Abstract
A new alkaline deep eutectic solvent (ChOH-TH) was developed using choline hydroxide and thiourea to pretreat reed straw for enhanced enzymatic hydrolysis. The study systematically examined the impact of ChOH-TH pretreatment on reed straw’s composition, microstructure, crystalline structure, and enzymatic saccharification efficiency to determine optimal conditions. Results showed that adding 70% deionized water to ChOH-TH and pretreating at 70 °C for 3 h effectively removed 70.73% of lignin from reed straw. This condition led to a 93.52% increase in reducing sugar output from the pretreated cellulose-enriched substrate, a 12.04-fold rise compared to untreated straw. Structural analysis revealed enhanced cellulose crystallinity to 51.38% in the residual biomass, along with surface modifications and changes in functional groups and components, contributing to improved enzymatic hydrolysis efficiency. Moreover, ChOH-TH maintained a 60.41% reducing sugar yield after five reuse cycles in the enzymatic hydrolysis of pretreated reed straw, highlighting its reusability and sustainability. It showed effective pretreatment performance on various lignocellulosic biomass types, indicating universality. The optimized ChOH-TH solvent is efficient, stable, and versatile for biomass pretreatment, improving enzymatic hydrolysis efficiency with economic and environmental benefits. This presents a green pathway for utilizing lignocellulosic waste with significant academic and industrial potential.
1. Introduction
Against the backdrop of dwindling global fossil fuel reserves and the ever-rising energy demand, the pursuit of alternative energy sources has become particularly pressing. The unsustainability of fossil fuels and their negative impact on the environment, especially in the context of global warming, have prompted policymakers and researchers worldwide to turn their attention to the development of renewable energy technologies [1,2,3,4]. Lignocellulosic biomass, by virtue of its widespread distribution in nature and inherent sustainability, is widely recognized as a viable alternative to fossil fuels [5,6]. Reed straw is a highly adaptable plant species characterized by vigorous growth patterns, rendering it well-suited for cultivation. It demonstrates a remarkable capacity to produce substantial biomass per unit area, positioning it as a reliable feedstock for energy generation. Notably, its harvest cycle is relatively short, thereby facilitating sustainable energy production [7].
Lignocellulosic biomass holds significant promise as a fossil fuel substitute, yet its complex structure and resistance to degradation impede its direct conversion into biofuels and chemicals [6,8]. Enzymes serve as a valuable catalyst for sustainable reactions in industry [9] and in particular for biomass valorisation for saccharification [10]. However, the crystallinity of lignocellulose, along with cellulose being encased by lignin and hemicellulose, poses substantial challenges to enzymatic saccharification [11,12]. Therefore, advancing pretreatment technologies to improve fermentable sugar yield and enzymatic hydrolysis efficiency in reed straw is crucial for optimizing its economic potential [13].
Conventional pretreatment methods for lignocellulosic biomass, including physical, chemical, physicochemical, and biological approaches, generally suffer from high cost, low efficiency, and environmental pollution [14,15]. Consequently, the development of environmentally benign pretreatment solvents has become a research focus. Deep eutectic solvents (DESs), composed of hydrogen bond donors and acceptors, possess advantages such as low melting points, low volatility, and biodegradability. They can disrupt the compact structure of lignocellulose and alter its crystalline morphology, making them promising green pretreatment agents [16,17,18,19,20,21]. Among them, alkaline deep eutectic solvents (ALDES) can efficiently remove glycation barriers, reduce equipment corrosion, and retain polysaccharides while extracting lignin, thereby improving the economic viability of biofuel production and the availability of substrates for enzymatic hydrolysis [22,23,24]. For instance, the choline chloride–monoethanolamine system increased the glucose yield of poplar wood by 6.6 times [25,26], and the K2CO3–ethylene glycol system achieved a lignin removal rate of 70.7% and a hemicellulose retention rate of 70.2% when pretreating coconut shells [27].
Thiourea, an inexpensive and low-toxicity organic compound, contains amino groups (-NH2) that can form hydrogen bonds with hydroxyl groups (-OH) in lignocellulose. This weakens the intermolecular forces of cellulose and reduces its crystallinity. When combined with urea or alkalis like NaOH, thiourea further enhances lignin removal and enzymatic hydrolysis efficiency [28,29,30]. However, traditional ALDES systems have high viscosity, requiring extended pretreatment time, elevated temperatures, or multi-step processes to improve lignin solubility and enzymatic hydrolysis efficiency, which increases operational costs and complexity.
In view of this, a novel alkaline deep eutectic solvent is designed in this study. On one hand, it retains the high polysaccharide retention property of ALDES and avoids the environmental and equipment corrosion issues associated with conventional pretreatment methods. On the other hand, it incorporates the hydrogen bonding mechanism of thiourea to specifically address the drawbacks of traditional ALDES, such as high viscosity and the need for harsh reaction conditions. By regulating the solvent components, efficient pretreatment under low-viscosity and mild conditions is achieved, balancing economy, environmental friendliness, and treatment efficiency, thus demonstrating clear technical rationality and application value.
The study assessed the influence of water addition, duration, and temperature on ChOH-TH effectiveness, correlating these parameters with reed straw composition changes and enzymatic saccharification efficiency. Analytical techniques (SEM, AFM, FTIR, XRD, and TGA) elucidated the impact of varying water addition in ChOH-TH on reed straw microstructure, composition, and thermal stability before and after pretreatment. Reusability of ChOH-TH was confirmed, highlighting its practicality and stability over multiple cycles. The study demonstrated ChOH-TH’s versatility in pretreating various biomass materials. Mass balance calculations evaluated material conversion efficiencies and losses. The research aims to support the development of efficient and cost-effective biomass pretreatment methods to facilitate the industrial adoption of renewable energy technologies.
2. Results and Discussion
2.1. Amount of Water Added
Water addition is crucial in the ChOH-TH pretreatment system. To elucidate the relationship between water content and pretreatment efficacy, we examined the impact of different water addition ratios on the chemical composition (polysaccharides and lignin), solid recovery, and enzymatic hydrolysis for reducing sugar yield from reed straw. The polysaccharide content peaked at 84.00% with minimal water, but dropped to 74.51% as water increased (Figure 1a). Maintaining optimal water levels effectively prevents the breakage of glycosidic bonds in cellulose due to excessive alkali hydrolysis by modulating the alkalinity of the ChOH-TH system, thereby preserving polysaccharides [21,31]. Lignin residue increased from 12.42% to 15.41%, while lignin removal decreased from 83.55% to 59.67%. The recovery of solids from the pretreated residue improved from 33.60% to 66.40% with more water, inversely affecting lignin removal (Figure 1b). At 90% water addition, reducing sugar yield dropped to 78.63%. Excess water in the ChOH-TH system dilutes the solution, hindering its ability to penetrate reed straw and disrupting the compact cell wall structure, thereby impairing lignin removal. Optimal pretreatment was achieved with 70% water, resulting in an 86.74% yield of reducing sugars through enzymatic hydrolysis, an 11.16-fold increase compared to the untreated yield of 7.77%. Thus, adding 70% water in the ChOH-TH system is most effective for reed straw pretreatment.
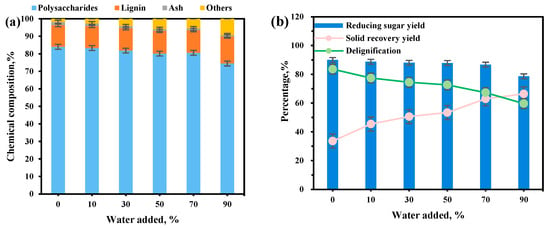
Figure 1.
The influence of water addition amounts on the ChOH-TH pretreatment of reed straw. (a) The chemical components with different water addition amounts; (b) The different water addition amounts on the yield of reducing sugars, solid recovery, and delignification.
2.2. Pretreatment Time
In the ChOH-TH pretreatment system, pretreatment time critically affects lignin removal and enzymatic hydrolysis efficiency by altering the interaction between reed straw and solvent. This study investigated the impact of pretreatment time on the composition of reed straw residue and enzymatic hydrolysis within a ChOH-TH system containing 70% water addition at 70 °C. Extending the pretreatment time from 0.5 to 3 h resulted in an increase in polysaccharide content in the reed straw residue, rising from 76.03% to 82.01% (Figure 2a). Concurrently, the lignin content decreased from 14.21% to 12.36%, and the lignin removal rate improved from 62.20% to 70.73%. However, the residue recovery rate declined from 67.50% to 60.10% (Figure 2b). The extended pretreatment time facilitated enhanced lignin removal, likely due to the penetration of the pretreatment solution into the straw matrix and the subsequent cleavage of lignin–hemicellulose linkages, such as ester and ether bonds, through alkaline hydrolysis. Additionally, lignin dissolution was further enabled by hydrogen bonding with thiourea. Concomitantly, the yield of reducing sugars increased from 77.95% to 93.52%, representing a 15.57% improvement. Consequently, a pretreatment time of 3 h was determined to be the optimal condition for this system.
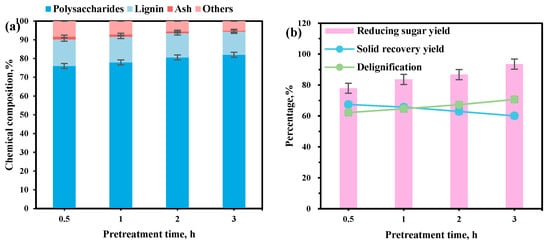
Figure 2.
The influence of pretreatment time on the ChOH-TH pretreatment of reed straw. (a) The chemical components with different pretreatment time; (b) The different pretreatment time on the yield of reducing sugars, solid recovery, and delignification.
2.3. Pretreatment Temperature
Elevating the pretreatment temperature enhances molecular kinetic energy, thereby facilitating the overcoming of the activation energy barrier and expediting the chemical reaction [32]. As depicted in Figure 3a, increasing the pretreatment temperature from 30 °C to 90 °C significantly boosted the thermal motion of reagent molecules, enhanced the permeability of reed straw, and expedited the swelling process. Concurrently, temperature elevation promotes the cleavage of ester and ether bonds between lignin and hemicellulose, and enhances the dissolution efficiency of thiourea and other lignin components. The rate of lignin removal increased rapidly, enhancing cellulose exposure. Compositional analysis (Figure 3a) showed a rise in polysaccharide content from 73.58% to 84.30% and a reduction in lignin content from 18.15% to 9.26%. The lignin removal rate increased significantly from 42.63% to 79.96%. Consequently, the recovery rate of reed straw residue declined from 80.20% to 54.90% (Figure 3b). The yield of reducing sugar gradually increased from 71.42% to 96.36% (Figure 3b). Although lignin removal was higher at 90 °C than at 70 °C, there was no significant difference in reducing sugar yield, with lower energy consumption at 70 °C. Therefore, 70 °C was the optimal temperature for ChOH-TH pretreatment of reed straw. Zhao et al. [25] developed an alkaline deep eutectic solvent (DES) composed of ethylene glycol and choline chloride for the pretreatment of wheat grass, achieving a high lignin removal efficiency of 71.4%. However, the excessive alkalinity of the system led to increased degradation of polysaccharides. The corncob underwent DES pretreatment at 130 °C for 2 h using a choline chloride–urea mixture (molar ratio 1:2) containing 40% water. Under these conditions, the saccharification efficiency reached only 65% [33]. Furthermore, in the aspect of organic solvent pretreatment, Xu et al. [34] used γ-pivalic acid (GVL), tetrahydrofuran (THF), and 2-methyltetrahydrofuran (2-MTHF) as three solvents to pretreat masson pine. It was found that when 75 mM H2SO4 was added at 150 °C, GVL performed the best in lignin removal from masson pine. The corresponding maximum glucose yield was 76.8%. Wu et al. [35] utilized the formic acid-methanesulfonic acid system to pretreat poplar trees at temperatures below 100 °C. When the sulfonic acid group concentration of the pretreatment solvent was 13.2 mmol/kg, the final glucose conversion rate could reach 45.9%, which was 89.3% higher than that of single formic acid treatment. Ionic liquids (IL) are also promising solvents for structurally stubborn lignocellulosic materials in enzymatic saccharification. Velmurugan et al. [36] studied the use of ionic liquid [C2mim]OAc under alkaline conditions to pretreat corn stalks for 2 h at 120 °C, and the cellulose-rich corn stalks showed a saccharification rate of 91%. Ahmad et al. [37] used ionic liquid [Emim]Glu to pretreat bamboo biomass at 120 °C for 19 h, and obtained a glucose yield of 86%. In this study, the temperature was reduced to 70 °C, substantially lower than the conventional range of 100–150 °C typically employed in most DES pretreatment processes. This reduction significantly decreased energy consumption during the heating phase while effectively minimizing non-specific degradation of polysaccharides associated with elevated temperatures. Furthermore, the incorporation of a high water addition (70%) did not markedly compromise pretreatment efficiency, and it facilitated process simplification, thereby contributing to cost reduction. These findings demonstrate notable theoretical innovation and practical application potential.
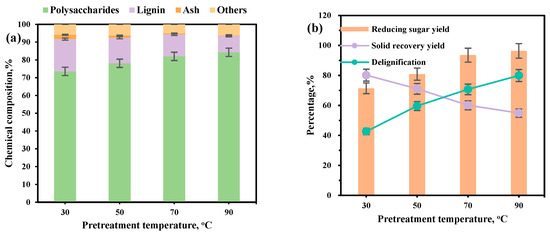
Figure 3.
The influence of pretreatment temperature on the ChOH-TH pretreatment of reed straw. (a) The chemical components with different pretreatment temperature; (b) The different pretreatment temperature on the yield of reducing sugars, solid recovery, and delignification.
2.4. Characterization
The reed straw was subjected to treatment with the ChOH-TH solution at 70 °C for 2 h under varying water addition levels (0%, 10%, 30%, 50%, 70%, 90%), and the resulting samples were labeled ChOH-TH-1 through ChOH-TH-6, respectively.
2.4.1. Surface Structure by SEM
SEM is a crucial technique for high-resolution surface morphology analysis, offering detailed images vital for examining biomass microstructure [38]. SEM analysis indicated that untreated reed straw has a smooth, densely continuous surface, with closely packed fiber bundles encased in a lignin and hemicellulose “protective layer,” exhibiting minimal pores or cracks (Figure 4a). After pretreatment with the ChOH-TH system, the surface transforms from its dense state to a rough, irregular morphology (Figure 4b). Initially, cellulose microfibers were tightly enveloped in a lignin–hemicellulose network, obscuring individual fiber morphology. Pretreatment dissolved lignin and hemicellulose, exposing cellulose microfibers, leading to fiber fracture, dispersion, and filamentous structures following fiber bundle disintegration in specific areas. The ChOH-TH pretreatment resulted in the formation of numerous micron-sized pores and channels within the lignocellulosic structure [39]. This structural modification facilitated improved cellulase penetration and adsorption, thereby enhancing the efficiency of enzymatic hydrolysis. Visual examination of the reed straw surface confirmed that the pretreatment reduced the inherent resistance of lignocellulose to degradation, laying the groundwork for improved enzymatic hydrolysis, in agreement with previous research findings [40].
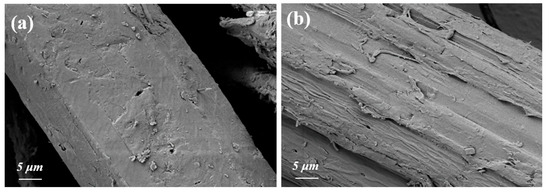
Figure 4.
SEM images of reed straw before (a) and after (b) pretreatment (70% water addition, 70 °C, 3 h).
2.4.2. Surface Roughness by AFM
AFM analysis demonstrated significant changes in the surface structure of reed straw before and after pretreatment, as shown in Figure 5. Table 1 lists key parameters, including root mean square roughness (Rq), average surface roughness (Ra), maximum vertical distance (Rmax), and surface area (SA). Initially, the untreated reed straw surface was smooth with few protrusions (Figure 5a). After pretreatment, however, the surface displayed marked roughness with numerous gullies and undulations (Figure 5b). Consequently, all roughness parameters (Rq, Ra, Rmax, and SA) increased significantly after pretreatment, consistent with SEM observations. A rough surface typically has a larger specific surface area, enhancing enzyme attachment and promoting enzymatic hydrolysis. AFM images can quantitatively assess the hydrophilicity of lignocellulose surfaces through their light and dark features. Lighter regions in AFM images usually indicate larger hydrophilic areas [41]. After pretreatment, the bright areas on straw residue surfaces increase, signifying enhanced hydrophilicity. This change likely results from the modification of certain hydrophobic components within the reed straw during pretreatment. Similar results by Yan et al. show increased hydrophilicity of lignocellulosic biomass after acid pretreatment. The increased surface hydrophilicity improved enzyme accessibility, facilitating enzymatic hydrolysis [42].
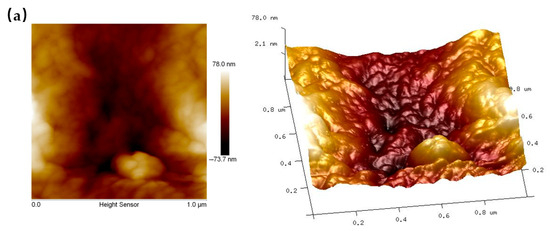
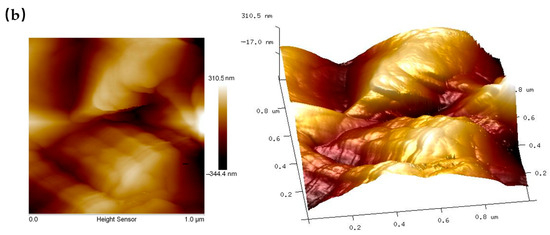
Figure 5.
AFM images of reed straw before (a) and after (b) pretreatment (70% water addition, 70 °C, 3 h).

Table 1.
The roughness parameters of reed straw before and after pretreatment.
2.4.3. Functional Groups by FTIR
FTIR spectroscopy is extensively employed to investigate structural changes in lignocellulose, offering direct insights into its molecular configuration. This study applied FTIR analysis to reed straw pretreated with ChOH-TH under different water addition conditions, scanning a range from 500 to 4000 cm−1, as illustrated in Figure 6. The spectral analysis identified the absorption peak at 3446 cm−1 as corresponding to the stretching vibration of hydroxyl (O-H) groups, while the peak at 2900 cm−1 pertained to hydrocarbon bond stretching. In untreated reed straw, the 1740 cm−1 absorption band was attributed to the C=O bond stretching in the acetyl ester structure (-OCOCH3) of hemicellulose [43]. After pretreatment, these peaks significantly diminished or nearly vanished, indicating a marked reduction in hemicellulose content. The lignin spectrum showed peaks at 1600 cm−1 and 1510 cm−1 from the C=C bond stretching in the aromatic ring skeleton [44], and at 1260 cm−1 from the guaiacol (G) benzene ring stretching [45]. The decreased intensity of these peaks after pretreatment indicates effective lignin removal. The increased relative intensity of cellulose peaks (1370 cm−1 for C-H bending and 1050 cm−1 for C-O-C stretching) after pretreatment indicated a higher cellulose concentration due to reduced lignin and hemicellulose, enhancing signal detection [43]. The absorption peak around 896 cm−1 corresponded to the β-glycosidic bond vibration between xylan and cellulose sugar units [20].
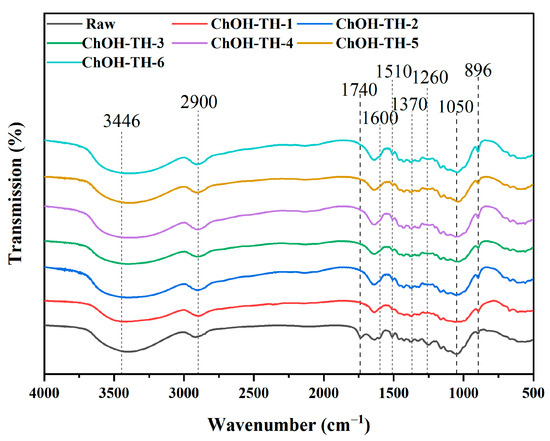
Figure 6.
FTIR spectra of raw materials and samples after pretreatment with varying water addition levels.
2.4.4. Crystallinity Index by XRD
Lignocellulose crystallization is typically assessed using XRD techniques [46], with the crystallinity index (CrI) of cellulose being crucial for enzymatic hydrolysis efficiency [39]. Thus, XRD was employed to examine the crystal characteristics of untreated reed straw and ChOH-TH pretreated residue with varying water additions. As illustrated in Figure 7, the XRD patterns revealed that the diffraction of reed straw residue, after ChOH-TH pretreatment with 0–90% water, closely resembled that of untreated material. Both exhibit typical cellulose type I diffraction peaks at 2θ ≈ 18° (cellulose (101) plane) and 2θ ≈ 22.17° (cellulose (002) plane), with symmetrical peak shapes and no significant peak position shift [47]. The pretreatment process did not alter the crystal morphology or structure of cellulose. The CrI of samples treated with ChOH-TH exceeded that of untreated reed straw (CrI = 46.4%). However, as water addition increased from 0% to 90%, the CrI of pretreated samples declined from 51.4% to 47.5%. This indicated that the removal of amorphous parts (hemicellulose, lignin and amorphous phase of cellulose) diminishes with increased water addition [39,48]. Excess water diluted the ChOH-TH concentration, reducing its ability to disrupt the amorphous components of reed straw.
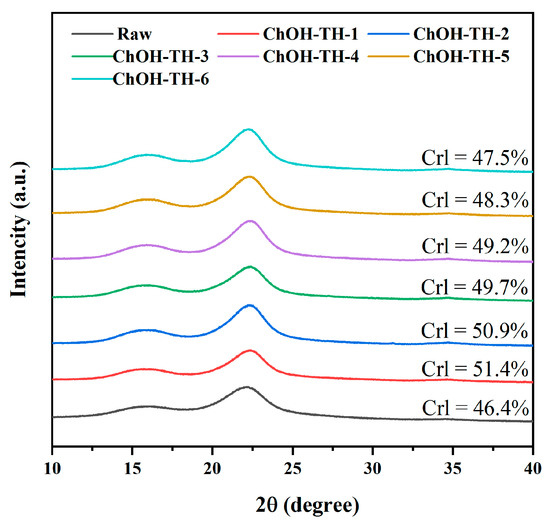
Figure 7.
XRD of raw materials and samples after pretreatment with varying water addition levels.
2.4.5. Thermal Stability by TGA
Thermal stability assessment using TGA technology involves monitoring material weight loss under varying temperature or time conditions. Derivative thermogravimetry (DTG), derived from TGA curves, elucidates the correlation between weight loss rate and temperature. Analysis of TGA/DTG curves unveils the thermal decomposition characteristics of materials and predicts their responses to diverse heat treatment conditions. Understanding the thermal stability of cellulose is crucial for elucidating the relationship between its chemical structure and properties. Figure 8a illustrates TGA curves of reed straw, including the raw material and ChOH-TH treated samples with varying water addition levels. These curves exhibited three distinct stages during pyrolysis: the initial stage (0~100 °C) primarily involved water evaporation, with minimal weight loss differences due to the samples being at a dry constant weight state before testing. Subsequent temperature increases lead to hemicellulose degradation, primarily attributed to its thermal instability. Figure 8b illustrates a gradual decrease in the DTG peak value of cellulose-rich substrate as the water content in ChOH-TH increased from 0% to 90%, aligning with the trend observed in polysaccharide content. Additionally, the DTG peak value of untreated reed straw surpassed that of ChOH-TH pretreated samples with varying water addition levels, underscoring the effective disruption of the dense lignocellulosic structure by ChOH-TH pretreatment [49]. Notably, cellulose exhibited its peak degradation rate around 350 °C (Figure 8b) without a significant change in residual mass during this phase, suggesting minimal impact of ChOH-TH pretreatment on the thermal stability of pure cellulose.
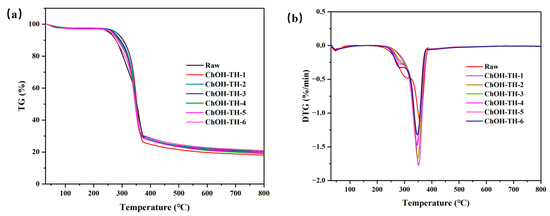
Figure 8.
TGA (a) and DTG (b) of raw materials and samples after pretreatment with varying water addition levels.
2.5. Applications to Other Types of Biomass
The efficacy of ChOH-TH pretreatment solvent was assessed on eight biomass types under optimal conditions (70% water addition, 70 °C, 3 h) determined for reed straw. Biomass categories were straw (corn straw, sorghum straw, rape straw), cortex (walnut green shell, wheat bran), shell (sunflower disk, rice hull), and bamboo powder. The results of changes in components and enzymatic saccharification before and after pretreatment are shown in Table 2.

Table 2.
Changes in components and saccharification rates before and after different biomass pretreatments.
Diverse biomass types exhibit varying responses to pretreatment methods due to differences in intrinsic composition and microstructural compactness. Corn straw and sorghum straw, with their hollow or vascular bundle structures featuring loose cell walls and high porosity, displayed notable saccharification efficiency following ChOH-TH pretreatment. This efficiency, evident in corn straw at 95.06% and sorghum straw at 90.75%, is attributed to the rapid penetration of the ChOH-TH system into the biomass, effectively disrupting lignin–hemicellulose cross-linking. Consequently, the reduction in lignin’s encasement of cellulose maximizes cellulose exposure for enzymatic hydrolysis, thereby enhancing saccharification efficiency.
Rape straw, due to its unique composition, exhibits lower reducing sugar content compared to other types of straw. During pretreatment, its thioglycosides transform into isothiocyanates, which slightly hinder cellulase activity [50], reducing the efficiency of enzymatic hydrolysis. Consequently, rape straw yields less reducing sugar than corn and sorghum straw. Despite this, rape straw pretreatment proves more efficient than that of bamboo powder and rice hull. Bamboo powder’s dense fiber structure, rich in lignin and cellulose, obstructs the penetration and removal of lignin by the ChOH-TH system. Rice hull, containing a silica-rich layer, forms a physical barrier to reagent penetration, adsorbing some reagents and reducing pretreatment efficacy, impeding cellulose accessibility [51].
Walnut green shell biomass demonstrated the lowest reducing sugar yield (29.22%) after pretreatment among the eight tested biomass types, which can be attributed to its less favorable compositional characteristics. Factors include low polysaccharide content (58.44%) and high lignin content (28.64%), limiting sugar production. Additionally, inhibitors such as juglone and tannins hinder enzyme activity by binding to cellulase active sites or altering enzyme structure, reducing saccharification efficiency compared to other biomass types [52].
Therefore, the aforementioned data indicated that ChOH-TH exhibited efficient and universal biomass pretreatment capabilities under mild conditions, making it a highly promising pretreatment method in the field of biomass refining.
2.6. Reuse of Pretreatment Solvents
Efficient solvent recycling is essential for the economic and environmental viability of lignocellulosic biomass processing [53]. This study evaluated the ChOH-TH system’s recycling feasibility, focusing on its effects on reed straw composition and enzymatic hydrolysis over multiple cycles. Although the system’s lignin removal and saccharification efficiency decrease with repeated use, its overall pretreatment effectiveness remains stable and reusable. Figure 9a showed a gradual decrease in polysaccharide content with more cycles, alongside an increase in residual lignin. The reducing sugar yield from enzymatic hydrolysis declined with each solvent reuse. However, even after the fifth use, the yield remained at 64.29% (Figure 9b). The viscosity of the ChOH-TH system increased with each reuse cycle, likely due to water evaporation and tar byproduct accumulation during pretreatment. This study examined how solvent reuse frequency affects pretreatment efficacy by analyzing the solid recovery rate, lignin removal rate, and enzymatic hydrolysis saccharification rate of reed straw. A significant linear correlation was found among these parameters. More recycling cycles reduced the lignin removal rate, increasing the residual lignin in straw and enhancing the solid recovery rate (Figure 9c). An inverse relationship was observed between solid recovery and lignin removal (y = −0.6675x + 109.55, R2 = 0.9746). Reduced lignin removal hinders cellulose exposure, impeding enzymatic saccharification. Conversely, a positive correlation existed between lignin removal rate and reducing sugar yield (y = 0.7841x + 35.495, R2 = 0.9725). The decline in lignin removal rate was crucial in enhancing solid recovery and reducing saccharification rate during solvent recycling. Notably, even after the fifth use, the ChOH-TH system maintained high lignin removal and saccharification efficiency, highlighting its practical utility. As shown in Figure 10, a broad and diffuse absorption band appears in the range of 3600–3000 cm−1, which is a comprehensive manifestation of the strong hydrogen bond network formed by the participation of both the N-H of thiourea and the O-H of choline. The 1620 cm−1 belongs to the N-H bending vibration of thiourea. The 1470 cm−1 and 1400 cm−1 belong to the C-H bending vibrations of the methyl and methylene groups in the choline cation. The 1080 cm−1 belongs to the C-N stretching vibration in the choline cation. This peak is one of the characteristic peaks of choline. The 950 cm−1 belongs to the C-C-O stretching vibration in the choline cation. The 730 cm−1 may belong to the C-S stretching vibration. After using the first and fifth times, the characteristic peak shapes of the functional groups basically remain unchanged, indicating that the ChOH-TH system has good stability.
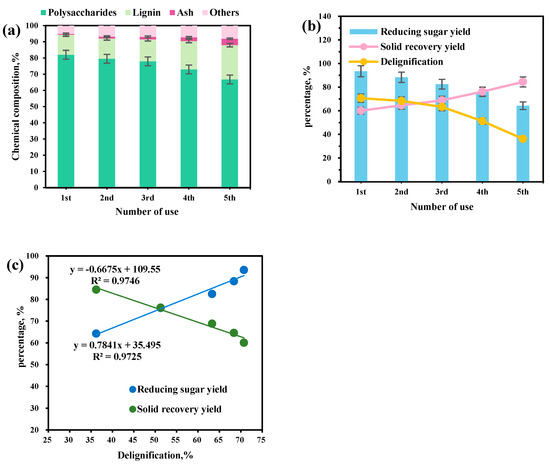
Figure 9.
The influence of using the pretreatment solvent continuously five times on the pretreatment effect of reed straw. (a) The chemical components with number of use; (b) The number of use on the yield of reducing sugars, solid recovery, and delignification; (c) The linear correlation between delignification and reducing sugar yield, as well as solid recovery yield.
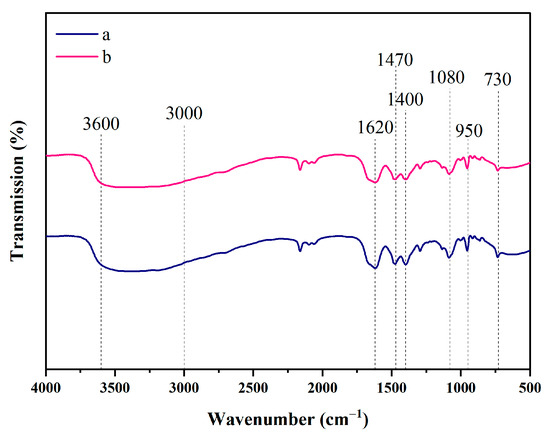
Figure 10.
ChOH-TH used the first time (a) and the fifth time (b).
2.7. Multidimensional Comprehensive Analysis
The study systematically compared CrI, polysaccharide content, lignin content, delignification, and reducing sugar yield of raw and pretreated reed straw residue (70% water addition, 70 °C, 3 h) using radar images (Figure 11).
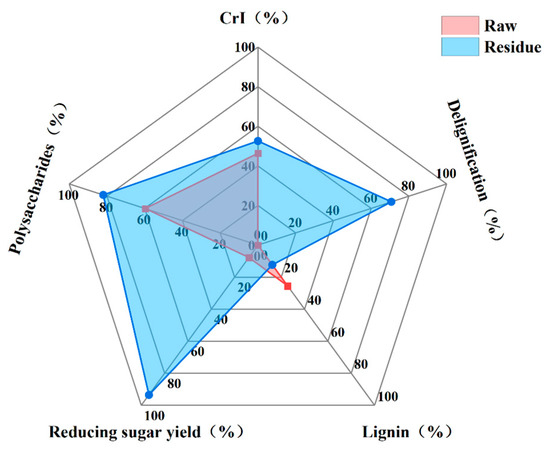
Figure 11.
Radar image of CrI, polysaccharide content, lignin content, delignification, and reducing sugar yield.
Reed straw pretreatment significantly increased crystallinity index by selectively restructuring cellulose, removing amorphous components like hemicellulose and lignin. This process enhanced enzyme binding to cellulose surfaces by reducing steric hindrance. Polysaccharide content and structural integrity were crucial for biomass saccharification efficiency. Pretreated reed straw showed significantly higher polysaccharide content than untreated straw, indicating that the ChOH-TH system pretreatment effectively preserved and enriched polysaccharide components. The delignification rate played a critical role in the evaluation of pretreatment effectiveness. Reed straw exhibited a substantial reduction in lignin content after pretreatment. Lignin hindered cellulase-substrate interaction by forming a barrier over cellulose, leading to non-productive enzyme adsorption and decreased hydrolysis efficiency. The ChOH-TH system disrupted lignin’s cross-linked structure through hydrogen bonding with choline cations, enhancing lignin dissolution. Thiourea acted as a hydrogen bond donor, promoting solvent-lignin interactions. Efficient lignin removal exposed enzymatic active sites on cellulose, reducing non-productive enzyme binding and enhancing saccharification efficiency.
Pretreatment significantly increased reducing sugar yield by removing lignin, enriching polysaccharides, and optimizing crystallinity, enhancing enzymatic hydrolysis and substrate concentration for efficient cellulose conversion.
2.8. Material Balance
Under optimal pretreatment conditions (70% water addition, 70 °C, 3 h), a material balance was performed on 100 g of reed straw (Figure 12). The enzymatic hydrolysis compared reducing sugar yields before and after pretreatment, demonstrating the advantages of this mild approach for reed straw biorefinery. Initially, the raw reed straw comprised 59.75 g of polysaccharides (59.75%), 25.37 g of lignin (25.37%), and 14.88 g of ash and extract impurities. After ChOH-TH pretreatment, polysaccharide residues were 49.29 g, and lignin residues were 7.43 g. The loss rate of solid residues was 39.9%, whereas the retention rate of polysaccharides reached 82.5%. Enzymatic hydrolysis of the pretreated material produced 46.10 g of reducing sugars, compared to 4.64 g from untreated reed straw, indicating a substantial increase in yield due to pretreatment. Thus, ChOH-TH pretreatment significantly enhanced reducing sugar production under mild conditions (70 °C), reducing energy consumption and improving the economic feasibility of reed straw biorefinery.
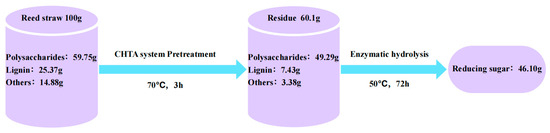
Figure 12.
Mass balance of reed straw before and after pretreatment.
3. Materials and Methods
3.1. Reagents and Materials
Reed straw collected from the suburbs of Changzhou City was crushed, soaked in water, and oven-dried at 80 °C. After drying, it was sieved through a 60-mesh sieve and stored in a dry place. The polysaccharide and lignin contents of the reed straw were 59.75% and 25.37%, respectively. Choline hydroxide (44%) was sourced from Shanghai Titan Science and Technology Co., Ltd. (Shanghai, China), thiourea from Shandong Keyuan Biochemical Co., Ltd. (Laizhou, China), and cellulase from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Preparation of ChOH-TH
A 1:2 molar mixture of choline hydroxide and thiourea was stirred at 140 rpm at 80 °C until a homogeneous liquid formed. The resulting DES was subsequently stored in a desiccator for pretreatment.
3.3. Lignocellulosic Biomass Pretreatment
To evaluate the influence of various parameters on the pretreatment efficacy of reed straw, experiments were conducted using the initial ChOH-TH protocol. Water was added in mass fractions of 0%, 10%, 30%, 50%, 70%, and 90%. The reed straw and ChOH-TH solution were mixed at a solid–liquid ratio of 1:20 (w/w) and processed at a set speed. The effects of different pretreatment durations (0.5 h, 1 h, 2 h, and 3 h) and temperatures (30 °C, 50 °C, 70 °C, and 90 °C) were also examined. After the reaction, samples were filtered through 300-mesh cloth bags, rinsed with water until the filtrate was neutral, and dried in an 80 °C oven to a constant weight, resulting in the pretreated reed straw residue.
Optimized parameters were utilized for pretreating reed straw to systematically evaluate the effectiveness of the CHOH-TH solvent across eight varieties of agricultural biomass waste, aiming to assess the universality of this solvent system. The selected biomass samples encompassed four categories—straw (corn straw, sorghum straw, rape straw), cortex (walnut green shell, wheat bran), shell (sunflower disk, rice hull), and bamboo powder—representing common lignocellulosic resources in agricultural waste. By comparing the composition and saccharification efficiency of untreated raw materials with pretreated residues, the study aimed to ascertain whether the solvent system could address the limitations associated with the use of individual raw materials.
3.4. Chemical Composition Analysis
Biomass residue samples were analyzed for polysaccharides, lignin, and ash content using established methods [54]. Cellobiose, glucose, xylose, and arabinose in enzymatic hydrolysis products of biomass were analyzed using an Agilent HPLC system (Santa Clara, CA, USA) equipped with Aminex HPX-87H columns. The mobile phase comprised 5 mmol/L sulfuric acid at a flow rate of 0.6 mL/min. Lignin content was assessed by treating biomass (0.3 g, dry weight) with 72% sulfuric acid (3.0 mL). The mixture was thoroughly combined in a glass bottle and incubated in a water bath at 30 °C for 1 h. Subsequently, 84.0 mL of water was added to dilute the acid, and the mixture was heated at 121 °C for 1 h to facilitate hydrolysis. Post-reaction, the hydrolysate was filtered, and the filtrate was used to measure acid soluble lignin (ASL). Filtration employed glass filters, and the residue was calcined in a muffle furnace at 550 °C to determine acid insoluble lignin (AIL) content. Solid recovery and delignification from various pretreatments were calculated as described by Wu et al. [55].
3.5. Enzymatic Hydrolysis
Enzymatic hydrolysis was performed in a 25 mL conical flask with 2 g of biomass residue. A 50 mM citrate buffer at pH 4.8 was added to moisten the residue, followed by 40 μL of a 10 g/L tetracycline solution and cellulase at 30 FPU/g residue. The total volume was adjusted to 10 mL with the same buffer, and the flask was sealed with plastic wrap. The reaction proceeded in a water bath shaker at 50 °C and 140 rpm for 72 h. Cellulase was deactivated by immersing the mixture in a boiling water bath for 5 min, followed by centrifugation at 12,000 rpm for 5 min. The supernatant was then analyzed for reducing sugar content to assess saccharification efficiency.
3.6. Characterization of Reed Straw
3.6.1. Scanning Electron Microscopy (SEM)
The morphology, surface structure, and microstructural changes of lignocellulose were analyzed using a Hitachi Regulus 810 SEM (Tokyo, Japan). Samples were dried and sputter-coated with gold to enhance electrical conductivity before imaging.
3.6.2. Atomic Force Microscopy (AFM)
The surface roughness variations of reed straw before and after pretreatment were examined utilizing the American Bruker Dimension ICON AFM (Billerica, MA, USA). The experiment was conducted in the standard peak force mode, capturing signal error graphs and topographic images at a scanning rate of 2.00 Hz. Subsequently, image processing and analysis were carried out using Nanoscope Analysis v3.00 software. Surface roughness parameters encompass root mean square roughness (Rq) and average roughness (Ra), as defined by Mendez-Vilas et al. [56].
Here, Zi is the height of the i-th point relative to the lowest point, and N is the total number of points in the image.
3.6.3. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectroscopy was conducted using a Nicolet IS50 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), scanning the spectral range from 500 to 4000 cm−1. Samples were prepared by combining 1.0 mg of the dried material with 0.1 g of potassium bromide (KBr), grinding the mixture to a 200-mesh fineness, and compressing the powder into tablets.
3.6.4. X-Ray Diffraction (XRD)
The crystallinity of both reprocessed and untreated reed straw was assessed using a Rigaku Ultima X-ray diffraction (XRD) instrument (Akishima City, Tokyo). The analysis covered a scanning range of 2θ = 5–90°. The crystallinity index (CrI) was determined using the formula:
where I002 represents the peak intensity at 2θ ≈ 22.17°, and Iam corresponds to the minimum intensity reflecting the amorphous content at 2θ ≈ 18°.
3.6.5. Thermogravimetric Analysis (TGA)
Thermal stability was assessed with a Netzsch TG209F3 thermogravimetric analyzer (Selb, Germany). Samples of approximately 3 mg were heated from room temperature to 800 °C at 10 °C/min under a nitrogen atmosphere.
3.7. Reuse of Pretreatment Solution
In the experimental study on recycling the pretreatment solution, reed straw was pretreated with a ChOH-TH solution containing the addition of 70% water at 70 °C for 3 h. After the reaction, the ChOH-TH solution and reed straw were separated by filtration. The recovered solution was reused for the pretreatment of reed straw in each cycle, while the filtered residue was collected, washed, dried, and subjected to further analysis of its components and saccharification efficiency. The entire cycle process was repeated continuously five times. Furthermore, considering that thiourea may decompose under alkaline conditions, generating by-products such as ammonia and isothiocyanates, which pose certain environmental emission risks. Subsequently, the experiment added the step of “activated carbon adsorption + pH adjustment”: 0.5 g/L activated carbon was added to the final DES system and shaken for 30 min. Then, 0.1 mol/L HCl was used to adjust the pH to neutral to avoid the environmental emission risks of the by-products.
3.8. Statistical Analysis
To ensure the reliability and statistical significance of the experimental results, all component analyses and enzymatic hydrolysis experiments were performed in triplicate (n = 3). The data reported in this study are presented as mean values ± standard deviation. SPSS software (version 22.0) was used for statistical analysis of the obtained data.
4. Conclusions
This study introduced a novel aqueous alkaline DES comprising choline hydroxide and thiourea. Incorporating 70% water addition, the yield of reducing sugar from reed straw increased markedly from 7.77% to 93.52% after pretreatment at 70 °C for 3 h. Characterization techniques demonstrated that this pretreatment disrupted the dense cell wall structure, enhancing biomass bioconversion. Furthermore, after five reuse cycles, the pretreatment solution still maintained a saccharification efficiency of 64.29%. These findings are pivotal for optimizing lignocellulosic biomass pretreatment strategies, advancing biorefinery processes towards reduced energy consumption, high conversion efficiency, and sustainability, with substantial theoretical and industrial implications.
Author Contributions
Conceptualization, L.G. and J.L.; methodology, L.G.; software, J.L.; validation, L.G., J.L. and M.L.; formal analysis, L.G. and J.C.; investigation, L.G. and J.L.; resources, L.G.; data curation, L.G., J.L. and M.L.; writing—original draft preparation, L.G. and J.L.; writing—review and editing, L.G.; visualization, J.L. and L.S.; supervision, J.Z.; project administration, L.G. and J.Z.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of Natural Science Research in Universities of Jiangsu Province (22KJB530004) and National Natural Science Foundation of China (22208030).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DESs | Deep eutectic solvents |
| ALDES | Alkaline deep eutectic solvent |
| SEM | Scanning electron microscope |
| AFM | Atomic force microscopy |
| FTIR | Fourier-transform Infrared |
| XRD | X-ray diffraction |
| CrI | crystallinity index |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
| ChOH-TH | Choline hydroxide and thiourea |
References
- Liu, J.P.; Xie, Y.; Xu, X.; Xiang, R.R.; Zhang, C.Y.; Ji, Z.; Yong, Q.; Ling, Z. Microwave assisted alkaline deep eutectic solvents treatment inducing deconstruction of recalcitrant moso bamboo cell walls for highly efficient dual-enzymatic saccharification. Chem. Eng. J. 2025, 508, 160909. [Google Scholar] [CrossRef]
- Zhu, R.N.; Yang, M.; Yin, J.; Wu, Z.; Li, M.R.; Zhao, L.H.; Wang, X.J.; Ren, J.L. Mild sodium carbonate pretreatment of wheat straw for improving cellulose and xylan enzymatic hydrolysis. Ind. Crops Prod. 2025, 227, 120728. [Google Scholar] [CrossRef]
- Liu, H.J.; Sun, D.Y.; Yang, L.; Ma, G.H.; Xia, R.Y.; Wang, Z.Q.; Yao, M.Z.; Gao, L.G.; Wei, R.P.; Pan, X.M.; et al. Pretreatment of enzymatic hydrolysis lignin based on deep eutectic solvent containing a reversibly-soluble base. Int. J. Biol. Macromol. 2025, 301, 140452. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, C.; Tang, W.; He, Y.C. Comprehensive understanding of enzymatic saccharification of Betaine: Lactic acid-pretreated sugarcane bagasse. Bioresour. Technol. 2023, 386, 129485. [Google Scholar] [CrossRef]
- Zhang, J.J.; Song, X.; Wu, Y.; Liang, J.; Lu, J.; Zhang, J.F. Waste to wealth: A novel low temperature eco-friendly lignocellulose pretreatment strategy for glucose production. Biochem. Eng. J. 2024, 209, 109384. [Google Scholar] [CrossRef]
- Tang, W.; Qian, H.J.; Wang, X.Y.; Huang, C.X.; He, Y.C. Adequately converting poplar sawdust’s carbohydrates to furfural and glucose by employing acid oxidation pretreatment and solid acid catalysis. Ind. Crops Prod. 2024, 221, 119374. [Google Scholar] [CrossRef]
- Semenova, M.V.; Rozhkova, A.M.; Osipov, D.O.; Telitsin, V.D.; Rubtsova, E.A.; Kondrat’eva, E.G.; Vasil’eva, I.S.; Morozova, O.V.; Yaropolov, A.I.; Sinitsyn, A.P. Methods for Preprocessing Reeds to Obtain Enzymatic Hydrolysates with a High Sugar Content. Appl. Biochem. Microbiol. 2024, 60, 931–941. [Google Scholar] [CrossRef]
- Li, R.L.; Zheng, Y.Y.; Zhao, X.X.; Yong, Q.; Meng, X.Z.; Ragauskas, A.; Huang, C.X. Recent advances in biomass pretreatment using biphasic solvent systems. Green. Chem. 2023, 25, 2505–2523. [Google Scholar] [CrossRef]
- Farhan, M.; Hasani, I.W.; Khafaga, D.S.R.; Ragab, W.M.; Kazi, R.N.A.; Aatif, M.; Muteeb, G.; Fahim, Y.A. Enzymes as catalysts in industrial biocatalysis: Advances in engineering, applications, and sustainable integration. Catalysts 2025, 15, 891. [Google Scholar] [CrossRef]
- Brunecky, R.; Li, Y.D.; Decker, S.R.; Himmel, M.E. Advancing continuous enzymatic hydrolysis for improved biomass saccharification. Biotechnol. Biofuels Bioprod. 2025, 18, 82. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, Y.; Wang, Y.Y.; Gong, W.H.; Ma, C.Y.; Li, S.; Wen, J.L. Promoting the disassemble and enzymatic saccharification of bamboo shoot shells via efficient hydrated alkaline deep eutectic solvent pretreatment. Int. J. Biol. Macromol. 2024, 264, 130702. [Google Scholar] [CrossRef]
- Guo, J.W.; Yu, G.C.; Wang, J.L. Comparative insight into biomass pretreatment by choline chloride-based deep eutectic solvents in relation to their physicochemical characteristics. J. Environ. Chem. Eng. 2025, 13, 118256. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Sun, Z.W.; Li, N.; Zhu, L.B.; Liu, Z.Q.; Xie, C.Y.; Zhang, B.X.; Zheng, Z.; Liang, S.Y.; Yan, J.; et al. Effects of Combination of Steam Explosion and Alkali Pretreatment on Enzymatic Hydrolysis, Sugar Yields, and Structural Properties of Reed Straw. Waste Biomass Valori. 2025, 16, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, A.; Li, X.H.; Xu, W.B.; Duan, X.X.; Shi, J.Y.; Li, X.Y. Research progress of deep eutectic solvents in lignocellulosic biomass pretreatment. Cellulose 2025, 32, 4637–4650. [Google Scholar] [CrossRef]
- Zhao, X.X.; Wang, J.H.; Lan, K.; Zhao, Z.C.; Lai, C.H.; Huang, C.X.; Yong, Q. Integrated Nondestructive Spectroscopic Technology to Reveal the Influence Mechanism of Lignins from Pretreated Corn Stover on Cellulose Saccharification. ACS Sustain. Chem. Eng. 2024, 12, 2871–2880. [Google Scholar] [CrossRef]
- Di, J.H.; Li, Q.; Ma, C.L.; He, Y.C. An efficient and sustainable furfurylamine production from biomass-derived furfural by a robust mutant co-transaminase biocatalyst. Bioresour. Technol. 2023, 369, 128425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, T.X.; Li, H.; Zhao, Q.S.; Zhao, B. Synergism of jet milling and deep eutectic solvent pretreatment on grapevine lignin fractionation and enhancing enzymatic hydrolysis. Int. J. Biol. Macromol. 2024, 269, 132144. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Cheng, L.; Zhou, X.; Ouyang, S.P. Comparative Evaluation of Dilute Acid, Alkaline, and Deep Eutectic Solvent Pretreatments on Enzymatic Hydrolysis of Sunflower Stalk Bark. Appl. Biochem. Biotechnol. 2025, 15, 1–16. [Google Scholar] [CrossRef]
- Li, Q.; Gao, R.Y.; Li, Y.C.; Fan, B.; Ma, C.L.; He, Y.C. Improved biotransformation of lignin-valorized vanillin into vanillylamine in a sustainable bioreaction medium. Bioresour. Technol. 2023, 384, 129292. [Google Scholar] [CrossRef]
- Wu, M.J.; Di, J.H.; Gong, L.; He, Y.C.; Ma, C.L.; Deng, Y. Enhanced adipic acid production from sugarcane bagasse by a rapid room temperature pretreatment. Chem. Eng. J. 2023, 452, 139320. [Google Scholar] [CrossRef]
- Xu, D.Z.; Ma, C.L.; Wu, M.J.; Deng, Y.; He, Y.C. Improved production of adipic acid from a high loading of corn stover via an efficient and mild combination pretreatment. Bioresour. Technol. 2023, 382, 129196. [Google Scholar] [CrossRef]
- Sha, H.; Cao, S.X.; Zhao, B.; Dong, Z.; Wang, G.; Duan, J. Effect of alkaline deep eutectic solvents pretreatment on CH4 yield from anaerobic digestion of corn stover. Energy 2024, 302, 131683. [Google Scholar] [CrossRef]
- Song, W.L.; Jiang, J.A.; Jiang, H.X.; Liu, C.T.; Dong, Y.; Chen, X.; Xiao, L.P. Acid/alkali-catalyzed deep eutectic solvent pretreatment of corn straw for enhanced biohydrogen production. Fuel 2023, 348, 128521. [Google Scholar] [CrossRef]
- Ortega-Sanhueza, I.; Albornoz-Palma, G.; Torres, C.; Andrade, A.; Pereira, M.; Marzialetti, T. The impact of humidity on fractionation of Eucalyptus globulus pinchips using alkaline DES of potassium carbonate and glycerol. Cellulose 2025, 32, 1–10. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.C.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G.R. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, Y.; Zhang, W.J.; Li, M.F.; Peng, F.; Bian, J. Alkaline deep eutectic solvents as novel and effective pretreatment media for hemicellulose dissociation and enzymatic hydrolysis enhancement. Int. J. Biol. Macromol. 2021, 193, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- He, C.J.; Li, X.L.; Luo, F.Q.; Mi, C.Y.; Zhan, A.; Ou, R.X.; Fan, J.J.; Clark, J.H.; Yu, Q. Glycol-based Alkaline Deep Eutectic Solvents for “Lignin-First” Dissolution from Coconut Shells. ACS Sustain. Chem. Eng. 2024, 12, 11327–11337. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J. Chem. Technol. Biotechnol. 2012, 87, 1209–1214. [Google Scholar] [CrossRef]
- Ul Haq, I.; Mustafa, Z.; Nawaz, A.; Rehman, A.U.; Mukhtar, H. Effect of physical and chemical pretreatment methods on sawdust for improved saccharification. Pak. J. Bot. 2020, 52, 1435–1440. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhu, L.L.; He, Y.C. Valorization of rapeseed straw through alkali-assisting deep eutectic solvent ChCl:Thiourea pretreatment at room temperature. Fuel 2024, 378, 132932. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhao, X.; Chen, L.; Zhang, X. Pretreatment with fermentable and recyclable deep eutectic solvent (DES) for improving resource utilization of biomass. Ind. Crops Prod. 2022, 190, 115868. [Google Scholar] [CrossRef]
- Piskulich, Z.A.; Mesele, O.O.; Thompson, W.H. Activation energies and beyond. Chem. A Eur. J. 2019, 123, 7185–7194. [Google Scholar] [CrossRef] [PubMed]
- Yanak, S.; Buyukkileci, A.O. Delignification of corncob by choline chloride-urea deep eutectic solvent for enzymatic production of xylooligosaccharides. Ind. Crops Prod. 2024, 217, 118894. [Google Scholar] [CrossRef]
- Xu, X.; Wang, K.; Zhou, Y.B.; Lai, C.H.; Zhang, D.H.; Xia, C.L.; Pugazhendhi, A. Comparison of organosolv pretreatment of masson pine with different solvents in promoting delignification and enzymatic hydrolysis efficiency. Fuel 2023, 338, 127361. [Google Scholar] [CrossRef]
- Wu, R.J.; Li, Y.Z.; Wang, X.D.; Fu, Y.J.; Qin, M.H.; Zhang, Y.C. In-situ lignin sulfonation for enhancing enzymatic hydrolysis of poplar using mild organic solvent pretreatment. Bioresour. Technol. 2023, 369, 128410. [Google Scholar] [CrossRef]
- Velmurugan, R.; Kuhad, R.C.; Gupta, R.; Chakraborty, S.; Ashokkumar, V.; Xia, C.L.; Pugazhendhi, A. Ionic liquid under alkaline aqueous conditions improves corncob delignification, polysaccharide recovery, enzymatic hydrolysis and ethanol fermentation. Biomass Bioenergy 2023, 178, 106980. [Google Scholar] [CrossRef]
- Ahmad, P.; Khalid, A.; Khan, A.; Muhammad, N. Evaluation of [Emim]Glu ionic liquid as a deconstruction solvent for lignocellulosic biomass to biobased materials. Biomass Bioenergy 2025, 204, 108380. [Google Scholar] [CrossRef]
- Jiang, C.X.; He, Y.C.; Chong, G.G.; Di, J.H.; Tang, Y.J.; Ma, C.L. Enzymatic in situ saccharification of sugarcane bagasse pretreated with low loading of alkalic salts Na2SO3/Na3PO4 by autoclaving. J. Biotechnol. 2017, 259, 73–82. [Google Scholar] [CrossRef]
- Kshirsagar, S.D.; Waghmare, P.R.; Loni, P.C.; Patil, S.A.; Govindwar, S.P. Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv. 2015, 5, 46525–46533. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Wu, C.Q.; Tang, W.; Ma, C.L.; He, Y.C. A novel cetyltrimethylammonium bromide-based deep eutectic solvent pretreatment of rice husk to efficiently enhance its enzymatic hydrolysis. Bioresour. Technol. 2023, 376, 128806. [Google Scholar] [CrossRef]
- Pang, C.S.; Xie, T.J.; Lin, L.; Zhuang, J.P.; Liu, Y.; Shi, J.B.; Yang, Q.L. Changes of the surface structure of corn stalk in the cooking process with active oxygen and MgO-based solid alkali as a pretreatment of its biomass conversion. Bioresour. Technol. 2012, 103, 432–439. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.R.; Zhang, K.J.; Si, M.Y.; Liu, M.R.; Chai, L.Y.; Liu, X.D. Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef]
- Dash, D.; Mund, N.K.; Upadhyay, D.; Mishra, P.; Dash, S.K.; Nayak, N.R. Evaluation of alkali and cellulose solvent pretreatments for fermentable sugar production from the biomass of Phragmites karka (Retz.) Trin. ex Steud.: A high biomass producing grass. Biomass Convers. Biorefin. 2021, 13, 7725–7736. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Xu, J.; Xie, J.X.; Zhu, S.Y.; Wang, B.; Li, J.; Chen, K.F. Physicochemical Transformation and Enzymatic Hydrolysis Promotion of Reed Straw after Pretreatment with a New Deep Eutectic Solvent. Carbohydr. Polym. 2022, 290, 119472. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Tang, W.; Ma, C.L.; He, Y.C. Improved Enzymatic Saccharification of Bulrush via an Efficient Combination Pretreatment. Bioresour. Technol. 2023, 383, 129369. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Z.; Tang, W.; Li, L.; Huang, M.H.; Ma, C.L.; He, Y.C. Enhancing enzymatic hydrolysis of waste sunflower straw by clean hydrothermal pretreatment. Bioresour. Technol. 2023, 383, 129236. [Google Scholar] [CrossRef] [PubMed]
- Sirvio, J.A.; Visanko, M.; Liimatainen, H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.X.; Huang, C.X.; Ling, Z.; Lai, C.H.; Yong, Q. Revealing the influence of metallic chlorides pretreatment on chemical structures of lignin and enzymatic hydrolysis of waste wheat straw. Bioresour. Technol. 2021, 342, 125983. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving enzymatic digestibility of sugarcane bagasse from different varieties of sugarcane using deep eutectic solvent pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef]
- Binczarski, M.J.; Zuberek, J.; Fraczyk, J.; Kolesinska, B.; Radojcin, M.; Pavkov, I.; Wiktorowska-Sowa, E.; Piotrowski, J.; Kaminski, Z.J.; Witonska, I.A. Application of Microorganisms for the Valorization of Side-Products of Rapeseed De-Oiling. Biomolecules 2025, 15, 917. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Characterization and alkaline pretreatment of rice husk varieties in Uganda for potential utilization as precursors in the production of activated carbon and other value-added products. Waste Manag. 2018, 81, 104–116. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Lourenço-Lopes, C.; Taofiq, O.; Otero, P.; Cao, H.; Xiao, J.B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Walnut (Juglans Regia) as a Source of Bioactive Compounds for the Formulation of Nutraceuticals and Functional Foods. Biol. Life Sci. Forum 2022, 12, 35. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.L.; Sharma, V.; Sun, P.P.; Nargotra, P.; Bajaj, B.K.; Chen, C.W.; Dong, C.D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2022, 10, 6. [Google Scholar] [CrossRef]
- Sluiter, A.D.; Hames, B.R.; Ruiz, O.R.; Scarlata, C.J.; Sluiter, J.; Templeton, D.W.; Crocker, D.P. Determination of structural carbohydrates and lignin in biomass national renewable. Energy Lab. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Wu, M.J.; Gong, L.; Ma, C.L.; He, Y.C. Enhanced enzymatic saccharification of sorghum straw by effective delignification via combined pretreatment with alkali extraction and deep eutectic solvent soaking. Bioresour. Technol. 2021, 340, 125695. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Vilas, A.; Bruque, J.M.; Gonzalez-Martin, M.L. Sensitivity of surface roughness parameters to changes in the density of scanning points in multi-scale AFM studies. application to a biomaterial surface. Ultramicroscopy 2007, 107, 617–625. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).