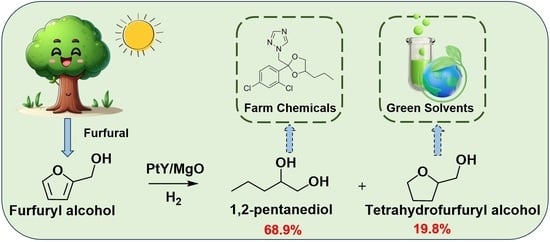
Hydrogenolysis of Biomass-Based Furfuryl Alcohol into 1,2-Pentanediol over Magnesium Oxide-Supported Pt-Y Bimetallic Catalysts
Abstract
1. Introduction
2. Results
2.1. Catalyst Screening
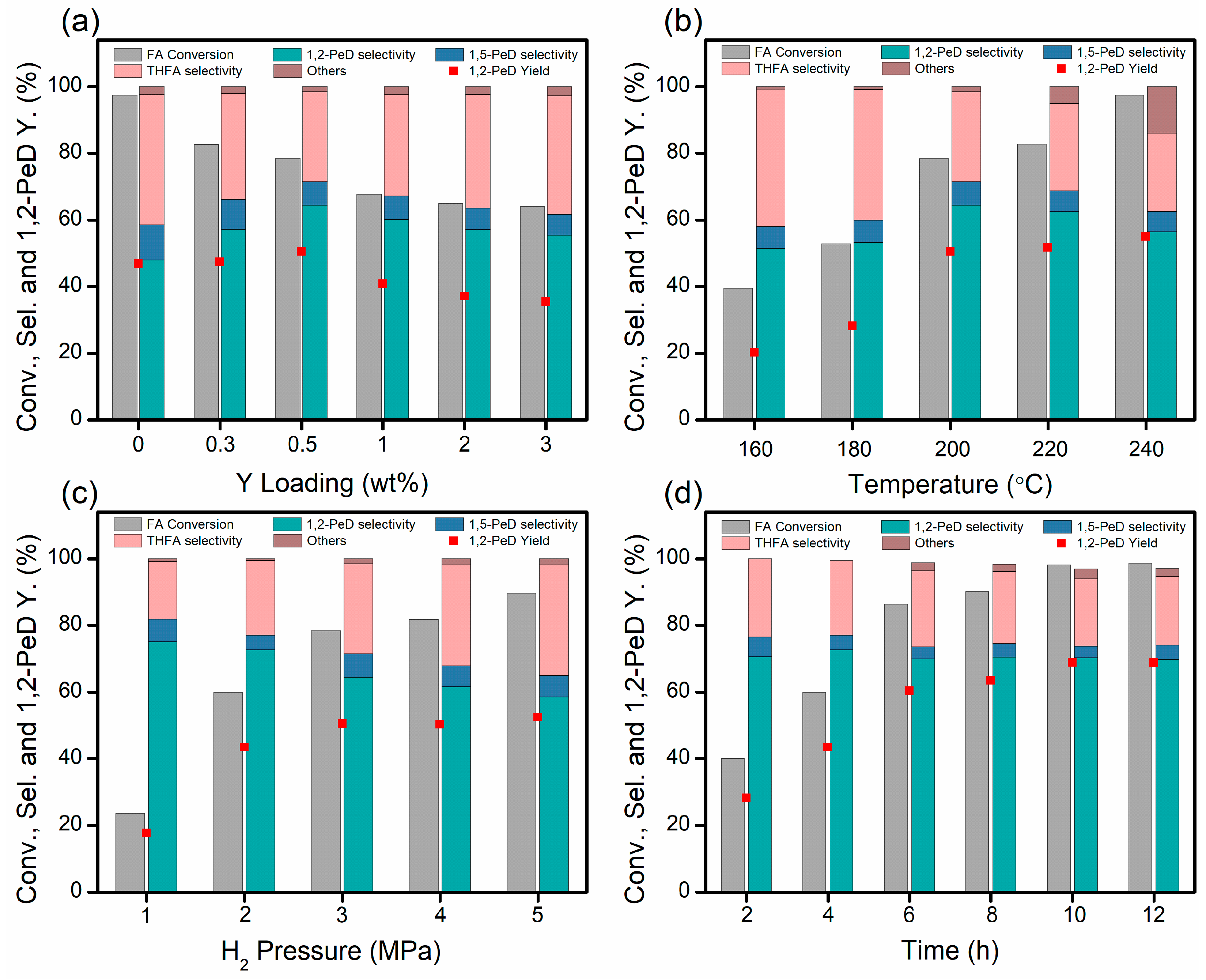
2.2. Hydrogenolysis of Furfuryl Alcohol over PtY/MgO
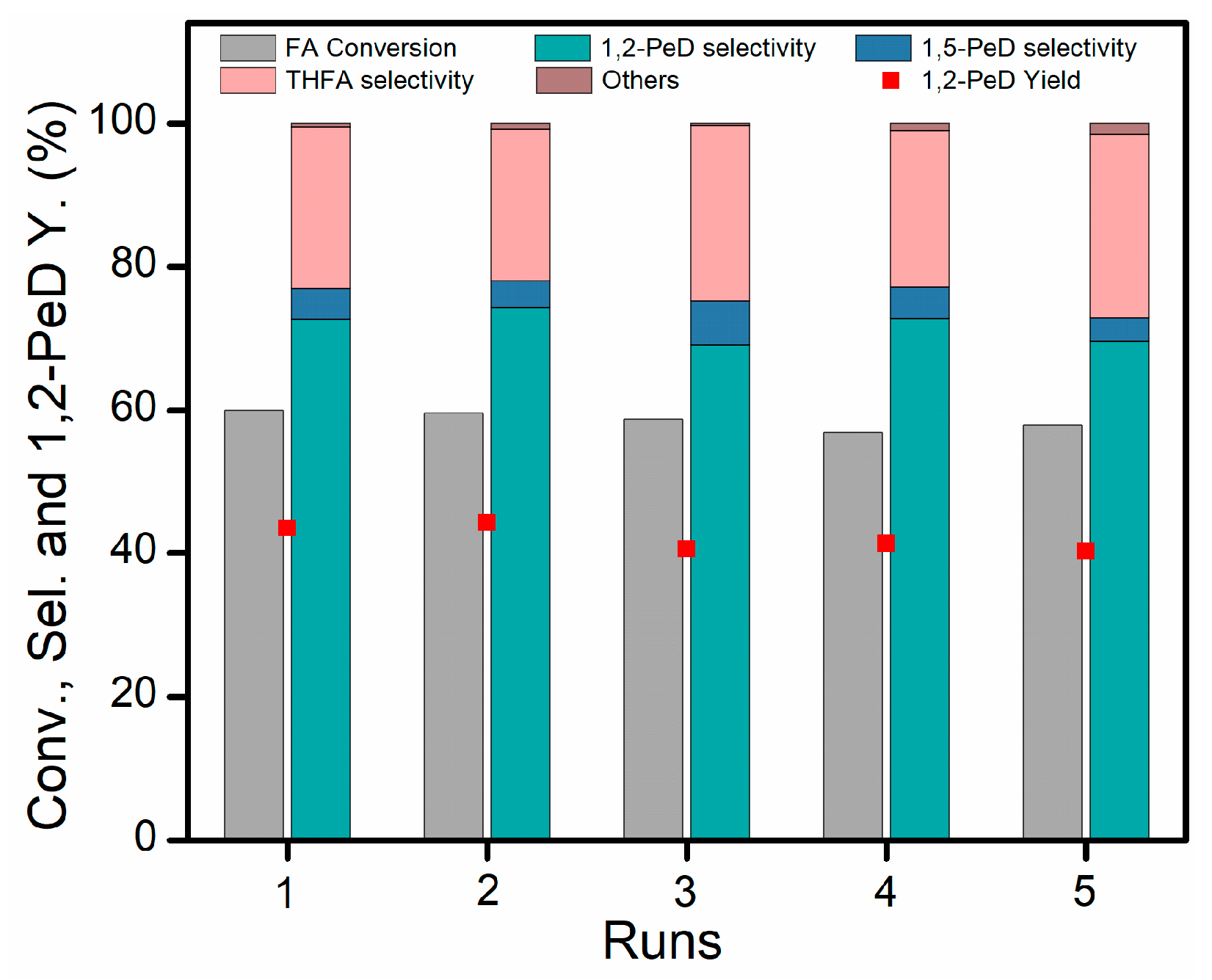
2.3. Catalyst Recyclability
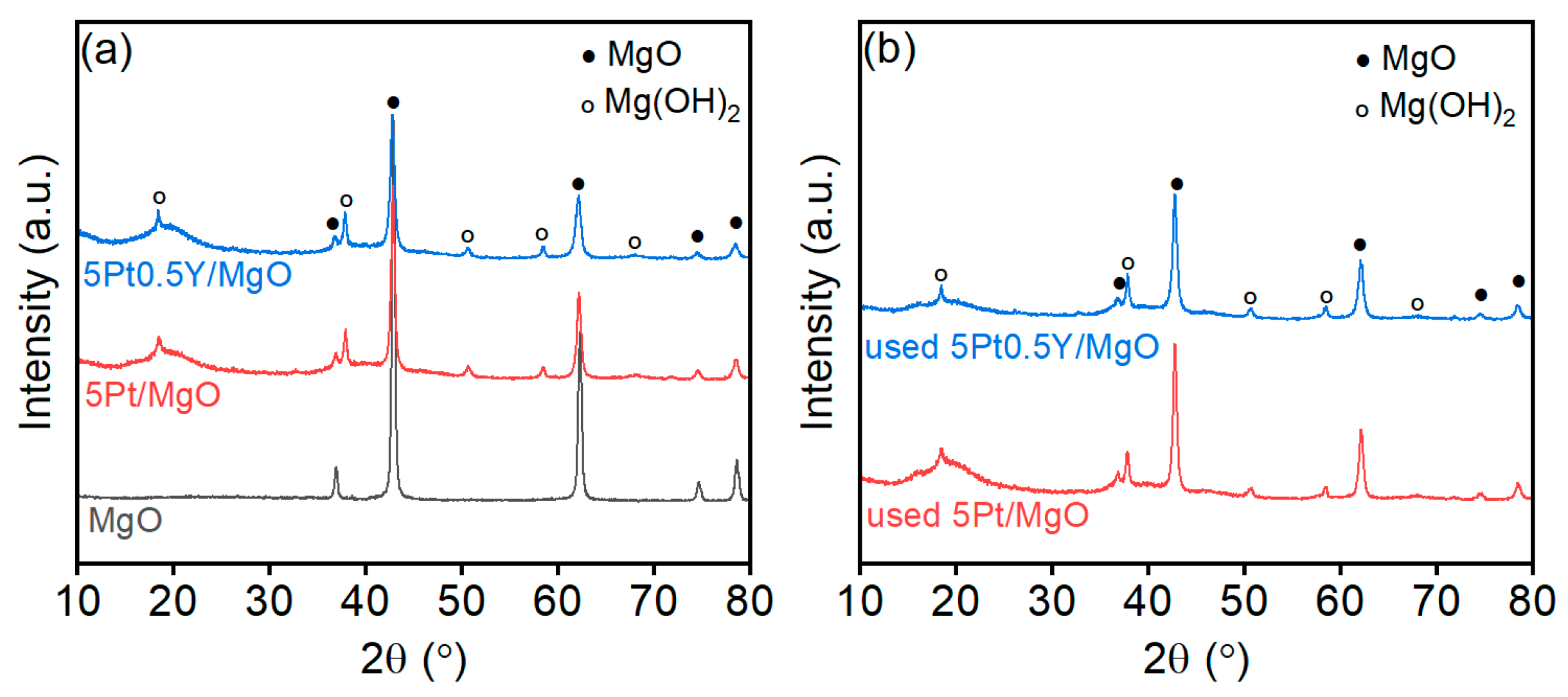
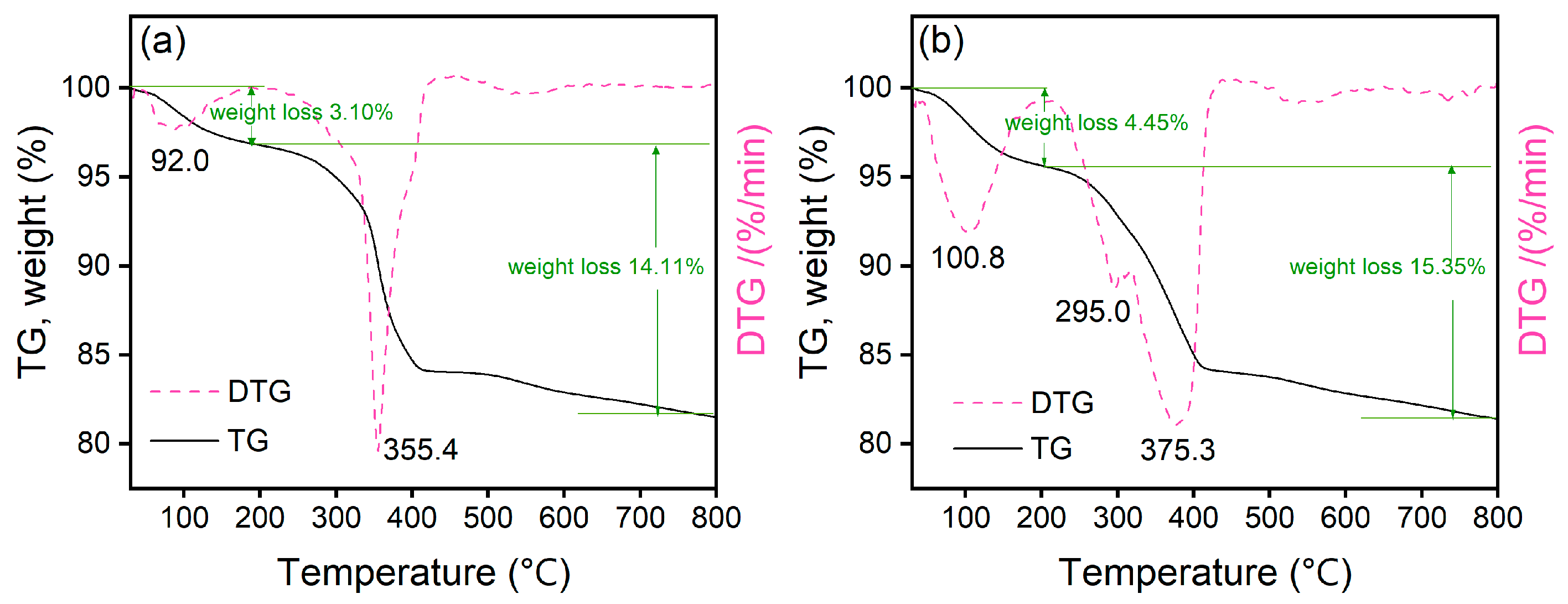
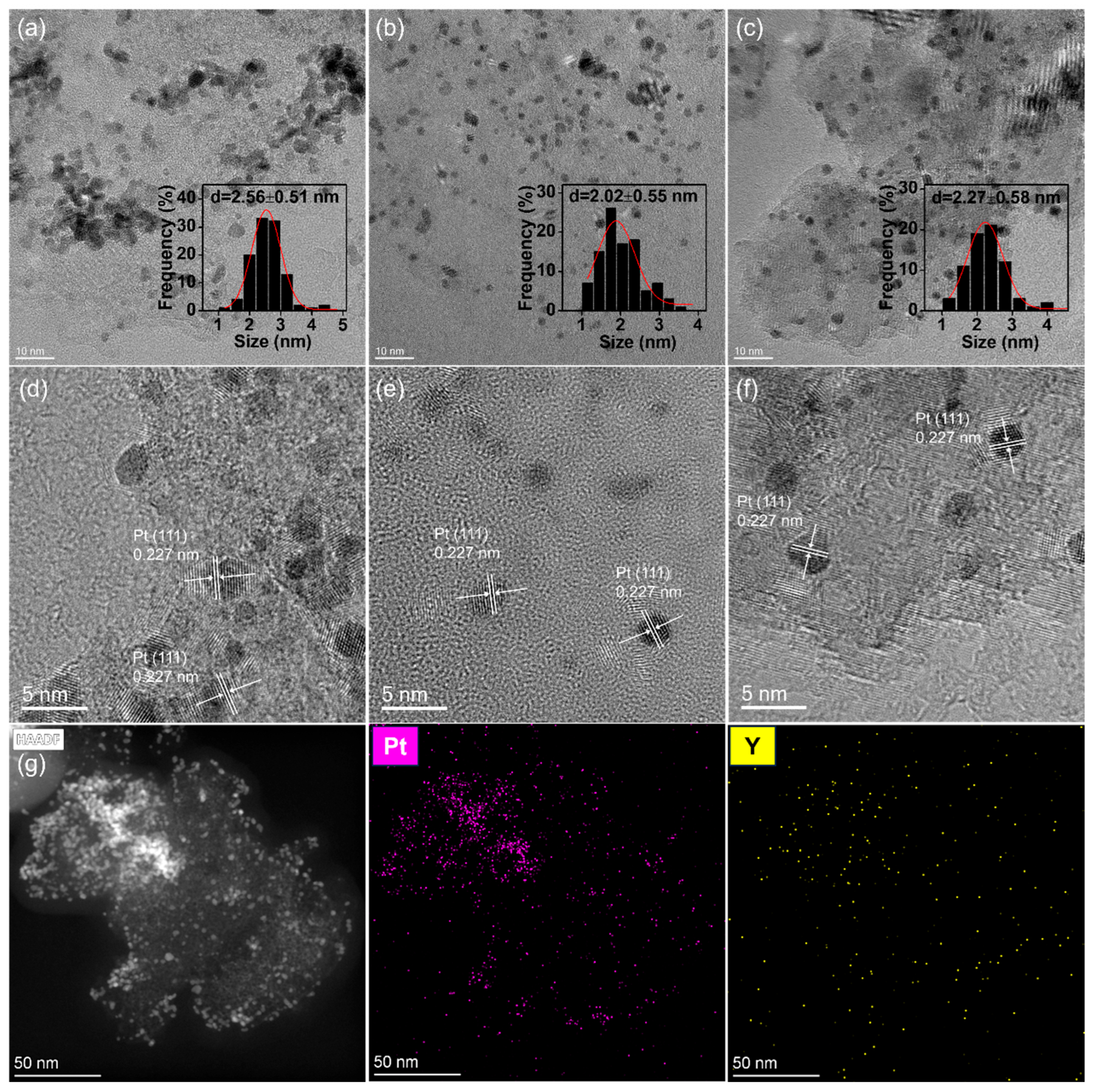
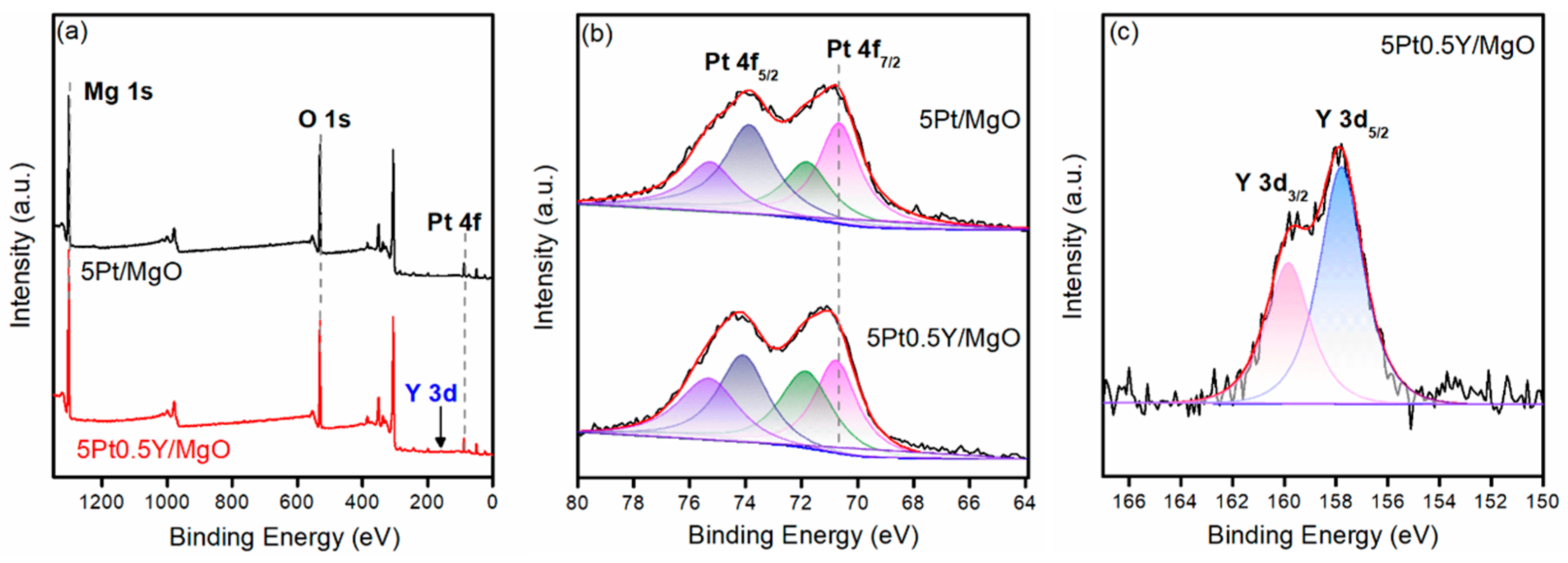
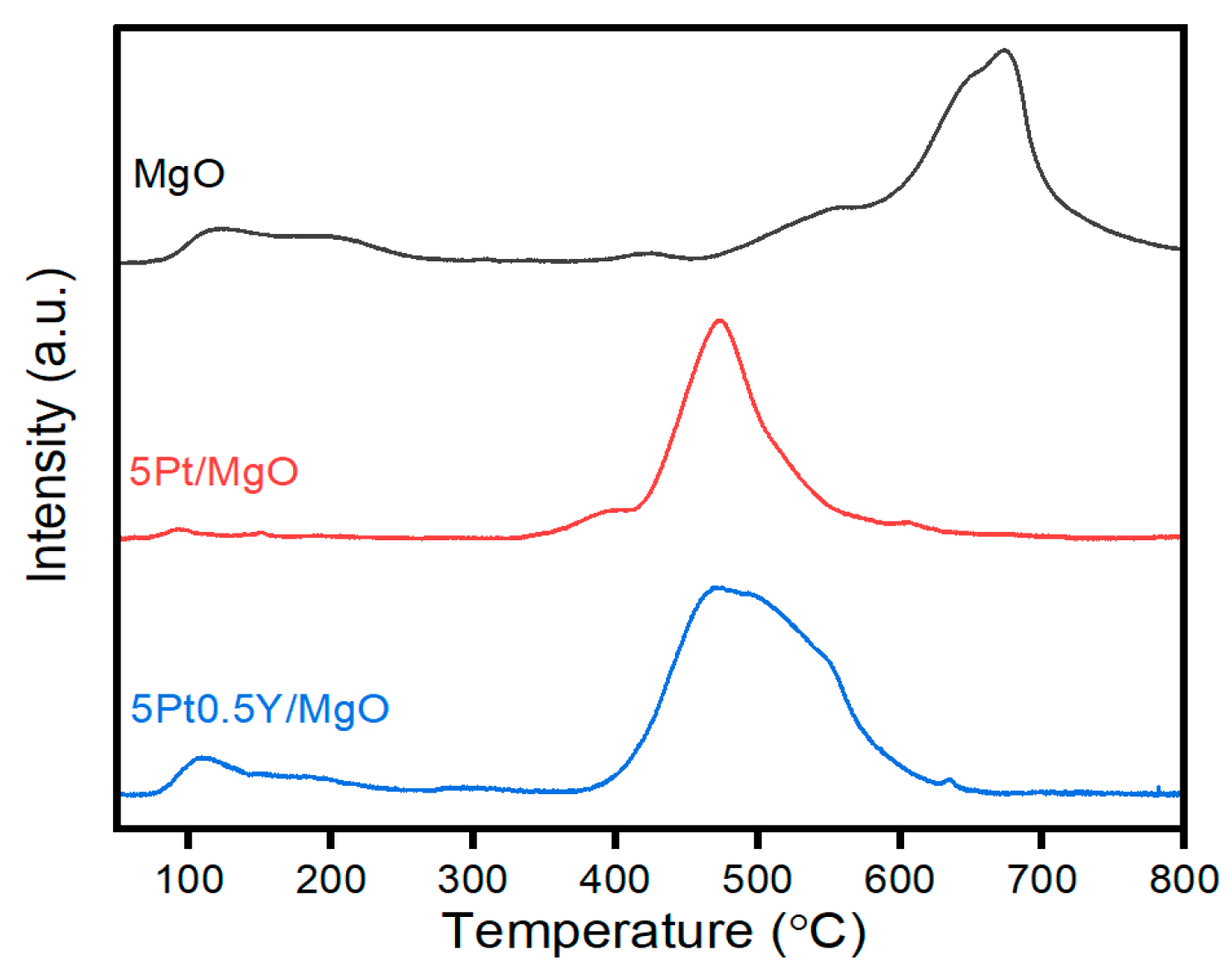
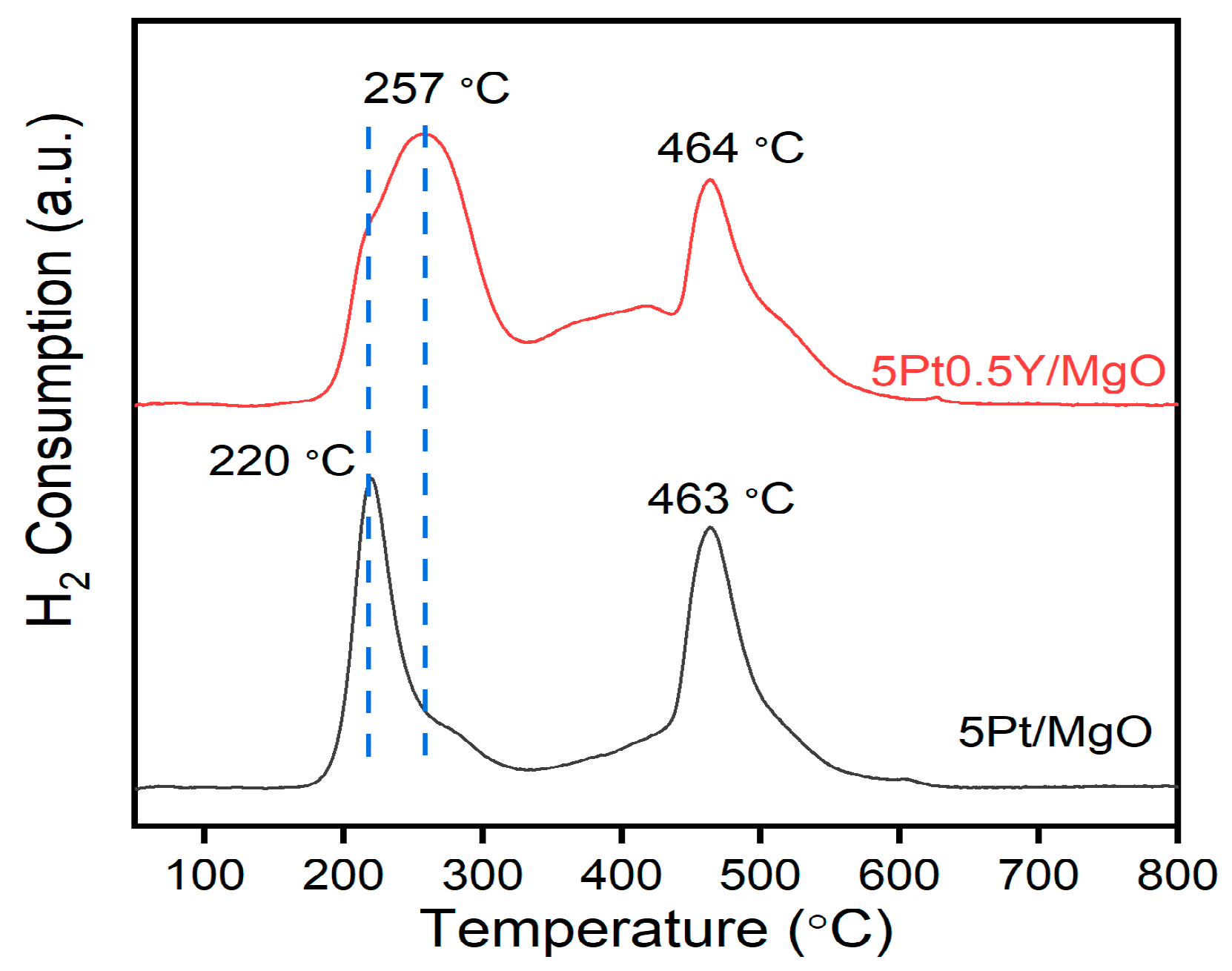
2.4. Characterization Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation and Characterization
4.3. Typical Procedures of Catalytic Reactions
4.4. Analytical Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, W.; Zhang, J.; An, Z.; Ma, X.; Zhang, Z.; Jiang, Y.; Zheng, L.; Shu, X.; Song, H.; et al. Selective Activation of C–OH, C–O–C, or C=C in Furfuryl Alcohol by Engineered Pt Sites Supported on Layered Double Oxides. ACS Catal. 2020, 10, 8032–8041. [Google Scholar] [CrossRef]
- Chen, L.; Ye, J.; Yang, Y.; Yin, P.; Feng, H.; Chen, C.; Zhang, X.; Wei, M.; Truhlar, D.G. Catalytic Conversion Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol and 2-Methylfuran at Terrace, Step, and Corner Sites on Ni. ACS Catal. 2020, 10, 7240–7249. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, J.; Cheng, Y.; Chen, Z.; Kang, S.; Cai, Z.; Xu, Y.; Wei, J. Enhanced Catalytic Transfer Hydrogenation of Biomass-Based Furfural into 2-Methylfuran over Multifunctional Cu–Re Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16624–16636. [Google Scholar] [CrossRef]
- Kalong, M.; Praikaew, W.; Ratchahat, S.; Chaiwat, W.; Koo-amornpattana, W.; Klysubun, W.; Limphirat, W.; Assabumrungrat, S.; Srifa, A. Continuous Furfural Hydrogenolysis into 2-Methylfuran and 2-Methyltetrahydrofuran over Cu/γ–Al2O3 with ReOx and WOx as Catalyst Boosters. Energy Fuels 2024, 38, 9836–9848. [Google Scholar] [CrossRef]
- Cao, C.; Guan, W.; Liu, Q.; Li, L.; Su, Y.; Liu, F.; Wang, A.; Zhang, T. Selective hydrogenolysis of furfural to 1,2-pentanediol over a Pt–Fe/MT catalyst under mild conditions. Green Chem. 2024, 26, 6511–6519. [Google Scholar] [CrossRef]
- Kurniawan, R.G.; Karanwal, N.; Park, J.; Verma, D.; Kwak, S.K.; Kim, S.K.; Kim, J. Direct conversion of furfural to 1,5-pentanediol over a nickel–cobalt oxide–alumina trimetallic catalyst. Appl. Catal. B Environ. 2023, 320, 121971. [Google Scholar] [CrossRef]
- Nimbalkar, A.S.; Oh, K.R.; Hong, D.Y.; Park, B.G.; Lee, M.; Hwang, D.W.; Awad, A.; Upare, P.P.; Han, S.J.; Hwang, Y.K. Continuous production of 1,2-pentanediol from furfuryl alcohol over highly stable bimetallic Ni–Sn alloy catalysts. Green Chem. 2024, 26, 11164–11176. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, B.; Zhao, C. Cu nanoparticles supported on core–shell MgO-La2O3 catalyzed hydrogenolysis of furfuryl alcohol to pentanediol. J. Catal. 2022, 410, 42–53. [Google Scholar] [CrossRef]
- Weng, Y.; Meng, S.; Zhu, W.; Zhang, M.; Sun, Q.; Zhang, Y. Synergistic hydrogenolysis of biomass furfuryl alcohol over Ru/solid base catalysts in hydrothermal reaction environment. J. Fuel Chem. Technol. 2021, 49, 1867–1874. [Google Scholar] [CrossRef]
- Sun, X.; Wen, B.; Wang, F.; Zhang, W.; Zhao, K.; Liu, X. Research advances on the catalytic conversion of biomass-derived furfural into pentanediols. Catal. Commun. 2024, 187, 106864. [Google Scholar] [CrossRef]
- Khemthong, P.; Yimsukanan, C.; Narkkun, T.; Srifa, A.; Witoon, T.; Pongchaiphol, S.; Kiatphuengporn, S.; Faungnawakij, K. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy 2021, 148, 106033. [Google Scholar] [CrossRef]
- Upare, P.P.; Kim, Y.; Oh, K.R.; Han, S.J.; Kim, S.K.; Hong, D.Y.; Lee, M.; Manjunathan, P.; Hwang, D.W.; Hwang, Y.K. A Bimetallic Ru3Sn7 Nanoalloy on ZnO Catalyst for Selective Conversion of Biomass-Derived Furfural into 1,2-Pentanediol. ACS Sustain. Chem. Eng. 2021, 9, 17242–17253. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, S.; He, Y.; Fan, G.; Li, X.; Jia, X.; Dong, M.; Fan, W. Pt/MgAlO bifunctional catalysts with various Mg/Al ratios for selective hydrogenation of furfural alcohol to 1,2-pentanediol. Catal. Today 2024, 433, 114647. [Google Scholar] [CrossRef]
- Ma, R.; Wu, X.P.; Tong, T.; Shao, Z.J.; Wang, Y.; Liu, X.; Xia, Q.; Gong, X.Q. The Critical Role of Water in the Ring Opening of Furfural Alcohol to 1,2-Pentanediol. ACS Catal. 2016, 7, 333–337. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, M.; Wang, J.; Sun, K.; Zhang, L.; Zhang, S.; Hu, G.; Xu, L.; Yuan, X.; Hu, X. Selective Conversion of Furfural into Diols over Co-Based Catalysts: Importance of the Coordination of Hydrogenation Sites and Basic Sites. Ind. Eng. Chem. Res. 2021, 60, 10393–10406. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Du, H.; Sun, K.; Zhang, Z.; Zhang, L.; Li, Q.; Zhang, S.; Liu, Q.; Hu, X. Importance of Magnesium in Cu-Based Catalysts for Selective Conversion of Biomass-Derived Furan Compounds to Diols. ACS Sustain. Chem. Eng. 2020, 8, 5217–5228. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Zhao, F.; Cui, F.; Li, X.; Xia, C.; Chen, J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2- and 1,5-pentanediols over a non-precious Cu–Mg3AlO4.5 bifunctional catalyst. Catal. Sci. Technol. 2016, 6, 668–671. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Kang, H.; Xia, C.; Chen, J. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts. Chin. J. Catal. 2016, 37, 700–710. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Zhang, J.; Li, X.; Jiang, Y.; Su, Y.; Li, W.; Zheng, M. Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols. Catalysts 2021, 11, 415. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Z. Selective Hydrogenolysis of Furfuryl Alcohol to Pentanediol over Pt Supported on MgO. Catalysts 2024, 14, 233. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase. Green Chem. 2012, 14, 3402–3409. [Google Scholar] [CrossRef]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct Transformation of Furfural to 1,2-Pentanediol Using a Hydrotalcite-Supported Platinum Nanoparticle Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, K.; Lu, M.; Wang, L.; Wei, Z.; Li, X. Acidic/Basic Oxides-Supported Cobalt Catalysts for One-Pot Synthesis of Isophorone Diamine from Hydroamination of Isophorone Nitrile. Ind. Eng. Chem. Res. 2015, 54, 9124–9132. [Google Scholar] [CrossRef]
- Kliewer, C.J.; Aliaga, C.; Bieri, M.; Huang, W.; Tsung, C.-K.; Wood, J.B.; Komvopoulos, K.; Somorjai, G.A. Furan Hydrogenation over Pt(111) and Pt(100) Single-Crystal Surfaces and Pt Nanoparticles from 1 to 7 nm: A Kinetic and Sum Frequency Generation Vibrational Spectroscopy Study. J. Am. Chem. Soc. 2010, 132, 13088–13095. [Google Scholar] [CrossRef]
- Luo, J.; Liang, C. Rhenium in Heterogeneous Catalysis: A Rising Star for Hydrogenation Reactions. ACS Catal. 2024, 14, 7032–7049. [Google Scholar] [CrossRef]
- Rukini, A.; Rhamdhani, M.A.; Brooks, G.A.; Van den Bulck, A. Metals Production and Metal Oxides Reduction Using Hydrogen: A Review. J. Sustain. Metall. 2022, 8, 1–24. [Google Scholar] [CrossRef]
- Sun, C.; Świrk Da Costa, K.; Wang, Y.; Li, L.; Fabbiani, M.; Hulea, V.; Rønning, M.; Hu, C.; Da Costa, P. Unraveling catalytic properties by yttrium promotion on mesoporous SBA-16 supported nickel catalysts towards CO2 methanation. Fuel 2022, 317, 122829. [Google Scholar] [CrossRef]
- Wang, X.; Weng, Y.; Zhao, X.; Xue, X.; Meng, S.; Wang, Z.; Zhang, W.; Duan, P.; Sun, Q.; Zhang, Y. Selective Hydrogenolysis and Hydrogenation of Furfuryl Alcohol in the Aqueous Phase Using Ru–Mn-Based Catalysts. Ind. Eng. Chem. Res. 2020, 59, 17210–17217. [Google Scholar] [CrossRef]
- Estrada, M.; Costa, V.V.; Beloshapkin, S.; Fuentes, S.; Stoyanov, E.; Gusevskaya, E.V.; Simakov, A. Aerobic oxidation of benzyl alcohol in methanol solutions over Au nanoparticles: Mg(OH)2 vs MgO as the support. Appl. Catal. A Gen. 2014, 473, 96–103. [Google Scholar] [CrossRef]
- Ding, J.; Li, Y.; Zhang, X.; Zhang, Z.; Zhang, Y.; Wang, Y.; Yu, G.; Wang, Z.; Guo, X.; Wang, Y.; et al. Study on the homologous effects on the performance of alkali metal-doping MgO-based adsorbents for CO2 capture. Sep. Purif. Technol. 2025, 355, 129673. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Li, C.; Li, S.; Tsang, C.; Liang, C. The role of oxophilic Mo species in Pt/MgO catalysts as extremely active sites for enhanced hydrodeoxygenation of dibenzofuran. Catal. Sci. Technol. 2020, 10, 2948–2960. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Fu, J.; Miao, C.; Jia, S.; Yan, P.; Huang, J. Highly selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan over metal-oxide supported Pt catalysts: The role of basic sites. Appl. Catal. A Gen. 2022, 643, 118762. [Google Scholar] [CrossRef]
- Newberg, J.T. Surface Thermodynamics and Kinetics of MgO(100) Terrace Site Hydroxylation. J. Phys. Chem. C 2014, 118, 29187–29195. [Google Scholar] [CrossRef]
- Bracco, J.N.; Camacho Meneses, G.; Colon, O.; Yuan, K.; Stubbs, J.E.; Eng, P.J.; Wanhala, A.K.; Einkauf, J.D.; Boebinger, M.G.; Stack, A.G.; et al. Reaction Layer Formation on MgO in the Presence of Humidity. ACS Appl. Mater. Interfaces 2024, 16, 712–722. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Wang, S. Influence of calcination temperature on the structure and hydration of MgO. Constr. Build. Mater. 2020, 262, 120776. [Google Scholar] [CrossRef]
- Puriwat, J.; Chaitree, W.; Suriye, K.; Dokjampa, S.; Praserthdam, P.; Panpranot, J. Elucidation of the basicity dependence of 1-butene isomerization on MgO/Mg(OH)2 catalysts. Catal. Commun. 2010, 12, 80–85. [Google Scholar] [CrossRef]
- Khamkongkaeo, A.; Mothaneeyachart, N.; Sriwattana, P.; Boonchuduang, T.; Phetrattanarangsi, T.; Thongchai, C.; Sakkomolsri, B.; Pimsawat, A.; Daengsakul, S.; Phumying, S.; et al. Ferromagnetism and diamagnetism behaviors of MgO synthesized via thermal decomposition method. J. Alloys Compd. 2017, 705, 668–674. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, L.; Liao, J.; Pei, A.; Yang, K.; Zhu, L.; Chen, B.H. Magnesium hydroxide–supported ruthenium as an efficient and stable catalyst for glycerol-selective hydrogenolysis without addition of base and acid additives. New J. Chem. 2020, 44, 16054–16061. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Niu, H.; Liang, C. Role of metal (Pt)–support (MgO) interactions in base-free glucose dehydrogenation. Catal. Sci. Technol. 2022, 12, 6849–6855. [Google Scholar] [CrossRef]
- Ye, J.; Chen, C.; Zheng, Y.; Zhou, D.; Liu, Y.; Chen, D.; Ni, L.; Xu, G.; Wang, F. Efficient conversion of cellulose to lactic acid over yttrium modified siliceous Beta zeolites. Appl. Catal. A Gen. 2021, 619, 118133. [Google Scholar] [CrossRef]
- Sandström, R.; Gracia-Espino, E.; Hu, G.; Shchukarev, A.; Ma, J.; Wågberg, T. Yttria stabilized and surface activated platinum (PtxYOy) nanoparticles through rapid microwave assisted synthesis for oxygen reduction reaction. Nano Energy 2018, 46, 141–149. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Zhang, T.; Liu, C.L.; Dong, W.S. One-pot conversion of xylose to 1,2-pentanediol catalyzed by an organic acid-assisted Pt/NC in aqueous phase. ChemSusChem 2024, 18, e202401109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pang, J.; Luo, W.; Zhao, Y.; Pan, X.; Zheng, M. Catalytic Conversion of Tetrahydrofurfuryl Alcohol over Stable Pt/MoS2 Catalysts. Catal. Lett. 2021, 151, 2734–2747. [Google Scholar] [CrossRef]
- Mansouri, A.; Semagina, N. Enhancement of palladium-catalyzed direct desulfurization by yttrium addition. Appl. Catal. A Gen. 2017, 543, 43–50. [Google Scholar] [CrossRef]
- Ilieva, L.; Venezia, A.; Petrova, P.; Pantaleo, G.; Liotta, L.; Zanella, R.; Kaszkur, Z.; Tabakova, T. Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation. Catalysts 2018, 8, 283. [Google Scholar] [CrossRef]
- Shen, H.; Quan, Y.; Ding, X.; Ren, J. CuLaAl ternary catalysts for the selective hydrogenolysis of furfuryl alcohol to 1,2-pentanediol prepared by cation-anion double hydrolysis method: New insights into La promoter. Int. J. Hydrogen Energy 2025, 140, 392–404. [Google Scholar] [CrossRef]
- Aramendía, M. Synthesis and characterization of Pt/MgO catalysts and their use in n-hexane conversion. Colloids Surf. A Physicochem. Eng. Asp. 2003, 225, 137–143. [Google Scholar] [CrossRef]
- Mao, B.; Xie, Y.; Qiu, X.; He, F.; Zhao, K.; Xu, E.; Lu, J. Impact of various metal promoters on the propane dehydrogenation performance of cobalt-based catalysts. Mol. Catal. 2024, 567, 114457. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P. Effect of a second metal (Y, K, Ca, Mn or Cu) addition on the carbon dioxide reforming of methane over nanostructured palladium catalysts. Appl. Catal. B Environ. 2012, 115–116, 190–200. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Liu, H.; You, C. Aluminum-yttrium oxides supported iron for effective catalytic reforming of toluene: Promotion roles of yttrium. Fuel Process. Technol. 2022, 235, 107344. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, K.; Wu, H.; Wang, J.; Cheng, Y.; Liu, Y.; Wei, Z. CoNi Alloy Nanoparticles Confined in N-Doped Porous Carbon as an Efficient and Versatile Catalyst for Reductive Amination of Levulinic Acid/Esters to N-Substituted Pyrrolidones. ACS Catal. 2023, 13, 12601–12616. [Google Scholar] [CrossRef]
- Liu, D.; Fu, J.; Wang, J.; Zhu, X.; Xu, J.; Zhao, Y.; Huang, J. Interfacial synergy within bimetallic oxide promotes selective hydrogenolysis of furfuryl alcohol to 1,5-pentanediol. Appl. Surf. Sci. 2024, 642, 158571. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2020, 63, 512–595. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, F.; Li, Z.; An, Y.; Lin, T.; Yang, Y.; Zhong, L.; Wang, H.; Sun, Y. Effect of Sodium on the Structure-Performance Relationship of Co/SiO2 for Fischer-Tropsch Synthesis. Chin. J. Chem. 2017, 35, 918–926. [Google Scholar] [CrossRef]
- Li, H.; Nie, X.; Du, H.; Zhao, Y.; Mu, J.; Zhang, Z.C. Understanding the Role of Base Species on Reversed Cu Catalyst in Ring Opening of Furan Compounds to 1,2-Pentanediol. ChemSusChem 2024, 17, e202300880. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Liu, X.; Guo, Y.; Norouzi Banis, M.; Hu, Y.; Wang, Y. The critical role of CeO2 crystal-plane in controlling Pt chemical states on the hydrogenolysis of furfuryl alcohol to 1,2-pentanediol. J. Catal. 2018, 365, 420–428. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Murakami, Y.; Imura, T.; Wakita, K. Hydrogenolysis of Furfuryl Alcohol to 1,2-Pentanediol Over Supported Ruthenium Catalysts. ChemistryOpen 2021, 10, 731–736. [Google Scholar] [CrossRef]
- Götz, D.; Lucas, M.; Claus, P. Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts. Catalysts 2017, 7, 50. [Google Scholar] [CrossRef]
- Götz, D.; Lucas, M.; Claus, P. C–O bond hydrogenolysis vs. C=C group hydrogenation of furfuryl alcohol: Towards sustainable synthesis of 1,2-pentanediol. React. Chem. Eng. 2016, 1, 161–164. [Google Scholar] [CrossRef]
- Lee, J.; Burt, S.P.; Carrero, C.A.; Alba-Rubio, A.C.; Ro, I.; O’Neill, B.J.; Kim, H.J.; Jackson, D.H.K.; Kuech, T.F.; Hermans, I.; et al. Stabilizing cobalt catalysts for aqueous-phase reactions by strong metal-support interaction. J. Catal. 2015, 330, 19–27. [Google Scholar] [CrossRef]

| Entry | Catalyst | Conv. (%) | Yield (Selectivity) (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
 | 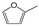 | 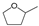 |  | 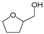 |  |  | |||
| 1 | 5Pt/Al2O3 | 100 | 0.3 | 6.7 | 20.9 | 28.0 | 31.0 | 9.8 (9.8) | 2.3 |
| 2 | 5Pt/CeO2 | 68.8 | 0.4 | 0.3 | 1.7 | 1.2 | 44.7 | 15.6 (22.7) | 4.9 |
| 3 | 5Pt/MgO | 61.5 | 0.6 | - | - | - | 32.6 | 20.4 (33.2) | 7.9 |
| 4 | 3Pt/MgO | 42.5 | 0.5 | - | - | - | 20.0 | 16.9 (39.8) | 5.1 |
| 5 | 1Pt/MgO | 14.9 | 0.4 | - | - | - | 5.4 | 6.9 (46.3) | 2.2 |
| 6 | 0.5Pt/MgO | 1.5 | - | - | - | - | 0.4 | 0.8 (53.3) | 0.3 |
| 7 | 5Pt1Re/MgO | 100 | 0.3 | - | - | - | 74.0 | 20.5 (20.5) | 5.2 |
| 8 | 5Pt1Fe/MgO | 49.5 | 0.8 | 27.2 | 1.4 | 1.0 | 3.4 | 7.8 (15.8) | 2.7 |
| 9 | 5Pt1Cu/MgO | 6.8 | 0.3 | - | - | - | 5.3 | 1.2 (17.9) | - |
| 10 | 5Pt1Ru/MgO | 100 | 0.2 | - | 0.4 | - | 89.8 | 3.9 (3.9) | 5.7 |
| 11 | 5Pt1Ni/MgO | 100 | 0.3 | - | 0.3 | - | 95.3 | 1.3 (1.3) | 2.8 |
| 12 | 5Pt1K/MgO | 17.8 | 0.5 | - | - | - | 11.0 | 4.7 (26.4) | 1.6 |
| 13 | 5Pt1La/MgO | 75.9 | 0.4 | - | - | - | 44.1 | 22.1 (29.4) | 8.8 |
| 14 | 5Pt1Y/MgO | 36.3 | 0.4 | - | - | - | 16.2 | 17.1 (47.2) | 2.6 |
| Sample | SBET [a] [m2·g−1] | Vp [b] [cm3·g−1] | Dp [c] [nm] | Base Sites [d] [mmol·g−1] | ||
|---|---|---|---|---|---|---|
| 50~300 °C | 300~700 °C | 500~800 °C | ||||
| MgO | 29.26 | 0.309 | 17.68 | 0.029 | - | 0.177 |
| 5Pt/MgO | 21.32 | 0.155 | 3.79 | 0.004 | 0.095 | - |
| 5Pt0.5Y/MgO | 28.48 | 0.152 | 3.82 | 0.010 | 0.175 | - |
| Used 5Pt0.5Y/MgO | 26.24 | 0.146 | 3.81 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Xu, J.; Guo, S.; Wu, H. Hydrogenolysis of Biomass-Based Furfuryl Alcohol into 1,2-Pentanediol over Magnesium Oxide-Supported Pt-Y Bimetallic Catalysts. Catalysts 2025, 15, 1005. https://doi.org/10.3390/catal15111005
Zhou K, Xu J, Guo S, Wu H. Hydrogenolysis of Biomass-Based Furfuryl Alcohol into 1,2-Pentanediol over Magnesium Oxide-Supported Pt-Y Bimetallic Catalysts. Catalysts. 2025; 15(11):1005. https://doi.org/10.3390/catal15111005
Chicago/Turabian StyleZhou, Kuo, Jialin Xu, Shengrong Guo, and Hongjun Wu. 2025. "Hydrogenolysis of Biomass-Based Furfuryl Alcohol into 1,2-Pentanediol over Magnesium Oxide-Supported Pt-Y Bimetallic Catalysts" Catalysts 15, no. 11: 1005. https://doi.org/10.3390/catal15111005
APA StyleZhou, K., Xu, J., Guo, S., & Wu, H. (2025). Hydrogenolysis of Biomass-Based Furfuryl Alcohol into 1,2-Pentanediol over Magnesium Oxide-Supported Pt-Y Bimetallic Catalysts. Catalysts, 15(11), 1005. https://doi.org/10.3390/catal15111005