Impact of Macroporosity on the Transesterification of Triglycerides over MgO/SBA-15
Abstract
1. Introduction
2. Results and Discussion
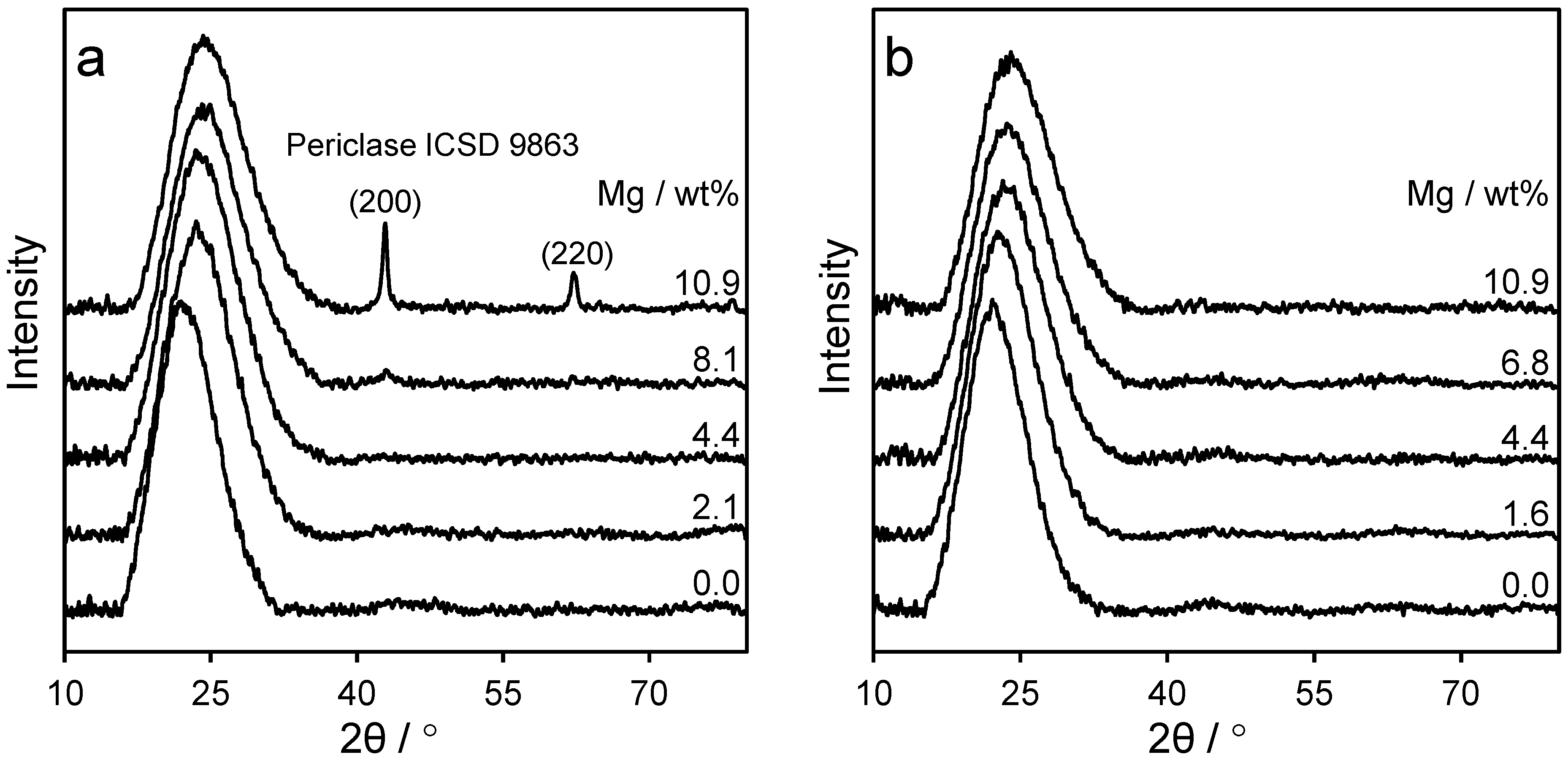
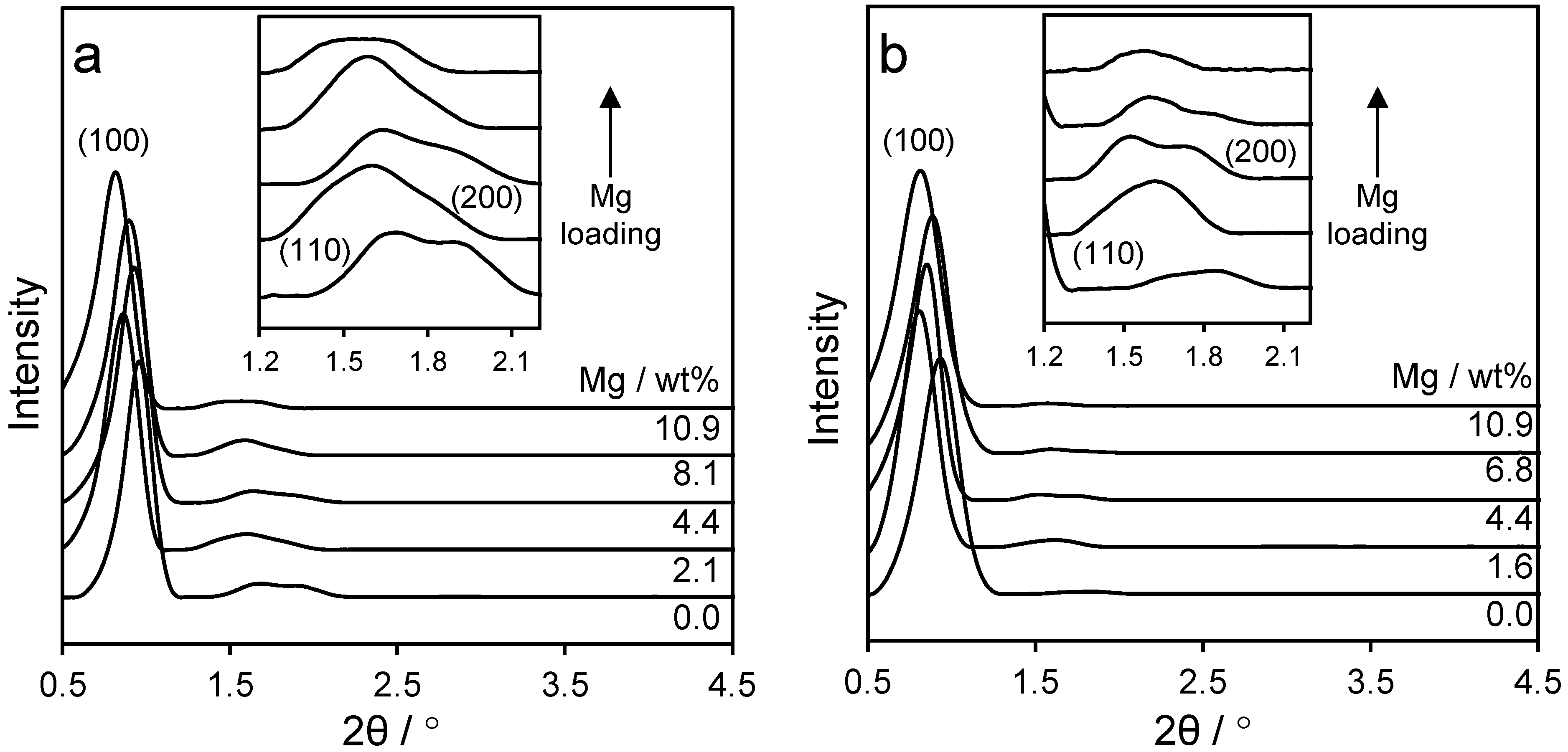
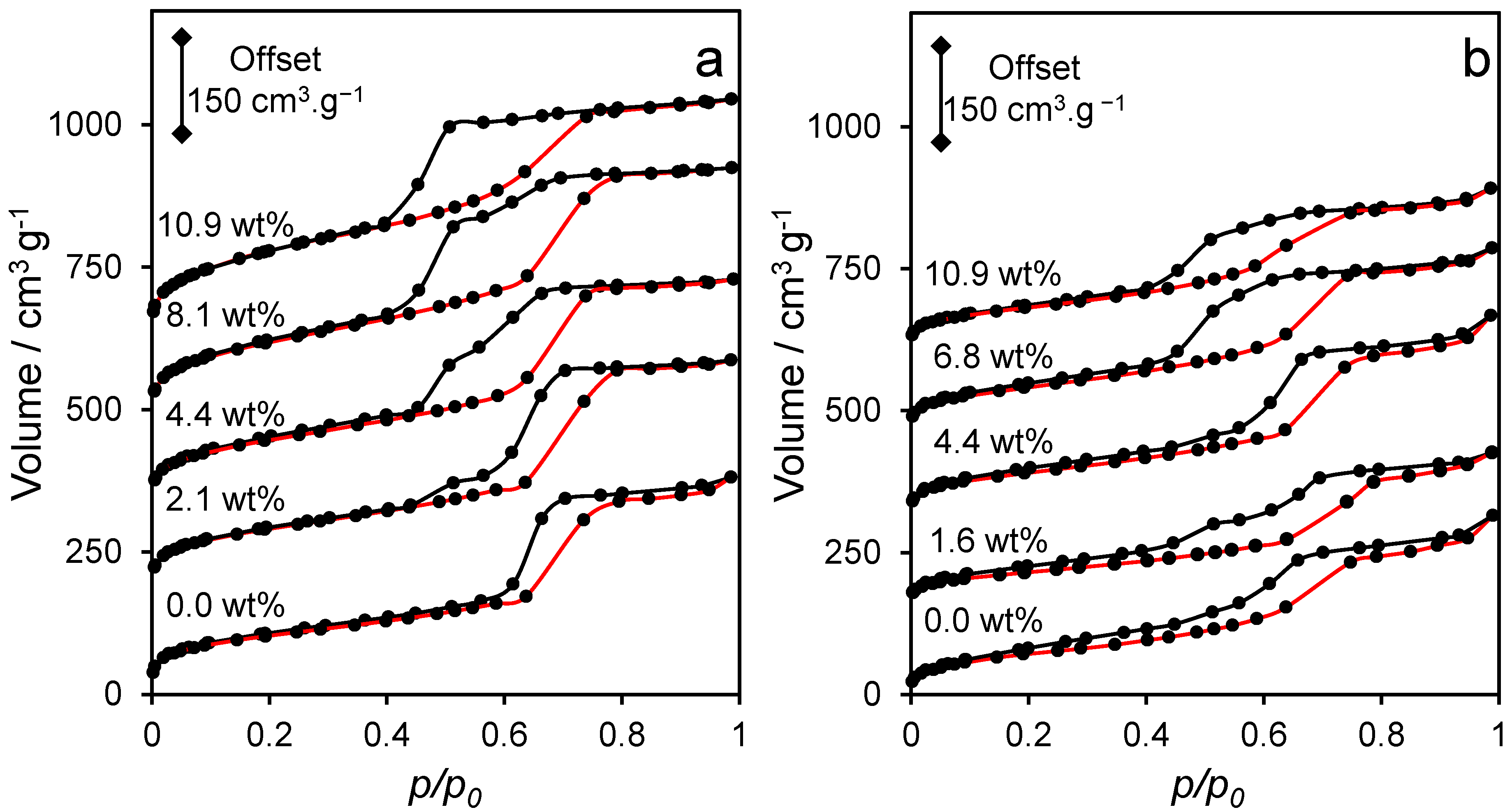
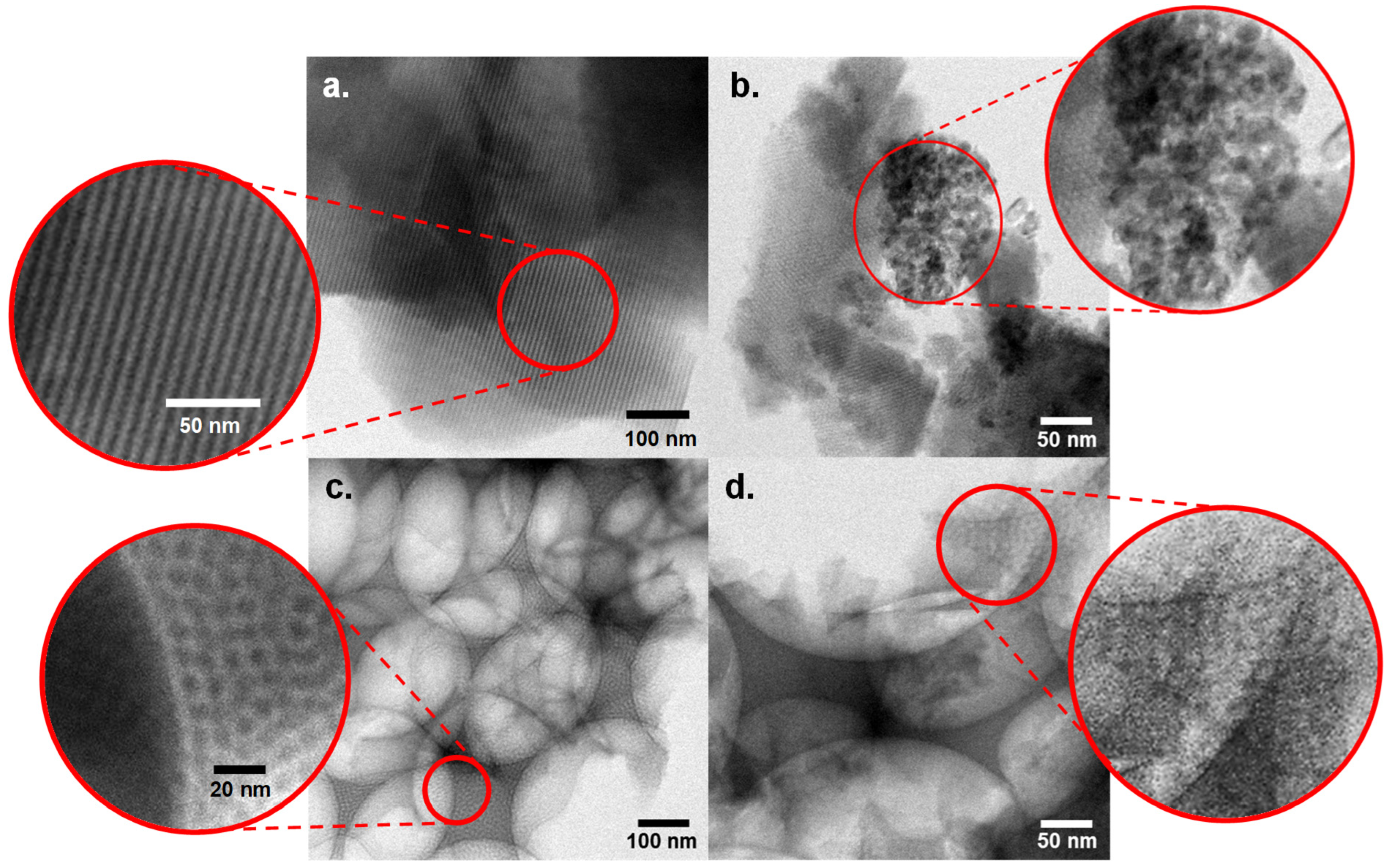
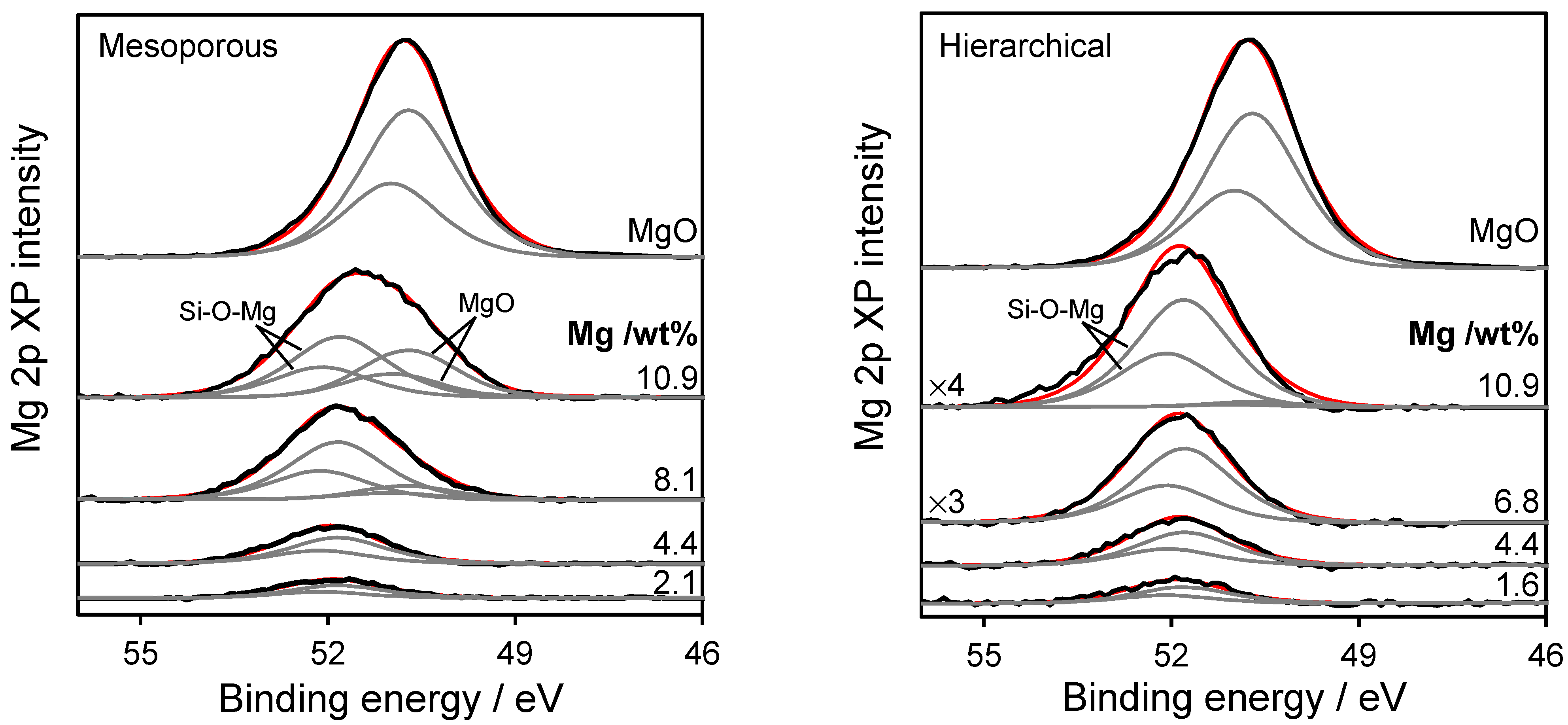
2.1. Catalyst Characterisation
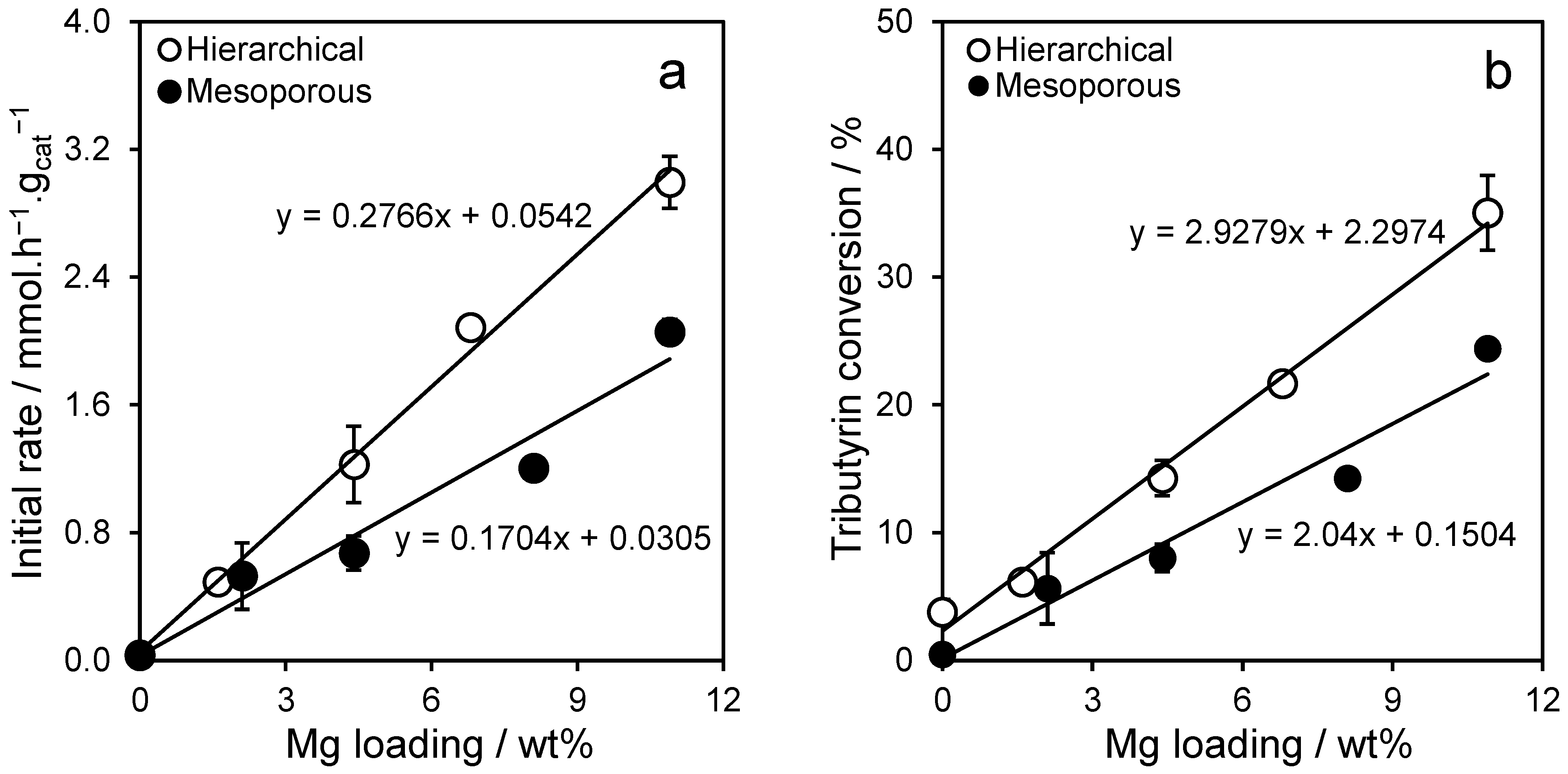
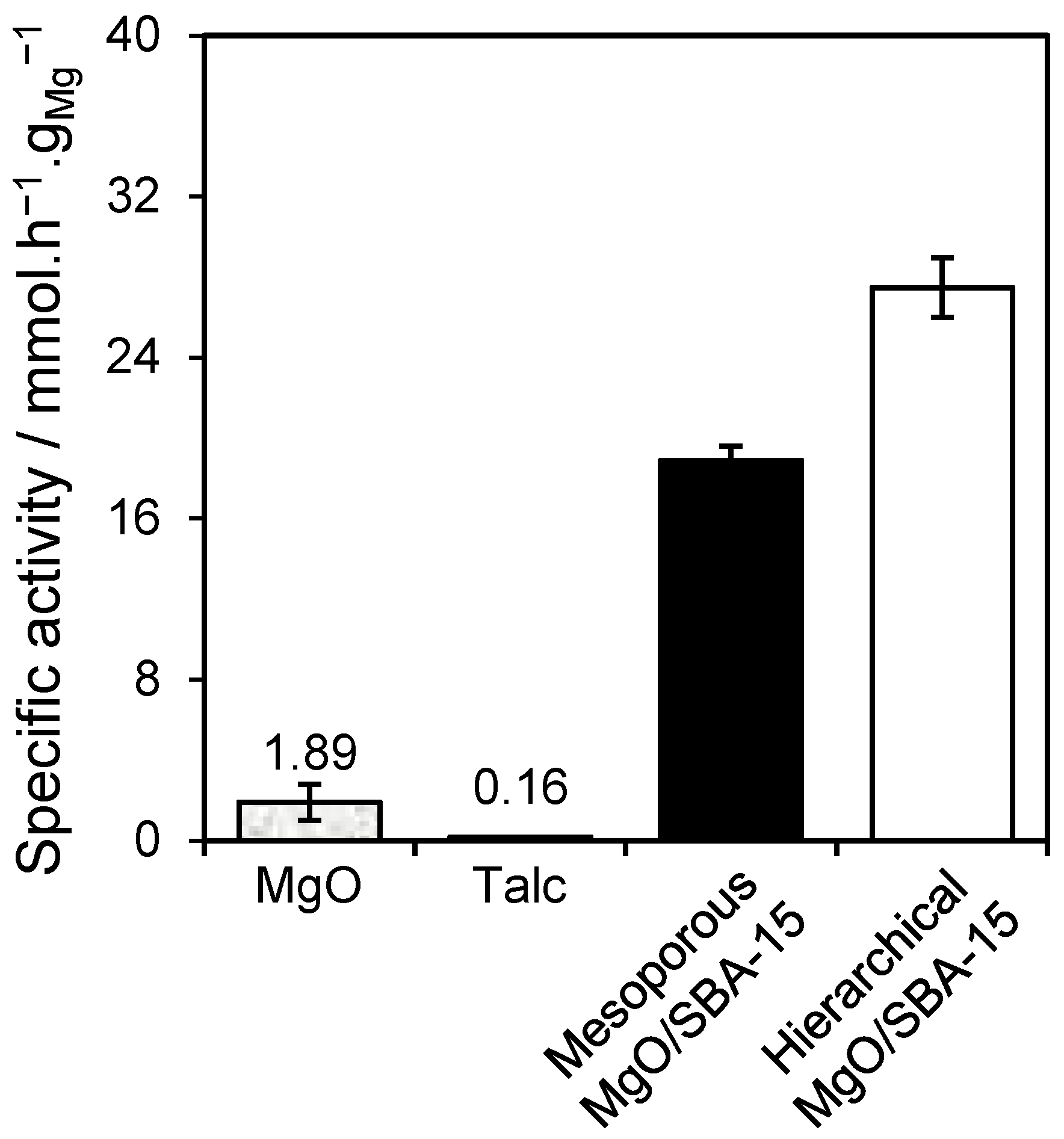
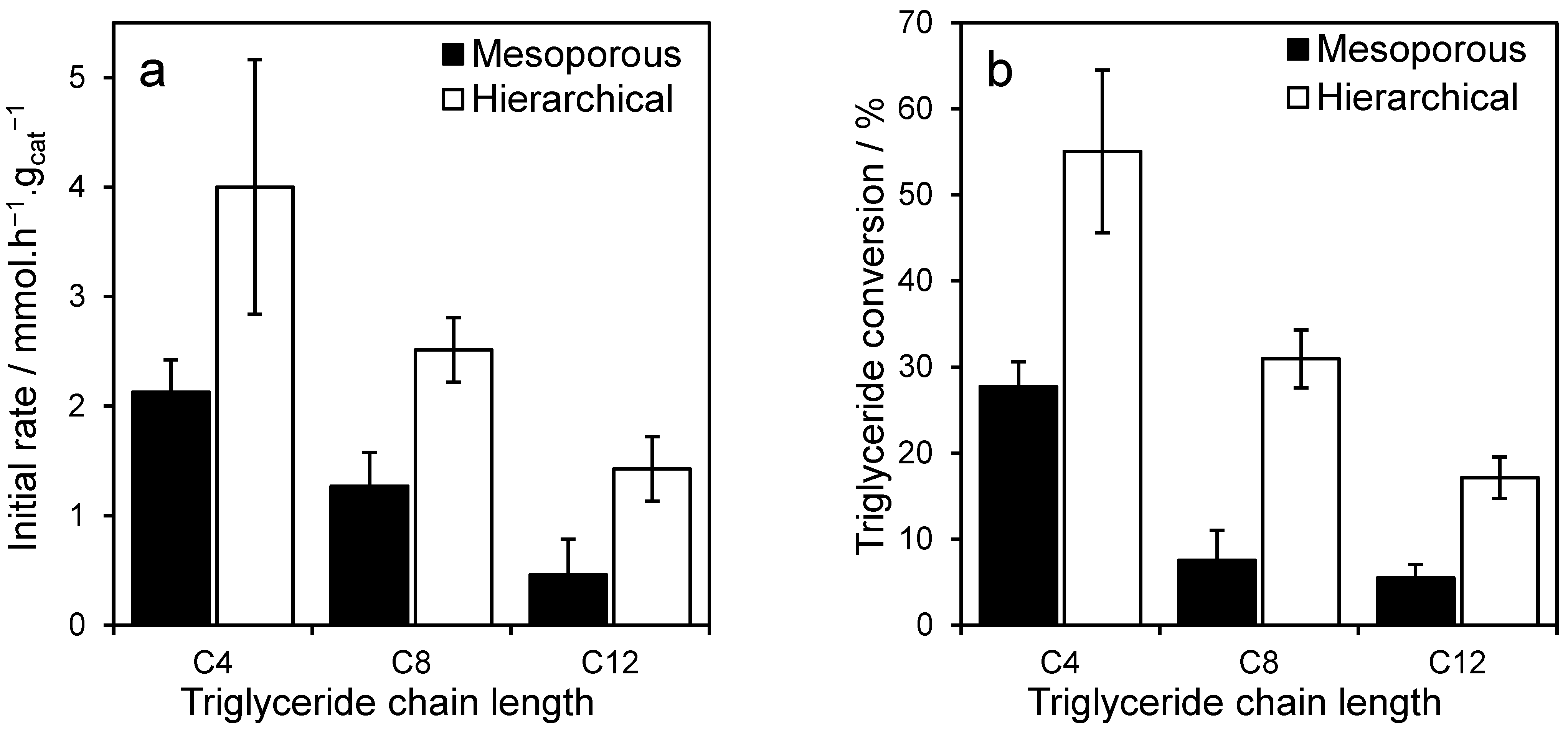
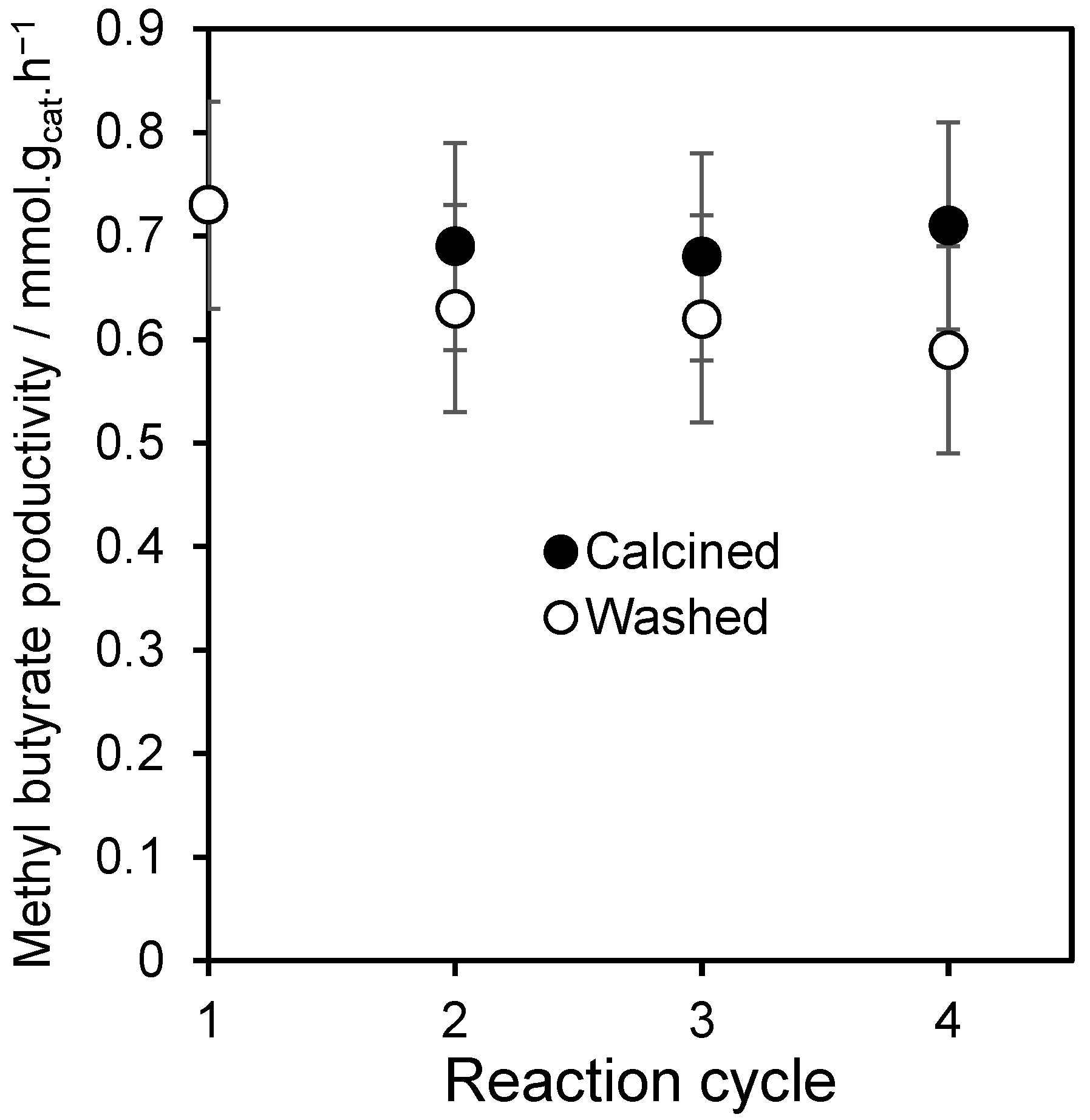
2.2. Triglyceride Transesterification
3. Materials and Methods
3.1. Synthesis of MgO-Functionalised SBA-15
3.2. Synthesis of Polystyrene Nanospheres
3.3. Synthesis of Hierarchical MgO/SBA-15
3.4. Material Characterisation
3.5. Triglyceride Transesterification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jaramillo, P.; Ribeiro, S.K.; Newman, P.; Dhar, S.; Diemuodeke, O.E.; Kajino, T.; Lee, D.S.; Nugroho, S.B.; Ou, X.; Strømman, A.H.; et al. Chapter 10 Transport. In IPCC 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Istrate, R.; Mas-Fons, A.; Beylot, A.; Northey, S.; Vaidya, K.; Sonnemann, G.; Kleijn, R.; Steubing, B. Decarbonizing lithium-ion battery primary raw materials supply chain. Joule 2024, 8, 2992–3016. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Christopher Selvam, D.; Raja, T.; Nagappan, B.; Upadhye, V.J.; Guntaj, J.; Devarajan, Y.; Mishra, R. The role of biodiesel in marine decarbonization: Technological innovations and ocean engineering challenges. Results Eng. 2025, 25, 103974. [Google Scholar] [CrossRef]
- Henriksen, Ø.S.P.E. Biofuels in Shipping Current Market and Guidance on Use and Reporting; DNV: Høvik, Norway, 2025; Available online: https://www.dnv.com/maritime/publications/biofuels-in-shipping-white-paper-2025-download/ (accessed on 19 October 2024).
- Mohammadi, H.; Saddler, J. Biofuel policies used by IEA Bioenergy Task 39 countries: The transition to using the carbon intensity (CI) of biofuels to set targets. Biofuels Bioprod. Biorefining 2025, 19, 929–941. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Rational design of heterogeneous catalysts for biodiesel synthesis. Catal. Sci. Technol. 2012, 2, 884. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. Chemsuschem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Felizardo, P.; Neiva Correia, M.J.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef]
- Larimi, A.; Harvey, A.P.; Phan, A.N.; Beshtar, M.; Wilson, K.; Lee, A.F. Aspects of Reaction Engineering for Biodiesel Production. Catalysts 2024, 14, 701. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- MacLeod, C.S.; Harvey, A.P.; Lee, A.F.; Wilson, K. Evaluation of the activity and stability of alkali-doped metal oxide catalysts for application to an intensified method of biodiesel production. Chem. Eng. J. 2008, 135, 63–70. [Google Scholar] [CrossRef]
- Jothiramalingam, R.; Wang, M.K. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 48, 6162–6172. [Google Scholar] [CrossRef]
- Yang, G.; Yu, J. Advancements in Basic Zeolites for Biodiesel Production via Transesterification. Chemistry 2023, 5, 438–451. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Honda, H.; Kuribayashi, H.; Toda, T.; Fukumura, T.; Yonemoto, T. Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2007, 98, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Wilson, K.; Hardacre, C.; Lee, A.F.; Montero, J.M.; Shellard, L. The application of calcined natural dolomitic rock as a solid base catalyst in triglyceride transesterification for biodiesel synthesis. Green Chem. 2008, 10, 654–659. [Google Scholar] [CrossRef]
- Montero, J.M.; Brown, D.R.; Gai, P.L.; Lee, A.F.; Wilson, K. In situ studies of structure–reactivity relations in biodiesel synthesis over nanocrystalline MgO. Chem. Eng. J. 2010, 161, 332–339. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Gandia, L.M. Heterogenization of the biodiesel synthesis catalysis: CaO and novel calcium compounds as transesterification catalysts. Chem. Eng. Res. Des. 2014, 92, 1519–1530. [Google Scholar] [CrossRef]
- Kouzu, M.; Yamanaka, S.-Y.; Hidaka, J.-S.; Tsunomori, M. Heterogeneous catalysis of calcium oxide used for transesterification of soybean oil with refluxing methanol. Appl. Catal. A Gen. 2009, 355, 94–99. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Gedanken, A. SiO2 Beads Decorated with SrO Nanoparticles for Biodiesel Production from Waste Cooking Oil Using Microwave Irradiation. Energy Fuels 2016, 30, 3151–3160. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaria, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Granados, M.L.; Alonso, D.M.; Sádaba, I.; Mariscal, R.; Ocón, P. Leaching and homogeneous contribution in liquid phase reaction catalysed by solids: The case of triglycerides methanolysis using CaO. Appl. Catal. B Environ. 2009, 89, 265–272. [Google Scholar] [CrossRef]
- Kouzu, M.; Tsunomori, M.; Yamanaka, S.; Hidaka, J. Solid base catalysis of calcium oxide for a reaction to convert vegetable oil into biodiesel. Adv. Powder Technol. 2010, 21, 488–494. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Alaei, S.; Haghighi, M.; Toghiani, J.; Vahid, B.R. Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: Influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crops Prod. 2018, 117, 322–332. [Google Scholar] [CrossRef]
- Montero, J.M.; Gai, P.; Wilson, K.; Lee, A.F. Structure-sensitive biodiesel synthesis over MgO nanocrystals. Green Chem. 2009, 11, 265–268. [Google Scholar] [CrossRef]
- Verziu, M.; Cojocaru, B.; Hu, J.; Richards, R.; Ciuculescu, C.; Filip, P.; Parvulescu, V.I. Sunflower and rapeseed oil transesterification to biodiesel over different nanocrystalline MgO catalysts. Green Chem. 2008, 10, 373–381. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.J.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Lee, A.F.; Wilson, K. Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; Crocker, M., Ed.; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Carrero, A.; Vicente, G.; Rodriguez, R.; Linares, M.; del Peso, G.L. Hierarchical zeolites as catalysts for biodiesel production from Nannochloropsis microalga oil. Catal. Today 2011, 167, 148–153. [Google Scholar] [CrossRef]
- Co, C.E.T.; Tan, M.C.; Diamante, J.A.R.; Yan, L.R.C.; Tan, R.R.; Razon, L.F. Internal mass-transfer limitations on the transesterification of coconut oil using an anionic ion exchange resin in a packed bed reactor. Catal. Today 2011, 174, 54–58. [Google Scholar] [CrossRef]
- Garg, S.; Soni, K.; Kumaran, G.M.; Bal, R.; Gora-Marek, K.; Gupta, J.K.; Sharma, L.D.; Dhar, G.M. Acidity and catalytic activities of sulfated zirconia inside SBA-15. Catal. Today 2009, 141, 125–129. [Google Scholar]
- Mbaraka, I.K.; Radu, D.R.; Lin, V.S.Y.; Shanks, B.H. Organosulfonic acid-functionalized mesoporous silicas for the esterification of fatty acid. J. Catal. 2003, 219, 329–336. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Melero, J.A.; Bautista, L.F.; Morales, G.; Iglesias, J.; Briones, D. Biodiesel Production with Heterogeneous Sulfonic Acid-Functionalized Mesostructured Catalysts. Energy Fuels 2009, 23, 539–547. [Google Scholar] [CrossRef]
- Li, E.; Rudolph, V. Transesterification of Vegetable Oil to Biodiesel over MgO-Functionalized Mesoporous Catalysts. Energy Fuels 2008, 22, 145–149. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Lee, A.F.; Pirez, C.; Wilson, K. Pore-expanded SBA-15 sulfonic acid silicas for biodiesel synthesis. Chem. Commun. 2012, 48, 212–214. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Dhainaut, J.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Hierarchical macroporous–mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Siles-Quesada, S.; Parlett, C.M.A.; Lamb, A.C.; Manayil, J.C.; Liu, Y.; Mensah, J.; Arandiyan, H.; Wilson, K.; Lee, A.F. Synthesis and catalytic advantage of a hierarchical ordered macroporous KIT-6 silica. Mater. Today Chem. 2023, 30, 101574. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Robinson, N.; Barbero, B.; Durndell, L.J.; Manayil, J.C.; Parlett, C.M.A.; D’Agostino, C.; Wilson, K.; Lee, A.F. Unravelling mass transport in hierarchically porous catalysts. J. Mater. Chem. A 2019, 7, 11814–11825. [Google Scholar] [CrossRef]
- Woodford, J.J.; Dacquin, J.-P.; Wilson, K.; Lee, A.F. Better by design: Nanoengineered macroporous hydrotalcites for enhanced catalytic biodiesel production. Energy Environ. Sci. 2012, 5, 6145–6150. [Google Scholar] [CrossRef]
- Creasey, J.J.; Parlett, C.M.A.; Manayil, J.C.; Isaacs, M.A.; Wilson, K.; Lee, A.F. Facile route to conformal hydrotalcite coatings over complex architectures: A hierarchically ordered nanoporous base catalyst for FAME production. Green Chem. 2015, 17, 2398–2405. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Jiang, Q.; Wang, Y.M.; Wang, H.J.; Sun, L.B.; Shi, L.Y.; Xu, J.H.; Wang, Y.; Chun, Y.; Zhu, J.H. Generating Superbasic Sites on Mesoporous Silica SBA-15. Chem. Mater. 2006, 18, 4600–4608. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Parlett, C.M.A.; Robinson, N.; Durndell, L.J.; Manayil, J.C.; Beaumont, S.K.; Jiang, S.; Hondow, N.S.; Lamb, A.C.; Jampaiah, D.; et al. A spatially orthogonal hierarchically porous acid–base catalyst for cascade and antagonistic reactions. Nat. Catal. 2020, 3, 921–931. [Google Scholar] [CrossRef]
- Lazdovica, K.; Kampars, V.; Gaile, A. Biodiesel production through the transesterification of rapeseed oil over CaO-MgO/SBA-15 catalysts. Environ. Prog. Sustain. Energy 2024, 43, e14273. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wu, Z.Y.; Wei, Y.L.; Zhu, J.H. In situ coating metal oxide on SBA-15 in one-pot synthesis. Microporous Mesoporous Mater. 2005, 84, 127–136. [Google Scholar] [CrossRef]
- Wei, Y.L.; Wang, Y.M.; Zhu, J.H.; Wu, Z.Y. In-Situ Coating of SBA-15 with MgO: Direct Synthesis of Mesoporous Solid Bases from Strong Acidic Systems. Adv. Mater. 2003, 15, 1943–1945. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; He, Y.; Yuan, Q.; Li, X.; Lu, G.; Zhang, T. The humidity-sensitive property of MgO-SBA-15 composites in one-pot synthesis. Sens. Actuators B Chem. 2010, 145, 386–393. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Wainwright, S.G.; Parlett, C.M.A.; Blackley, R.A.; Zhou, W.Z.; Lee, A.F.; Wilson, K.; Bruce, D.W. True liquid crystal templating of SBA-15 with reduced microporosity. Microporous Mesoporous Mater. 2013, 172, 112–117. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Isaacs, M.A.; Beaumont, S.K.; Bingham, L.M.; Hondow, N.S.; Wilson, K.; Lee, A.F. Spatially orthogonal chemical functionalization of a hierarchical pore network for catalytic cascade reactions. Nat. Mater. 2016, 15, 178–182. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase Diagrams and Aggregation Behavior of Poly(oxyethylene)-Poly(oxypropylene)-Poly(oxyethylene) Triblock Copolymers in Aqueous Solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. New J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Khouchaf, L.; Boulahya, K.; Das, P.P.; Nicolopoulos, S.; Kis, V.K.; Lábár, J.L. Study of the Microstructure of Amorphous Silica Nanostructures Using High-Resolution Electron Microscopy, Electron Energy Loss Spectroscopy, X-ray Powder Diffraction, and Electron Pair Distribution Function. Materials 2020, 13, 4393. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- O’Connell, K.; Regalbuto, J.R. High Sensitivity Silicon Slit Detectors for 1 nm Powder XRD Size Detection Limit. Catal. Lett. 2015, 145, 777–783. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; Garcia-Martinez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Parlett, C.M.A.; Keshwalla, P.; Wainwright, S.G.; Bruce, D.W.; Hondow, N.S.; Wilson, K.; Lee, A.F. Hierarchically Ordered Nanoporous Pd/SBA-15 Catalyst for the Aerobic Selective Oxidation of Sterically Challenging Allylic Alcohols. ACS Catal. 2013, 3, 2122–2129. [Google Scholar] [CrossRef]
- Putz, F.; Waag, A.; Balzer, C.; Braxmeier, S.; Elsaesser, M.S.; Ludescher, L.; Paris, O.; Malfait, W.J.; Reichenauer, G.; Hüsing, N. The influence of drying and calcination on surface chemistry, pore structure and mechanical properties of hierarchically organized porous silica monoliths. Microporous Mesoporous Mater. 2019, 288, 109578. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.; Cox, C.; Bosman, M.; Qian, X.; Li, N.; Zhao, H.; Du, Y.; Li, J.; Nocera, D.G. Room temperature stable COx-free H2 production from methanol with magnesium oxide nanophotocatalysts. Sci. Adv. 2016, 2, e1501425. [Google Scholar] [CrossRef]
- Seal, S.; Krezoski, S.; Hardcastle, S.E.; Barr, T.L.; Petering, D.H.; Cheng, C.F.; Klinowski, J.; Evans, P.H. Investigations of the surface chemistry of pathogenic silicates. J. Vac. Sci. Technol. A 1995, 13, 1260–1266. [Google Scholar] [CrossRef]
- Bortz, M.; Ohuchi, F.S. An x-ray photoelectron spectroscopy study of the interfacial relations between titanium and cordierite-based ceramic thin films. J. Appl. Phys. 1988, 64, 2054–2058. [Google Scholar] [CrossRef]
- Jin, Y.S.; Yan, Q.J.; Yin, Z.R.; Chen, Y. Secondary ion mass spectrometry and X-ray photoelectron spectroscopy of Na2MoO4/SiO2 catalysts for methane oxidative coupling. J. Chem. Soc. Faraday Trans. 1995, 91, 381–384. [Google Scholar] [CrossRef]
- Seal, S.; Krezoski, S.; Barr, T.L.; Petering, D.H.; Klinowski, J.; Evans, P.H. Surface chemistry and biological pathogenicity of silicates: An X-ray photoelectron spectro-scopic study. Proc. R. Soc. B Biol. Sci. 1996, 263, 943–951. [Google Scholar]
- Paparazzo, E. XPS analysis of oxides. Surf. Interface Anal. 1988, 12, 115–118. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Bonding-state characterization of the constitutent elements of silicate minerals by X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1985, 81, 485–495. [Google Scholar] [CrossRef]
- Haider, N.C.; Alonso, J., Jr.; Swartz, W.E., Jr. Valence and Core Electron Spectra of Mg in MgO in Evoporated Thin Films. Z. Für Naturforschung A 1975, 30a. [Google Scholar]
- Schüth, F.; Ward, M.D.; Buriak, J.M. Common Pitfalls of Catalysis Manuscripts Submitted to Chemistry of Materials. Chem. Mater. 2018, 30, 3599–3600. [Google Scholar] [CrossRef]
- Jurado, E.; Camacho, F.; Luzón, G.; Fernández-Serrano, M.; García-Román, M. Kinetic model for the enzymatic hydrolysis of tributyrin in O/W emulsions. Chem. Eng. Sci. 2006, 61, 5010–5020. [Google Scholar] [CrossRef]
- Jeong, J.; Cho, K.; Kim, S.H.; Lee, D.; Park, J.-I.; Bae, D.; Cho, K.; Kim, J.-C. Zeolite beta nanosponge with high acidity on mesopores for efficient tributyrin transesterification. Fuel 2025, 402, 135965. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renew. Energy 2009, 34, 766–768. [Google Scholar] [CrossRef]
- Jiang, W.; Niu, X.; Yuan, F.; Zhu, Y.; Fu, H. Preparation of KF–La2O2CO3 solid base catalysts and their excellent catalytic activities for transesterification of tributyrin with methanol. Catal. Sci. Technol. 2014, 4, 2957–2968. [Google Scholar] [CrossRef]
- Hsieh, L.-S.; Kumar, U.; Wu, J.C.S. Continuous production of biodiesel in a packed-bed reactor using shell–core structural Ca(C3H7O3)2/CaCO3 catalyst. Chem. Eng. J. 2010, 158, 250–256. [Google Scholar] [CrossRef]
- Chantrasa, A.; Phlernjai, N.; Goodwin, J.G. Kinetics of hydrotalcite catalyzed transesterification of tricaprylin and methanol for biodiesel synthesis. Chem. Eng. J. 2011, 168, 333–340. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Davison, T.J.; Okoli, C.; Wilson, K.; Lee, A.F.; Harvey, A.; Woodford, J.; Sadhukhan, J. Multiscale modelling of heterogeneously catalysed transesterification reaction process: An overview. RSC Adv. 2013, 3, 6226–6240. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Xiao, G.; Lv, J. Kinetics of the transesterification reaction catalyzed by solid base in a fixed-bed reactor. Energy Fuels 2010, 24, 5829–5833. [Google Scholar] [CrossRef]
- Ilgen, O. Reaction kinetics of dolomite catalyzed transesterification of canola oil and methanol. Fuel Process. Technol. 2012, 95, 62–66. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.-F.; Marin, G.B. Kinetics of heterogeneously MgO-catalyzed transesterification. Appl. Catal. B Environ. 2006, 62, 35–45. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Yang, H.; Meng, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding Effect of Wall Structure on the Hydrothermal Stability of Mesostructured Silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, Y.-T.; Lee, J.-J.; Cheng, S. Tuning pore diameter of platelet SBA-15 materials with short mesochannels for enzyme adsorption. J. Mater. Chem. 2011, 21, 5693–5703. [Google Scholar] [CrossRef]
- Sen, T.; Tiddy, G.J.T.; Casci, J.L.; Anderson, M.W. Synthesis and Characterization of Hierarchically Ordered Porous Silica Materials. Chem. Mater. 2004, 16, 2044–2054. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Creasey, J.J.; Chieregato, A.; Manayil, J.C.; Parlett, C.M.A.; Wilson, K.; Lee, A.F. Alkali- and nitrate-free synthesis of highly active Mg–Al hydrotalcite-coated alumina for FAME production. Catal. Sci. Technol. 2014, 4, 861–870. [Google Scholar] [CrossRef]
- Martyanov, I.N.; Sayari, A. Comparative study of triglyceride transesterification in the presence of catalytic amounts of sodium, magnesium, and calcium methoxides. Appl. Catal. A Gen. 2008, 339, 45–52. [Google Scholar] [CrossRef]
- Rabee, A.I.M.; Manayil, J.C.; Isaacs, M.A.; Parlett, C.M.A.; Durndell, L.J.; Zaki, M.I.; Lee, A.F.; Wilson, K. On the Impact of the Preparation Method on the Surface Basicity of Mg–Zr Mixed Oxide Catalysts for Tributyrin Transesterification. Catalysts 2018, 8, 228. [Google Scholar] [CrossRef]
- Kozlowski, J.T.; Aronson, M.T.; Davis, R.J. Transesterification of tributyrin with methanol over basic Mg:Zr mixed oxide catalysts. Appl. Catal. B Environ. 2010, 96, 508–515. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, L.; Fan, G.; Li, F. Enhanced base-catalyzed activity and structural stability of nitrogen-doped carbon modified MgO–MgFe2O4 magnetic composites as catalysts for transesterification of tributyrin. Dalton Trans. 2017, 46, 6324–6332. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T.; Bawaked, S.; Basahel, S. Effect of Si precursor on structural and catalytic properties of nanosize magnesium silicates. Appl. Catal. A Gen. 2014, 488, 208–218. [Google Scholar] [CrossRef]

| Mesoporous MgO/SBA-15 | Hierarchical MgO/SBA-15 | ||||||
|---|---|---|---|---|---|---|---|
| Mg Content a /wt% | Surface Area b /m2·g−1 | Pore Size c /nm | Mg Content a /wt% | Surface Area b /m2·g−1 | Pore Size c /nm | ||
| Total | Micropore | Total | Micropore | ||||
| 0.0 | 370 ± 37 | 72 ± 7 | 5.2 | 0.0 | 265 ± 27 | 0 | 5.0 |
| 2.1 | 502 ± 50 | 142 ± 14 | 5.2 | 1.6 | 232 ± 23 | 0 | 5.0 |
| 4.4 | 525 ± 53 | 126 ± 13 | 4.5 | 4.4 | 322 ± 32 | 14 ± 1 | 4.9 |
| 8.1 | 604 ± 60 | 130 ± 13 | 4.0 | 6.8 | 327 ± 33 | 0 | 4.2 |
| 10.9 | 650 ± 65 | 143 ± 14 | 3.9 | 10.9 | 294 ± 29 | 0 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, T.A.; Damptey, L.; Isaacs, M.A.; Parlett, C.M.A.; Durndell, L.J.; Granollers Mesa, M.; Kyriakou, G.; Wilson, K.; Lee, A.F. Impact of Macroporosity on the Transesterification of Triglycerides over MgO/SBA-15. Catalysts 2025, 15, 1054. https://doi.org/10.3390/catal15111054
Bryant TA, Damptey L, Isaacs MA, Parlett CMA, Durndell LJ, Granollers Mesa M, Kyriakou G, Wilson K, Lee AF. Impact of Macroporosity on the Transesterification of Triglycerides over MgO/SBA-15. Catalysts. 2025; 15(11):1054. https://doi.org/10.3390/catal15111054
Chicago/Turabian StyleBryant, Thomas A., Lois Damptey, Mark A. Isaacs, Christopher M. A. Parlett, Lee J. Durndell, Marta Granollers Mesa, Georgios Kyriakou, Karen Wilson, and Adam F. Lee. 2025. "Impact of Macroporosity on the Transesterification of Triglycerides over MgO/SBA-15" Catalysts 15, no. 11: 1054. https://doi.org/10.3390/catal15111054
APA StyleBryant, T. A., Damptey, L., Isaacs, M. A., Parlett, C. M. A., Durndell, L. J., Granollers Mesa, M., Kyriakou, G., Wilson, K., & Lee, A. F. (2025). Impact of Macroporosity on the Transesterification of Triglycerides over MgO/SBA-15. Catalysts, 15(11), 1054. https://doi.org/10.3390/catal15111054








