Polymerization of Ethylene and 1,3-Butadiene Using Methylaluminoxane-Phosphine Catalyst Systems
Abstract
1. Introduction
2. Results and Discussions
2.1. Polymerization of Ethylene Catalyzed by Phosphine/MAO Systems
2.2. Polymerization of 1,3-Butadiene Catalyzed by MAO/Phosphine Systems
2.3. Characterization of Species Active for Polymerization
3. Materials and Methods
3.1. General
3.2. General Procedure for Ethylene Polymerization
3.3. General Procedure for 1,3-Butadiene Polymerization
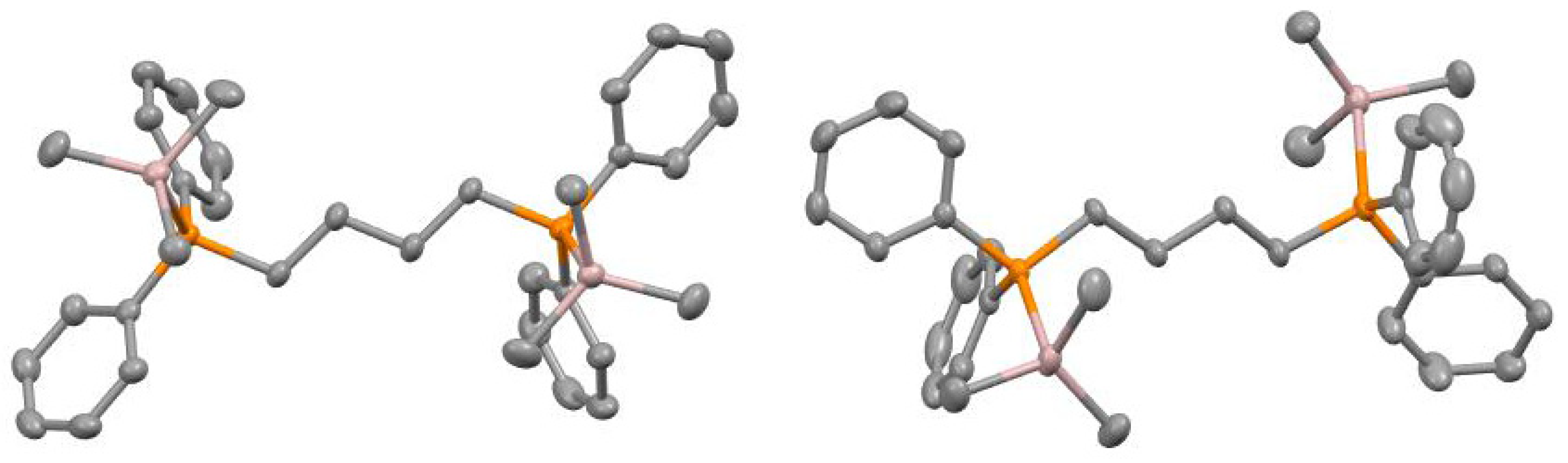
3.4. Synthesis of [(Me3Al)2(dppb)]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Capacchione, C.; Grisi, F.; Lamberti, M.; Lamberti, M.; Mazzeo, M.; Milani, B.; Milione, S.; Pappalardo, D.; Zuccaccia, C.; Pellecchia, C. Metal Catalyzed Polymerization: From Stereoregular Poly(α-Olefins) to Tailor-Made Biodegradable/Biorenewable Polymers and Copolymers. Eur. Inorg. Chem. 2023, 26, e202200644. [Google Scholar] [CrossRef]
- Birajdar, R.S.; Bodkhe, D.; Gupta, P.; Shaikh, M.H.; Ramekar, R.; Chikkali, S.H. Emerging trends in olefin polymerization: A perspective. J. Macromol. Sci. A 2023, 60, 731–750. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Marks, T.J. Early Transition Metal Catalysis for Olefin-Polar Monomer Copolymerization. Angew. Chem. Int. Ed. 2020, 59, 14726–14735. [Google Scholar] [CrossRef]
- Desert, X.; Carpetier, J.-F.; Kirillov, E. Quantification of active sites in single-site group 4 metal olefin polymerization catalysis. Coord. Chem. Rev. 2019, 386, 50–68. [Google Scholar] [CrossRef]
- Elkin, T.; Eisen, M.S. Amidinate group 4 complexes in the polymerization of olefins. Catal. Sci. Technol. 2015, 5, 82–95. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of Vanadium Complex Catalysts for Precise Olefin Polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes Polymerization: Where We Are and What Lies Ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Peng, D.; Yan, X.; Yu, C.; Zhang, S.; Li, X. Transition metal complexes bearing tridentate ligands for precise olefin polymerization. Polym. Chem. 2016, 7, 2601–2634. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C. Immobilisation of homogeneous olefin polymerization catalysts. Factors influencing activity and stability. Dalton Trans. 2013, 42, 8979–8987. [Google Scholar] [CrossRef]
- Takeuchi, D. Recent progress in olefin polymerization catalyzed by transition metal complexes: New catalysts and new reactions. Dalton Trans. 2010, 39, 311–328. [Google Scholar] [CrossRef]
- Wu, R.; Wu, W.K.; Stieglitz, L.; Gaan, S.; Rieger, B.; Heuberger, M. Recent advances on α-diimine Ni and Pd complexes for catalyzed ethylene (Co)polymerization: A comprehensive review. Coord. Chem. Rev. 2023, 474, 214844. [Google Scholar] [CrossRef]
- Mahmood, Q.; Li, X.; Qin, L.; Wang, L.; Sun, W.-H. Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo)polymerization. Dalton Trans. 2022, 51, 14375–14407. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Hu, X.; Wang, C.; Jian, Z. Advances on Controlled Chain Walking and Suppression of Chain Transfer in Catalytic Olefin Polymerization. ACS Catal. 2022, 12, 14304–14320. [Google Scholar] [CrossRef]
- Zhou, G.; Cui, L.; Mu, H.; Jian, Z. Custom-made polar monomers utilized in nickel and palladium promoted olefin copolymerization. Polym. Chem. 2021, 12, 3878–3892. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Carrow, B.P.; Nozaki, K. Transition-Metal-Catalyzed Functional Polyolefin Synthesis: Effecting Control through Chelating Ancillary Ligand Design and Mechanistic Insights. Macromolecules 2014, 47, 2541–2555. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.N.W.; Nozaki, K. Ortho-Phosphinobenzenesulfonate: A Superb Ligand for Palladium-Catalyzed Coordination-Insertion Copolymerization of Polar Vinyl Monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, A.; D’Agosto, F.; Boisson, C. Rare-Earth Metallocenes for Polymerization of Olefins and Conjugated Dienes: From Fundamental Studies to Olefin Block Copolymers. ChemPlusChem 2024, 89, e202400262. [Google Scholar] [CrossRef]
- Wang, H.; Cue, J.M.O.; Calubaquib, E.L.; Kularatne, R.N.; Miller, S.J.T.; Stefan, M.C. Neodymium catalysts for polymerization of dienes, vinyl monomers, and ε-caprolactone. Polym. Chem. 2021, 12, 6790–6823. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2018, 10, 42–61. [Google Scholar]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef]
- Coles, M.P.; Jordan, R.F. Cationic Aluminum Alkyl Complexes Incorporating Amidinate Ligands. Transition-Metal-Free Ethylene Polymerization Catalysts. J. Am. Chem. Soc. 1997, 119, 8125–8126. [Google Scholar]
- Ihara, E.; Young, V.G., Jr.; Jordan, R.F. Cationic Aluminum Alkyl Complexes Incorporating Aminotroponiminate Ligands. J. Am. Chem. Soc. 1998, 120, 8277–8278. [Google Scholar] [CrossRef]
- Bruce, M.; Gibson, V.C.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Cationic alkyl aluminium ethylene polymerization catalysts based on monoanionic N,N,N-pyridyliminoamide ligands. Chem. Commun. 1998, 2523–2524. [Google Scholar]
- Cameron, P.A.; Gibson, V.C.; Redshaw, C.; Segal, J.A.; Bruce, M.D.; White, A.J.P.; Williams, D.J. Pendant arm Schiff base complexes of aluminium as ethylene polymerization catalysts. Chem. Commun. 1999, 1883–1884. [Google Scholar]
- Cavell, R.G.; Aparna, K.; Kamalesh Babu, R.P.; Wang, Q. Aluminum bis(iminophosphorano)methanide and methandiide complexes—Transition metal-free ethylene polymerization cationic catalyst precursors. J. Mol. Catal. A Chem. 2002, 189, 137–143. [Google Scholar]
- Martin, H.; Bretinger, H. High-molecular-weight polyethylene: Growth reactions at bis(dichloroaluminium)ethane and trialkylaluminium. Makromol. Chem. 1992, 193, 1283–1288. [Google Scholar]
- Kim, J.S.; Wojcinski II, L.M.; Liu, S.; Sworen, J.C.; Sen, A. Novel Aluminum-Based, Transition Metal-Free, Catalytic Systems for Homo- and Copolymerization of Alkenes. J. Am. Chem. Soc. 2000, 122, 5668–5669. [Google Scholar]
- Tang, J.; Xu, Z.; Liu, Z.; Fu, Y.; Hua, J. Pyridine-amido aluminum catalyst precursors for 1,3-butadiene transition-metal-free stereospecific polymerization. Polym. Chem. 2023, 14, 980–989. [Google Scholar]
- Kimura, D.; Takeuchi, N.; Ogura, S.; Takazawa, A.; Kakiage, M.; Yamanobe, T.; Uehara, H. Ethylene polymerization using N-Heterocyclic carbene complexes of silver and aluminum. Des. Monom. Polym. 2023, 26, 182–189. [Google Scholar]
- Reinhold, M.; McGrady, J.E.; Meier, R.J. Simulation of ethylene insertion in an aluminium catalyst. J. Chem. Soc. Dalton Trans. 1999, 487–488. [Google Scholar] [CrossRef]
- Ghorbani, N.; Ghohe, N.M.; Torabi, S.; Yates, B.F.; Ariafard, A. Theoretical Investigation into the Mechanism of Ethylene Polymerization by a Cationic Dinuclear Aluminum Complex: A Longstanding Unresolved Issue. Organometallics 2013, 32, 1687–1693. [Google Scholar] [CrossRef]
- Tabatabaie, E.S.; Dehghanpour, S.; Mosaddegh, E.; Babaahmadi, R.; Chipman, A.; Yates, B.F.; Ariafard, A. Rationale for the reactivity differences between main group and d0 transition metal complexes toward olefin polymerization. Dalton Trans. 2019, 48, 6997–7005. [Google Scholar] [CrossRef] [PubMed]
- Ghitto, F.; Pateraki, C.; Tanskanen, J.; Severn, J.R.; Luehmann, N.; Kusmin, A.; Stellbrink, J.; Linnolahti, M.; Bochmann, M. Probing the Structure of Methylalunoxane (MAO) by a Combined Chemical, Spectroscopic, Neutron Scattering, and Computational Approach. Organometallics 2013, 32, 3354–3362. [Google Scholar]
- Urciuoli, G.; Vittoria, A.; Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Tensi, L.; Ehm, C.; Macchioni, A.; Busico, V. Borate Salts of Aluminum-Alkyl Cations Stabilized by P-, O-, and C-Donors: Synthesis, Characterization and Application as Cocatalysts. Inorg. Chem. 2025, 64, 9225–9236. [Google Scholar]
- Yamakawa, S.; Takeuchi, D.; Osakada, K.; Takano, S.; Kaita, S. Polymerization of 1,3-butadiene catalyzed by Co(II) and Ni(II) complexes of 6,6′-dihydroxy-2,2′-bipyridine ligands: 1,4-cis-polymerization versus isospecific 1,2-polymerization. RSC Adv. 2025, 15, 12557. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, 1–6. [Google Scholar]
- Neese, F. Software update: The ORCA program system, version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar]
- Neese, F. Software Update: The ORCA Program System—Version 6.0. WIREs Comput. Mol. Sci. 2025, 15, e70019. [Google Scholar]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. The SHARK Integral Generation and Digestion System. J. Comp. Chem. 2022, 4, 381–396. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, L224108. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comp. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree-Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree-Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An improved chain of spheres for exchange algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef]
- Izsak, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Izsak, R.; Hansen, A.; Neese, F. The resolution of identity and chain of spheres approximations for the LPNO-CCSD singles Fock term. Mol. Phys. 2012, 110, 2413–2417. [Google Scholar] [CrossRef]
- Izsak, R.; Neese, F.; Klopper, W. Robust fitting techniques in the chain of spheres approximation to the Fock exchange: The role of the complementary space. J. Chem. Phys. 2013, 139, 094111. [Google Scholar] [CrossRef] [PubMed]


| Run | Phosphine (/μmol) | Al (/mmol) | Toluene/mL | Yield/g | Activity/g·mmol[P]−1·h−1 | Mn b | Mw/Mn b |
|---|---|---|---|---|---|---|---|
| 1 | PPh3 (20) | MAO (30) | 20 | 0.0038 | 0.010 | - | - |
| 2 | PCy3 (20) | MAO (30) | 20 | 0.0081 | 0.020 | - | - |
| 3 | CyJohnPhos (20) | MAO (30) | 20 | 0.0441 | 0.110 | 92,000 | 50.4 |
| 4 | JohnPhos (20) | MAO (30) | 20 | 0.0016 | 0.004 | - | - |
| 5 | XPhos (20) | MAO (30) | 20 | trace | - | - | - |
| 6 | - | MAO (30) | 20 | trace | - | - | - |
| 7 | CyJohnPhos (40) | MAO (30) | 20 | 0.0811 | 0.101 | 254,000 | 172 |
| 8 | CyJohnPhos (20) | MAO (16) | 20 | 0.0296 | 0.148 | 248,000 | 144 |
| 9 | CyJohnPhos (40) | MMAO (30) | 20 | 0.0024 | 0.012 | - | - |
| 10 | CyJohnPhos (40) | MAO (16) | 10 | trace | - | - | - |
| 11 | DPPM (20) | MAO (30) | 20 | 0.0087 | 0.022 | - | - |
| 12 | DPPE (20) | MAO (30) | 20 | 0.0218 | 0.055 | - | - |
| 13 | DPPP (20) | MAO (30) | 20 | 0.0057 | 0.014 | - | - |
| 14 | DPPB (20) | MAO (30) | 20 | 1.1188 | 2.797 | 737,000 | 40 |
| 15 | DPPPen (20 | MAO (30) | 20 | 0.0062 | 0.150 | - | - |
| 16 | DPPHex (20) | MAO (30) | 20 | 0.0105 | 0.026 | - | - |
| 17 | rac-BINAP (20) | MAO (30) | 20 | 0.0325 | 0.081 | 97,000 | 64 |
| 18 | DPPB (20) | MAO (16) | 10 | 0.1313 | 0.328 | - | - |
| 19 | DPPB (20) | MAO (30) | 10 | 0.1746 | 0.437 | - | - |
| Run | Phosphine (/μmol) | Al (/μmol) | Toluene /mL | Time /h | Yield (%) | cis-1,4 (%) b | trans-1,4 (%) b | 1,2 (%) b | Mn c | Mw/Mn c |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CyJohnPhos (20) | MAO (30,000) | 20 | 20 | trace | - | - | - | - | - |
| 2 | CyJohnPhos (20) | MAO (4000) | 10 | 28 | 1.4 | 84.9 | 15.1 | - | - | |
| 3 | DPPE (20) | MAO (4000) | 10 | 72 | trace | - | - | - | - | - |
| 4 | DPPP (20) | MAO (4000) | 10 | 72 | trace | 32.3 | 50.9 | 16.8 | - | - |
| 5 | DPPB (20) | MAO (4000) | 10 | 72 | 1.4 | 78.9 | 21.1 | - | - | |
| 6 | DPPHex (20) | MAO (4000) | 10 | 72 | 0.55 | 67.0 | 33.0 | - | - | |
| 7 | rac-BINAP (20) | MAO (4000) | 10 | 72 | 10.3 | 76.9 | 17.0 | 6.1 | 24,000 | 3.45 |
| 8 | BIPHEP (20) | MAO (4000) | 10 | 72 | 3.1 | 93.8 | 1.1 | 5.1 | 19,000 | 1.74 |
| 9 | BIPHEP (40) | MAO (4000) | 10 | 72 | 5.2 | 90.1 | 4.8 | 5.1 | - | - |
| 10 | BIPHEP (60) | MAO (4000) | 10 | 72 | trace | - | - | - | - | - |
| 11 | BIPHEP (20) | Me3Al (40) [Ph3C][B(C6F5)4] (20) | 10 | 72 | trace | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, N.; Takeuchi, D. Polymerization of Ethylene and 1,3-Butadiene Using Methylaluminoxane-Phosphine Catalyst Systems. Catalysts 2025, 15, 942. https://doi.org/10.3390/catal15100942
Kimura N, Takeuchi D. Polymerization of Ethylene and 1,3-Butadiene Using Methylaluminoxane-Phosphine Catalyst Systems. Catalysts. 2025; 15(10):942. https://doi.org/10.3390/catal15100942
Chicago/Turabian StyleKimura, Nanako, and Daisuke Takeuchi. 2025. "Polymerization of Ethylene and 1,3-Butadiene Using Methylaluminoxane-Phosphine Catalyst Systems" Catalysts 15, no. 10: 942. https://doi.org/10.3390/catal15100942
APA StyleKimura, N., & Takeuchi, D. (2025). Polymerization of Ethylene and 1,3-Butadiene Using Methylaluminoxane-Phosphine Catalyst Systems. Catalysts, 15(10), 942. https://doi.org/10.3390/catal15100942







