Abstract
Efficient and sustainable catalytic processes are crucial for advancing green chemical manufacturing. Here, we describe the synthesis of novel silver artificial metalloenzymes in colloidal form in aqueous media and room temperature. The strategy is based on the in situ generation of silver nanoparticles by a genetically modified Geobacillus thermocatenulatus lipase (GTL) in the active site as an inducer and scaffold protein, producing an enzyme–Ag bioconjugate. Using a structural analysis of the formation of silver nanoparticles by XRD and UV spectra, we found the formation of Ag2O species with nanoparticles of around 11 nm average diameter size. Gel filtration chromatography demonstrated the presence of single protein molecules in the bioconjugates, although silver nanoparticles were initially formed by cysteine coordination in the active site but later were formed in other parts of the protein (five AgNPs per molecules, which is in concordance with the UV size). The enzyme structure was altered after nanoparticle formation and Ag-S interaction, which was observed in fluorescence analysis. This new enzyme showed reductive activity against p-nitrophenol to p-amino and a high conversion > 99% in the reduction of acetophenone to phenylethanol, although the enantioselective was quite moderate but higher in water that in the presence of co-solvents. Finally, oxidase-like activity was evaluated in the direct oxidation of phenylethanol to acetophenone in water, obtained at around a 23% yield of ketone after 60 h.
1. Introduction
The development of efficient and sustainable catalytic processes stands as a cornerstone in the pursuit of greener chemical manufacturing [1,2,3]. Catalysis, especially biocatalysis, plays a pivotal role in enhancing reaction rates and selectivity under environmentally benign conditions. However, the inherent limitations of natural enzymes, such as their narrow substrate scope and sensitivity to operational conditions, have prompted the exploration of artificial catalytic systems [4,5]. Among these, artificial metalloenzymes (ArMEs) have emerged as a promising class of biocatalysts, offering the unique opportunity to combine the versatile catalytic mechanisms of transition metals with the high selectivity and specificity of biological macromolecules. One of the main challenges in that field lies in the development of methodologies that allow for the creation of these catalysts in a simple, efficient, and sustainable way [6,7,8,9,10].
Previously, our group pioneered a straightforward and eco-friendly methodology for generating enzyme–metal nanoparticle hybrids. The essence of this method lies in its simplicity and effectiveness, wherein the enzyme’s three-dimensional structure serves as a scaffold for the in situ formation of nanoparticles. The enzymes act as the stabilizer, reducing agent, and inductor of very well-dispersed small NP formations, effectively circumventing common challenges such as aggregation [11,12,13,14]. This work extends the framework of nanobiohybrids and demonstrates its applicability beyond the synthesis of solid nanobiohybrids to the creation of colloidal ArMEs.
In this work, we created a silver metalloenzyme following the concept process of bionanohybrid synthesis [11] but by targeting and limiting the metal coordination to specific cavities within the enzyme’s three-dimensional structure in order to obtain colloidal soluble artificial enzymes. This could allow us to influence in the size and shape of the nanoparticle formation with a key effect of the second coordination sphere of the enzyme. This could therefore be a key parameter affecting substrate selectivity or allowing for chiral discrimination [5,15,16]. To direct the ionic metal to coordinate at the desired sites, the enzyme’s surface was modified by introducing an anchoring point for the metal. For this purpose, we employed a genetic introduction of cysteine, known for its strong silver-binding affinity, by substituting catalytic serine in the Geobacillus thermocatenulatus lipase (GTL) (Ser114Cys) and therefore losing its esterase and lipase activity [17].
In this way, the formation of the metal nanoparticles is first coordinated by the metal ions with the groups of the protein, cysteine, for a subsequent generation of a small cluster of atoms with a final growth of the same influenced by the protein structure. In this case, the main effect would be on the position of the active site, which would be affected by a coordination in the environment of an oxyanion, where its cavity is around 28 Armstrong in size (Figure 1a). While silver is traditionally not as prevalent in catalytic applications as palladium, they offer unique advantages, providing a distinct set of reactivities that are less accessible through other metal catalysts [18,19,20,21,22]. They demonstrate the ability to oxidize and reduce back to their metallic state under mild conditions [23,24]. The unique redox chemistry and high reactivity have been employed in versatile catalytic applications in the form of complexes, nanoparticles, and single-atom catalysts [25,26]. These new Ag artificial metalloenzymes were tested in redox processes in aromatic compounds in aqueous media and room temperature to mimic redox enzyme activities (Video S1).
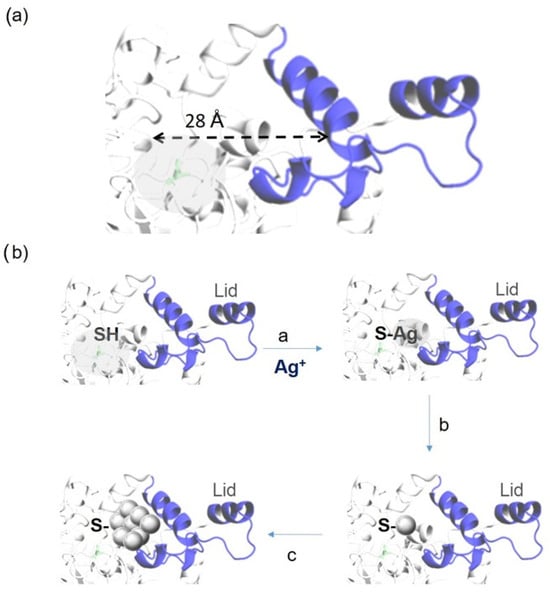
Figure 1.
(a) Structure of GTL in open conformation, including the size of the oxyanion hole. The picture was made using Pymol 3.0.3 software with pdb file 2w22. (b) General scheme of the formation of enzyme–AgNPs artificial metalloenzymes: a—enzyme–metal coordination; b—nanoparticle formation; c—nanoparticle growth.
2. Results and Discussion
2.1. Synthesis and Characterization of AgNC-GTL Bioconjugate Metalloenzyme
The enzyme variant (GTL114) enabled a targeted anchoring of metal ions (Figure 2A). The synthesis was performed combining metal salt and the enzyme at pH 8.5 at room temperature. Enzymes purified in the presence of detergent ensure the open conformation of the lipase. This is a key element, especially in the case of this enzyme, which has a large active site cavity involving a large number of hydrophobic groups [27]. The progress of nanoparticle formation on the enzyme was monitored via UV–visible spectroscopy. Control solutions of enzyme or metal salts alone showed no significant changes. However, combining them resulted in observable emission band increases and colour changes, indicating in situ nanoparticle formation induced by the protein scaffold. Colloidal silver nanoparticles exhibit characteristic peaks in the 400–500 nm range due to surface plasmon vibration, which also is related to the final size in nanometres. UV–visible spectra obtained during ArME preparation showed an initial change after one hour, with a distinct peak indicating nanoparticle formation after five hours, starting with the appearance of a peak around a wavelength of 399 nm, which has been demonstrated to be related to nanoparticle sizes around 10 nm [28]. At 6 h, the clear formation of this size of silver nanoparticles was observed (Figure 2B). At 24 h, the peak broadened and shifted to 495 nm, which is consistent with a nanoparticle size of around 60 nm in diameter [28] (Figure 2B). While variations were observed, no clear trends between starting conditions and spectral shifts were identified. The subsequent purification step removed excess unconjugated reagent, i.e., free Ag in the solution.

Figure 2.
(A) Scheme of the synthesis of Ag metalloenzyme. (B) UV–visible spectrum of the synthesis of artificial Ag metalloenzyme at pH 8.5 and r.t. After one hour, the first changes are visible (red), the formation of the ArMEs becomes more visible after five hours (blue), and the second shoulder peak appears after 24 h (green). (C) Fluorescence spectrum of Ag metalloenzyme compared to the free enzyme (280 nm peak corresponds to excitation peak). Experiments were performed in triplicate.
After preparation of the ArMEs, additional characterization was conducted. Initially, the stability of the metalloenzymes was visually inspected. Stable batches remained colourful and homogeneous over weeks, while others precipitated immediately post-synthesis. The increase in the silver concentration to 6 mM consistently led to precipitation. Lowering the concentration appeared to yield more stable species, the optimal one using 1.5 mM of Ag salt. The synthesis temperature to 50 °C resulted in a similar result. To confirm metal–protein coupling, fluorescence spectroscopy was employed (Figure 2C). The optical properties of metal nanoparticles undergo significant changes due to coupling with fluorescent molecules and surface plasmon resonance.
A comparison with spectra from free enzyme samples revealed a shift in emission peaks in 2 nm towards longer wavelengths for ArMEs. Moreover, the presence of metal nanoparticles typically leads to fluorescent quenching, a phenomenon observed in our experiments (Figure 2C) [29].
Subsequently, powder X-ray diffraction (XRD) analysis was performed to characterize the silver structures formed in conjugation with the protein (Figure 3). The XRD pattern of the sample shows a signature mainly for silver (i) oxide (Ag2O) species. The peaks for 2θ at 27° (100), 32° (110), 38 ° (111), 46° (200), 54 and 56° (211), 67° (220), and 76° (311) matched well the with JCPDS no. 00-076-1393 for Ag2O.

Figure 3.
XRD pattern of Ag-GTL114 metalloenzyme.
Furthermore, from XRD peaks, the average crystallite size allows us to calculate a theoretical size of the nanoparticle using the Scherrer formula (see Section 3). This allowed us to determine an average size of AgNPs of around 110 ± 1 Å (11 nm). This means that the metal nanoparticles generated are smaller than observed by UV.
At this point, evaluating the enzyme structure by bioinformatics studies showed that in the protein structure, the oxyanion hole and positions surrounding cysteine 114 are mainly hydrophobic groups with the presence of Asp 318, Pro165, Tyr30 and Trp20, and Trp31 residues [29]. Metal can coordinate on this area at 8.5, with a greater influence on the coordination with Cys. As we observed experimentally, the growth of the silver nanoparticle after 6 h generates a nanoparticle with a larger size, such that this large protein pocket (2.78 nm) increases in the solvent, also generating a reorganization of the structure, changes which can be observed in the fluorescence signal. Longer incubation generated a slight increase in the Ag2O nanoparticles, although this is coordinated to the protein.
Recently, it has been demonstrated that a longer incubation of silver ions in aqueous media display an increase in the number of coordination points with the binding preference of Ag+ to the donor atoms and residues in the order of S > N > O and Cys > Met > His > Lys > Val, respectively [30].
In order to understand this process and determine the structure of the bioconjugate, gel filtration chromatography of the purified artificial enzyme was performed (Figure 4). The eluent used was 10 mM phosphate buffer, at pH 7. Under these conditions, the chemical modification with silver was able to change the form of the native enzyme as a different elution profile, and four different bioconjugates sizes were obtained, even in the presence of 0.5% (v/v) Triton X-100 (which avoids the generation of dimeric protein formation). In this way, 36% of the total molecules of Ag metalloenzymes were Ag–enzyme bioconjugate, whereas 8% were protein dimers, 27% protein trimers, and even 28% of tetramers of GTL114 were observed. This multimeric formation was induced by the silver coordination with more than one group in different protein molecules. The molecular weight of Ag metalloenzymes was estimated from a calibration curve plotted using standard proteins (GTL114, 43 kDa, CAL-B, 33 kDa, BSA, 65 kDa, and Laccase 80 kda).

Figure 4.
Elution profile in gel filtration chromatography of Ag metalloenzyme.
Therefore, considering the results by gel filtration, where a monomeric GTL was obtained and this ratio, it is possible to think that AgNPs were generated around the protein structure instead of the active site. Thus, this final structure could explain the size around 60 nm for silver nanoparticles in UV (which could correspond to five different AgNPs of 11 nm).
2.2. Determination of Metallic Activity in Reductive Processes
Once the silver binding to the scaffold was confirmed and the properties of each condition characterized, the next step was to evaluate the metallic activity of the newly generated artificial metalloenzymes. This was achieved through nitroarene reduction, specifically the reduction of p-nitrophenol (pNP) to p-aminophenol (pAP) in the presence of a reducing agent, which serves as a benchmark for catalyst nanoparticle testing. This method is advantageous for metalloenzymes, as it operates in an aqueous medium and can be monitored by UV–visible spectrophotometry.
Upon the addition of control solutions containing either only dissolved silver salt or only the mutant enzyme to nitrophenol, no significant change in concentration was observed (Figure 5). However, the addition of the metalloenzyme resulted in a measurable decrease in pNP over time, with almost 30% conversion in 1 min (92% in 10 min) (Figure 5). This demonstrates the metallic capacity of the Ag–enzyme hybrid.
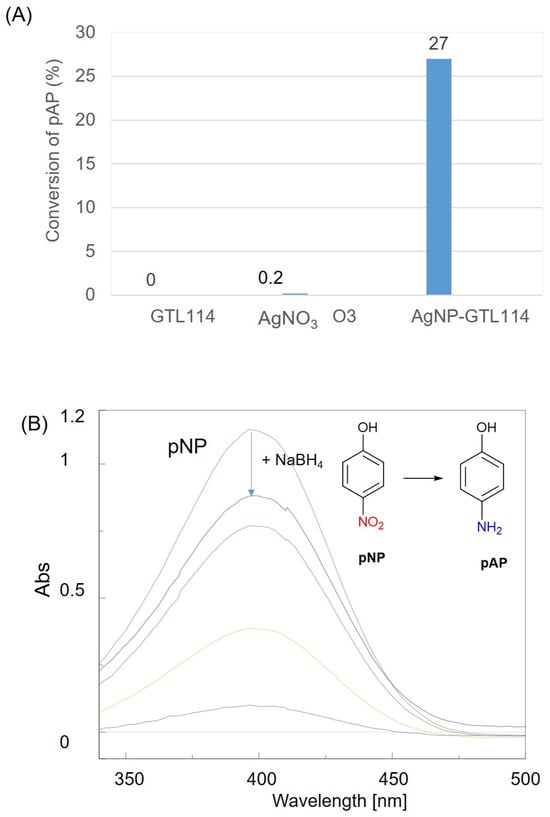
Figure 5.
(A) Conversion (%) in the reduction of pNP to pAP in 1 min. (B) UV spectrophotometer profile of pNP transformation after 30 min [0 min (green), 1 min (blue), 3 min (red), 5 min (light green), purple (10 min)]. See Section 3.
Thus, considering this result, we evaluated the potential applicability of this colloidal Ag metalloenzyme in the specific reduction of ketones to chiral alcohols, particularly in the reduction of acetophenone (Figure 6). This reaction was performed evaluating at different conditions, in pure water, the presence of solvents, or even the presence of additives. While free enzymes did not show any oxidation activity, the metalloenzyme was able to catalyze the complete reduction of acetophenone in 2 h at 25 °C, whereas the conversion was slightly lower at higher T, with a final 83% conversion (Figure 6A). The enantioselectivity of the process was also evaluated, observing a moderate selectivity to the S enantiomer (ee: 2.6%).

Figure 6.
Reduction of acetophenone to phenylethanol catalyzed by AgNP-GTL114 metalloenzyme. (A) Scheme of the reaction. (B) Conversion process. Conditions: acetophenone 1 mM, 0.05 mg/mL (50 μL) NaBH4, 50 μL of catalyst, 2 h. Conversion was calculated by HPLC. (C) Enantiomeric excess of S enantiomer at different reaction conditions. Enantiomeric excess (ee) was calculated by chiral HPLC.
In order to modify the selectivity, the addition of different co-solvents or increasing the T was used; however, no improvement in the selectivity values were observed (Figure 6B). This could be due to the change in morphology in the Ag nanoparticles, as we observed at 50 °C, but this is also reported to be by the solvent [31].
2.3. AgNPs-GTL114 Metalloenzyme as Oxidase-like Enzyme
In order to demonstrate the redox capacity of this metalloenzyme without the presence of any cofactor, the catalytic oxidation of phenyl alcohol was tested (Figure 7) using hydrogen peroxide as a green oxidant in aqueous media and room T for 2 h. At these conditions, the Ag metalloenzyme showed around 6% conversion in acetophenone yield, whereas the native scaffold enzyme did not show any oxidative activity (Figure 7B). Also, the addition of radical agents such as TEMPO or catalase (enzymes able to produce oxygen from hydrogen peroxide degradation) were added to the reaction, but in any case, the conversion improved. Even the increase in the T of the reaction did not work (Figure 7B). Then, reactions in water were evaluated at longer incubation times, obtaining a maximum yield of acetopehenone of 22.8% after 24 h.

Figure 7.
Oxidation of phenylethanol to acetophenone catalyzed by AgNP-GTL114 metalloenzyme. (A) Scheme of the reaction. (B) Conversion process. Conditions: racemic phenylethanol 1 mM, (20 μL) H2O2, 50 μL of catalyst, 2 h, (TEMPO 20 mg (2.11 mmol/g solid), catalase 10 μL, at 25 °C). Conversion and yield were calculated by HPLC. (C) Reaction profile of the catalyzed oxidation using AgNPs-GTL114 in aqueous media; yield (blue), conversion (orange).
However, a further extension of the reaction time revealed that more than one product was formed. About half of the substrate was consumed after three days, while only 22.4% of the expected product was formed (Figure 6C).
After six days, the yield of acetophenone slightly decreased (21.5%) at 66% conversion (Figure 6C). The literature confirms that silver-containing catalysts, indeed, are capable of decomposing aromatic compounds into small fragments [32]. Additional analysis would be required to elucidate this process for the Ag metalloenzyme.
3. Materials and Methods
3.1. General
Silver nitrate, p-nitrophenol, Triton® X-100, sodium phosphate, and dialysis tubing made of cellulose (with an average diameter of 33 mm) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetophenone, R-, and S-Phenylethanol were from Fluka (Buchs, Switzerland). Butyl-Sepharose® 4 Fast Flow was from GE Healthcare (Uppsala, Sweden). Geobacillus thermocatenulatus lipase variant (Cys65Ser/Cys296Ser: G114) was produced by Dr. de las Rivas (ICTAN, CSIC, Madrid, Spain) [26].
3.2. Instrumentation
The synthesis of metalloenzymes was performed in a Opa Q incubator equipped with an OL30 ME orbital. Spectrophotometric analyses were conducted on a V-730 spectrophotometer manufactured by JASCO (Tokyo, Japan). Inductively coupled plasma–optical emission spectrometry (ICP-OES) was carried out using an OPTIMA 2100 DV instrument produced by PerkinElmer (Waltham, MA, USA). Chromatographic analyses were run at 25 °C using an HPLC spectrum P100 (Thermo Separation Products, Waltham, MA, USA). Analyses were run using an L-7300 column oven and a UV6000LP detector. Acetonitrile HPLC grade was provided by Scharlab (Barcelona, Spain).
3.3. Purification of GTL114
The E. coli crude extract (5 mL) containing the GTL114 (7 mgprotein/mL) was added to 20 mL of 25 mM sodium phosphate buffer at pH 7.0 (obtaining a final enzyme concentration of 7 mg/mL determined by the Bradford assay). Ten grammes of the butyl-Sepharose support was incubated with 10 mL of the enzyme solution (7 mg/mL) and 190 mL of 25 mM sodium phosphate buffer pH 7 for 24 h. The mixture was then filtered under vacuum and the recovered solid was washed with water to obtain the desired catalyst. Finally, the solid was incubated in a 1:10 (w/v) ratio in a solution of sodium phosphate buffer (25 mM pH 7) containing 0.5% (v/v) Triton X-100 for 1 h at room temperature. After the indicated time, the support was removed by centrifugation and the soluble enzyme was collected in the supernatant and stored at 4 °C.
3.4. Synthesis of Ag-GTL Metalloenzymes
The protocol of preparation of the metalloenzymes is described in Figure 8. A total of 100 μL of GTL114 (70 μg protein) was added to 600 μL of distilled water. An amount of 200 μL of this solution was added to 1.8 mL of silver salt solution (0.25 mg/mL AgNO3). This mixture was then adjusted to pH 8.5 using a 1 mM NaOH solution. Initially, the solution had a pH of approximately 5.5 and was transparent. On adjusting the pH, the solution changed in colour (light purple). This solution was kept for 24 h under constant stirring at 130 rpm at room temperature or in an Opa Q incubator with an OL30 ME orbital at room temperature or 50 °C. The formation of the silver metal nanoparticles was followed using a JASCO UV-Vis spectrophotometer to observe band increases and colour changes. Finally, the colloidal silver metalloenzymes were purified by dialysis using a membrane with a molecular weight cut-off of 14 kDa. The metalloenzymes were kept under stirring for four hours (refreshing the water every 30 min) at room temperature in a beaker containing 1 L of distilled water. After that, the ArME solution (grey colour) was stored in a fridge at 4 °C.

Figure 8.
General procedure for the preparation of ArMEs from mutant enzymes and silver.
3.5. Fluorescence Spectroscopy
In order to confirm the binding of silver metal to the protein, the excitation–emission spectra of the free GTL mutants and (a selection of) the synthesized silver metalloenzymes were determined using a Horiba Scientific Fluoromax spectrofluorometer (Irvine, CA, USA). Measurements were conducted at room temperature using 1 cm optical pitch quartz cuvettes. The excitation wavelength was set to 280 nm, and the excitation and emission bandwidths were set to 5 nm. The fluorescence emission spectra were recorded between 200 and 500 nm. All spectroscopic measurements were performed using an NDQ-50-50 × 50 M filter.
3.6. Average Crystallite Size of AgNPs from XRD Peaks
The average size of metal nanoparticles was determined using the Scherrer formula.
where D represents crystallite size, k is a shape-dependent constant (commonly 0.9), λ is the X-ray wavelength, β is the full width at half-maximum (FWHM), and θ is the Bragg angle. The average size of AgNPs was estimated to be 11 nm (Table 1).
Dhkl = kλ/βdcosθ

Table 1.
Average crystallite size of synthesized AgNPs from XDR data.
3.7. Gel Filtration of GTL–Ag Metalloenzyme
Gel filtration analyses were carried out using a plastic column packed with beaded agarose-4BCL (column size 15 mm × 160 mm; column bed volume 8 mL). The eluting buffer was 10 mM sodium phosphate, at pH 7.0. All separations were carried out at 25 °C with a flow rate of 1.2 mL/min, where 0.1 mL of AgNP-GTL114 was added to the column and eluted with 15 mL of the indicated buffer. The eluate was collected in 0.5 mL aliquots and the reductase-like activity was determined by the pNP assay for the metalloenzyme (see below). The molecular weight of the metalloenzyme was valued using standard proteins, GTL-114 containing 0.5% (v/v) Triton X-100 (43 kDa), laccase solution from M. thermophila expressed in A. oryzae (Novozym 51003) (85 kDa), and Lipase B from Candida antarctica (CALB) (33 KDa).
3.8. Metal Activity Test: Reduction of 4-Nitrophenol to 4-Aminophenol
To verify the catalytic activity of the silver and gold on the artificial metalloenzyme, a p-nitrophenol (pNP) assay was performed. A solution of 2.7 mg pNP in 2 mL of distilled water was prepared to yield a concentration of 1 mM. Subsequently, 3.2 mg of solid NaBH4 was added to the solution, resulting in a transformation of the solution from light yellow to intense yellow due to the formation of 4-nitrophenolate ions (the UV peak of the substrate underwent an immediate shift from 317 to 400 nm). Subsequently, 50 μL of the metalloenzyme was added under gentle stirring at room temperature to initiate the reaction. The progression of the reaction was then monitored by the periodic extraction of an aliquot (200 μL) at various time points (2, 5, 10, 15, 20, 25, and 30 min). This extraction was conducted by diluting the sample in 2 mL of distilled water, and the UV-Vis absorption spectrum was subsequently measured between 500 and 250 nm in a quartz cuvette with a 1 cm optical path length.
3.9. Selective Reduction of Acetophenone to 1-Phenylethanol
The reductive ability and stereoselectivity of the metalloenzymes were evaluated in the selective reduction of acetophenone to 1-phenylethanol. To this end, a 1 mM acetophenone solution was prepared in 2 mL of distilled water. Subsequently, under constant agitation, 50 μL of a 0.5 mg/mL NaBH4 solution and 50 μL of the corresponding silver metalloenzyme were added. The progression of the reaction was then monitored by normal-phase HPLC after the indicated time (2 h or 24 h) using a Phenomenex Lux 3u Cellulose column (250 × 460 mm) (Phenomenex, Torrance, CA, USA) that allowed for chiral recognition. The flow rate was set to 0.5 mL/min, the mobile phase was 90:10 hexane/isopropanol, and UV was detected at a wavelength of 220 nm. Prior to injection, each sample was extracted with hexane at a 1:1 (v/v) ratio. The extraction coefficients (Kd) for acetophenone, (R)-1-phenylethanol, and (S)-1-phenylethanol were determined to be 0.52, 0.72, and 0.79, respectively. Under these conditions, the retention times of acetophenone, (R)-1-phenyl ethanol, and (S)-1-phenyl ethanol were 10.5 min, 13.4 min, and 15.1 min, respectively. Substrates of commercially available purity were utilized as standards.
3.10. Selective Oxidation of 1-Phenylethanol to Acetophenone
The oxidative ability and stereoselectivity of the metalloenzymes were evaluated in the selective oxidation of 1-phenylethanol to acetophenone. The procedure described in the selective reduction of acetophenone to 1-phenylethanol was applied with the following changes: first, a 1 mM (R/S)-1-phenylethanol solution was prepared in 2 mL of distilled water. Then, under constant stirring, 20 μL of 33% w/v H2O2 and 50 μL of the corresponding silver metalloenzyme were added. The procedure for HPLC analysis remained unchanged.
4. Conclusions
In this work, a novel colloidal Ag metalloenzyme has been prepared based on the in situ formation of silver nanoparticles. The method is based on a great affinity to thiol and Ag, introducing by genetic tools cysteine in the active site of GTL as an anchoring point. However, experimental conditions generate a novel Ag metalloenzyme, where silver nanoparticles were formed initially in the cysteine but were also able to coordinate to different residues generating different silver clusters. In all cases, the nanoparticles were Ag2ONPs with an average dimeter size of around 11 nm, with a final size of 60 nm for the bioconjugate. This Ag-GTL114 metalloenzyme showed excellent redox activity instead of the esterase one (loss by the genetic modification), a reductive activity against nitroarenes (>90% conversion in 10 min) and against acetophenone to phenyl ethanol (>95% in 2 h). However, the enantioselectivity of the enzyme in the latter process was moderate, ee:2.5%, against S enantiomer in aqueous media, probably considering the effect of the AgNPs in the different part of the enzymes or the presence of oligomer systems. However, the efficiency in the oxidation of phenyl ethanol to ketone was high, with a >50% conversion with 25% yield. These results demonstrate the capacity to create a new type of silver metalloenzyme, transforming a lipase in a redox enzyme, although future experiments focused on generating the nanoparticle exclusively on the active site and therefore being 100% affected by the second coordination sphere of the protein structure to improve selectivity, favouring one enantiomer against the another, will be performed.
Additional experiments looking for other positions where the cysteine can be introduced, allowing the lipase active site to remain intact, could be interesting for metal–enzymatic cascade processes [33].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15010061/s1, Video S1: Colloidal metalloenzyme production and catalytic application.
Author Contributions
Conceptualization, J.M.P.; methodology G.B. and C.G.-S., software, G.B.; validation, J.M.P., G.B. and C.G.-S.; formal analysis, J.M.P., G.B. and C.G.-S.; investigation, J.M.P., G.B. and C.G.-S.; resources, J.M.P.; data curation, J.M.P. and G.B.; writing—original draft preparation, J.M.P. and G.B.; writing—review and editing, J.M.P., G.B. and C.G.-S.; supervision, J.M.P.; funding acquisition, J.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the funding support from the Spanish National Research Council (CSIC) and the European Commission for funding the project, grant no. 101060130, HORIZON-WIDERA-2021-ACCESS-02-01.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the European Commission for funding the TwinPrebioEnz project. We thank de las Rivas (ICTAN-CSIC) for the technical preparation of the enzyme variant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cornils, B.; Herrmann, W.A.; Wong, C.H.; Zanthoff, H.-W. Catalysis from A to Z; John Wiley & Sons: Hoboken, NJ, USA, 2020; 2952p, ISBN 9783527343119. [Google Scholar]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2007; Print ISBN 9783527307159; Online ISBN 9783527611003. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J. (Eds.) Handbook of Green Chemistry and Technology; Blackwell Science Ltd.: Hoboken, NJ, USA, 2002; Print ISBN 9780632057153; Online ISBN 9780470988305. [Google Scholar] [CrossRef]
- Murakami, Y.; Kikuchi, J.I.; Hisaeda, Y.; Hayashida, O. Artificial enzymes. Chem. Rev. 1996, 96, 721–758. [Google Scholar] [CrossRef]
- Davis, H.J.; Ward, T.R. Artificial Metalloenzymes: Challenges and Opportunities. ACS. Cent. Sci. 2019, 5, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Hanreich, S.; Bonandi, E.; Drienovská, I. Design of Artificial Enzymes: Insights into Protein Scaffolds. ChemBioChem 2023, 24, e20220056. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Bucholz, K. Highlights in biocatalysis—Historical landmarks and current trends. Eng. Life Sci. 2005, 5, 309–323. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- Wang, M.; Si, T.; Zhao, H. Biocatalyst Development by Directed Evolution. Bioresour. Technol. 2012, 115, 117–125. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [PubMed]
- Naapuri, J.M.; Losada-Garcia, N.; Deska, J.; Palomo, J.M. Synthesis of silver and gold nanoparticles–enzyme–polymer conjugate hybrids as dual-activity catalysts for chemoenzymatic cascade reactions. Nanoscale 2022, 14, 5701–5715. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Romero, O.; Guitiérrez-Fernández, J.; de las Rivas, B.; Hermoso, J.A.; Palomo, J.M. Synthesis of a heterogeneous artificial metallolipase with chimeric catalytic activity. Chem. Commun. 2015, 51, 9324–9327. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M. Artificial enzymes with multiple active sites. Curr. Opin. Green. Sustain. Chem. 2021, 29, 100452. [Google Scholar] [CrossRef]
- Klein, A.S.; Zeymer, C. Design and engineering of artificial metalloproteins: From de novo metal coordination to catalysis. PEDS 2021, 34, gzab003. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.D.; Serrano-Plana, J.; Peterson, R.L.; Ward, T.R. Artificial Metalloenzymes Based on the Biotin-Streptavidin Technology: Enzymatic Cascades and Directed Evolution. Acc. Chem. Res. 2019, 52, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; de las Rivas, B.; Lopez-Tejedor, D.; Palomo, J.M. Effect of Site-Specific Peptide-Tag Labeling on the Biocatalytic Properties of Thermoalkalophilic Lipase from Geobacillus thermocatenulatus. ChemBioChem 2018, 19, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, Y.; Wang, L.; Mu, X.; Zhao, Q.; Si, R.; Zhu, X.; Chen, S.; Zhang, B.; Chen, D.; et al. Gold catalysts containing interstitial carbon atoms boost hydrogenation activity. Nat. Commun. 2020, 11, 4600. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castillo, J.E.; Gallo-Villanueva, R.C.; Madou, M.J.; Perez-Gonzalez, V.H. Anisotropic gold nanoparticles: A survey of recent synthetic methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Y.; Zhang, Y.; Tang, P. Silver-catalyzed dibromotrifluoromethoxylation of terminal alkynes. ACS Catal. 2021, 11, 3218–3223. [Google Scholar] [CrossRef]
- Dong, X.Y.; Gao, Z.W.; Yang, K.F.; Zhang, W.Q.; Xu, L.W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; Morales, M.D.P.; Palomo, J.M. Synthesis of heterogeneous enzyme–metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef]
- Sekine, K.; Yamada, T. Silver-catalyzed carboxylation. Chem. Soc. Rev. 2016, 45, 4524–4532. [Google Scholar] [CrossRef]
- Li, A.Y.; Gellé, A.; Segalla, A.; Moores, A. Silver Nanoparticles in Organic Transformations. Silver Catal. Org. Synth. 2018, 1, 723–793. [Google Scholar]
- Naodovic, M.; Yamamoto, H. Asymmetric silver-catalyzed reactions. Chem. Rev. 2008, 108, 3132–3148. [Google Scholar] [CrossRef]
- Pagliaro, M.; Della Pina, C.; Mauriello, F.; Ciriminna, R. Catalysis with Silver: From Complexes and Nanoparticles to MORALs and Single-Atom Catalysts. Catalysts 2020, 10, 1343. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Godoy, C.; de Las Rivas, B.; Fernández-Lorente, G.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R.; Martínez-Ripolla, M.; Hermoso, J.A. Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, C.; de las Rivas, B.; Palomo, J.M. Design of a gold nanoparticle-site in an engineered lipase: An artificial metalloenzyme with enantioselective reductase-like activity. Nanoscale 2024, 16, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Wang, M.; Hu, M.; Xu, X.; Yan, A.; Hao, Q.; Lia, H.; Sun, H. Atomic differentiation of silver binding preference in protein targets: Escherichia coli malate dehydrogenase as a paradigm. Chem. Sci. 2020, 11, 11714–11719. [Google Scholar] [CrossRef]
- Khan, K.; AL-Thabaiti, S.A.; Obaid, A.Y.; Khan, Z.A.; Al-Youbi, A.O. Effects of solvents on the stability and morphology of CTAB-stabilized silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 120–125. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans. Plasma Sci. 2006, 34, 984–995. [Google Scholar] [CrossRef]
- Losada-García, N.; Urriolabeitia, E.; Palomo, J.M. Solid-phase lipase-CuNPs biohybrids as catalysts for one-pot parallel synthesis of 2,3,4-tryacetyl-D-gluconic acid. ChemCatChem 2023, 15, e202201632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).