Abstract
The effectiveness of periodate-assisted photocatalysis in removing the cationic dye Safranin O (SO) was evaluated using a UV/TiO2/IO4− process operated at room temperature under near-neutral pH conditions. Under base conditions ([IO4−] = 0.15 mM, [TiO2] = 0.4 g/L, [SO] = 10 mg/L), the ternary system achieved a pseudo-first-order rate constant of 0.6212 min−1, outperforming the UV/TiO2 and UV/IO4− processes by approximately 21- and 29-fold, respectively. This yielded a synergy ratio of about 12 compared to the sum of the binary processes. Targeted quenching experiments revealed the operative pathways. Strong inhibition by ascorbic acid and phenol indicates that interfacial holes and •OH are key oxidants. Methanol caused a moderate slowdown, consistent with •OH and hole scavenging. Benzoquinone and oxalate suppressed removal by intercepting the electron and O2•− pathways, respectively. Dichromate markedly inhibited the process via optical screening and competition for electrons. Azide had little effect, suggesting a minor role for singlet oxygen. Matrix studies showed progressively slower kinetics from deionized water to mineral water to seawater. This was due to halides, sulfate, alkalinity, and TiO2 aggregation driven by ionic strength. Additional tests confirmed that the dominant modulators of performance were humic acid (site fouling and light screening), chloride and sulfate (radical speciation and surface effects), nitrite (near-diffusion radical quenching), and bicarbonate at pH 8.3 (conversion of •OH to CO3•−). Nonionic surfactants (Tween 80, Triton X-100) also depressed SO removal through micellar sequestration and competitive adsorption on TiO2. The study confirms the potential of UV/TiO2/IO4− as a tunable AOP capable of delivering rapid and reliable dye degradation under a wide range of water quality conditions. The mechanistic mapping unifies two roles for IO4−, an electron acceptor that inhibits recombination and a photochemical precursor of iodine centered and •OH radicals and connect these roles to the observed synergy and to the trend across deionized water, mineral water, and seawater. The scavenger outcomes assign the main oxidant flux to holes and •OH radicals with a contributory electron or O2•− branch from IO4− reduction.
Keywords:
UV/TiO2/IO4− technique; Safranin O; degradation; salts; surfactants; humic acid; radical probe 1. Introduction
Advanced oxidation processes (AOPs) based on semiconductor photocatalysis are becoming mainstream in water treatment because they effectively break down dyes, pharmaceuticals, and other micropollutants without excessive chemical use [1]. Titanium dioxide (TiO2) is the gold standard photocatalyst; however, its performance is often limited by fast electron-hole recombination and sensitivity to background water chemistry during interfacial reactions. Therefore, strategies that pair TiO2 with suitable electron acceptors or photochemically activatable co-oxidants show promise in achieving higher quantum efficiency [2].
Among these co-oxidants, periodate (IO4−) is a promising alternative to H2O2 and persulfate [3]. Under UV light, periodate is photoactivated to produce highly reactive intermediates, such as IO3•, IO4•, and •OH, while scavenging conduction-band electrons. This process suppresses recombination and broadens the oxidant spectrum. These dual functions result in significant rate enhancements across various organics and over a relatively broad pH range, including neutral conditions [3].
Although IO4− activation is understood to generate both iodine-centered radicals and hydroxyl radicals, the present experiments did not measure their individual lifetimes or proportional contributions. Established approaches can differentiate these routes. Transient absorption spectroscopy or pulse radiolysis directly observes iodine intermediates with distinct spectra and decay kinetics, which differ from the signals associated with hydroxyl radicals. In parallel, electron paramagnetic resonance (EPR) with spin trapping provides selective detection of hydroxyl radicals in illuminated TiO2 suspensions. Competition kinetics with orthogonal scavengers then apportions reactivity. Diagnostic products add a final line of evidence, since iodinated products rise with iodine radical flux while hydroxylation products track the hydroxyl radical probes. These complementary measurements provide a practical route to separate the two pathways [4].
However, the oxidant speciation and charge-transfer pathways that make UV/TiO2/ IO4− effective are vulnerable to constituents commonly found in natural and engineered waters. Specifically, halides divert •OH into reactive chlorine species with lower second-order rate constants toward organics. For quantitative context, •OH reacts with typical aromatic and oxygenated organic molecules at about 109–1010 M−1·s−1, whereas Cl2•− most often reacts at about 106–108 M−1·s−1 and approaches 109 M−1·s−1 only for strongly electron-rich substrates. Alkalinity converts •OH to the more selective carbonate radical; natural organic matter (humics) screens light and fouls TiO2 surfaces; and common anions/cations shift colloidal stability and adsorption equilibria [5]. Depending on the matrix, the net result is often a departure from ideal laboratory behavior. This underscores the need to determine which species matter and why under realistic conditions.
Recent studies have firmly established that combining TiO2 photocatalysis with periodate (UV/TiO2/IO4−) greatly accelerates the breakdown of various organic contaminants and increases activity at neutral pH levels. This process outperforms bare UV/TiO2 or UV/IO4− systems when it comes to dyes and pharmaceuticals. Benchmarks on Rhodamine B, Safranin O (SO), and other recalcitrant compounds demonstrate the process’s rapid kinetics, reduced effluent toxicity, and comparatively weak pH dependence [3,6]. Mechanistic analyses attribute these improvements to the dual role of IO4− as a conduction-band electron acceptor, which suppresses electron/hole recombination, and as a photochemically activatable precursor of iodine-centered and hydroxyl radicals. This broadens the operative oxidant pool.
Diagnostic scavengers are powerful tools for deconvoluting these pathways. Established probes such as benzoquinone (an electron/superoxide interceptor), oxalate (a hole scavenger), and methanol and phenol (hydroxyl radical/hole scavengers) enable targeted testing of mechanistic hypotheses in heterogeneous photocatalysis. Other probes include azide, which quenches singlet oxygen, as well as strong oxidizing and reducing agents. Examples of the latter include dichromate and ascorbic acid, respectively.
Safranin O (SO) is best known as a biological counterstain in histology and cytology, where its use is generally confined to small, controlled volumes with regulated disposal in clinical and research laboratories. Consequently, reports of widespread environmental occurrences from staining applications are scarce. Nevertheless, SO is a synthetic cationic dye of the phenazine family that also finds use in textiles, food coloring, and polymerization processes, where larger-scale discharge into aquatic environments is possible [7,8,9]. Like synthetic dyes as a class, their release raises environmental concerns: even at low concentrations, dyes reduce light penetration, impair photosynthetic activity, and elevate COD and BOD in receiving waters. SO itself is highly soluble, resistant to biodegradation, and capable of persisting in natural waters, where it can disrupt aquatic ecosystems [10,11,12]. Toxicological studies further report irritation, allergic responses, and potential mutagenic effects associated with exposure [10,11,12]. Taken together with the broad evidence base on the impacts of dyes, these features make SO a pragmatic model contaminant for assessing advanced oxidation in water.
In this study, the UV/TiO2/IO4− process was evaluated for the degradation of the cationic dye SO under ambient, near-neutral conditions. Particular emphasis was placed on the influence of canonical radical scavengers and representative water matrices (deionized, mineral, and seawater) in modulating the distribution of operative oxidants and charge carriers. By coupling kinetic analyses with a structured scavenger panel and matrix tests, the respective contributions of interfacial holes, •OH radicals, and electron-driven periodate pathways were clarified. The investigation further delineates the principal constituents of real water matrices that interfere with these oxidative routes and thereby attenuate the overall efficiency of UV/TiO2/IO4− treatment.
Prior works show that coupling TiO2 photocatalysis with periodate under ultraviolet irradiation can accelerate transformation rates by accepting conduction band electrons, thereby mitigating recombination, and by photochemical formation of iodine-centered oxidants alongside hydroxyl radicals, which broadens the operative oxidant pool [6,13,14]. Across dyes and pharmaceuticals at near neutral pH, these studies generally report faster kinetics than UV/TiO2 or UV/IO4− alone [6,14]. The present study contributes data that resolve how a structured panel of diagnostic scavengers and representative water matrices, including deionized water, mineral water, and seawater, redistributes reactive pathways in UV/TiO2/IO4−. The analysis identifies conditions under which interfacial holes, hydroxyl radicals, and an electron-driven periodate branch dominate, with each attribution tied either to literature or to measurements in this study [15,16].
Our earlier contribution on SO removal by UV/TiO2/IO4− focused on operating conditions and mineralization in simplified media [3]. The present study builds on that work in two complementary ways. First, a mechanistic dissection of the UV/TiO2/IO4− system was provided using a structured seven-scavenger panel that apportions the relative roles of valence-band holes/•OH and the electron/periodate branch. Second, these insights were translated to realistic matrices by systematically comparing deionized water, mineral water, and seawater and by isolating the most consequential interferents (humic acid, Cl−, SO42−, NO2−, HCO3−) as well as nonionic surfactants (Tween 80, Triton X-100). Quantitatively, the three-way synergy of the UV/TiO2/IO4− process was also reported, confirming both strong coupling among UV, TiO2, and IO4− and high intrinsic reactivity under near-neutral pH. The primary innovation of this study is mechanistic; it provides a scavenger-based pathway map with direct performance implications that clarify matrix tolerance and guide application in real waters.
2. Results and Discussion
2.1. Synergistic UV/TiO2/IO4− Treatment
The photocatalytic degradation of SO (10 mg/L) was investigated using TiO2 (0.4 g/L) in the presence of IO4− ions (0.15 mM) under UV irradiation at pH 6. Preliminary tests were performed under IO4− alone, TiO2/IO4−, UV/IO4−, and classical photocatalysis (UV/TiO2) conditions to assess the contribution of each component. The resulting concentration–time data were analyzed using the pseudo-first-order kinetic model, which provides a quantitative means of determining the apparent rate constants for the different reaction systems (Figure 1). Residual analysis confirmed that deviations from the pseudo-first-order model were random and exhibited no systematic trends, thereby supporting the validity of the applied kinetic treatment. The complete concentration–time profiles have been published in the earlier study [3]. Across all conditions, including elevated scavenger concentrations, no departure from pseudo-first-order behavior was detected. The coefficients of determination (R2) for the kinetic fits consistently exceeded 0.98, underscoring the robustness of the model and the reliability of the derived rate constants. The UV/TiO2/IO4− system exhibited a first-order rate constant dramatically larger than any binary combination. These results establish an unequivocal super-additive response when UV, TiO2, and periodate act concurrently. Literature documents IO4− as a conduction band electron acceptor that limits recombination and as a photochemically activatable precursor of iodine-centered oxidants and hydroxyl radicals in UV/TiO2/IO4− and related UV/IO4− systems [6,13]. Evidence in this manuscript for operative oxidants, including interfacial holes, hydroxyl radicals, and an electron or superoxide branch, derives from the structured scavenger panel and the kinetic analyses described later. Interpretation integrates established aqueous radical rate constants and selectivity compendia with trends in inhibition strength and matrix dependence observed in this dataset, with explicit acknowledgment of caveats on scavenger selectivity in heterogeneous photocatalysis [15,16,17]. The synergy ratio (SR3) of the ternary system relative to the sum of the binary baselines was evaluated to quantify the interaction:
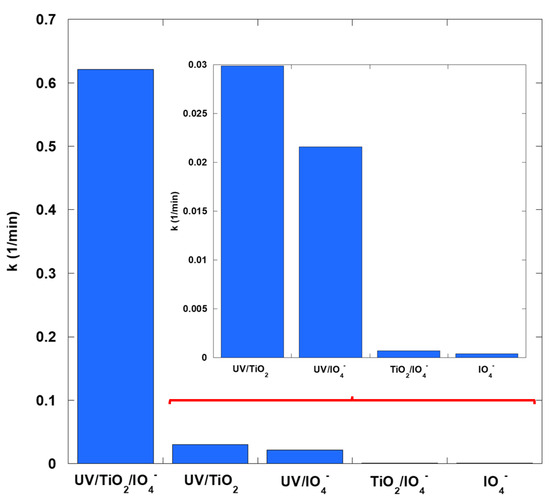
Figure 1.
Enhanced rate constants of SO removal by UV/TiO2/IO4− compared with individual and binary processes (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
The calculated synergy ratio is 11.9.
To formalize the baseline, the ternary synergy based on an additive expectation is defined as SR3 equals the observed pseudo-first order rate constant in the ternary system divided by the sum of the pseudo-first order rate constants measured for the binary systems studied here. This baseline reflects independent, parallel oxidation channels and is the analog of Bliss additivity used to screen for supra-additive behavior. For completeness, a conservative single process benchmark is also reported as SRmax equals the observed pseudo-first order rate constant in the ternary system divided by the largest pseudo-first order rate constant among the binary systems. Because the sum in the denominator of SR3 is greater than or equal to the maximum term, SR3 is less than or equal to SRmax for the same dataset. Reporting both values prevents overinterpretation and enables comparison with the broader advanced oxidation literature, where some studies adopt the sum baseline while others reference the highest single binary process.
For comparison, the enhancement relative to the best binary processes is substantial, with SRmax1 = () ≈ 20.8 and SRmax2 = () ≈ 28.8. This demonstrates that three-way coupling offers significantly greater benefits than any two-component combination. This level of synergy is consistent with the well-established role of periodate in photo-TiO2 systems. IO4− acts as a conduction-band electron scavenger. This suppresses electron/hole recombination and increases the flux of valence-band holes, which form •OH radicals from surface H2O/OH−. This electron sink function is widely recognized as the primary means of achieving higher apparent rates in TiO2 photocatalysis. Additionally, UV activation of periodate and the reduction of IO4− by photogenerated electrons open iodine-centered radical and high-valent iodine pathways (e.g., IO4•, IO3•, and I2•−). These pathways complement •OH/O2•− chemistry, broadening the oxidative spectrum to include cationic dyes, such as SO. Moreover, combining UV/TiO2 with IO4− creates a redox shuttle (I(VII) ⇌ I(V)/I(III)), accelerating interfacial charge transfer and sustaining radical production.
Under UVA irradiation at 365 nm and pH 6 in the presence of TiO2 and dissolved oxygen, IO4− functions as an electron acceptor and photochemical oxidant. In this matrix, periodate activation yields iodine-centered radicals IO3• and IO4• together with reactive oxygen species, and concomitantly undergoes reduction to iodate, which accumulates as the stable iodine sink. Transient spectroscopy on iodate and periodate in water has identified short-lived iodine(VI) intermediates that connect iodine(VII) to lower oxidation states during irradiation, supporting cycling within the iodine redox manifold under aqueous photochemical conditions [6,18]. Reports of homogeneous and heterogeneous UV- or UVA-activated periodate at circumneutral pH corroborate these steps and the dominance of iodate as the product under oxic conditions [18,19,20]. Iodine speciation was not resolved in this dataset. Reports on UV or photocatalytic periodate under oxic conditions identify iodate as the dominant product and document iodine-centered radicals and transient iodine intermediates, consistent with I(VII) to I(VI) to I(V) cycling under the used conditions [13].
The UV/TiO2/IO4− system yielded a pseudo-first order rate coefficient of 0.6212 min−1. Homogeneous UV–periodate systems on azo dyes report first-order fits with rate coefficients typically on the order of 10−2 to 10−1 min−1 at comparable ultraviolet fields and millimolar periodate [13]. Catalyst-activated periodate systems frequently report higher values; representative examples include values near 5 × 10−1 min−1 for pharmaceutical surrogates, subject to pollutant identity and matrix [21]. These ranges place the present value at the upper end of periodate AOP performance, consistent with the dual role of periodate as an electron acceptor that suppresses recombination and as a photochemically activatable oxidant precursor.
The weak performance of IO4− alone and TiO2/IO4− in the dark is also mechanistically coherent. Without photons, there are no TiO2 e−/h+ pairs, and periodate lacks an efficient activation channel. Thus, only slow direct oxidation or limited background homolysis occurs. Upon UV illumination, both direct photoactivation of IO4− and photocatalytic routes become available and reinforce each other. This explains the order-of-magnitude increase in k for the ternary system.
To illustrate, the nearly 21-fold improvement compared with the most effective binary systems, together with an SR3 value of 11.9, demonstrates that the ternary configuration harnesses photons, the catalyst, and the oxidant in both an additive and synergistic fashion. This outcome highlights the UV/TiO2/IO4− process as an exceptionally powerful advanced oxidation pathway for SO degradation.
2.2. Scavenger Impact
Understanding which oxidants and charge carriers govern SO degradation in the UV/TiO2/IO4− process is essential for mechanism-driven design. Seven mechanistic scavengers were employed to identify the dominant oxidant species within the UV/TiO2/IO4− process: ascorbic acid (AA), methanol (MeOH), benzoquinone (BQ), oxalate (OX), sodium dichromate (Na2Cr2O7), sodium azide (NaN3), and phenol (Ph).
To guide interpretation without over-committing to absolute energy placements, a concise band-centered narrative is adopted. Under pristine conditions, photoexcitation promotes electrons to the conduction band and creates holes in the valence band. Electrons reduce adsorbed oxygen to superoxide, and holes oxidize interfacial water or hydroxide to hydroxyl radicals, while band-to-band recombination remains a major loss channel. At intermediate additive levels where an electron acceptor is present, periodate traps conduction band electrons and forms iodine-centered oxidants, recombination is suppressed, and holes continue to generate hydroxyl radicals with modest competition from hole scavengers. At high additive levels, scavenging and trapping dominate, diverting both electrons and holes from productive routes, radical formation declines, and recombination regains prominence. This qualitative progression matches the measured trends and is consistent with the known pH-dependent and interface-sensitive positions of TiO2 band edges.
As illustrated in Figure 2, the inhibition patterns correspond to the expected redox and radical-scavenging chemistry of the UV/TiO2/IO4− system. Specifically, the rate of SO removal slows progressively as AA or MeOH is added. In the additive-free control, SO decays rapidly, reflecting the efficient separation of charges on TiO2 and the rapid generation of reactive intermediates (•OH, IO3•, and IO4•) from photoactivated periodate. These intermediates complement surface h+/•OH pathways.
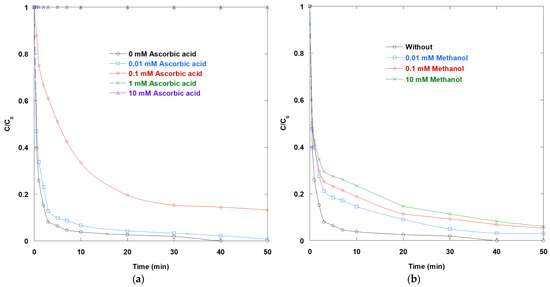
Figure 2.
Impact of (a) AA and (b) MeOH on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
Introducing AA results in strong, dose-dependent suppression (Figure 2a). It should be noted that complete inhibition of SO degradation was already observed at 1 mM AA. The kinetic profile at this concentration was superposed with that obtained at 10 mM, indicating that the threshold for total quenching is reached at or below 1 mM. Three concurrent mechanisms explain this potency. First, the reactive ascorbate monoanion (AH−) consumes •OH radicals at diffusion-controlled rates (k = 109–1010 M−1·s−1 [15]), diverting oxidants directly away from SO. Second, AA efficiently reduces photogenerated iodine radicals and oxidants from IO4− photolysis. This short-circuits the intended IO7−/IO4−/IO3− redox cycling. This erodes the electron-acceptor role of periodate, which normally suppresses e−/h+ recombination. Third, AA behaves as a fast hole/electron transfer substrate at illuminated TiO2, competing with SO for photogenerated holes and reducing the dye’s accessible oxidizing capacity. Together, these pathways explain why submillimolar AA decelerates SO degradation and why 1 mM AA largely quenches the process.
MeOH inhibits SO degradation in a clear, monotonic fashion, albeit less dramatically than AA at the same molarity (Figure 2b). This difference is mechanistically reasonable. MeOH is a well-known •OH and hole scavenger. It reacts with •OH with a reaction rate constant of (9–10) × 108 M−1·s−1 [15]. These reactions remove oxidizing equivalents from SO and reduce the effective radical/charge density near the semiconductor interface. Unlike AA, however, methanol does not strongly reduce iodine intermediates or periodate itself. Therefore, ternary synergy is primarily weakened by radical and hole quenching rather than by the collapse of the IO4− electron-acceptor cycle. This explains the milder inhibition at equal concentrations.
Viewed as a whole, the data emphasize that the remarkable performance of UV/TiO2/IO4− relies on three factors: access of SO to illuminated TiO2 surfaces, the rapid scavenging of photogenerated electrons by IO4−, and an undisturbed radical inventory from IO4− photolysis.
Adding either BQ or OX suppresses SO removal, and this inhibition is clearly dose-dependent (Figure 3). In the BQ series, concentrations of 0.01–0.1 mM result in modest slowing. However, concentrations of 1–10 mM leave a significant amount of residual SO after 50 min. This pattern aligns with p-BQ’s established role as a superoxide (O2•−) scavenger and electron acceptor in photocatalytic systems. BQ intercepts conduction-band electrons and O2•−, thereby diminishing the O2•−-pathway and the associated downstream oxidants (e.g., H2O2/•OH). These oxidants normally complement hole/•OH chemistry in TiO2 photocatalysis. Thus, the observed deceleration occurs. Note that BQ reacts rapidly with •OH (k = 6.6 × 109 M−1·s−1 [15]). Therefore, at mM doses, BQ competes with the dye for hydroxyl radicals and reduces the available oxidant. Together, these mechanisms provide a basis for the strong, monotonic inhibition observed. However, BQ can undergo side photochemistry (e.g., photogenerated 1O2 or altered •OH formation) and may not be perfectly selective for O2•− under UV conditions. Nevertheless, the results in Figure 3 show consistent suppression across the full BQ range. Under the present conditions (UV/TiO2/IO4−, pH 6), the net effect is inhibitory. This finding supports the meaningful contribution of O2•−/electron-mediated steps in the ternary process.
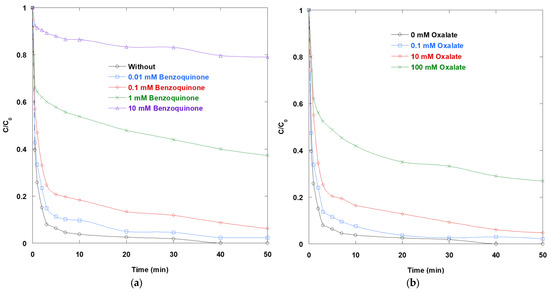
Figure 3.
Impact of (a) BQ and (b) OX on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
Similarly, OX impedes SO degradation (Figure 3b). It has minimal effects at 0.1 mM but substantially inhibits degradation at 10–100 mM. As a classic hole scavenger, oxalate binds to TiO2 and undergoes photodecarboxylation [22,23,24]. This process consumes valence-band holes, thereby reducing the formation of •OH at the interface. It also competes with dye oxidation. Strong adsorption near the TiO2 point of zero charge can block reactive sites, thereby reducing the reaction rate at higher concentrations.
In the context of the UV/TiO2/IO4− mechanism, these scavenger tests reinforce the cooperative roles of holes, •OH, and electron-derived species. Periodate typically acts as an efficient electron acceptor, suppressing e−/h+ recombination and participating in iodine-centered radical chemistry. The introduction of BQ competes for those electrons and removes superoxide anion radicals, while OX removes holes before they can oxidize surface water or adsorbates. The net result is slower SO removal in both series, highlighting that both charge-carrier channels are operative and disruption of either channel degrades performance.
Figure 4a shows that Cr2O72− markedly inhibits SO degradation, with stronger inhibition occurring at higher concentrations (0.1 mM, 1 mM, and 10 mM, in that order). In contrast, azide produces only a slight and nearly negligible slowdown at these concentrations (Figure 4b). These contrasting behaviors are consistent with the distinct roles of the two additives in photoinduced redox and radical chemistry.
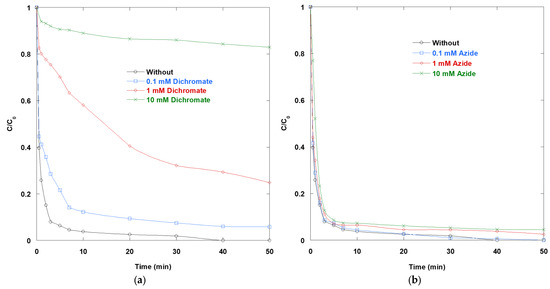
Figure 4.
Impact of (a) Cr2O72− and (b) N3− on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
Three effects act together for Cr2O72−. First, Cr2O72− strongly absorbs in the near-UV range (notably around 257 and 350 nm), creating an inner filter that reduces the number of photons reaching TiO2 and periodate. This optical penalty increases with concentration. Second, Cr2O72− is a potent electron acceptor at TiO2, forming surface complexes that are photoreduced to Cr(III). The resulting Cr(III) hydroxide/oxide deposits and related surface species are known to passivate active sites and promote recombination, progressively deactivating the photocatalyst. Third, in the UV/TiO2/IO4− system specifically, periodate functions as the intended electron sink and source of iodine-centered and •OH oxidants under UV light. However, introducing a competing electron acceptor (Cr2O72−) diverts electrons away from the reduction/activation of periodate. This weakens the synergy that normally accelerates dye removal. Under different conditions, Cr2O72− can promote photocatalysis by pairing electron consumption with hole-driven oxidation of organics. However, the present data show the opposite, likely because optical screening and surface deactivation outweigh the benefits of charge separation at pH 6 and the tested concentrations.
The minimal impact of N3− suggests that singlet oxygen (1O2) likely plays a minor role in the removal of SO. Azide is a classic quencher of singlet oxygen, with rate constants ranging from 108 to 109 M−1·s−1 [15]. If singlet oxygen were a dominant oxidant, strong inhibition would be evident. Additionally, azide reacts with •OH to form the azidyl radical (N3•), which oxidizes aromatics. This reaction pathway can partially compensate for •OH scavenging and help explain the weak net effect observed.
Collectively, these scavenger tests reinforce the idea that the impressive performance of UV/TiO2/IO4− hinges on maintaining photon delivery to TiO2, preserving periodate-mediated electron scavenging and radical formation, and avoiding surface passivation. Strongly absorbing/electron-accepting species, such as Cr2O72−, undermine these requirements (optical filtering, site blocking, and competition for electrons). However, azide’s quenching of 1O2 is largely irrelevant because 1O2 contributes minimally under these conditions.
Ph clearly inhibits SO degradation in a concentration-dependent manner (Figure 5). A concentration as low as 0.01 mM slows decay, while a concentration between 0.1 and 1.0 mM results in significant kinetic penalties. A concentration of 10 mM leaves a substantial residual after 50 min. This behavior is consistent with Ph’s well-documented reactivity toward oxidizing species and charge carriers that drive the UV/TiO2/IO4− process. Ph is an exceptionally fast scavenger of •OH in water, with a typical k = 109–1010 M−1·s−1 [15]. Thus, increasing Ph directly diverts hydroxyl radicals away from SO. Additionally, at pH 6, Ph is neutral (pKa = 10) and adsorbs onto TiO2 near its point of zero charge, where surface complexation and hydrogen bonding are favored. This adsorption competitively blocks catalytic sites and limits SO’s access to photogenerated holes, which are prerequisites for rapid photocatalytic oxidation. In addition, Ph acts as an efficient hole scavenger on illuminated TiO2; the rapid oxidation of Ph to phenoxyl/semiquinone radicals competes with dye oxidation. This reduces the effective hole flux available to initiate surface •OH and direct electron-transfer reactions. These inhibitory pathways are essential to the UV/TiO2/IO4− system because IO4− scavenges conduction-band electrons. This process suppresses recombination and generates iodine-centered oxidants and •OH upon photoactivation. However, Ph erodes these three advantages by consuming •OH, competing for holes, and occupying TiO2 surface sites. This explains the strong, monotonic slowdown in SO removal as Ph concentrations increase.
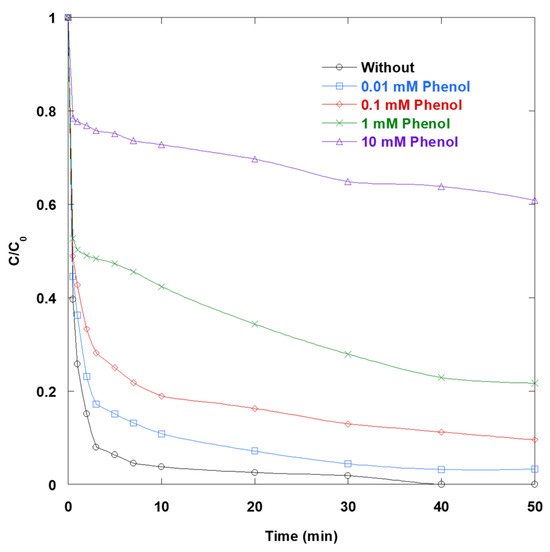
Figure 5.
Impact of Ph on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
In summary, scavenger tests demonstrate that SO removal in the UV/TiO2/IO4− process depends on the presence of interfacial holes, •OH radicals, and electron-driven pathways. The strong inhibition by AA and Ph suggests rapid •OH (and hole) quenching and competitive interfacial electron transfer. Meanwhile, MeOH produces a milder slowdown, consistent with its role as a •OH/hole scavenger. The pronounced effects of BQ and OX highlight the importance of the e−/O2•− branch and valence-band holes, respectively. BQ intercepts conduction-band electrons and O2•−, and OX acts as a classic TiO2 hole scavenger. Cr2O72− suppresses performance optically due to strong UV absorption and inner filtering, as well as kinetically by competing for electrons. This undermines the periodate-assisted charge separation that drives the ternary synergy. In contrast, azide has little impact, suggesting that 1O2 is, at most, a minor contributor under these conditions, despite azide’s high quenching rate for singlet oxygen.
Selectivity limits of the scavenger panel warrant clarification. BQ intercepts conduction band electrons and superoxide, yet also reacts rapidly with hydroxyl radicals in water, with a reported second-order rate constant of 6.6 × 109 M−1·s−1. Therefore, inhibition by BQ reflects joint interception of electrons, superoxide, and hydroxyl radicals. Ph and MeOH each remove hydroxyl radicals and also consume photogenerated holes at the TiO2 surface, which complicates assignment based on a single quencher. Under these conditions, interpretation benefits from orthogonal diagnostics. EPR with spin trapping can be used to confirm the presence of radical intermediates, including superoxide adducts that are stabilized by traps such as 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DEPMPO) or 5-(diisopropoxyphosphoryl)-5-methyl-1-pyrroline N-oxide (DIPPMPO). Independent singlet oxygen readout can be obtained from furfuryl alcohol loss or from the conversion of 2,2,6,6-tetramethylpiperidine (TEMP) to 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) with appropriate controls. The combined approach reduces misattribution and supports the conclusion that holes and hydroxyl radicals are the predominant oxidants, while the electron and superoxide branches serve as contributory pathways.
The inference of iodine-centered radicals from scavenger outcomes and matrix effects is consistent with direct observations reported for periodate systems. In UV/TiO2/IO4−, EPR with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) shows hydroxyl and superoxide signals in agreement with the probe trends, supporting a mixed oxidant pool in this photocatalytic matrix [6]. In ozone activated periodate, EPR resolves an iodate radical and confirms concurrent reactive oxygen species, demonstrating that iodine species can be captured by spin trapping under aqueous conditions [25]. Transient spectroscopic studies on photoactivated periodate further identify short lived iodine intermediates that connect periodate to lower iodine oxidation states, which is compatible with the proposed pathway [20].
2.3. Humic Acid Impact
Figure 6 shows that humic acid (HA) inhibits the degradation of SO via UV/TiO2/IO4− process. Higher HA concentrations (5–20 mg/L) result in greater inhibition. HA acts as an inner UV filter that competes for photons in the near-UV range. This reduces the fluence available to excite TiO2 and activate periodate photochemically. This optical penalty is widely observed in natural organic matter (NOM)-rich waters. Additionally, HA strongly adsorbs onto TiO2 at around pH 6, altering the surface charge, aggregation, and occlusion of reactive sites. This suppresses hole-driven interfacial oxidation and promotes carrier recombination. Furthermore, HA’s phenolic and electron-rich moieties scavenge •OH and related oxidants at high composite rate constants, diverting radicals away from the dye. These mechanisms erode the synergy that makes the UV/TiO2/IO4− combination effective. This synergy includes fast electron scavenging by IO4−, which limits e−/h+ recombination, and multi-reactive oxygen species generation under irradiation. By lowering photon delivery, blocking TiO2 sites, and consuming radicals, HA shifts the system toward slower, less efficient pathways, even in the presence of periodate.
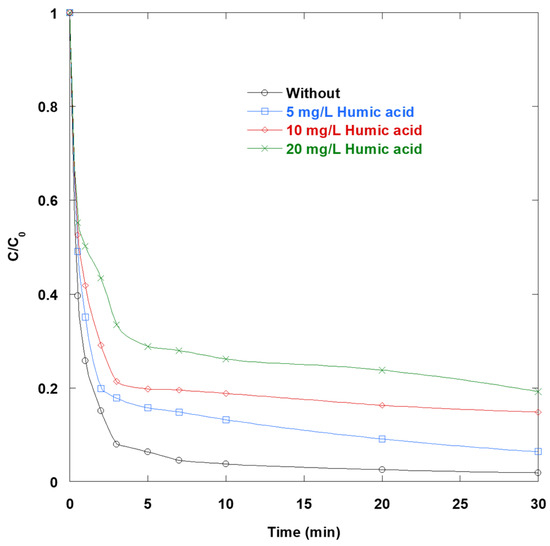
Figure 6.
Impact of HA on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
In practice, these results imply that matrices rich in NOM will require countermeasures such as pretreatment to reduce HA, a higher UV fluence or IO4− dose, or surface modifications that prevent HA adsorption.
2.4. Salt Impact
Figure 7a shows how chloride affects SO removal in the UV/TiO2/IO4− process. As the NaCl concentration increases from 0.1 to 100 mM, SO decay progressively slows. This is consistent with three chloride-driven effects. First, chloride converts •OH into reactive chlorine species (RCS) through rapid reactions yielding Cl2•−/ClOH•−. These RCS are longer-lived; however, they react with organics much more slowly than •OH. Typical kCl2•− values range from 105 to 108 M−1·s−1 [26], whereas k•OH values range from 109 to 1010 M−1·s−1 [15]. This reduces the effective oxidant strength experienced by SO. Second, chloride competes with photogenerated holes on illuminated TiO2 and blocks adsorption sites. This lowers interfacial •OH formation and hinders dye access to reactive centers. Third, photocatalytic rates decrease further at higher salinity levels due to a reduction in active surface area, as well as an alteration of the double layer caused by increased ionic strength, TiO2 aggregation, and anion coverage.
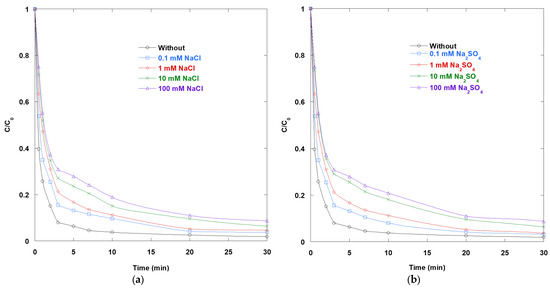
Figure 7.
Impact of (a) NaCl and (b) Na2SO4 on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
The UV/TiO2/IO4− process relies on fast hole/•OH chemistry at the TiO2 interface, periodate-mediated electron scavenging, and multi-radical generation. The shift from •OH to the less reactive RCS, coupled with surface and site effects, explains the consistent decline in performance in the presence of chloride. Some removal persists at moderate chloride concentrations due to the residual activity of RCS and non-radical pathways. However, the net influence is clearly inhibitory.
Figure 7b shows that increasing the concentration of Na2SO4 from 0.1 to 100 mM progressively slows the decay of SO. This suggests that sulfate interferes with both interfacial charge transfer and oxidant availability. Sulfate competitively consumes •OH to form SO4•− (•OH + SO42− → SO4•− + OH−) [16]. For the aqueous matrices examined, the reaction of hydroxyl radical with sulfate is not expected to be a controlling sink. Using a representative second-order rate constant of about 1.2 × 107 M−1·s−1 and a sulfate level of approximately 28–30 mM in seawater, the corresponding pseudo-first order loss rate is about 3.4–3.6 × 105 s−1. In the same matrices, chloride attains about 0.5–0.6 M and reacts with the hydroxyl radical at 4.3 × 109 M−1·s−1, giving pseudo-first-order loss rates ≥2 × 109 s−1. Consequently, the chloride pathway dominates hydroxyl radical scavenging by roughly four orders of magnitude, whereas conversion to sulfate radical via sulfate remains a minor channel under the conditions used. Since SO4•− oxidizes aromatics more slowly than •OH, the radical pool becomes less effective as sulfate increases. In parallel, elevated ionic strength and the divalent character of SO42− promote TiO2 aggregation and compress the electrical double layer. These effects reduce accessible surface area and mass transfer. Additionally, sulfate can specifically adsorb onto metal oxide surfaces, altering surface charge and blocking reactive sites. This type of inner-/outer-sphere complexation is well-established on oxides and helps explain the rate losses observed. Together with the known sensitivity of TiO2 photocatalysis to inorganic anions, these factors help explain the monotonic inhibition observed here. Sulfate shifts the system away from fast hole/•OH-driven oxidation at illuminated TiO2 toward less reactive pathways, simultaneously diminishing the effective catalytic surface area.
Figure 8a shows that bicarbonate produced only a modest and gradual decrease in SO removal in the UV/TiO2/IO4− system at pH 8.3, even at concentrations up to 100 mM. This muted effect is mechanistically consistent with radical speciation and interfacial chemistry under these conditions. At pH 8.3, the carbonate system is dominated by HCO3− (pK1 = 6.35; pK2 = 10.33). Thus, •OH is primarily diverted to the carbonate radical (CO3•−) via the reaction •OH + HCO3− (k = 107 M−1·s−1 [15]) rather than being strongly quenched. CO3•− is less reactive than •OH; however, it remains a competent and selective oxidant toward electron-rich aromatics (k typically 105–108 M−1·s−1 [27]). This helps sustain dye decay. Surface processes further explain the resilience. At this pH, the TiO2 surface becomes negatively charged, favoring the electrostatic adsorption of cationic dyes and promoting hole-driven oxidation near the semiconductor interface. Bicarbonate and carbonate can adsorb onto titania, forming (bi)carbonate surface species [28]. However, under our conditions, the benefits of the strong SO-TiO2 interaction outweigh this adsorption. Periodate continues to act as an efficient electron acceptor and photochemically activatable oxidant source (•OH, IO3•, IO4•, etc.), so the ternary synergy that accelerates degradation remains largely intact despite bicarbonate buffering. Overall, the data suggest that at circum-alkaline pH, bicarbonate moderates, but does not impede, the performance of UV/TiO2/IO4−. Some •OH is converted to CO3•−, and some sites are covered by (bi)carbonate. Nevertheless, SO adsorption at negatively charged TiO2 and robust periodate-mediated charge separation maintain high removal rates.
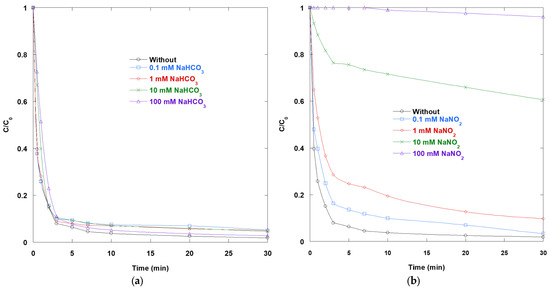
Figure 8.
Impact of (a) NaHCO3 and (b) NaNO2 on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 8.3 for (a) and pH 6 for (b)).
The apparent difference in pH between baseline conditions (6) and bicarbonate-containing systems (8.3) arises from the intrinsic buffering capacity of bicarbonate rather than from an imposed adjustment. Importantly, our previous study [3] demonstrated that the degradation efficiency of the UV/TiO2/IO4− system is nearly identical at pH 6 and 8, confirming that this modest shift does not significantly influence photocatalytic performance. Within this range, TiO2 exhibits a surface charge that is nearly neutral at pH 6 and slightly negative at pH 8.3, conditions that remain favorable for adsorption of the cationic dye. Periodate speciation is unaffected, with IO4− prevailing as the dominant form.
Figure 8b illustrates the effect of nitrite on SO removal in the UV/TiO2/IO4− process. Nitrite causes strong, dose-dependent inhibition. There is a slight deceleration at concentrations of 0.1–1 mM, which becomes more pronounced at 10 mM and essentially stops the reaction at 100 mM. Three effects explain this trend. Nitrite reacts with •OH at a near-diffusion-controlled rate of k = 1.0 × 1010 M−1·s−1 [15], converting these potent oxidants into NO2•. NO2• is a moderate oxidant with an E° = 1.03 V/NHE, and it is far less effective toward many aromatics. The resulting drop in oxidative strength directly slows SO decay. Additionally, nitrite is readily oxidized at illuminated TiO2 to nitrate and related intermediates. This consumes valence-band holes that would otherwise drive interfacial •OH formation and dye oxidation. Moreover, NO2− absorbs in the 300–400 nm range (ε = 22 M−1·cm−1 at ~354–355 nm). At the studied concentration levels, this significantly reduces the number of photons that reach TiO2 and periodate, thereby increasing kinetic losses. Although nitrite photolysis can generate •OH under UVA, the combined radical/hole scavenging and optical screening outweigh such production. This leads to the observed net inhibition. Since high activity in UV/TiO2/IO4− relies on periodate-assisted charge separation and multi-radical formation, nitrite emerges as a critical interferent.
Overall, the kinetic limitations observed in saline matrices arise from two interrelated mechanisms. First, radical speciation shifts divert •OH into less reactive oxidants, with Cl− generating longer-lived chlorine radicals (Cl2•−/ClOH•−) and HCO3− producing CO3•−, thereby lowering the effective second-order reactivity toward SO. Second, interfacial and colloidal effects induced by elevated ionic strength and divalent cations (e.g., Ca2+, Mg2+) compress the electrical double layer, promote TiO2 aggregation, and enhance the adsorption of anions that block catalytic sites. Collectively, these pathways attenuate the periodate-assisted synergy that otherwise accelerates UV/TiO2/IO4− performance in low-salinity water.
2.5. Water Matrix Impact
Safranin O degradation via the UV/TiO2/IO4− process was investigated in mineral water and seawater, and the results were compared with those obtained in deionized water (Figure 9).
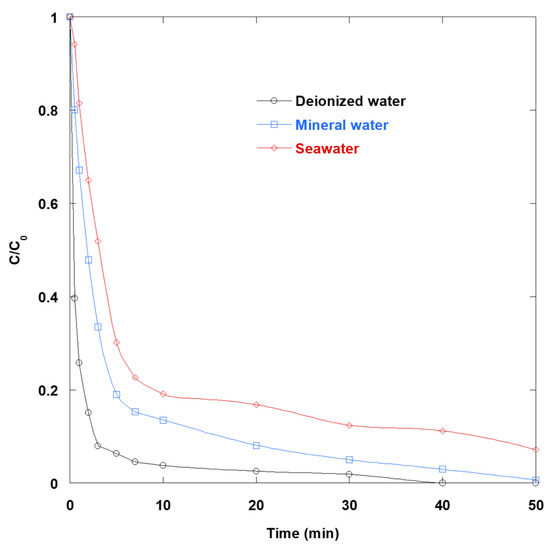
Figure 9.
SO removal via UV/TiO2/IO4− process in various water matrices (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C).
The principal characteristics of the two water sources employed are summarized as follows:
- –
- Mineral water: pH 7.2; salinity: 561 mg/L; Ca2+: 99 mg/L; Mg2+: 24 mg/L; K+: 2.1 mg/L; Na+: 15.8 mg/L; HCO3−: 265 mg/L; SO42−: 68 mg/L; Cl−: 72 mg/L; NO3−: 15 mg/L; NO2− <0.02 mg/L; total organic carbon: 0.33 mg/L.
- –
- Seawater: pH 7.5; salinity: 35.0 g/L; Ca2+: 0.4 g/L; Mg2+: 1.3 g/L; Na+: 11.0 g/L; SO42−: 3.0 g/L; Cl−: 20.0 g/L; total organic carbon: 0.97 mg/L.
Although the present study demonstrates that the compositions of mineral water and seawater encompass multiple concurrently present species, the individual contributions of chloride, sulfate, ionic strength, and divalent cations cannot be fully separated under these complex conditions. The current findings suggest that chloride predominantly affects radical scavenging, whereas sulfate contributes mainly through ionic strength effects. However, synergistic or antagonistic interactions among these species cannot be excluded. A systematic series of single-salt investigations across environmentally relevant concentration ranges, ideally coupled with advanced mechanistic probes, will be required in future work to corroborate and refine the processes postulated in this work.
Figure 9 shows that SO disappeared fastest in deionized water, slowed in mineral water, and disappeared slowest in seawater. This hierarchy reflects the effects of background ions and natural constituents on interfacial charge transfer on TiO2, as well as on the available oxidant pool.
In deionized water, the ternary process operates near optimally. Periodate efficiently scavenges photogenerated electrons, and under UV light, it yields iodine-centered species together with •OH. These features make the UV/TiO2/IO4− system highly active and comparatively pH-tolerant. In mineral water, moderate levels of HCO3−, Cl−, SO42−, and divalent cations (Ca2+/Mg2+) introduce penalties that are well known. Bicarbonate converts •OH to the less reactive and selective CO3•− radical, chloride diverts •OH to reactive chlorine species (Cl2•−/ClOH•−), which generally oxidize organics more slowly than •OH, and higher ionic strength with Ca2+/Mg2+ compresses the electrical double layer. This promotes TiO2 aggregation and decreases the accessible surface area. The net result is a moderate slowdown in kinetics compared to deionized water. The seawater matrix exacerbates all of these effects. Very high Cl− (and SO42−) shifts the oxidant speciation further from •OH toward halogen- and sulfate-derived radicals. The brine-level ionic strength accelerates TiO2 aggregation and sedimentation and alters interfacial adsorption. Light screening by concentrated salts can also reduce the effective UVA fluence. Consequently, the synergistic effect of UV/TiO2/IO4− is reduced, and SO removal is slowest in seawater.
2.6. Surfactant Impact
The impact of non-ionic surfactants Triton X-100 and Tween 80 on SO degradation via UV/TiO2/IO4− process was shown in Figure 10a,b. The results clearly indicate that the presence of non-ionic surfactants consistently hinders the degradation of SO. The degree of inhibition increases with the amount of surfactant added. Without surfactants, SO is nearly depleted within 10 min. In contrast, after 30 min, a significant amount of residual SO remains when 10 mM of either Triton X-100 or Tween 80 is present. Intermediate concentrations (0.1–1 mM) produce graded, dose-dependent slowdowns. This behavior is expected for TiO2 photocatalysis at pH 6 (near the point of zero charge of TiO2), where efficient removal relies on access to photogenerated holes/•OH and the electron acceptor periodate at the interface. Surface-active organics impede these pathways by occupying active sites and altering the interfacial microenvironment. Two concentration regimes explain these trends. Below or near the critical micelle concentration (CMC), individual surfactant molecules adsorb onto the TiO2-water interface. There, they compete with SO and IO4− for surface sites. This lowers the density of reactive centers and suppresses •OH formation and dye adsorption. Nonionic ethoxylates and polysorbates are known to adsorb appreciably onto TiO2. The result is a modest but measurable kinetic penalty at 0.01 mM. Above the CMC, micellization dominates. SO partitions into the palisade/hydrophobic region of micelles, spatially separating the chromophore from the catalyst surface and reactive radicals. Concomitantly, micelles and surfactant layers can screen light and further reduce surface access. In micellar media, •OH radicals are less able to attack solutes buried within aggregates. This results in lower photocatalytic rates, a pattern also observed at 0.1–10 mM.
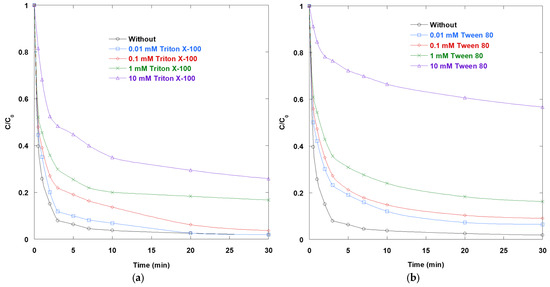
Figure 10.
Impact of non-ionic surfactants (a) Triton X-100 and (b) Tween 80 on the removal of SO via UV/TiO2/IO4− process (0.15 mM periodate, 0.4 g/L catalyst, 10 mg/L SO, 25 °C, pH 6).
The contrasting CMCs of the two surfactants help explain the differences observed in the panels. The CMC of Triton X-100 in water ranges from 0.22 to 0.30 mM. In contrast, the CMC of Tween 80 ranges from 0.011 to 0.015 mM, about one order of magnitude lower. Consequently, 0.1 mM of Tween 80 is well above its CMC and strongly micellizing, while 0.1 mM of Triton X-100 remains below its CMC. At concentrations of 1–10 mM, both are well above their respective CMCs and strongly inhibitory. It should be noted that the CMC values referenced here are derived from measurements in pure water, whereas the actual micellization threshold may shift in the presence of TiO2, NaIO4, and at pH 6. In this study, the real CMC values under the experimental conditions were not quantified, and the discussion is therefore based on literature values as a conceptual framework. Given that ionic strength, divalent cations, and nanoparticle surfaces can influence micellization, future investigations should include direct CMC determinations in the relevant matrices to validate and refine the proposed interpretation. Typically, Tween 80 has a greater effect at equal molarity because it is deeper into the micellar regime.
Beyond sequestration and site blocking, chemical competition also contributes. Poly(ethylene oxide) chains and alkylphenol ethoxylates react rapidly with •OH and other oxidants. In the present photocatalytic AOP, the surfactants divert reactive species away from SO and the TiO2 surface, compounding the aforementioned physical effects.
Inhibition is particularly significant in the UV/TiO2/IO4− process because IO4− acts as an efficient electron acceptor that inhibits e−/h+ recombination. However, surfactant adsorption or micelle formation can reduce access of periodate to the catalyst surface. Consequently, electron scavenging becomes less effective, and the synergy of the ternary system diminishes. This is consistent with the observed convergence of the curves toward slower kinetics as the surfactant concentration increases.
When considered jointly, the data suggest that nonionic surfactants hinder SO degradation in three ways. First, they competitively adsorb onto TiO2, thereby blocking reactive sites. Second, at concentrations above the CMC, the surfactants sequester SO in micelles, limiting interfacial contact and radical attack. Third, they scavenge radicals with surfactant chains. To maintain the significant enhancement provided by periodate-assisted TiO2 photocatalysis, matrices containing detergents require one of the following strategies: a higher photon fluence or oxidant dose; pretreatment to remove surfactants; or methods to prevent interfacial fouling. These strategies include adsorptive pre-concentration, flow regimes that shear off soft layers, and catalyst modifications.
3. Materials and Methods
Safranin O (SO) and sodium periodate (NaIO4) were obtained from Fluka (Morris Plains, NJ, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. The photocatalyst was TiO2 (Evonik P25; 80% anatase/20% rutile; mean particle size = 30 nm; BET surface area = 50 m2/g). All stock solutions were prepared in deionized water.
Photocatalytic experiments were conducted in a jacketed cylindrical Pyrex reactor (inner diameter 6 cm, height 15 cm, working volume 200 mL) equipped with a water-circulating jacket connected to a thermostatic bath to maintain 25 ± 1 °C. Illumination was supplied by a low-pressure mercury lamp (Oriel 6035 (UVP), Irvine, CA, USA); 15 mW/cm2, principal emission at 365 nm) housed in a quartz sleeve and positioned vertically at the reactor center, 2 cm above the base. The distance between the lamp and the reactor wall was 3 cm, ensuring uniform photon distribution. Continuous magnetic stirring at 300 rpm maintained a homogeneous suspension of TiO2 particles and uniform irradiation. The reactor was covered with an aluminum foil shield to prevent stray light and minimize photolysis outside the intended volume.
A suspension containing 200 mL of SO (10 mg/L) and TiO2 (0.4 g/L) was stirred in the dark for 30 min to establish adsorption/desorption equilibrium. For the conventional UV/TiO2 runs, irradiation commenced immediately after dark equilibration. For UV/TiO2/IO4− experiments, an appropriate amount of NaIO4 was added at the instant the lamp was switched on. Samples withdrawn at defined intervals were centrifuged (10,000 rpm, 30 min) to remove photocatalyst and analyzed at 519 nm with a UV–Vis spectrophotometer (Biochrom, Cambridgeshire, UK) using 1 cm quartz cuvettes. The experimental conditions were established from insights gained in our prior parametric studies [3]. Complete mixing was validated by a salt-pulse conductivity tracer (global mixing time) and by the Villermaux–Dushman test (micromixing) [29], with the impeller Reynolds number reported to document turbulent conditions. Finally, adsorptive loss to tubes was checked by processing dye-only standards through the same centrifugation protocol. The results indicated that no adsorption occurred onto the centrifuge tubes.
BQ functions as an interceptor of conduction band electrons and superoxide, rapidly coupling with O2•− and accepting electrons, which modulates the electron or superoxide branch in photocatalysis [17,30,31]. OX primarily acts as a valence-band hole scavenger on TiO2 and is widely used to suppress hole-driven oxidation while promoting electron availability [32,33]. MeOH and Ph are standard probes for hydroxyl radicals and holes in water, with second-order rate constants for reaction with hydroxyl radicals in the range of 108 to 1010 M−1·s−1 [15,16]. Azide quenches singlet oxygen in water with rate constants near 3 to 5 × 108 M−1·s−1, so a weak response indicates at most a minor singlet oxygen contribution under the present conditions [34,35].
Scavenger molecules can retard the observed kinetics through site blocking on TiO2 surfaces and through consumption of reactive intermediates in solution or at the interface. To distinguish these channels, the procedure first measures equilibrium adsorption of SO and each probe onto TiO2 in the dark at the experimental ionic strength and pH and reports the adsorption coefficients. Rates are also normalized by the measured photon fluence from ferrioxalate actinometry to remove any light screening by the probe solution. Selection of probes emphasizes minimal adsorption for bulk hydroxyl radical quenching with MeOH, electron or superoxide interception with BQ, and hole interception with OX.
Spectral analysis confirmed that the concentrated salts employed exhibit negligible absorbance at 365 nm, excluding their contribution to optical screening. In contrast, dichromate displays strong absorption bands extending into the near-UV, while humic acids show broad absorption tails across 300–400 nm. Accordingly, the attenuation observed in dichromate- and humic acid-containing systems reflects not only radical scavenging but also partial photon absorption, whereas the effects of inorganic salts are restricted to solution chemistry without measurable optical interference.
Under the present conditions (pH 6), IO4− is the predominant iodine species. Although the experiments were designed to minimize direct photolysis of IO4− at 365 nm, redox cycling among I(VII), I(V), and I(III) cannot be excluded, and partial accumulation of iodate or iodide may occur [18]. In this study, iodine speciation was not directly monitored, and the discussion is therefore based on the dominant role of IO4− as an electron acceptor.
Varying the SO solution pH between 1 and 10 did not alter λmax or the initial absorbance at λmax, ensuring robust spectrophotometric quantification.
All trials were conducted in triplicate, and mean values are reported. Differences between conditions were evaluated using two-tailed Student t tests, with p < 0.03 taken as statistically significant.
4. Conclusions
UV/TiO2/IO4− is a highly effective AOP for SO under ambient conditions. With a first-order rate constant of 0.6212 min−1 and a ternary synergy ratio of 11.9, it is approximately 20 times faster than the most efficient binary system (UV/TiO2). Data converge on a mechanism in which valence-band holes and hydroxyl radicals at the TiO2 interface interact with electron-periodate chemistry (iodine-centered radicals and related species). Periodate serves as an efficient electron sink, suppressing recombination. Targeted scavenger tests mapped the operative pathways and the most consequential interferents. AA and Ph produced the strongest inhibition by rapidly consuming •OH/holes and competing at the TiO2 surface. Meanwhile, MeOH caused a milder yet consistent slowdown through •OH/hole quenching. BQ (an electron/superoxide interceptor) and OX (a hole scavenger) reduced performance, confirming the importance of both charge-carrier branches. Dichromate reduced removal via optical screening and competition for electrons. Azide had a negligible effect, suggesting that 1O2 played a minor role under these conditions. Water-matrix constituents modulated outcomes in predictable ways. Humic acid impeded removal through light screening, surface fouling, and radical consumption. Chloride and sulfate shifted oxidant speciation from •OH toward more stable halogen and sulfate radicals. They also promoted colloidal and charge-layer effects that reduced the active surface area. At pH 8.3, bicarbonate converted some of the •OH to CO3•−, yet substantial activity remained. Performance consistently ranked as follows: deionized water > mineral water > seawater. These results highlight the cumulative impact of halides, sulfate, alkalinity, and ionic strength in real waters. In addition, the nonionic surfactants Tween 80 and Triton X-100 were found to suppress SO degradation by promoting micelle-mediated solubilization and blocking TiO2 active sites, thereby diverting reactive species and restricting interfacial access. These results identify surfactants as practical interferents that must be considered in real-water applications.
Because interpretations based on chemical scavengers remain indirect, future studies that integrate spin-trapping EPR with selective probes at controlled concentrations are anticipated to more clearly distinguish the hole, hydroxyl radical, and superoxide pathways under the reported conditions.
In practice, these insights provide clear design guidelines. Favor conditions that maintain strong dye-TiO2 contact and rapid electron scavenging by periodate. Anticipate losses in matrices with high concentrations of reducing agents, phenolic compounds, surfactants, halides, sulfates, or NOM. Mitigate these losses through pretreatment, increased UV fluence or periodate dose, or surface or particle engineering that resists site blocking and halide or NOM adsorption. The demonstrated activity and pH tolerance of UV/TiO2/IO4−, coupled with its mechanistically grounded sensitivities, provide a robust foundation for scaling up and for future work on catalyst architectures tailored to saline and NOM-laden waters.
In saline effluents, performance declines with increasing ionic strength and chloride because oxidant speciation shifts away from hydroxyl radicals, and aggregation and charge-layer compression reduce the accessible TiO2 surface area, which explains the observed order: deionized water > mineral water > seawater. In surfactant-rich effluents, nonionic surfactants such as Tween 80 and Triton X-100 sequester dyes into micelles and occupy TiO2 sites that divert reactive species and restrict interfacial access, so treatment outcomes favor matrices with limited surfactant loads or conditions that reduce micelles before photocatalysis.
Although the UV/TiO2/IO4− process exhibits strong mechanistic and kinetic advantages, the relatively high cost of periodate represents a limitation for large-scale applications. Practical implementation will require strategies that reduce oxidant demand or offset reagent cost, including catalyst modifications that enhance electron transfer efficiency, coupling with renewable UV sources, or selective deployment for high-value or recalcitrant effluents where conventional oxidants are less effective. Consideration of these economic and scalability factors is essential for translating the laboratory performance of UV/TiO2/IO4− into viable treatment technologies.
Author Contributions
Investigation, validation, data curation, visualization, M.B.; conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition, O.H.; validation, visualization, supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the Ongoing Research Funding Program, (ORFFT-2025-086-2), King Saud University, Riyadh, Saudi Arabia for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hamdaoui, O. Innovative and Hybrid Advanced Oxidation Processes for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2025; ISBN 978-0-443-14100-3. [Google Scholar]
- Chakravorty, A.; Roy, S. A Review of Photocatalysis, Basic Principles, Processes, and Materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Bendjama, M.; Hamdaoui, O.; Ferkous, H.; Alghyamah, A. Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization. Catalysts 2022, 12, 1460. [Google Scholar] [CrossRef]
- Mezyk, S.P.; Elliot, A.J. Pulse Radiolysis of Iodate in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1994, 90, 831–836. [Google Scholar] [CrossRef]
- Lei, Y.; Lei, X.; Westerhoff, P.; Zhang, X.; Yang, X. Reactivity of Chlorine Radicals (Cl• and Cl2•−) with Dissolved Organic Matter and the Formation of Chlorinated Byproducts. Environ. Sci. Technol. 2021, 55, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kamali, M.; Uleners, T.; Symus, J.; Zhang, S.; Liu, Z.; Costa, M.E.V.; Appels, L.; Cabooter, D.; Dewil, R. UV/TiO2/Periodate System for the Degradation of Organic Pollutants—Kinetics, Mechanisms and Toxicity Study. Chem. Eng. J. 2022, 449, 137680. [Google Scholar] [CrossRef]
- Mohan, C.; Kumari, P.; Kumari, N.; Negi, A. Fabrication of Colored Polymeric Membrane Using Clay-Based Nano Pigments of Safranin O (SO) Dye. Membranes 2023, 13, 619. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S.A. Safranin-O Removal from Aqueous Solutions Using Lignin Nanoparticle-g-Polyacrylic Acid Adsorbent: Synthesis, Properties, and Application. Adsorpt. Sci. Technol. 2018, 36, 1422–1440. [Google Scholar] [CrossRef]
- Ghosh, I.; Kar, S.; Chatterjee, T.; Bar, N.; Das, S.K. Adsorptive Removal of Safranin-O Dye from Aqueous Medium Using Coconut Coir and Its Acid-Treated Forms: Adsorption Study, Scale-up Design, MPR and GA-ANN Modeling. Sustain. Chem. Pharm. 2021, 19, 100374. [Google Scholar] [CrossRef]
- Nejadebrahim, A.; Ebrahimi, M.; Allonas, X.; Croutxé-Barghorn, C.; Ley, C.; Métral, B. A New Safranin Based Three-Component Photoinitiating System for High Resolution and Low Shrinkage Printed Parts via Digital Light Processing. RSC Adv. 2019, 9, 39709–39720. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Karthika, A.; Seenivasagan, R.; Kasimani, R.; Sudhakara Rao, J.; Poonkuzhali, K. Microbial Technologies for Sustainable Textile Effluent Treatment: A Review. J. Environ. Chem. Eng. 2024, 12, 113275. [Google Scholar] [CrossRef]
- Chia, L.; Tang, X.; Weavers, L.K. Kinetics and Mechanism of Photoactivated Periodate Reaction with 4-Chlorophenol in Acidic Solution. Environ. Sci. Technol. 2004, 38, 6875–6880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, C.S. Effect of Hydrogen Peroxide, Periodate and Persulfate on Photocatalysis of 2-Chlorobiphenyl in Aqueous TiO2 Suspensions. Water Res. 1999, 33, 2031–2036. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B.; Buxton, G.V.; Greenstock, C.L.; Helman, P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (•OH/O•−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Carlsson, C.; Fégeant, B.; Svensson, E.; Wiklund, L.; Jonsson, M. On the Selectivity of Radical Scavengers Used To Probe Hydroxyl Radical Formation in Heterogeneous Systems. J. Phys. Chem. C 2022, 126, 12435–12440. [Google Scholar] [CrossRef]
- Kläning, U.K.; Sehested, K.; Wolff, T. Laser Flash Photolysis and Pulse Radiolysis of Iodate and Periodate in Aqueous Solution. Properties of Iodine(VI). J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 1707–1718. [Google Scholar] [CrossRef]
- Tian, F.X.; Hu, X.J.; Xu, B.; Zhang, T.Y.; Gao, Y.Q. Phototransformation of Iodate by UV Irradiation: Kinetics and Iodinated Trihalomethane Formation during Subsequent Chlor(Am)Ination. J. Hazard. Mater. 2017, 326, 138–144. [Google Scholar] [CrossRef]
- Nessaibia, M.; Ghodbane, H.; Ferkous, H.; Merouani, S.; Alam, M.; Balsamo, M.; Benguerba, Y.; Erto, A. Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water. Water 2023, 15, 758. [Google Scholar] [CrossRef]
- Jian, J.; Zhang, S.; Chen, P.; Liu, D.; Wang, Y.; Liu, L.; Xiao, Z.; Xu, Z.; Pan, Y.; Lv, W.; et al. Innovative Spherical Fe-Mn Layered Double Hydroxides (LDH) for the Degradation of Sulfisoxazole through Activated Periodate: Efficacy and Mechanistic Insights. Environ. Pollut. 2025, 367, 125598. [Google Scholar] [CrossRef]
- Mendive, C.B.; Blesa, M.A.; Bahnemann, D. The Adsorption and Photodegradation of Oxalic Acid at the TiO2 Surface. Water Sci. Technol. 2007, 55, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Roncaroli, F.; Blesa, M.A. Kinetics of Adsorption of Oxalic Acid on Different Titanium Dioxide Samples. J. Colloid. Interface Sci. 2011, 356, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Y.; Zhang, H.; Liu, P.; Zhao, H. Adsorption and Oxidation of Oxalic Acid on Anatase TiO2 (001) Surface: A Density Functional Theory Study. J. Colloid. Interface Sci. 2015, 454, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, T.; Chen, H. Exploration of Low-Dose Ozone-Activated Periodate (O3/IO4−) for Rapid Degradation of the β-Blocker Pindolol: Efficiency and Radical Mechanism. Water Res. 2025, 285, 124116. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate Constants of Dichloride Radical Anion Reactions with Molecules of Environmental Interest in Aqueous Solution: A Review. Environ. Sci. Pollut. Res. 2021, 28, 41552–41575. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M.; Pétrier, C. Influence of Bicarbonate and Carbonate Ions on Sonochemical Degradation of Rhodamine B in Aqueous Phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar] [CrossRef]
- Henderson, M.A. Evidence for Bicarbonate Formation on Vacuum Annealed TiO2(110) Resulting from a Precursor-Mediated Interaction between CO2 and H2O. Surf. Sci. 1998, 400, 203–219. [Google Scholar] [CrossRef]
- Commenge, J.M.; Falk, L. Villermaux–Dushman Protocol for Experimental Characterization of Micromixers. Chem. Eng. Process. Process Intensif. 2011, 50, 979–990. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, J.; Hu, Y.; Liu, Y.; Hu, S.; Zhu, C. Photochemical Reactions between 1,4-Benzoquinone and O2•−. Environ. Sci. Pollut. Res. 2020, 27, 31289–31299. [Google Scholar] [CrossRef]
- Ariza-Pérez, A.; Martín-Gómez, J.; Herrera-Beurnio, M.C.; López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Marinas, A.; Urbano, F.J. Role of P-Benzoquinone in the Photocatalytic Production of Solketal. Molecules 2025, 30, 3339. [Google Scholar] [CrossRef]
- Li, Y.; Wasgestian, F. Photocatalytic Reduction of Nitrate Ions on TiO2 by Oxalic Acid. J. Photochem. Photobiol. A Chem. 1998, 112, 255–259. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, M.C. Enhanced Photocatalytic Reduction of Cr(VI) by Combined Magnetic TiO2-Based NFs and Ammonium Oxalate Hole Scavengers. Catalysts 2019, 9, 72. [Google Scholar] [CrossRef]
- Haag, W.R.; Mill, T. Rate Constants for Interaction of 1O21Δg) with Azide Ion in Water. Photochem. Photobiol. 1987, 45, 317–321. [Google Scholar] [CrossRef]
- Mashiko, S.; Suzuki, N.; Koga, S.; Nakano, M.; Goto, T.; Ashino, T.; Mizumoto, I.; Inaba, H. Measurement of Rate Constants for Quenching Singlet Oxygen with a Cypridina Luciferin Analog (2-Methyl-6-[p-Methoxyphenyl]-3,7-Dihydroimidazo[1,2-a]Pyrazin-3-One) and Sodium Azide. J. Biolumin. Chemilumin. 1991, 6, 69–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).