Platinum Nanoparticles Supported on Atomic Layer Deposited SnO2 Decorated Multiwalled Carbon Nanotubes as the Electrocatalyst for the Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results and Discussion
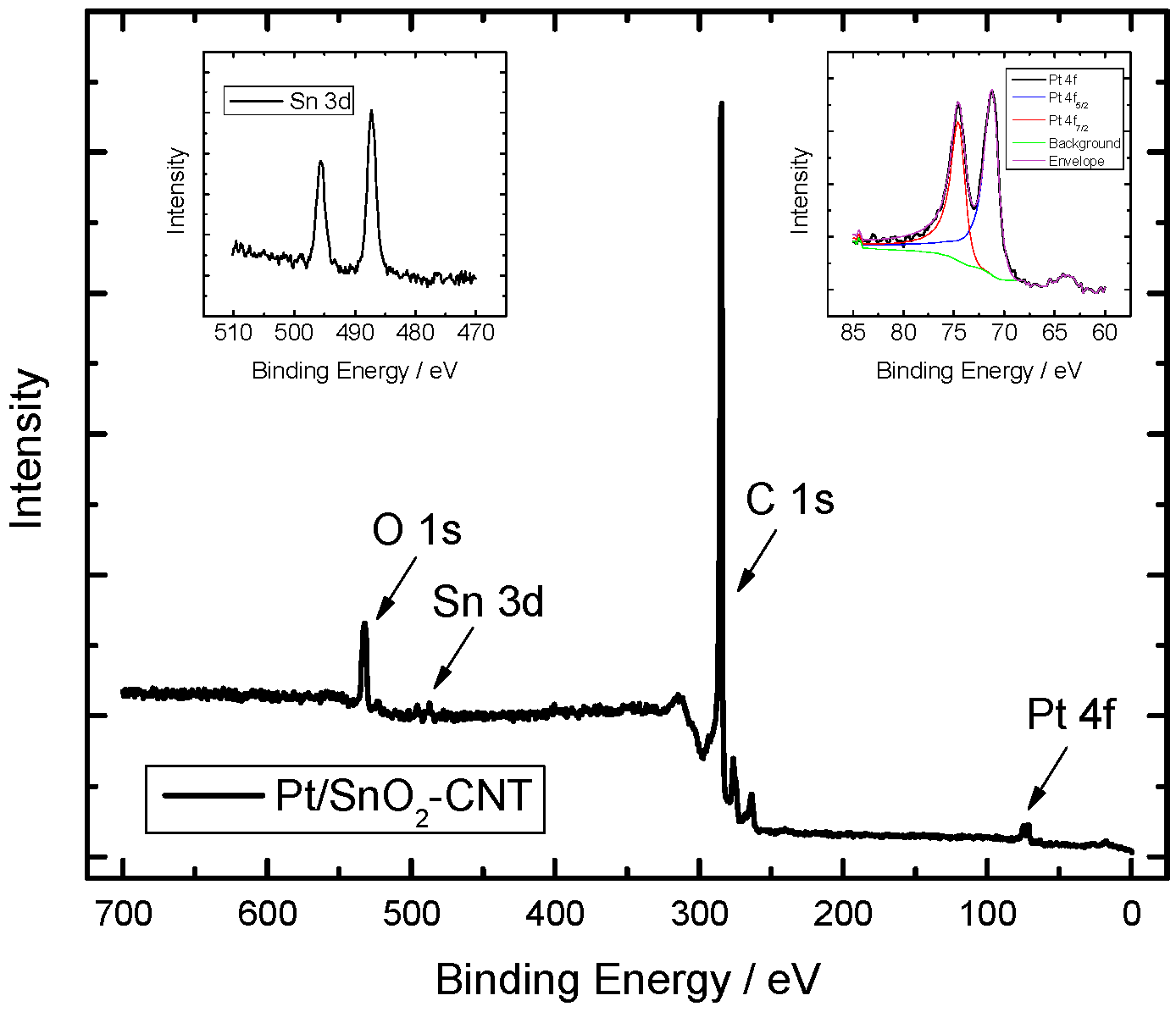
2.1. Physico-Chemical Characterization of Catalyst Materials
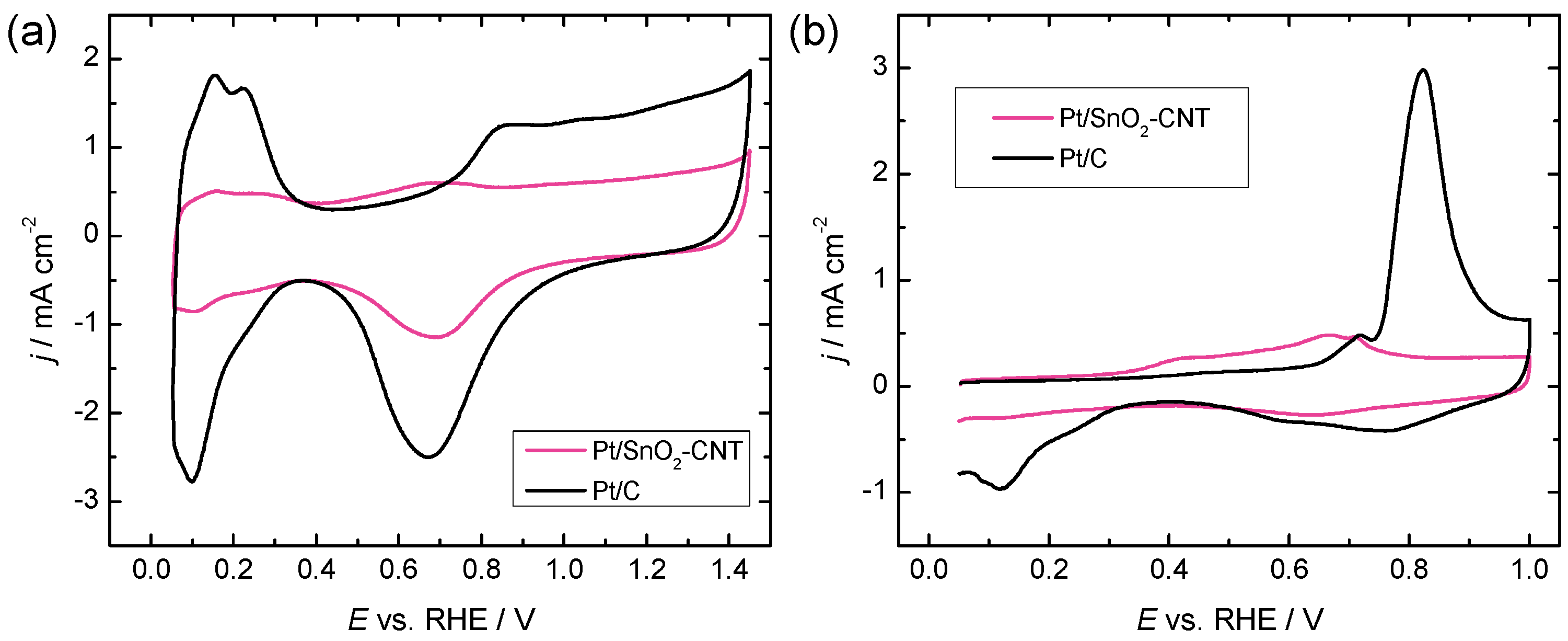
2.2. Electrochemical Characterization of Catalyst Materials
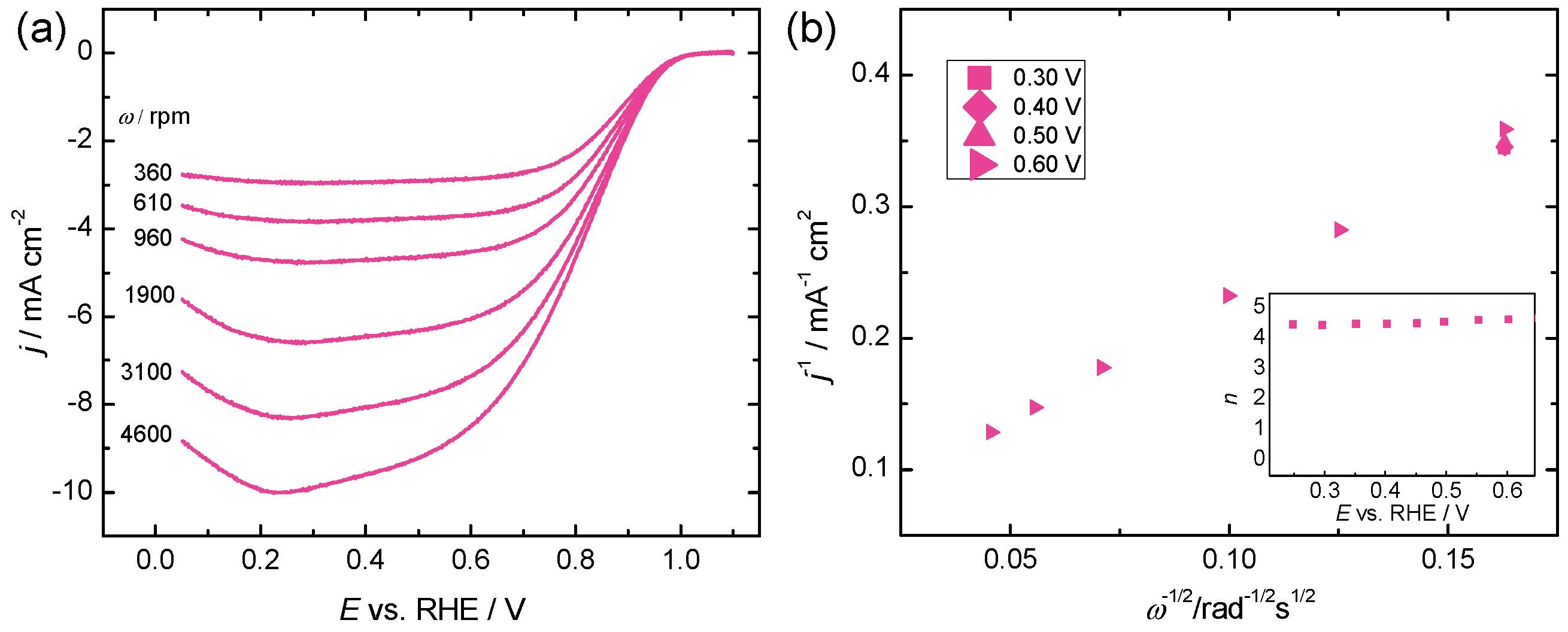
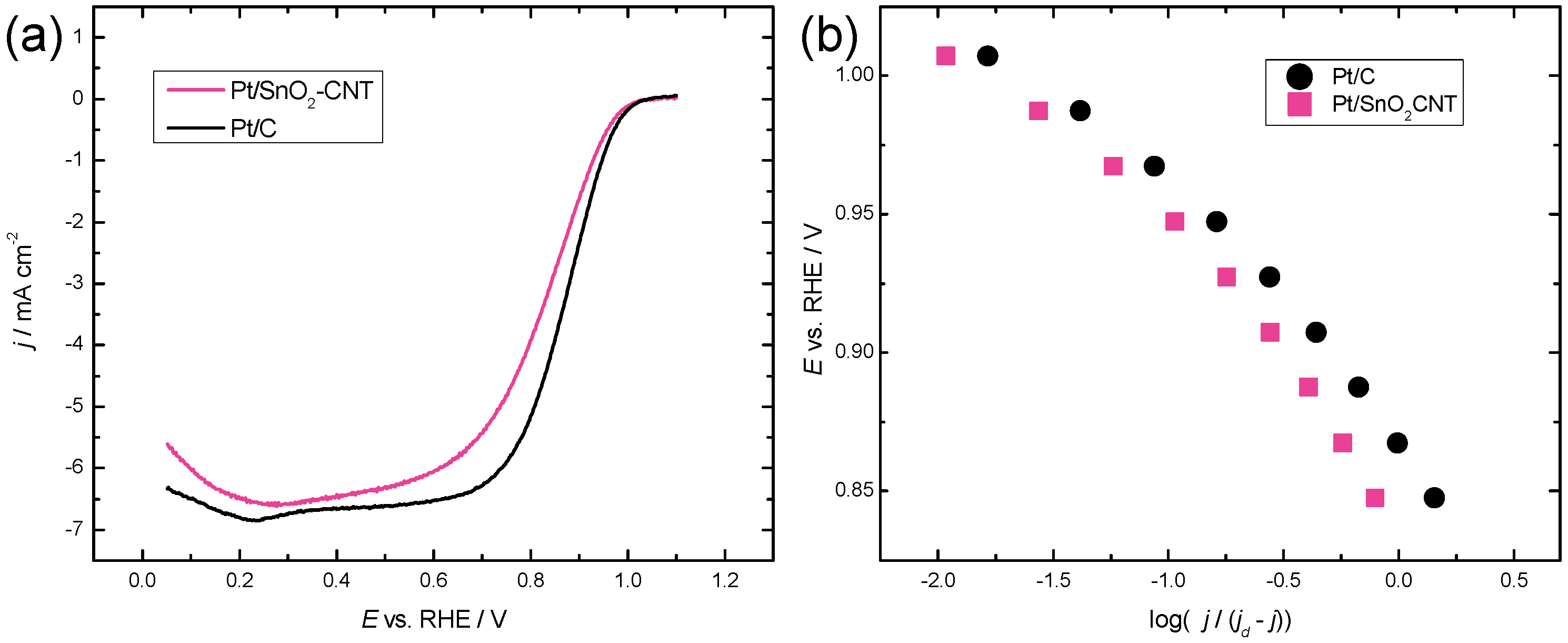
2.3. Oxygen Reduction Reaction Study
| Catalyst | Pt (wt%) | ECSA (cm2) | E1/2 (V) | Tafel Slope (mV) | SA @ 0.9 V (mA cm−2) | MA @ 0.9 V (A g−1) |
|---|---|---|---|---|---|---|
| Pt/SnO2–CNT | 15 ± 3 1 | 0.96 ± 0.02 | 0.83 ± 0.01 | −61 ± 2 | 0.15 ± 0.03 | 44 ± 9 |
| Pt/C | 20 | 4.7 ± 0.2 | 0.88 ± 0.01 | −61 ± 1 | 0.05 ± 0.01 | 61 ± 6 |
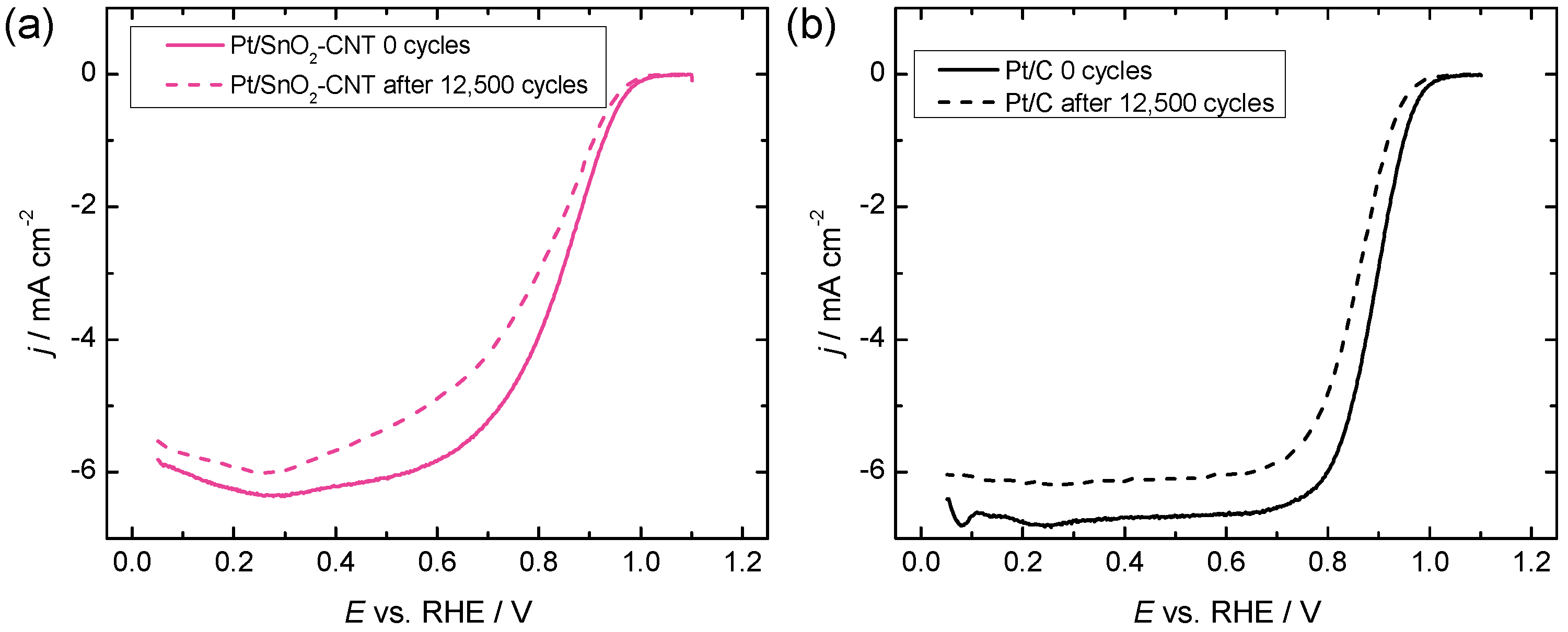
2.4. Accelerated Durability Testing
3. Materials and Methods
3.1. Synthesis of Catalyst
3.2. Preparation of Electrode
3.3. Material Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Daalen, K.R.; Tonne, C.; Semenza, J.C.; Rocklöv, J.; Markandya, A.; Dasandi, N.; Jankin, S.; Achebak, H.; Ballester, J.; Bechara, H.; et al. The 2024 Europe Report of the Lancet Countdown on Health and Climate Change: Unprecedented Warming Demands Unprecedented Action. Lancet Public Health 2024, 9, e495–e522. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 13 June 2025).
- Morfeldt, J.; Davidsson Kurland, S.; Johansson, D.J.A. Carbon Footprint Impacts of Banning Cars with Internal Combustion Engines. Transport. Res. Part. D Transport. Environ. 2021, 95, 102807. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The Role of Platinum in Proton Exchange Membrane Fuel Cells. Platin. Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Parekh, A. Recent Developments of Proton Exchange Membranes for PEMFC: A Review. Front. Energy Res. 2022, 10, 956132. [Google Scholar] [CrossRef]
- Mukerjee, S. Particle Size and Structural Effects in Platinum Electrocatalysis. J. Appl. Electrochem. 1990, 20, 537–548. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on Platinum Nanoparticles: Particle Size Effect on Oxygen Reduction Reaction Activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The Impact of Geometric and Surface Electronic Properties of Pt-Catalysts on the Particle Size Effect in Electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Sakthivel, M.; Drillet, J.-F. An Extensive Study about Influence of the Carbon Support Morphology on Pt Activity and Stability for Oxygen Reduction Reaction. Appl. Catal. B Environ. 2018, 231, 62–72. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Nagar, R.; Ramaprabhu, S. Synthesis and Investigation of Mechanism of Platinum–Graphene Electrocatalysts by Novel Co-Reduction Techniques for Proton Exchange Membrane Fuel Cell Applications. J. Mater. Chem. 2012, 22, 25325. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Palafox-Segoviano, J.A.; Pérez-Díaz, P.J.; Medina-Ramírez, A. Synthesis of Supported Pt Nanoparticles by Sonication for ORR: Effect of the Graphene Oxide-Carbon Composite. Int. J. Hydrogen Energy 2021, 46, 26027–26039. [Google Scholar] [CrossRef]
- Chambers, R.; Hussain, S.; Kozlova, J.; Kukli, K.; Ritslaid, P.; Kikas, A.; Kisand, V.; Erikson, H.; Tammeveski, K. Pt Nanoparticles Electrochemically Deposited onto Heteroatom-Doped Graphene Supports as Electrocatalysts for ORR in Acid Media. J. Electrochem. Soc. 2024, 171, 096506. [Google Scholar] [CrossRef]
- Chambers, R.; Piirsoo, H.-M.; Tamm, A.; Kozlova, J.; Kukli, K.; Ritslaid, P.; Treshchalov, A.; Erikson, H.; Tammeveski, K. Heteroatom-Doped Graphene-Supported Pt Nanoparticles as Electrocatalysts for Proton Exchange Membrane Fuel Cells. ACS Appl. Nano Mater. 2024, 7, 5943–5955. [Google Scholar] [CrossRef]
- Kongkanand, A.; Kuwabata, S.; Girishkumar, G.; Kamat, P. Single-Wall Carbon Nanotubes Supported Platinum Nanoparticles with Improved Electrocatalytic Activity for Oxygen Reduction Reaction. Langmuir 2006, 22, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Alexeyeva, N.; Tammeveski, K.; Lopez-Cudero, A.; Solla-Gullón, J.; Feliu, J.M. Electroreduction of Oxygen on Pt Nanoparticle/Carbon Nanotube Nanocomposites in Acid and Alkaline Solutions. Electrochim. Acta 2010, 55, 794–803. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bates, A.; Lee, S.C.; Lee, D.-H.; Park, S. A Review of the Application of CNTs in PEM Fuel Cells. Int. J. Green Energy 2015, 12, 787–809. [Google Scholar] [CrossRef]
- Jukk, K.; Kozlova, J.; Ritslaid, P.; Sammelselg, V.; Alexeyeva, N.; Tammeveski, K. Sputter-Deposited Pt Nanoparticle/Multi-Walled Carbon Nanotube Composite Catalyst for Oxygen Reduction Reaction. J. Electroanal. Chem. 2013, 708, 31–38. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.W.; Carlton, C.; Shao-Horn, Y. Pt-Covered Multiwall Carbon Nanotubes for Oxygen Reduction in Fuel Cell Applications. J. Phys. Chem. Lett. 2011, 2, 1332–1336. [Google Scholar] [CrossRef]
- Lee, G.; Choi, H.; Tak, Y. In Situ Durability of Various Carbon Supports against Carbon Corrosion during Fuel Starvation in a PEM Fuel Cell Cathode. Nanotechnology 2019, 30, 085402. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.G.; Wei, Z.D.; Qi, X.Q.; Xia, M.R.; Wang, Y.Q. Experimental and DFT Study of Thiol-Stabilized Pt/CNTs Catalysts. Phys. Chem. Chem. Phys. 2012, 14, 16581. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Rähn, M.; Merisalu, M.; Tamm, A.; Sammelselg, V.; Alonso-Vante, N.; Tammeveski, K. High Oxygen Reduction Reaction Activity and Durability of Pt Catalyst Photo-Deposited on SnO2-Coated and Uncoated Multi-Walled Carbon Nanotubes. J. Electroanal. Chem. 2021, 896, 115147. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Z.; Dou, M.; Wang, F. Highly Dispersed and Crystalline Ta2O5 Anchored Pt Electrocatalyst with Improved Activity and Durability Toward Oxygen Reduction: Promotion by Atomic-Scale Pt–Ta2O5 Interactions. ACS Catal. 2019, 9, 3278–3288. [Google Scholar] [CrossRef]
- Guan, J.; Zan, Y.; Shao, R.; Niu, J.; Dou, M.; Zhu, B.; Zhang, Z.; Wang, F. Phase Segregated Pt–SnO2/C Nanohybrids for Highly Efficient Oxygen Reduction Electrocatalysis. Small 2020, 16, 2005048. [Google Scholar] [CrossRef]
- Montero-Ocampo, C.; Garcia, J.R.V.; Estrada, E.A. Comparison of TiO2 and TiO2-CNT as Cathode Catalyst Supports for ORR. Int. J. Electrochem. Sci. 2013, 8, 12780–12800. [Google Scholar] [CrossRef]
- Niu, F.; Pang, Y.; Liu, W.; Shi, Z.; Cui, Y.; Yang, Z.; Yang, S.; Yin, Y. Strong Metal-Support Interaction Induced by Oxygen Vacancies to Enhance the Activity and Durability of Pt/TiO2/Hollow Carbon Nanospheres ORR Catalyst. J. Alloys Comp. 2025, 1010, 177039. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liang, J.; Lin, Z.; Liu, X.; Chen, Y.; Lu, G.; Wang, C.; Wei, P.; Han, J.; et al. Tuning Oxygen Vacancy in SnO2 Inhibits Pt Migration and Agglomeration towards High-Performing Fuel Cells. Appl. Catal. B Environ. 2023, 320, 122017. [Google Scholar] [CrossRef]
- Kakinuma, K.; Kobayashi, R.; Iiyama, A.; Uchida, M. Influence of Ionomer Content on Both Cell Performance and Load Cycle Durability for Polymer Electrolyte Fuel Cells Using Pt/Nb-SnO2 Cathode Catalyst Layers. J. Electrochem. Soc. 2018, 165, J3083–J3089. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Rähn, M.; Matisen, L.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Pt Nanoparticles Sputter-Deposited on TiO2/MWCNT Composites Prepared by Atomic Layer Deposition: Improved Electrocatalytic Activity towards the Oxygen Reduction Reaction and Durability in Acid Media. Int. J. Hydrogen Energy 2018, 43, 4967–4977. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Kook, M.; Rähn, M.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Improved ORR Activity and Long-Term Durability of Pt Nanoparticles Deposited on TiO2 -Decorated Multiwall Carbon Nanotubes. J. Electrochem. Soc. 2019, 166, F1284–F1291. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Kikas, A.; Kisand, V.; Kozlova, J.; Aarik, J.; Tamm, A.; et al. Platinum Sputtered on Nb-Doped TiO2 Films Prepared by ALD: Highly Active and Durable Carbon-Free ORR Electrocatalyst. J. Electrochem. Soc. 2020, 167, 164505. [Google Scholar] [CrossRef]
- Shi, G.; Tano, T.; Tryk, D.A.; Iiyama, A.; Uchida, M.; Kakinuma, K. Temperature Dependence of Oxygen Reduction Activity at Pt/Nb-Doped SnO2 Catalysts with Varied Pt Loading. ACS Catal. 2021, 11, 5222–5230. [Google Scholar] [CrossRef]
- Jukk, K.; Kongi, N.; Tarre, A.; Rosental, A.; Treshchalov, A.B.; Kozlova, J.; Ritslaid, P.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Electrochemical Oxygen Reduction Behaviour of Platinum Nanoparticles Supported on Multi-Walled Carbon Nanotube/Titanium Dioxide Composites. J. Electroanal. Chem. 2014, 735, 68–76. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, S.; Liu, M.; Luo, J.; Liu, X.; Liao, H.; Liu, F.; Tan, P.; Pan, J. Anti-Corrosive Tin Oxide Modified Carbon Support for Platinum Nanoparticles Enables Robust Oxygen Reduction Reaction. J. Colloid Interface Sci. 2025, 692, 137511. [Google Scholar] [CrossRef] [PubMed]
- Takei, C.; Kobayashi, R.; Mizushita, Y.; Hiramitsu, Y.; Kakinuma, K.; Uchida, M. Platinum Anti-Dissolution Mechanism of Pt/Nb-SnO2 Cathode Catalyst Layer during Load Cycling in the Presence of Oxygen for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2018, 165, F1300–F1311. [Google Scholar] [CrossRef]
- Kakinuma, K.; Suda, K.; Kobayashi, R.; Tano, T.; Arata, C.; Amemiya, I.; Watanabe, S.; Matsumoto, M.; Imai, H.; Iiyama, A.; et al. Electronic States and Transport Phenomena of Pt Nanoparticle Catalysts Supported on Nb-Doped SnO2 for Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 34957–34963. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.-H.; Chiu, K.-Y.; Lee, S.-W.; Chen, T.-Y.; Jia, Y.; Wang, J.-H.; Wang, K.-W.; Dai, S. Surface SnO2 Decoration: An Economical and Efficient Alternative to Pt Shells in Pt-Co Catalysts for Enhanced Oxygen Reduction Reaction. Chem. Eng. J. 2025, 511, 161971. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Zhao, C.; Hu, A.; Sun, X.; Mei, B.; Long, J. Carbothermal Reduction-Assisted Synthesis of a Carbon-Supported Highly Dispersed PtSn Nanoalloy for the Oxygen Reduction Reaction. Inorg. Chem. 2024, 63, 19322–19331. [Google Scholar] [CrossRef]
- Gu, S.; Huang, H.-Y.; Lin, Y.-C.; Hsieh, C.-A.; Panda, P.K.; Chang, J.-K.; Hsieh, C.-T. High-Entropy Alloys with Low Platinum Content: Novel Catalysts for Oxygen Reduction and Evolution Reactions. Emergent Mater. 2025, 8, 3649–3662. [Google Scholar] [CrossRef]
- Panda, P.K.; Huang, H.-Y.; Dash, P.; Hsieh, C.-T.; Chang, J.-K.; Liu, W.-R. Liquid-Phase Microwave Synthesis of Platinum-Based High-Entropy Alloy Catalysts on Carbon Supports for Electrochemical Hydrogen Adsorption/Desorption and Oxygen Evolution/Reduction Reactions. Int. J. Hydrogen Energy 2025, 111, 536–545. [Google Scholar] [CrossRef]
- Ma, J.; Habrioux, A.; Alonso-Vante, N. The Effect of Substrates at Cathodes in Low-temperature Fuel Cells. ChemElectroChem 2014, 1, 37–46. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen Reduction Reaction on Nanostructured Pt-Based Electrocatalysts: A Review. Int. J. Hydrogen Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Hoque, M.A.; Higgins, D.C.; Hassan, F.M.; Choi, J.-Y.; Pritzker, M.D.; Chen, Z. Tin Oxide-Mesoporous Carbon Composites as Platinum Catalyst Supports for Ethanol Oxidation and Oxygen Reduction. Electrochim. Acta 2014, 121, 421–427. [Google Scholar] [CrossRef]
- Cao, W.; Mao, Y.; Hu, B.; Yang, Y.; Zhou, W.; Shao, Z. Significantly Improved Stability and Water Retention for Pt Supported on W-Doped SnO2 to Catalyse the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A 2024, 12, 10799–10807. [Google Scholar] [CrossRef]
- Murase, R.; Inaba, M.; Matsuoka, Y.; Kamitaka, Y.; Yoshino, S.; Koiwai, A.; Takeshita, T.; Kodama, K. Influence of Ionomer Content in Pt/Mesoporous SnO2 Electrodes on PEM Fuel Cell Performance. ECS Adv. 2025, 4, 014503. [Google Scholar] [CrossRef]
- Choudhury, D.; Das, B.; Sarma, D.D.; Rao, C.N.R. XPS Evidence for Molecular Charge-Transfer Doping of Graphene. Chem. Phys. Lett. 2010, 497, 66–69. [Google Scholar] [CrossRef]
- Izydorczyk, W.; Izydorczyk, J. Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere. Sensors 2021, 21, 5741. [Google Scholar] [CrossRef]
- Bayindir, Z.; Duchesne, P.N.; Cook, S.C.; MacDonald, M.A.; Zhang, P. X-Ray Spectroscopy Studies on the Surface Structural Characteristics and Electronic Properties of Platinum Nanoparticles. J. Chem. Phys. 2009, 131, 244716. [Google Scholar] [CrossRef]
- Tammeveski, K.; Tenno, T.; Rosental, A.; Talonen, P.; Johansson, L.-S.; Niinistö, L. The Reduction of Oxygen on Pt-TiO2 Coated Ti Electrodes in Alkaline Solution. J. Electrochem. Soc. 1999, 146, 669–676. [Google Scholar] [CrossRef]
- López-Cudero, A.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. CO Electrooxidation on Carbon Supported Platinum Nanoparticles: Effect of Aggregation. J. Electroanal. Chem. 2010, 644, 117–126. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Mastronardi, V.; Pompa, P.P.; Moglianetti, M.; Malitesta, C. Bare Platinum Nanoparticles Deposited on Glassy Carbon Electrodes for Electrocatalytic Detection of Hydrogen Peroxide. ACS Appl. Nano Mater. 2021, 4, 7650–7662. [Google Scholar] [CrossRef]
- Prass, S.; St-Pierre, J.; Klingele, M.; Friedrich, K.A.; Zamel, N. Hydrogen Oxidation Artifact During Platinum Oxide Reduction in Cyclic Voltammetry Analysis of Low-Loaded PEMFC Electrodes. Electrocatalysis 2021, 12, 45–55. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity Benchmarks and Requirements for Pt, Pt-Alloy, and Non-Pt Oxygen Reduction Catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Hussain, S.; Kongi, N.; Erikson, H.; Rähn, M.; Merisalu, M.; Matisen, L.; Paiste, P.; Aruväli, J.; Sammelselg, V.; Estudillo-Wong, L.A.; et al. Platinum Nanoparticles Photo-Deposited on SnO2-C Composites: An Active and Durable Electrocatalyst for the Oxygen Reduction Reaction. Electrochim. Acta 2019, 316, 162–172. [Google Scholar] [CrossRef]
- Ganassin, A.; Colic, V.; Tymoczko, J.; Bandarenka, A.S.; Schuhmann, W. Non-Covalent Interactions in Water Electrolysis: Influence on the Activity of Pt(111) and Iridium Oxide Catalysts in Acidic Media. Phys. Chem. Chem. Phys. 2015, 17, 8349–8355. [Google Scholar] [CrossRef] [PubMed]
- Spasov, D.D.; Ivanova, N.A.; Pushkarev, A.S.; Pushkareva, I.V.; Presnyakova, N.N.; Chumakov, R.G.; Presnyakov, M.Y.; Grigoriev, S.A.; Fateev, V.N. On the Influence of Composition and Structure of Carbon-Supported Pt-SnO2 Hetero-Clusters onto Their Electrocatalytic Activity and Durability in PEMFC. Catalysts 2019, 9, 803. [Google Scholar] [CrossRef]
- Rabis, A.; Binninger, T.; Fabbri, E.; Schmidt, T.J. Impact of Support Physicochemical Properties on the CO Oxidation and the Oxygen Reduction Reaction Activity of Pt/SnO2 Electrocatalysts. J. Phys. Chem. C 2018, 122, 4739–4746. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of Oxygen Reduction Activities via the Rotating Disc Electrode Method: From Pt Model Surfaces to Carbon-Supported High Surface Area Catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Geng, D.; Chen, Y.; Banis, M.N.; Li, R.; Cai, M.; Sun, X. Direct Growth of Single-Crystal Pt Nanowires on Sn@CNT Nanocable: 3D Electrodes for Highly Active Electrocatalysts. Chem. Eur. J. 2010, 16, 829–835. [Google Scholar] [CrossRef]
- Li, S.-Z.; Lin, Z.-J.; Chen, Q.-A.; Cai, Z.; Li, Q. Electrochemical-Method-Induced Strong Metal-Support Interaction in Pt-CNT@SnO2 for CO-Tolerant Hydrogen Oxidation Reaction. J. Electrochem. 2024, 30, 2404121. [Google Scholar] [CrossRef]
- Higuchi, E.; Miyata, K.; Takase, T.; Inoue, H. Ethanol Oxidation Reaction Activity of Highly Dispersed Pt/SnO2 Double Nanoparticles on Carbon Black. J. Power Sources 2011, 196, 1730–1737. [Google Scholar] [CrossRef]
- Borbáth, I.; Gubán, D.; Pászti, Z.; Sajó, I.E.; Drotár, E.; De La Fuente, J.L.G.; Herranz, T.; Rojas, S.; Tompos, A. Controlled Synthesis of Pt3Sn/C Electrocatalysts with Exclusive Sn–Pt Interaction Designed for Use in Direct Methanol Fuel Cells. Top. Catal. 2013, 56, 1033–1046. [Google Scholar] [CrossRef]
- Ðurasović, I.; Peter, R.; Dražić, G.; Faraguna, F.; Anelić, R.; Marciuš, M.; Jurkin, T.; Mohaček Grošev, V.; Gracheva, M.; Klencsár, Z.; et al. Catalytically Active Oxidized PtOx Species on SnO2 Supports Synthesized via Anion Exchange Reaction for 4-Nitrophenol Reduction. Nanomaterials 2025, 15, 1159. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Vidal-Iglesias, F.J.; Herrero, E.; Feliu, J.M.; Aldaz, A. CO Monolayer Oxidation on Semi-Spherical and Preferentially Oriented (100) and (111) Platinum Nanoparticles. Electrochem. Commun. 2006, 8, 189–194. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Gómez-Marín, A.M.; Schouten, K.J.P.; Koper, M.T.M.; Feliu, J.M. Interaction of Hydrogen Peroxide with a Pt(111) Electrode. Electrochem. Commun. 2012, 22, 153–156. [Google Scholar] [CrossRef]
- Ruiz Camacho, B.; Morais, C.; Valenzuela, M.A.; Alonso-Vante, N. Enhancing Oxygen Reduction Reaction Activity and Stability of Platinum via Oxide-Carbon Composites. Catal. Today 2013, 202, 36–43. [Google Scholar] [CrossRef]
- Jia, J.; Wang, H.; Ji, S.; Yang, H.; Li, X.; Wang, R. SnO2-Embedded Worm-like Carbon Nanofibers Supported Pt Nanoparticles for Oxygen Reduction Reaction. Electrochim. Acta 2014, 141, 13–19. [Google Scholar] [CrossRef]
- Erikson, H.; Lüsi, M.; Sarapuu, A.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Oxygen Electroreduction on Carbon-Supported Pd Nanocubes in Acid Solutions. Electrochim. Acta 2016, 188, 301–308. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal–Support Interaction in Platinum and Palladium Nanoparticles Loaded on Nitrogen-Doped Mesoporous Carbon for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Elezovic, N.R.; Radmilovic, V.R.; Kovac, J.; Babic, B.M.; Gaijic-Krstajic, L.M.; Krstajic, N.V. Pt Nanoparticles on Tin Oxide Based Support as a Beneficial Catalyst for Oxygen Reduction in Alkaline Solutions. RSC Adv. 2015, 5, 15923–15929. [Google Scholar] [CrossRef]
- Tiido, K.; Alexeyeva, N.; Couillard, M.; Bock, C.; MacDougall, B.R.; Tammeveski, K. Graphene–TiO2 Composite Supported Pt Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2013, 107, 509–517. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, C.; He, B.; Dong, H.; Dai, W.; Lu, H.; Zhang, X. An Advanced Electrocatalyst of Pt Decorated SnO2/C Nanofibers for Oxygen Reduction Reaction. J. Electroanal. Chem. 2016, 781, 198–203. [Google Scholar] [CrossRef]
- Rivera Rocabado, D.S.; Ishimoto, T.; Koyama, M. The Effect of SnO2(110) Supports on the Geometrical and Electronic Properties of Platinum Nanoparticles. SN Appl. Sci. 2019, 1, 1485. [Google Scholar] [CrossRef]
- Krasnova, A.; Nechitailov, A.A.; Pelageikina, A.; Glebova, N.V. Differences in the Electrochemical Behavior of Vulcan XC-72 Carbon Black and Glassy Carbon after Prolonged Potential Cycling. Electrochem. Commun. 2023, 155, 107578. [Google Scholar] [CrossRef]
- Castanheira, L.; Dubau, L.; Mermoux, M.; Berthomé, G.; Caqué, N.; Rossinot, E.; Chatenet, M.; Maillard, F. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: From Model Experiments to Real-Life Operation in Membrane Electrode Assemblies. ACS Catal. 2014, 4, 2258–2267. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Cavaliere, S.; Jones, D.; Rozière, J. Strong Metal–Support Interaction Improves Activity and Stability of Pt Electrocatalysts on Doped Metal Oxides. Phys. Chem. Chem. Phys. 2018, 20, 8765–8772. [Google Scholar] [CrossRef]
- Promsawan, N.; Saipanya, S.; Rattanakansang, S.; Themsirimongkon, S.; Inceesungvorn, B.; Waenkaew, P. Modification of Various Carbons with Various Metal Oxides and Noble Metal Compositions as Electrocatalysts for Ethanol Oxidation. Compos. Interfaces 2020, 27, 1023–1045. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Kiisler, A.-A.; Uustare, T.; Sammelselg, V. Influence of Substrate Temperature on Atomic Layer Growth and Properties of HfO2 Thin Films. Thin Solid Films 1999, 340, 110–116. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical Characterisation of Platinum Nanoparticles Prepared by Microemulsion: How to Clean Them without Loss of Crystalline Surface Structure. J. Electroanal. Chem. 2000, 491, 69–77. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambers, R.; Tarre, A.; Otsus, M.; Kozlova, J.; Kukli, K.; Kikas, A.; Kisand, V.; Erikson, H.; Tammeveski, K. Platinum Nanoparticles Supported on Atomic Layer Deposited SnO2 Decorated Multiwalled Carbon Nanotubes as the Electrocatalyst for the Oxygen Reduction Reaction. Catalysts 2025, 15, 1052. https://doi.org/10.3390/catal15111052
Chambers R, Tarre A, Otsus M, Kozlova J, Kukli K, Kikas A, Kisand V, Erikson H, Tammeveski K. Platinum Nanoparticles Supported on Atomic Layer Deposited SnO2 Decorated Multiwalled Carbon Nanotubes as the Electrocatalyst for the Oxygen Reduction Reaction. Catalysts. 2025; 15(11):1052. https://doi.org/10.3390/catal15111052
Chicago/Turabian StyleChambers, Raegan, Aivar Tarre, Markus Otsus, Jekaterina Kozlova, Kaupo Kukli, Arvo Kikas, Vambola Kisand, Heiki Erikson, and Kaido Tammeveski. 2025. "Platinum Nanoparticles Supported on Atomic Layer Deposited SnO2 Decorated Multiwalled Carbon Nanotubes as the Electrocatalyst for the Oxygen Reduction Reaction" Catalysts 15, no. 11: 1052. https://doi.org/10.3390/catal15111052
APA StyleChambers, R., Tarre, A., Otsus, M., Kozlova, J., Kukli, K., Kikas, A., Kisand, V., Erikson, H., & Tammeveski, K. (2025). Platinum Nanoparticles Supported on Atomic Layer Deposited SnO2 Decorated Multiwalled Carbon Nanotubes as the Electrocatalyst for the Oxygen Reduction Reaction. Catalysts, 15(11), 1052. https://doi.org/10.3390/catal15111052







