Q.; Jia, R.; Xiang, P.; et al. Critical review in electrocatalytic nitrate reduction to ammonia towards a sustainable nitrogen utilization. Chem. Eng. J. 2024, 483, 148952. [Google Scholar] [CrossRef]
- Wu, Z.; Song, Y.; Liu, Y.; Luo, W.; Li, W.; Yang, J. Electrocatalytic nitrate reduction: Selectivity at the crossroads between ammonia and nitrogen. Chem Catal. 2023, 3, 100786. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Bi, X. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies. Chem. Eng. J. 2020, 382, 123034. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Wang, H.; Wei, H.; Tang, C.; Li, G.; Dou, Y.; Liu, H.K.; Dou, S.X. Electrocatalytic nitrogen cycle: Mechanism, materials, and momentum. Energy Environ. Sci. 2024, 17, 9027–9050. [Google Scholar] [CrossRef]
- Huang, H.; Russell, A.E. Approaches to achieve surface sensitivity in the in situ XAS of electrocatalysts. Curr. Opin. Electrochem. 2021, 27, 100681. [Google Scholar] [CrossRef]
- Shen, W.; Ye, Y.; Xia, Q.; Xi, P. Progress in in situ characterization of electrocatalysis. EES Catal. 2024, 3, 10–31. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Wang, M.; Liu, S.; Wang, Z.; Qian, T.; Yan, C. Optimizing Intermediate Adsorption via Heteroatom Ensemble Effect over RuFe Bimetallic Alloy for Enhanced Nitrate Electroreduction to Ammonia. Adv. Energy Mater. 2023, 13, 2301409. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Liu, H.; Liu, S.; Yuan, Y.; Meng, Y.; Wang, M.; Shen, C.; Peng, Q.; Chen, J.; et al. Breaking Local Charge Symmetry of Iron Single Atoms for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2023, 62, e202308044. [Google Scholar] [CrossRef]
- Long, X.; Huang, F.; Zhong, T.; Zhao, H.; Li, P.; Fang, J.; Tian, S.; Shu, D.; He, C. One-Step Strategy to Maximize Single-Atom Catalyst Utilization in Nitrate Reduction via Bidirectional Optimization of Mass Transfer and Electron Supply. Environ. Sci. Technol. 2025, 59, 8555–8567. [Google Scholar] [CrossRef]
- Bordiga, S.; Groppo, E.; Agostini, G.; van Bokhoven, J.A.; Lamberti, C. Reactivity of Surface Species in Heterogeneous Catalysts Probed by In Situ X-ray Absorption Techniques. Chem. Rev. 2013, 113, 1736–1850. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, J.; Roldan Cuenya, B. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.; Shen, P.; Zhang, H.; Ma, D.; Chu, K. Lewis Acid Fe-V Pairs Promote Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2211537. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.-Y.; Shi, Y.-M.; Liu, C.-B.; Yu, Y.-F.; Zhang, B. In Situ structural reconstruction of NiMo alloy as a versatile organic oxidation electrode for boosting hydrogen production. Rare Met. 2022, 41, 836–843. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, Z.; Xu, J.; Shen, W.; Zhang, Q.; Yang, X.; Xu, T.; Dang, D.; Hu, H.; Zhao, B.; et al. In Situ Raman study of nickel bicarbonate for high-performance energy storage device. Nano Energy 2019, 64, 103919. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Dong, H.; Zhou, L.; Xu, H.; Wang, H.-R.; Yan, X.; Jiang, Y.; Zhang, J.; Gu, Z.-G. Fe single-atom catalysts with pre-organized coordination structure for efficient electrochemical nitrate reduction to ammonia. Appl. Catal. B Environ. 2022, 317, 121750. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Dieckhöfer, S.; Varhade, S.; Brix, A.C.; Lielpetere, A.; Seisel, S.; Junqueira, J.R.C.; Schuhmann, W. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 2022, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, S.; Gulati, S.; Strain, J.M.; Liang, Y.; Mulvehill, M.C.; Spurgeon, J.M. In Situ Analytical Techniques for the Investigation of Material Stability and Interface Dynamics in Electrocatalytic and Photoelectrochemical Applications. Small Methods 2021, 5, 2100322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Wen, W.; Yu, X.; Du, Y.; Ni, K.; Zhu, Y.; Zhu, M. Atomically Dispersed Unsaturated Cu-N3 Sites on High-Curvature Hierarchically Porous Carbon Nanotube for Synergetic Enhanced Nitrate Electroreduction to Ammonia. Adv. Funct. Mater. 2023, 33, 2302651. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Ma, H.; Koper, M.T.M. Surface Modification of Pt(100) for Electrocatalytic Nitrate Reduction to Dinitrogen in Alkaline Solution. Langmuir 2015, 31, 3277–3281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, X.; Zhang, Z.; Chen, L.; Liu, W.; Tong, L.; Gao, X.; Zhang, J. Graphdiyne Enabled Nitrogen Vacancy Formation in Copper Nitride for Efficient Ammonia Synthesis. J. Am. Chem. Soc. 2024, 146, 14898–14904. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Q.; Shi, L.; Liu, Z. Electron spin resonance studies of coals and coal conversion processes: A review. Fuel Process. Technol. 2019, 188, 212–227. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z.; Zhang, G.; Wang, Y. On-Demand Atomic Hydrogen Provision by Exposing Electron-Rich Cobalt Sites in an Open-Framework Structure toward Superior Electrocatalytic Nitrate Conversion to Dinitrogen. Environ. Sci. Technol. 2022, 56, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, P.; Li, X.; Ma, D.; Chu, K. Sub-nm RuOx Clusters on Pd Metallene for Synergistically Enhanced Nitrate Electroreduction to Ammonia. ACS Nano 2023, 17, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Lim, D.-H. DFT investigation into efficient transition metal single-atom catalysts supported on N-doped graphene for nitrate reduction reactions. Chem. Eng. J. 2023, 468, 143466. [Google Scholar] [CrossRef]
- Gao, X.; Tse, E.C.M. Theoretical Insights into Designing Single-Atom Catalysts on Defective MXenes for Efficient Reduction of Nitrate into Ammonia. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Lv, L.; Shen, Y.; Liu, J.; Gao, X.; Zhou, M.; Zhang, Y.; Meng, X.; Yang, X.; Gong, D.; Zheng, Y.; et al. Revealing the origin of activity and selectivity for Ti/g-C3N4 to ammonia production via nitrate reduction electrocatalysis: A first-principles study. Appl. Catal. A: Gen. 2022, 645, 118846. [Google Scholar] [CrossRef]
- Zuo, Y.; Sun, M.; Li, T.; Sun, L.; Han, S.; Chai, Y.; Huang, B.; Wang, X. Capturing Copper Single Atom in Proton Donor Stimulated O-End Nitrate Reduction. Adv. Mater. 2025, 37, 2415632. [Google Scholar] [CrossRef]
- Zhou, B.; Tong, Y.; Yao, Y.; Zhang, W.; Zhan, G.; Zheng, Q.; Hou, W.; Gu, X.-K.; Zhang, L. Reversed I1Cu4 single-atom sites for superior neutral ammonia electrosynthesis with nitrate. Proc. Natl. Acad. Sci. USA 2024, 121, e2405236121. [Google Scholar] [CrossRef]
- Ke, Z.; He, D.; Yan, X.; Hu, W.; Williams, N.; Kang, H.; Pan, X.; Huang, J.; Gu, J.; Xiao, X. Selective NOx–Electroreduction to Ammonia on Isolated Ru Sites. ACS Nano 2023, 17, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Duy, L.T.; Truong, D.C.; Nguyen Le, B.T.; Phan, B.T.; Cho, Y.; Liu, X.; Lee, H. Efficient ammonia synthesis via electroreduction of nitrite using single-atom Ru-doped Cu nanowire arrays. Chem. Commun. 2022, 58, 5257–5260. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yu, Y.; Zhao, Z.; Mushtaq, M.A.; Ji, Q.; Yasin, G.; Rehman, L.N.U.; Liu, X.; Cai, X.; Tsiakaras, P.; et al. N, O trans-coordinating silver single-atom catalyst for robust and efficient ammonia electrosynthesis from nitrate. Appl. Catal. B Environ. 2023, 331, 122687. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wang, W.; Yin, L.; Crittenden, J.C. Electrocatalytic nitrate reduction to ammonia on defective Au1Cu (111) single-atom alloys. Appl. Catal. B Environ. 2022, 310, 121346. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Cai, W.; Zhao, H.; Zhang, Y.; Sun, Y.; Hu, Z.; Li, S.; Lai, J.; Wang, L. Facet-controlled palladium nanocrystalline for enhanced nitrate reduction towards ammonia. J. Colloid Interface Sci. 2021, 600, 620–628. [Google Scholar] [CrossRef]
- Su, Z.; Chen, T. Porous Noble Metal Electrocatalysts: Synthesis, Performance, and Development. Small 2021, 17, 2005354. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Fu, X.; Huang, H. Descriptors for the Evaluation of Electrocatalytic Reactions: D-Band Theory and Beyond. Adv. Funct. Mater. 2022, 32, 2107651. [Google Scholar] [CrossRef]
- Yu, J.; Yong, X.; Cao, A.; Lu, S. Bi-Layer Single Atom Catalysts Boosted Nitrate-to-Ammonia Electroreduction with High Activity and Selectivity. Acta Phys.-Chim. Sin. 2024, 40, 2307015. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Xu, L.; Zhang, Y.; Wei, W.; Zhao, E.; Wu, Y.; Chen, C. Challenges and Perspectives of Single-Atom-Based Catalysts for Electrochemical Reactions. JACS Au 2023, 3, 736–755. [Google Scholar] [CrossRef]
- Cai, X.; Yang, F.; An, L.; Fu, C.; Luo, L.; Shen, S.; Zhang, J. Evaluation of Electrocatalytic Activity of Noble Metal Catalysts Toward Nitrogen Reduction Reaction in Aqueous Solutions Under Ambient Conditions. ChemSusChem 2022, 15, e202102234. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, S.; Lee, H. Heterogeneous Atomic Catalysts Overcoming the Limitations of Single-Atom Catalysts. ACS Nano 2020, 14, 14355–14374. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Sheng, L.; Wang, M.; Fu, W.; Gao, S.; Zhang, Z.; Chen, S.; Si, R.; Wang, L.; et al. Copper single-atom catalyst as a high-performance electrocatalyst for nitrate-ammonium conversion. J. Hazard. Mater. 2022, 434, 128892. [Google Scholar] [CrossRef]
- Kment, Š.; Bakandritsos, A.; Tantis, I.; Kmentová, H.; Zuo, Y.; Henrotte, O.; Naldoni, A.; Otyepka, M.; Varma, R.S.; Zbořil, R. Single Atom Catalysts Based on Earth-Abundant Metals for Energy-Related Applications. Chem. Rev. 2024, 124, 11767–11847. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tse, E.C.M. Unraveling the Performance Descriptors for Designing Single-Atom Catalysts on Defective MXenes for Exclusive Nitrate-To-Ammonia Electrocatalytic Upcycling. Small 2024, 20, 2306311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Zhang, H.; Chen, X.; Xu, J.; Yang, J.; Zhang, H.; Hu, G. Atomic-dispersed copper simultaneously achieve high-efficiency removal and high-value-added conversion to ammonia of nitrate in sewage. J. Hazard. Mater. 2022, 424, 127319. [Google Scholar] [CrossRef]
- Zhao, D.; Jia, Y.; Wang, H.; Liu, S.; Zhang, W.-X.; Fan, J.; Li, Q.; Luo, L.; Guo, X.; Wang, R.; et al. Modulation of the Second-Beyond Coordination Structure in Single-Atom Electrocatalysts for Confirmed Promotion of Ammonia Synthesis. J. Am. Chem. Soc. 2025, 147, 1884–1892. [Google Scholar]
- Gao, Q.; Yao, B.; Pillai, H.S.; Zang, W.; Han, X.; Liu, Y.; Yu, S.-W.; Yan, Z.; Min, B.; Zhang, S.; et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat. Synth. 2023, 2, 624–634. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, X.; He, Y.; Zhang, H.; Zhou, X.; Zhang, H.; Zhang, S.; Dong, Y.; Hu, X.; Kuklin, A.V.; et al. Two-dimensional BCN matrix inlaid with single-atom-Cu driven electrochemical nitrate reduction reaction to achieve sustainable industrial-grade production of ammonia. Appl. Mater. Today 2021, 25, 101206. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, H.; Dong, F.; Zhao, X.; Qu, Y.; Wang, L.; Peng, Y.; Wang, D.; Fang, W.; Li, J. N-Coordinated Cu–Ni Dual-Single-Atom Catalyst for Highly Selective Electrocatalytic Reduction of Nitrate to Ammonia. Small 2023, 19, 2207695. [Google Scholar] [CrossRef]
- Lu, X.; Wei, J.; Lin, H.; Li, Y.; Li, Y.-y. Boron Regulated Fe Single-Atom Structures for Electrocatalytic Nitrate Reduction to Ammonia. ACS Appl. Nano Mater. 2024, 7, 14654–14664. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, C.; Zheng, L.; Petit, E.; Qi, K.; Zhang, Y.; Wu, H.; Wang, W.; Tiberj, A.; et al. 3.4% Solar-to-Ammonia Efficiency from Nitrate Using Fe Single Atomic Catalyst Supported on MoS2 Nanosheets. Adv. Funct. Mater. 2022, 32, 2108316. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, C.; Jia, Y.; Nie, G.; Yang, G. Spin modulation of single Fe atoms with thiolate-poisoned Pd nanoclusters for highly efficient ammonia synthesis. AIChE J. 2024, 70, e18449. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Fang, Z.; Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 2021, 14, 3522–3531. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Hou, C.-C.; Zou, L.; Sun, L.; Zhang, K.; Liu, Z.; Li, Y.; Li, C.; Zou, R.; Yu, J.; Xu, Q. Single-Atom Iron Catalysts on Overhang-Eave Carbon Cages for High-Performance Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 7384–7389. [Google Scholar] [CrossRef]
- Zheng, X.; Hao, J.; Zhuang, Z.; Kang, Q.; Wang, X.; Lu, S.; Duan, F.; Du, M.; Zhu, H. Emerging electrospinning platform toward nanoparticle to single atom transformation for steering selectivity in ammonia synthesis. Nanoscale 2024, 16, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiang, J.; Zhang, G.; Chen, K.; Chu, K. Single-atom Co alloyed Ru for electrocatalytic nitrite reduction to ammonia. Nano Res. 2024, 17, 3660–3666. [Google Scholar] [CrossRef]
- Ni, J.; Yan, J.; Li, F.; Qi, H.; Xu, Q.; Su, C.; Sun, L.; Sun, H.; Ding, J.; Liu, B. Atomic Co–P Catalytic Pair Drives Efficient Electrochemical Nitrate Reduction to Ammonia. Adv. Energy Mater. 2024, 14, 2400065. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; An, N.; Zhang, S.; Song, Q.; Yang, Y.; Li, J.; Liu, X. Boosted ammonium production by single cobalt atom catalysts with high Faradic efficiencies. Proc. Natl. Acad. Sci. USA 2022, 119, e2123450119. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Ruzybayev, I.; Shah, I.; Huang, C.P. The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold. Appl. Catal. B Environ. 2016, 180, 199–209. [Google Scholar] [CrossRef]
- Xie, S.; Ruan, W.; Liu, Q.; Zhang, Y.; Guo, X.; Ding, K. Theoretical insight into electrochemical nitrate reduction on transition metal iron doped graphdiyne. Int. J. Quantum Chem. 2024, 124, e27379. [Google Scholar] [CrossRef]
- Wu, T.; Kong, X.; Tong, S.; Chen, Y.; Liu, J.; Tang, Y.; Yang, X.; Chen, Y.; Wan, P. Self-supported Cu nanosheets derived from CuCl-CuO for highly efficient electrochemical degradation of NO3−. Appl. Surf. Sci. 2019, 489, 321–329. [Google Scholar] [CrossRef]
- Yin, H.; Dong, F.; Wang, Y.; Su, H.; Li, X.; Peng, Y.; Duan, H.; Li, J. Understanding the Activity Trends in Electrocatalytic Nitrate Reduction to Ammonia on Cu Catalysts. Nano Lett. 2023, 23, 11899–11906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Q.; Liao, P.; Duan, W.; Liang, S.; Yan, Z.; Feng, C. Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production. Small 2020, 16, 2004526. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Feng, T.; Ma, M.; Li, Z.; Tang, L. Emerging Advances in Cu-based electrocatalysts for electrochemical nitrate reduction (NO3RR). Surf. Interfaces 2024, 48, 104294. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Y.; Zhan, J.; Jia, Y.; Yao, T.; Zhang, L.-H.; Yu, F. P-Modified Single-Atom Cu Catalyst Boosting Electrocatalytic Performance of NO3− Reduction to NH3. ChemCatChem 2023, 15, e202201633. [Google Scholar] [CrossRef]
- Cheng, X.-F.; He, J.-H.; Ji, H.-Q.; Zhang, H.-Y.; Cao, Q.; Sun, W.-J.; Yan, C.-L.; Lu, J.-M. Coordination Symmetry Breaking of Single-Atom Catalysts for Robust and Efficient Nitrate Electroreduction to Ammonia. Adv. Mater. 2022, 34, 2205767. [Google Scholar] [CrossRef]
- Zhang, W.; Chao, Y.; Zhang, W.; Zhou, J.; Lv, F.; Wang, K.; Lin, F.; Luo, H.; Li, J.; Tong, M.; et al. Emerging Dual-Atomic-Site Catalysts for Efficient Energy Catalysis. Adv. Mater. 2021, 33, 2102576. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X.; Su, Y.; Qin, X.; Chen, S.; Yu, H. Enhanced Chlorinated Pollutant Degradation by the Synergistic Effect between Dechlorination and Hydroxyl Radical Oxidation on a Bimetallic Single-Atom Catalyst. Environ. Sci. Technol. 2021, 55, 14194–14203. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, K.; Chen, Y. Breaking the Scaling Relationship Limit: From Single-Atom to Dual-Atom Catalysts. Acc. Mater. Res. 2022, 3, 584–596. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, P.; Qi, C.; Li, M.; Xie, M.; Zhu, G.; Luo, W. Synergistic electroreduction of nitrate to ammonia via bimetallic Fe-Cu sites supported on hierarchical porous carbon. Trans. Mater. Res. 2025, 1, 100030. [Google Scholar] [CrossRef]
- Singh, B.; Gawande, M.B.; Kute, A.D.; Varma, R.S.; Fornasiero, P.; McNeice, P.; Jagadeesh, R.V.; Beller, M.; Zbořil, R. Single-Atom (Iron-Based) Catalysts: Synthesis and Applications. Chem. Rev. 2021, 121, 13620–13697. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Mu, J.; Liu, B. Transition metal single-atom electrocatalytic reduction catalyst for nitrate to ammonia. J. Electroanal. Chem. 2024, 969, 118533. [Google Scholar] [CrossRef]
- Li, J.; Zhong, W.; Wu, K.; Petit, E.; Lajaunie, L.; Qi, K.; Zhang, Y.; Wu, H.; Liu, J.; Heng, J.; et al. Efficient solar-driven electrocatalytic nitrate-to-ammonia conversion by 2D ultrathin Fe single-atom catalysts. J. Mater. Chem. A 2024, 12, 26103–26112. [Google Scholar] [CrossRef]
- Song, Q.; Li, M.; Hou, X.; Li, J.; Dong, Z.; Zhang, S.; Yang, L.; Liu, X. Anchored Fe atoms for NO bond activation to boost electrocatalytic nitrate reduction at low concentrations. Appl. Catal. B Environ. 2022, 317, 121721. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, T.; Fu, H.; Chen, Z.; Qu, X.; Zheng, S. Construction and identification of highly active single-atom Fe1-NC catalytic site for electrocatalytic nitrate reduction. Appl. Catal. B Environ. 2023, 323, 122181. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Li, L.; Hui, K.S.; Dinh, D.A.; Wu, S.; Kumar, S.; Chen, F.; Shao, Z.; Hui, K.N. High-throughput identification of highly active and selective single-atom catalysts for electrochemical ammonia synthesis through nitrate reduction. Nano Energy 2022, 100, 107517. [Google Scholar] [CrossRef]
- Song, B.; Xiang, D.; Chen, C.; Xu, J.; Ma, Y.; Yang, L.; Lee, M.-H.; Zhao, D.; Han, K.; Wang, N. Enhancing nitrate reduction reaction electroactivity of Fe single atom catalyst by modification of Fe nanoclusters and further application in self-powered ammonia synthesis from air. Chem. Eng. J. 2025, 521, 166374. [Google Scholar] [CrossRef]
- Zhao, H.; Xiang, J.; Sun, Z.; Shang, S.; Chu, K. Electroreduction of Nitrite to Ammonia over a Cobalt Single-Atom Catalyst. ACS Sustain. Chem. Eng. 2024, 12, 2783–2789. [Google Scholar] [CrossRef]
- Xiang, T.; Liang, Y.; Zeng, Y.; Deng, J.; Yuan, J.; Xiong, W.; Song, B.; Zhou, C.; Yang, Y. Transition Metal Single-Atom Catalysts for the Electrocatalytic Nitrate Reduction: Mechanism, Synthesis, Characterization, Application, and Prospects. Small 2023, 19, 2303732. [Google Scholar] [CrossRef]
- Xu, S.; Shi, Y.; Wen, Z.; Liu, X.; Zhu, Y.; Liu, G.; Gao, H.; Sun, L.; Li, F. Polystyrene spheres-templated mesoporous carbonous frameworks implanted with cobalt nanoparticles for highly efficient electrochemical nitrate reduction to ammonia. Appl. Catal. B Environ. 2023, 323, 122192. [Google Scholar] [CrossRef]
- Liu, P.; Yan, J.; Huang, H.; Song, W. Cu/Co bimetallic conductive MOFs: Electronic modulation for enhanced nitrate reduction to ammonia. Chem. Eng. J. 2023, 466, 143134. [Google Scholar] [CrossRef]
- Chao, G.; Wang, J.; Zong, W.; Fan, W.; Xue, T.; Zhang, L.; Liu, T. Single-atom catalysts for electrocatalytic nitrate reduction into ammonia. Nanotechnology 2024, 35, 432001. [Google Scholar] [CrossRef]
- Ajmal, S.; Huang, J.; Guo, J.; Tabish, M.; Mushtaq, M.A.; Alam, M.M.; Yasin, G. Substrate Engineering of Single Atom Catalysts Enabled Next-Generation Electrocatalysis to Power a More Sustainable Future. Catalysts 2025, 15, 137. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Lian, S.; Li, J.; Sun, K.; Zhao, S.; Kim, Y.D.; Ren, Y.; Zhang, M.; Liu, Q.; et al. Engineering the oxygen vacancies enables Ni single-atom catalyst for stable and efficient C-H activation. Appl. Catal. B Environ. 2022, 314, 121516. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Yang, L.; Gao, Q.; Li, P.; Zhang, Y.; Xiong, Y.; Fan, Z.; Quan, X.; Liu, Y. Single-Atom Cu and Zn Vacancy Synergy in NiFe-LDH Boosts Metal–Support Interaction for High-Efficiency Nitrate-to-Ammonia Electroreduction. Environ. Sci. Technol. 2025, 59, 11414–11425. [Google Scholar] [CrossRef]
- Tian, H.; Song, A.; Zhang, P.; Sun, K.; Wang, J.; Sun, B.; Fan, Q.; Shao, G.; Chen, C.; Liu, H.; et al. High Durability of Fe–N–C Single-Atom Catalysts with Carbon Vacancies toward the Oxygen Reduction Reaction in Alkaline Media. Adv. Mater. 2023, 35, 2210714. [Google Scholar] [CrossRef]
- Senthamaraikannan, T.G.; Kaliaperumal, S.; Krishnamurty, S. Role of Chemical Structure of Support in Enhancing the Catalytic Activity of a Single Atom Catalyst Toward NRR: A Computational Study. Front. Chem. 2021, 9, 733422. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liang, T.; You, J.; Huo, Q.; Qi, S.; Zhao, J.; Meng, N.; Liao, J.; Shang, C.; Yang, H.; et al. Coordination environment-tailored electronic structure of single atomic copper sites for efficient electrochemical nitrate reduction toward ammonia. Energy Environ. Sci. 2024, 17, 8360–8367. [Google Scholar] [CrossRef]
- Chang, G.; Chen, X.; Lv, J.-J.; Kong, Z.; Wang, Z.-J. Cobalt-Based Electrocatalysts for Sustainable Nitrate Conversion: Structural Design and Mechanistic Advancements. Nano-Micro Lett. 2025, 18, 73. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Cao, D.; Cheng, D. Carbon-based material-supported single-atom catalysts for energy conversion. iScience 2022, 25, 104367. [Google Scholar] [CrossRef]
- Leverett, J.; Tran-Phu, T.; Yuwono, J.A.; Kumar, P.; Kim, C.; Zhai, Q.; Han, C.; Qu, J.; Cairney, J.; Simonov, A.N.; et al. Tuning the Coordination Structure of Cu–N–C Single Atom Catalysts for Simultaneous Electrochemical Reduction of CO2 and NO3− to Urea. Adv. Energy Mater. 2022, 12, 2201500. [Google Scholar] [CrossRef]
- Zhao, D.; Jia, Y.; Wang, H.; Liu, S.; Zhang, W.-X.; Fan, J. Electrocatalytic nitrate reduction to ammonia over single-atom catalysts: Focus on support materials. Sep. Purif. Technol. 2025, 377, 134420. [Google Scholar] [CrossRef]
- Fan, B.; Wang, W.; Liu, Z.; Guo, J.; Yuan, H.; Tan, Y. Recent progress in single atomic catalysts for electrochemical N2 fixation. Microstructures 2024, 4, 2024025. [Google Scholar] [CrossRef]
- Wu, C.; Shen, Y.; Lv, L.; Meng, X.; Yang, X.; Wang, X.; Jiang, X.; Ai, Q.; Shuai, Y.; Zhou, Z. Multi-orbital engineering of single-atom catalysts: Unlocking high-efficiency nitrate reduction. J. Mater. Chem. A 2025, 13, 6631–6643. [Google Scholar] [CrossRef]
- Yin, H.; Peng, Y.; Li, J. Electrocatalytic Reduction of Nitrate to Ammonia via a Au/Cu Single Atom Alloy Catalyst. Environ. Sci. Technol. 2023, 57, 3134–3144. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Choi, H.; Kong, Y.; Oh, J. Tandem Electroreduction of Nitrate to Ammonia Using a Cobalt–Copper Mixed Single-Atom/Cluster Catalyst with Synergistic Effects. Adv. Sci. 2024, 11, 2407250. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tang, S.; Li, Z.; Wang, M.; Jin, Z.; Li, P.; Zhan, X.; Zhou, H.; Yu, G. Intermetallic Single-Atom Alloy In–Pd Bimetallene for Neutral Electrosynthesis of Ammonia from Nitrate. J. Am. Chem. Soc. 2023, 145, 13957–13967. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Selective Nitrate-to-Ammonia Transformation on Surface Defects of Titanium Dioxide Photocatalysts. ACS Catal. 2017, 7, 3713–3720. [Google Scholar] [CrossRef]
- Hiramatsu, W.; Shiraishi, Y.; Ichikawa, S.; Tanaka, S.; Kawada, Y.; Hiraiwa, C.; Hirai, T. Surface Oxygen Vacancies on Copper-Doped Titanium Dioxide for Photocatalytic Nitrate-to-Ammonia Reduction. J. Am. Chem. Soc. 2025, 147, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-W.; Song, H.; Yoo, J.; Chipoco Haro, D.A.; Lee, H.M.; Medford, A.J.; Hatzell, M.C. Impact of Local Microenvironments on the Selectivity of Electrocatalytic Nitrate Reduction in a BPM-MEA System. Adv. Energy Mater. 2024, 14, 2304202. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Z.; Han, S.; Deng, L.; Yu, J.; Lin, Y.; Long, X.; Gu, M.; Yang, S. Identifying the Active Sites of a Single Atom Catalyst with pH-Universal Oxygen Reduction Reaction Activity. Cell Rep. Phys. Sci. 2020, 1, 100115. [Google Scholar] [CrossRef]
- Meng, Z.; Priyadarsini, A.; Shi, K.; Ren, Z.; Subedi, D.; Israel, D.; Kaden, W.E.; Feng, X.; Kattel, S. pH-Dependent Electroreduction of Nitrate on Fe Single-Atom Catalyst. ChemSusChem 2025, 18, e202500717. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xu, H.; Chang, L.; Lin, A.; Cheng, D. Revealing the pH-dependent mechanism of nitrate electrochemical reduction to ammonia on single-atom catalysts. Nanoscale 2022, 14, 15422–15431. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ye, C.; Shen, Y. Effects of electrolyte pHs, temperatures, potentials and oxalate ions on the electrocatalytic reduction of nitrates. J. Electroanal. Chem. 2024, 957, 118143. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Xie, J.; Ding, L.; Zhu, J.; Wei, W.; Yan, Y.-M.; Chu, D.; Ni, B.-J. Recent trends and prospects in electrochemical nitrate reduction to ammonia with an emphasis on cobalt catalysts. Coord. Chem. Rev. 2025, 539, 216751. [Google Scholar] [CrossRef]
- Guo, J.; Brimley, P.; Liu, M.J.; Corson, E.R.; Muñoz, C.; Smith, W.A.; Tarpeh, W.A. Mass Transport Modifies the Interfacial Electrolyte to Influence Electrochemical Nitrate Reduction. ACS Sustain. Chem. Eng. 2023, 11, 7882–7893. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, S.L.; Yang, J.; Hu, S.; Duan, D.; Heng, J.Z.X.; Wang, Z.; Yang, W.; Liu, X.; Yan, Q.; et al. Phase-Regulatable Synthesis of Single-Atom Alloy Nanocages for Efficient Alkaline Hydrogen Evolution. J. Am. Chem. Soc. 2025, 147, 35293–35303. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, G.; Cheng, X.; Xu, F.; Huang, H.; Wang, X.; Yang, L.; Wu, Q.; Hu, Z. Self-enhanced localized alkalinity at the encapsulated Cu catalyst for superb electrocatalytic nitrate/nitrite reduction to NH3 in neutral electrolyte. Sci. Adv. 2024, 10, eadm9325. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Nazemi, M. Understanding Potential Losses and pH Distribution in the Electrochemical Nitrate Reduction Reaction to Ammonia. Ind. Eng. Chem. Res. 2024, 63, 9315–9328. [Google Scholar] [CrossRef]
- Park, D.; Jung, Y. Atomic-scale understanding of alkali metal cation effects on electro-catalytic reactions. Chem Catal. 2024, 4, 100823. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Westerhoff, P.; Garcia-Segura, S.; Sánchez-Sánchez, C.M. Selectivity modulation during electrochemical reduction of nitrate by electrolyte engineering. Sep. Purif. Technol. 2023, 321, 124233. [Google Scholar] [CrossRef]
- Wan, K.; Cui, Y.; Francisco, J.S.; Shi, X.; Zeng, X.C. Unravelling the Effects of Anions on the Vicinal H-Bonding Network near an Electrode and Activity of Single-Atom Electrocatalysts. J. Am. Chem. Soc. 2025, 147, 38301–38310. [Google Scholar] [CrossRef]
- Fan, J.; Arrazolo, L.K.; Du, J.; Xu, H.; Fang, S.; Liu, Y.; Wu, Z.; Kim, J.-H.; Wu, X. Effects of Ionic Interferents on Electrocatalytic Nitrate Reduction: Mechanistic Insight. Environ. Sci. Technol. 2024, 58, 12823–12845. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Ji, X.; Wang, S.-C.; Jing, P.; Xu, X.; Liu, B.; Zhang, J. Stabilization of Electron-Deficient Cu1δ+ Species by Cl–Doped CeO2 Constructed via Electrochemical Reconstruction for Electroreduction of Nitrate to Ammonia Over 2500 Hours. Adv. Funct. Mater. 2025, 35, 2502073. [Google Scholar] [CrossRef]
- Zheng, S.-J.; Dong, X.-Y.; Chen, H.; Huang, R.-W.; Cai, J.; Zang, S.-Q. Unveiling Ionized Interfacial Water-Induced Localized H* Enrichment for Electrocatalytic Nitrate Reduction. Angew. Chem. Int. Ed. 2025, 64, e202413033. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Chen, Z.; Zhang, G.; Chen, W.; Peng, C.; Yang, X.; Zheng, L.; Li, Y.; Ren, X.; Cao, H.; et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 2022, 15, 760–770. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Wang, C.; Liu, Y.; Yang, Y.; Wang, C.; Jiang, W.; Fu, L.; Xu, J. CoP nanowires on carbon cloth for electrocatalytic NOx− reduction to ammonia. J. Electroanal. Chem. 2022, 910, 116171. [Google Scholar] [CrossRef]
- Liu, J.-C.; Luo, F.; Li, J. Electrochemical Potential-Driven Shift of Frontier Orbitals in M–N–C Single-Atom Catalysts Leading to Inverted Adsorption Energies. J. Am. Chem. Soc. 2023, 145, 25264–25273. [Google Scholar] [CrossRef]
- Yang, J.; Qi, H.; Li, A.; Liu, X.; Yang, X.; Zhang, S.; Zhao, Q.; Jiang, Q.; Su, Y.; Zhang, L.; et al. Potential-Driven Restructuring of Cu Single Atoms to Nanoparticles for Boosting the Electrochemical Reduction of Nitrate to Ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Z.; Ou, Y.; Zhong, L.; Yan, C.; Zhang, C.; Song, K.; Liu, H.; Liu, D.; Song, P.; et al. Pulsed Electrocatalysis Driven Efficient Ammonia Synthesis by Facilitating *NOOH Formation and Balancing *H Supply. Angew. Chem. Int. Ed. 2025, 64, e202510287. [Google Scholar] [CrossRef] [PubMed]
| Catalysts | Voltage | Electrolyte | FENH3 (%) | Ammonia Yield Rate | Ref. |
|---|---|---|---|---|---|
| BCN@Cu/CNT | −0.6 V | 0.1 M KOH + 50 mg L−1 NO3− | 95.32 | 10.13 mmol h−1 mgcat−1 | [74] |
| Cu12-NDI-H | −1.1 V | 0.1 M KHCO3 + 0.5 M KNO3 | 98.7 | 35.1 mg mgh−1 mgcat−1 | [75] |
| Cu/CuAu SAA | −0.5 V | 1 M KOH + 1 M KNO3 | 85.5 | 8470 m mol h−1 g−1 | [76] |
| Cu-SACs | −0.6 V | 0.1 M KOH + 100 mM NO3− | 97.37 | 3.36 mg h−1 cm−2 | [77] |
| Cu/Ni-NC | −0.7 V | 0.5 M Na2SO4 + 100 ppm NO3− | 97.28 | 322.35 mmol h−1 mgcat−1 cm−2 | [78] |
| Fe-BCN | −0.3 V | 0.1 M KOH + 0.5 M KNO3 | 97.48 | 0.128 mmol h−1 cm−2 | [79] |
| Fe-MoS2-SACs | −0.48 V | 0.1 M Na2SO4 + 0.1 M NaNO3 | 98 | 0.432 mg h−1 cm−2 | [80] |
| Fe-N-C/PdNC | −0.5 V | 1 M KOH + 0.5 M KNO3 | 98.6 | 392.16 mmol h−1 gcat−1 | [81] |
| Fe-PPy SACs | −0.7 V/ −0.3 V | 0.1 M KOH + 0.1 M KNO3 | ~100 | 0.162 mmol h−1 cm−2 | [82] |
| Fe-SACs | −0.66 V | 0.1 M K2SO4 + 0.5 M NO3− | 75 | 0.46 mmol h−1 cm−2 | [83] |
| Fe-V2O5-SACs | −0.7 V | 1.0 M KOH + 0.1 M KNO3 | 98 | 12.5 mg h−1 cm−2 | [84] |
| Co SAs/CNFs | −0.7 V | 0.1 M K2SO4 + 0.1 M KNO3 | 91.3 | 0.79 mmol h−1 cm−2 | [85] |
| Co1Ru | −0.7 V | 0.5 M Na2SO4 + 0.1 M NaNO2 | 92 | 2379.2 μmol h−1 cm−2 | [86] |
| Co2-P/NPG | −0.7 V | 0.5 M K2SO4 + 0.1 M KNO3 | 93.8 | 0.506 mmol h−1 mgcat−1 | [87] |
| Co-SACs | −0.69 V | 100 mgL−1 NO3− + 0.02 M Na2SO4 | 92 | 0.433 mg h−1 cm−2 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Wang, Y. Single-Atom Catalysts for Electrochemical Nitrate Reduction to Ammonia: Rational Design, Mechanistic Insights, and System Perspectives. Catalysts 2025, 15, 1084. https://doi.org/10.3390/catal15111084
Yin S, Wang Y. Single-Atom Catalysts for Electrochemical Nitrate Reduction to Ammonia: Rational Design, Mechanistic Insights, and System Perspectives. Catalysts. 2025; 15(11):1084. https://doi.org/10.3390/catal15111084
Chicago/Turabian StyleYin, Shupeng, and Yinglong Wang. 2025. "Single-Atom Catalysts for Electrochemical Nitrate Reduction to Ammonia: Rational Design, Mechanistic Insights, and System Perspectives" Catalysts 15, no. 11: 1084. https://doi.org/10.3390/catal15111084
APA StyleYin, S., & Wang, Y. (2025). Single-Atom Catalysts for Electrochemical Nitrate Reduction to Ammonia: Rational Design, Mechanistic Insights, and System Perspectives. Catalysts, 15(11), 1084. https://doi.org/10.3390/catal15111084






