Colloidal Protein–Silver Nanoparticle Metalloenzyme as Artificial Redox Biocatalyst
Abstract
1. Introduction
2. Results and Discussion
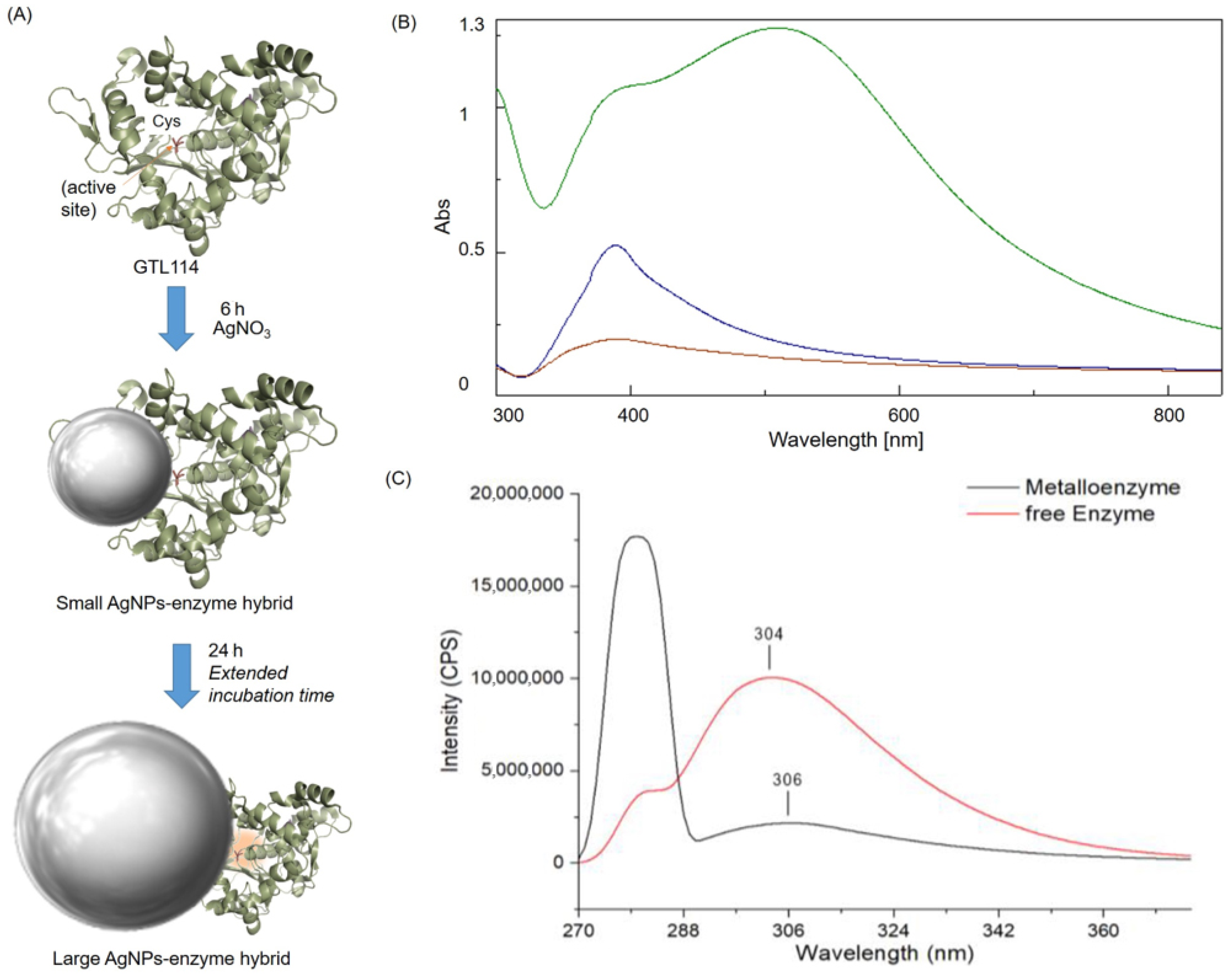
2.1. Synthesis and Characterization of AgNC-GTL Bioconjugate Metalloenzyme
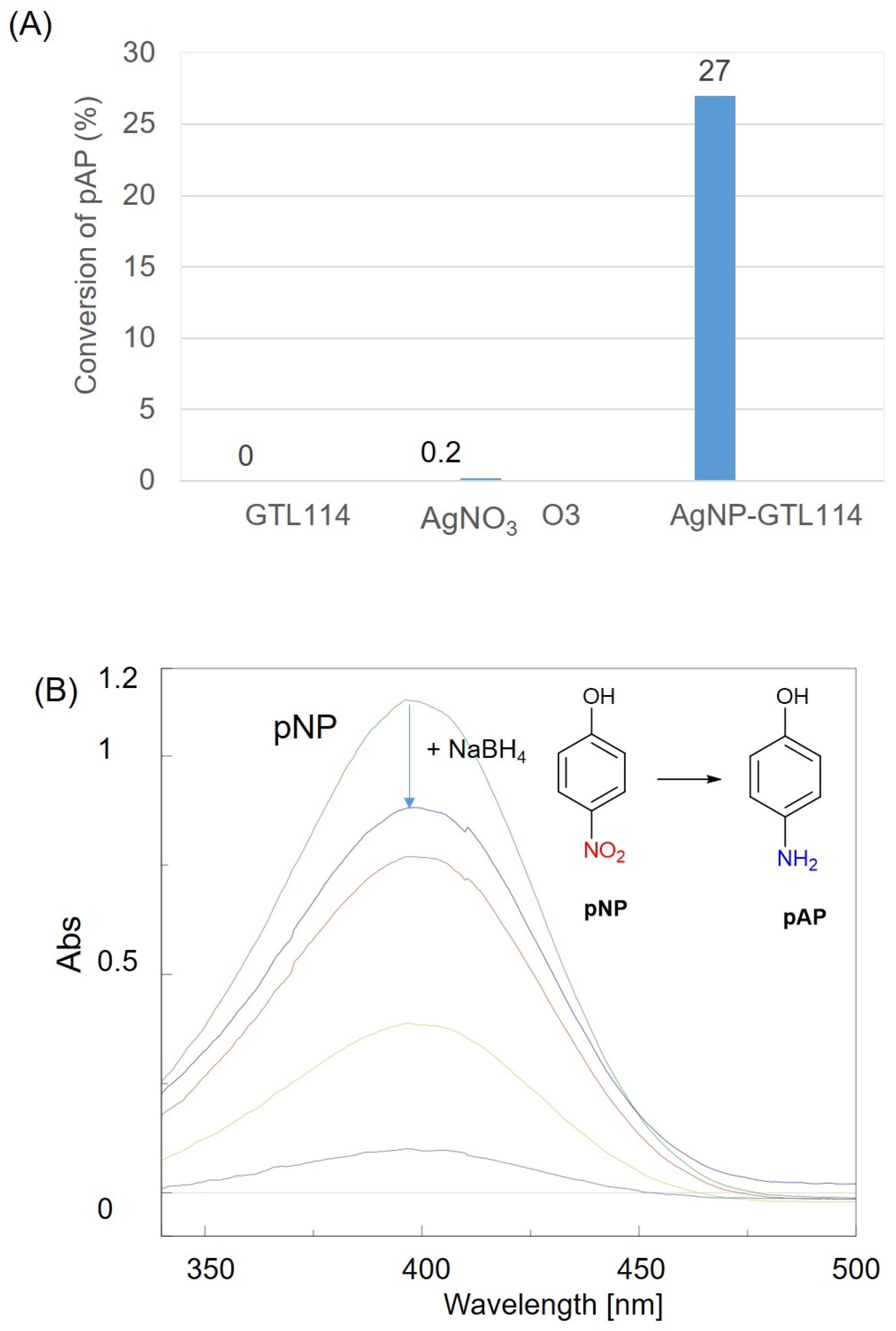
2.2. Determination of Metallic Activity in Reductive Processes
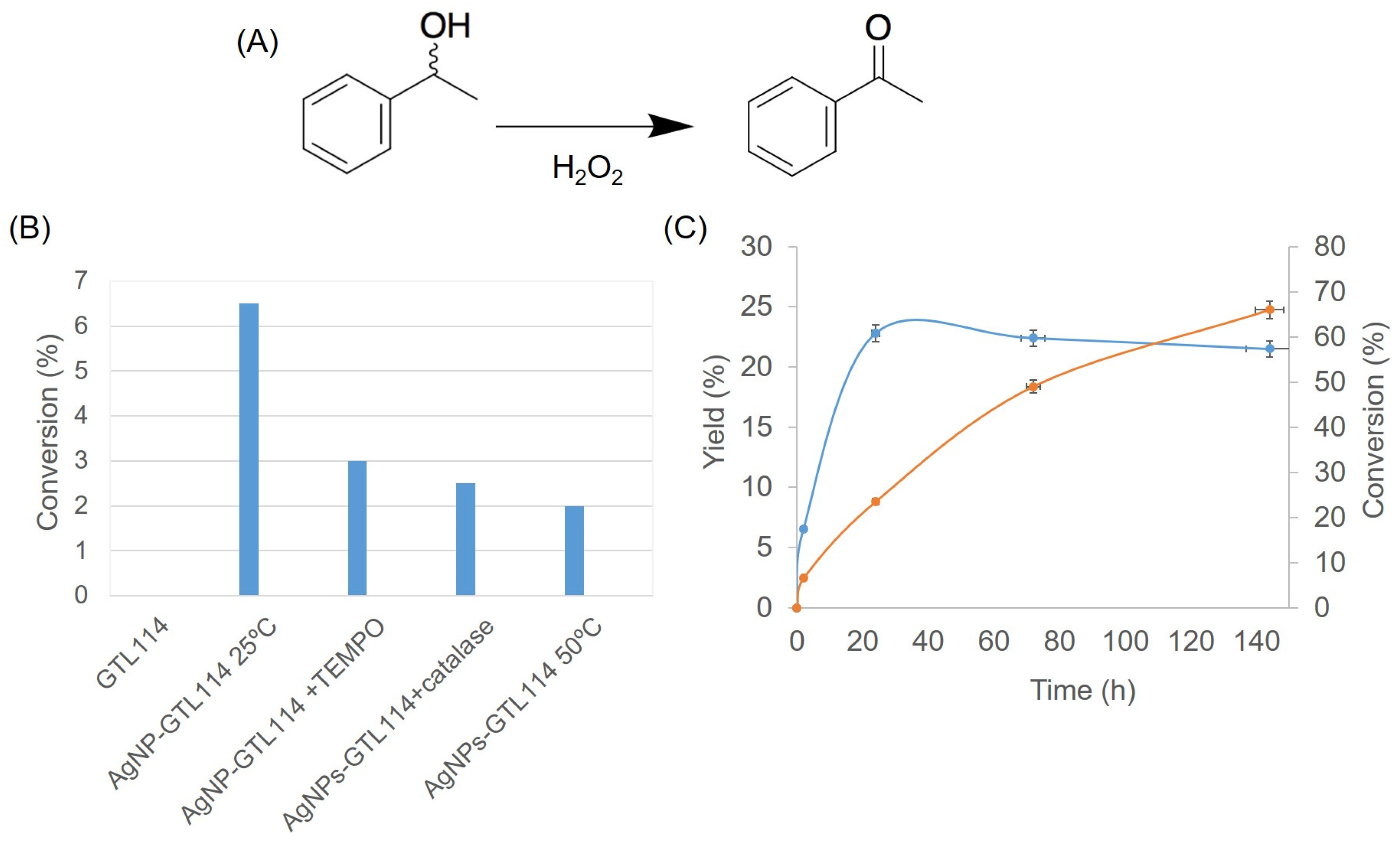
2.3. AgNPs-GTL114 Metalloenzyme as Oxidase-like Enzyme
3. Materials and Methods
3.1. General
3.2. Instrumentation
3.3. Purification of GTL114
3.4. Synthesis of Ag-GTL Metalloenzymes
3.5. Fluorescence Spectroscopy
3.6. Average Crystallite Size of AgNPs from XRD Peaks
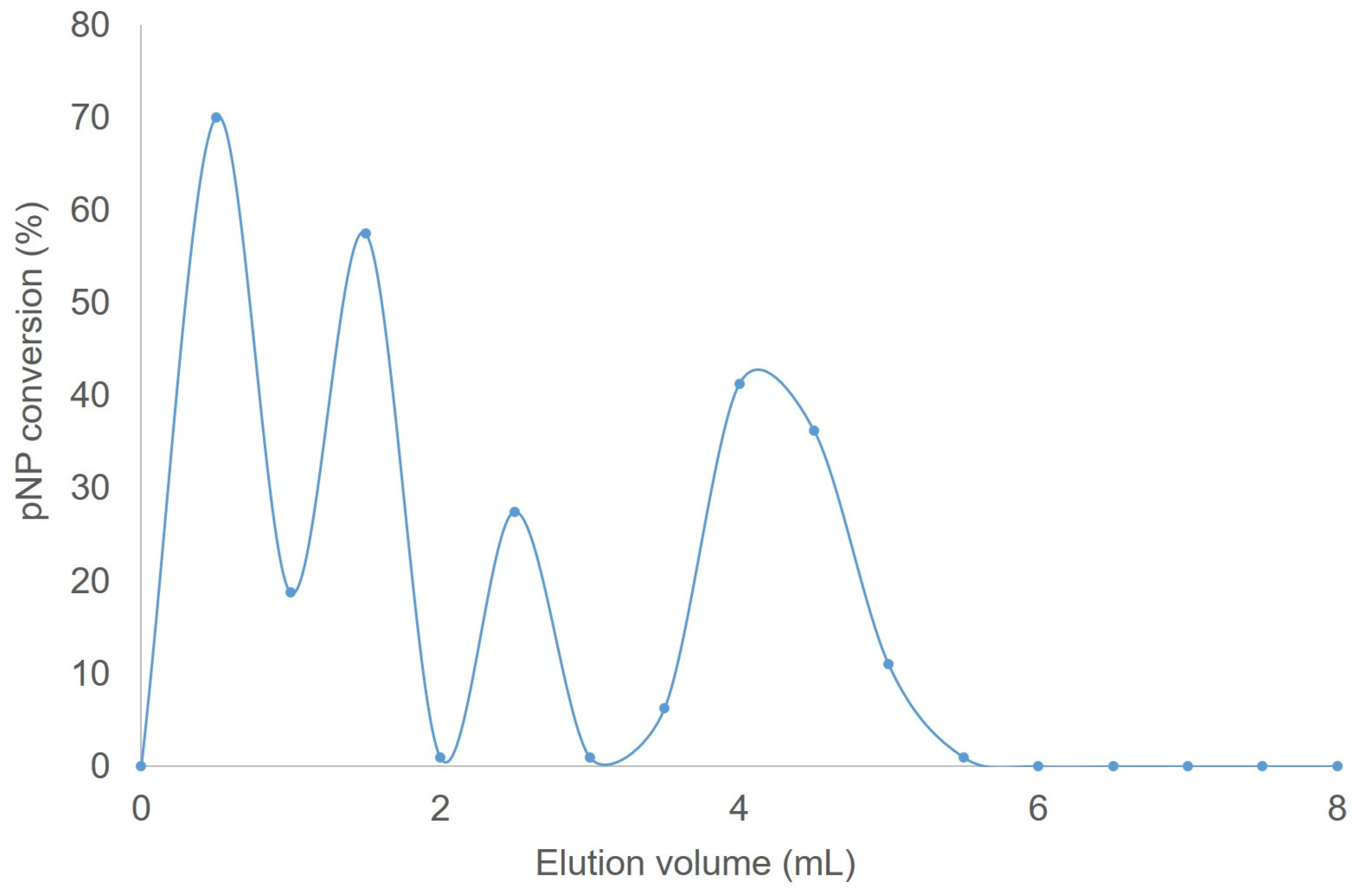
3.7. Gel Filtration of GTL–Ag Metalloenzyme
3.8. Metal Activity Test: Reduction of 4-Nitrophenol to 4-Aminophenol
3.9. Selective Reduction of Acetophenone to 1-Phenylethanol
3.10. Selective Oxidation of 1-Phenylethanol to Acetophenone
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornils, B.; Herrmann, W.A.; Wong, C.H.; Zanthoff, H.-W. Catalysis from A to Z; John Wiley & Sons: Hoboken, NJ, USA, 2020; 2952p, ISBN 9783527343119. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2007; Print ISBN 9783527307159; Online ISBN 9783527611003. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J. (Eds.) Handbook of Green Chemistry and Technology; Blackwell Science Ltd.: Hoboken, NJ, USA, 2002; Print ISBN 9780632057153; Online ISBN 9780470988305. [Google Scholar] [CrossRef]
- Murakami, Y.; Kikuchi, J.I.; Hisaeda, Y.; Hayashida, O. Artificial enzymes. Chem. Rev. 1996, 96, 721–758. [Google Scholar] [CrossRef]
- Davis, H.J.; Ward, T.R. Artificial Metalloenzymes: Challenges and Opportunities. ACS. Cent. Sci. 2019, 5, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Hanreich, S.; Bonandi, E.; Drienovská, I. Design of Artificial Enzymes: Insights into Protein Scaffolds. ChemBioChem 2023, 24, e20220056. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Bucholz, K. Highlights in biocatalysis—Historical landmarks and current trends. Eng. Life Sci. 2005, 5, 309–323. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- Wang, M.; Si, T.; Zhao, H. Biocatalyst Development by Directed Evolution. Bioresour. Technol. 2012, 115, 117–125. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [PubMed]
- Naapuri, J.M.; Losada-Garcia, N.; Deska, J.; Palomo, J.M. Synthesis of silver and gold nanoparticles–enzyme–polymer conjugate hybrids as dual-activity catalysts for chemoenzymatic cascade reactions. Nanoscale 2022, 14, 5701–5715. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Romero, O.; Guitiérrez-Fernández, J.; de las Rivas, B.; Hermoso, J.A.; Palomo, J.M. Synthesis of a heterogeneous artificial metallolipase with chimeric catalytic activity. Chem. Commun. 2015, 51, 9324–9327. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M. Artificial enzymes with multiple active sites. Curr. Opin. Green. Sustain. Chem. 2021, 29, 100452. [Google Scholar] [CrossRef]
- Klein, A.S.; Zeymer, C. Design and engineering of artificial metalloproteins: From de novo metal coordination to catalysis. PEDS 2021, 34, gzab003. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.D.; Serrano-Plana, J.; Peterson, R.L.; Ward, T.R. Artificial Metalloenzymes Based on the Biotin-Streptavidin Technology: Enzymatic Cascades and Directed Evolution. Acc. Chem. Res. 2019, 52, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; de las Rivas, B.; Lopez-Tejedor, D.; Palomo, J.M. Effect of Site-Specific Peptide-Tag Labeling on the Biocatalytic Properties of Thermoalkalophilic Lipase from Geobacillus thermocatenulatus. ChemBioChem 2018, 19, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, Y.; Wang, L.; Mu, X.; Zhao, Q.; Si, R.; Zhu, X.; Chen, S.; Zhang, B.; Chen, D.; et al. Gold catalysts containing interstitial carbon atoms boost hydrogenation activity. Nat. Commun. 2020, 11, 4600. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castillo, J.E.; Gallo-Villanueva, R.C.; Madou, M.J.; Perez-Gonzalez, V.H. Anisotropic gold nanoparticles: A survey of recent synthetic methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Y.; Zhang, Y.; Tang, P. Silver-catalyzed dibromotrifluoromethoxylation of terminal alkynes. ACS Catal. 2021, 11, 3218–3223. [Google Scholar] [CrossRef]
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; Morales, M.D.P.; Palomo, J.M. Synthesis of heterogeneous enzyme–metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef]
- Sekine, K.; Yamada, T. Silver-catalyzed carboxylation. Chem. Soc. Rev. 2016, 45, 4524–4532. [Google Scholar] [CrossRef]
- Li, A.Y.; Gellé, A.; Segalla, A.; Moores, A. Silver Nanoparticles in Organic Transformations. Silver Catal. Org. Synth. 2018, 1, 723–793. [Google Scholar]
- Naodovic, M.; Yamamoto, H. Asymmetric silver-catalyzed reactions. Chem. Rev. 2008, 108, 3132–3148. [Google Scholar] [CrossRef]
- Pagliaro, M.; Della Pina, C.; Mauriello, F.; Ciriminna, R. Catalysis with Silver: From Complexes and Nanoparticles to MORALs and Single-Atom Catalysts. Catalysts 2020, 10, 1343. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Godoy, C.; de Las Rivas, B.; Fernández-Lorente, G.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R.; Martínez-Ripolla, M.; Hermoso, J.A. Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, C.; de las Rivas, B.; Palomo, J.M. Design of a gold nanoparticle-site in an engineered lipase: An artificial metalloenzyme with enantioselective reductase-like activity. Nanoscale 2024, 16, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Wang, M.; Hu, M.; Xu, X.; Yan, A.; Hao, Q.; Lia, H.; Sun, H. Atomic differentiation of silver binding preference in protein targets: Escherichia coli malate dehydrogenase as a paradigm. Chem. Sci. 2020, 11, 11714–11719. [Google Scholar] [CrossRef]
- Khan, K.; AL-Thabaiti, S.A.; Obaid, A.Y.; Khan, Z.A.; Al-Youbi, A.O. Effects of solvents on the stability and morphology of CTAB-stabilized silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 120–125. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Losada-García, N.; Urriolabeitia, E.; Palomo, J.M. Solid-phase lipase-CuNPs biohybrids as catalysts for one-pot parallel synthesis of 2,3,4-tryacetyl-D-gluconic acid. ChemCatChem 2023, 15, e202201632. [Google Scholar] [CrossRef]




| 2θ (in Degrees) | FWHM | Cristallite Size (Å) |
|---|---|---|
| 27.623 | 0.779 | 108 |
| 36.404 | 0.779 | 110 |
| 46.068 | 0.779 | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojanov, G.; Garcia-Sanz, C.; Palomo, J.M. Colloidal Protein–Silver Nanoparticle Metalloenzyme as Artificial Redox Biocatalyst. Catalysts 2025, 15, 61. https://doi.org/10.3390/catal15010061
Bojanov G, Garcia-Sanz C, Palomo JM. Colloidal Protein–Silver Nanoparticle Metalloenzyme as Artificial Redox Biocatalyst. Catalysts. 2025; 15(1):61. https://doi.org/10.3390/catal15010061
Chicago/Turabian StyleBojanov, Glenn, Carla Garcia-Sanz, and Jose M. Palomo. 2025. "Colloidal Protein–Silver Nanoparticle Metalloenzyme as Artificial Redox Biocatalyst" Catalysts 15, no. 1: 61. https://doi.org/10.3390/catal15010061
APA StyleBojanov, G., Garcia-Sanz, C., & Palomo, J. M. (2025). Colloidal Protein–Silver Nanoparticle Metalloenzyme as Artificial Redox Biocatalyst. Catalysts, 15(1), 61. https://doi.org/10.3390/catal15010061








