Exploring the Evolution of Co-Deposited Copper and Iron Nanostructures on Hydroxyapatite: Implications in NH3-SCR Reaction
Abstract
1. Introduction
2. Results
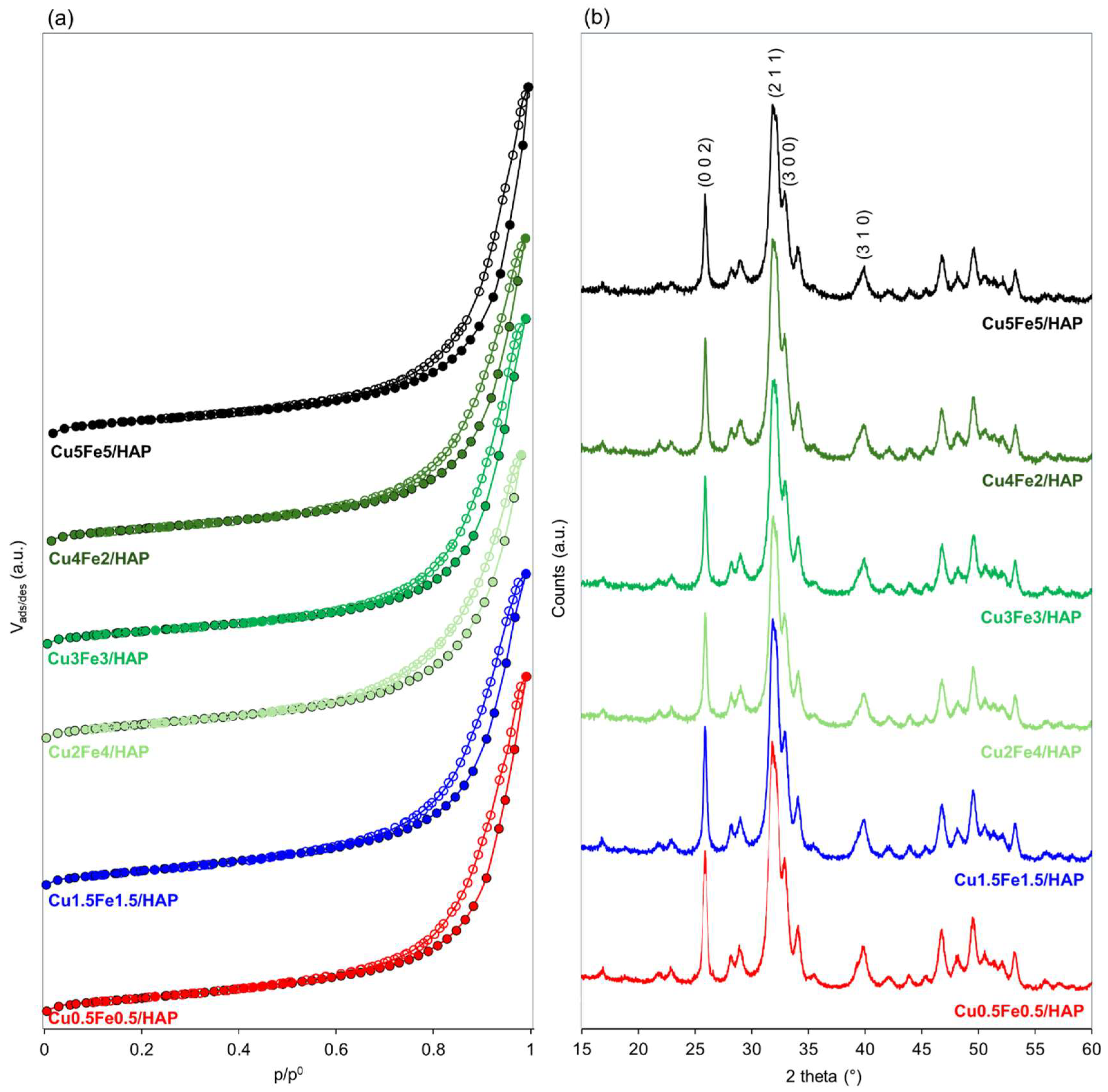
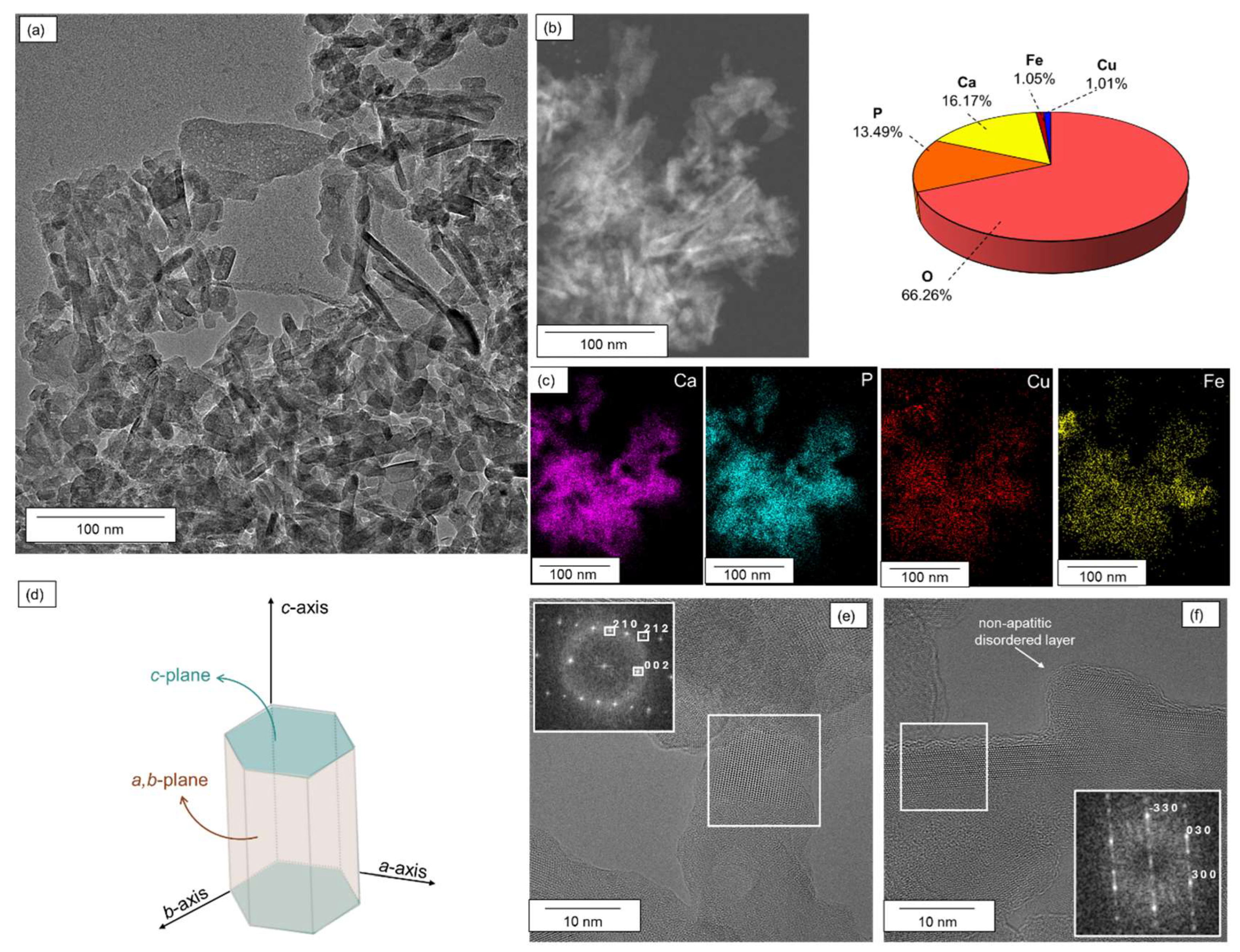
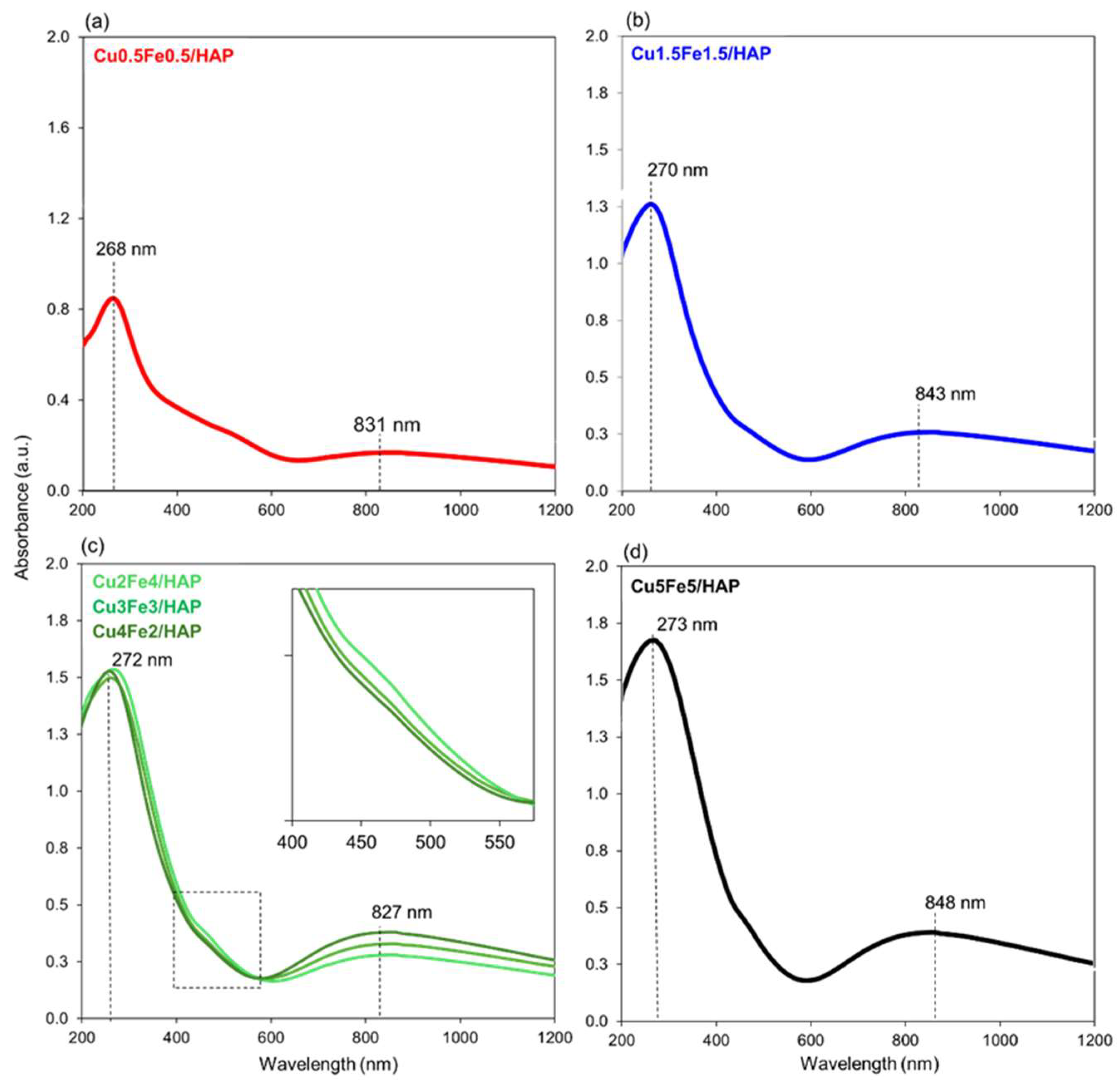
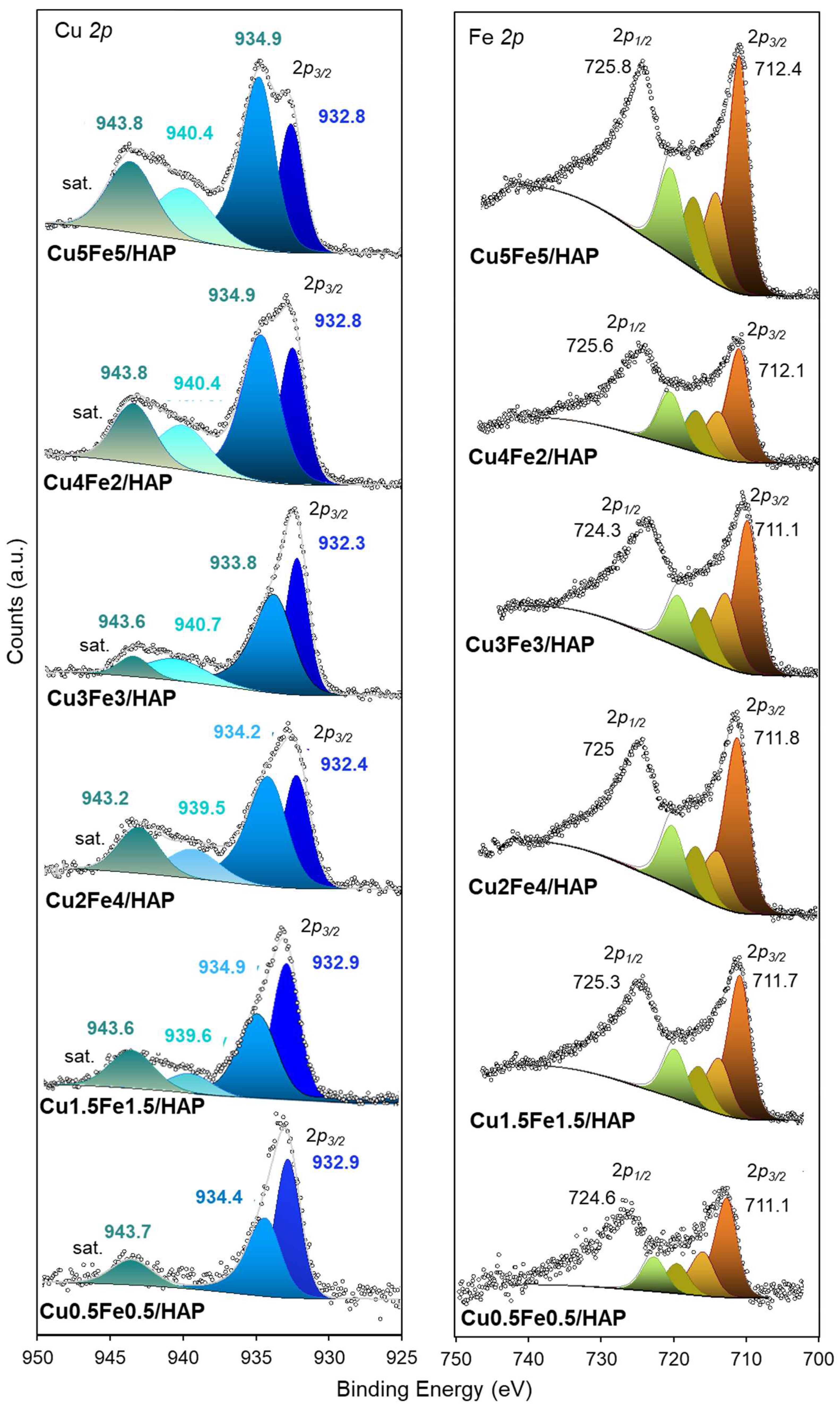
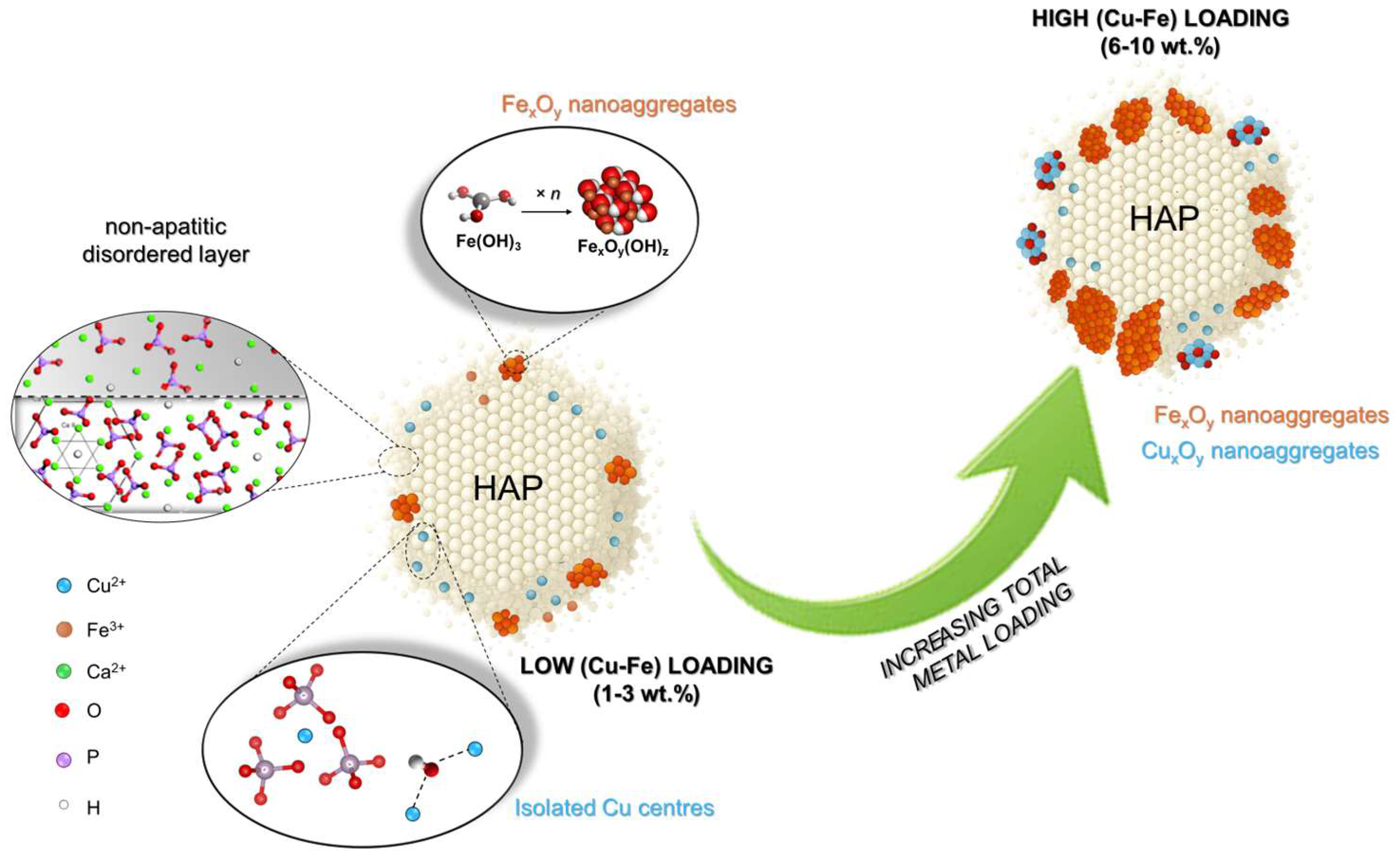
2.1. Structure and Surface Properties of Copper–Iron HAP Samples
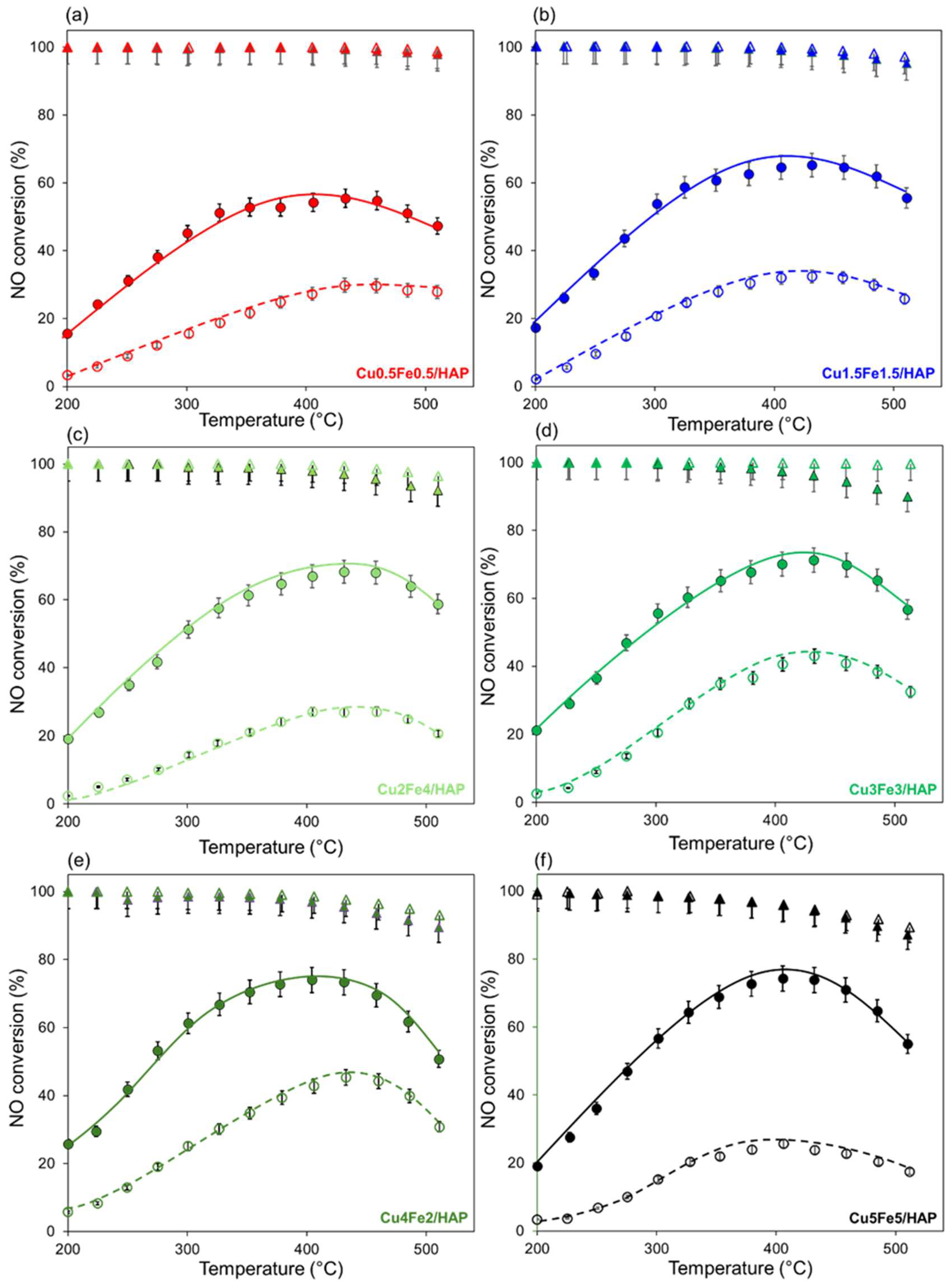
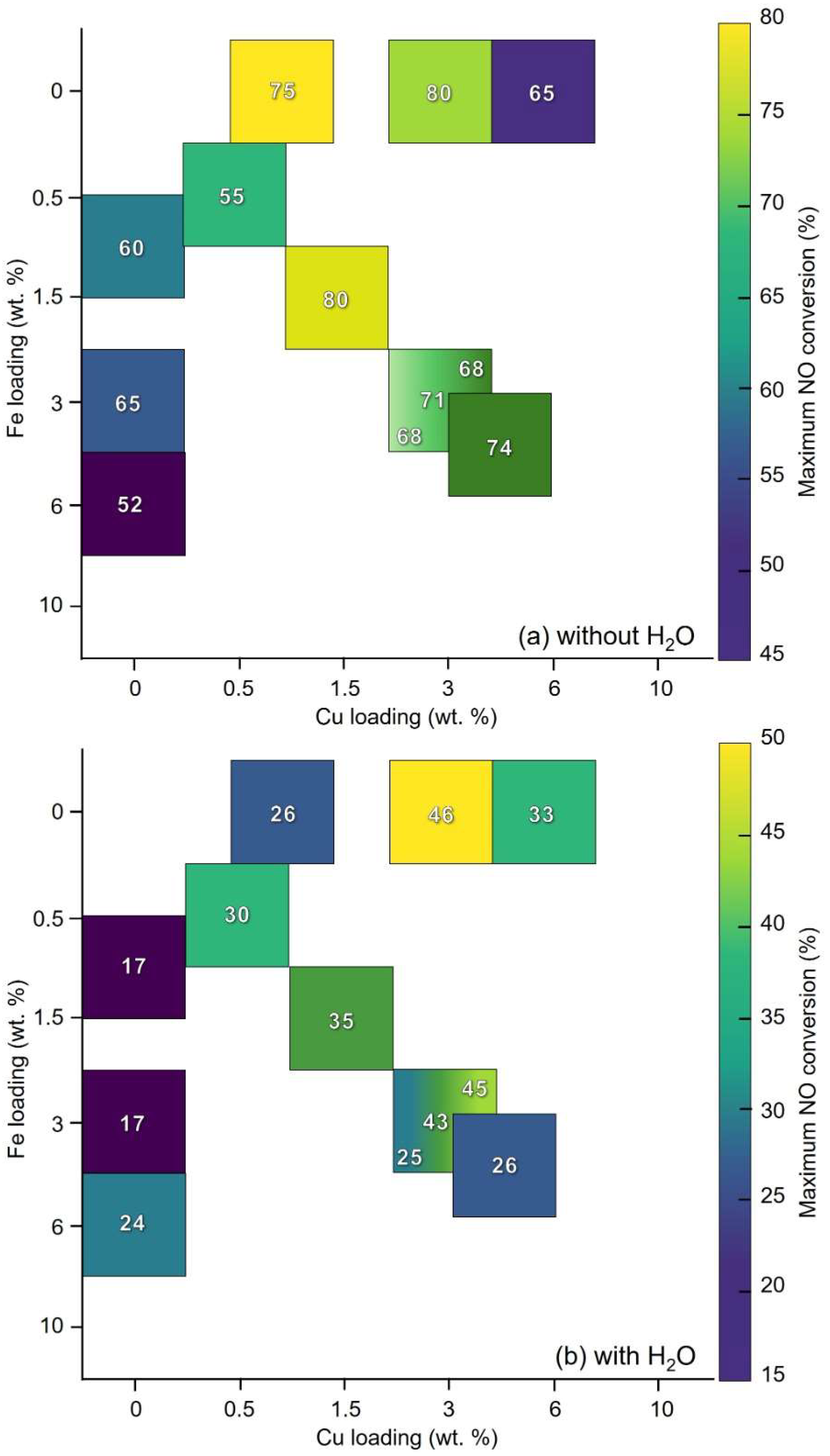
2.2. Reactivity in NH3-SCR Reaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Hydroxyapatite Modification
4.3. Material Characterization
4.4. Reactivity Tests in NH3-SCR Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer, Emmet, Teller |
| BJH | Barret, Joyner, Halenda |
| DR-UV | Diffuse Reflectance UV-vis |
| DTGS | Deuterated Triglycine Sulfate |
| EDX | Energy-Dispersive X-ray |
| FFT/FT | Fast Fourier Transform/Fourier Transform |
| GHSV | Gas Hourly Space Velocity |
| HAP | Hydroxyapatite |
| HR | High-resolution |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| IR/NIR | Infrared/Near Infrared |
| µ-GC | micro gas phase chromatograph |
| SAED | Selected Area Electron Diffraction |
| SCR | Selective Catalytic Reduction |
| TEM | Transmission Electron Microscopy |
| VOC | Volatile Organic Compounds |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-Ray Diffraction |
References
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a Multifunctional Material for Air, Water and Soil Pollution Control: A Review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A Journey from Biomaterials to Advanced Functional Materials. Adv. Colloid. Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Avola, T.; Campisi, S.; Polito, L.; Arici, S.; Ferruti, L.; Gervasini, A. Addressing the Issue of Surface Mechanisms and Competitive Effects in Cr(VI) Reductive-Adsorption on Tin-Hydroxyapatite in the Presence of Co-Ions. Sci. Rep. 2023, 13, 18913. [Google Scholar] [CrossRef]
- Campisi, S.; Evangelisti, C.; Postole, G.; Gervasini, A. Combination of Interfacial Reduction of Hexavalent Chromium and Trivalent Chromium Immobilization on Tin-Functionalized Hydroxyapatite Materials. Appl. Surf. Sci. 2021, 539, 148227. [Google Scholar] [CrossRef]
- Kiani, D.; Baltrusaitis, J. Surface Chemistry of Hydroxyapatite for Sustainable N-Butanol Production from Bio-Ethanol. Chem. Catal. 2021, 1, 782–801. [Google Scholar] [CrossRef]
- Ben Osman, M.; Krafft, J.M.; Thomas, C.; Yoshioka, T.; Kubo, J.; Costentin, G. Importance of the Nature of the Active Acid/Base Pairs of Hydroxyapatite Involved in the Catalytic Transformation of Ethanol to n-Butanol Revealed by Operando DRIFTS. ChemCatChem 2019, 11, 1765–1778. [Google Scholar] [CrossRef]
- Agbeboh, N.I.; Oladele, I.O.; Daramola, O.O.; Adediran, A.A.; Olasukanmi, O.O.; Tanimola, M.O. Environmentally Sustainable Processes for the Synthesis of Hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A Review of Syntheses, Structure and Applications in Heterogeneous Catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Minh, D.P. Design and Applications of Hydroxyapatite-Based Catalysts, 1st ed.; Minh, D.P., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2022; ISBN 978-3-527-83020-6. [Google Scholar]
- Ying Kei, C.L. Iron-Substituted Hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–282. ISBN 978-0-08-102834-6. [Google Scholar]
- Nikitina, Y.O.; Petrakova, N.V.; Ashmarin, A.A.; Titov, D.D.; Shevtsov, S.V.; Penkina, T.N.; Kuvshinova, E.A.; Barinov, S.M.; Komlev, V.S.; Sergeeva, N.S. Preparation and Properties of Copper-Substituted Hydroxyapatite Powders and Ceramics. Inorg. Mater. 2019, 55, 1061–1067. [Google Scholar] [CrossRef]
- Amedlous, A.; Amadine, O.; Essamlali, Y.; Maati, H.; Semlal, N.; Zahouily, M. Copper Loaded Hydroxyapatite Nanoparticles as Eco-Friendly Fenton-like Catalyst to Effectively Remove Organic Dyes. J. Environ. Chem. Eng. 2021, 9, 105501. [Google Scholar] [CrossRef]
- Khachani, M.; Kacimi, M.; Ensuque, A.; Piquemal, J.Y.; Connan, C.; Bozon-Verduraz, F.; Ziyad, M. Iron-Calcium-Hydroxyapatite Catalysts: Iron Speciation and Comparative Performances in Butan-2-Ol Conversion and Propane Oxidative Dehydrogenation. Appl. Catal. A Gen. 2010, 388, 113–123. [Google Scholar] [CrossRef]
- Escolano Casado, G.; Ivanchenko, P.; Paul, G.; Bisio, C.; Marchese, L.; Ashrafi, A.M.; Milosavljevic, V.; Degli Esposti, L.; Iafisco, M.; Mino, L. Surface and Structural Characterization of Cu-Exchanged Hydroxyapatites and Their Application in H2O2 Electrocatalytic Reduction. Appl. Surf. Sci. 2022, 595, 153495. [Google Scholar] [CrossRef]
- Dos Santos Silva, D.; Villegas, A.E.C.; Bonfim, R.D.P.F.; Salim, V.M.M.; De Resende, N.S. Iron-Substituted Hydroxyapatite as a Potential Photocatalyst for Selective Reduction of CO2 with H2. J. CO2 Util. 2022, 63, 102102. [Google Scholar] [CrossRef]
- Chlala, D.; Giraudon, J.-M.; Nuns, N.; Labaki, M.; Lamonier, J.-F. Highly Active Noble-Metal-Free Copper Hydroxyapatite Catalysts for the Total Oxidation of Toluene. ChemCatChem 2017, 9, 2275–2283. [Google Scholar] [CrossRef]
- Galloni, M.G.; Campisi, S.; Gervasini, A.; Morandi, S.; Manzoli, M. How Hydroxyapatite Governs Surface Cu(II) and Fe(III) Structuring: Effects in the N2O Decomposition under Highly Oxidant Atmosphere. Appl. Catal. A Gen. 2023, 655, 119101. [Google Scholar] [CrossRef]
- Campisi, S.; Galloni, M.G.; Marchetti, S.G.; Auroux, A.; Postole, G.; Gervasini, A. Functionalized Iron Hydroxyapatite as Eco-Friendly Catalyst for NH3-SCR Reaction: Activity and Role of Iron Speciation on the Surface. ChemCatChem 2020, 12, 1676–1690. [Google Scholar] [CrossRef]
- Schiavoni, M.; Campisi, S.; Carniti, P.; Gervasini, A.; Delplanche, T. Focus on the Catalytic Performances of Cu-Functionalized Hydroxyapatites in NH3-SCR Reaction. Appl. Catal. A Gen. 2018, 563, 43–53. [Google Scholar] [CrossRef]
- Galloni, M.G.; Campisi, S.; Marchetti, S.G.; Gervasini, A. Environmental Reactions of Air-Quality Protection on Eco-Friendly Iron-Based Catalysts. Catalysts 2020, 10, 1415. [Google Scholar] [CrossRef]
- Campisi, S.; Galloni, M.G.; Bossola, F.; Gervasini, A. Comparative Performance of Copper and Iron Functionalized Hydroxyapatite Catalysts in NH3-SCR. Catal. Commun. 2019, 123, 79–85. [Google Scholar] [CrossRef]
- Campisi, S.; Leone, M.; Papacchini, M.; Evangelisti, C.; Polito, L.; Postole, G.; Gervasini, A. Multifunctional Interfaces for Multiple Uses: Tin(II)-Hydroxyapatite for Reductive Adsorption of Cr(VI) and Its Upcycling into Catalyst for Air Protection Reactions. J. Colloid. Interface Sci. 2023, 630, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Delafontaine, L.; Guo, S.; Asset, T.; Cristiani, P.; Campisi, S.; Gervasini, A.; Atanassov, P. Steering Cu-Based CO2RR Electrocatalysts’ Selectivity: Effect of Hydroxyapatite Acid/Base Moieties in Promoting Formate Production. ACS Energy Lett. 2022, 7, 2304–2310. [Google Scholar] [CrossRef]
- Campisi, S.; Galloni, M.G.; Gervasini, A. Unlocking Catalytic Efficiency: How Preparation Strategies and Copper Loading Enhance Hydroxyapatite Catalysts for NH3 Oxidation. Catalysts 2025, 15, 405. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Avakyan, L.; Paramonova, E.; Bystrov, V.; Coutinho, J.; Gomes, S.; Renaudin, G. Iron in Hydroxyapatite: Interstitial or Substitution Sites? Nanomaterials 2021, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kagawa, S.; Ogasawara, M.; Kato, S. Multiple Incorporation of Copper and Iron Ions into the Channel of Hydroxyapatite. J. Solid State Chem. 2023, 317, 123673. [Google Scholar] [CrossRef]
- Kalita, J.; Das, B.; Dhar, S.S. Synergistic Effect of Iron and Copper in Hydroxyapatite Nanorods for Fenton-like Oxidation of Organic Dye. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128750. [Google Scholar] [CrossRef]
- Costentin, G.; Drouet, C.; Salles, F.; Sarda, S. Structure and Surface Study of Hydroxyapatite-Based Materials: Experimental and Computational Approaches. In Design and Applications of Hydroxyapatite-Based Catalysts; Wiley: Hoboken, NJ, USA, 2022; pp. 73–140. [Google Scholar]
- Goldberg, M.A.; Antonova, O.S.; Donskaya, N.O.; Fomin, A.S.; Murzakhanov, F.F.; Gafurov, M.R.; Konovalov, A.A.; Kotyakov, A.A.; Leonov, A.V.; Smirnov, S.V.; et al. Effects of Various Ripening Media on the Mesoporous Structure and Morphology of Hydroxyapatite Powders. Nanomaterials 2023, 13, 418. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, H.; Djemal, S.; Petitto, C.; Delahay, G. Copper Loaded Hydroxyapatite Catalyst for Selective Catalytic Reduction of Nitric Oxide with Ammonia. Appl. Catal. B 2011, 107, 158–163. [Google Scholar] [CrossRef]
- Mondal, S.; De Anda Reyes, M.E.; Pal, U. Plasmon Induced Enhanced Photocatalytic Activity of Gold Loaded Hydroxyapatite Nanoparticles for Methylene Blue Degradation under Visible Light. RSC Adv. 2017, 7, 8633–8645. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Bao, S.; Zhang, W.; Liang, T.; Zheng, S.; Yi, L.; Guo, L.; Wu, X. A Review on the Characterization of Metal Active Sites over Cu-Based and Fe-Based Zeolites for NH3-SCR. RSC Adv. 2022, 12, 27746–27765. [Google Scholar] [CrossRef] [PubMed]
- Pestryakov, A.N.; Petranovskii, V.P.; Kryazhov, A.; Ozhereliev, O.; Pfänder, N.; Knop-Gericke, A. Study of Copper Nanoparticles Formation on Supports of Different Nature by UV-Vis Diffuse Reflectance Spectroscopy. Chem. Phys. Lett. 2004, 385, 173–176. [Google Scholar] [CrossRef]
- Sherman, D.M.; Waite, T.D. Electronic Spectra of Fe3+ Oxides and Oxide Hydroxides in the near IR to near UV. Am. Miner. 1985, 70, 1262–1269. [Google Scholar]
- Centi, G.; Perathoner, S.; Biglino, D.; Giamello, E. Adsorption and Reactivity of NO on Copper-on-Alumina Catalysts: I. Formation of Nitrate Species and Their Influence on Reactivity in No and NH3 Conversion. J. Catal. 1995, 152, 75–92. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Subramaniam, B.; Chaudhari, R.V. Ultraviolet-Visible Spectroscopy and Temperature-Programmed Techniques as Tools for Structural Characterization of Cu in CuMgAlO x Mixed Metal Oxides. J. Phys. Chem. C 2012, 116, 18207–18221. [Google Scholar] [CrossRef]
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface Characterization of Hydroxyapatite and Related Calcium Phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Raikar, G.N.; Ong, J.L.; Lucas, L.C. Hydroxyapatite Characterized by XPS. Surf. Sci. Spectra 1996, 4, 9–13. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; López, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 Nm) Pulsed Laser Deposition Produces Crystalline Hydroxyapatite Thin Coatings at Room Temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Bazin, T.; Gaudon, M.; Champion, E.; Julien, I.; Prestipino, C.; Figueroa, S.J.A.; Duttine, M.; Demourgues, A. Copper Versatility in Hydroxyapatite: Valence States, Clusters, and Optical Absorption Properties. Inorg. Chem. 2024, 63, 22181–22193. [Google Scholar] [CrossRef]
- Guo, J.; Duchesne, P.N.; Wang, L.; Song, R.; Xia, M.; Ulmer, U.; Sun, W.; Dong, Y.; Loh, J.Y.Y.; Kherani, N.P.; et al. High-Performance, Scalable, and Low-Cost Copper Hydroxyapatite for Photothermal CO2 Reduction. ACS Catal. 2020, 10, 13668–13681. [Google Scholar] [CrossRef]
- Mansour, A.N.; Brizzolara, R.A. Characterization of the Surface of γ-Fe2O3 Powder by XPS. Surf. Sci. Spectra 1996, 4, 351–356. [Google Scholar] [CrossRef]
- Wang, Y.; Sherwood, P.M.A. Iron (III) Phosphate (FePO4) by XPS. Surf. Sci. Spectra 2002, 9, 99–105. [Google Scholar] [CrossRef]
- Bengoa, J.F.; Campisi, S.; Gervasini, A.; Marchetti, S.G. Mössbauer Spectroscopy as a Tool to Predict the Catalytic Activity of the Fe3+ Sites in an Exchanged Fe/Hydroxyapatite System. J. Nanopart. Res. 2023, 25, 100. [Google Scholar] [CrossRef]
- Lee, G.; Deka, D.J.; Rappé, K.G.; Szanyi, J. Water Effects on NH3-SCR over Cu-Based Small-Pore Zeolite Catalysts: A Review. Appl. Catal. A Gen. 2025, 706, 120491. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Zoubeir, L.; Adeline, S.; Laurent, C.S.; Yoann, C.; Truc, H.T.; Benoît, L.G.; Federico, A. The Use of the Novosol Process for the Treatment of Polluted Marine Sediment. J. Hazard. Mater. 2007, 148, 606–612. [Google Scholar] [CrossRef] [PubMed]
| Code 1 | Surface Area 2 (m2·g−1) | Bulk Composition 3 (wt.%) | Surface Composition 4 (atomic %) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Fe | Ca/P | Cu | Fe | Ca/P | ||
| HAP | 75 | - | - | 1.81 | - | - | 1.5 |
| Cu0.5Fe0.5/HAP | 74 | 0.51 ± 0.01 | 0.44 ± 0.01 | 1.62 | 0.5 (1.4 wt.%) | 1.3 (3.2 wt.%) | 1.4 |
| Cu1.5Fe1.5/HAP | 81 | 1.54 ± 0.05 | 1.37 ± 0.02 | 1.67 | 1.4 (3.7 wt.%) | 3.9 (8.9 wt.%) | 1.2 |
| Cu2Fe4/HAP | 77 | 2.03 ± 0.04 | 3.75 ± 0.05 | 1.55 | 1.4 (3.7 wt.%) | 4.8 (11 wt.%) | 1.2 |
| Cu3Fe3/HAP | 72 | 2.99 ± 0.01 | 2.75 ± 0.01 | 1.60 | 2.2 (5.7 wt.%) | 4.9 (11 wt.%) | 1.2 |
| Cu4Fe2/HAP | 73 | 3.86 ± 0.01 | 1.81 ± 0.01 | 1.61 | 3.2 (8.1 wt.%) | 3.7 (8.3 wt.%) | 1.2 |
| Cu5Fe5/HAP | 73 | 5.28 ± 0.04 | 5.21 ± 0.04 | 2.36 | 3.6 (8.9 wt.%) | 6.5 (14 wt.%) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloni, M.G.; Zhang, W.; Giroir-Fendler, A.; Campisi, S.; Gervasini, A. Exploring the Evolution of Co-Deposited Copper and Iron Nanostructures on Hydroxyapatite: Implications in NH3-SCR Reaction. Catalysts 2025, 15, 929. https://doi.org/10.3390/catal15100929
Galloni MG, Zhang W, Giroir-Fendler A, Campisi S, Gervasini A. Exploring the Evolution of Co-Deposited Copper and Iron Nanostructures on Hydroxyapatite: Implications in NH3-SCR Reaction. Catalysts. 2025; 15(10):929. https://doi.org/10.3390/catal15100929
Chicago/Turabian StyleGalloni, Melissa Greta, Weidong Zhang, Anne Giroir-Fendler, Sebastiano Campisi, and Antonella Gervasini. 2025. "Exploring the Evolution of Co-Deposited Copper and Iron Nanostructures on Hydroxyapatite: Implications in NH3-SCR Reaction" Catalysts 15, no. 10: 929. https://doi.org/10.3390/catal15100929
APA StyleGalloni, M. G., Zhang, W., Giroir-Fendler, A., Campisi, S., & Gervasini, A. (2025). Exploring the Evolution of Co-Deposited Copper and Iron Nanostructures on Hydroxyapatite: Implications in NH3-SCR Reaction. Catalysts, 15(10), 929. https://doi.org/10.3390/catal15100929












