Effect of a Grinding Method in the Preparation of CuO-ZnO-Al2O3@HZSM-5 Catalyst for CO2 Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
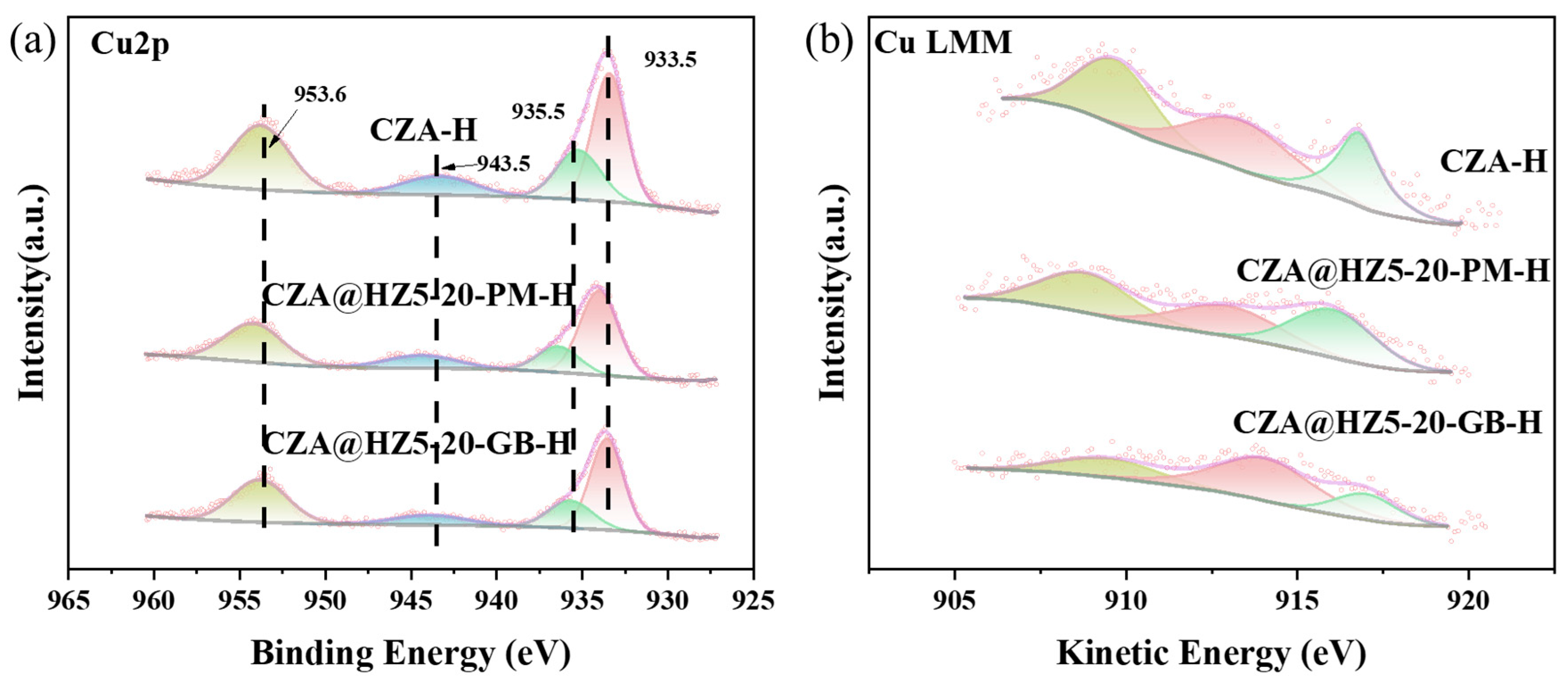
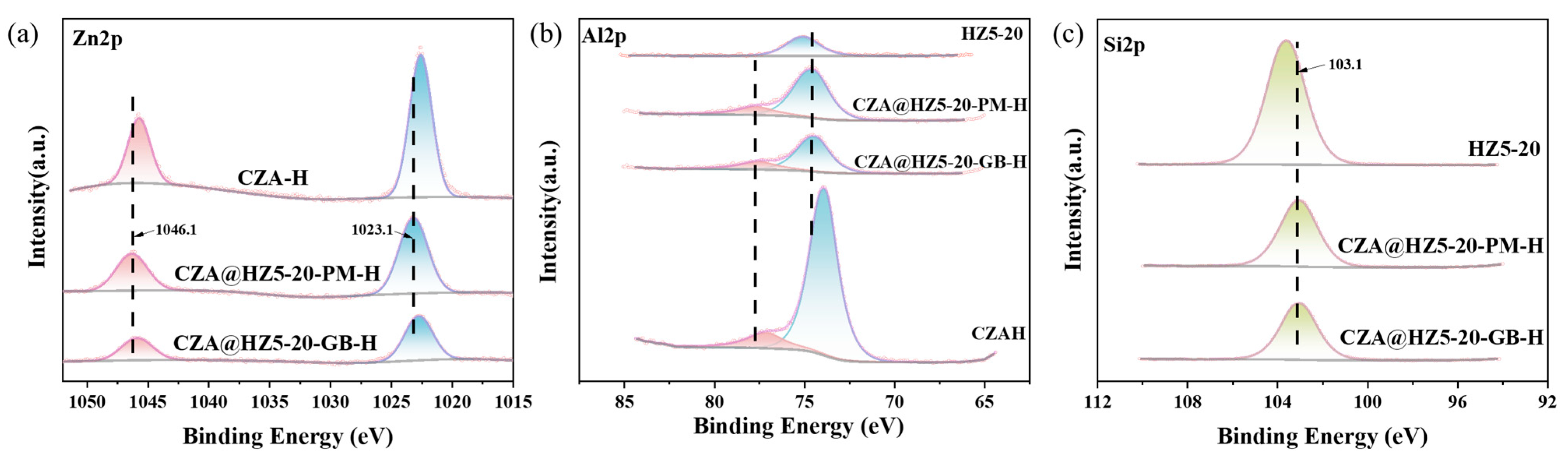
2.1. Characterizations
2.2. Catalytic Performance Test of Catalysts
2.2.1. Gas Velocity
2.2.2. Reaction Temperature
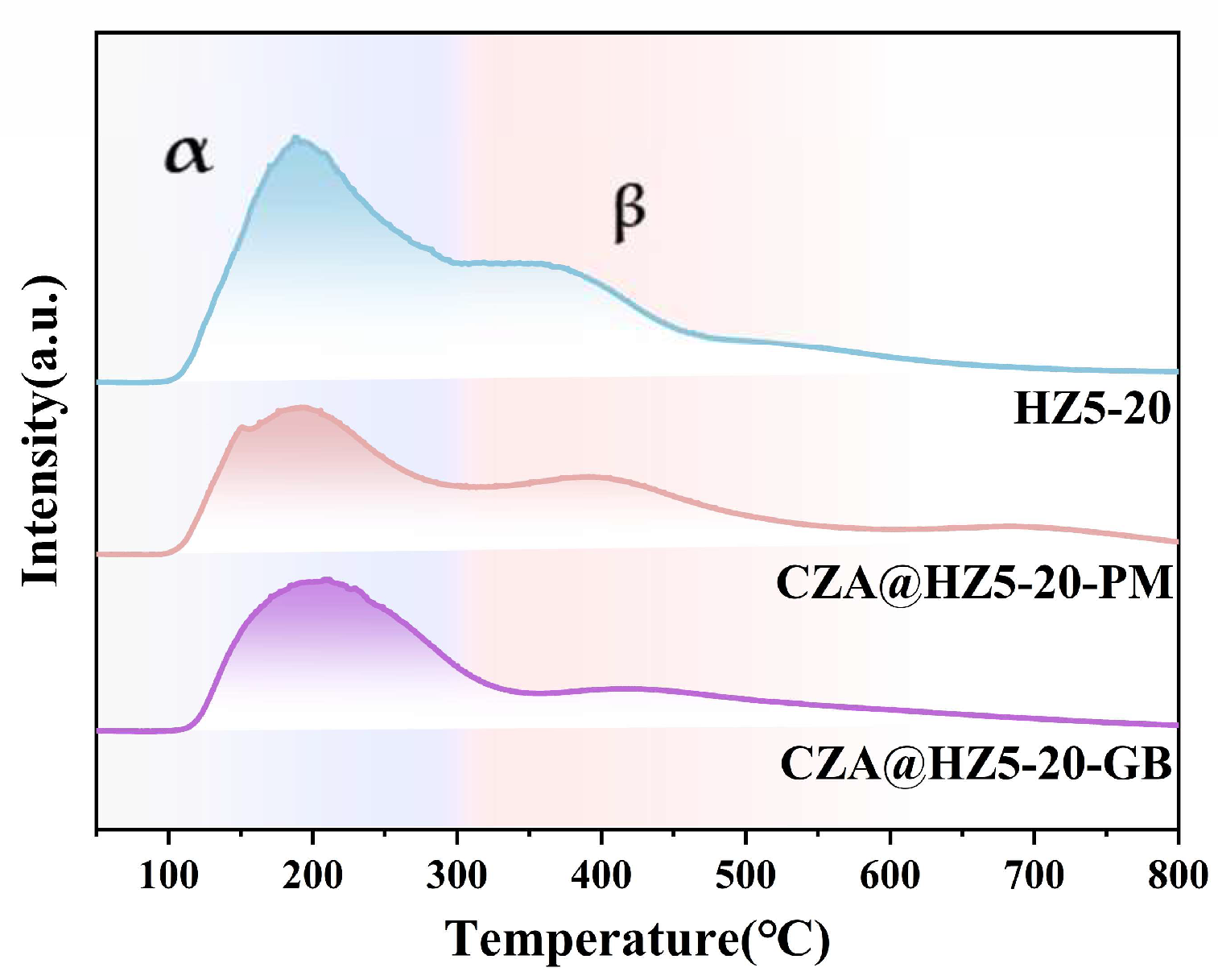
2.2.3. Acidic Sites
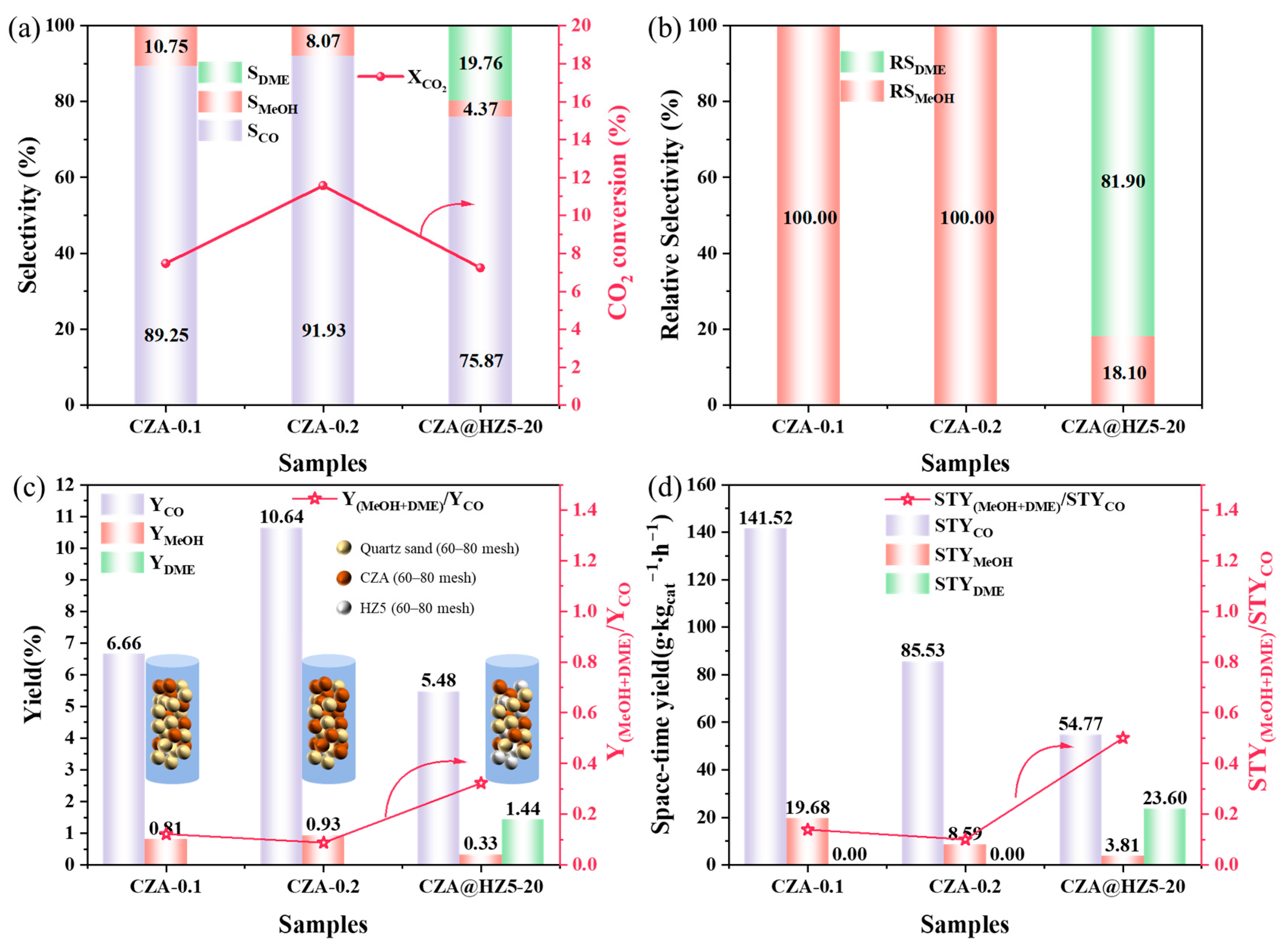
2.2.4. Filling Amount of Active Component
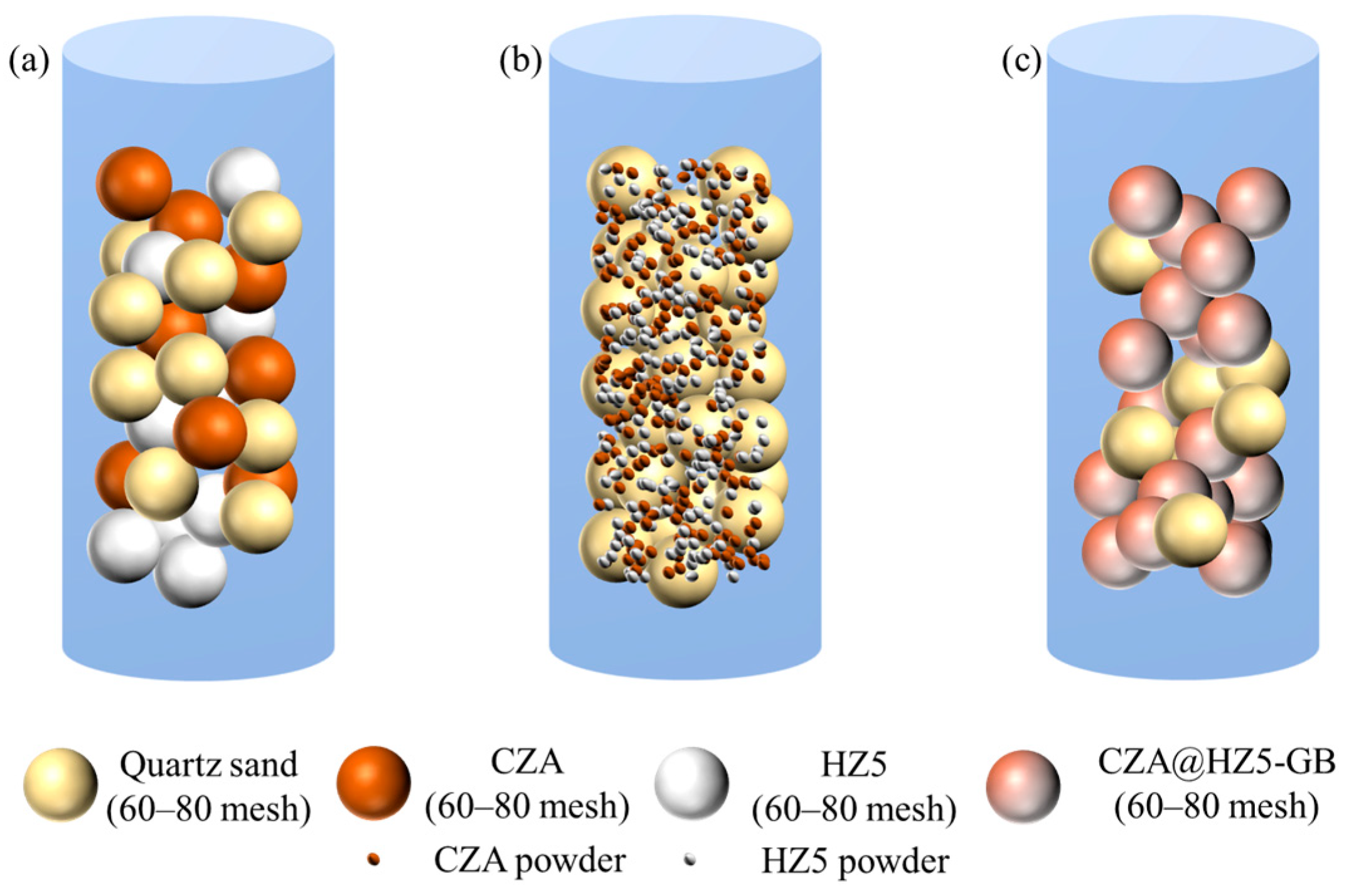
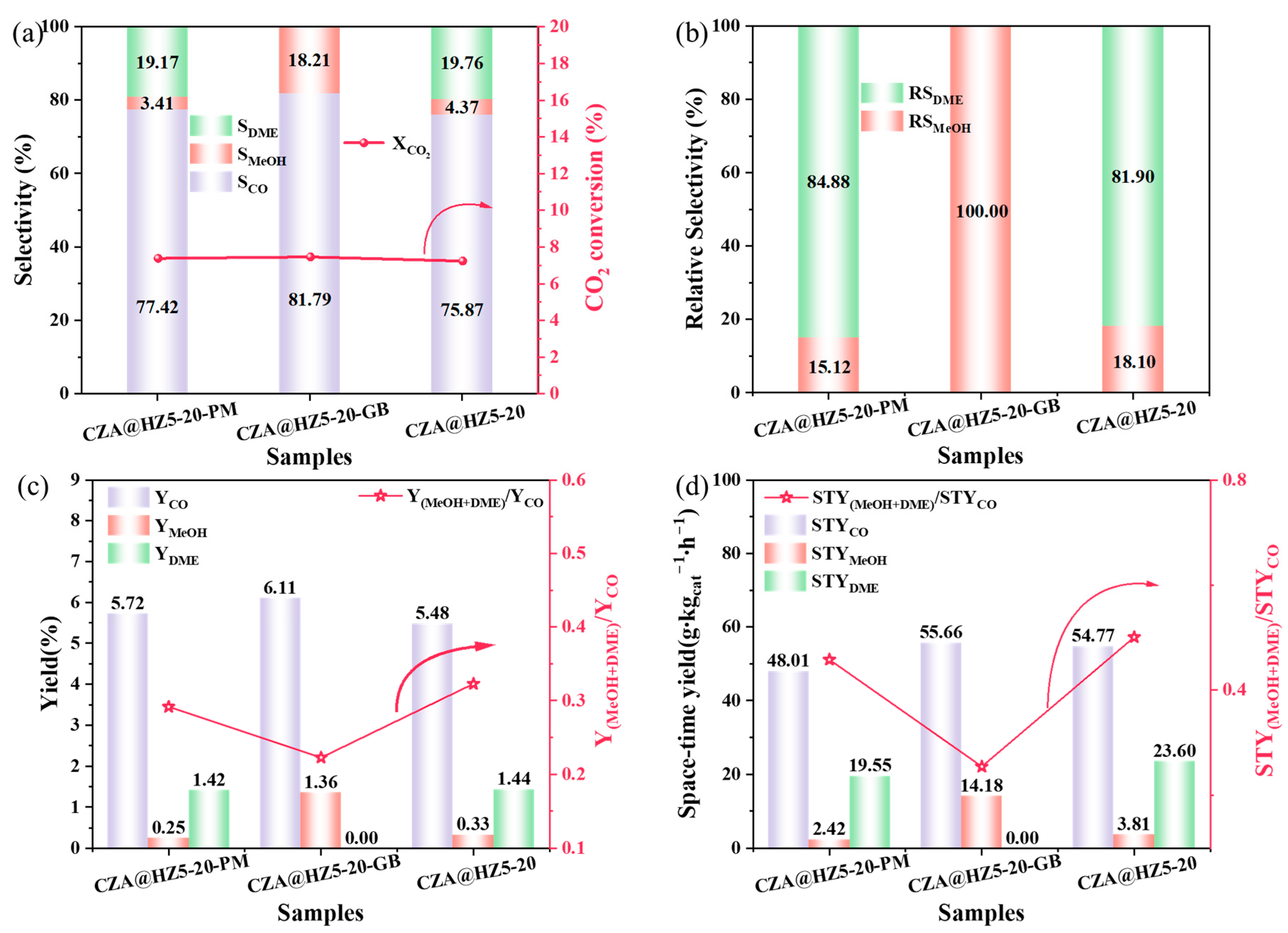
2.2.5. Physical Mixing Mode of the Active Component and the Carrier
2.3. Study on Catalytic Mechanism of Catalysts
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization Methods
3.3. Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Overa, S.; Feric, T.G.; Park, A.H.A.; Jiao, F. Tandem and Hybrid Processes for Carbon Dioxide Utilization. Joule 2021, 5, 8–13. [Google Scholar] [CrossRef]
- Nie, X. Application Prospect of Carbon Dioxide Hydrogenation to Methanol Technology in the New Electric Power Systems. AJEE 2024, 12, 17–25. [Google Scholar] [CrossRef]
- Lin, T.C.; Bhan, A. Rates and Reversibility of CO2 Hydrogenation on Cu-Based Catalysts. J. Catal. 2024, 429, 115214. [Google Scholar] [CrossRef]
- Sun, J.; Liu, F.; Salahuddin, U.; Wu, M.; Zhu, C.; Lu, X.; Zhang, B.; Zhao, B.; Xie, Z.; Ding, Y.; et al. Optimization and Understanding of ZnO Nanoarray Supported Cu-ZnO-Al2O3 Catalyst for Enhanced CO2 -Methanol Conversion at Low Temperature and Pressure. Chem. Eng. J. 2023, 455, 140559. [Google Scholar] [CrossRef]
- Liu, Z.; An, X.; Song, M.; Wang, Z.; Wei, Y.; Mintova, S.; Giordano, G.; Yan, Z. Dry Gel Assisting Crystallization of Bifunctional CuO–ZnO–Al2O3/SiO2–Al2O3 Catalysts for CO2 Hydrogenation. Biomass Bioenergy 2022, 163, 106525. [Google Scholar] [CrossRef]
- Cui, X.; Luo, M.; Yang, Z.; Rahman, R.; Li, Z.; Yang, W.; Xia, L. Efficient Cu–Zn–Al/LDH Catalysts for CO2 -to-Methanol Conversion. Energy Fuels 2025, 39, 2675–2687. [Google Scholar] [CrossRef]
- Li, X.; Eliasson, H.; Dachraoui, W.; Erni, R. Growth and Mobility of Copper in Industrial Cu/ZnO/Al2O3 Hydrogenation Catalyst Investigated by in Situ Gas-Cell Scanning Transmission Electron Microscopy. Appl. Surf. Sci. 2025, 688, 162321. [Google Scholar] [CrossRef]
- Shao, S.; Cui, C.; Tang, Z.; Li, G. Recent Advances in Metal-Organic Frameworks for Catalytic CO2 Hydrogenation to Diverse Products. Nano Res. 2022, 15, 10110–10133. [Google Scholar] [CrossRef]
- Bao, C.; Li, Y.T.; Zhang, Q.; Hu, T.L. Copper Nanoparticles Supported on Metal-Organic Framework with Topological Defects for CO2 Hydrogenation to Methanol. J. Colloid Interface Sci. 2025, 686, 1147–1156. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wu, D.; Zhang, J.; Ma, Q.; Gao, X.; Lai, X.; Xia, H.; Fan, S.; Zhao, T.S. Hydrogenation of CO2 to Light Olefins on CuZnZr@(Zn-)SAPO-34 Catalysts: Strategy for Product Distribution. Fuel 2019, 239, 44–52. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.; Meng, F.; Zhao, B.; Kanitkar, S.; Tang, Y. Catalytic Conversion of Chloromethane to Olefins and Aromatics Over Zeolite Catalysts. Catal. Lett. 2021, 151, 1038–1048. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Gao, M.; Shao, X.; Dai, W.; Wu, G.; Guan, N.; Xu, Z.; Ye, M.; Li, L. Directional Construction of Active Naphthalenic Species within SAPO-34 Crystals toward More Efficient Methanol-to-Olefin Conversion. J. Am. Chem. Soc. 2022, 144, 21408–21416. [Google Scholar] [CrossRef]
- Ali, M.; Zafar, F.; Shen, D.; Wang, X.; Wook Bae, J. Contributions of ZSM-5 Morphology over Hybridized ZnO-ZrO2/ZSM-5 for Direct CO2 Hydrogenation Activity to Aromatics. Fuel 2024, 378, 132925. [Google Scholar] [CrossRef]
- Kosari, M.; Lee, K.; Wang, C.; Rimaz, S.; Zhou, S.; Hondo, E.; Xi, S.; Seayad, A.M.; Zeng, H.C.; Borgna, A. Optimizing Hollow ZSM-5 Spheres (hZSM5) Morphology and Its Intrinsic Acidity for Hydrogenation of CO2 to DME with Copper–Aluminum. Chem. Eng. J. 2023, 470, 144196. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Mezzapica, A.; Spadaro, L.; Arena, F.; Frusteri, F. Catalytic Behaviour of a Bifunctional System for the One Step Synthesis of DME by CO2 Hydrogenation. Catal. Today 2014, 228, 51–57. [Google Scholar] [CrossRef]
- Mohamed, A.T.; Ahmad, Y.H.; Anwer, A.H.; Soliman, A.; Saad, M.A.H.; Aroua, M.K.; Al-Qaradawi, S.Y.; Benamor, A. CO2 Conversion to Dimethyl Ether on Cu/ZnO/Al2O3-ZSM-5 Tandem Catalysts in a Double-Bed Reactor: Tuning the ZSM-5 Catalyst Acidity and Porosity. Energy Fuels 2025, 39, 2059–2074. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced Catalytic Performance of Zr Modified CuO/ZnO/Al2O3 Catalyst for Methanol and DME Synthesis via CO2 Hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Yang, G.; Tsubaki, N.; Shamoto, J.; Yoneyama, Y.; Zhang, Y. Confinement Effect and Synergistic Function of H-ZSM-5/Cu-ZnO-Al2O3 Capsule Catalyst for One-Step Controlled Synthesis. J. Am. Chem. Soc. 2010, 132, 8129–8136. [Google Scholar] [CrossRef]
- Dokania, A.; Dutta Chowdhury, A.; Ramirez, A.; Telalovic, S.; Abou-Hamad, E.; Gevers, L.; Ruiz-Martinez, J.; Gascon, J. Acidity Modification of ZSM-5 for Enhanced Production of Light Olefins from CO2. J. Catal. 2020, 381, 347–354. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F. A Review on CO2 Hydrogenation to Lower Olefins: Understanding the Structure-Property Relationships in Heterogeneous Catalytic Systems. J. CO2 Util. 2021, 47, 101506. [Google Scholar] [CrossRef]
- Ding, X.; Duan, J.; Jia, M.; Fan, H.; Lyu, Y.; Fu, J.; Liu, X. Advanced Zeolite-Based Catalysts for CO2 Hydrogenation to Targeted High-Value Chemicals and Fuels. Chem. Asian J. 2025, 20, e202401703. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Zhan, G.; Huang, J.; Li, Q. Design and Synthesis of Bioinspired ZnZrOx&Bio-ZSM-5 Integrated Nanocatalysts to Boost CO2 Hydrogenation to Light Olefins. ACS Sustain. Chem. Eng. 2021, 9, 6446–6458. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Li, S.; Zhang, J.; Bu, X.; Wang, H.; Ji, T.; Li, J.; Chang, C.R.; Shi, Y.; et al. Low-Temperature CO2 Hydrogenation to Aromatics over ZnZrO Integrated with Boron-Modified ZSM-5. Appl. Catal. B Environ. Energy 2025, 377, 125523. [Google Scholar] [CrossRef]
- Xin, Q.; Guo, H.; Wang, Y.; Xiao, L.; Wang, W.; Wu, W. Indium-Promoted ZnZrOx/Nano-ZSM-5 for Efficient Conversion of CO2 to Aromatics with High Selectivity. J. Environ. Chem. Eng. 2022, 10, 108032. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z. Ethanol as a Binder to Fabricate a Highly-Efficient Capsule-Structured CuO−ZnO−Al2O3@HZSM-5 Catalyst for Direct Production of Dimethyl Ether from Syngas. ChemCatChem 2020, 12, 999–1006. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Zhao, Z. Direct Coating Copper–Zinc–Aluminum Oxalate with H-ZSM-5 to Fabricate a Highly Efficient Capsule-Structured Bifunctional Catalyst for Dimethyl Ether Production from Syngas. Catal. Sci. Technol. 2019, 9, 3763–3770. [Google Scholar] [CrossRef]
- Sibi, M.G.; Verma, D.; Kim, J. Direct Conversion of CO2 into Aromatics over Multifunctional Heterogeneous Catalysts. Catal. Rev. 2024, 66, 863–922. [Google Scholar] [CrossRef]
- Lin, M.; Jiang, D.; Yan, Y.; Zhan, L.; Song, X.; Li, R.; Wu, Y. Selective Regulation of Products for Guaiacol Hydrodeoxygenation by Adjusting Type and Acidity of Supports. Bioresour. Technol. 2024, 413, 131478. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fan, S.; Wang, H.; Wang, S.; Qin, Z.; Dong, M.; Fan, W.; Wang, J. Selective Conversion of CO2 to Trimethylbenzene and Ethene by Hydrogenation over a Bifunctional ZnCrOx/H-ZSM-5 Composite Catalyst. ACS Catal. 2024, 14, 271–282. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, J.; Liu, F.; Ma, J.; Yao, M.; Geng, S.; Cao, J.; Li, Z. Direct Conversion of Carbon Dioxide into Light Olefins over ZnZrOx/ZSM-5@n-ZrO2 Tandem Catalyst. Fuel 2024, 357, 129727. [Google Scholar] [CrossRef]
- García-Trenco, A.; Martínez, A. A Rational Strategy for Preparing Cu–ZnO/H-ZSM-5 Hybrid Catalysts with Enhanced Stability during the One-Step Conversion of Syngas to Dimethyl Ether (DME). Appl. Catal. A Gen. 2015, 493, 40–49. [Google Scholar] [CrossRef]
- Baracchini, G.; Machoke, A.G.F.; Klumpp, M.; Wen, R.; Arnold, P.; Schwieger, W.; Dittmeyer, R. Structured Catalysts for the Direct Synthesis of Dimethyl Ether from Synthesis Gas: A Comparison of Core@shell versus Hybrid Catalyst Configuration. Catal. Today 2020, 342, 46–58. [Google Scholar] [CrossRef]
- Jia, H.; Du, T.; Fang, X.; Gong, H.; Qiu, Z.; Li, Y.; Wang, Y. Synthesis of Template-Free ZSM-5 from Rice Husk Ash at Low Temperatures and Its CO2 Adsorption Performance. ACS Omega 2021, 6, 3961–3972. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Xu, X. Clarifying the Methanol Synthesis Mechanism via CO2 Hydrogenation on the Cu(111) Surface: Insights from Accurate Doubly Hybrid Density Functionals. ACS Catal. 2025, 15, 5039–5045. [Google Scholar] [CrossRef]
- Ren, H.; Xu, C.H.; Zhao, H.-Y.; Wang, Y.-X.; Liu, J.; Liu, J.Y. Methanol Synthesis from CO2 Hydrogenation over Cu/γ-Al2O3 Catalysts Modified by ZnO, ZrO2 and MgO. J. Ind. Eng. Chem. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Dintzer, T.; Luo, J.; Parkhomenko, K.; Roger, A.C. Tuning the Highly Dispersed Metallic Cu Species via Manipulating Brønsted Acid Sites of Mesoporous Aluminosilicate Support for CO2 Hydrogenation Reactions. Appl. Catal. B Environ. 2020, 269, 118804. [Google Scholar] [CrossRef]
- Van Schagen, T.N.; Keestra, H.; Brilman, D.W.F. Improved Kinetic Model for Methanol Synthesis with Cu/ZnO/Al2O3 Catalysts Based on an Extensive State-of-the-Art Dataset. Chem. Eng. J. 2025, 507, 159953. [Google Scholar] [CrossRef]
- Grabow, L.C.; Mavrikakis, M. Mechanism of Methanol Synthesis on Cu through CO2 and CO Hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Krim, K.; Sachse, A.; Le Valant, A.; Pouilloux, Y.; Hocine, S. One Step Dimethyl Ether (DME) Synthesis from CO2 Hydrogenation over Hybrid Catalysts Containing Cu/ZnO/Al2O3 and Nano-Sized Hollow ZSM-5 Zeolites. Catal. Lett. 2023, 153, 83–94. [Google Scholar] [CrossRef]
- Varimalla, S.; Manda, K.; Boggala, S.; Nappuni, R.C.; Inkollu, S.; Aytam, H.P.; Akula, V. Effect of Method of Preparation of Ni and/or Cu Supported on ZSM-5 Catalysts for the Aqueous Phase Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Today 2024, 441, 114916. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Liu, L.; Du, T.; Webley, P.A. Highly Dispersed Cu-ZnO-ZrO2 Nanoparticles on Hydrotalcite Adsorbent as Efficient Composite Catalysts for CO2 Hydrogenation to Methanol. Korean J. Chem. Eng. 2021, 38, 747–755. [Google Scholar] [CrossRef]
- Xue, H.; Meng, T.; Liu, F.; Guo, X.; Wang, S.; Mao, D. Enhanced Resistance to Calcium Poisoning on Zr-Modified Cu/ZSM-5 Catalysts for the Selective Catalytic Reduction of NO with NH3. RSC Adv. 2019, 9, 38477–38485. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Wang, Y.; Hu, Y.; Nian, Y.; Li, W.; Zhang, J. Pyrrolidone Ligand Improved Cu-based Catalysts with High Performance for Acetylene Hydrochlorination. Appl. Organom. Chem. 2021, 35, e6066. [Google Scholar] [CrossRef]
- Kamsuwan, T.; Guntida, A.; Praserthdam, P.; Jongsomjit, B. Differences in Deterioration Behaviors of Cu/ZnO/Al2O3 Catalysts with Different Cu Contents toward Hydrogenation of CO and CO2. ACS Omega 2022, 7, 25783–25797. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Du, T.; Li, Y.; Wang, H.; Yue, Q.; Zhou, L.; Wang, Y. Preparation of Catalyst for CO2 Hydrogenation Reaction Based on the Idea of Element Sharing and Preliminary Exploration of Catalytic Mechanism. Environ. Sci. Pollut. Res. 2024, 31, 48014–48026. [Google Scholar] [CrossRef]
- Adasho Achomo, M.; Kumar, A.; Muthukumar, P.; Peela, N.R. Experimental Studies on Hydrogen Production from Steam Reforming of Methanol Integrated with Metal Hydride-Based Hydrogen Purification System. Int. J. Hydrog. Energy 2024, 76, 28–43. [Google Scholar] [CrossRef]
- Ojeda, M.; Osterman, N.; Dražić, G.; Fele Žilnik, L.; Meden, A.; Kwapinski, W.; Balu, A.M.; Likozar, B.; Novak Tušar, N. Conversion of Palmitic Acid Over Bi-Functional Ni/ZSM-5 Catalyst: Effect of Stoichiometric Ni/Al Molar Ratio. Top. Catal. 2018, 61, 1757–1768. [Google Scholar] [CrossRef]
- Xu, D.; Fan, H.; Liu, K.; Hou, G.; Qin, C.; Xu, Y.; Li, R.; Wang, J.; Ding, M. Impacts of Interaction between Active Components on Catalyst Deactivation over KFe/ZSM-5 Bifunctional Catalyst. ACS Sustain. Chem. Eng. 2023, 11, 10441–10452. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, W.; Li, G.K.; Wang, C.; Yang, H.; Zhang, D. Effect of SiO2/Al2O3 Ratio on the Performance of Nanocrystal ZSM-5 Zeolite Catalysts in Methanol to Gasoline Conversion. Appl. Catal. A Gen. 2016, 523, 312–320. [Google Scholar] [CrossRef]
- Sun, K.; Lu, W.; Wang, M.; Xu, X. Low-temperature synthesis of DME from CO2/H2 over Pd-modified CuO–ZnO–Al2O3–ZrO2/HZSM-5 catalysts. Catal. Commun. 2004, 5, 367–370. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Du, J.; Li, C.; Wang, K.; Liu, L.; Yu, X.; Wang, K.; Liu, N. The influence of composition on the functionality of hybrid CuO–ZnO–Al2O3/HZSM-5 for the synthesis of DME from CO2 hydrogenation. RSC Adv. 2018, 8, 30387–30395. [Google Scholar] [CrossRef] [PubMed]
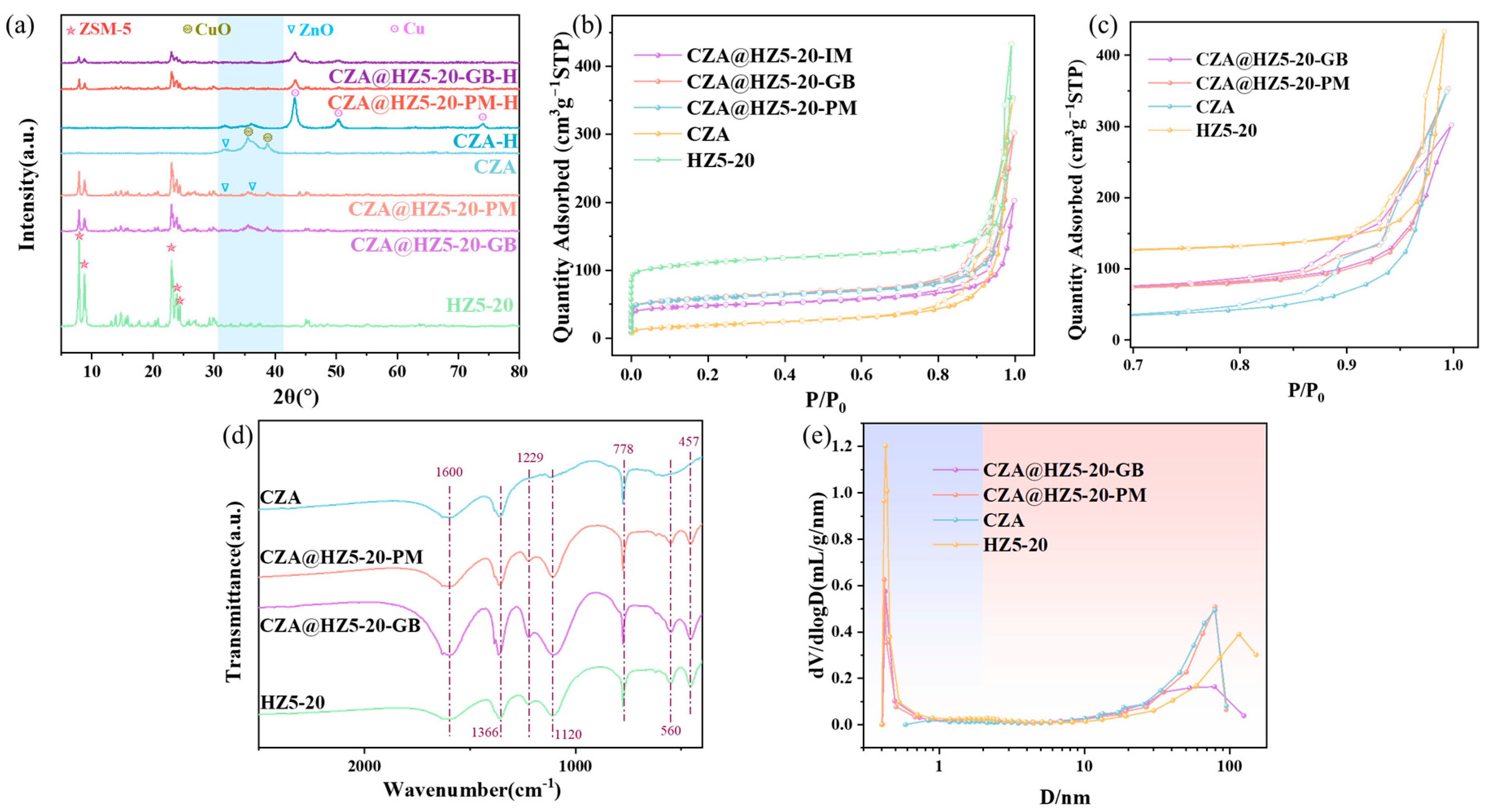



| Catalysts | SBET (m2/g) | t-Plot SMicro (m2/g) | Vtotal (cm3/g) | Vmicropore (cm3/g) | Rmicropore (%) |
|---|---|---|---|---|---|
| HZ5-20 | 348.4 | 220.8 | 0.5471 | 0.1715 | 31.35 |
| CZA@HZ5-20-GB | 189.5 | 138.5 | 0.3853 | 0.0918 | 23.83 |
| CZA@HZ5-20-PM | 186.8 | 104.6 | 0.4537 | 0.0900 | 19.84 |
| CZA | 66.7 | 13.9 | 0.4480 | 0.0289 | 6.45 |
| Samples | XCO2 (%) | Selectivity (%) | Yield (%) | Space–Time Yield (g∙kgcat−1∙h−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MeOH | DME | CO | MeOH | DME | CO | MeOH | DME | CO | ||
| CZA-0.1 | 7.47 | 10.75 | 0.00 | 89.25 | 0.81 | 0.00 | 6.66 | 19.68 | 0.00 | 141.52 |
| CZA-0.2 | 11.57 | 8.07 | 0.00 | 91.93 | 0.93 | 0.00 | 10.64 | 8.59 | 0.00 | 85.53 |
| CZA@HZ5-20 | 7.24 | 4.37 | 19.76 | 75.87 | 0.33 | 1.44 | 5.48 | 3.81 | 23.60 | 54.77 |
| Samples | XCO2 (%) | Selectivity (%) | Yield (%) | Space–Time Yield (g∙kgcat−1∙h−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MeOH | DME | CO | MeOH | DME | CO | MeOH | DME | CO | ||
| CZA@HZ5-20-PM | 7.39 | 3.41 | 19.17 | 77.42 | 0.25 | 1.42 | 5.72 | 2.42 | 19.55 | 48.01 |
| CZA@HZ5-20-GB | 7.46 | 18.21 | 0.00 | 81.79 | 1.36 | 0.00 | 6.11 | 14.18 | 0.00 | 55.66 |
| CZA@HZ5-20 | 7.24 | 4.37 | 19.76 | 75.87 | 0.33 | 1.44 | 5.48 | 3.81 | 23.60 | 54.77 |
| Samples | Peak α (%) | DANH3-α (mmol/g) | Peak β (%) | DANH3-β (mmol/g) |
|---|---|---|---|---|
| CZA@HZ5-20-GB | 68.00 | 0.68 | 32.00 | 0.32 |
| CZA@HZ5-20-PM | 58.18 | 0.64 | 41.82 | 0.46 |
| HZ5-20 | 58.91 | 0.95 | 41.09 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Du, T.; Li, Y.; Chen, P.; Xiang, R.; Sun, Z.; Yang, B.; Wang, Y. Effect of a Grinding Method in the Preparation of CuO-ZnO-Al2O3@HZSM-5 Catalyst for CO2 Hydrogenation. Catalysts 2025, 15, 1068. https://doi.org/10.3390/catal15111068
Jia H, Du T, Li Y, Chen P, Xiang R, Sun Z, Yang B, Wang Y. Effect of a Grinding Method in the Preparation of CuO-ZnO-Al2O3@HZSM-5 Catalyst for CO2 Hydrogenation. Catalysts. 2025; 15(11):1068. https://doi.org/10.3390/catal15111068
Chicago/Turabian StyleJia, He, Tao Du, Yingnan Li, Peng Chen, Rui Xiang, Zhaoyi Sun, Bowen Yang, and Yisong Wang. 2025. "Effect of a Grinding Method in the Preparation of CuO-ZnO-Al2O3@HZSM-5 Catalyst for CO2 Hydrogenation" Catalysts 15, no. 11: 1068. https://doi.org/10.3390/catal15111068
APA StyleJia, H., Du, T., Li, Y., Chen, P., Xiang, R., Sun, Z., Yang, B., & Wang, Y. (2025). Effect of a Grinding Method in the Preparation of CuO-ZnO-Al2O3@HZSM-5 Catalyst for CO2 Hydrogenation. Catalysts, 15(11), 1068. https://doi.org/10.3390/catal15111068






