Abstract
Selective oxidation of benzyl alcohol to benzaldehyde is crucial for sustainable chemical synthesis, which provides the atom-economical and environmentally benign pathways. In this work, we used the in situ reduction immobilization to synthesize a series of Au nanoparticles supported by CoAlOx support with spinel structure for alkali-free oxidation of benzyl alcohol. The synthesis methodology was preliminarily optimized and the influence of Co/Al molar ratio in Au/CoAlOx on the catalytic performances was subsequently revealed based on characterizations. Results suggested that the electronic interaction between Au and CoAlOx can be regulated and maximized under the Co/Al ratio of 3. It became a main factor to modulate the dispersion of Au nanoparticles, surface chemical composition, as well as the oxygen adsorption/activation ability. Benefiting from such synergistic interaction, the optimized Au/Co3AlOx catalyst achieved 86.1% BnOH conversion under 99.9% benzaldehyde selectivity with well-maintained structural stability under recycle tests. This work provides a rational design strategy for developing highly efficient gold catalysts with well-constructed Au-support interfaces for the alkali-free oxidation of alcohol.
1. Introduction
Benzaldehyde is one of the most widely used platform chemicals for high value-added downstream products. It is a key intermediate in the production of antibiotics, antifungal drugs and other organic compounds, which has a huge market demand for pharmaceutical synthesis [1]. Over recent decades, significant emphasis has been placed on advancing catalytic technologies for the aerobic oxidation of alcohols, driven by the growing demand for sustainable chemical processes [2]. Most of the research focus on the rational design of efficient catalytic systems to achieve high activity, selectivity, and stability under environmentally benign conditions [3,4,5]. Dong et al. [6] exploited liquid-phase oxidation of benzyl alcohol using molecular oxygen, representing an environmentally benign approach for sustainable chemical synthesis that aligns with green chemistry principles. Although alkaline additives (e.g., KOH, NaOH) enhance oxidation efficiency [7], their inevitable generation of salt-containing wastewater introduces environmental burdens that ultimately constrain the industrial viability. Consequently, recent efforts have focused on the efficient production of benzaldehyde in an alkali-free system [8,9]. The oxidation process of benzyl alcohol is closely related to the type of support, the design of interfaces between support and the d-valence orbital of precious metals.
In the past decades, research on gold catalysts for the selective oxidation of benzyl alcohol to benzaldehyde has primarily focused on catalyst structure modulation, support optimization, and reaction mechanism. Two typical strategies are commonly involved. Firstly, Au-based bimetallic catalysts have been most extensively investigated because of their high catalytic activity and selectivity [10,11]. Liu et al. [12] synthesized the AuRu/ZIF-67 by different synthesis methods. The addition of Ru to the Au catalyst reduced the oxidation activity of benzyl alcohol while considerably improving the yield of benzaldehyde. Another efficient strategy is to modulate the surface properties of the supporting materials. Secondary metal oxide was dispersed on the surface of supporting materials to promote the surface acidic sites and the oxygen vacancy. Reports suggested that TiO2-modified supports significantly enhance the selectivity of benzaldehyde of Au-Pd/TiO2-SiO2 by adjusting the number of surface Lewis acid sites [13], while excessive TiO2 leads to performance degradation due to the formation of Brønsted acid sites. Nakayama et al. [14] synthesized the MgAlOx nanocrystals modified Au/CeO2. Results suggested that the sheared dimension of MgAlOx benefited the dispersion of Au particles and provided basic surface OH groups for deprotonation of alcohols. Both ideas facilitate the construction of new Au–metal (oxide) interfaces, where the adsorption and activation balance between benzyl alcohol and oxygen can be optimized.
Spinel oxide (AB2O4) features inherent stability and small crystallite sizes, enabling the spinel oxides by adjusting tetrahedral-to-octahedral coordination ratios or metal element compositions [15]. Synergistic effects between support and adjacent Au sites can be modulated to optimize the reactant activation and the selective bond cleavage. Notably, Zhang et al. promoted the Au catalyst on spinel promoted with trace amounts of residual carbon in the skeleton [16]. It puts forward the influence of surface carbon-containing functionalities on the deposition of metal centers and the diffusion effect of reactants. Appropriate modulation on the structure of support increased the surface area and decreased the overall work function of the catalyst with an accelerated charge transfer between Au and the adsorbate. Paul et al. [17] also demonstrated the developed BiVO4 spinel with abundant oxygen vacancies and porosity by virtue of calcination. As it was discussed their work, the defected spinel structure is stable, which displayed high adsorption energy efficient charge transferability with reactant. Yang et al. also illustrated the easier modification of CuMn2O4 spinel by either acid or alkali treatment, that oxygen activation engineering could be tuned in terms of the surface Mn3+/Mn4+ ratrio [18]. Our previous work designed the Au/MgAl-LDH catalysts with defective gold nanoparticles, which is regulated according to the preferential crystal plane constructed via the precise crystallization of the support [19]. The construction of Au–spinel interface is further emphasized. Under reciprocal contacting interface, its designed architecture demonstrated remarkable catalytic performance in base-free aerobic oxidation of benzyl alcohol, with a TOF reaching 1008 h−1.
Here in this work, a series of catalysts supported on spinel structure were synthesized and used for the aerobic catalytic oxidation of benzyl alcohol. In order to explore the relationship between the structure of gold nanocatalysts and the oxidative catalytic performance of benzyl alcohol, characterizations including Thermogravimetry/Derivative Thermogravimetry (TG/DTG), X-ray diffraction (XRD), N2 adsorption-desorption, X-ray photo electronic spectroscopy (XPS) and O2-temperature programmed desorption (O2-TPD) were applied to study the structure and chemical properties of the catalysts. Gold catalysts can be activated and utilized under alkali-free conditions to obtain a high yield of benzaldehyde, and after consecutive cycle reactions, the catalyst is basically stable without deactivation on activity or structure change.
2. Results and Discussion
2.1. Fast Selection for Catalyst Design and Synthesis
In this work, a series of Au-based catalysts were synthesized. Layered double hydroxides (Co3AlOx-LDH) were prepared as the precursor of the supporting materials. Before the modulation of the catalyst, the synthesis details were studied and optimized in terms of the thermal treatment and deposition procedure. After the deposition of gold nanoparticles and thermal calcination, Co3AlOx is likely to be formed depending the detailed temperature during thermal treatment, which has an important influence on the dispersion of metal species and the Au–support interaction. Herein, the TG/DTG curves of the Au/Co3Al-LDH precursor is preliminarily analyzed in Figure 1 to understand the possible phase transition during calcination. Several continuous steps of weight loss are observed. The first step at 120–185 °C is caused by the release of adsorbed water and the water molecules between the hydrotalcite layers, which gradually decomposes under increasing temperature. Intensified weight loss at 230–285 °C can be ascribed to the decomposition of carbonate and transformation of hydrotalcite in the catalyst precursors [20,21]. A subsequent weight loss happens at even higher temperature until 450 °C, which should be correlated with the decomposition of PVA bonding with the Au nanoparticles [22]. The TG/DTG curves suggest that most of the chemical composition can be decomposed at 300 °C. During this process, hydrotalcite is gradually transformed into spinel and the Au nanoparticles are stabilized and exposed on surface. The catalysts were designed for the selective hydrogenation of benzyl alcohol. The schematic illustration of the reaction is shown in Scheme S2. Benzaldehyde is the target product and benzene, benzoic acid and toluene could be produced under unqualified catalyst system. A simple test by calcination of the Au/Co3Al-LDH precursor at different temperature also confirmed the superiority by thermal treating the sample at 300 °C in Figure 1b and Table S1 (supporting information). The catalytic performances by catalysts going through different pre-treatments suggest that one-pot calcination is necessary to make efficient gold catalysts (Figure S1). Herein, the calcination temperature is fixed at 300 °C for subsequent study.
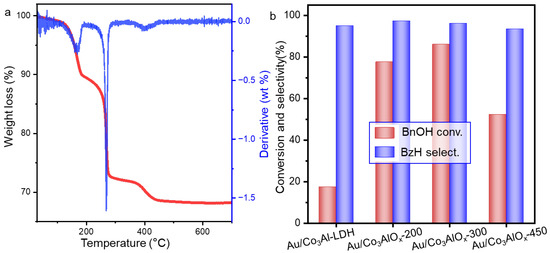
Figure 1.
(a) TG/DTG curves of Au/Co3Al-LDH catalyst, and (b) Catalytic performances for alkali-free oxidation of BnOH by Au/Co3Al-LDH precursor and Au/Co3AlOx catalysts calcined at different temperature. Reaction condition: O2 flow rate of 20 mL/min, atmospheric pressure, 80 °C, 4 h, nBnOH/nAu = 220.
The in situ reduction immobilization was used to synthesize the supported Au nanoparticles (Au NPs) as we previously reported [19]. Two additional references prepared by traditional impregnation and deposition-precipitation method are also tested for comparison in Figure 2a. Comparing with the Au/Co3AlOx by in situ reduction immobilization, the Au/Co3AlOx-I displays poor BnOH conversion of only 48.5% with 90.3% selectivity to BzH. The Au/Co3AlOx-D shows mean activity with 86.1% BnOH conversion and 99.9% BzH selectivity. The Au/Co3AlOx by in-situ reduction immobilization method displays greatly improved BnOH conversion of 86.1% with 89.3% selectivity of BzH. The XRD patterns in Figure 2b,c suggest the similar crystal phases by different synthesis methods. Characteristic peaks of the Co2AlO4 spinel phase with 2θ of 31.2°, 36.8°, 44.8°, 59.3° and 65.2° [23], corresponding to the Co2AlO4 (220), (311), (400), (511), (440) crystal planes. However, there are not any clear diffraction peaks of Au crystal phase, probably due to the overlapping or covering of the Co2AlO4 (311) with the Au (111) plane. The magnified XRD patterns in Figure 2c display more details that a shoulder peak becomes apparent in both the Au/Co3AlOx-I and Au/Co3AlOx-D, which should be ascribed to the overlapping Au (111) plane at 38.2°. The absence of shoulder peak in Au/Co3AlOx by reduction immobilization evidences the smaller particles with fine distribution than the other samples, which should be one of the reasons for the better performance of BnOH oxidation. According to the preliminary discussion of the catalysts with optimized synthesis method, a series of Au/ConAlOx catalysts with different Co/Al ratio are designed using the in-situ reduction immobilization method.
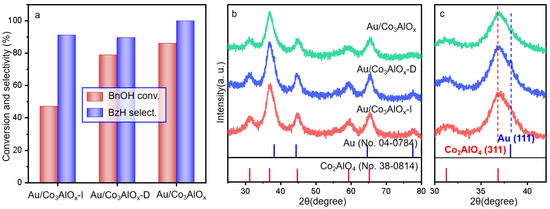
Figure 2.
(a) Catalytic performances by Au/Co3AlOx catalysts by different synthesis methods, (b) XRD patterns by different Au catalysts in the 2θ range of 25–80°, and (c) Magnified XRD patterns in the 2θ range of 30–45°. ‘-I’ and ‘-D’ in ‘b’ denoted the catalyst synthesized by impregnation and deposition-precipitation method. Reaction condition in ‘a’: O2 flow rate of 20 mL/min, atmospheric pressure, 80 °C, 4 h, nBnOH/nAu = 220.
2.2. Catalytic Performances of Au/ConAlOx Catalysts for Alcohol Oxidation
The Au/ConAlOx catalysts with different Co/Al ratio were subsequently used for the alkali-free oxidation reaction of benzyl alcohol as displayed in Figure 3 and Table S1. Two references by Au NPs supported on cobalt oxide and alumina were also tested for comparison. As shown in Figure S2, both the Au/CoOx and Au/Al2O3 displayed inferior catalytic performances compared with the Au particles supported by CoAlOx spinel phase. The conversion of benzyl alcohol (BnOH) is only 42.5% by Au/Al2O3. If the series of Au/ConAlOx is used, the transformation of BnOH is obviously promoted under the same reaction condition that a volcanic trend can be observed with the increasing Co/Al ratio. BnOH conversion increases from 32.5% by Au/Co1AlOx to a maximum of 86.1% by Au/Co3AlOx. The selectivity of benzaldehyde (BzH) is generally maintained in the range of 90.3–99.9%. An additional reference by Au loading on a physical mixture of CoOx and Al2O3 (Au/CoOx-Al2O3) is also carried out in Figure 3a, which displays the lower BnOH conversion of only 27.4%. The much higher BnOH conversion by the series of Au/ConAlOx with spinel phase indicates that the possible synergistic interaction between Au and the CoAlOx spinel phase should be significant for the activation and adsorption of BnOH during the reactions. It can be seen that the BnOH conversion is more significantly influenced by the Au catalysts modulated by the Co/Al ratio. It might result from the different chemical structure and surface distribution of Au active sites, for which we will give more evidence in the following characterization section.
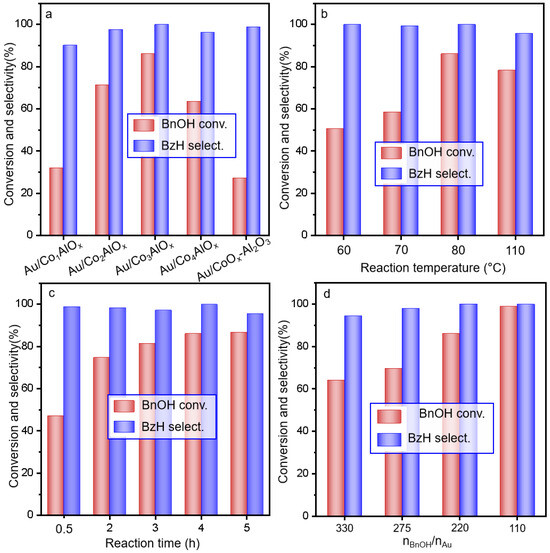
Figure 3.
(a) Catalytic performances for the selective oxidation of benzyl alcohol by Au/ConAlOx catalysts. (b) Effect of reaction temperature, (c) reaction time and (d) the molar ratio between BnOH and gold on the performances of Au/Co3AlOx catalyst. Reaction conditions: Oxygen flow rate at atmospheric pressure is 20 mL/min, atmospheric pressure, nBnOH/nAu = 220, 80 °C, 4 h, if not specified.
The effect of reaction temperature and reaction time are further optimized using the Au/Co3AlOx as a typical catalyst in Figure 3b–d and Table S2. At low temperature, the conversion of BnOH is only 50.3% at 60 °C, with a high BnOH selectivity of 99.9%. As the reaction temperature increases, the BnOH conversion exhibits an increasing trend from 60 to 80 °C, while the BzH selectivity remains nearly 100%. Further increasing the temperature, both the conversion of BnOH and the selectivity of BzH decrease, probably due to the by-products generated by condensation between reactants. Figure 3c reveals the influence of reaction time on the catalytic performances. The BnOH steadily increases with the prolonged reaction time and reaches the saturation at 3–4 h. The catalyst dosage also impacts the BnOH conversion in terms of the molar ratio between BnOH and catalyst (Figure 3d). The BnOH conversion reaches 64.5% under nBnOH/nAu of 330, which steadily increases with the nBnOH/nAu ratio. The BnOH conversion and the BzH selectivity reach 98.5% and 99.1% under nBnOH/nAu of 110.
2.3. Chemical Structure and Composition of Au/ConAlOx Catalysts
The chemical composition in the Au/ConAlOx catalysts were tested by Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) as listed in Table S3. The metal contents including the Au loading and the calculated Co/Al atomic ratio in typical samples are generally consistent with the predicted value during synthesis. The crystal structure of the series of Au/ConAlOx catalysts are first analyzed by the XRD patterns (Figure 4). Under higher proportion of Co2+ in the original synthesis, the diffraction peaks of Co2AlO4 spinel phase are clearly observed in the Au/ConAlOx (n = 2, 3, 4) at 31.2°, 36.8°, 44.8°, 59.3° and 65.2°, respectively. They can be ascribed to the Co2AlO4 (220), (311), (400), (511), (440) crystal planes. It suggests the well-crystallized phase in these catalysts caused by the occupation of tetrahedral sites by Co2+ in the spinel structure. However, as the Co/Al ratio decreases, the diffraction peaks of the Au/Co1AlOx become dispersive and broadened, suggesting the immature crystal phase generated under low Co/Al ratio. There is not any detectable shoulder peak at 38.2° resulted from the Au (111) crystal plane, indicating the small sizes of Au NPs formed in these catalysts by in situ reduction immobilization method.
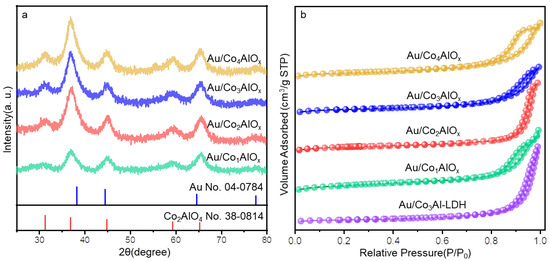
Figure 4.
(a) XRD patterns and (b) N2 adsorption-desorption by different Au/ConAlOx catalysts.
The morphology of Au/Co1AlOx and Au/Co3AlOx is displayed in Figure S3. Cloud-like surfaces can be observed in these samples. Compared with Au/Co3AlOx, the Au/Co1AlOx with lower Co/Al ratio is more heterogeneous with surface roughness. The specific surface area and pore size distribution of catalysts are measured by the N2 adsorption-desorption curves in Figures 4b and S4. All the tested samples exhibit typical IV isotherms with a pronounced H3-type hysteresis loop [24,25]. The pore size distribution calculated using the Barret-Joyner-Halenda (BJH) model is in the range of 2–14 nm as mesoporous adsorbent materials. The specific surface area of Au/Co3Al-LDH precursor before calcination is only 53 m2/g with the average pore size of 14.0 nm (Table S4). After calcination, the specific surface area of the Au/ConAlOx increases to 86–106 m2/g with the reduced Co/Al ratio. The specific surface area and pore volume of Au/Co3AlOx and Au/Co4AlOx reach the maximum of 102–106 m2/g and 0.38–0.39 cm3/g. The porous structure may further influence the distribution of Au NPs and the interacting synergy between Au and the supporting material [26].
The morphology of the catalysts is subsequently revealed by the Transmission electron microscope (TEM) images. At higher Co/Al ratio, the thin layer of Au/Co4AlOx is observed and should result from the original structure of the hydrotalcite. Au NPs are random and disordered dispersing on the surface of support with the average particle size of 6.2 nm (Figure S5). Lattice fringes of 2.44 and 2.86 Å belonging to the Co2AlO4 (311) and (220) crystal faces are observed surrounding the Au NPs in the Au/Co4AlOx. The morphology of Au/Co3AlOx is displayed in Figure 5. As the Co/Al decreases to 3, the average size of Au NPs is only 2.8 nm with uniform size distribution. Small Au NPs are highly dispersed on the thin edges of Co3AlOx. Au NPs are surrounded by Co2AlO4 (311) crystal plane with the lattice fringes of 0.244 nm. The fine dispersion of the Co and Al element signals in Figure 5c–g confirm the uniformity of metal species on the support. However, the morphology of the Au/Co1AlOx displays indistinct edges in Figure S6, resulting from the immature structure by lower calcination temperature and is consistent with the broadened peaks in XRD patterns. The average size of Au NPs increases to 10.9 and large spherical particles with rough surface can be observed.
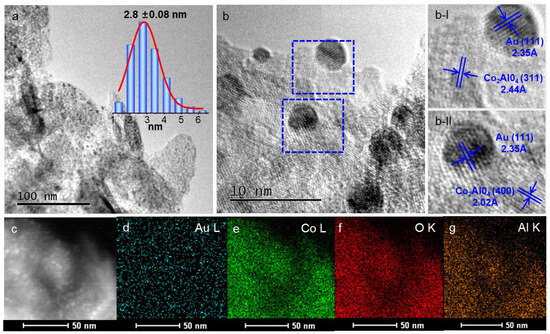
Figure 5.
(a,b) TEM images of Au/Co3AlOx, (c–g) HAADF-STEM elemental mappings of Au/Co3AlOx. The inset picture in ‘a’ is the size distribution of Au nanoparticles based on more than 200 particles. ‘b-I’ and ‘b-II’ are the magnified pictures in the dotted box of ‘b’.
If combining with the catalytic performances by different Au/CoAlOx catalysts, the Au/Co3AlOx with smaller particles displays the higher BnOH conversion under the same reaction condition. Whilst the Au/Co1AlOx with larger Au NPs displays inferior activity and only 32.5% at 80 °C. For better confirmation, the specific rates by three typical catalysts are calculated in terms of the produced mole of BzH per gram Au per hour. The specific rate of Au/Co3AlOx reaches 1290.8 molBzH·gAu−1·h−1. It is also very interesting to mention that there is a clear correlation between the specific rate and average particles sizes of Au as displayed in Figure S7. The clear size effect is a direct result by modulating the surface and chemical structure of the CoAlOx support, which results in the distinctive Au-CoAlOx interaction in terms of the Co/Al stoichiometric ratio.
The surface chemical states of metal and oxygen species are further explored by the XPS spectra in Figure 6. The Au 4f XPS spectra of the Au/Co3Al-LDH precursor without calcination displays a pair of deconvolution peaks at 83.9 eV and 87.5 eV, which are ascribed to the presence of surface Au0 species. It is a direct result of the formation of Au NPs by in situ reduction during synthesis, which are deposited on the Co3Al-LDH without great interaction before calcination. After calcination, a pair of additional characteristic peaks at 84.7 and 88.3 eV are observed, related with the Auδ+ (0 < δ < 1) species in oxidative states. Reports confirmed such Auδ+ species as direct evidence for the strong interaction between Au and metal oxide by Au atoms bonding with the oxygen species on support [27,28]. Santra et al. [29] also suggested that the creation of Auδ+-V0-Ce3+ sites was induced by the increasing O vacancy on ceria and was responsible for the better activity of Au catalysts. Surprisingly, obvious peak shifts of the Au0 species towards higher B.E. value are detected in the calcined Au/ConAlOx catalysts with the increasing Co/Al ratio, indicating the electrons escaping from the surface of Au NPs. Quantitative analysis demonstrates that the Au/Co3AlOx and Au/Co4AlOx catalysts possesses the much-higher Auδ+ content of 39.4% and 31.5% (Table S5), respectively. Liu et al. [30] reported that the Au roasted in air easily reacted with the lattice oxygen of support and was conducive to the selective oxidation of many primary alcohols. Previous research also suggested the formation of Auδ+ species on the adsorption and activation of reactants during oxidation reactions. Oxygen adsorption and dissociation may also facilitated on these small Au nanoparticles, where defect sites are predominantly formed with better modulated electronic structure, as depicted by TEM images and XPS spectra [31].
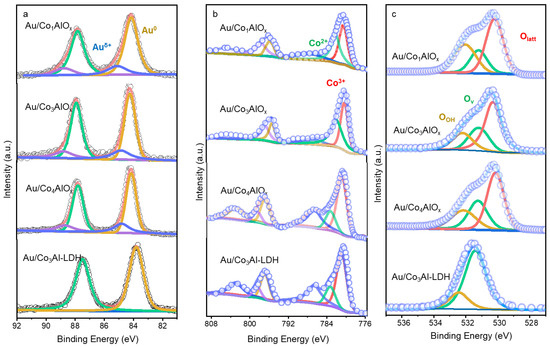
Figure 6.
XPS spectra of Au 4f (a), Co 2p (b) and O 1s (c) core level of different catalysts.
Figure 5b displays the Co 2p XPS spectra resolved into Co 2p 3/2 and Co 2p 5/2 spin-orbit components. The Co 2p 3/2 envelope was deconvoluted into three peaks at 780.3 eV, 782.2 eV, and 778.0 eV, assigned to surface Co3+, Co2+ and a satellite feature, respectively [15]. The metallic Co0 (B.E. at 780.0 eV) species is not detected, which is consistent with the formation of Co2AlO4 spinel [32]. Quantitative analysis of surface Co species indicates that the Co2+ content is significantly influenced by the Co/Al ratio and calcination conditions. And the variation of Co2+/Co3+ was reported as a typical sign to estimate the formation of oxygen vacancy, which promotes the activation of oxygen molecules and facilitates the reaction procedure. Notably, the B.E. value of Co species by different Au/ConAlOx catalysts shift to higher position compared with the fresh Au/Co3Al-LDH precursor without calcination. Combining with the simultaneous shift of the Au0 peaks, a specific metal-support interaction can be confirmed by electron transferring from Au atoms to ConAlOx support.
The XPS spectra of O 1s core level are also displayed in Figure 6c. Three characteristic peaks at 530.2 eV, 531.2 eV and 532.3 eV correspond to the lattice oxygen in metal oxides (Olatt), the oxygen vacancies (Ov), and the surface adsorbed -OH (OOH), respectively [33]. It has to be noted that the Au/Co3Al-LDH before calcination displays huge characteristic peaks at 531.2 eV. This should be the result of the overlapped characteristic peaks of Ov species with the C=O group due to the residual CO32− before calcination [34]. The absence of Olatt species in the fresh Au/Co3Al-LDH precursor should be attributed to the immature structure of the support before calcination. After calcination, lattice oxygen becomes apparent in these Au/ConAlOx catalysts, consistent with the formation of spinel oxide. The proportion of oxygen vacancies in the Au/Co3AlOx slightly increases with the decreasing Co/Al ratio.
The O2-TPD profiles are further operated to reveal the migration capacity of oxygen species by different catalysts. As shown in Figures 7a and S8, the detected catalysts exhibit sharp desorption peak in 200–400 °C, indicating the presence of moderate-strong chemisorbed oxygen species (O2−/O−) on the support surface [35]. It could originate from the oxygen vacancies formed accompanying the Co2+/Co3+ redox pairs. Reports also suggested that the strongly bound lattice oxygen (O2−) or deeply defective structure appeared in the temperature range of 600–800 °C [36]. However, only weak and overlapped peaks can be observed at high temperatures by the tested samples. It suggests the stable structure in the main skeleton of catalyst [37]. It is interesting that the number of active oxygen species in the series of Au/ConAlOx catalysts increases with the raising Co/Al ratio and becomes steady after the Co/Al reaching 3, which is consistent with the XPS O1s spectra. The Au/ConAlOx with higher Co ratio facilitates the O2 activation and dissociation, which results in boosting catalytic performances for alkali-free benzyl alcohol oxidation.
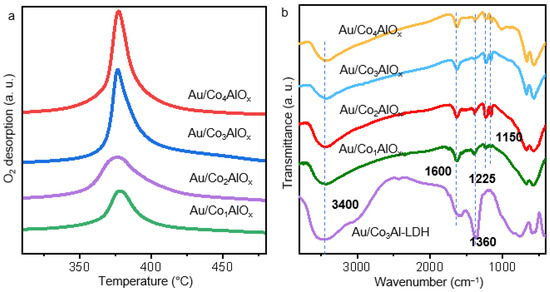
Figure 7.
(a) O2-TPD profiles and (b) FT-IR spectra by Au/Co3Al-LDH and different Au/ConAlOx catalysts.
The FT-IR spectra in Figure 7b also elucidate the structural evolution and the synergistic interplay between compositions. The absorption band around 3400 cm−1 and 1660 cm−1 is attributed to the hydroxyl group and stretching vibration of H2O molecules, which are observed in all the tested samples due to the humidity of sample surface [38]. They should peak at 1580 cm−1, and the strong peak at 1360 cm−1 in the Au/Co3Al-LDH precursor can be ascribed to the asymmetric stretching vibration peak of C-O in CO32− [39]. After calcination, the stretching vibration peak of C-O greatly weakens in the series of Au/ConAlOx catalysts, indicating the partial removal of CO32−. Compared with the Au/Co3Al-LDH catalyst, the Au/ConAlOx catalyst exhibits lattice vibration peaks of metal oxides at 1225 cm−1 and 1150 cm−1. The vibration peak at 670 cm−1 is a typical characteristic peak of layered double oxides, indicating that the spinel-type mixed oxide has indeed been formed. With the increase of the cobalt/aluminum ratio, the stretching vibration peaks of Co-O and Al-O at 570 cm−1 appear and shift to lower wavenumbers, indicating an enhancement in the electron cloud density of Co and Al on the catalyst surface [37]. It is also consistent with the electron transfer from Au to support as revealed by the XPS data.
2.4. Discussion on the Catalytic Performance-Structure Relationship
According to the above characterizations and analysis, it is indicated that the specific interaction between Au and the CoAlOx support becomes an essential factor to regulate the electronic environment and the size distribution of Au NPs. The surface composition such as metal species and defected oxygen vacancy simultaneously changed according to the Co/Al ratio in the supporting materials. It was previously reported that the formation of oxygen vacancy is frequently correlated with the oxygen activation ability and adjacent α-H of benzyl alcohol interacts with oxygen vacancies [40], which may promote the catalytic performances for many oxidation reactions. As revealed by the O2-TPD profiles in Figure 7a, the oxygen adsorption and activation ability are greatly enhanced by the incremental Co/Al ratio. Herein, two additional references are synthesized, by thermal treating the Au/Co3Al-LDH precursor under both Ar and H2 atmosphere, to understand the possible influence of oxygen vacancy on the catalytic performances of BnOH oxidation. Thermal treatment in Ar instead of air commonly correlates with the formation of oxygen vacancy due to the surface migration of lattice oxygen. Whilst reports suggested that thermal reduction in H2 results in the reaction of protons with lattice oxygen in metal oxide and facilitates the creation of oxygen vacancy [41]. Both thermal treatment in Ar or H2 facilitates the formation of oxygen vacancy compared with that treated in air, especially the latter one (in H2).
Subsequent analysis by XRD patterns in Figure 8a suggests the similar XRD patterns by Au/Co3AlOx and Au/Co3AlOx-Ar, indicating the maintain of crystallization and crystal sizes independent of the gas atmosphere in air or Ar. The XPS spectra in the O 1s and Au 4f core level (Figure S9) further confirm the higher surface ratio of Au0 (72.9%) with slightly promoted lattice oxygen amount (52.6%) after Ar thermal treatment. The lattice oxygen was reported to be easily migrated to create defected oxygen vacancy. The lower Auδ+ ratio of 27.1% in Au/Co3AlOx-Ar compared with 39.4% Auδ+ ratio in Au/Co3AlOx might be the direct result of the weakened Au-CoAlOx interaction formed in the non-oxidative Ar atmosphere. However, the crystal phase obviously changed in the Au/Co3AlOx-H2. Only broad diffraction peaks of CoO in partially reduced state are observed in the XRD patterns of Au/ConAlOx-H2, suggesting the phase segregation from the spinel structure to a complex mixture of CoO and Al2O3. Surprisingly, the BnOH conversion by both the Au/Co3AlOx-Ar and Au/Co3AlOx-H2 obviously decreases to 68.5% and 19.4% in Figure 8b, although the BzH selectivity is kept at higher than 90%. The variation of support structure in Au/Co3AlOx-H2 explains the deactivation of the catalytic performance of Au/ConAlOx-H2 for BnOH oxidation. The inferior catalytic performance by Au/Co3AlOx-Ar also excludes the single influence by oxygen vacancy on the catalytic performance. It also evidences the fact that the structural integrity of the spinel phase and the strong Au-support interaction with abundant Auδ+ species is a prerequisite for sustaining transformation of BnOH with high BzH selectivity.
Based on the above characterizations, the catalyst synthesized via in situ reduction immobilization results in the modulated Au–CoAlOx interface with the electron-deficient Auδ+ and electron-rich CoAlOx counterpart. Au particles are highly dispersed on the support, thereby sustaining high activity during cyclic reactions. Notably, the Co3AlOx support not only served as a scaffold for the uniform dispersion of Au nanoparticles but also provided abundant Ov and Co3+/Co2+ redox couples. Moderate oxygen vacancies in the Au/Co3AlOx catalyst also facilitates the O2 activation without destructing the spinel structure, thereby avoiding deactivation caused by phase collapse. The electron transfer between gold and Co3AlOx regulates the electron density of Au and facilitates the transfer of interfacial electrons from the benzyl alcohol substrate to oxygen via the Au-Co sites on the catalyst surface, thereby accelerating the entire oxidation cycle.
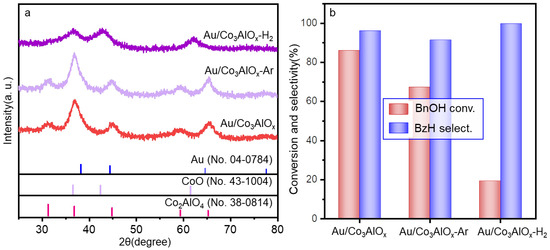
Figure 8.
(a) Oxidation reaction performances of benzyl alcohol and (b) XRD patterns by catalysts under different calcination atmospheres. Reaction conditions: Oxygen flow rate of 20 mL/min, atmospheric pressure, 80 °C, 4 h, nBnOH/nAu = 220.
The stability and reusability of catalysts are crucial for the development of sustainable chemistry and are performed by using the Au/Co3AlOx catalyst (Figure 9a), which could be reused at least five times without significant loss of activity. The conversion of BnOH maintains at 79.2–86.1%. The XRD patterns after reaction display the similar crystal structure of the fresh Au/Co3AlOx catalyst. XPS spectra in the Au 4f core level also suggested the surface percentage of Auδ+ as 40.3% in Figure S10, which is similar to the 39.4% in the fresh Au/Co3AlOx catalyst, suggesting the stable surface composition of metal species after tests. The as-synthesized Au/Co3AlOx catalyst can also be used for the oxidation processes of other alcohols such as cinnamyl alcohol and 1-hexanol (Table S6). It can be seen that conversion of 1-hexanol could be more facile than cinnamyl alcohol under the same reaction condition. Very high selectivity of the target aldehydes can be obtained (95.0–99.9%). The turnover frequency of Au/Co3AlOx catalysts is 532 h−1 at only 80 °C in Table S7 with the reported literature. Considering the trade-off between catalytic performance and energy consumption, the Au/Co1Al-C is among the most efficient Au catalysts in the literature.
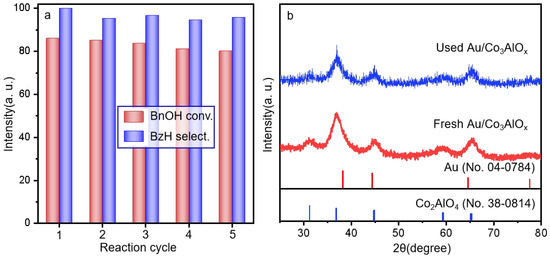
Figure 9.
(a) Stability test by Au/Co3AlOx and (b) the XRD patterns of the fresh and used Au/Co3AlOx after reaction. Reaction conditions: Oxygen flow rate at atmospheric pressure is 20 mL/min, atmospheric pressure, 80 °C, 4 h, nBnOH/nAu = 220.
3. Materials and Methods
3.1. Chemical Reagents
Cobalt nitrate (Co(NO3)2·6H2O, AR), aluminum nitrate (Al(NO3)3·9H2O, 99%), sodium hydroxide (NaOH, AR), sodium carbonate (Na2CO3, 99.8%), chloroauric acid (HAuCl4·3H2O, AR), sodium borohydride (NaBH4, AR), benzyl alcohol (BnOH, C7H8O, AR), benzaldehyde (BzH, C7H6O, AR), paraxylene (C8H10, AR), biphenyl (C12H10, 99.5%), urea(CO(NH2)2, AR), polyvinyl alcohol (PVA, AR).
3.2. Catalyst Synthesis
Synthesis of Co3Al-LDH. The Co3Al-LDH precursor was synthesized by using the classical coprecipitation method. The synthesis was operated at room temperature. The sum of cobalt and aluminum nitrate was 0.015 mol with molar ratio of 3:1 in 80 mL water, denoted as solution A. Simultaneously, Na2CO3 and 0.024 mol of NaOH at a ratio of [CO32−] = 2.0 [Al3+] were added to 80 mL of water to form Solution B. Subsequently, 50 mL water was added in a beaker, and the pH value was adjusted to 9 using Solution B. Then, solutions A and B were co-added to solution C at a constant pH value of 9 by two drops per second under stirring. The mixture was aged for 12 h under constant stirring at 80 °C. After cooling down and filtration, the precipitate was washed 3~5 times with deionized water until the filtrate reached pH 7. The precipitate was dried overnight at 60 °C and designated as Co3Al-LDH. The ConAl-LDH (n = 1, 2, 4) samples with different Co/Al ratios were prepared by the same method.
Preparation of the Au/ConAlOx catalyst. Gold nanoparticles were loaded onto the ConAl-LDH support by in situ reduction immobilization in Scheme S1 (supporting information). The Au loading was fixed at 1.5 wt%. The HAuCl4·3H2O (5.08 × 10−2 mol/L) aqueous solution (0.3 mL) was mixed with PVA (0.1 g/L) in 15 mL deionized water for 30 min at room temperature. The weight ratio of PVA/Au was kept at 5:1. A freshly prepared 0.1 mol/L NaBH4 solution (NaBH4/Au molar ratio of 5:1) was added under rapid agitation. Subsequently, 0.2 g ConAl-LDH support was added to the above mixed solution and stirred at room temperature for 12 h. After washing and drying at 60 °C overnight, the Au/ConAl-LDH precursor was obtained. The catalyst precursor was calcined in air at 300 °C for 3 h to obtain the Au/ConAlOx catalyst.
Preparation of the Au/Co3AlOx-I catalyst. The Au/Co3AlOx-I catalyst with 1.5 wt% Au loading was synthesized at room temperature by modified impregnation method. 0.2 g Co3Al-LDH support was dispersed in 15 mL of water. 0.3 mL aqueous solution of HAuCl4·3H2O was then added into the above mixture. After half an hour, 0.1 mol/L NaBH4 (molar ratio NaBH4/Au of 5:1) was added to reduce the gold precursor. After stirring at room temperature for 12 h, the Au/Co3AlOx-I reference sample can be obtained after wash, dry and calcination in air at 300 °C for 3 h.
Preparation of the Au/Co3AlOx-D catalyst. The Au/Co3AlOx-D catalyst with 1.5 wt% Au loading was synthesized at room temperature by deposition-precipitation with urea. Urea was used as the precipitator with the urea/Au molar ratio of 400. Deposition process was operated under 80 °C for 6 h and aged at 25 °C for 12 h. The subsequent wash, dry and calcination was the same as other gold catalysts. The final catalyst was denoted as Au/Co3AlOx-D.
3.3. Alkali-Free Oxidation of Benzyl Alcohol
Benzyl alcohol oxidation was performed in a 25 mL three-necked flask. 100 mL benzyl alcohol (BnOH) was dissolved in paraxylene with the concentration of 0.0316 mol/L. For example, 8 mL volume of the above solution was added in a flask; 15 mg of catalyst was added into the flask, which was placed in a magnetic stirrer equipped with a reflux condenser and vigorously stirred at a constant speed. Oxygen was passed into the flasks at a rate of 20 mL/min. The carbon balance was calculated to ensure the availability of the reaction data. For each piece of available data, the carbon balance should be higher than 95% to ensure the stable and rational reaction procedure. After the reaction, the solution was cooled to room temperature and then filtered. Then, gas phase analysis was performed on a gas chromatograph (Agilent GC-7890B, Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and an HP-5 capillary column (30 m × 0.32 mm × 0.25 μm). With biphenyl as the internal standard, the conversion rate of benzyl alcohol (BnOH) and the selectivity of benzaldehyde (BzH) can be calculated as follows:
where, n0, nBnOH and nBzH represent the initial moles of benzyl alcohol, and the final moles of benzyl alcohol and benzaldehyde, respectively. The above data was calculated by gas chromatography and calculated by the internal standard method.
3.4. Characterization
Thermal gravimetric analysis (TGA, METTLER TOLEDO, Greifensee, Switzerland) was obtained from DSC testing under 25–900 °C and heating rate of 5°/min. An X-ray powder diffractor (XRD) was used to test samples with Cu K α (λ = 1.5418 A) on Lab XRD7000S (Shimadzu, Kotyo, Japan), under the operating voltage and current of 40 kV and 30 mA at a scanning speed of 5°/min. TEM images and Energy-dispersive X-ray spectroscopy (EDS) imaging images were obtained using the FEI TEM device Tecnai G2 F30 (Waltham, MA, USA) at an acceleration voltage of 120 KV. The average particle size was calculated on the basis of over 200. Under constant liquid nitrogen temperature and certain pressure range, Beijing JWGB JK W100 model instrument was used for the nitrogen adsorption-desorption test. Specific surface area, pore size distribution, and pore volumes of samples were determined by the Brunauer–Emmett–Teller (BET) and Barrett, Joyner, and Halenda (BJH) methods. X-ray photoelectron spectroscopy (XPS) was measured on an ESCALABTM250 Xi’s ESCALABTM250 Xi-type X-ray (Thermo Fisher Scientific, Waltham, MA, USA) photoelectron spectrometer from ThermoFisher, using a monochromatic excitation light source AlK α, and calibrated with C 1s at a charge-corrected energy spectrum peak of 284.6 eV. During O2-TPD, the catalyst was pretreated at 120 °C for 60 min in Ar atmosphere. After being cooled down to 50 °C, 5% O2/Ar (30 mL/min) was adsorbed for 1 h and then switched to O2/Ar for 30 min. The temperature was then increased to 750 °C at a rate of 10 °C/min to make the data collection. Fourier transform infrared (FT-IR) spectroscopy was employed to characterize the different functional groups present in the catalysts. For the measurements in this study, a Nicolet iN10 spectrometer (manufactured by Hitachi High-Technologies Corporation, Tokyo, Japan) was utilized.
4. Conclusions
In this work, we systematically synthesized a series of Au nanoparticles supported by CoAlOx support with spinel structure. An in situ reduction immobilization method was used and optimized by the deposition strategy, thermal treatment details, and the gas atmosphere. The influence of Co/Al molar ratio in Au/CoAlOx on the catalytic performances for alkali-free oxidation of benzyl alcohol was subsequently revealed based on characterizations. Results suggested that the electronic interaction between Au and CoAlOx can be regulated and maximized under a Co/Al ratio of 3. It became a main factor to modulate the dispersion of Au nanoparticles. The average sizes of Au particles can be lowered to 2.8 nm under appropriate Au-CoAlOx interaction. As a result, the surface ratio of Auδ+ and the oxygen adsorption/activation ability were promoted in terms of the constructed Au-CoAlOx interface. Benefiting from such synergistic interaction, the optimized Au/Co3AlOx catalyst achieved 86.1% BnOH conversion under 99.9% BzHselectivity with well-maintained structural stability under recycle tests. This work provides a rational design strategy for developing highly efficient gold catalysts with well-defined Au-support interfaces for the alkali-free oxidation of alcohol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15111053/s1, Scheme S1: Schematic illustration o the continuous synthesis of CoxAl-LDH and the Au/ConAlOx catalyst; Scheme S2: Reaction scheme for the selective oxidation of benzyl alcohol to produce benzaldehyde; Figure S1: Catalytic performances for alkali-free oxidation of BnOH by different Au/Co3AlOx catalysts; Figure S2: Catalytic performances for alkali-free oxidation of BnOH by Au/Co3AlOx and reference samples; Figure S3: SEM images of (a,b) Au/Co1AlOx and (c,d) Au/Co3AlOx; Figure S4: Pore size distribution by different catalysts. (a) Au/Co4AlOx, (b) Au/Co3AlOx, (c) Au/Co1AlOx, (d) Au/Co3Al-LDH. Figure S5. (a,b) TEM images and (c) Particle size distribution of the Au/Co4AlOx catalyst; Figure S6: (a,b) TEM images and (c) Particle size distribution of the Au/Co1AlOx catalyst; Figure S7: The specific rate by typical catalysts changes with the average particle sizes; Figure S8: O2-TPD profiles by different catalysts in the range of 50–800 °C; Figure S9: XPS spectra of Au 4f (a) and O 1s (b) core level by Au/Co3AlOx and Au/Co3AlOx-Ar by Ar treatment; Figure S10. XPS spectra of Au 4f (a) and O 1s (b) core level by fresh Au/Co3AlOx catalyst and used Au/Co3AlOx catalyst after reaction; Table S1: Experimental data by different Au catalysts in Figure 1b, Figure 2a and Figure 3a under the same reaction condition; Table S2: Experimental data by Au/Co3AlOx catalyst under different conditions in Figure 2b–d; Table S3: Metal loading in typical catalysts by ICP-OES; Table S4: The specific surface area, pore volume (micropore, mesoporous) and pore diameter of different catalysts; Table S5: Surface information in terms of XPS spectra by different catalysts; Table S6: Catalytic performances for alkali-free oxidation of BnOH, cinnamyl alcohol, and 1-hexanol by Au/Co3AlOx; Table S7: Comparison of turnover frequency (TOF) in the current work and the reported literature. The Supplementary Materials contain 7 references [42,43,44,45,46,47,48].
Author Contributions
Conceptualization, C.L. and J.L.; methodology, J.L.; validation, M.B. and S.Z.; formal analysis, M.B. and S.Z.; investigation, M.B. and S.Z.; data curation, W.Y., H.L. and S.L.; writing—original draft preparation, M.B.; writing—review and editing, C.L. and J.L.; visualization, C.L. and J.L.; supervision, C.L. and J.L.; project administration, C.L. and J.L.; funding acquisition, C.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Liaoning Province, grant number 2023-MSBA-002, 2023-BSBA-073; National Natural Science Foundation of China, grant number 22172016, 21978031.
Data Availability Statement
The original data presented in the study are openly available in the supporting information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, Z.; Li, K.; Lin, L.; Jiang, Z.; Wang, Y.; Yan, K. Recent advances on the electrocatalytic oxidation of biomass-derived aldehydes. Green Energy Environ. 2025, 10, 898–916. [Google Scholar] [CrossRef]
- Van der Ham, M.P.J.M.; Creus, J.; Bitter, J.H.; Koper, M.T.M.; Pescarmona, P.P. Electrochemical and Non-Electrochemical Pathways in the Electrocatalytic Oxidation of Monosaccharides and Related Sugar Alcohols into Valuable Products. Chem. Rev. 2024, 124, 11915–11961. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Cheng, X.; Zhao, K.; Gutman, K.; Li, T.; Zhang, L. Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev. 2021, 121, 8979–9038. [Google Scholar] [CrossRef]
- Asmaul Hoque, M.; Schweinzer, C.; Martinez, J.J.; Tewes, F.; Sacchetti, V.; Grützmacher, H.; Stahl, S.S. Heterogeneous Fe-N-C Cocatalyst for Hydroquinone Oxidation Enables Aerobic Oxidation of Primary Alcohols to Aldehydes. Angew. Chem. Int. Ed. 2025, 64, e202424778. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Liu, W.; Xie, B.; Zhang, J. TEMPO immobilization on activated carbon by a novel surface-formylation tactic for long-term aerobic oxidation of alcohols. Chem. Eng. J. 2023, 471, 144454. [Google Scholar] [CrossRef]
- Dong, Y.; Luo, J.; Li, S.; Liang, C. CeO2 decorated Au/CNT catalyst with constructed Au-CeO2 interfaces for benzyl alcohol oxidation. Catal. Commun. 2020, 133, 105843. [Google Scholar] [CrossRef]
- Kwon, Y.; Lai, S.C.S.; Rodriguez, P.; Koper, M.T.M. Electrocatalytic Oxidation of Alcohols on Gold in Alkaline Media: Base or Gold Catalysis? J. Am. Chem. Soc. 2011, 133, 6914–6917. [Google Scholar] [CrossRef]
- Liu, J.; Zou, S.; Wu, J.; Kobayashi, H.; Zhao, H.; Fan, J. Green catalytic oxidation of benzyl alcohol over Pt/ZnO in base-free aqueous medium at room temperature. Chin. J. Catal. 2018, 39, 1081–1089. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Y.; Yang, S.; Zhu, W.; Li, S.; Liang, C. Structural Construction of Au–Pd Nanocomposite for Alkali-Free Oxidation of Benzyl Alcohol. ACS Appl. Mater Interf. 2023, 15, 22025–22035. [Google Scholar] [CrossRef]
- Zhang, X.H.; Sun, Z.H.; Jin, R.; Zhu, C.W.; Zhao, C.L.; Lin, Y.; Guan, Q.Q.; Cao, L.N.; Wang, H.W.; Li, S.; et al. Conjugated dual size effect of core-shell particles synergizes bimetallic catalysis. Nat. Commun. 2023, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Douthwaite, M.; Wang, K.; Akdim, O.; Daniel, I.T.; Oh, R.; Liu, L.; Wang, Z.; Meng, F.; et al. Solvent-Free Benzyl Alcohol Oxidation Using Spatially Separated Carbon-Supported Au and Pd Nanoparticles. ACS Catal. 2024, 14, 16551–16561. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, L.L.; Wang, L.Y.; Zang, M.L.; Zhou, X.J.; Azam, M.; Tai, X.S. Bimetallic Au-Ru nanoparticles supported on zeolitic imidazolate framework-67 as highly efficient catalysts for the selective oxidation of benzyl alcohol. Sci. Rep. 2025, 15, 12145. [Google Scholar] [CrossRef]
- Jin, Q.; Bao, J.; Sakiyama, H.; Tsubaki, N. Preparation, structure and performance of TS-1 zeolite-coated Au–Pd/TiO2–SiO2 capsule catalyst for propylene epoxidation with oxygen and hydrogen. Res. Chem. Intermediat. 2011, 37, 177–184. [Google Scholar] [CrossRef]
- Nakayama, A.; Yoshida, A.; Aono, C.; Honma, T.; Sakaguchi, N.; Taketoshi, A.; Fujita, T.; Murayama, T.; Shimada, T.; Takagi, S.; et al. Preparation and Catalytic Properties of Gold Single-Atom and Cluster Catalysts Utilizing Nanoparticulate Mg-Al Layered Double Hydroxides. ChemPlusChem 2025, 90, e202400465. [Google Scholar] [CrossRef]
- Bo, S.; Zhang, X.; Wang, C.; Wang, H.; Chen, X.; Zhou, W.; Cheng, W.; Liu, Q. Inorganic–organic hybrid cobalt spinel oxides for catalyzing the oxygen evolution reaction. Nat. Commun. 2025, 16, 2483. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Bao, M.; Gao, N.; Luo, J.; Liang, C. Catalytic Oxidation of Benzyl Alcohol by Gold Nanoparticles on Carbon Residue–Modified CoAlOx Spinel. Ind. Eng. Chem. Res. 2024, 63, 16154–16163. [Google Scholar] [CrossRef]
- Paul, R.; Maity, N.; Das, B.; Rani, S.; Ghosh, K.; Lisenkov, S.; Ponomareva, I.; Ghosh, R. Controllable oxygen vacancy defect engineering of BiVO4 porous structures for room temperature NH3 detection. Chem. Eng. J. 2025, 515, 163814. [Google Scholar] [CrossRef]
- Yang, Y.; Si, W.; Peng, Y.; Chen, J.; Wang, Y.; Chen, D.; Tian, Z.; Wang, J.; Li, J. Oxygen vacancy engineering on copper-manganese spinel surface for enhancing toluene catalytic combustion: A comparative study of acid treatment and alkali treatment. Appl. Catal. B-Environ. 2024, 340, 123142. [Google Scholar] [CrossRef]
- Luo, J.; Yang, S.; Ling, Y.; Yang, W.; Niu, H.; Li, W.; Liu, H.; Liang, C. Defective Au nanoparticles manipulated by Au-MgxAl-LDH interplay for alkali-free oxidation of benzyl alcohol. Chem. Eng. J. 2023, 473, 145171. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Cheng, Z.; Hu, Z.; Liu, K.; Wang, Y.; Chang, Q. Research on the low-temperature synthesis of cobalt aluminum spinel type blue pigments. J. Alloys Comp. 2021, 864, 158625. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, H.; Kim, S.; Han, J.W.; Lee, K.B. Structural changes of hydrotalcite-based Co-containing mixed oxides with calcination temperature and their effects on NOx adsorption: A combined experimental and DFT study. Chem. Eng. J. 2022, 437, 135209. [Google Scholar] [CrossRef]
- Luo, J.J.; Shan, F.X.; Yang, S.H.; Zhou, Y.X.; Liang, C.H. Boosting the catalytic behavior and stability of a gold catalyst with structure regulated by ceria. RSC Adv. 2022, 12, 1384–1392. [Google Scholar] [CrossRef]
- Menezes, J.P.d.S.Q.; Duarte, K.R.; Manfro, R.L.; Souza, M.M.V.M. Effect of niobia addition on cobalt catalysts supported on alumina for glycerol steam reforming. Renew. Energy 2020, 148, 864–875. [Google Scholar] [CrossRef]
- Bergadà, O.; Vicente, I.; Salagre, P.; Cesteros, Y.; Medina, F.; Sueiras, J.E. Microwave effect during aging on the porosity and basic properties of hydrotalcites. Microp. Mesop. Mater. 2007, 101, 363–373. [Google Scholar] [CrossRef]
- Song, J.; Yu, G.; Li, X.; Yang, X.; Zhang, W.; Yan, W.; Liu, G. Oxidative coupling of alcohols and amines to an imine over Mg-Al acid-base bifunctional oxide catalysts. Chin. J. Catal. 2018, 39, 309–318. [Google Scholar] [CrossRef]
- Du, E.; Yang, J.; Huai, L.; Hao, P.; Lv, M.; Chen, Z.; Chen, Y.; Zhang, J. Quantifying Interface-Dependent Active Sites Induced by Strong Metal–Support Interactions on Au/TiO2 in 2,5-Bis(hydroxymethyl)furan Oxidation. ACS Catal. 2025, 15, 54–62. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, M.; Vinothkumar, G.; Suresh Babu, K. Synergistic effect of gold supported on redox active cerium oxide nanoparticles for the catalytic hydrogenation of 4-nitrophenol. New J. Chem. 2017, 41, 6720–6729. [Google Scholar] [CrossRef]
- Santra, C.; Pramanik, M.; Bando, K.K.; Maity, S.; Chowdhury, B. Gold nanoparticles on mesoporous Cerium-Tin mixed oxide for aerobic oxidation of benzyl alcohol. J. Mol. Catal. A Chem. 2016, 418–419, 41–53. [Google Scholar] [CrossRef]
- Liu, Y.R.; Chen, Y.; Li, Y.W.; Guan, W.; Xia, Q.H.; Cao, M.X.; Huo, P.W.; Zhang, Y.L. Oxygen vacancy-driven strong metal-support interactions on AuPd/TiO2 catalysts for high-efficient air-oxidation of 5-hydroxymethylfurfural. Chem. Eng. J. 2023, 476, 146874. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Xu, Y.; Liu, Q.; Bahri, M.; Zhang, L.; Browning, N.D.; Cowan, A.J.; Tang, J. Efficient hole abstraction for highly selective oxidative coupling of methane by Au-sputtered TiO2 photocatalysts. Nat. Energy 2023, 8, 1013–1022. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, Q.; Zhang, H.; Zhang, X.; Tan, H.; Zhao, Y. Supercritical CO2-driven mechanochemical synthesis for spinel oxides with high electrochemical performance. J. Mater Sci. Technol. 2025, 243, 321–330. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Photo-accelerated Co3+/Co2+ transformation on cobalt and phosphorus co-doped g-C3N4 for Fenton-like reaction. J. Mater Chem. A 2021, 9, 22399–22409. [Google Scholar] [CrossRef]
- Luo, J.J.; Dong, Y.N.; Yang, S.H.; Shan, F.X.; Jiang, Q.; Ma, Y.; Liang, C.H. Au Nanoparticles Anchored on Sulfonated Carbon Nanotubes for Benzyl Alcohol Oxidation. ACS Appl. Nano Mater. 2022, 5, 4887–4895. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering Cobalt Oxide with Coexisting Cobalt Defects and Oxygen Vacancies for Enhanced Catalytic Oxidation of Toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Lv, X.L.; Hao, X.H.; Xu, W.J.; Shao, S.T.; Li, X.L.; Zhang, Y.; Jia, H.P. Constructing robust Ru/CoAlOx catalyst with superior lead resistance and thermal stability for chlorobenzene catalytic oxidation utilizing the anchoring effect of surface Al sites. Appl. Catal. B-Environ. 2025, 366, 125010. [Google Scholar] [CrossRef]
- Tao, L.G.; Zhang, Z.Q.; Chen, P.J.; Zhao, G.F.; Liu, Y.; Lu, Y. Thin-felt Al-fiber-structured Pd-Co-MnOx/Al2O3 catalyst with high moisture resistance for high-throughput O3 decomposition. Appl. Surf. Sci. 2019, 481, 802–810. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, W.Y.; Ji, S.L.; Lu, W.M.Z.; Hua, X.J.; He, M.Y.; Chen, Q.; Liu, Y.P. CoAl metahydrotalcite-B efficiently catalyzes the aerobic oxidative cleavage of 1,2-diols without any additives. Appl. Catal. A-Gen. 2023, 649, 118944. [Google Scholar] [CrossRef]
- Shen, W.; Hu, T.; Liu, X.; Zha, J.; Meng, F.; Wu, Z.; Cui, Z.; Yang, Y.; Li, H.; Zhang, Q.; et al. Defect engineering of layered double hydroxide nanosheets as inorganic photosensitizers for NIR-III photodynamic cancer therapy. Nat. Commun. 2022, 13, 3384. [Google Scholar] [CrossRef]
- An, Z.; Ma, H.; Han, H.; Huang, Z.; Jiang, Y.; Wang, W.; Zhu, Y.; Song, H.; Shu, X.; Xiang, X.; et al. Insights into the Multiple Synergies of Supports in the Selective Oxidation of Glycerol to Dihydroxyacetone: Layered Double Hydroxide Supported Au. ACS Catal. 2020, 10, 12437–12453. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Patel, S.B.; Ye, S.; Wu, Y.; Zhou, Z.; Qiao, L.; Wang, Y.; Marinkovic, N.; Li, M.; et al. Atomic dynamics of gas-dependent oxide reducibility. Nature 2025, 644, 927–932. [Google Scholar] [CrossRef]
- Marelli, M.; Jouve, A.; Villa, A.; Psaro, R.; Balerna, A.; Prati, L.; Evangelisti, C. Hybrid Au/CuO Nanoparticles: Effect of Structural Features for Selective Benzyl Alcohol Oxidation. J. Phys. Chem. C 2019, 123, 2864–2871. [Google Scholar] [CrossRef]
- Stucchi, M.; Cattaneo, S.; Cappella, A.; Wang, W.; Wang, D.; Villa, A.; Prati, L. Catalytic Oxidation of Methoxy Substituted Benzyl Alcohols as Model for Lignin Valorisation. Catal. Today 2020, 357, 15–21. [Google Scholar] [CrossRef]
- Hong, Y.; Jing, X.; Huang, J.; Sun, D.; Odoom-Wubah, T.; Yang, F.; Du, M.; Li, Q. Biosynthesized Bimetallic Au–Pd Nanoparticles Supported on TiO2 for Solvent-Free Oxidation of Benzyl Alcohol. Acs Sustain. Chem. Eng. 2014, 2, 1752–1759. [Google Scholar] [CrossRef]
- Cao, E.; Sankar, M.; Nowicka, E.; He, Q.; Morad, M.; Miedziak, P.J.; Taylor, S.H.; Knight, D.W.; Bethell, D.; Kiely, C.J.; et al. Selective suppression of disproportionation reaction in solvent-less benzyl alcohol oxidation catalysed by supported Au–Pd nanoparticles. Catal. Today 2012, 203, 146–152. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhou, J.; Liu, Y.; Wei, Z.; Zhang, H. Layered double hydroxides supported atomically precise Aun nanoclusters for air oxidation of benzyl alcohol: Effects of size and active site structure. J. Catal. 2020, 389, 409–420. [Google Scholar] [CrossRef]
- Liu, M.; Fan, G.; Yu, J.; Yang, L.; Li, F. Defect–rich Ni–Ti layered double hydroxide as a highly efficient support for Au nanoparticles in base–free and solvent–free selective oxidation of benzyl alcohol. Dalton Trans. 2018, 47, 5226–5235. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S.; Yang, S.; Yang, W.; Luo, J.; Liang, C. Promotion of Au nanoparticles on carbon frameworks for alkali-free aerobic oxidation of benzyl alcohol. Front. Chem. Eng. 2023, 4, 1116366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).