Biowaste-to-Catalyst: Magnetite Functionalized Potato-Shell as Green Magnetic Biochar Catalyst (PtS200–Fe3O4) for Efficient Procion Blue Textile Wastewater Dye Abatement
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of PtS200–Fe3O4 Material
2.1.1. XRD Pattern
2.1.2. SEM Morphology
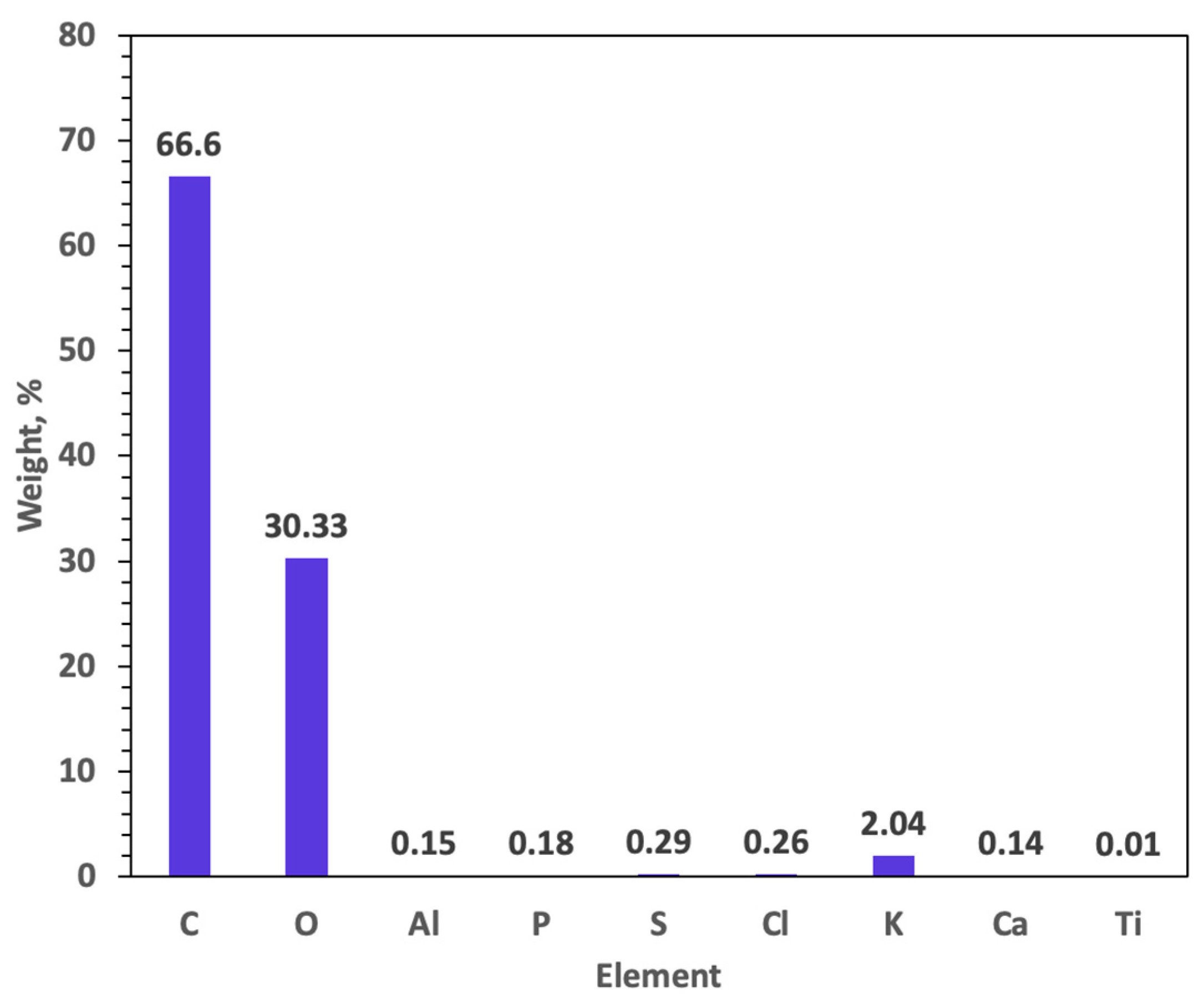
2.1.3. EDX
2.1.4. Surface Elemental Mapping of PtS200–Fe3O4 Composite
2.2. Dye Photo-Fenton Oxidation
2.2.1. Effect of Different Treatment Systems
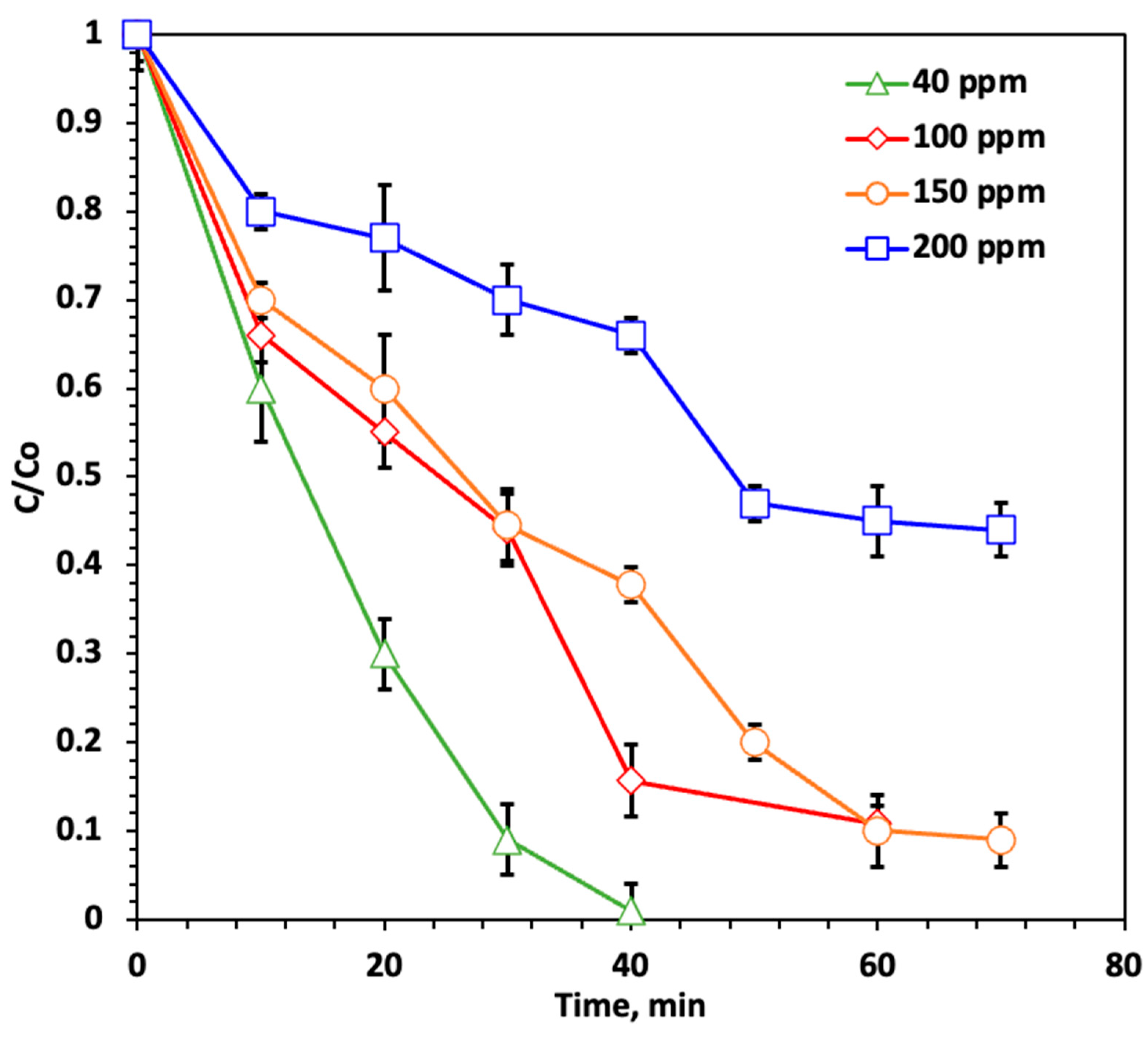
2.2.2. Effect of Dye Loadings
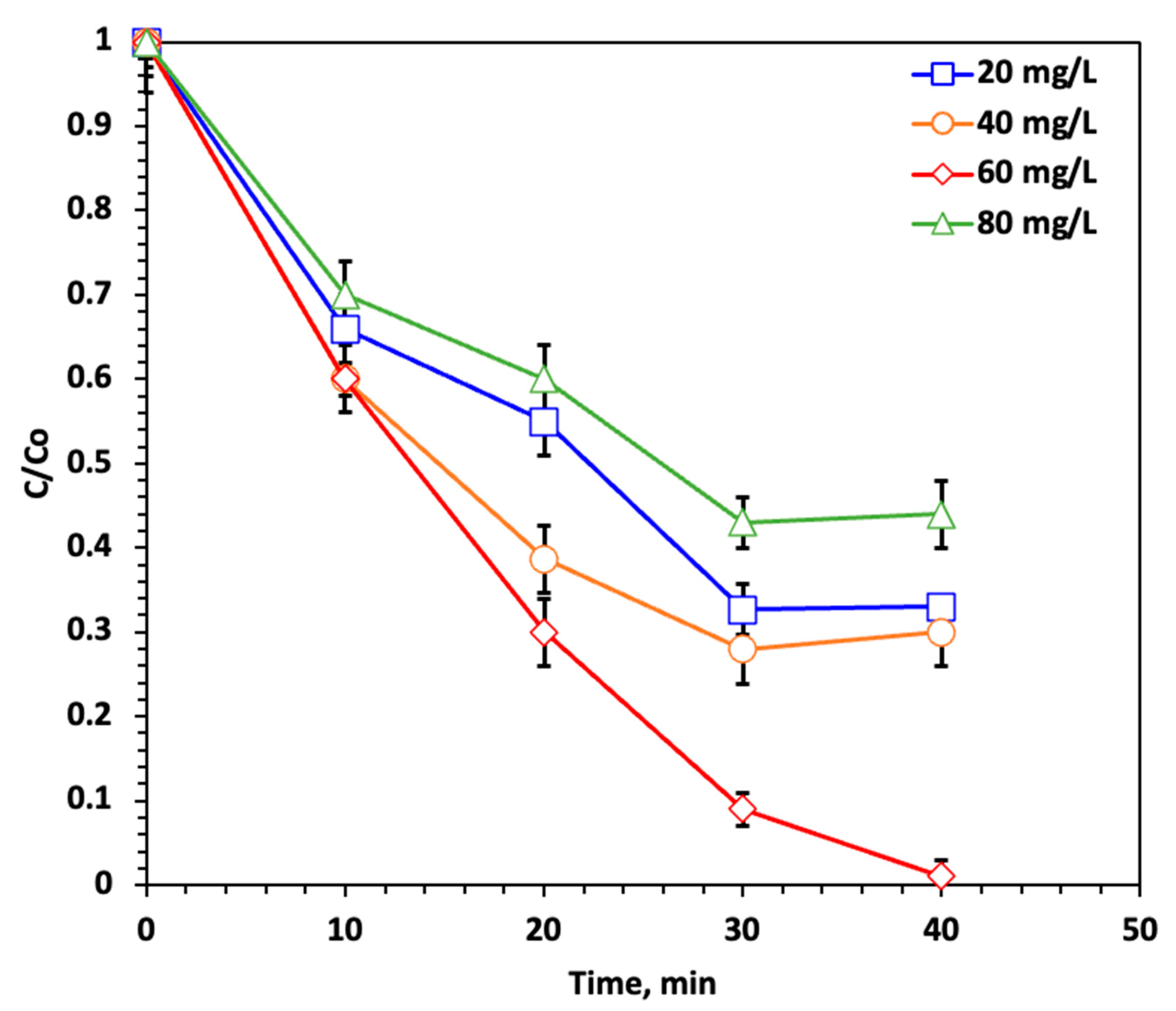
2.2.3. Effect of Catalyst (PtS200–Fe3O4) Concentration
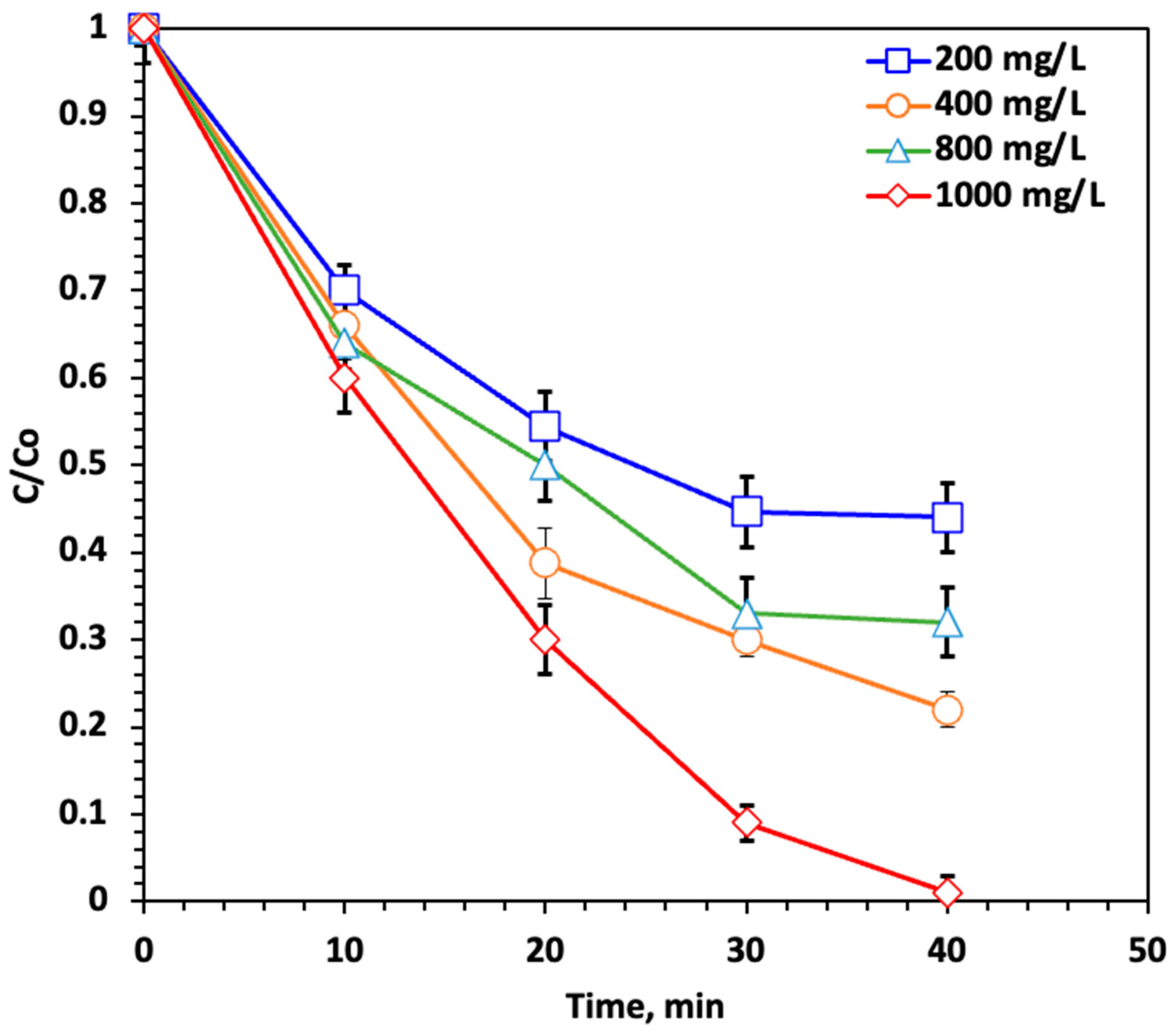
2.2.4. Effect of H2O2 Concentration
2.2.5. Effect of pH
2.2.6. Effect of Temperature
2.2.7. Kinetic Analysis
2.2.8. Reaction Thermodynamics Analysis
2.2.9. Recyclability and Sustainable Approach
2.2.10. Comparative Investigation with Literature
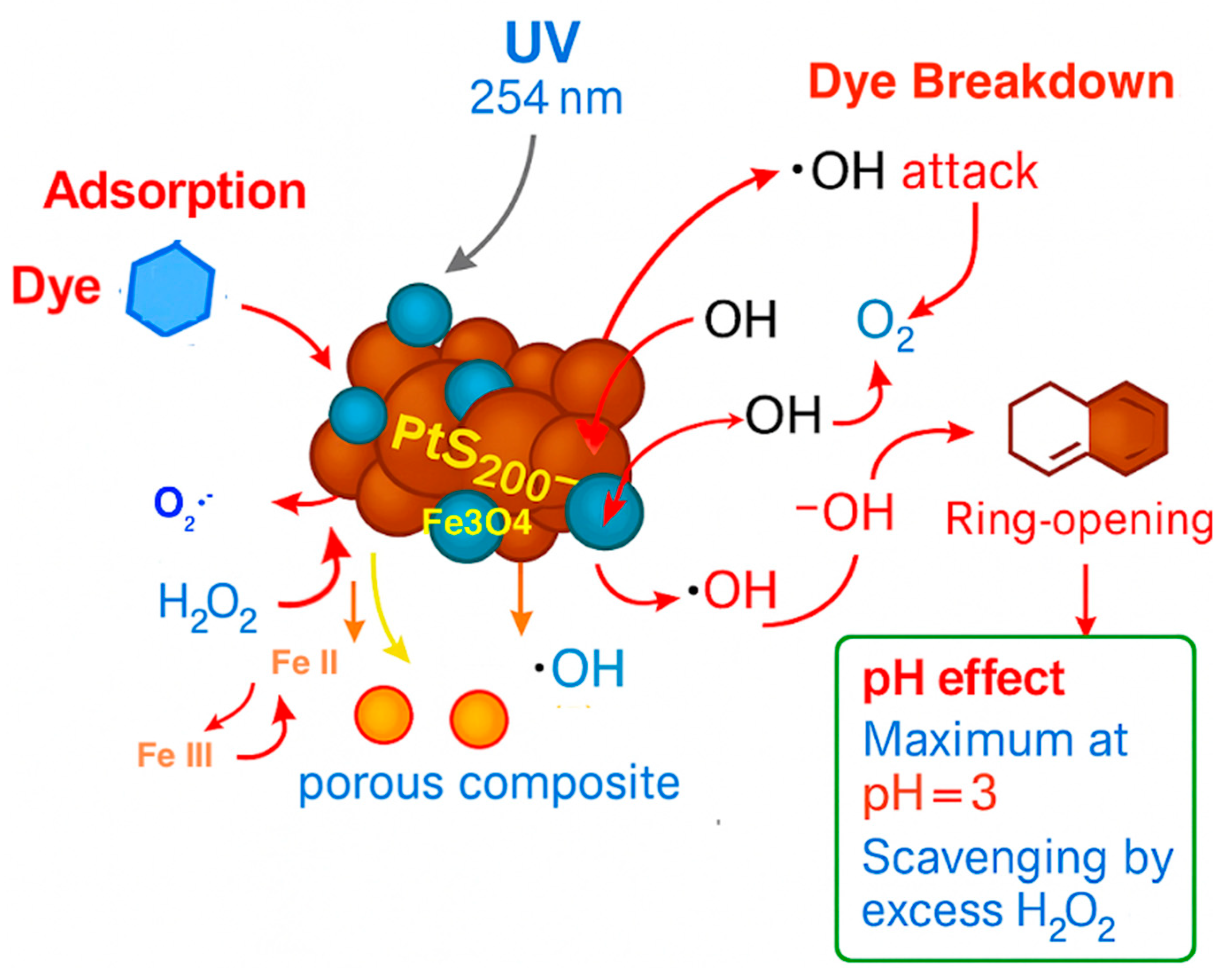
2.2.11. Mechanistic Investigation
3. Experimental Investigation:
3.1. Materials
3.1.1. Preparation of Biochar from Potato Shell (PtS200)
3.1.2. Preparation of Magnetite (Fe3O4) Nanoparticles
3.1.3. Photocatalyst (PtS200–Fe3O4) Preparation
3.1.4. Wastewater
3.2. Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, L.; Zhou, X.; Wang, J.; Chen, Y.; Zhang, H.; Liu, Q. Iron-based biochar catalyst derived from Fenton sludge for advanced oxidation of phenolic and arsenite pollutants. Water 2025, 17, 765. [Google Scholar] [CrossRef]
- Masud, A.; Rahman, M.; Kim, S.; Lee, C. Fe–biochar composites for advanced oxidation: Enhanced ROS generation and catalytic performance. npj Mater. Degrad. 2025, 9, 71. [Google Scholar]
- Li, H.; Sun, Y.; Gao, L.; Chen, J. Partially oxidized FeS/biochar composites for efficient photo-Fenton degradation of organic pollutants. Environ. Pollut. 2024, 336, 122753. [Google Scholar]
- Hashemi, S. Magnetic biochar for catalytic removal of textile dyes via Fenton and Fenton-like reactions: A review. Environ. Res. 2024, 250, 118768. [Google Scholar]
- Leichtweis, J.; Silva, T.; Pereira, R.; Rocha, J. CuFe2O4/biochar composite for photo-Fenton degradation of synthetic dyes. J. Environ. Chem. Eng. 2022, 10, 108247. [Google Scholar]
- Ashour, E.A.; Tony, M.A. Equilibrium and Kinetic Studies on Biosorption of Iron (II) and Iron (III) Ions onto Eggshell Powder from Aqueous Solution. Appl. Eng. 2017, 1, 65–73. [Google Scholar]
- Mangood, A.H.; Salama, E.S.; El-Sayed, I.E.T.; Fouad, M.K.; Tony, M.A. Valorizing alum sludge waste augmented ferrite as a sustainable magnetic pathway for treating Indigo carmine effluent. Sci. Rep. 2025, 15, 29450. [Google Scholar] [CrossRef]
- Khan, M.; Ali, I.; Han, D.; Zhao, Y. Magnetite-based heterogeneous Fenton systems for dye wastewater treatment. Chemosphere 2021, 270, 128608. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Wang, Y.; Liu, H. Fe-doped TiO2 photocatalyst for the photo-Fenton degradation of reactive dyes. Appl. Catal. B Environ. 2022, 307, 121151. [Google Scholar] [CrossRef]
- Rodríguez-Rasero, C.; Montes-Jimenez, V.; Alexandre-Franco, M.F.; Fernández-González, C.; Píriz-Tercero, J.; Cuerda-Correa, E.M. Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water—A Review. Water 2024, 16, 1607. [Google Scholar] [CrossRef]
- Al-Kdasi, A.; Idris, A.; Saed, K.; Guan, C.T. Treatment of textile wastewater by advanced oxidation processes—A review. Glob. Nest Int. J. 2004, 6, 222–230. [Google Scholar]
- Kang, S.F.; Liao, C.H.; Po, S.T. Decolorization of textile wastewater by photo-Fenton oxidation technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Tizaoui, C.; Hilal, N. Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination 2011, 266, 1–16. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Iron recovery from acid mine drainage sludge as Fenton source for municipal wastewater treatment. Int. J. Environ. Anal. Chem. 2020, 102, 1245–1260. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- Amelia, S.; Sediawan, W.B.; Prasetyo, I.; Munoz, M.; Ariyanto, T. Role of the pore structure of Fe/C catalysts on heterogeneous Fenton oxidation. J. Environ. Chem. Eng. 2020, 8, 102921. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Recent advances in heterogeneous Fenton-like catalysis for water treatment. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar]
- Mangood, A.H.; Salama, E.S.; El-Sayed, I.E.T.; Fouad, M.K.; Tony, M.A. Construction of heterojunction superparamagnetic Cd0.5Ag0.5Fe2O4 nanocomposite photocatalyst for simulated textile effluent oxidation. J. Phys. Conf. Ser. 2023, 2830, 012018. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Lui, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-derived porous carbon materials for energy and environmental applications. J. Mater. Chem. A. 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; Ali, I.A.; El Sherbiney, S.A.; Tony, M.A. Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int. J. Environ. Anal. Chem. 2021, 103, 2636–2658. [Google Scholar] [CrossRef]
- Guye, M.E.; Dabaro, M.D.; Kim, H. Biomass-derived graphitic-like hierarchical porous carbon for electrochemical supercapacitor application. J. Energy Storage 2025, 115, 116037. [Google Scholar] [CrossRef]
- Vandana, T.U.; Tripathy, B.K.; Mishra, R.K.; Sharma, A.; Mohanty, K. A review on waste biomass-derived biochar: Production, characterisation, and advanced analytical techniques for pollutants assessment in water and wastewater. Process Saf. Environ. Prot. 2025, 201, 10750. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Martins, V.; Rocha, F.; Soković, M.D.; Barata, A.M.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Valorisation of table tomato crop by-products: Phenolic profiles and in vitro antioxidant and antimicrobial activities. Food Bioprod. Process. 2020, 124, 307–319. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef]
- Khelifi, S.; Choukchou-Braham, A.; Sbihi, H.M.; Azam, M.; Al-Resayes, S.I.; Ayari, F. Treatment of textile dyeing wastewater using advanced photo-oxidation processes for decolorization and COD reduction. Desalin. Water Treat. 2021, 217, 350–357. [Google Scholar] [CrossRef]
- Gavril Rațu, R.N.; Stoica, F.; Lipșa, F.D.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Pumpkin and Pumpkin By-Products: A Comprehensive Overview of Phytochemicals, Extraction, Health Benefits, and Food Applications. Foods 2024, 13, 2694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nour, M.M.; Tony, M.A.; Nabwey, H.A. Immobilization of magnetic nanoparticles on cellulosic wooden sawdust for competitive Nudrin elimination from environmental waters as a green strategy: Box–Behnken design optimization. Int. J. Environ. Res. Public Health 2022, 19, 15397. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Sustainable use of low-cost adsorbents prepared from waste fruit peels for the removal of selected reactive and basic dyes found in wastewaters. Environ. Sci. Pollut. Res. Int. 2024, 31, 14662–14689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wan, J.; Li, Q.; Sun, L.; Zhang, Y.; Li, Z.; Dang, C.; Fu, J. Degradation of Reactive Brilliant Red X-3B by Photo-Fenton-like Process: Effects of Water Chemistry Factors and Degradation Mechanism. Water 2022, 14, 380. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Bidira, F.; Al-Senani, G.M.; Bekele, E.A.; Garomsa, F.S.; Vigneshwaran, S.; Al-Qahtani, S.D.; Asaithambi, P. Synergistic pollutant removal from brewery wastewater using electrocoagulation-assisted pumpkin seed-activated carbon. Process Saf. Environ. Prot. 2025, 200, 107429. [Google Scholar] [CrossRef]
- Mangood, A.H.; Salama, E.S.; El Sayed, I.E.T.; Fouad, M.K.; Tony, M.A. Efficient Augmentation of Superparamagnetic Ferrite with Alum Sludge as a Sustainable Nanoadsorbent Matrix for Promoting Dye Removal: Preparation, Characterization and Application. Int. J. Environ. Res. 2025, 19, 76. [Google Scholar] [CrossRef]
- Yusuf, K.; Benek, V.; Teğin, İ.; Önal, Y.; Erol, K.; Alacabey, I. Reactive Blue 19 Adsorption on Activated Carbon from Pumpkin (Cucurbita Pepo) Seed Waste: Kinetic, Isotherm and Thermodynamic Studies. J. Environ. Res. Eng. Manag. 2024, 80, 7–20. [Google Scholar] [CrossRef]
- Nour, M.M.; Tony, M.A.; Lee, W.H.; Nabwey, H.A. Opportunities of aluminum sludge dredging with soft magnetic conditioner for dewaterability enrichment. Sci. Rep. 2025, 15, 7796. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; Wiley-VCH: Zurich, Switzerland, 2003. [Google Scholar]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as adsorbents. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewater: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Res. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J. Environ. Manag. 2011, 92, 749–755. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A. New treatment of stabilized leachate by ozone/Fenton in the advanced oxidation process. Waste Manag. 2012, 32, 1693–1698. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Fan, M.-H.; Guo, H.-Q.; Lee, Y.-F.; Sun, R.-X. Oxidative decomposition of p-nitroaniline in water by solar photo-Fenton advanced oxidation process. J. Hazard. Mater. 2008, 153, 187–193. [Google Scholar] [CrossRef]
- Beltrán, F.J.; González, M.; Ribas, F.J.; Alvarez, P. Fenton Reagent Advanced Oxidation of Polynuclear Aromatic Hydrocarbons in Water. Water Air Soil Pollut. 1998, 105, 685–700. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Levchuk, I.; Manzano, M.; Sillanpää, M. Toxicity Reduction of Industrial and Municipal Wastewater by Advanced Oxidation Processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): A Review. Catalysts 2020, 10, 612. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Advanced oxidation of Reactive Blue 181 solution: A comparison between Fenton and Sono-Fenton Process. Ultrason. Sonochemistry 2014, 21, 1881–1885. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G. Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci. 2006, 5, 81–135. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Trellu, C.; Vargas, H.O.; Mousset, E.; Ganiyu, S.O.; Oturan, M.A. Electro-Fenton process in combination with other advanced oxidation processes: Challenges and opportunities. Curr. Opin. Electrochem. 2022, 37, 101171. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.; Senthilnathan, J. Nanocatalysts in Fenton Based Advanced Oxidation Process for Water and Wastewater Treatment. J. Bionanoscience 2016, 10, 356–368. [Google Scholar] [CrossRef]
- Mahtab, M.S.; Islam, D.T.; Farooqi, I.H. Optimization of the process variables for landfill leachate treatment using Fenton based advanced oxidation technique. Eng. Sci. Technol. Int. J. 2021, 24, 428–435. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Tony, M.A.; Purcell, P.J.; Zhao, Y.Q. Fenton oxidation for the removal of dyes from textile wastewater. J. Environ. Sci. Health Part A 2012, 47, 1743–1752. [Google Scholar]
- Sangeetha Piriya, R.; Jayabalakrishnan, R.M.; Maheswari, M.; Boomiraj, K.; Oumabady, S. Coconut shell derived ZnCl2 activated carbon for malachite green dye removal. Water Sci. Technol. 2021, 83, 1167–1182. [Google Scholar] [CrossRef]
- Smith, A. Advanced Fenton processes for the degradation of reactive textile dyes. J. Environ. Chem. Eng. 2019, 7, 103292. [Google Scholar]
- Giannakis, S.; Samoili, S.; Rodríguez-Chueca, J. A meta-analysis of the scientific literature on (photo)Fenton and persulfate advanced oxidation processes: Where do we stand and where are we heading to? Curr. Opin. Green Sustain. Chem. 2021, 29, 100456. [Google Scholar] [CrossRef]
- Brillas, E. A review on the application of single and combined Fenton, photo-Fenton, and electrochemical advanced oxidation processes to remove diclofenac from aqueous media. J. Environ. Chem. Eng. 2025, 13, 115443. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, Y.; Huang, L.; Chen, Y.; Bian, Z. In situ Fe(III)-doped TiO2 mesocrystals catalyzed visible light photo-Fenton system. Catal. Today 2023, 410, 309–316. [Google Scholar] [CrossRef]
- Watwe, V.S.; Kulkarni, S.D.; Kulkarni, P.S. Valorizing Cr(III) in a Microwave-Assisted Fenton-like System for Sustainable Dye Degradation and Resource-Efficient Wastewater Treatment. ACS Sustain. Resour. Manag. 2025, 2, 1580–1592. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Zhao, M.; Wang, X.; Wang, S.; Gao, M. Creating an energy-efficient electrochemical advanced oxidation pathway combining anodic oxidation and electro-Fenton via a potential alternating mode. Chem. Eng. J. 2024, 496, 153934. [Google Scholar] [CrossRef]
- Bencheqroun, Z.; Sahin, N.E.; Soares, O.S.; Pereira, M.F.; Zaitan, H.; Nawdali, M.; Rombi, E.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Fe(III)-exchanged zeolites as efficient electrocatalysts for Fenton-like oxidation of dyes in aqueous phase. J. Environ. Chem. Eng. 2022, 10, 107891. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Li, Z.-H.; Xiao, R.-S.; Wang, X.-G.; Dai, H.-L.; Tang, S.; Zheng, J.-Z.; Yang, M.; Yuan, S.-S. Real-time electrochemical monitoring sensor for pollutant degradation through galvanic cell system. Rare Met. 2025, 44, 1800–1812. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Yang, H.; Li, Y. Adsorption of pollutants from wastewater by biochar: Mechanisms and applications. Environ. Adv. 2023, 9, 100317. [Google Scholar]
- Liu, C.; Chen, Y.; Wang, J.; Xu, H. Removal of methylene blue by porous biochar obtained by KOH activation from bamboo biochar. Bioresour. Bioprocess. 2023, 10, 67. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Sun, X. Progress in porous carbon-based adsorbents for the removal of dyes. Inorg. Chem. Front. 2025, 12, 952–970. [Google Scholar]
- Wang, J.; Sun, M.; Zhao, Y. Oxalic acid-assisted photo-Fenton catalysis using Fe3O4 nanoparticles for dye degradation. Chem. Proc. 2024, 8, 67. [Google Scholar]
- Singh, R.; Sharma, P.; Verma, A. Fe3O4–CdO nanocomposite for photocatalytic degradation of organic dyes: Activity and stability. Catalysts 2024, 14, 71. [Google Scholar]







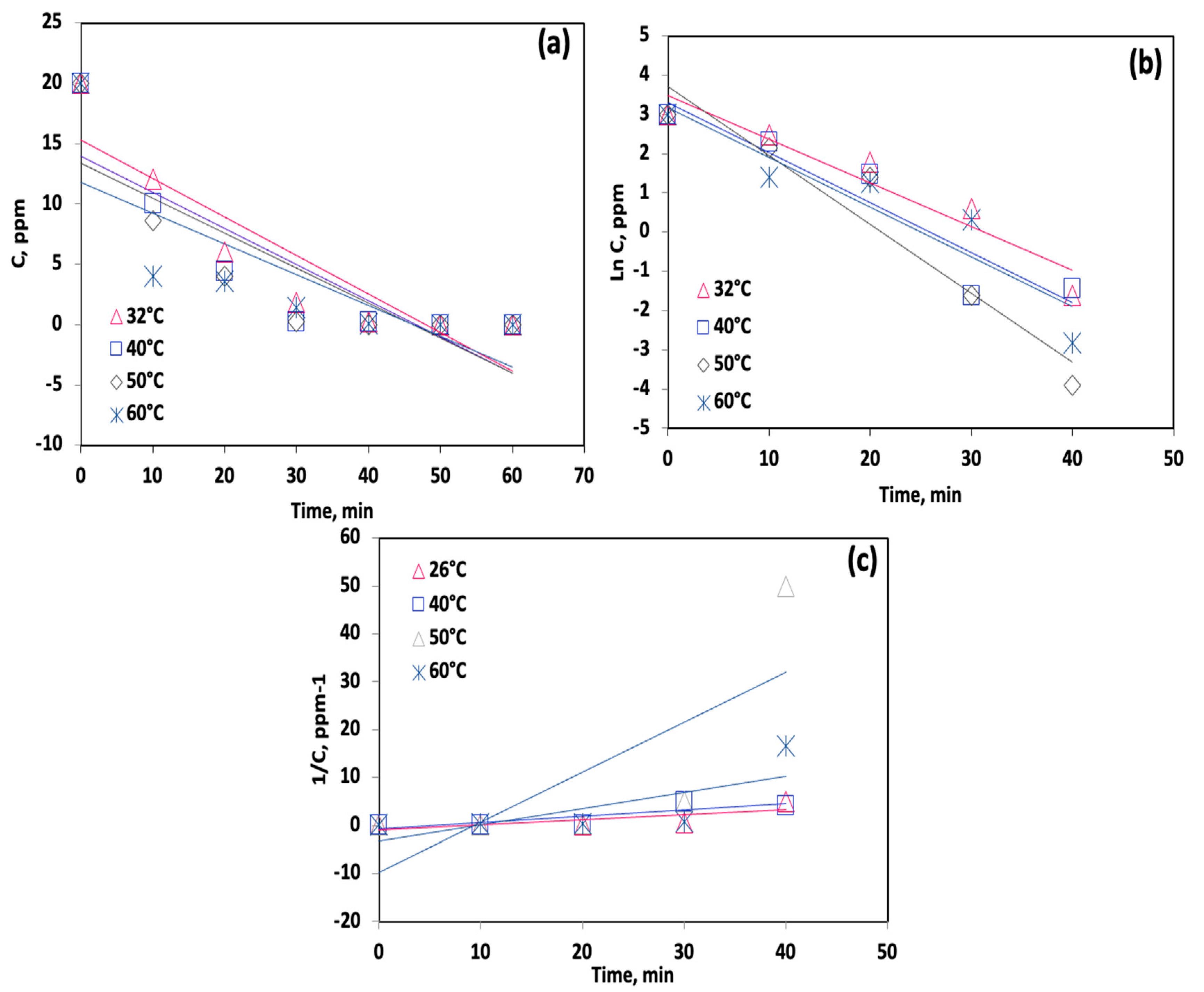



| Kinetic Model/ Linearized Equation | T (°C) | k | Units | R2 | t½ (min) |
|---|---|---|---|---|---|
| Zero-order | 32 | 0.32 | mg·L−1·min−1 | 0.81 | 31.25 |
| 40 | 0.30 | mg·L−1·min−1 | 0.73 | 33.33 | |
| 50 | 0.25 | mg·L−1·min−1 | 0.70 | 35.71 | |
| 60 | 0.28 | mg·L−1·min−1 | 0.58 | 40.00 | |
| First-order | 32 | 0.11 | min−1 | 0.92 | 4.08 |
| 40 | 0.12 | min−1 | 0.89 | 5.33 | |
| 50 | 0.13 | min−1 | 0.93 | 5.78 | |
| 60 | 0.17 | min−1 | 0.87 | 6.30 | |
| Second-order | 32 | 0.10 | L·mg−1·min−1 | 0.58 | 0.49 |
| 40 | 0.13 | L·mg−1·min−1 | 0.71 | 0.38 | |
| 50 | 0.33 | L·mg−1·min−1 | 0.57 | 0.15 | |
| 60 | 0.41 | L·mg−1·min−1 | 0.53 | 0.12 |
| T (°C) | Ea (kJ·mol−1) | ΔG (kJ·mol−1) | ΔH (J·mol−1) | ΔS (J·mol−1·k−1) |
|---|---|---|---|---|
| 32 | −9.97 | 79.26 | −12.47 | −304.79 |
| 40 | 82.31 | −12.57 | −303.13 | |
| 50 | 84.79 | −12.66 | −301.73 | |
| 60 | 86.76 | −12.74 | −298.83 |
| Catalyst/Source | Fenton Reaction Type | Optimal Operating Parameters Conditions | Removal Efficiency (%)/Oxidation Time | Reusability | Ref. |
|---|---|---|---|---|---|
| Biochar-supported with Fe3O4 (BC/Fe3O4) | Photo-Fenton | pH ~ 5.7, [H2O2]: 200 mg/L, UV 254 nm | 99% in 30 min | 72% after 6th cycle | Current work |
| FeSO4 + H2O2 (Homogeneous Fenton) | Classical Fenton | pH ~ 3, [H2O2]: 200 mg/L | 95% in 60 min | Not reusable (sludge formation) | [59] |
| Fe3O4 nanoparticles (Heterogeneous Fenton) | Fenton-like | pH ~ 5–6, [H2O2]: 200 mg/L, UV-assisted | 92% in 45 min | Up to 5 cycles (~70% after 5th) | [60] |
| FeSO4 + H2O2 (Homogeneous Fenton) | Classical Fenton | pH ~ 3, [H2O2]: 200 mg/L | 95% in 60 min | Not reusable (sludge formation) | [61] |
| Fe3O4 nanoparticles (Heterogeneous Fenton) | Fenton-like | pH ~ 5–6, [H2O2]: 200 mg/L, UV-assisted | 92% in 45 min | Up to 5 cycles (~70% after 5th) | [62] |
| Zero-valent iron (ZVI) + H2O2 | Fenton-like | pH ~ 4, [H2O2]: 150 mg/L | 85% in 60 min | Limited due to iron passivation | [63] |
| Fe-doped TiO2 photocatalyst | Photo-Fenton | pH 5, UV-Vis irradiation | 96% in 40 min | Up to 4 cycles (~80%) | [64] |
| Fe-exchanged zeolite + H2O2 | Heterogeneous Fenton | pH ~ 6, [H2O2]: 200 mg/L | 88% in 50 min | Stable for 3–4 cycles | [65] |
| Fe-doped TiO2 photocatalyst | Photo-Fenton | pH ~ 5, UV-Vis irradiation | 96% in 40 min | Up to 4 cycles (~80%) | [66] |
| Fe-exchanged zeolite + H2O2 | Heterogeneous Fenton | pH ~ 6, [H2O2]: 200 mg/L | 88% in 50 min | Stable for 3–4 cycles | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nour, M.M.; Tony, M.A.; Fouad, M.K.; Nabwey, H.A. Biowaste-to-Catalyst: Magnetite Functionalized Potato-Shell as Green Magnetic Biochar Catalyst (PtS200–Fe3O4) for Efficient Procion Blue Textile Wastewater Dye Abatement. Catalysts 2025, 15, 997. https://doi.org/10.3390/catal15100997
Nour MM, Tony MA, Fouad MK, Nabwey HA. Biowaste-to-Catalyst: Magnetite Functionalized Potato-Shell as Green Magnetic Biochar Catalyst (PtS200–Fe3O4) for Efficient Procion Blue Textile Wastewater Dye Abatement. Catalysts. 2025; 15(10):997. https://doi.org/10.3390/catal15100997
Chicago/Turabian StyleNour, Manasik M., Maha A. Tony, Mai K. Fouad, and Hossam A. Nabwey. 2025. "Biowaste-to-Catalyst: Magnetite Functionalized Potato-Shell as Green Magnetic Biochar Catalyst (PtS200–Fe3O4) for Efficient Procion Blue Textile Wastewater Dye Abatement" Catalysts 15, no. 10: 997. https://doi.org/10.3390/catal15100997
APA StyleNour, M. M., Tony, M. A., Fouad, M. K., & Nabwey, H. A. (2025). Biowaste-to-Catalyst: Magnetite Functionalized Potato-Shell as Green Magnetic Biochar Catalyst (PtS200–Fe3O4) for Efficient Procion Blue Textile Wastewater Dye Abatement. Catalysts, 15(10), 997. https://doi.org/10.3390/catal15100997









