A Study on Maximizing the Performance of a Concrete-Based TiO2 Photocatalyst Using Hydrophilic Polymer Dispersion
Abstract
1. Introduction
2. Results
2.1. Analysis of TiO2 Properties
2.1.1. XRD Analysis
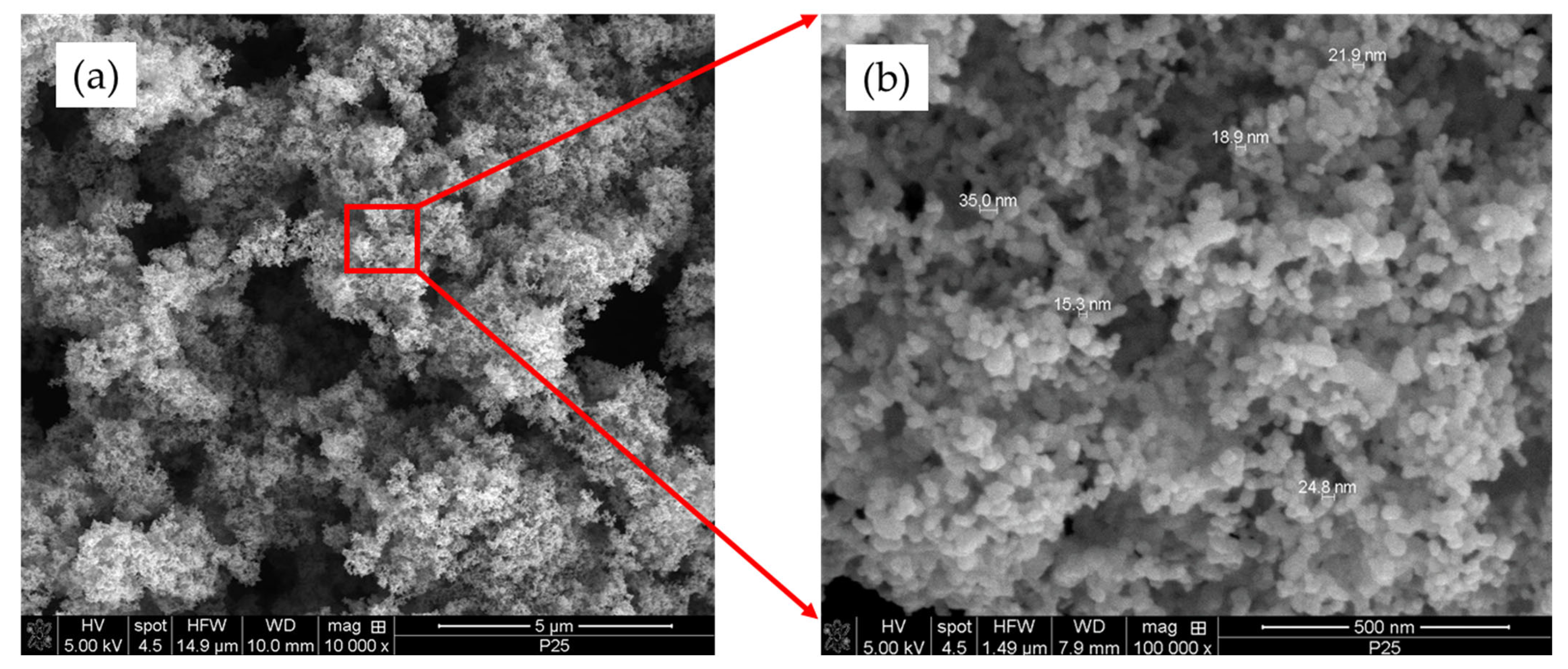
2.1.2. SEM Analysis
2.1.3. BET and BJH Analysis
2.2. Comparison of Polymer Preprocessing Dispersion Stability
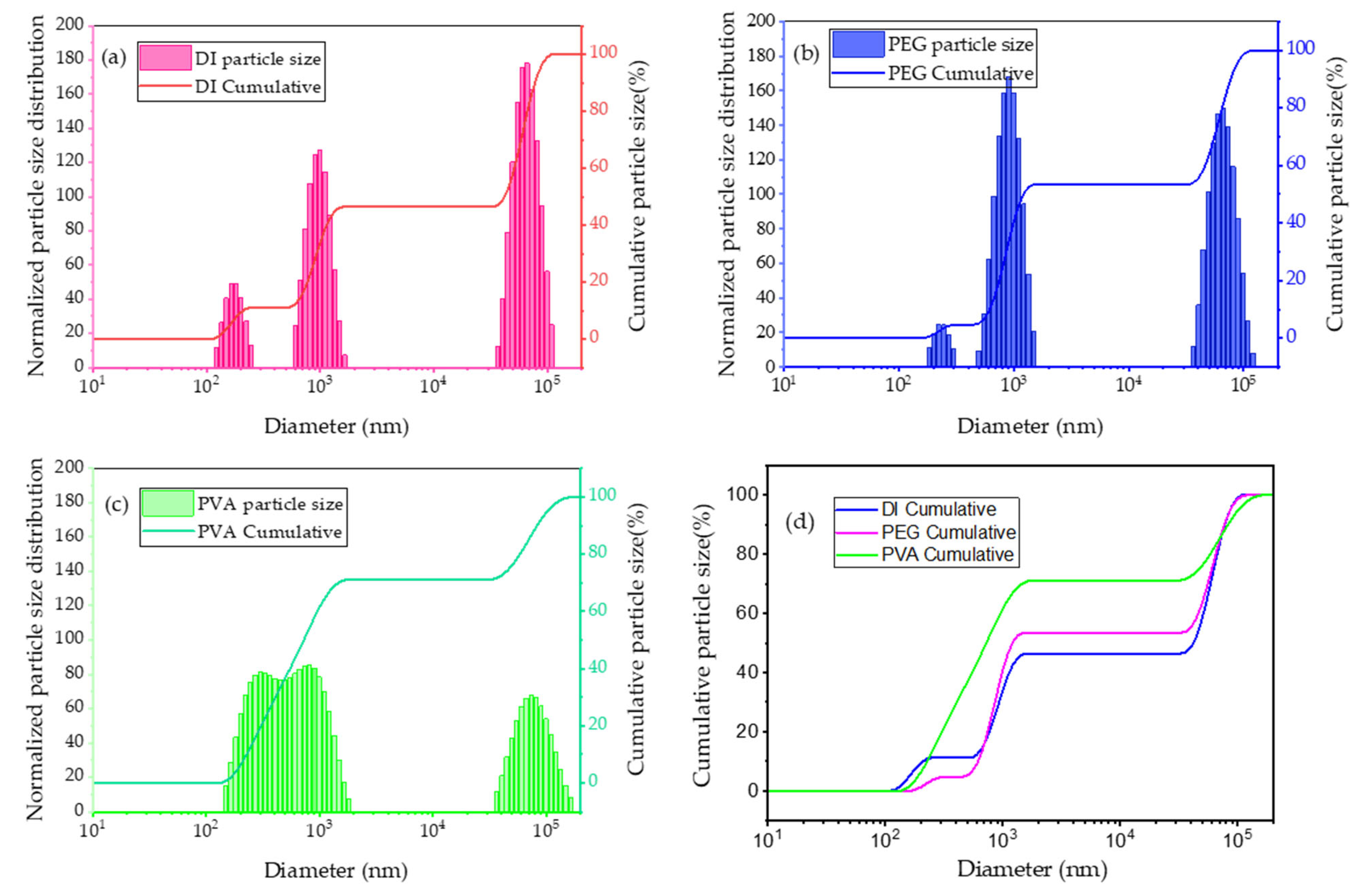
2.2.1. Particle Size Ratio Analysis by Distribution Range with PVA, PEG, and PEGME
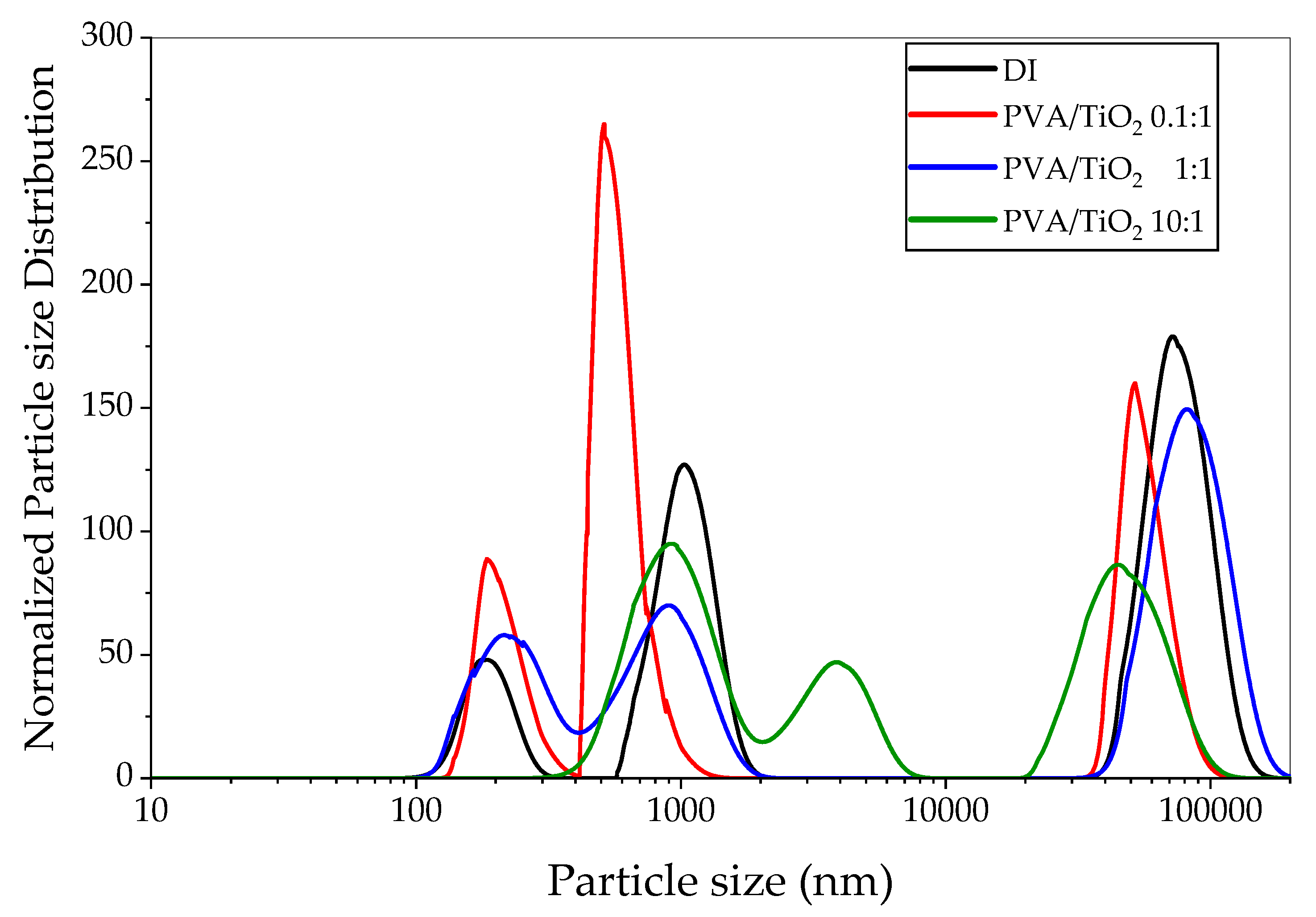
2.2.2. Analysis of Dispersion Characteristics According to the Polymer/TiO2 Ratio
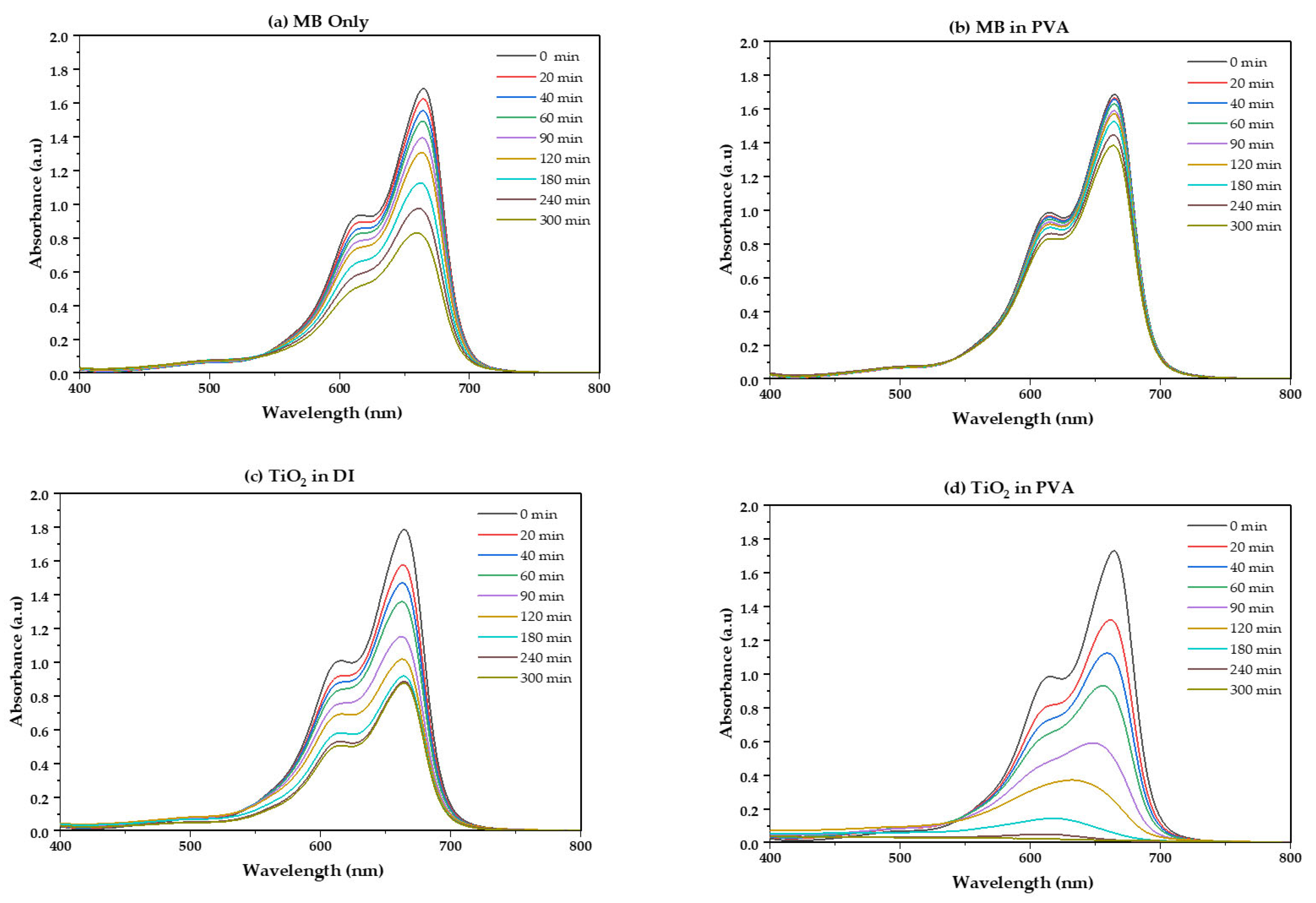
2.2.3. UV-Vis Analysis of TiO2 Dispersion
2.2.4. Comparison of TiO2 Dispersion and Photoreaction Rate
2.2.5. Comparison of Concrete Surface Powder Photoreaction
2.2.6. Evaluation of Time and Alkali Stability of TiO2 Dispersion
2.3. Comparison of the Self-Cleaning Performance of Concrete Specimens
2.4. Comparative Analysis and Mechanism
3. Discussion
3.1. Correlation of Dispersion and Photoactivity According to Polymer Type
3.2. Impact of Mixing Ratio (TiO2:PVA) and Re-Aggregation Threshold
3.3. UV–Vis Spectrum Analysis
3.4. Pseudo-Primary (Langmuir–Hinshelwood) Velocity and Half-Life
3.5. Causes of Degradation in Concrete Matrix
3.6. Literature Comparison and Performance Positioning
3.7. Uncertainty and Reproducibility
3.8. Mechanism Schematic and Design Implications
4. Materials and Methods
4.1. Materials
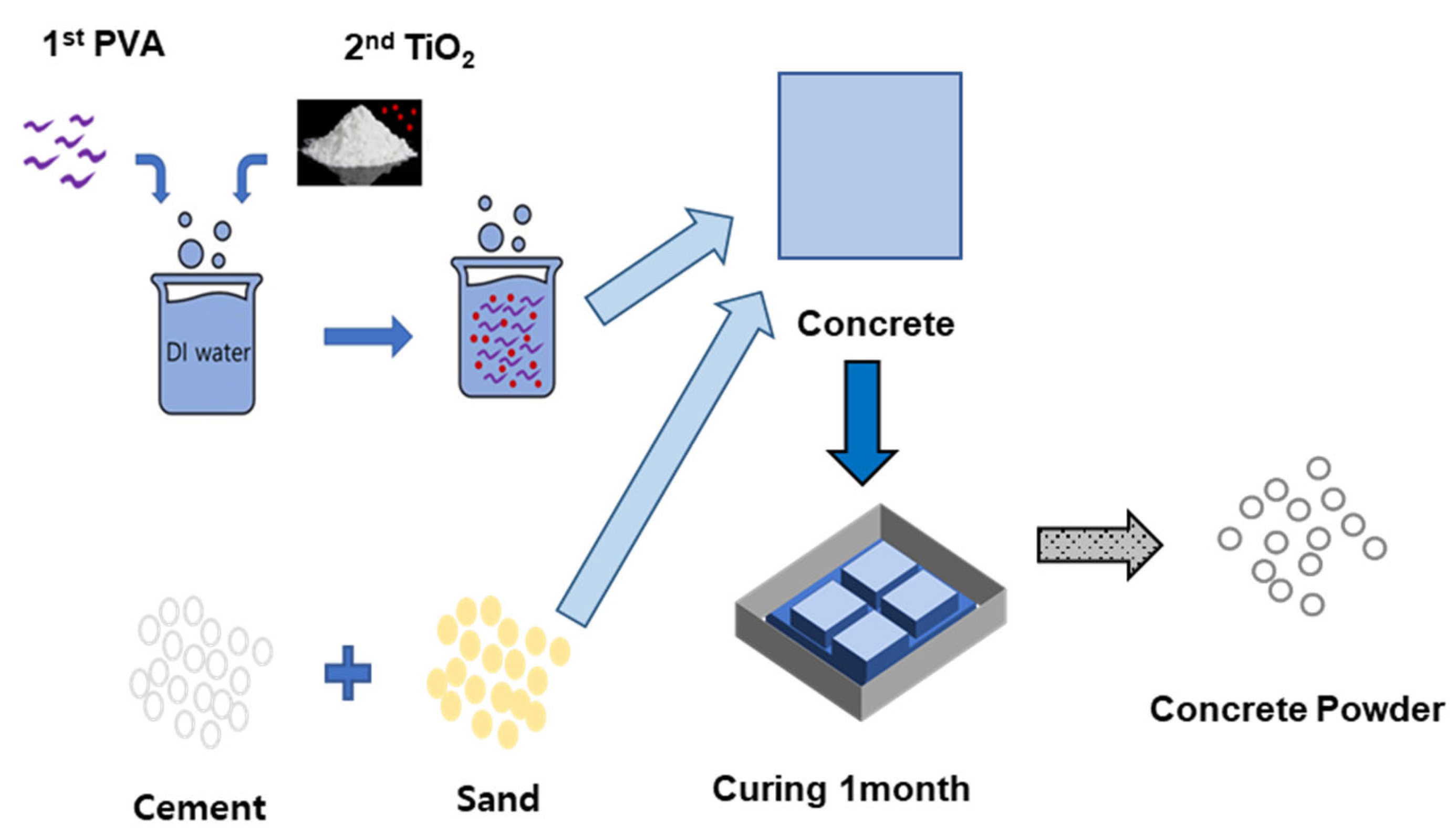
4.2. Dispersion Preparation Procedure
4.3. Physical Properties Analysis Equipment and Methods
4.4. Methylene Blue Photolysis Test in Aqueous Solution
4.5. Concrete Specimen Manufacturing and Self-Purification Performance Evaluation
5. Conclusions
- The characteristics of photocatalytic TiO2 (P25) were examined using BET, SEM, and XRD. With a particle size of 10–30 nm and a surface area of approximately 55–57 cm2/g, the anatase–rutile ratio was 81:19. Furthermore, it was found that this powder aggregated in the aqueous solution when it was dispersed there, with the particle size being more than five times larger.
- In order to investigate dispersibility, water-soluble polymers such as PVA, PEG, and PEGMA were used. PVA showed great dispersibility.
- The PVA:TiO2 concentration ratio of 0.1:1 achieved the best dispersibility.
- When compared to TiO2 dispersed in DI, TiO2 dispersed in PVA showed better MB photodegradation ability.
- PVA-dispersed TiO2 samples demonstrated a stronger photodegradation effect in concrete powder examination, maintaining about 1.9 times the performance increase compared to DI. PVA increases activity by a different mechanism than better colloidal stability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Terpiłowski, K.; Chibowski, S.; Urban, T.; Zarko, V.I.; Gun’ko, V.M. Effect of polyacrylic acid (PAA) adsorption on stability of mixed alumina-silica oxide suspension. Powder Technol. 2012, 224, 241–247. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase–Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Kujawa, W.; Didyk-Mucha, A.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Rudawska, A. Synergistic Effect of Combined Polymorphs Anatase-Rutile Nano-Modified Lightweight Concrete on Photocatalytic Reduction of NOx, Self-Cleaning Performance, and Antimicrobial Properties. Buildings 2023, 13, 1736. [Google Scholar] [CrossRef]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Appl. Surf. Sci. 2002, 199, 368–371. [Google Scholar] [CrossRef]
- Zerga, A.Y.; Tahir, M.; Alias, H.; Mohamed, A.R. Sludge-derived biochar nanotexture to construct BC/TiO2 composite with metallic elements influential effect for efficient photocatalytic hydrogen evolution. Fuel 2024, 362, 131678. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Zhu, N.; Lou, Z.; Yuan, H. Facile synthesis of porous TiO2 photocatalysts using waste sludge as the template. Appl. Surf. Sci. 2015, 359, 944–952. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2−xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Hanif, M.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Kim, Y.S. Photocatalytic VOCs Degradation Efficiency of Polypropylene Membranes by Incorporation of TiO2 Nanoparticles. Membranes 2023, 13, 50. [Google Scholar] [CrossRef]
- Hanif, M.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Han, S.-W.; Kang, S.-S.; Kim, Y.S. Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber. J. Compos. Sci. 2024, 8, 86. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Wen, J.; Wan, Y.; Zhao, Q.; Cao, X.; Ding, Y.; Wang, Z.L.; Li, H.; Bian, Z. Selective Recovery of Precious Metals through Photocatalysis. Nat. Sustain. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, S.; Ge, H.; Chen, X.; Xu, Z.; Yue, Y.; Yamashita, H.; Yu, H.; Li, H.; Bian, Z. Photocatalytic Dissolution of Precious Metals by TiO2 through Photogenerated Free Radicals. Angew. Chem. Int. Ed. 2022, 61, e202213640. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Q.; Zhou, Y.; Zhang, L.; Zhao, Y. Enhanced Photocatalytic Activity of TiO2 Nanoparticles Modified with Polyacrylamide via Surface Grafting for Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 1981. [Google Scholar]
- Ifang, S.; Gallus, M.; Liedtke, S.; Kurtenbach, R.; Wiesen, P.; Kleffmann, J. Standardization methods for testing photocatalytic air remediation materials: Problems and solution. Atmos. Environ. 2014, 91, 154–161. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering photocatalytic cements: Understanding TiO2 surface chemistry to control and modulate photocatalytic performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- Hanif, M.A.; Kim, Y.S.; Ameen, S.; Kim, H.G.; Kwac, L.K. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings 2022, 12, 579. [Google Scholar] [CrossRef]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Tailoring the Effects of Titanium Dioxide (TiO2) and Polyvinyl Alcohol (PVA) in the Separation and Antifouling Performance of Thin-Film Composite Polyvinylidene Fluoride (PVDF) Membrane. Membranes 2021, 11, 241. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, T.-G.; Hwangbo, S.-A.; Jeong, J.-R. Effect of the TiO2 Colloidal Size Distribution on the Degradation of Methylene Blue. Nanomaterials 2023, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Surkatti, R.; van Loosdrecht, M.C.M.; Hussein, I.A.; El-Naas, M.H. PVA-TiO2 Nanocomposite Hydrogel as Immobilization Carrier for Gas-to-Liquid Wastewater Treatment. Nanomaterials 2024, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Rasteiro, M.G.; Garcia, F.A.; Hunkeler, D.; Pinheiro, I. Evaluation of the Performance of Dual Polyelectrolyte Systems on the Re-Flocculation Ability of Calcium Carbonate Aggregates in Turbulent Environment. Polymers 2016, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Mohamed, A.A. Advances in Ultrasound-Assisted Synthesis of Photocatalysts and Sonophotocatalytic Processes: A Review. iScience 2023, 26, 108583. [Google Scholar] [CrossRef]
- Alshabander, B.; Abd-Alkader, M.B. Photocatalytic Degradation of Methyl blue by TiO2 Nanoparticles Incorporated in Cement. Int. J. Phys. 2023, 2, 1042. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, L.; Zhao, R.; Wu, J.; Gao, J.; Li, L.; Chen, J.; Wang, W.; Galloni, M.G.; Scesa, F.M.; et al. Recent Advances in Photocatalysis on Cement-Based Materials. Cem. Concr. Res. 2022, 161, 106984. [Google Scholar] [CrossRef]
- KS L 5201; Portland Cement. Korean Agency for Technology and Standards (KATS): Seoul, Republic of Korea, 2021.
- KS L ISO 679; Methods of Testing Cements-Determination of Strength. Korean Agency for Technology and Standards (KATS): Seoul, Republic of Korea, 2022.
- Fregni, A.; Venturi, L.; Franzoni, E. Evaluation of the Performance and Durability of Self-Cleaning Treatments Based on TiO2 Nanoparticles Applied to Cement-Based Renders and Boards. Coatings 2023, 13, 990. [Google Scholar] [CrossRef]
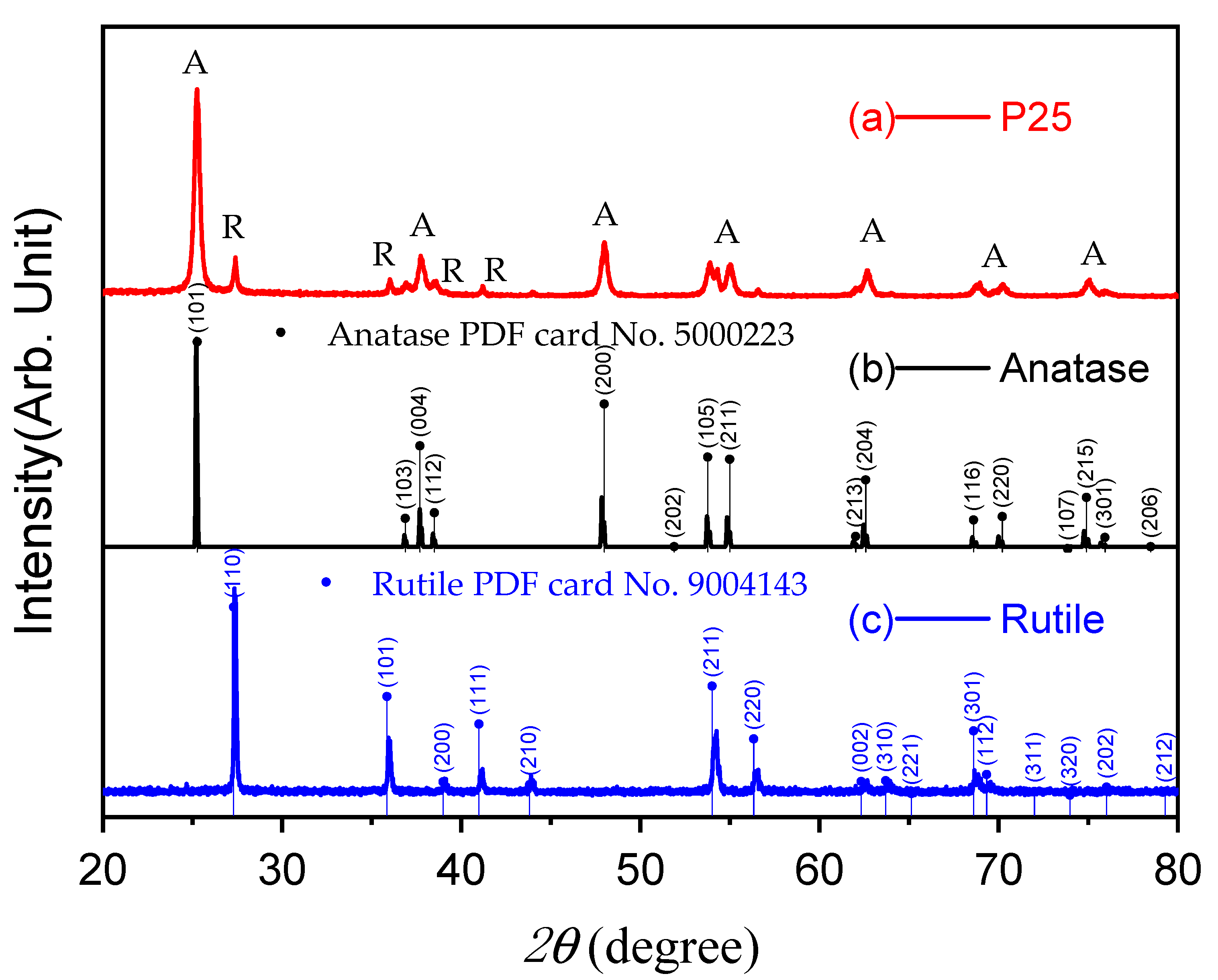


| TiO2 | Anatase | Rutile |
|---|---|---|
| wt.% | 81.33 | 19.06 |
| Particle Size (nm) | DI | PEG | PVA |
|---|---|---|---|
| 100~2000 | 46.5 | 53.4 | 66.7 |
| 20,000~200,000 | 53.5 | 46.6 | 33.3 |
| PVA/TiO2 | DI | 0.1:1 | 1:1 | 10:1 |
|---|---|---|---|---|
| Particle size (nm) ± SD | 1715.7 ± 37.5 | 1395.5 ± 52.9 | 1813.2 ± 46.1 | 3351.6 ± 379.1 |
| PDI | 0.504 | 0.174 | 0.631 | 0.963 |
| Zeta potential (mV) ± SD | −5.84 ± 0.37 | −10.90 ± 0.37 | −9.27 ± 0.48 | −9.27 ± 0.81 |
| Sample | k_app (10−2 min−1) | R2 | Relative Performance |
|---|---|---|---|
| DI | 0.15 | 0.85 | 1.0 |
| PVA 0.1% | 1.71 | 0.98 | 11.4 |
| Sample | k_app (×10−3 min−1) | R2 | Relative Performance |
|---|---|---|---|
| DI | 2.7 | 0.90 | 1.0 |
| PVA 0.1% | 5.2 | 0.98 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Song, K.; Kim, J.; Kang, H.-J.; Yu, D.; Kim, H.G.; Kim, Y.S. A Study on Maximizing the Performance of a Concrete-Based TiO2 Photocatalyst Using Hydrophilic Polymer Dispersion. Catalysts 2025, 15, 935. https://doi.org/10.3390/catal15100935
Kim JS, Song K, Kim J, Kang H-J, Yu D, Kim HG, Kim YS. A Study on Maximizing the Performance of a Concrete-Based TiO2 Photocatalyst Using Hydrophilic Polymer Dispersion. Catalysts. 2025; 15(10):935. https://doi.org/10.3390/catal15100935
Chicago/Turabian StyleKim, Jung Soo, Kanghyeon Song, Jiwon Kim, Hyun-Ju Kang, Dayoung Yu, Hong Gun Kim, and Young Soon Kim. 2025. "A Study on Maximizing the Performance of a Concrete-Based TiO2 Photocatalyst Using Hydrophilic Polymer Dispersion" Catalysts 15, no. 10: 935. https://doi.org/10.3390/catal15100935
APA StyleKim, J. S., Song, K., Kim, J., Kang, H.-J., Yu, D., Kim, H. G., & Kim, Y. S. (2025). A Study on Maximizing the Performance of a Concrete-Based TiO2 Photocatalyst Using Hydrophilic Polymer Dispersion. Catalysts, 15(10), 935. https://doi.org/10.3390/catal15100935









