Research Progress on Zeolite-Type High-Temperature NH3-SCR Catalysts
Abstract
1. Introduction
2. Structural and Functional Comparison of Zeolite Support
3. ZSM-5 Support
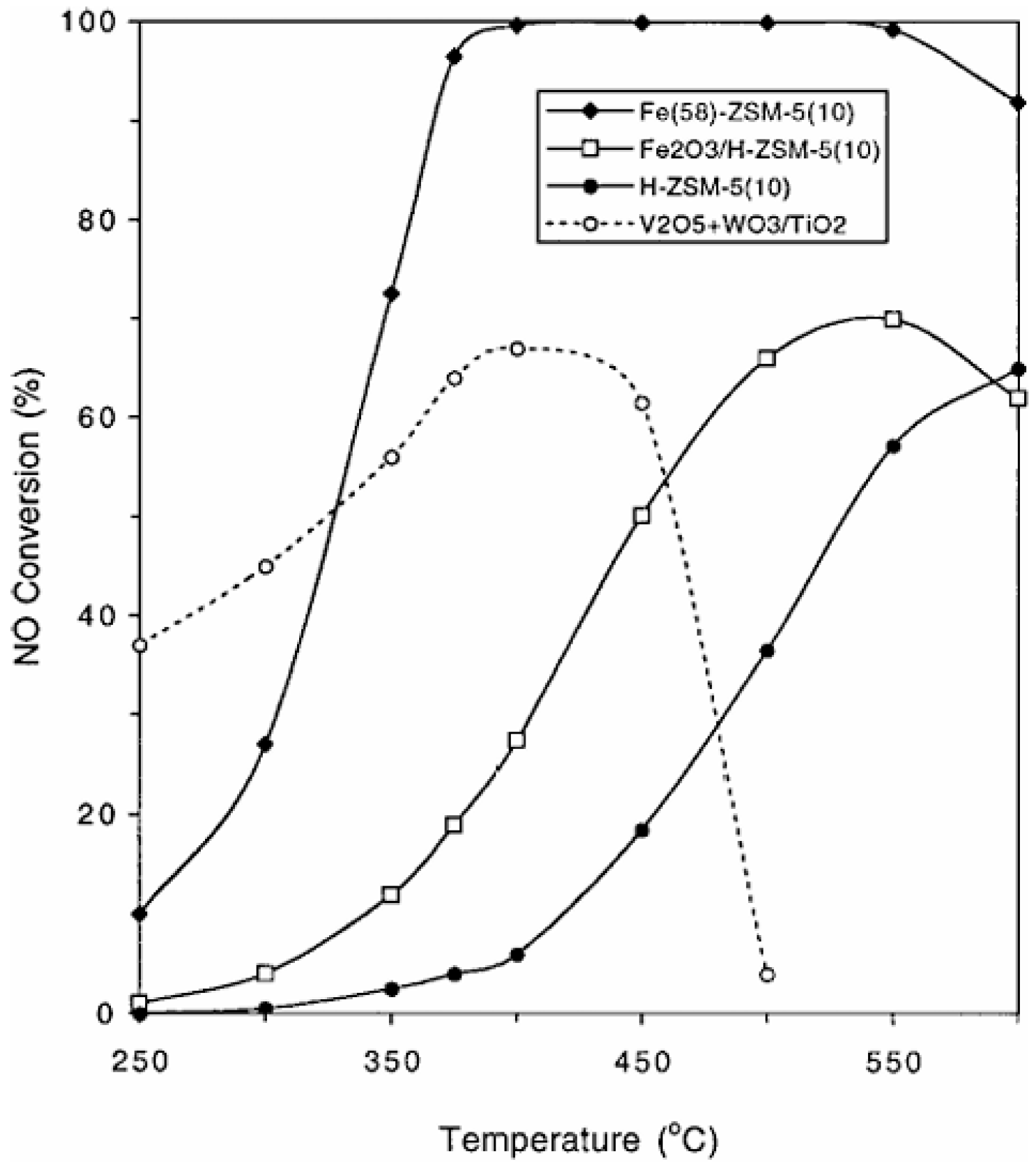
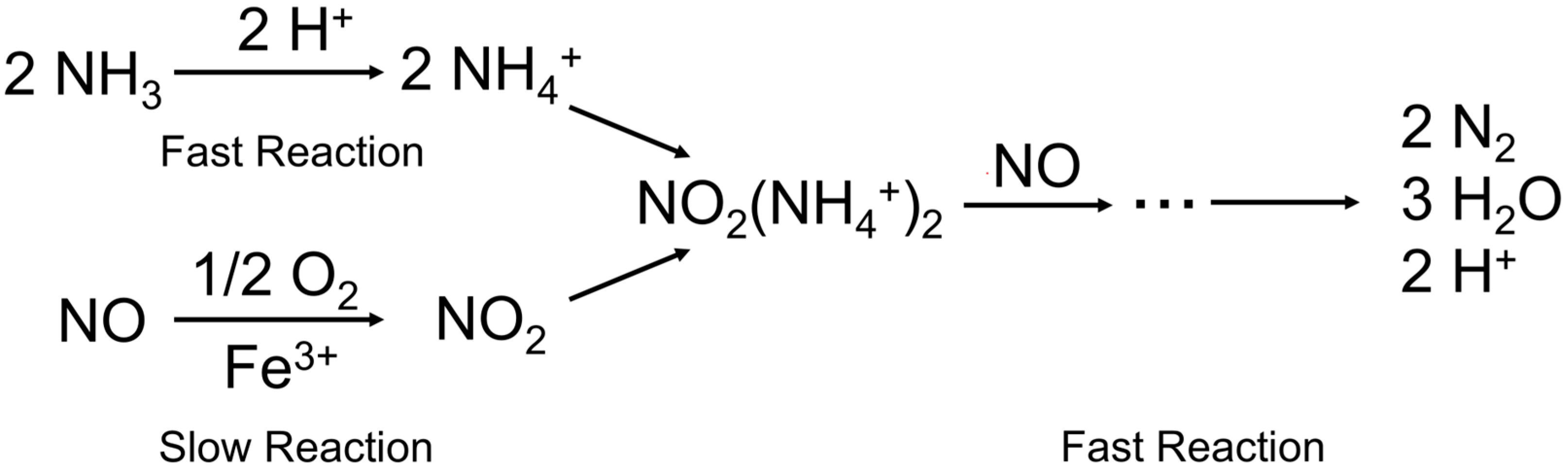
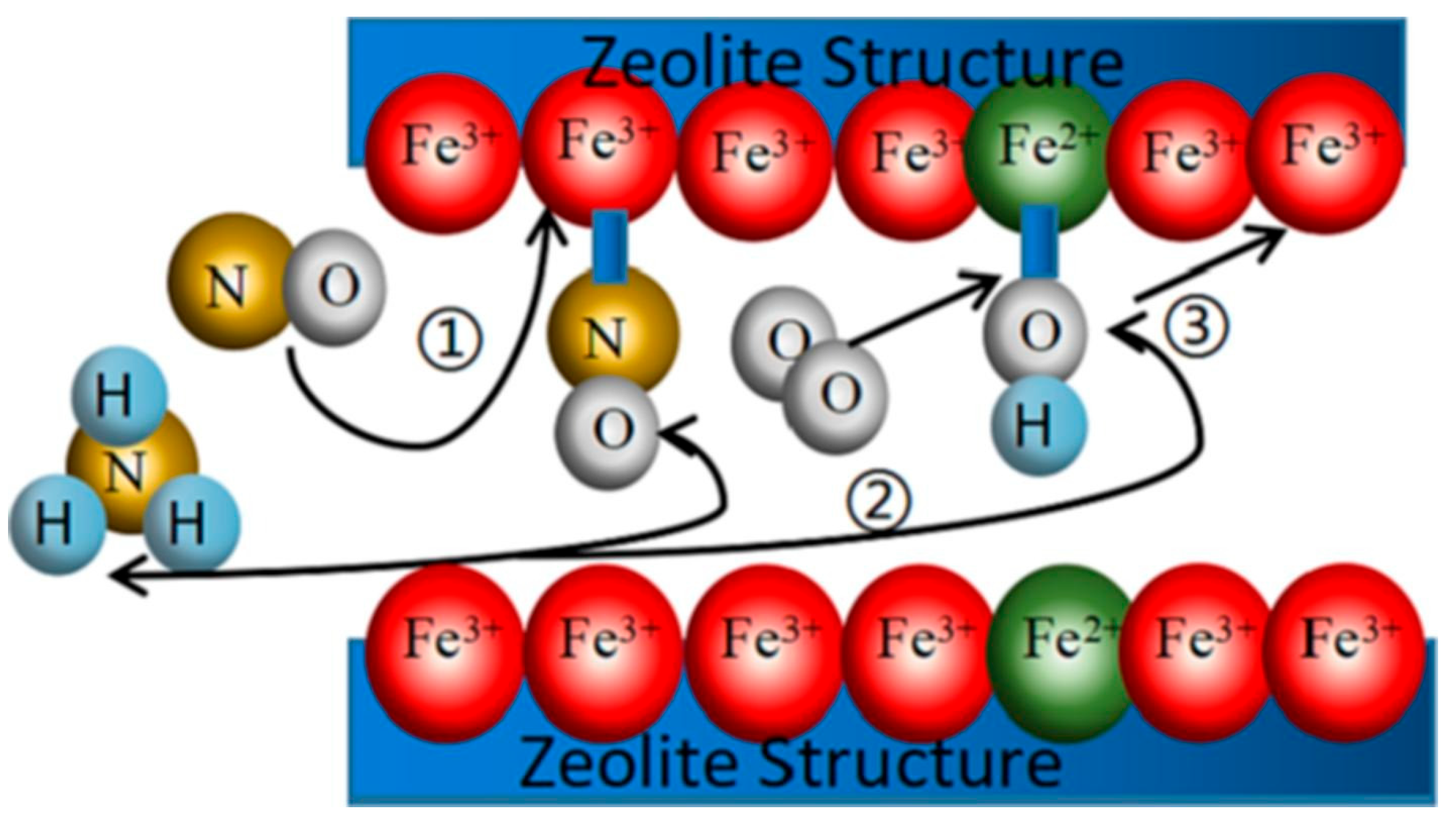
3.1. Single Metal-Doped ZSM-5 Catalyst
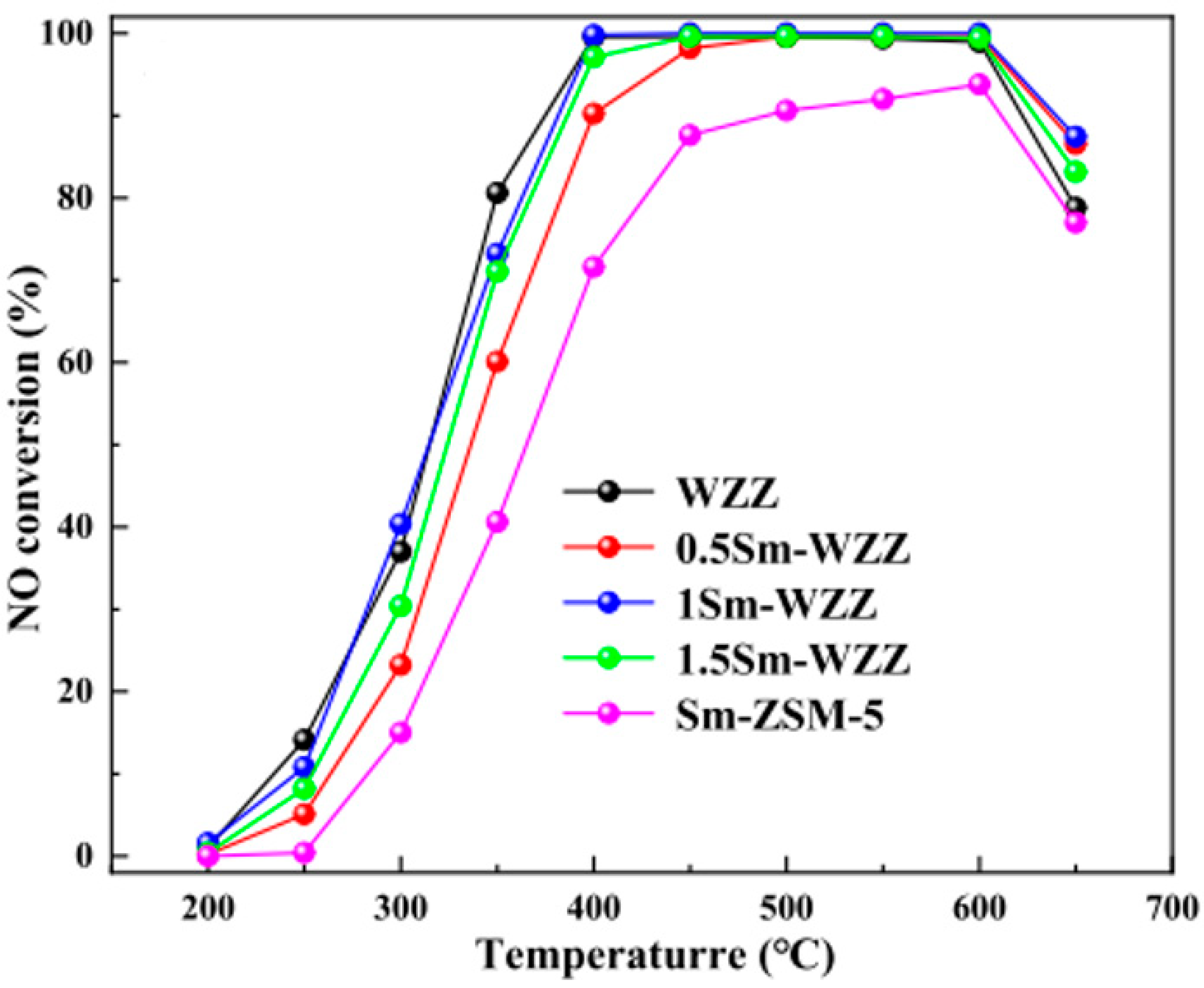
3.2. Composite Metal-Doped ZSM-5 Catalyst
4. Beta Support
4.1. Single Metal-Doped Beta Catalyst
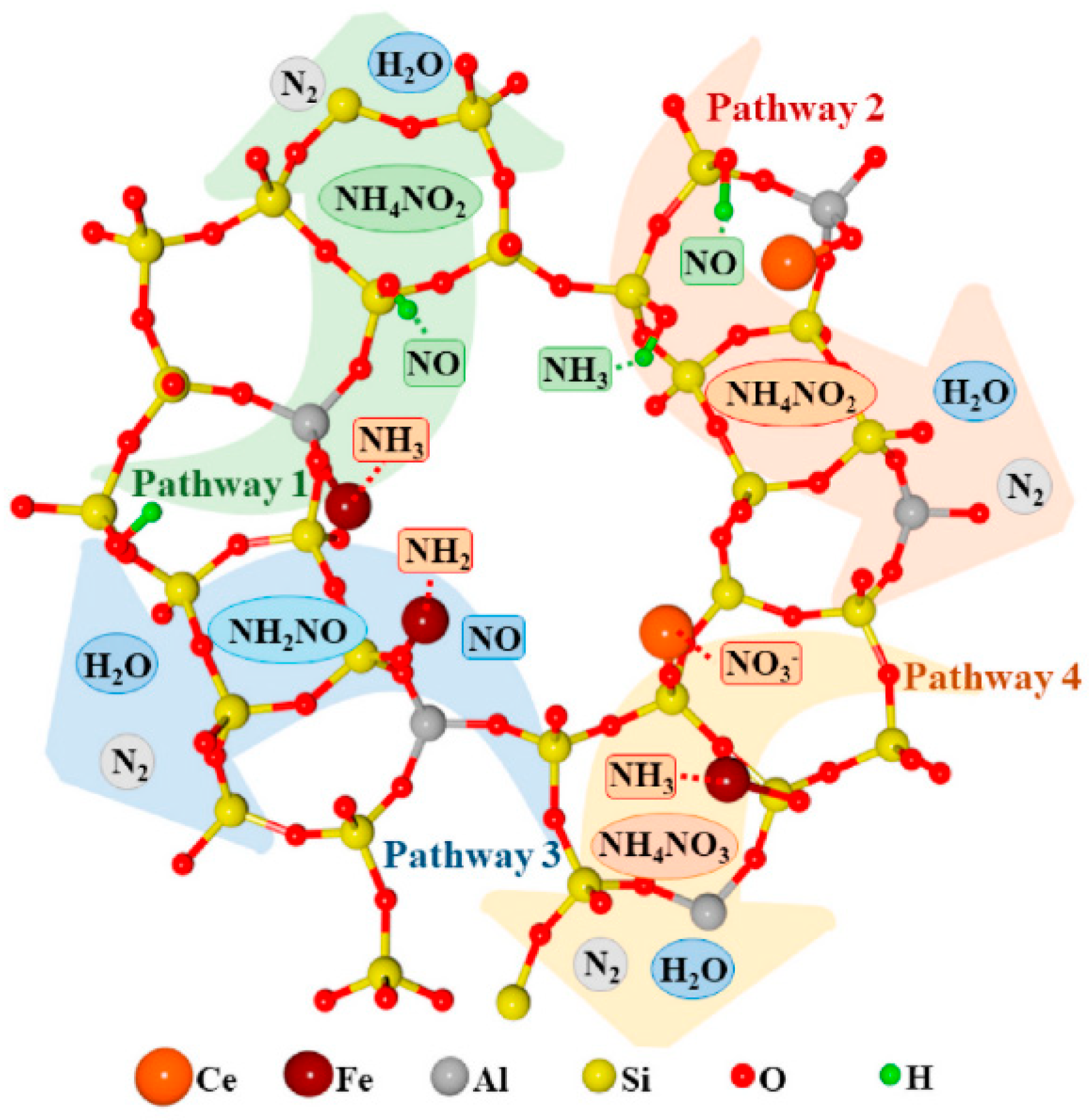
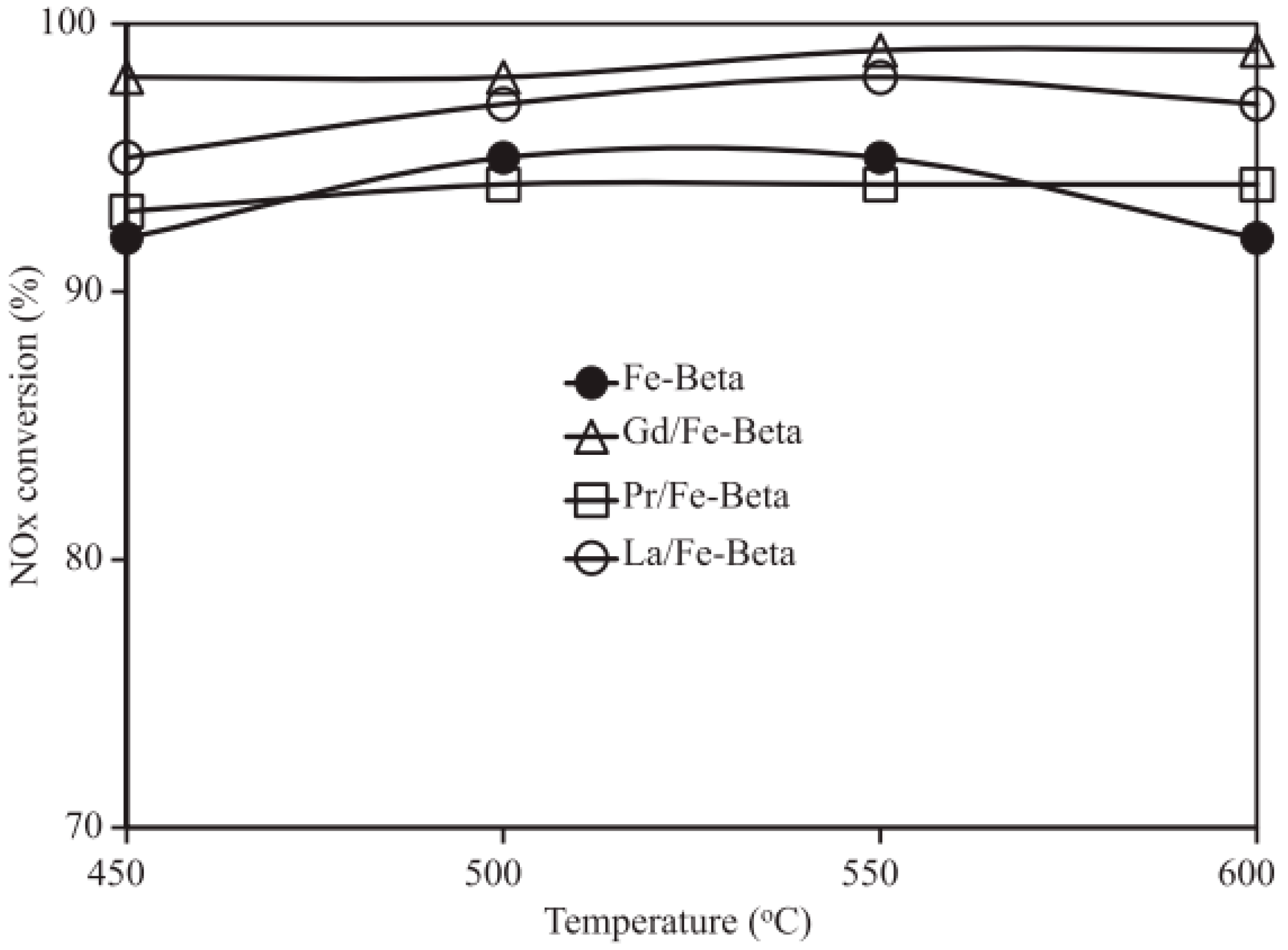
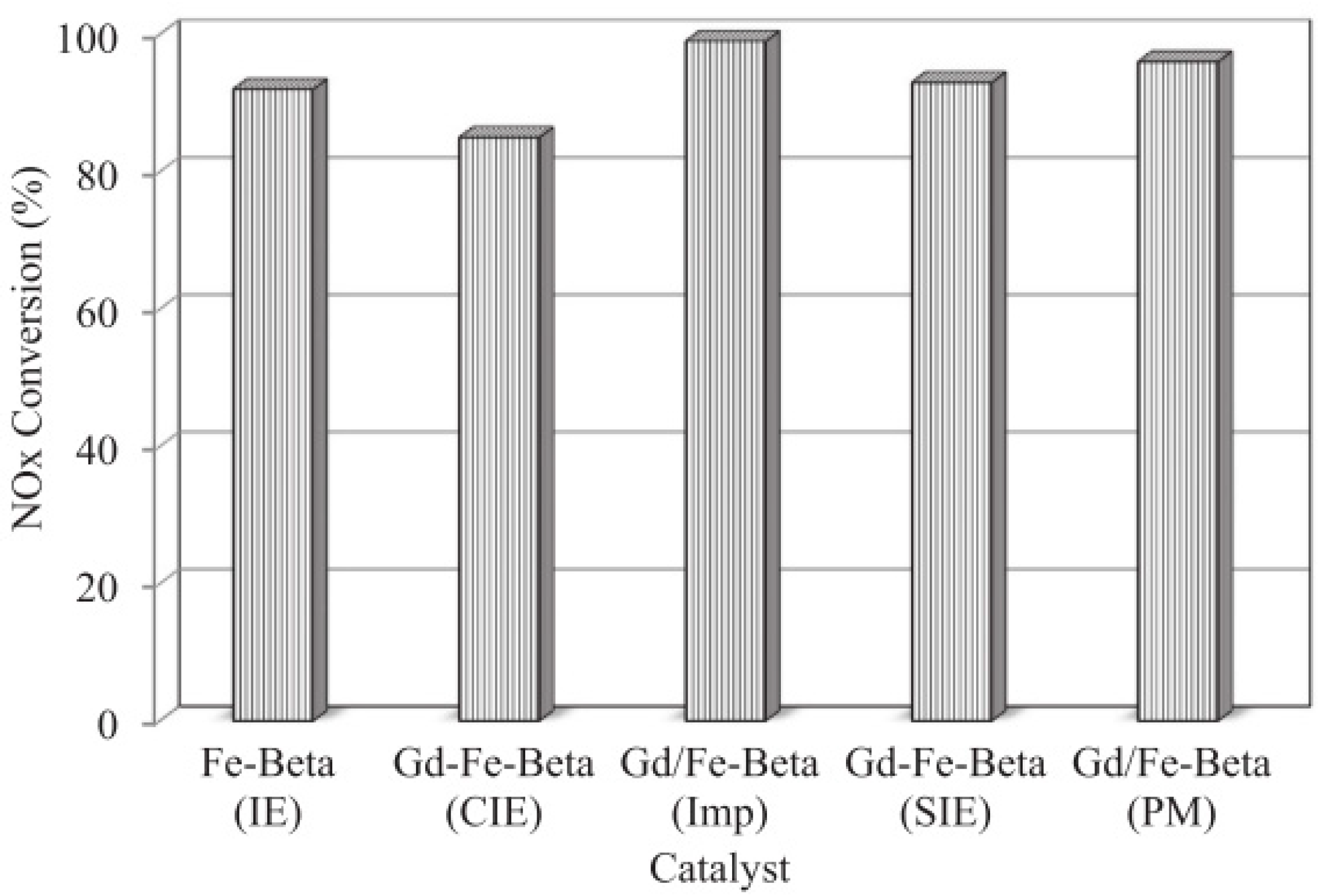
4.2. Composite Metal-Doped Beta Catalyst
5. SSZ-13 Support
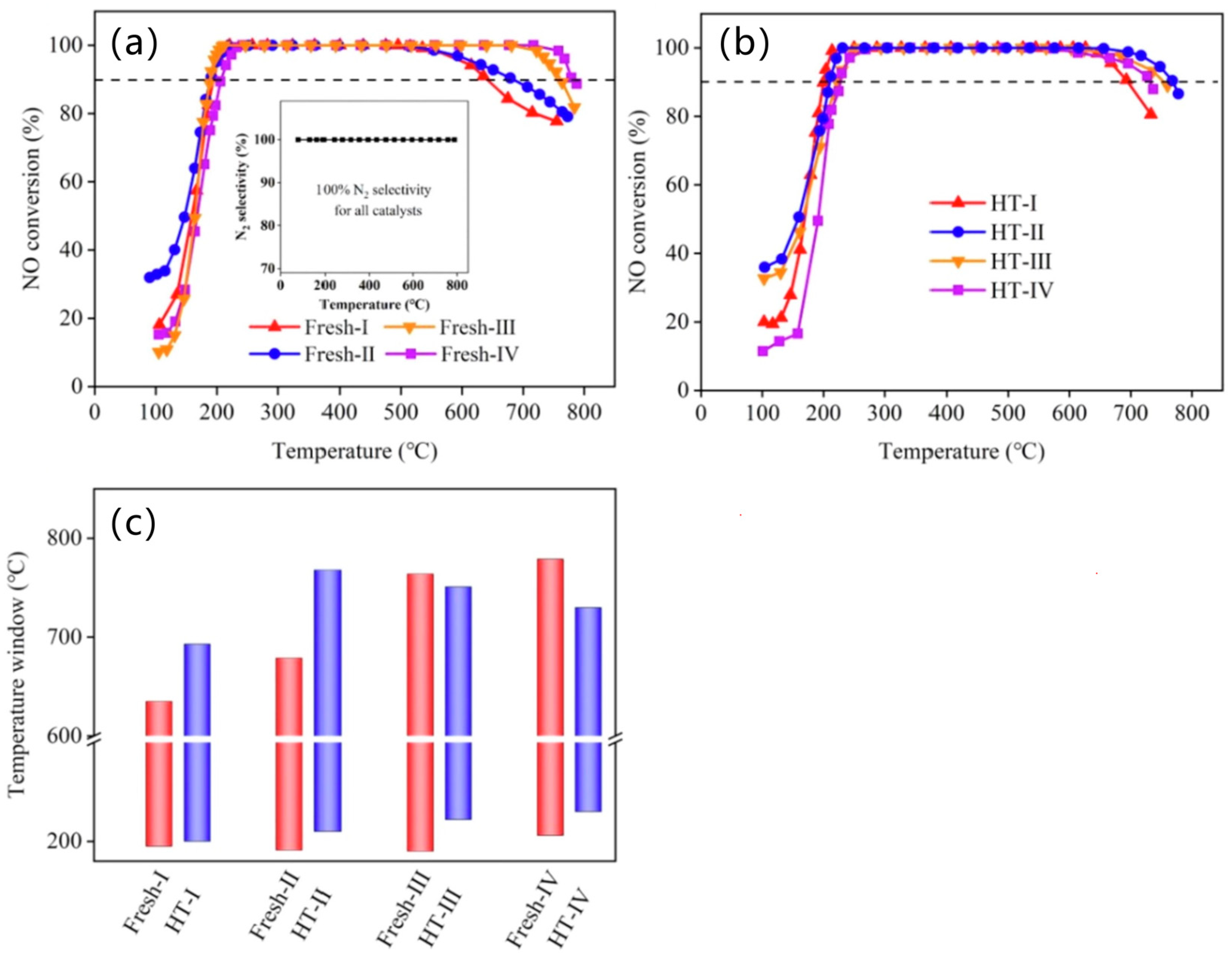
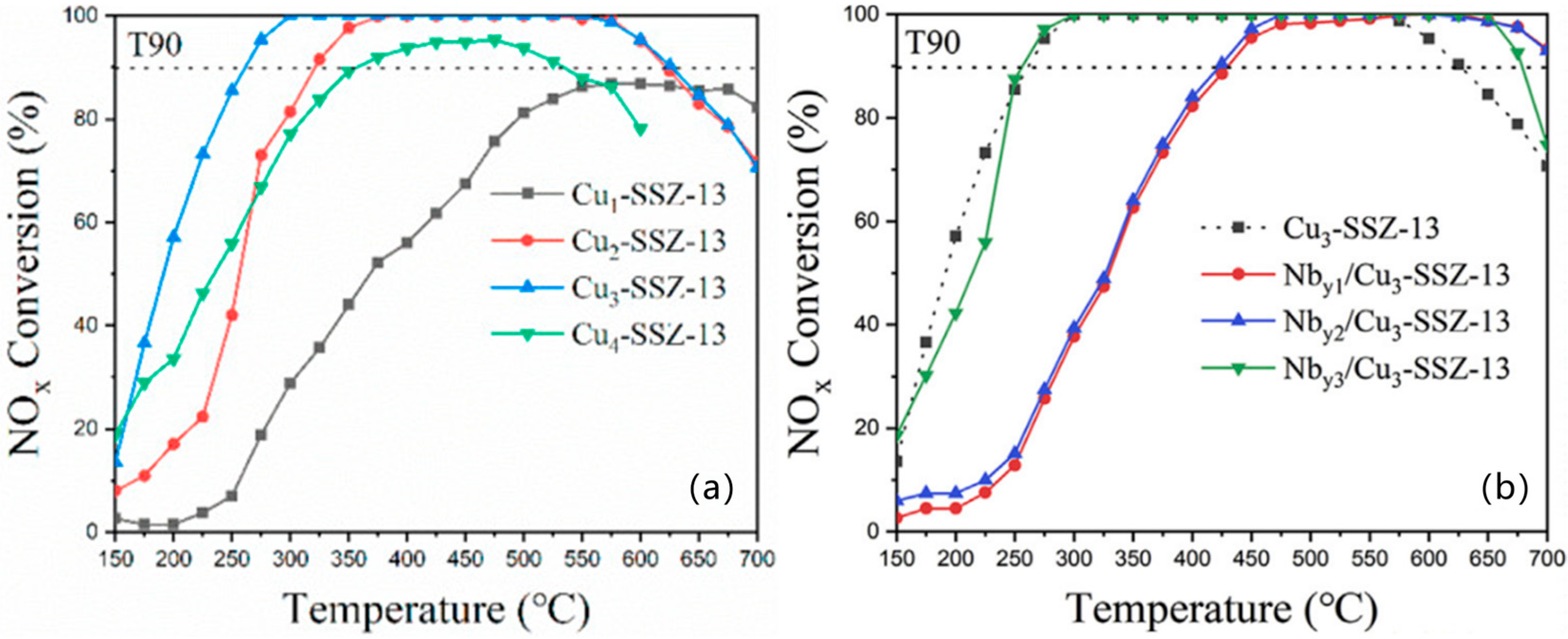
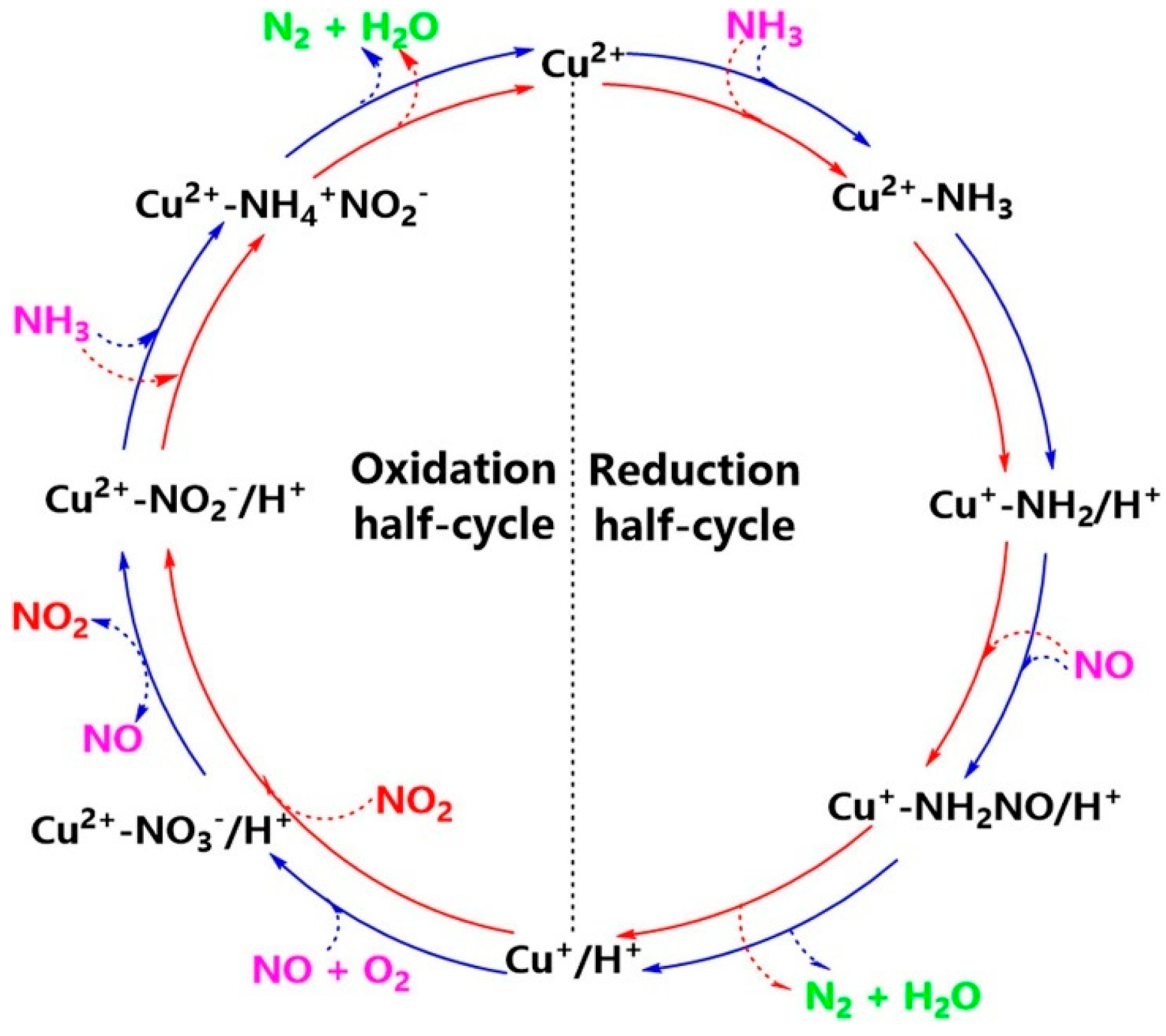
5.1. Single Metal-Doped SSZ-13 Catalyst
5.2. Composite Metal-Doped SSZ-13 Catalyst
6. SAPO-34 Support
6.1. Single Metal-Doped SAPO-34 Catalyst
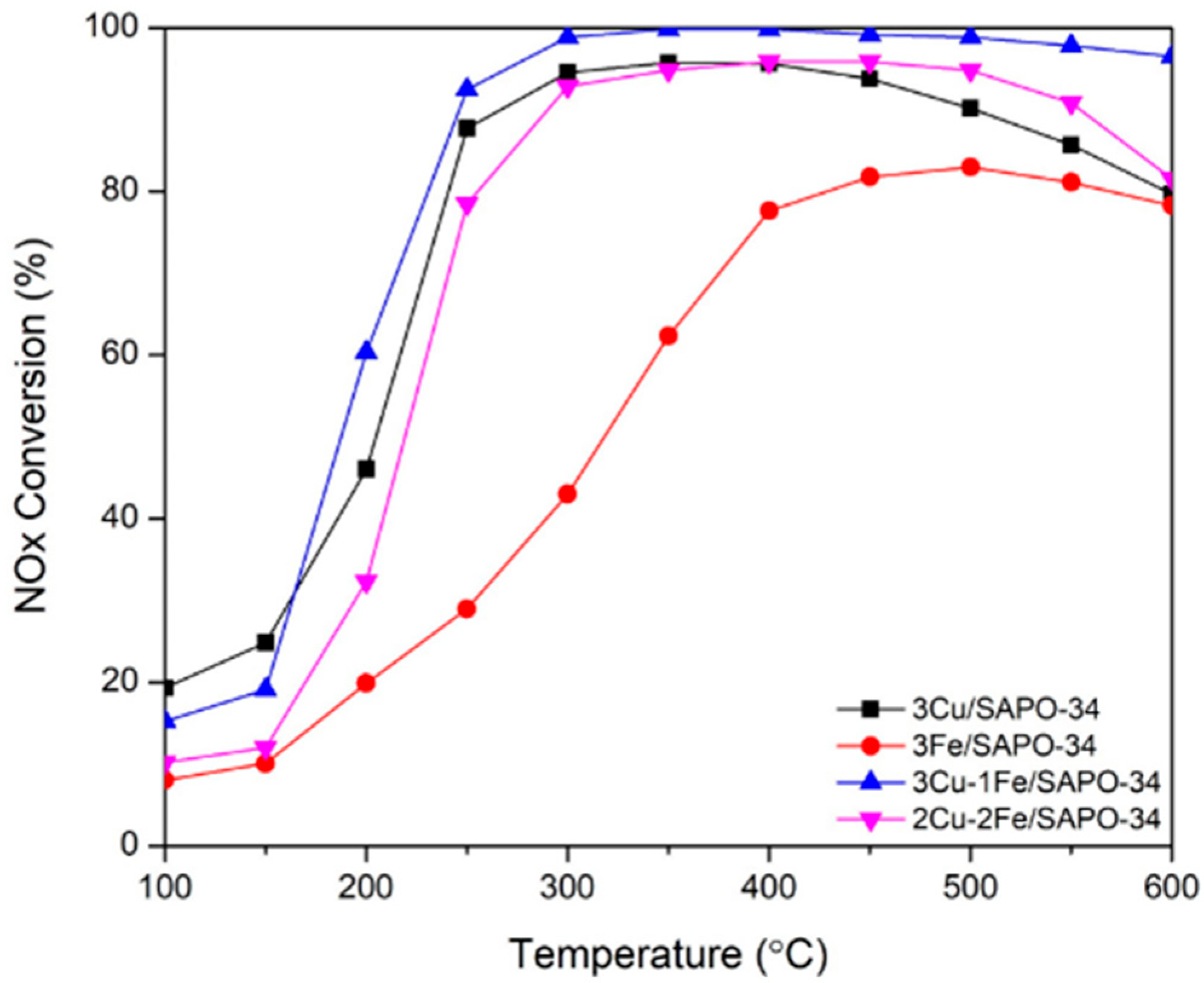
6.2. Composite Metal-Doped SAPO-34 Catalyst
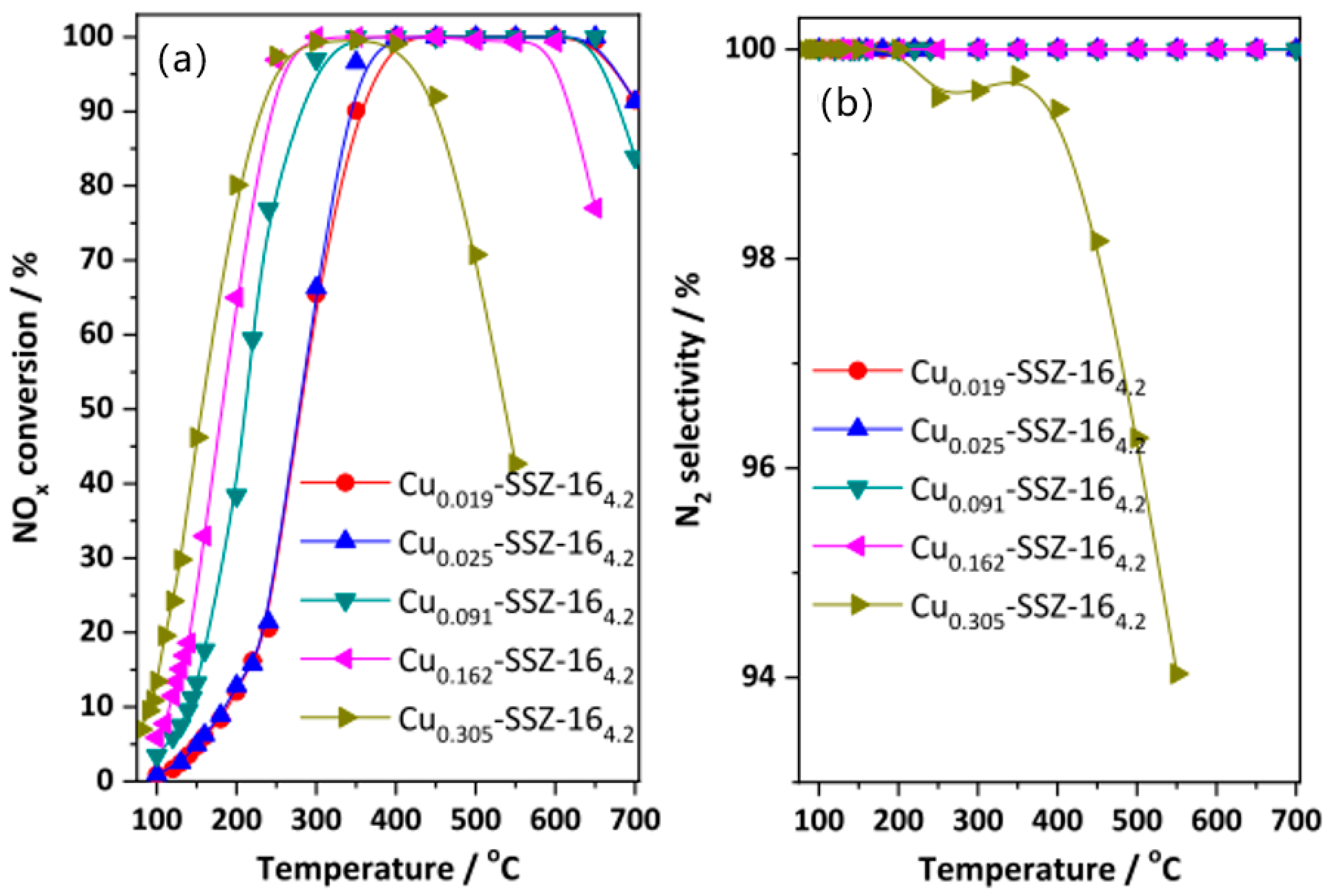
7. Other Support
8. Comparison of Catalytic Characteristics and Mechanisms for Fe/Cu-Based Zeolite Catalysts with Doping Modification in High-Temperature NH3-SCR
9. Outlook and Future Research Directions
9.1. Deepening Mechanistic Understanding Through Ultra-High-Temperature In Situ Characterization
9.2. Advancing Rational Catalyst Design Using Density Functional Theory and Machine Learning
9.3. Reducing Synthesis Costs and Advancing Green Manufacturing
9.4. Verification of Catalyst Performance Under Actual Gas Turbine Operating Conditions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gülen, S.C.; Curtis, M. Gas Turbine’s Role in Energy Transition. J. Eng. Gas Turbines Power 2024, 146, 101008. [Google Scholar] [CrossRef]
- Banihabib, R.; Assadi, M. The Role of Micro Gas Turbines in Energy Transition. Energies 2022, 15, 8084. [Google Scholar] [CrossRef]
- Genc, T.S. Energy Transition and the role of new natural gas turbines for power production: The case of GT11N2 M generators. Energy Econ. 2024, 131, 107412. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Wu, Y. Advanced combustion technologies for future gas turbines. J. Tsinghua Univ. Sci. Technol. 2021, 61, 1423–1437. [Google Scholar]
- Liu, Z.; Yao, J.; Zhuang, K.; Yu, L.; Zhou, K. Comparison of Characteristics of SCR-DeNOx Catalyst for Gas-Turbine Units and Coal-Fired Units. Electr. Power 2021, 54, 145–152. [Google Scholar]
- Xue, H.; Yuan, J.; Wu, M.; Gu, Y.; Ding, D.; Zhang, L. Current Status of Nitrogen Oxide Emissions from Gas Turbine Units and Suggestions for Standard Revision. Energy Environ. 2022, 4, 90–92. [Google Scholar] [CrossRef]
- Qing, M.; Lei, S.; Kong, F.; Liu, L.; Zhang, W.; Wang, L.; Guo, T.; Su, S.; Hu, S.; Wang, Y.; et al. Analysis of ammonium bisulfate/sulfate generation and deposition characteristics as the by-product of SCR in coal-fired flue gas. Fuel 2022, 313, 122790. [Google Scholar] [CrossRef]
- Ma, S.; Guo, M.; Song, H.; Chen, G.; Yang, J.; Zang, B.; Li, Z. Formation mechanism and influencing factors of ammonium bisulfate during the selective catalytic reduction process. Therm. Power Gener. 2014, 43, 75–78+86. [Google Scholar]
- Jiang, Y.; Gao, X.; Wu, W.; Zhang, Y. Review of the Deactivation of Selective Catalytic Reduction DeNOx Catalysts. Proc. CSEE 2013, 33, 18–31+13. [Google Scholar]
- Chapman, D.M. Behavior of titania-supported vanadia and tungsta SCR catalysts at high temperatures in reactant streams: Tungsten and vanadium oxide and hydroxide vapor pressure reduction by surficial stabilization. Appl. Catal. A-Gen. 2011, 392, 143–150. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Abrams, B.L. Fundamental chemistry of V-SCR catalysts at elevated temperatures. Catal. Today 2017, 297, 60–63. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, Y.; Xu, M.; Qu, Y.; Wang, H.; Pei, X.; Ouyang, H. Progress in the Development of SCR Denitration Catalysts with Resistance of High-temperature Deactivation. Mater. Rep. 2022, 36, 68–76. [Google Scholar]
- Jin, P.; Chen, Z.; Jin, R.; Chen, Y.; Liu, Z.; Zhuang, K.; Yu, L.; Sheng, Z. Study on deNOx Performance of Cu-based Zeolites Catalyst in the Wide Temperature NH3-SCR. Saf. Environ. Eng. 2023, 30, 233–240. [Google Scholar]
- Wang, Y.; Tang, T.; Zhou, Q.; Xu, L.; Tang, C. Research progress in high-temperature SCR denitration: From catalyst innovations to reaction mechanism exploration. Electr. Power Technol. Environ. Prot. 2025, 41, 343–356. [Google Scholar]
- Sa, A.; Zubaidy, S. Gas turbine performance at varying ambient temperature. Appl. Therm. Eng. 2011, 31, 2735–2739. [Google Scholar] [CrossRef]
- Chen, Q. Treatment of Large Exhaust Temperature Deviation of Siemens F-Class Gas Turbine in a Factory. Power Energy 2017, 38, 354–357. [Google Scholar]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3 single bond CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Luca, L.; Isabella, N.; Gianguido, R.; Lorenzo, D.; Guido, B.; Elio, G.; Pio, F.; Fiorenzo, B. Characterization and Reactivity of V2O5–MoO3/TiO2 De-NOx SCR Catalysts. J. Catal. 1999, 187, 419–435. [Google Scholar]
- Casagrandea, L.; Liettia, L.; Novaa, I.; Forzattia, P.; Baiker, A. SCR of NO by NH3 over TiO2-supported V2O5–MoO3 catalysts: Reactivity and redox behavior. Appl. Catal. B Environ. 1999, 22, 63–77. [Google Scholar] [CrossRef]
- Zhang, Q.-M.; Song, C.-L.; Lv, G.; Bin, F.; Pang, H.-T.; Song, J.-O. Effect of metal oxide partial substitution of V2O5 in V2O5–WO3/TiO2 on selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2015, 24, 79–86. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Q.; Tao, X.; Pan, Y.; Gu, S.; Shen, Y. Novel W-Zr-Ox/TiO2 catalyst for selective catalytic reduction of NO by NH3 at high temperature. Catal. Today 2020, 358, 254–262. [Google Scholar] [CrossRef]
- Bian, X.; Xu, Q.; Cai, M.; Cen, P.; Wu, W. The SCR reaction and mechanism of a silicic acid modified Ce-W/TiO2 catalyst. New J. Chem. 2022, 46, 15596–15604. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process. Appl. Catal. B Environ. 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Xu, T.; Weng, D.; Si, Z.; Ran, R. Effects of silica additive on the NH3-SCR activity and thermal stability of a V2O5/WO3-TiO2 catalyst. Chin. J. Catal. 2016, 37, 1340–1346. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, J.; Ling, H.; Liu, W.; Jiao, F. Deactivation Mechanism of Spent TiO2-Based Selective Catalytic Reduction (SCR) Catalysts and State-of-the-Art Recycling Technologies. Langmuir 2024, 40, 26371–26386. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, H.; Luo, X.; Shi, K.; Zhao, H.; Wang, W.; Chen, Q.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2019, 245, 743–752. [Google Scholar] [CrossRef]
- Shi, A.; Wang, X.; Yu, T.; Shen, M. The effect of zirconia additive on the activity and structure stability of V2O5/WO3-TiO2 ammonia SCR catalysts. Appl. Catal. B Environ. 2011, 106, 359–369. [Google Scholar] [CrossRef]
- Si, W.; Liu, H.; Yan, T.; Wang, H.; Fan, C.; Xiong, S.; Zhao, Z.; Peng, Y.; Chen, J.; Li, J. Sn-doped rutile TiO2 for vanadyl catalysts: Improvements on activity and stability in SCR reaction. Appl. Catal. B Environ. 2020, 269, 118797. [Google Scholar] [CrossRef]
- Kwon, D.W.; Lee, S.; Kim, J.; Lee, K.-Y.; Ha, H.P. Influence of support composition on enhancing the performance of Ce-V on TiO2 comprised tungsten-silica for NH3-SCR. Catal. Today 2021, 359, 112–123. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A-Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Guo, L.; Zheng, W.; Wang, G.; Zheng, S.; Wu, X. Research progress on NH3-SCR mechanism of metal-supported zeolite catalysts. J. Fuel Chem. Technol. 2021, 49, 1294–1315. [Google Scholar] [CrossRef]
- Yuan, Y.; Guan, B.; Chen, J.; Zhuang, Z.; Zheng, C.; Zhou, J.; Su, T.; Zhu, C.; Guo, J.; Dang, H.; et al. Research status and outlook of molecular sieve NH3-SCR catalysts. Mol. Catal. 2024, 554, 113846. [Google Scholar] [CrossRef]
- Simancas, R.; Chokkalingam, A.; Elangovan, S.P.; Liu, Z.; Sano, T.; Iyoki, K.; Wakihara, T.; Okubo, T. Recent progress in the improvement of hydrothermal stability of zeolites. Chem. Sci. 2021, 12, 7677–7695. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Wang, P.; Yu, D.; Zhang, L.; Ren, Y.; Jin, M.; Lei, L. Evolution mechanism of NOx in NH3-SCR reaction over Fe-ZSM-5 catalyst: Species-performance relationships. Appl. Catal. A-Gen. 2020, 607, 117806. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Li, X.; Zhao, H.; Lv, X. Study on the role of Fe species and acid sites in NH3-SCR over the Fe-based zeolites. Fuel 2023, 336, 126759. [Google Scholar] [CrossRef]
- Liu, J.; Du, Y.; Liu, J.; Zhao, Z.; Cheng, K.; Chen, Y.; Wei, Y.; Song, W.; Zhang, X. Design of MoFe/Beta@CeO2 catalysts with a core−shell structure and their catalytic performances for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2017, 203, 704–714. [Google Scholar] [CrossRef]
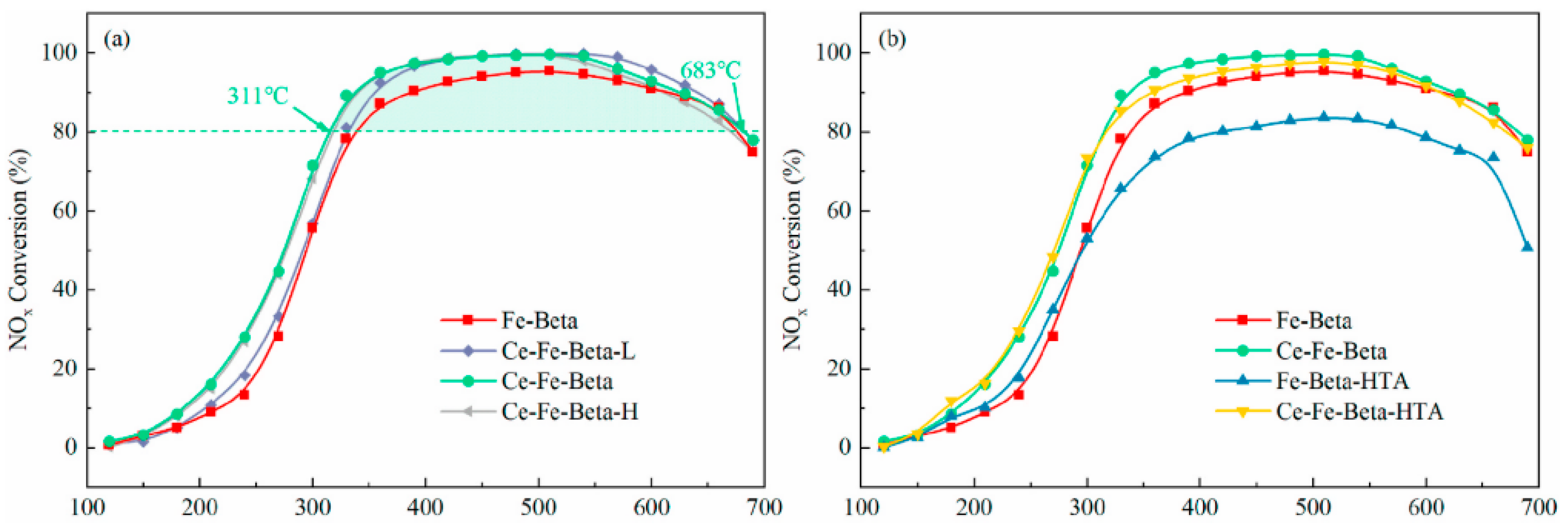
- Zhang, Y.; Wang, P.; Zhao, H.; Lyu, X.; Lei, L.; Zhang, L. The promoting mechanism of Ce on the hydrothermal stability of Fe-Beta catalyst for NH3-SCR reaction. Microporous Mesoporous Mater. 2022, 338, 111937. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Shan, Y.; Du, J.; Liu, K.; He, H. Hydrothermal Stability Enhancement of Al-Rich Cu-SSZ-13 for NH3 Selective Catalytic Reduction Reaction by Ion Exchange with Cerium and Samarium. Ind. Eng. Chem. Res. 2020, 59, 6416–6423. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Chen, Y.; Bao, W.; Chang, L.; Feng, G. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3. Chem. Eng. J. 2015, 263, 9–19. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NO with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Xu, H.; Lin, C.; Lin, Q.; Feng, X.; Zhang, Z.; Wang, Y.; Chen, Y. Grain size effect on the high-temperature hydrothermal stability of Cu/SAPO-34 catalysts for NH3-SCR. J. Environ. Chem. Eng. 2020, 8, 104559. [Google Scholar] [CrossRef]
- Jabłońska, M. Recent progress in the selective catalytic reduction of NO with NH3 on Cu-SAPO-34 catalysts. Mol. Catal. 2022, 518, 112111. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Nova, I.; Tronconi, E.; Collier, J.E.; York, A.P.E. Structure–Activity Relationship of Different Cu–Zeolite Catalysts for NH3-SCR. Top. Catal. 2016, 59, 875–881. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Lu, G.; Tang, Z. Recent Progress on Establishing Structure–Activity Relationship of Catalysts for Selective Catalytic Reduction (SCR) of NOx with NH3. Catal. Surv. Asia 2017, 22, 1–19. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Ding, J.; Huang, H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst. Chem. Eng. J. 2019, 361, 578–587. [Google Scholar] [CrossRef]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Damma, D.; Ettireddy, P.; Reddy, B.; Smirniotis, P. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Elkaee, S.; Phule, A.D.; Yang, J.H. Advancements in (SCR) technologies for NOx reduction: A comprehensive review of reducing agents. Process Saf. Environ. Prot. 2024, 184, 854–880. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective Transformation of Various Nitrogen-Containing Exhaust Gases toward N2 over Zeolite Catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ottinger, N.A.; Cremeens, C.M. Vanadium and tungsten release from V-based selective catalytic reduction diesel aftertreatment. Atmos. Environ. 2015, 104, 154–161. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- International Zeolite Association. Available online: https://www.iza-online.org/ (accessed on 9 September 2025).
- Valentin, V.; Mintova, S. Layer-by-layer preparation of zeolite coatings of nanosized crystals. Microporous Mesoporous Mater. 2001, 43, 41–49. [Google Scholar]
- Tang, G.; Li, Y.; Wang, Y.; Chai, Y.; Liu, C. A review on the synthesis, structural modification and application of two-dimensional MFI zeolite. J. Porous Mater. 2022, 29, 1649–1666. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Jin, Y.; Zhang, R. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts. Catal. Today 2019, 327, 295–307. [Google Scholar] [CrossRef]
- Newsam, J.M.; Treacy, M.M.; Koetsier, W.T.; Gruyter, C.D. Structural characterization of zeolite beta. Proc. R. Soc. Lond A 1988, 420, 375–405. [Google Scholar]
- Zhang, J.; Shan, Y.; Zhang, L.; Du, J.; He, H.; Han, S.; Lei, C.; Wang, S.; Fan, W.; Feng, Z.; et al. Importance of controllable Al sites in CHA framework by crystallization pathways for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 277, 119193. [Google Scholar] [CrossRef]
- Chen, L.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Jansson, J.; Skoglundh, M.; Grönbeck, H. A Complete Multisite Reaction Mechanism for Low-Temperature NH3-SCR over Cu-CHA. ACS Catal. 2020, 10, 5646–5656. [Google Scholar] [CrossRef]
- Pu, X.; Liu, X.; Wang, Y.; Jia, Y.; Zhang, P. Research progress on Cu-SAPO-34 denitration catalyst Cu-SAPO-34. Ind. Catal. 2023, 31, 12–17. [Google Scholar]
- Zones, S.I. Conversion of Faujasites to High-silica Chabazite SSZ-13 in the Presence of N,N,N-Trimethyl-1-adamantammonium Iodide. J. Chem. Soc. Faraday Trans. 1991, 87, 3709–3716. [Google Scholar] [CrossRef]
- Khana, N.; Yooa, D.; Bhadraa, B.; Junb, J.; Kimb, T.; Kimb, C.; Jhunga, S. Preparation of SSZ-13 zeolites from beta zeolite and their application in the conversion of ethylene to propylene. Chem. Eng. J. 2019, 377, 119546. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.; He, H. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Fickel, D.W.; Lobo, R.F. Copper Coordination in Cu-SSZ-13 and Cu-SSZ-16 Investigated by Variable-Temperature XRD. J. Phys. Chem. C 2010, 114, 1633–1640. [Google Scholar] [CrossRef]
- Tsunoji, N.; Tsuchiya, K.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Nishitoba, T.; Yokoi, T.; Ohnishi, T.; Ogura, M.; Sadakane, M.; et al. Multiple templating strategy for the control of aluminum and phosphorus distributions in AFX zeolite. Microporous Mesoporous Mater. 2021, 321, 111124. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y.; Liu, C.; Liu, Q. The Challenges and Comprehensive Evolution of Cu-Based Zeolite Catalysts for SCR Systems in Diesel Vehicles: A Review. Catal. Surv. Asia 2022, 27, 181–206. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Corma, A. Direct synthesis design of Cu-SAPO-18, a very efficient catalyst for the SCR of NOx. J. Catal. 2014, 319, 36–43. [Google Scholar] [CrossRef]
- Moliner, M.; Franch, C.; Palomares, E.; Grill, M.; Corma, A. Cu–SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx. Chem. Commun. 2012, 48, 8264–8266. [Google Scholar] [CrossRef]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B Environ. 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.-Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.-S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef]
- Long, R.; Yang, R. Catalytic Performance of Fe–ZSM-5 Catalysts for Selective Catalytic Reduction of Nitric Oxide by Ammonia. J. Catal. 1999, 188, 332–339. [Google Scholar] [CrossRef]
- Ma, A.-Z.; Grünert, W. Selective catalytic reduction of NO by ammonia over Fe-ZSM-5 catalysts. Chem. Commun. 1999, 1999, 71–72. [Google Scholar] [CrossRef]
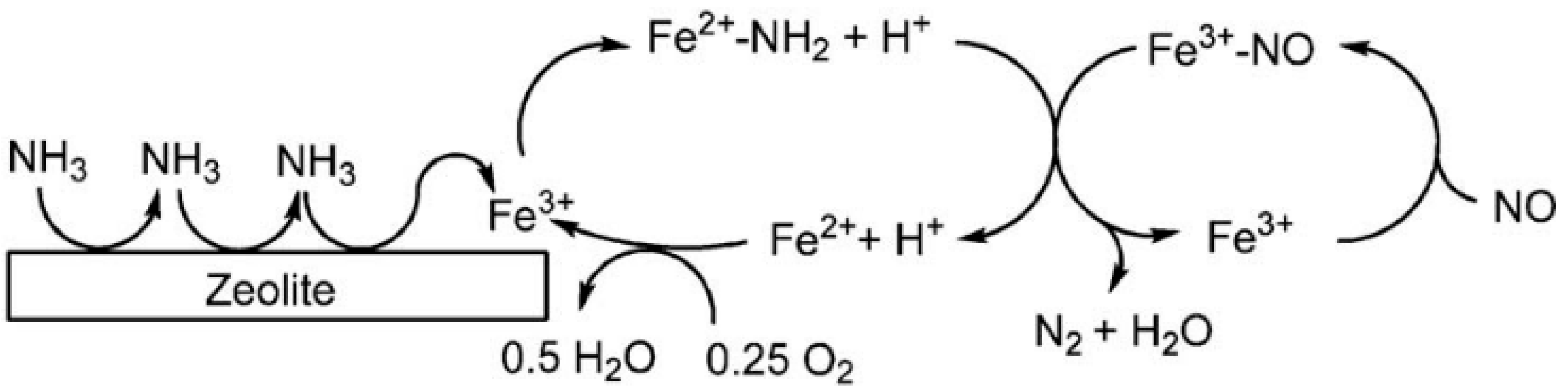
- Long, R.Q.; Yang, R.T. Reaction Mechanism of Selective Catalytic Reduction of NO with NH3 over Fe-ZSM-5 Catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Yang, R.T. Selective Catalytic Reduction of Nitric Oxide with Ammonia over ZSM-5 Based Catalysts for Diesel Engine Applications. Catal. Lett. 2007, 121, 111–117. [Google Scholar] [CrossRef]
- Shi, X.; Liu, F.; Shan, W.; He, H. Selective Catalytic Reduction of NOx with NH3 over Fe-ZSM-5 Catalysts. In Abstracts of Session 2, 28th Annual Meeting of the Chinese Chemical Society; Chinese Chemical Society: Beijing, China; Available online: https://kns.cnki.net/kcms2/article/abstract?v=ZHE1803t14tVnDFTXmxRBogUw2nC36yJPbxJy0j5GvviUqAXIURKp5CC6DtoenvICuHu8VY_l5GC6OSYTiT61GJu1ir7a8zMhH6NlKhuH1AYL1mwKBOFX1ib_4ajO8saDhjNfDleYkU8evPgcyTnRIQQIUPjC96wTt6fYvlw_EVB-ygjgAMKTw==&uniplatform=NZKPT&language=CHS (accessed on 3 November 2025).
- Shi, X.; Liu, F.; Shan, W.; He, H. Hydrothermal Deactivation of Fe-ZSM-5 Prepared by Different Methods for the Selective Catalytic Reduction of NOx with NH3. Chin. J. Catal. 2012, 33, 454–464. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, T.; Liu, Z.; Cao, Y.; Gong, M.; Chen, Y. Influence of Ce and La modified Fe-ZSM-5 catalyst on NH3-SCR reaction property. Chem. Res. Appl. 2013, 25, 1065–1068. [Google Scholar]
- Long, R.Q.; Yang, R.T. Superior Fe-ZSM-5 Catalyst for Selective Catalytic Reduction of Nitric Oxide by Ammonia. J. Am. Chem. Soc. 1999, 121, 5595–5596. [Google Scholar] [CrossRef]
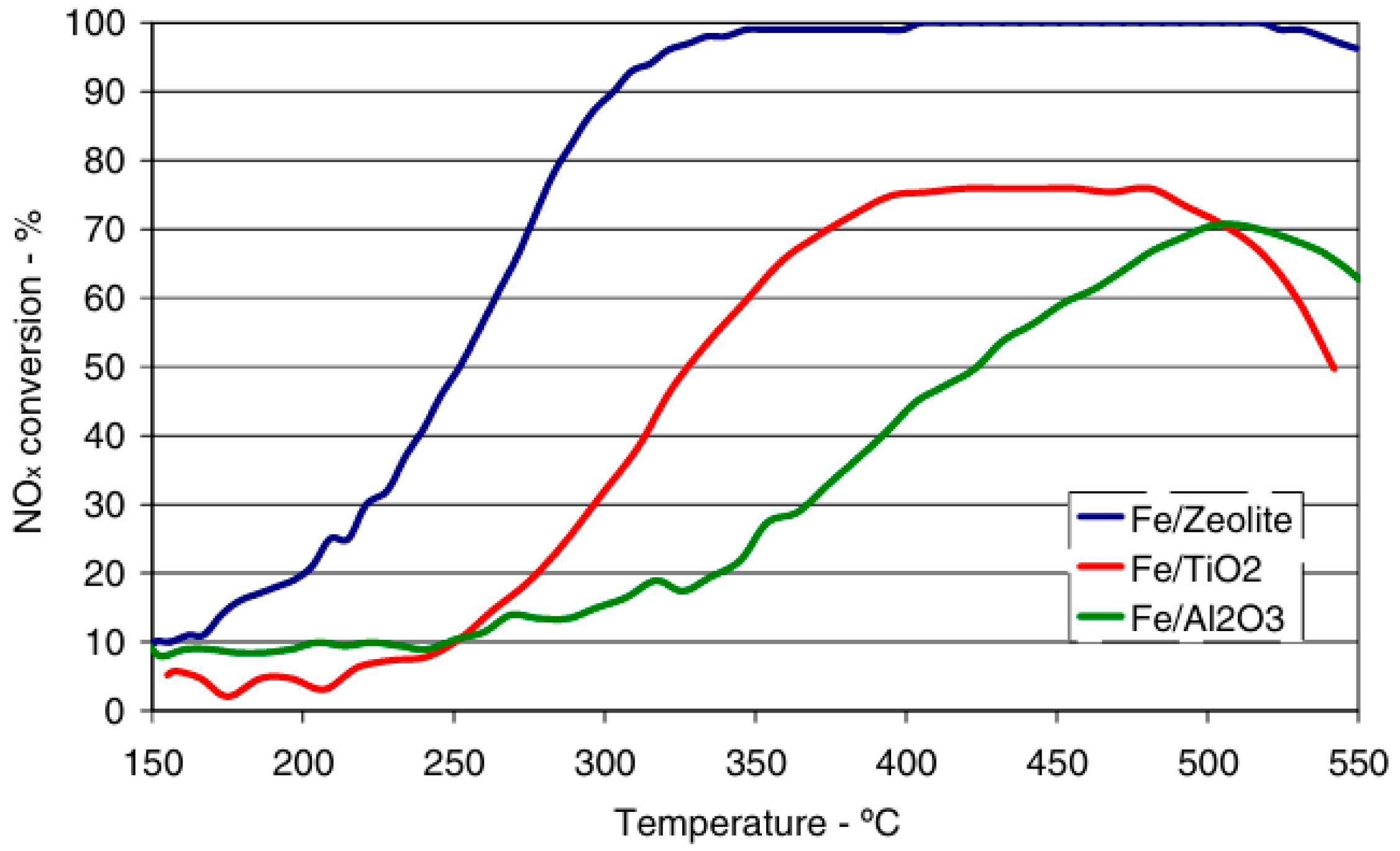
- Wang, D.; Wang, L.; Gao, G.; Xue, X.; Wu, P.; Zhang, Y. Performance of selective catalytic reduction of NOx over Ce-Fe/ZSM-5 catalysts for denitrification of gas turbines. Chin. J. Process Eng. 2020, 20, 718–727. [Google Scholar]
- Feng, S.; Li, Z.; Shen, B.; Yuan, P.; Wang, B.; Liu, L.; Wang, Z.; Ma, J.; Kong, W. High activity of NH3-SCR at high temperature over W-Zr/ZSM-5 in the exhaust gas of diesel engine. Fuel 2022, 323, 124337. [Google Scholar] [CrossRef]
- Feng, S.; Kong, W.; Wang, Y.; Xing, Y.; Wang, Z.; Ma, J.; Shen, B.; Chen, L.; Yang, J.; Li, Z.; et al. Mechanistic investigation of Sm doping effects on SO2 resistance of W-Zr-ZSM-5 catalyst for NH3-SCR. Fuel 2023, 353, 129139. [Google Scholar] [CrossRef]
- Liu, H.; You, C.; Wang, H. Experimental and Density Functional Theory Studies on the Zeolite-Based Fe–Ni–W Trimetallic Catalyst for High-Temperature NOx Selective Catalytic Reduction: Identification of Active Sites Suppressing Ammonia Over-oxidation. ACS Catal. 2021, 11, 1189–1201. [Google Scholar] [CrossRef]
- Liu, Z.; Millington, P.J.; Bailie, J.E.; Rajaram, R.R.; Anderson, J.A. A comparative study of the role of the support on the behaviour of iron based ammonia SCR catalysts. Microporous Mesoporous Mater. 2007, 104, 159–170. [Google Scholar] [CrossRef]
- Frey, A.M.; Mert, S.; Due-Hansen, J.; Fehrmann, R.; Christensen, C.H. Fe-BEA Zeolite Catalysts for NH3-SCR of NOx. Catal. Lett. 2009, 130, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhao, R.; Zhao, Q.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Fe-doped Beta zeolite from organotemplate-free synthesis for NH3-SCR of NOx. Catal. Sci. Technol. 2016, 6, 6581–6592. [Google Scholar] [CrossRef]
- Ma, L.; Chang, H.; Yang, S.; Chen, L.; Fu, L.; Li, J. Relations between iron sites and performance of Fe/HBEA catalysts prepared by two different methods for NH3-SCR. Chem. Eng. J. 2012, 209, 652–660. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Yu, T.; Fan, S.; Shen, M. The role of various iron species in Fe-β catalysts with low iron loadings for NH3-SCR. Catal. Sci. Technol. 2014, 4, 1350–1356. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhang, Z.; Fan, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Water promotion mechanism on the NH3-SCR over Fe-BEA catalyst. Catal. Commun. 2018, 115, 59–63. [Google Scholar] [CrossRef]
- Klukowski, D.; Balle, P.; Geiger, B.; Wagloehner, S.; Kureti, S.; Kimmerle, B.; Baiker, A.; Grunwaldt, J.D. On the mechanism of the SCR reaction on Fe/HBEA zeolite. Appl. Catal. B Environ. 2009, 93, 185–193. [Google Scholar] [CrossRef]
- Sultana, A.; Fujitani, T.; Iki, N. Development and validation of rare earth modified Fe-BEA SCR catalyst for mitigation of NOx from NH3 gas turbine. Clean. Mater. 2022, 4, 100096. [Google Scholar] [CrossRef]
- Kielland, B. Individual Activity Coefficients of Ions in Aqueous Solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Gao, F.; Kollár, M.; Kukkadapu, R.K.; Washton, N.M.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Fe/SSZ-13 as an NH3-SCR catalyst: A reaction kinetics and FTIR/Mössbauer spectroscopic study. Appl. Catal. B Environ. 2015, 164, 407–419. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H.F. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure–Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Jin, Y.; Pang, L.; Chen, Z.; Li, T. Influence of calcination temperature on the evolution of Fe species over Fe-SSZ-13 catalyst for the NH3-SCR of NO. Catal. Today 2022, 388–389, 158–167. [Google Scholar] [CrossRef]
- Chen, M.; Bi, J.; Liu, C.; Ren, S.-B. Enhancing performance of Fe-SSZ-13 and Cu-Fe-SSZ-13 zeolites for selective catalytic reduction reaction via post-treatment method. Mater. Lett. 2023, 349, 134872. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Han, M.-J.; Jiao, Y.-L.; Zhou, C.-H.; Guo, Y.-L.; Guo, Y.; Lu, G.-Z.; Wang, L.; Zhan, W.-C. Catalytic activity of Cu–SSZ-13 prepared with different methods for NH3-SCR reaction. Rare Met. 2018, 38, 210–220. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Qin, M.; Zhao, Z.; Fan, X.; Li, Q. Insight into the effects of Cu2+ ions and CuO species in Cu-SSZ-13 catalysts for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2022, 622, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hou, C.; Yang, S.; Mao, D.; Liu, J. Research progress in transition metals modified Cu-SSZ-13 zeolite denitration catalyst. Chem. Ind. Eng. Prog. 2023, 42, 2963–2974. [Google Scholar]
- Chen, M.; Li, J.; Xue, W.; Wang, S.; Han, J.; Wei, Y.; Mei, D.; Li, Y.; Yu, J. Unveiling Secondary-Ion-Promoted Catalytic Properties of Cu-SSZ-13 Zeolites for Selective Catalytic Reduction of NOx. J. Am. Chem. Soc. 2022, 144, 12816–12824. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Tang, X.; Liu, J.; Ju, M. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas. Chem. Ind. Eng. Prog. 2023, 42, 4636–4648. [Google Scholar]
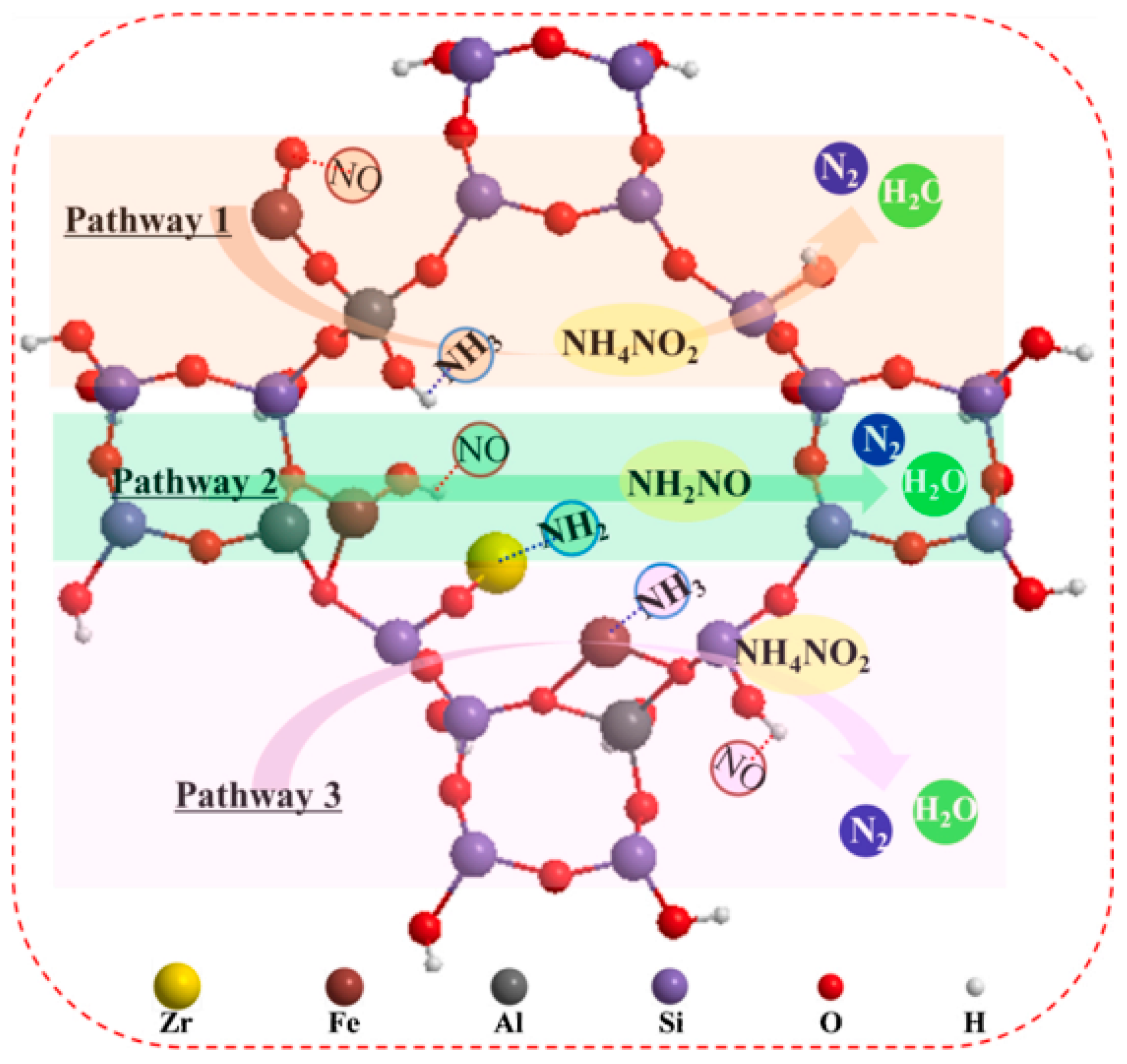
- Chen, Z.; Liu, Q.; Guo, L.; Zhang, S.; Pang, L.; Guo, Y.; Li, T. The promoting mechanism of in situ Zr doping on the hydrothermal stability of Fe-SSZ-13 catalyst for NH3-SCR reaction. Appl. Catal. B Environ. 2021, 286, 119816. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, G.; Chang, L.; Bao, W.; Wang, J. Study on denitrification performance of titanium doped Cu-SSZ-13 prepared by in-situ method. Mod. Chem. Ind. 2019, 39, 89–94. [Google Scholar]
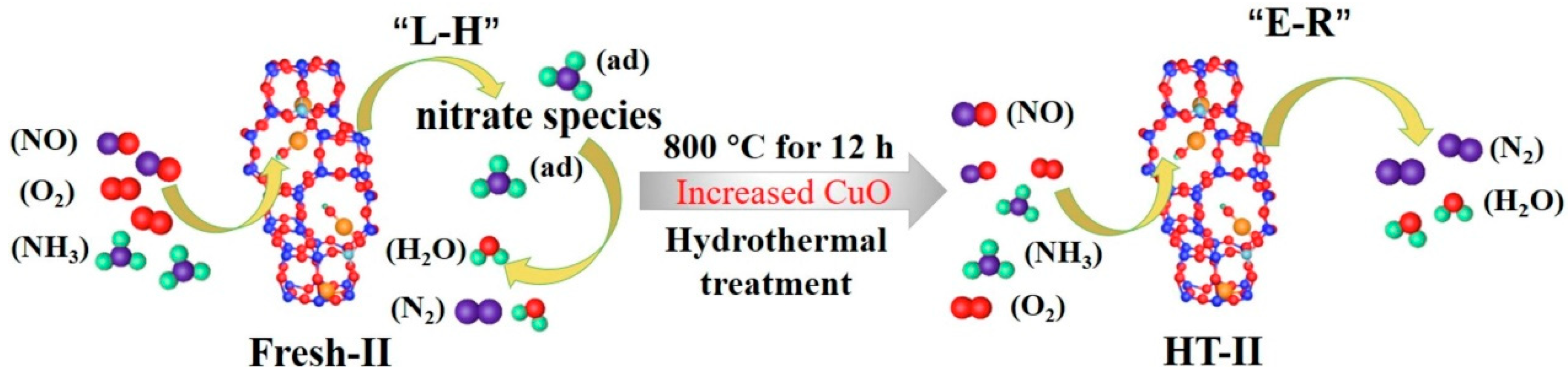
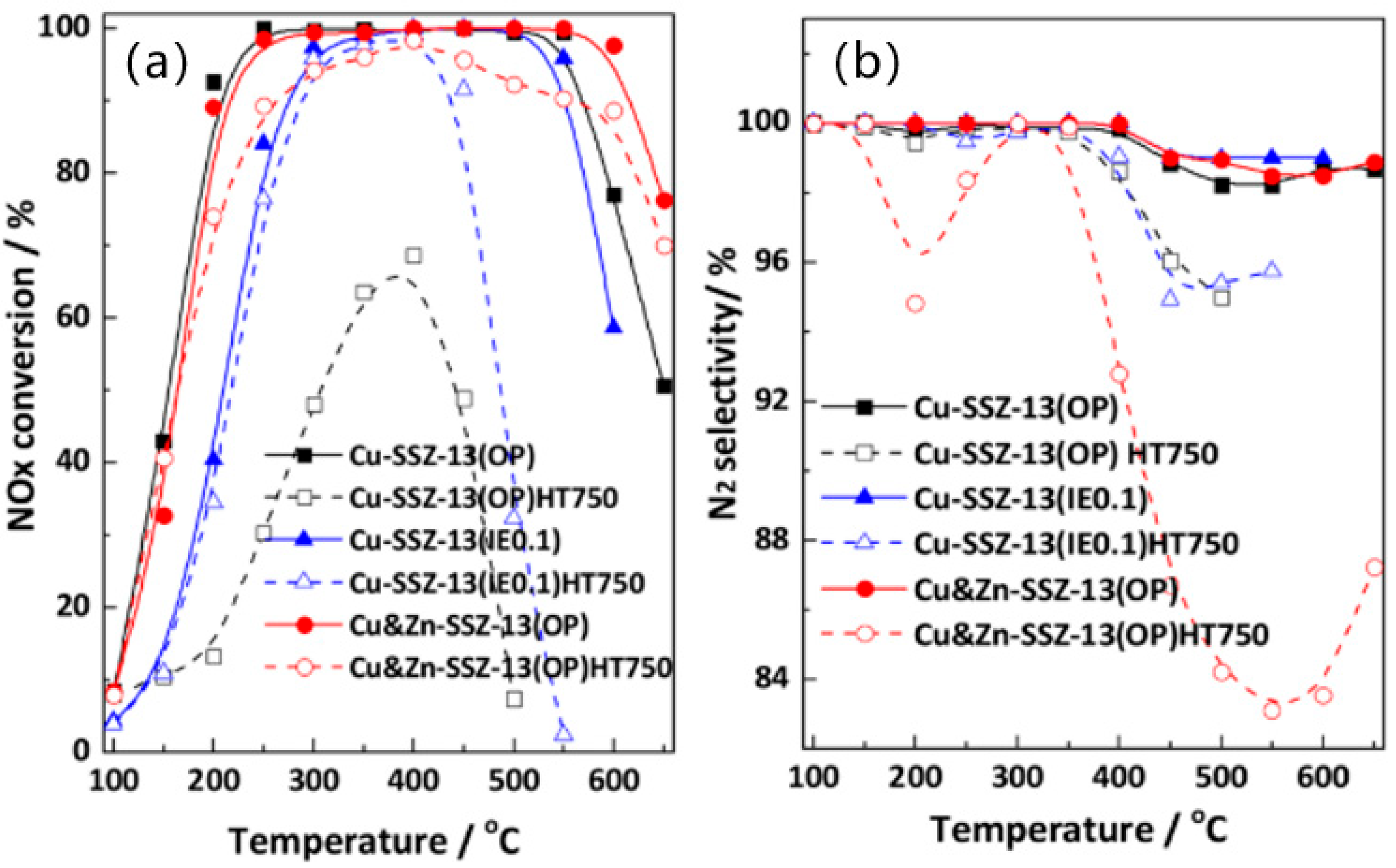
- Xu, R.; Wang, Z.; Liu, N.; Dai, C.; Zhang, J.; Chen, B.-H. Understanding Zn Functions on Hydrothermal Stability in a One-Pot-Synthesized Cu&Zn-SSZ-13 Catalyst for NH3 Selective Catalytic Reduction. ACS Catal. 2020, 10, 6197–6212. [Google Scholar]
- Zhao, Z.; Yu, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; Mcguire, R.; et al. Rare-earth ion exchanged Cu-SSZ-13 zeolite from organotemplate-free synthesis with enhanced hydrothermal stability in NH3-SCR of NOx. Catal. Sci. Technol. 2019, 9, 241–251. [Google Scholar] [CrossRef]
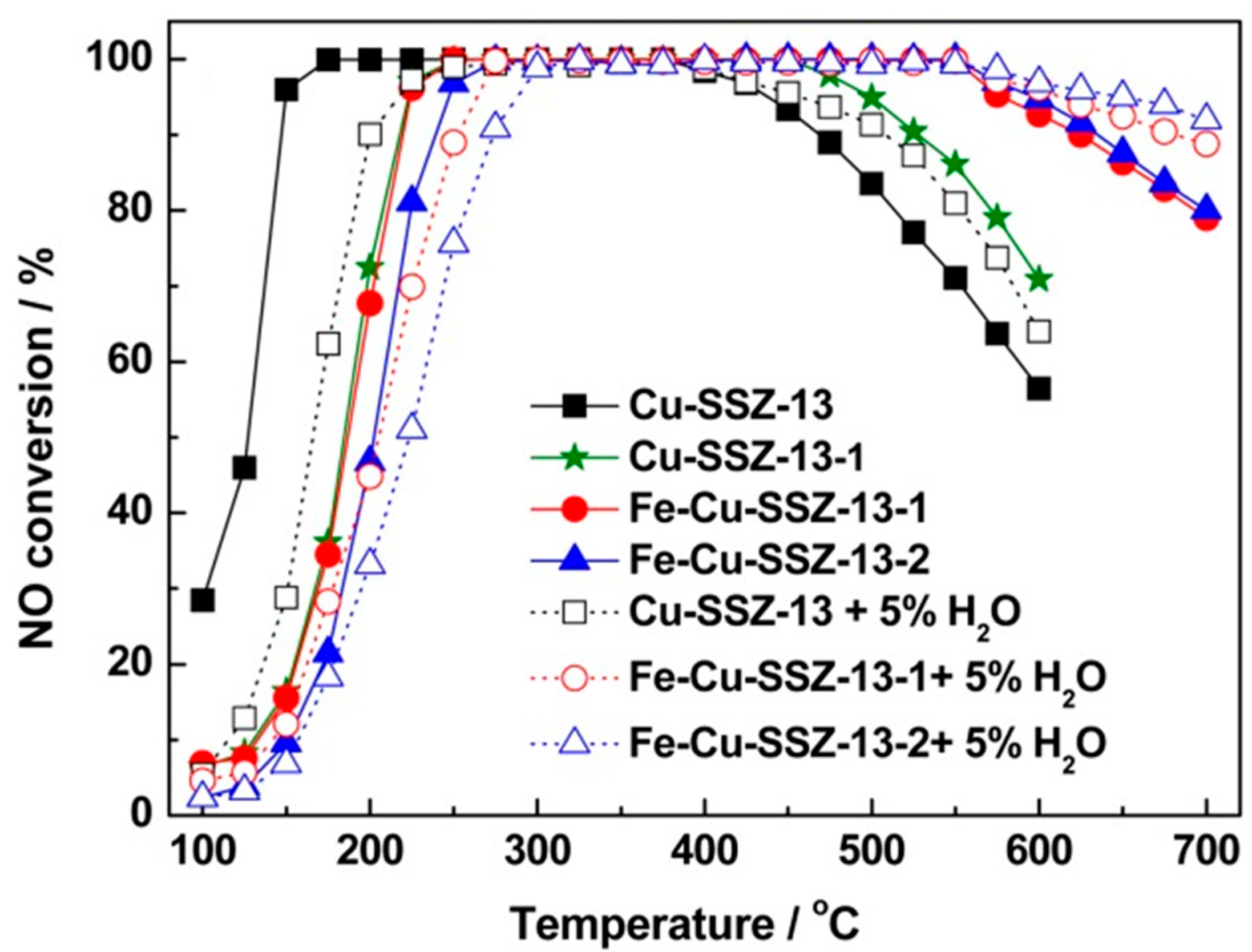
- Zhang, T.; Li, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. High activity and wide temperature window of Fe-Cu-SSZ-13 in the selective catalytic reduction of NO with ammonia. AIChE J. 2015, 61, 3825–3837. [Google Scholar] [CrossRef]
- Wan, J.; Chen, J.; Zhao, R.; Zhou, R. One-pot synthesis of Fe/Cu-SSZ-13 catalyst and its highly efficient performance for the selective catalytic reduction of nitrogen oxide with ammonia. J. Environ. Sci. 2021, 100, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Li, G. One-pot synthesis of FeCu–SSZ-13 using Cu–TEPA as the template by adding iron complexes. Catal. Sci. Technol. 2021, 11, 7467–7474. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Tang, X.; Xing, C.; Jin, T. The promotion effect of niobium on the low-temperature activity of Al-rich Cu-SSZ-13 for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2021, 418, 129433. [Google Scholar] [CrossRef]
- Liu, J.; Tang, X.; Xing, C.; Jin, T.; Yin, Y.; Wang, J. Niobium modification for improving the high-temperature performance of Cu-SSZ-13 in selective catalytic reduction of NO by NH3. J. Solid State Chem. 2021, 296, 122028. [Google Scholar] [CrossRef]
- Usman, M.; Ghanem, A.S.; Niaz Ali Shah, S.; Garba, M.D.; Yusuf Khan, M.; Khan, S.; Humayun, M.; Laeeq Khan, A. A Review on SAPO-34 Zeolite Materials for CO2 Capture and Conversion. Chem. Rec. 2022, 22, e202200039. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qi, G.; Weng, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Wang, J.; Wang, C.; Shen, M.; Li, W. The influence of crystallite size on the structural stability of Cu/SAPO-34 catalysts. Appl. Catal. B Environ. 2019, 248, 430–440. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Varga, T.; Wang, Y.; Szanyi, J.; Wang, Y.; Peden, C.H.F.; et al. Unraveling the mysterious failure of Cu/SAPO-34 selective catalytic reduction catalysts. Nat. Commun. 2019, 10, 1137. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B Environ. 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Kamasamudram, K.; Epling, W.S. In Situ-DRIFTS Study of Selective Catalytic Reduction of NOx by NH3 over Cu-Exchanged SAPO-34. ACS Catal. 2013, 3, 871–881. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Meng, Y.; Pang, L.; Guo, Y.; Luo, Z.; Fang, Y.; Dong, Y.; Cai, W.; Li, T. Insight into solid-state ion-exchanged Cu-based zeolite (SSZ-13, SAPO-18, and SAPO-34) catalysts for the NH3-SCR reaction: The promoting role of NH4-form zeolite substrates. Appl. Surf. Sci. 2022, 571, 151328. [Google Scholar] [CrossRef]
- Tang, J.; Xu, M.; Yu, T.; Ma, H.; Shen, M.; Wang, J. Catalytic deactivation mechanism research over Cu/SAPO-34 catalysts for NH3-SCR (II): The impact of copper loading. Chem. Eng. Sci. 2017, 168, 414–422. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chen, Y.; Tang, L.; Bao, W.-R.; Chang, L.-P.; Han, L.-N. One-step hydrothermal synthesis of Cu-SAPO-34/cordierite and its catalytic performance on NOx removal from diesel vehicles. Trans. Nonferrous Met. Soc. China 2013, 23, 3330–3336. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Li, X.; Liu, M.; Li, Y. The promotion effect of Fe to Cu-SAPO-34 for selective catalytic reduction of NOx with NH3. Catal. Today 2017, 297, 84–91. [Google Scholar] [CrossRef]
- Doan, T.; Dam, P.; Nguyen, K.; Vuong, T.H.; Le, M.T.; Pham, T.H. Copper-Iron Bimetal Ion-Exchanged SAPO-34 for NH3-SCR of NOx. Catalysts 2020, 10, 321. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Q.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. Effect of Metal Modification on NH3-SCR Reaction Performance of Cu-SAPO-34 Catalyst. Rare Met. 2020, 44, 159–165. [Google Scholar]
- Mao, J.-W.; Xu, B.; Hu, Y.-K.; Zhang, C.-Y.; Meng, H.-M. Effect of Ce metal modification on the hydrothermal stability of Cu-SAPO-34 catalyst. J. Fuel Chem. Technol. 2020, 48, 1208–1216. [Google Scholar] [CrossRef]
- Chen, B.; Cheng, J.; Qiao, J.; Dai, C.; Xu, R.; Yu, G.; Wang, N.; Xing, J.; Liu, N. Understanding of low- and high-temperature DeNOx efficiency for NH3-SCR via comparison on Cu modified CHA and AFX zeolites. Fuel 2023, 348, 128501. [Google Scholar] [CrossRef]
- Li, R.; Jiang, X.; Lin, J.; Zhang, Z.; Huang, Q.; Fu, G.; Zhu, Y.; Jiang, J. Understanding the influence of hydrothermal treatment on NH3-SCR of NO activity over Cu-SSZ-16. Chem. Eng. J. 2022, 441, 136021. [Google Scholar] [CrossRef]
- Wei, X.; Ke, Q.; Cheng, H.; Guo, Y.; Yuan, Z.; Zhao, S.; Sun, T.; Wang, S. Seed-assisted synthesis of Cu-(Mn)-UZM-9 zeolite as excellent NO removal and N2O inhibition catalysts in wider temperature window. Chem. Eng. J. 2020, 391, 123491. [Google Scholar] [CrossRef]
- Fu, G.; Yang, R.; Liang, Y.; Yi, X.; Li, R.; Yan, N.; Zheng, A.; Yu, L.; Yang, X.; Jiang, J. Enhanced hydrothermal stability of Cu/SSZ-39 with increasing Cu contents, and the mechanism of selective catalytic reduction of NO. Microporous Mesoporous Mater. 2021, 320, 111060. [Google Scholar] [CrossRef]
- Du, J.; Han, S.; Huang, C.; Shan, Y.; Zhang, Y.; Shan, W.; He, H. Comparison of precursors for the synthesis of Cu-SSZ-39 zeolite catalysts for NH3-SCR reaction. Appl. Catal. B Environ. 2023, 338, 123072. [Google Scholar] [CrossRef]
- Han, S.; Tang, X.; Wang, L.; Ma, Y.; Chen, W.; Wu, Q.; Zhang, L.; Zhu, Q.; Meng, X.; Zheng, A.; et al. Potassium-directed sustainable synthesis of new high silica small-pore zeolite with KFI structure (ZJM-7) as an efficient catalyst for NH3-SCR reaction. Appl. Catal. B Environ. 2021, 281, 119480. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, D.; Wei, Y.; Han, J.; Li, D.; Chen, M.; Yu, J. Copper-exchanged SUZ-4 zeolite catalysts for selective catalytic reduction of NOx. Mater. Chem. Front. 2024, 8, 2142–2148. [Google Scholar] [CrossRef]
- Ryu, T.; Ahn, N.H.; Seo, S.; Cho, J.; Kim, H.; Jo, D.; Park, G.T.; Kim, P.S.; Kim, C.H.; Bruce, E.L.; et al. Fully Copper-Exchanged High-Silica LTA Zeolites as Unrivaled Hydrothermally Stable NH3-SCR Catalysts. Angew. Chem. 2017, 129, 3304–3308. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Xu, L.; Ohnishi, T.; Yanaba, Y.; Ogura, M.; Wakihara, T.; Okubo, T. Understanding the high hydrothermal stability and NH3-SCR activity of the fast-synthesized ERI zeolite. J. Catal. 2020, 391, 346–356. [Google Scholar] [CrossRef]
- Martín, N.; Vennestrøm, P.N.R.; Thøgersen, J.R.; Moliner, M.; Corma, A. Iron-Containing SSZ-39 (AEI) Zeolite: An Active and Stable High-Temperature NH3-SCR Catalyst. ChemCatChem 2017, 9, 1754–1757. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, S.; Hong, S.B. Facile one-pot synthesis of Fe-UZM-35 catalysts for ammonia selective catalytic reduction. Appl. Catal. B Environ. 2023, 329, 122552. [Google Scholar] [CrossRef]
- Grzybek, J.; Gil, B.; Roth, W.J.; Skoczek, M.; Kowalczyk, A.; Chmielarz, L. Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: An in situ FTIR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 196, 281–288. [Google Scholar] [CrossRef]
- Ming, S.; Chen, Z.; Fan, C.; Pang, L.; Guo, W.; Albert, K.B.; Liu, P.; Li, T. The effect of copper loading and silicon content on catalytic activity and hydrothermal stability of Cu-SAPO-18 catalyst for NH3-SCR. Appl. Catal. A-Gen. 2018, 559, 47–56. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Ce doping to Cu-SAPO-18: Enhanced catalytic performance for the NH3-SCR of NO in simulated diesel exhaust. Microporous Mesoporous Mater. 2019, 276, 133–146. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Anderson, J.A.; Zhang, Z. The Potential of Cu-SAPO-44 in the Selective Catalytic Reduction of NOx with NH3. ChemCatChem 2016, 8, 3740–3745. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Wang, C.; Li, K.; Liu, S.; Li, X.; Chen, J.; Li, J. Selective Catalytic Reduction of NOx with Ammonia over Copper Ion Exchanged SAPO-47 Zeolites in a Wide Temperature Range. ChemCatChem 2018, 10, 2481–2487. [Google Scholar] [CrossRef]
- Zhang, S.; Ming, S.; Guo, L.; Bian, C.; Meng, Y.; Liu, Q.; Dong, Y.; Bi, J.; Li, D.; Wu, Q.; et al. Controlled synthesis of Cu-based SAPO-18/34 intergrowth zeolites for selective catalytic reduction of NOx by ammonia. J. Hazard. Mater. 2021, 414, 125543. [Google Scholar] [CrossRef]
- Gao, Q.; Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines. Catalysts 2020, 10, 336. [Google Scholar] [CrossRef]
- Lin, J.; Hu, X.; Tan, X.; Lin, C.; Zhang, Y.; Shan, W.; He, H. Y-Doped Cu-SSZ-39 Zeolite with Only Cu2+-2Z Species: An NH3-SCR Catalyst with Extremely High Hydrothermal Stability. Environ. Sci. Technol. 2025, 59, 15515–15525. [Google Scholar] [CrossRef]
- He, S.; Lin, Z.; Shi, J.; Zhou, L.; Cui, Q.; Li, T.; Liu, J.; Yue, Y. Interface-engineered ZnO/FeCu-SSZ-13 with robust SO2 and H2O resistance for low-temperature NH3-SCR. Chem. Eng. J. 2025, 522, 167119. [Google Scholar] [CrossRef]
- Feng, Y.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Jansson, J.; Skoglundh, M.; Grönbeck, H. High-Temperature Reaction Mechanism of NH3-SCR over Cu-CHA: One or Two Copper Ions? J. Phys. Chem. C 2024, 128, 6689–6701. [Google Scholar] [CrossRef]
- Han, J.; Li, J.; Zhao, W.; Li, L.; Chen, M.; Ge, X.; Wang, S.; Liu, Q.; Mei, D.; Yu, J. Cu-OFF/ERI Zeolite: Intergrowth Structure Synergistically Boosting Selective Catalytic Reduction of NOx with NH3. J. Am. Chem. Soc. 2024, 146, 7605–7615. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Shin, J.S.; Kim, Y.; Lee, J.M. Decision tree analysis on the performance of zeolite-based SCR catalysts. IFAC-Pap. 2021, 54, 55–60. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.S. Green routes for synthesis of zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef]
- Nanda, A.S.F.; Kadja, G.T.M. Bio-based templates for generating hierarchical zeolites: An overview for greener synthesis pathway. J. Porous Mater. 2024, 31, 1155–1173. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Yip, A.C.K. Advances in the Green Synthesis of Microporous and Hierarchical Zeolites: A Short Review. Catalysts 2019, 9, 274. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, L.; Yang, C.; Chen, Y.; Sun, Q.; Zhang, H.; Li, C.; Nawaz, F.; Meng, X.; Xiao, F.S. Designed copper–amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef]
- Li, Q.; Cong, W.; Zhang, J.; Xu, C.; Wang, F.; Han, D.; Wang, G.; Bing, L. Rapid synthesis of hierarchical nanosized SSZ-13 zeolite with excellent MTO catalytic performance. Microporous Mesoporous Mater. 2022, 331, 111649. [Google Scholar] [CrossRef]
- Miao, M.; Qiu, Y.; Yao, L.; Wu, Q.; Ruan, H.; Van Der Bruggen, B.; Shen, J. Preparation of N,N,N-trimethyl-1-adamantylammonium hydroxide with high purity via bipolar membrane electrodialysis. Sep. Purif. Technol. 2018, 205, 241–250. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, L.; Guo, A.; Chen, D.; Shan, Y.; Fu, M.; Wu, J.; Ye, D.; Chen, P. Economical and Sustainable Synthesis of Small-Pore Chabazite Catalysts for NOx Abatement by Recycling Organic Structure-Directing Agents. Environ. Sci. Technol. 2023, 57, 655–665. [Google Scholar] [CrossRef]
- Debost, M.; Clatworthy, E.B.; Grand, J.; Barrier, N.; Nesterenko, N.; Gilson, J.-P.; Boullay, P.; Mintova, S. Direct synthesis of nanosized CHA zeolite free of organic template by a combination of cations as structure directing agents. Microporous Mesoporous Mater. 2023, 358, 112337. [Google Scholar] [CrossRef]
- Hrabanek, P.; Zikanova, A.; Supinkova, T.; Drahokoupil, J.; Fila, V.; Lhotka, M.; Dragounova, H.; Laufek, F.; Brabec, L.; Jirka, I.; et al. Static in-situ hydrothermal synthesis of small pore zeolite SSZ-16 (AFX) using heated and pre-aged synthesis mixtures. Microporous Mesoporous Mater. 2016, 228, 107–115. [Google Scholar] [CrossRef]
- Bossche, A.V.D.; Rodriguez, N.R.; Riaño, S.; Dehaen, W.; Binnemans, K. Dissolution behavior of precious metals and selective palladium leaching from spent automotive catalysts by trihalide ionic liquids. RSC Adv. 2021, 11, 10110–10120. [Google Scholar] [CrossRef] [PubMed]


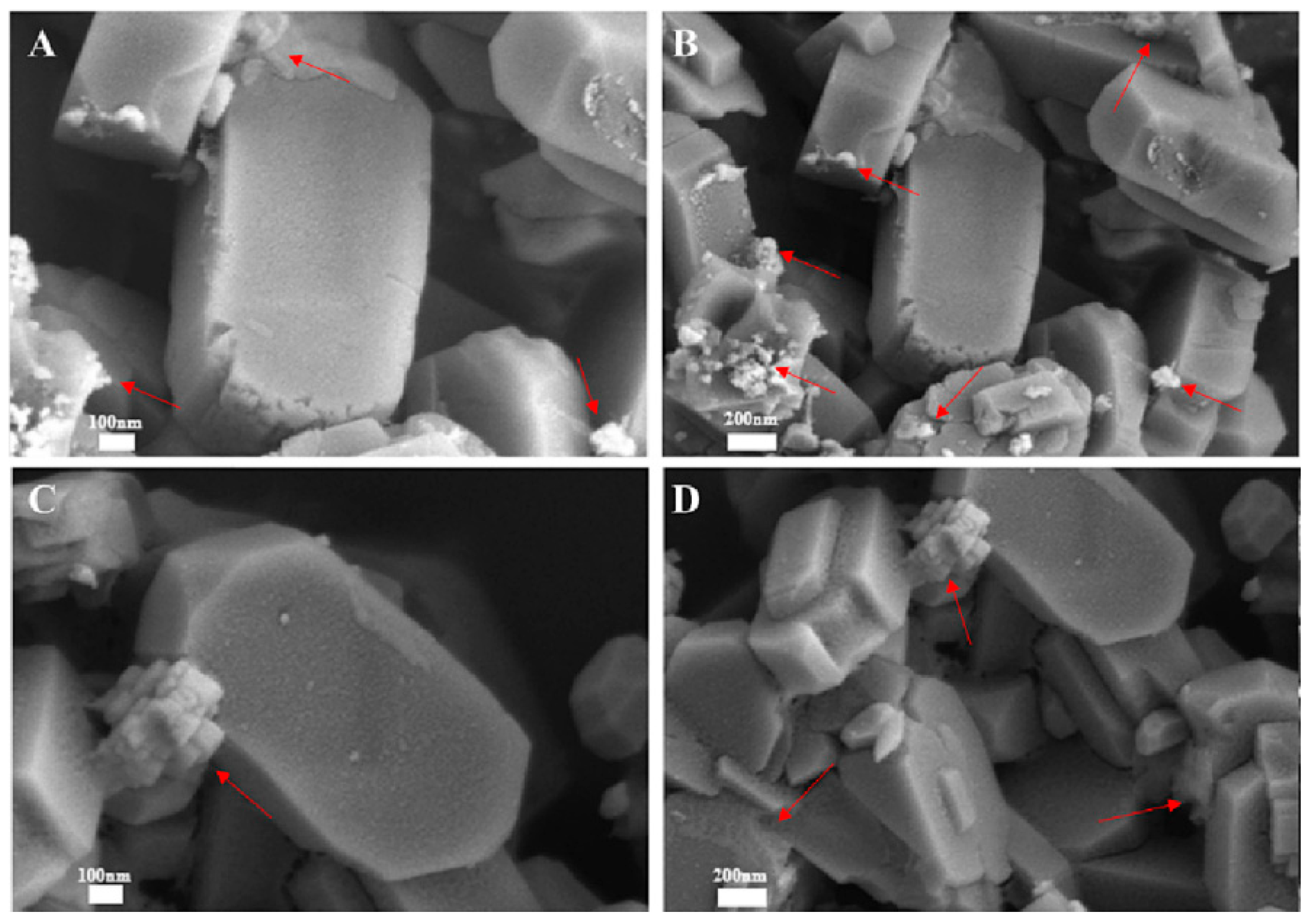




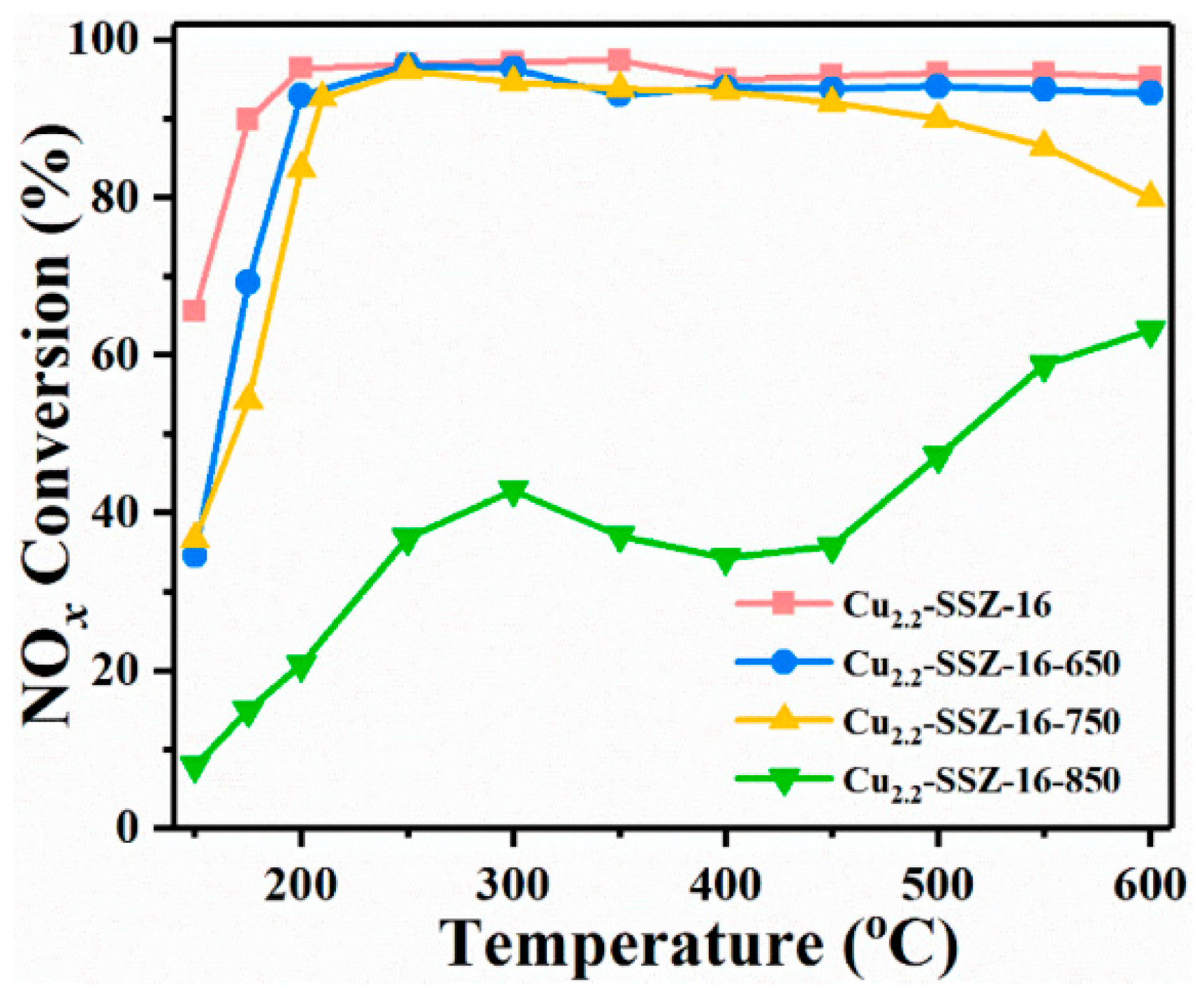
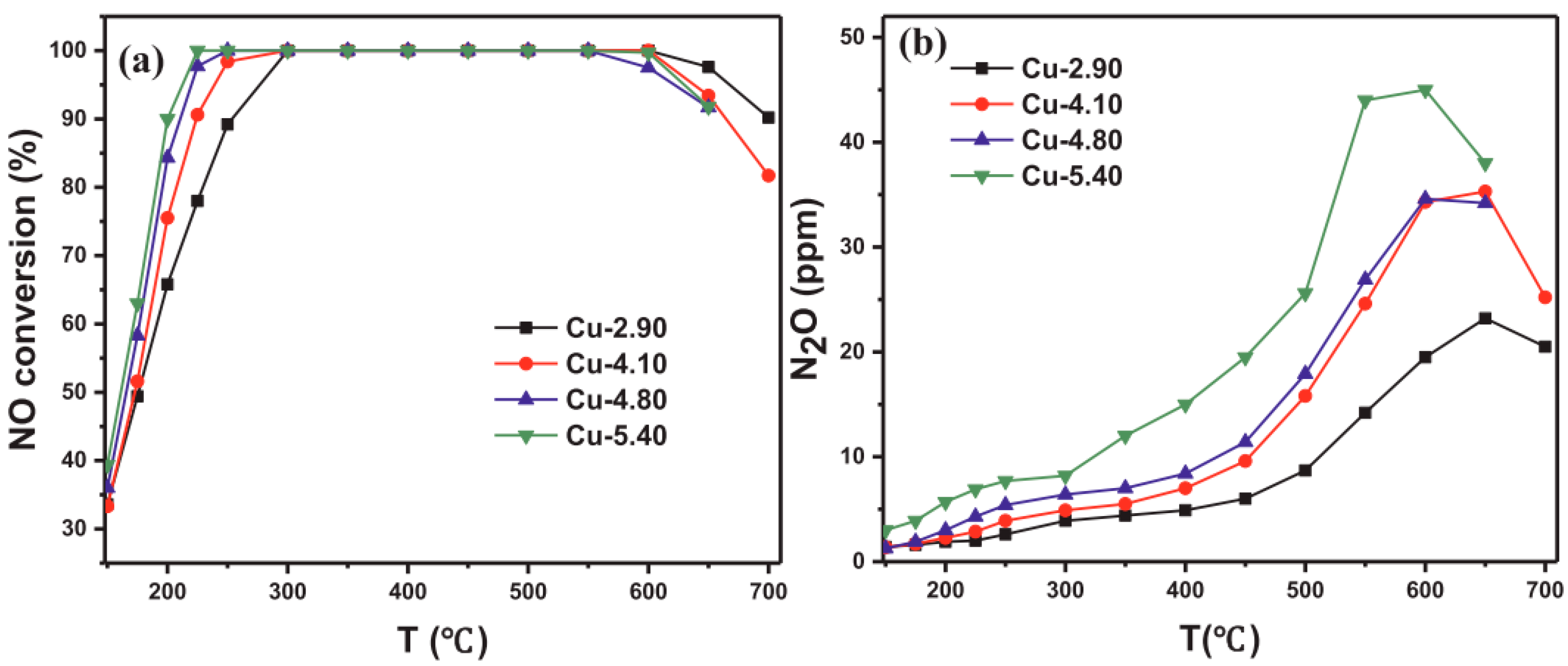
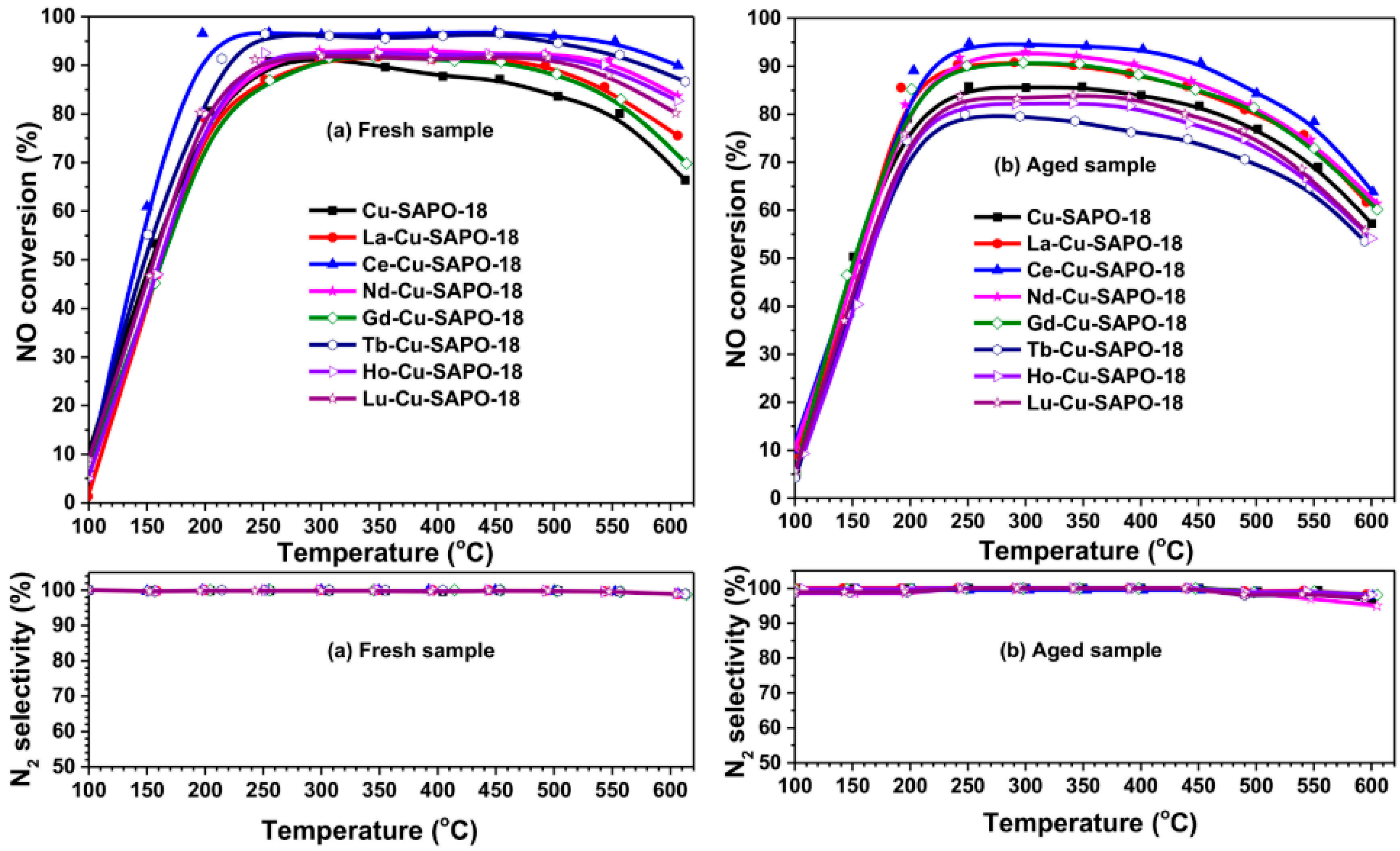
| Topology | Name | Maximum Diameter (Å) | Channel System | Ring Member | Synthesis Cost | Hydrothermal Stability |
|---|---|---|---|---|---|---|
| MFI | ZSM-5 | 5.1 × 5.5 | 3-dimensional | 10/6/5/4 | Medium | Low |
| 5.3 × 5.6 | ||||||
| BEA | Beta | 6.6 × 6.7 | 3-dimensional | 12/6/5/4 | Medium | Medium |
| 5.6 × 5.6 | ||||||
| CHA | SSZ-13 | 3.8 × 3.8 | 3-dimensional | 8/6/4 | High | Excellent |
| SSZ-62 | ||||||
| SAPO-34 | ||||||
| SAPO-47 | ||||||
| AFX | SSZ-16 | 3.4 × 3.6 | 3-dimensional | 8/6/4 | High | Excellent |
| SAPO-56 | ||||||
| AEI | SSZ-39 | 3.8 × 3.8 | 3-dimensional | 8/6/4 | High | Excellent |
| SAPO-18 | ||||||
| LTA | UZM-9 | 4.1 × 4.1 | 3-dimensional | 8/6/4 | High | Good |
| SAPO-42 |
| Catalyst | Preparation Method | Reaction Condition | Denitrification Activity | Refs. |
|---|---|---|---|---|
| Cu/SSZ-16 | ion-exchange | [NO] = [NH3] = 600 ppm, [O2] = 6%, [H2O] = 5%, GHSV = 400,000 h−1 | (300~670 °C) > 95% | [132] |
| Cu/SSZ-16 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 100,000 h−1 | (175~600 °C) > 90% | [133] |
| Cu/UZM-9 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 10%, GHSV = 100,000 h−1 | (250~700 °C) > 90% | [134] |
| Cu/SSZ-39 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 190,000 h−1 | (250~550 °C) > 90% | [135] |
| Cu/SSZ-39 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 200,000 h−1 | (200~550 °C) > 90% | [136] |
| Cu/ZJM-7 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 80,000 h−1 | (200~550 °C) > 90% | [137] |
| Cu/SUZ-4 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 200,000 h−1 | (250~550 °C) > 90% | [138] |
| Cu/LTA | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 10%, GHSV = 100,000 h−1 | (220~600 °C) > 90% | [139] |
| Cu/ERI | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 3%, GHSV = 50,000 h−1 | (250~600 °C) > 90% | [140] |
| Fe/SSZ-39 | one-pot | [NO] = 50 ppm, [NH3] = 60 ppm, [O2] = 10%, [H2O] = 10% | (330~550 °C) > 90% | [141] |
| Fe/UZM-35 | one-pot | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 10%, GHSV = 100,000 h−1 | (250~600 °C) > 90% | [142] |
| Fe/MCM-56 | ion-exchange | [NO] = [NH3] = 2500 ppm, [O2] = 2.5% | (430~600 °C) > 80% | [143] |
| Cu/SAPO-18 | incipient wetness impregnation | [NO] = 1000 ppm, [NH3] = 1100 ppm, [O2] = 5%, [H2O] = 10%, GHSV = 30,000 h−1 | (230~575 °C) > 80% | [144] |
| Ce-Cu/SAPO-18 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 14%, [H2O] = 5%, GHSV = 130,000 h−1 | (200~600 °C) > 90% | [145] |
| Cu/SAPO-44 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5.3%, [H2O] = 10%, GHSV = 100,000 h−1 | (200~550 °C) > 90% | [146] |
| Cu/SAPO-47 | ion-exchange | [NO] = [NH3] = 500 ppm, [O2] = 5%, [H2O] = 5%, GHSV = 100,000 h−1 | (250~600 °C) > 90% | [147] |
| Cu/SAPO-18/34 | incipient wetness impregnation | [NO] = 1000 ppm, [NH3] = 1100 ppm, [O2] = 5%, [H2O] = 10%, GHSV = 30,000 h−1 | (200~575 °C) > 90% | [148] |
| Primary Active Metals | Doped Modifying Elements | Reaction Mechanism | Advantages After Doping | Core Limitations | Typical Zeolite Support | References |
|---|---|---|---|---|---|---|
| Fe | — | E-R or L-H | — | Severe oxidation of NH3 at high temperatures, with poor activity above 600 °C | ZSM-5, Beta, SSZ-13, SAPO-34 | [38,39,91,92,93,94,95,96,100,101,102,123] |
| Zr | E-R | Enhance the dispersion of iron species, increase acidic sites, promote the formation of intermediates, and improve hydrothermal stability | The preparation method is complex and may clog the channels | SSZ-13 | [110] | |
| Ce | E-R or L-H | Promote NOx adsorption and activation, enhancing high-temperature SCR activity and hydrothermal stability | Severe oxidation of NH3 at high temperatures and poor hydrothermal stability | ZSM-5, Beta | [41,85,86,87] | |
| Gd | E-R or L-H | Inhibit the agglomeration of iron species and enhance the stability of the zeolite framework | The high cost of the rare earth element Gd | Beta | [98] | |
| Ni, W | E-R or L-H | Significantly inhibits the oxidation of NH3 | The preparation method is complex | ZSM-5 | [90] | |
| Cu | — | E-R or L-H | — | Poor high-temperature activity | ZSM-5, Beta, SSZ-13, SAPO-34 | [104,124,125,126] |
| — | E-R or L-H | — | The preparation method is complex and costly, and it produces the byproduct N2O | SSZ-16, UZM-9 | [132,133,134] | |
| Fe | E-R or L-H | Significantly enhanced high-temperature activity | The preparation method is complex | SSZ-13, SAPO-34 | [114,128,129] | |
| Nb | L-H | The formed Nb=O can increase acidity, optimize the species distribution of Cu, and enhance redox capability | The optimal load capacity of Nb is critical, but it comes at a high cost | SSZ-13 | [117,118] | |
| Zn | E-R or L-H | Inhibit Cu2+ migration and sintering to enhance hydrothermal stability | Zn may be toxic under certain conditions, and its framework may collapse at extremely high temperatures | SSZ-13 | [112] | |
| Ti | E-R or L-H | Protect the molecular sieve framework to enhance hydrothermal stability | Ti modification is costly and requires post-processing | SSZ-13 | [111] | |
| Ce | E-R and L-H | Enhance high-temperature performance and inhibit backbone hydrolysis | The preparation method is complex | SAPO-18 | [145] | |
| Y | E-R or L-H | Stabilize the molecular sieve framework and protect Brønsted acid sites | The cost of the rare earth element Y is high | SSZ-13 | [113] |
| Zeolite Type (Topology) | Active Metal | Denitrification Activity | Hydrothermal Stability | Key Advantages | Main Limitations | Synthesis Cost | Industrial Potential |
|---|---|---|---|---|---|---|---|
| ZSM-5 (MFI) | Fe | (425~680 °C) > 80% | Low | Low cost, tunable acidity, mature synthesis | Severe oxidation of NH3 at high temperatures | Low | Medium (for mid-temperature, cost-sensitive scenarios) |
| Ce, Fe | (370~600 °C) > 90% | Excellent | |||||
| W, Zr | (350~630 °C) > 90% | Moderate | |||||
| Sm, W, Zr | (380~640 °C) > 90% | Moderate | |||||
| Fe, Ni, W | (480~750 °C) > 90% | Excellent | |||||
| Beta (BEA) | Fe | (310~600 °C) > 90% | Moderate | 3D large pores, favorable mass transfer, high surface area | Structural defects, prone to dealumination at high temperatures | Medium | Medium (requires enhanced high-temp stability) |
| Ce, Fe | (311~683 °C) > 80% | Excellent | |||||
| Gd, Fe | (450~600 °C) > 90% | Excellent | |||||
| SSZ-13 (CHA) | Cu | (200~680 °C) > 90% | Excellent | Outstanding hydrothermal stability, superior shape selectivity | Expensive template, high synthesis cost | High | High (Top candidate for high-temperature use, pending cost reduction) |
| Zr, Fe | (430~575 °C) > 90% | ||||||
| Ti, Cu | (250~640 °C) > 90% | ||||||
| Zn, Cu | (200~600 °C) > 90% | ||||||
| Fe, Cu | (200~625 °C) > 90% | ||||||
| Nb, Cu | (200~625 °C) > 90% | ||||||
| SAPO-34 (CHA) | Cu | (300~670 °C) > 90% | Good | Good hydrothermal stability, mild acidity suppresses NH3 oxidation | Activity decline at very high temperatures, complex synthesis control | Medium-High | Medium-High (A viable alternative to SSZ-13) |
| Fe, Cu | (250~600 °C) > 90% | Good | |||||
| SSZ-16 (AFX) | Cu | (300~670 °C) > 90% | Good | Ultra-wide temperature window, high N2 selectivity | Expensive template, potential framework collapse at very high temperature | High | Medium (Potential for ultra-wide temperature scenarios) |
| UZM-9 (LTA) | Cu | (250~700 °C) > 90% | Good | Broad activity window, seed-assisted synthesis possible | Increased N2O byproduct formation at high Cu loadings | Medium | Medium (require solving N2O selectivity issue) |
| SAPO-18 (AEI) | Ce, Cu | (200~600 °C) > 90% | Good | High N2 selectivity | Lower Brønsted acidity than SAPO-34 | Medium-High | Medium-High (require solving low-cost synthetic routes issue) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, X.; Bian, X.; Bai, Y.; Zha, M.; Huang, Y.; Wei, J. Research Progress on Zeolite-Type High-Temperature NH3-SCR Catalysts. Catalysts 2025, 15, 1060. https://doi.org/10.3390/catal15111060
Mu X, Bian X, Bai Y, Zha M, Huang Y, Wei J. Research Progress on Zeolite-Type High-Temperature NH3-SCR Catalysts. Catalysts. 2025; 15(11):1060. https://doi.org/10.3390/catal15111060
Chicago/Turabian StyleMu, Xuewen, Xue Bian, Yuting Bai, Meng Zha, Yu Huang, and Jing Wei. 2025. "Research Progress on Zeolite-Type High-Temperature NH3-SCR Catalysts" Catalysts 15, no. 11: 1060. https://doi.org/10.3390/catal15111060
APA StyleMu, X., Bian, X., Bai, Y., Zha, M., Huang, Y., & Wei, J. (2025). Research Progress on Zeolite-Type High-Temperature NH3-SCR Catalysts. Catalysts, 15(11), 1060. https://doi.org/10.3390/catal15111060







