Molybdenum Telluride-Promoted BiOCl Photocatalysts for the Degradation of Sulfamethoxazole Under Solar Irradiation: Kinetics, Mechanism, and Transformation Products
Abstract
1. Introduction
2. Results
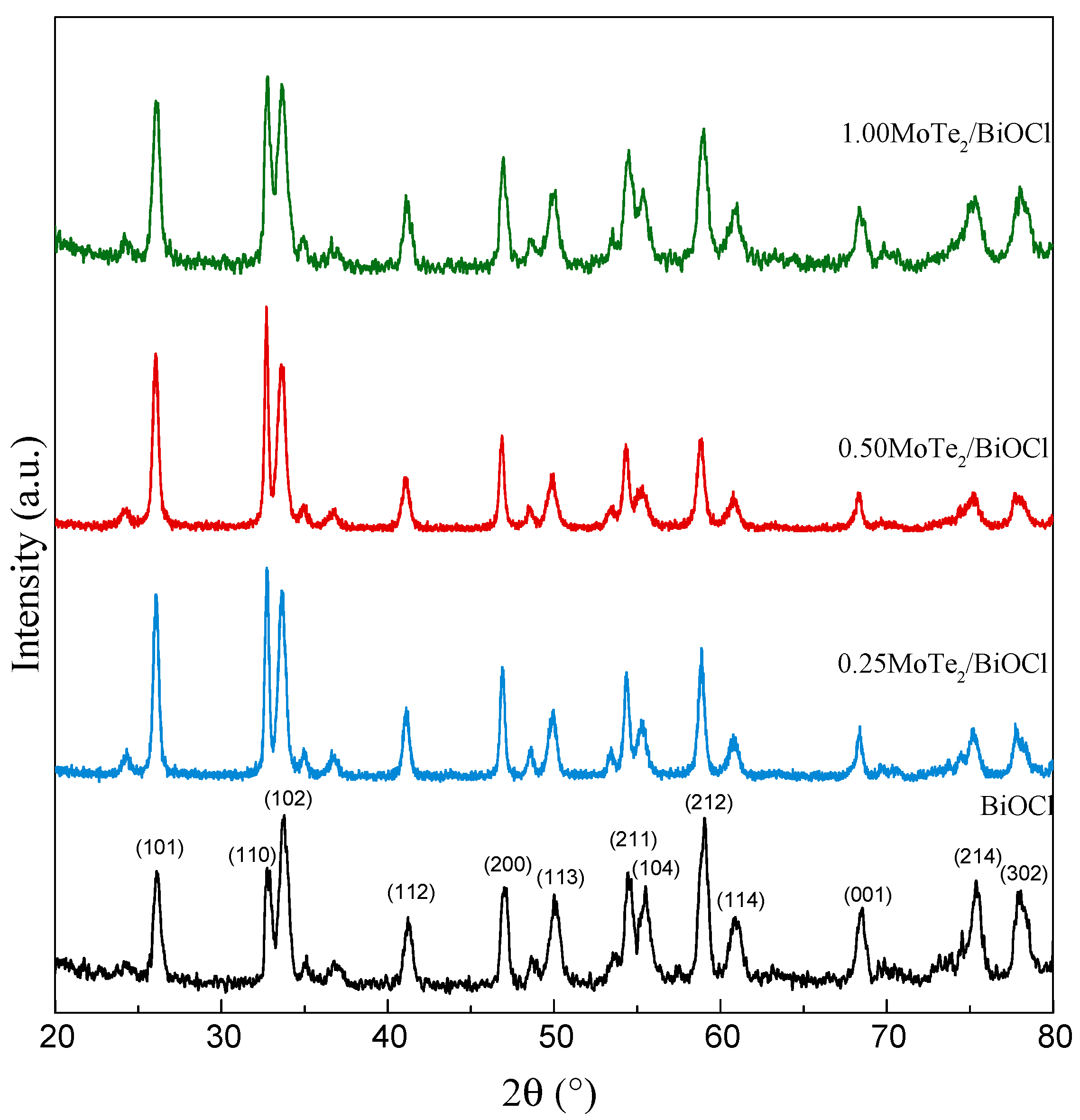
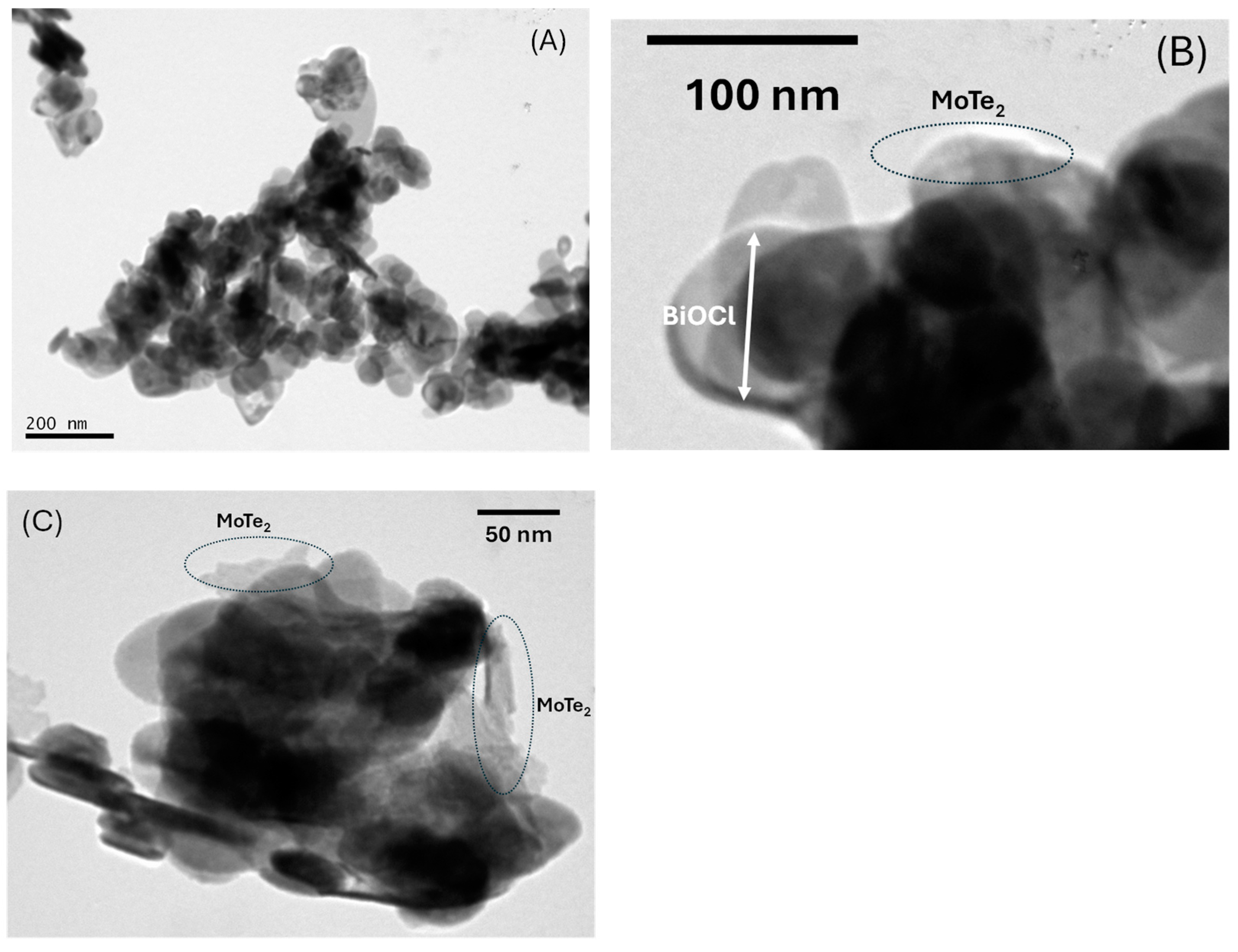
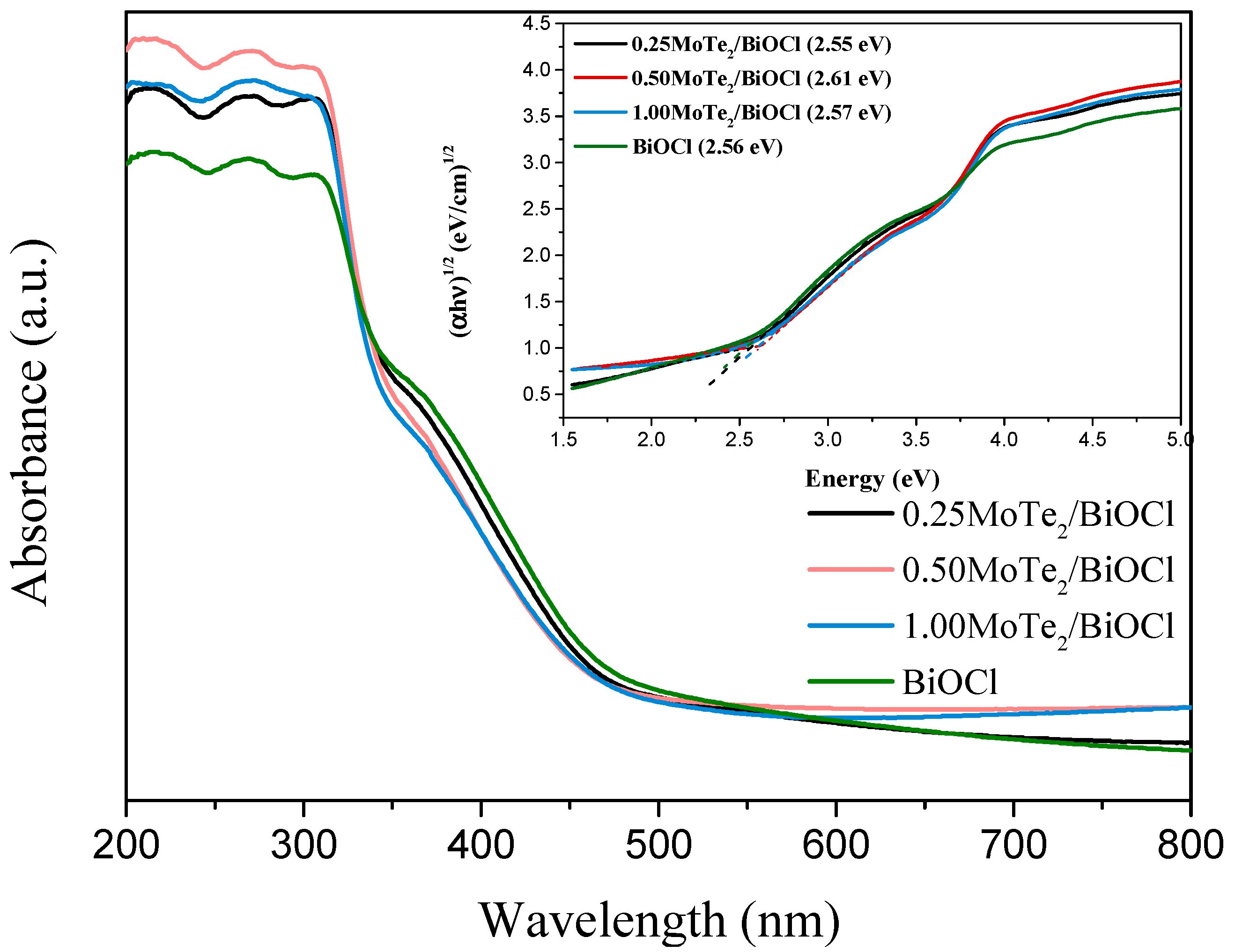
2.1. Physicochemical and Optical Characterization
2.2. Photocatalytic Results
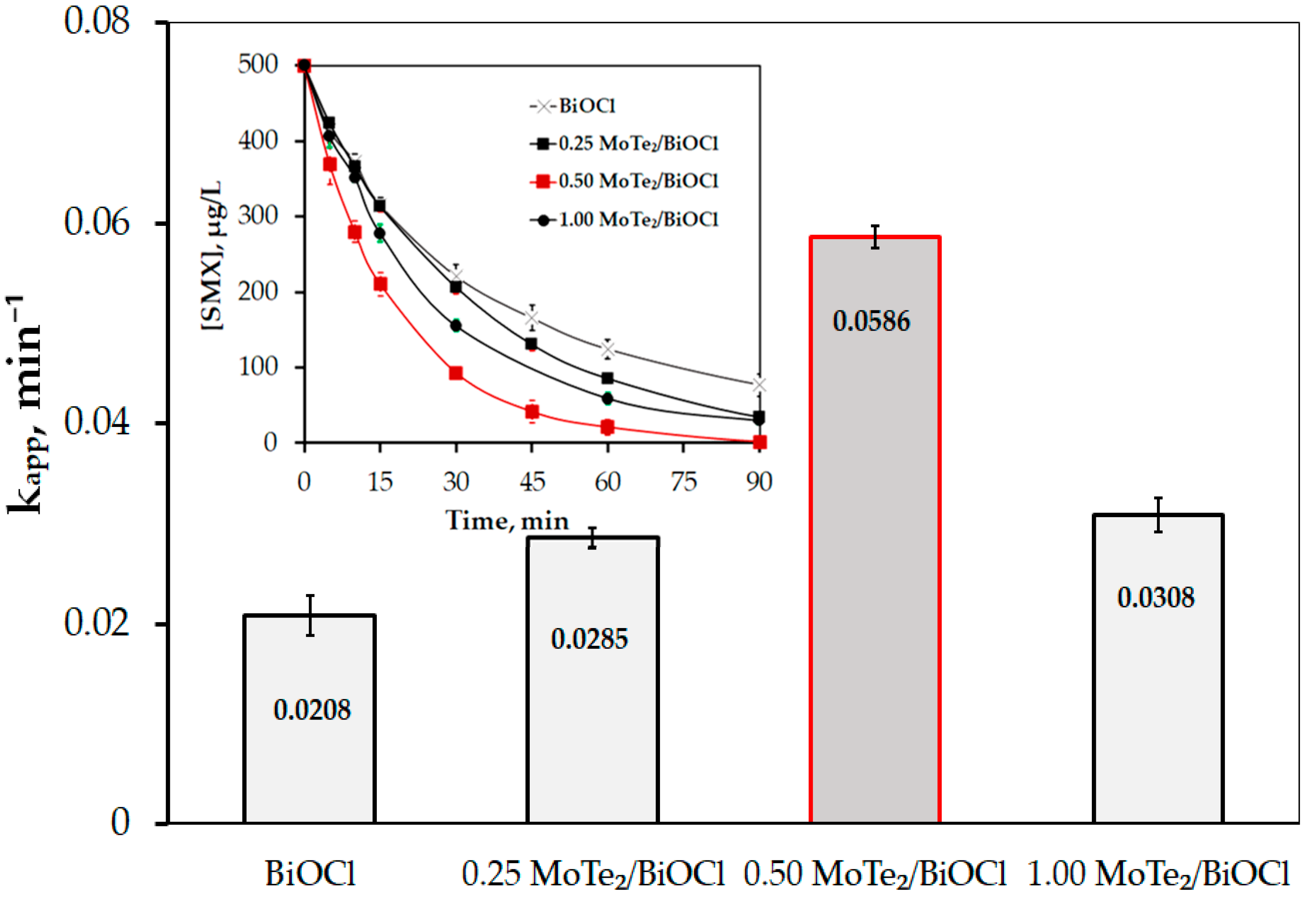
2.2.1. Photocatalytic Performance of MoTe2/BiOCl
2.2.2. Effect of Initial Concentration of 0.5 MoTe2/BiOCl and SMX
2.2.3. Effect of Initial pH and Evaluation of Reactive Species Contribution Using Scavengers
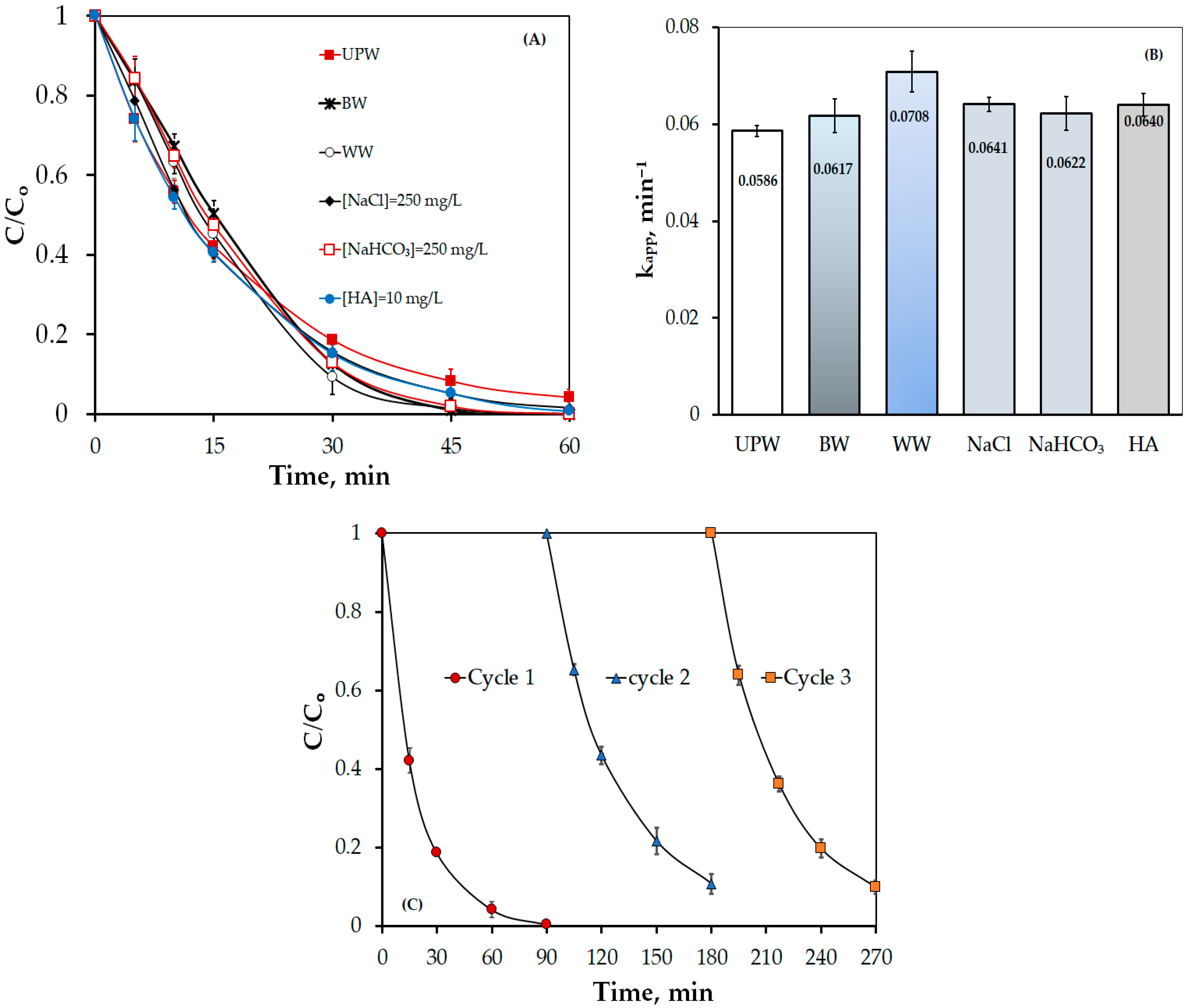
2.2.4. Effect of Water Matrix
2.2.5. Reuse of Photocatalysts
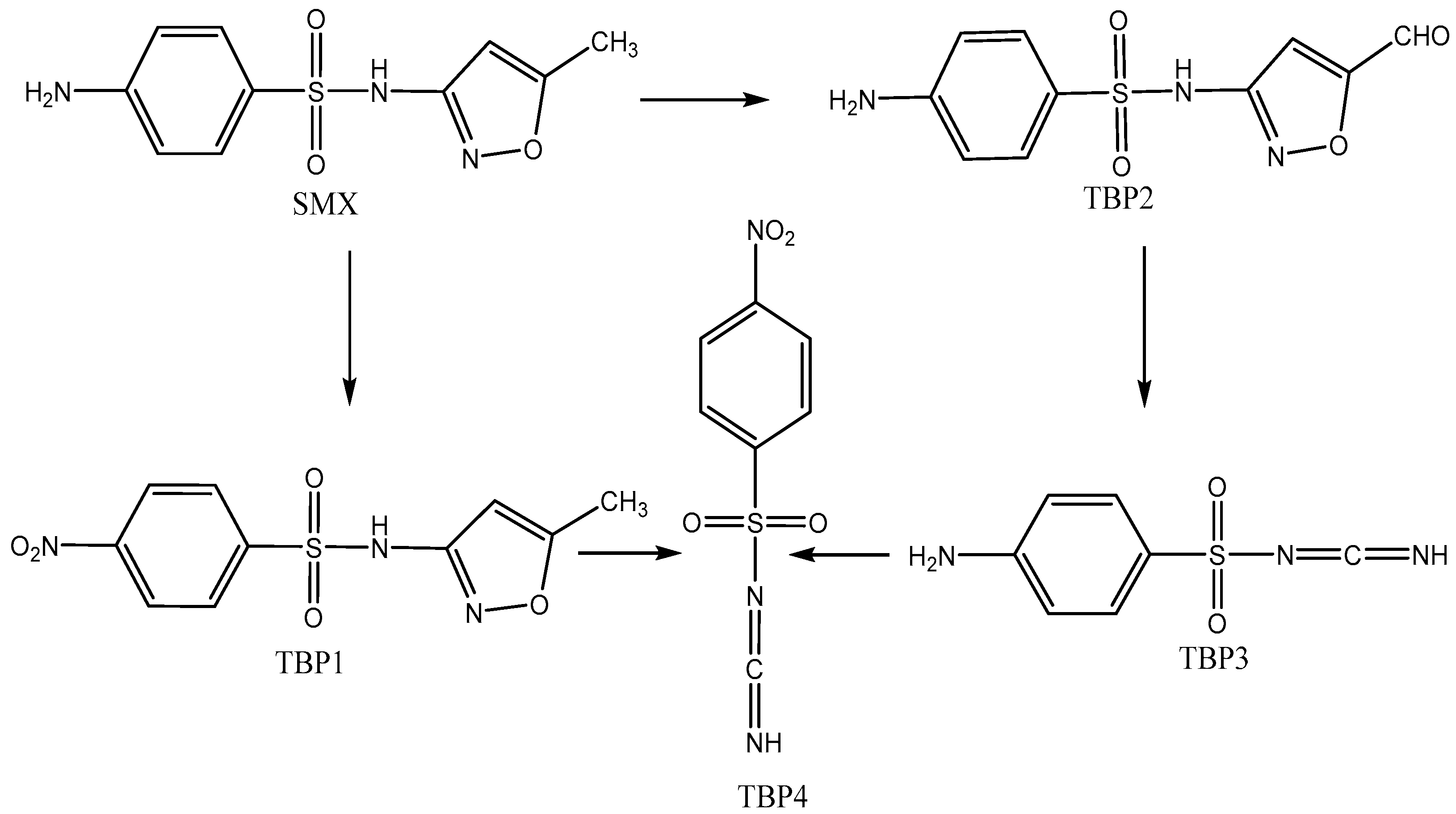
2.3. Proposed Transformation Pathways
3. Materials and Methods
3.1. Chemical Reagents
3.2. Photocatalysts Preparation Procedure and Characterization
3.3. Analytical Determination
3.4. Photocatalytic Tests
3.5. Identification of Transformation By-Products (TBPs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and Removal of Pharmaceutical and Personal Care Products from Aquatic Systems Using Advanced Treatment—A Review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Deo, R.P. Pharmaceuticals in the Surface Water of the USA: A Review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- Vatovec, C.; Kolodinsky, J.; Callas, P.; Hart, C.; Gallagher, K. Pharmaceutical Pollution Sources and Solutions: Survey of Human and Veterinary Medication Purchasing, Use, and Disposal. J. Environ. Manag. 2021, 285, 112106. [Google Scholar] [CrossRef] [PubMed]
- Letsinger, S.; Kay, P.; Rodríguez-Mozaz, S.; Villagrassa, M.; Barceló, D.; Rotchell, J.M. Spatial and Temporal Occurrence of Pharmaceuticals in UK Estuaries. Sci. Total Environ. 2019, 678, 74–84. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals in the Marine Environment: A Review. Environ. Rev. 2018, 27, 151–165. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and Antibiotic Resistant Genes (ARGs) in Groundwater: A Global Review on Dissemination, Sources, Interactions, Environmental and Human Health Risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Batt, A.L.; Benson, R.; Boone, J.S.; Conerly, O.; Donohue, M.J.; King, D.N.; Kostich, M.S.; et al. Nationwide Reconnaissance of Contaminants of Emerging Concern in Source and Treated Drinking Waters of the United States. Sci. Total Environ. 2017, 581–582, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chang, J.; Fu, B.; Wang, H. Insight into Advanced Oxidation Processes for the Degradation of Fluoroquinolone Antibiotics: Removal, Mechanism, and Influencing Factors. Sci. Total Environ. 2023, 857, 159172. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Falara, P.P.; Likodimos, V. Photocatalytic Degradation of Pharmaceuticals and Organic Contaminants of Emerging Concern Using Nanotubular Structures. Curr. Opin. Green Sustain. Chem. 2021, 29, 100470. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of Solar Light-Induced Photocatalytic Degradation of Azo Dyes in Aqueous TiO2 Suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient Production of Hydrogen by Photo-Induced Reforming of Glycerol at Ambient Conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2019, 378, 7. [Google Scholar] [CrossRef] [PubMed]
- Roslan, N.N.; Lau, H.L.; Suhaimi, N.A.A.; Shahri, N.N.M.; Verinda, S.B.; Nur, M.; Lim, J.-W.; Usman, A. Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review. Catalysts 2024, 14, 189. [Google Scholar] [CrossRef]
- Serra-Pérez, E.; Dražić, G.; Takashima, M.; Ohtani, B.; Kovačič, S.; Žerjav, G.; Tušar, N.N. Influence of the Surface Structure of the TiO2 Support on the Properties of the Au/TiO2 Photocatalyst for Water Treatment under Visible Light. Catal. Today 2024, 437, 114764. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Cui, Y.; Fu, X.; Wang, Y. Recent Progress in Copper-Based Inorganic Nanostructure Photocatalysts: Properties, Synthesis and Photocatalysis Applications. Mater. Today Sustain. 2023, 21, 100276. [Google Scholar] [CrossRef]
- Monfort, O.; Pop, L.-C.; Sfaelou, S.; Plecenik, T.; Roch, T.; Dracopoulos, V.; Stathatos, E.; Plesch, G.; Lianos, P. Photoelectrocatalytic Hydrogen Production by Water Splitting Using BiVO4 Photoanodes. Chem. Eng. J. 2016, 286, 91–97. [Google Scholar] [CrossRef]
- Sajjad, S.; Leghari, S.A.K.; Zhang, J. Nonstoichiometric Bi2O3: Efficient Visible Light Photocatalyst. RSC Adv. 2013, 3, 1363–1367. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, D.; Liu, C.; Zhou, X.; Yang, H.; Qu, J.; He, D. Efficient Photocatalytic Water Disinfection by a Novel BP/BiOBr S-Scheme Heterojunction Photocatalyst. Chem. Eng. J. 2023, 468, 143581. [Google Scholar] [CrossRef]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent Advances in Bismuth-Based Photocatalysts: Environment and Energy Applications. Green Energy Environ. 2022, 8, 1232. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Zhang, Y.; Gu, Y.; An, Y. BiOCl-Based Photocatalysts: Synthesis Methods, Structure, Property, Application, and Perspective. Inorg. Chem. Commun. 2022, 138, 109277. [Google Scholar] [CrossRef]
- Dellinger, T.M.; Braun, P. V BiOCl Nanoparticles Synthesized in Lyotropic Liquid Crystal Nanoreactors. Scr. Mater. 2001, 44, 1893–1897. [Google Scholar] [CrossRef]
- Ye, P.; Xie, J.; He, Y.; Zhang, L.; Wu, T.; Wu, Y. Hydrolytic Synthesis of Flowerlike BiOCl and Its Photocatalytic Performance under Visible Light. Mater. Lett. 2013, 108, 168–171. [Google Scholar] [CrossRef]
- Cao, S.; Guo, C.; Lv, Y.; Guo, Y.; Liu, Q. A Novel BiOCl Film with Flowerlike Hierarchical Structures and Its Optical Properties. Nanotechnology 2009, 20, 275702. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhao, X.; Liu, M.; Gao, Z.; Yang, D.; Shi, L.; Tang, Y.; Song, H. BiOCl/TiO2 Composite Photocatalysts Synthesized by the Sol–Gel Method for Enhanced Visible-Light Catalytic Activity toward Methyl Orange. J. Mater. Res. 2020, 35, 3067–3078. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Liu, Y.; Zhang, L. Photocatalytic Properties BiOCl and Bi2O3 Nanofibers Prepared by Electrospinning. Scr. Mater. 2008, 59, 332–335. [Google Scholar] [CrossRef]
- Ye, L.; Deng, Y.; Wang, L.; Xie, H.; Su, F. Bismuth-Based Photocatalysts for Solar Photocatalytic Carbon Dioxide Conversion. ChemSusChem 2019, 12, 3671–3701. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, Y.; Wu, S.; Zhu, Y.; Chen, H.; Yu, X.; Zhang, Y. Facile Fabrication of BiOI/BiOCl Immobilized Films with Improved Visible Light Photocatalytic Performance. Front. Chem. 2018, 6, 58. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.; Zhang, Z.; Jiang, C.; Zuojiao, K.; Liu, J.; Wang, Y. Kinetics and Mechanism Insights into the Photodegradation of Tetracycline Hydrochloride and Ofloxacin Mixed Antibiotics with the Flower-like BiOCl/TiO2 Heterojunction. J. Photochem. Photobiol. A Chem. 2019, 378, 114–124. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhao, Y.; Sun, C.; Xu, H.; Zhao, J. PVP-Induced Bi2S3/BiOCl Photocatalyst with Open Hollow Structures for the Removal of Ciprofloxacin under Visible-Light Irradiation. J. Alloys Compd. 2021, 861, 157995. [Google Scholar] [CrossRef]
- Shinde, P.V.; Hussain, M.; Moretti, E.; Vomiero, A. Advances in Two-Dimensional Molybdenum Ditelluride (MoTe2): A Comprehensive Review of Properties, Preparation Methods, and Applications. SusMat 2024, 4, e236. [Google Scholar] [CrossRef]
- Yu, S.; Wu, X.; Wang, Y.; Guo, X.; Tong, L. 2D Materials for Optical Modulation: Challenges and Opportunities. Adv. Mater. 2017, 29, 1606128. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Yang, P. WS2/MoSe2 van der Waals Heterojunctions Applied to Photocatalysts for Overall Water Splitting. J. Colloid Interface Sci. 2023, 650, 1312–1318. [Google Scholar] [CrossRef]
- Singla, S.; Singh, P.; Basu, S.; Devi, P. BiVO4/MoSe2 Photocatalyst for the Photocatalytic Abatement of Tetracycline and Photoelectrocatalytic Water Splitting. Mater. Chem. Phys. 2023, 295, 127111. [Google Scholar] [CrossRef]
- Yao, C.; Wu, D.; Yuan, C.; Xue, X.; Liu, L.; Zhang, X. Design, Preparation, and Photocatalytic Performance of MoSe2 Quantum Dots Modified BiOCl Composite Photocatalysts. Opt. Mater. 2024, 147, 114745. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Xu, Y.; Wang, S.-T.; Li, S.-L.; Yamamoto, M.; Aparecido-Ferreira, A.; Li, W.; Sun, H.; Nakaharai, S.; Jian, W.-B.; et al. Ambipolar MoTe2 Transistors and Their Applications in Logic Circuits. Adv. Mater. 2014, 26, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lin, Q.; Xiao, S.; Li, J.; Fang, H. Optically Active Telecom Defects in MoTe2 Fewlayers at Room Temperature. Nanomaterials 2023, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; Jindal, A.; Yuan, N.F.Q.; Jung, Y.; Antony, A.; Wang, H.; Kim, B.; Chiu, Y.-C.; Taniguchi, T.; Watanabe, K.; et al. Enhanced Superconductivity in Monolayer Td-MoTe2. Nano Lett. 2021, 21, 2505–2511. [Google Scholar] [CrossRef]
- Octon, T.J.; Nagareddy, V.K.; Russo, S.; Craciun, M.F.; Wright, C.D. Fast High-Responsivity Few-Layer MoTe2 Photodetectors. Adv. Opt. Mater. 2016, 4, 1750–1754. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Wang, P.; Kuang, A.; Zhou, T.; Yuan, H.; Chen, H. Bilayer MoTe2/XS2 (X = Hf,Sn,Zr) Heterostructures with Efficient Carrier Separation and Light Absorption for Photocatalytic Water Splitting into Hydrogen. Appl. Surf. Sci. 2021, 544, 148842. [Google Scholar] [CrossRef]
- Yuan, Z.; Xiang, Y.; Jian, X.; Zhang, H.; Liu, M.; Cao, R.; Hu, Y.; Gao, X. Zn0.5Cd0.5S/MoTe2 Z-Scheme Heterojunction with Space-Separated Oxidation-Reduction Catalytic Sites for Photocatalytic CO2 Reduction. Sep. Purif. Technol. 2025, 359, 129836. [Google Scholar] [CrossRef]
- Kouvelis, K.; Karavaka, E.E.; Panagiotaras, D.; Papoulis, D.; Frontistis, Z.; Petala, A. Photocatalytic Degradation of Losartan with BiOCl/Sepiolite Nanocomposites. Catalysts 2024, 14, 433. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chang, M.-J.; Sun, M.; Zhang, C.-M.; Yang, L.-Q.; Du, H.-L.; Luo, Z.-M. Facile Synthesis of BiOCl with Extremely Superior Visible Light Photocatalytic Activity Synergistically Enhanced by Co Doping and Oxygen Vacancies. Sep. Purif. Technol. 2022, 301, 121953. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Giannakopoulos, S.; Petala, A.; Frontistis, Z.; Mantzavinos, D. Fabrication of a Novel MoB/BiOCl Photocatalyst for Losartan and Escherichia coli Removal. Catal. Today 2024, 430, 114510. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Chang, M.; Song, Y.; Sun, Q.; Wang, F.; Zou, H.; Shi, Z. Photoluminescence Properties and Photocatalytic Activities of SiO2@TiO2:Sm3+ Nanomaterials. J. Phys. Chem. Solids 2021, 149, 109775. [Google Scholar] [CrossRef]
- Ma, D.; Zhong, J.; Peng, R.; Li, J.; Duan, R. Effective Photoinduced Charge Separation and Photocatalytic Activity of Hierarchical Microsphere-like C60/BiOCl. Appl. Surf. Sci. 2019, 465, 249–258. [Google Scholar] [CrossRef]
- Ioannidi, A.; Petala, A.; Frontistis, Z. Copper Phosphide Promoted BiVO4 Photocatalysts for the Degradation of Sulfamethoxazole in Aqueous Media. J. Environ. Chem. Eng. 2020, 8, 104340. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, X.; Ma, C.; Huang, H.; Yu, K.; Liu, Y.; Lu, Z.; Li, C.; Zhu, Z.; Huo, P. A 2D Mesoporous Photocatalyst Constructed by the Modification of Biochar on BiOCl Ultrathin Nanosheets for Enhancing the TC-HCl Degradation Activity. New J. Chem. 2020, 44, 79–86. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p–n Heterojunction with Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Abellán, M.N.; Giménez, J.; Esplugas, S. Photocatalytic Degradation of Antibiotics: The Case of Sulfamethoxazole and Trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Kanigaridou, Y.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Solakidou, M.; Konstantinou, I.; Deligiannakis, Y.; Mantzavinos, D.; Kondarides, D.I. Solar Photocatalytic Degradation of Bisphenol A with CuOx/BiVO4: Insights into the Unexpectedly Favorable Effect of Bicarbonates. Chem. Eng. J. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Outsiou, A.; Frontistis, Z.; Ribeiro, R.S.; Antonopoulou, M.; Konstantinou, I.K.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T.; Mantzavinos, D. Activation of sodium persulfate by magnetic carbon xerogels (CX/CoFe) for the oxidation of bisphenol A: Process variables effects, matrix effects and reaction pathways. Water Res. 2017, 124, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Ioannidi, A.; Frontistis, Z.; Antonopoulou, M.; Tselios, C.; Tsikritzis, D.; Konstantinou, I.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Correlating the Properties of Hydrogenated Titania to Reaction Kinetics and Mechanism for the Photocatalytic Degradation of Bisphenol A under Solar Irradiation. Appl. Catal. B Environ. 2016, 188, 65–76. [Google Scholar] [CrossRef]
- Petala, A.; Arvaniti, O.S.; Travlou, G.; Mantzavinos, D.; Frontistis, Z. Solar Light Induced Photocatalytic Removal of Sulfamethoxazole from Water and Wastewater Using BiOCl Photocatalyst. J. Environ. Sci. Health Part A 2021, 56, 963–972. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Andrews, N.L.P.; Fan, J.Z.; Forward, R.L.; Chen, M.C.; Loock, H.-P. Determination of the Thermal, Oxidative and Photochemical Degradation Rates of Scintillator Liquid by Fluorescence EEM Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange by ZnO:Eu Nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Bancirova, M. Sodium Azide as a Specific Quencher of Singlet Oxygen during Chemiluminescent Detection by Luminol and Cypridina Luciferin Analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef]
- Lee, J.; Kok, S.H.W.; Ng, B.-J.; Kong, X.Y.; Putri, L.K.; Chai, S.-P.; Tan, L.-L. Hole Scavenger-Free Nitrogen Photofixation in Pure Water with Non-Metal B-Doped Carbon Nitride: Implicative Importance of B Species for N2 Activation. J. Environ. Chem. Eng. 2023, 11, 109511. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. A Critical View about Use of Scavengers for Reactive Species in Heterogeneous Photocatalysis. Appl. Catal. A Gen. 2024, 685, 119879. [Google Scholar] [CrossRef]
- Ali, S.A.; Majumdar, S.; Chowdhury, P.K.; Alshehri, S.M.; Ahmad, T. Ultrafast Charge Transfer Dynamics in Multifaceted Quaternary Te–MoTe2–MoS2/ZnO S-Scheme Heterostructured Nanocatalysts for Efficient Green Hydrogen Energy. ACS Appl. Energy Mater. 2024, 7, 7325–7337. [Google Scholar] [CrossRef]
- Lan, Y.; Xia, L.-X.; Huang, T.; Xu, W.; Huang, G.-F.; Hu, W.; Huang, W.-Q. Strain and Electric Field Controllable Schottky Barriers and Contact Types in Graphene-MoTe2 van Der Waals Heterostructure. Nanoscale Res. Lett. 2020, 15, 180. [Google Scholar] [CrossRef]
- Jones, L.A.H.; Xing, Z.; Swallow, J.E.N.; Shiel, H.; Featherstone, T.J.; Smiles, M.J.; Fleck, N.; Thakur, P.K.; Lee, T.-L.; Hardwick, L.J.; et al. Band Alignments, Electronic Structure, and Core-Level Spectra of Bulk Molybdenum Dichalcogenides (MoS2, MoSe2, and MoTe2). J. Phys. Chem. C 2022, 126, 21022–21033. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Blaise, C.; André, C. Occurrence of Pharmaceutical Products in a Municipal Effluent and Toxicity to Rainbow Trout (Oncorhynchus mykiss) Hepatocytes. Ecotoxicol. Environ. Saf. 2006, 64, 329–336. [Google Scholar] [CrossRef]
- Hou, J.; Dai, D.; Wei, R.; Wu, X.; Wang, X.; Tahir, M.; Zou, J.-J. Narrowing the Band Gap of BiOCl for the Hydroxyl Radical Generation of Photocatalysis under Visible Light. ACS Sustain. Chem. Eng. 2019, 7, 16569–16576. [Google Scholar] [CrossRef]
- Shen, T.; Shi, X.; Guo, J.; Li, J.; Yuan, S. Photocatalytic Removal of NO by Light-Driven Mn3O4/BiOCl Heterojunction Photocatalyst: Optimization and Mechanism. Chem. Eng. J. 2021, 408, 128014. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Petala, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Copper Phosphide and Persulfate Salt: A Novel Catalytic System for the Degradation of Aqueous Phase Micro-Contaminants. Appl. Catal. B Environ. 2019, 244, 178–187. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Lee, K.; Chen, N.; Kim, M.S.; Seo, J.; Lee, D.; Cho, H.; Kim, H.; Lee, J.; et al. Bicarbonate-Enhanced Generation of Hydroxyl Radical by Visible Light-Induced Photocatalysis of H2O2 over WO3: Alteration of Electron Transfer Mechanism. Chem. Eng. J. 2022, 432, 134401. [Google Scholar] [CrossRef]
- Tomara, T.; Frontistis, Z.; Petala, A.; Mantzavinos, D. Photocatalytic Performance of Ag2O towards Sulfamethoxazole Degradation in Environmental Samples. J. Environ. Chem. Eng. 2019, 7, 103177. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of water matrix on the photocatalytic removal of pharmaceuticals by visible light active materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Wu, J.-H.; Yu, H.-Q. Confronting the Mysteries of Oxidative Reactive Species in Advanced Oxidation Processes: An Elephant in the Room. Environ. Sci. Technol. 2024, 58, 18496–18507. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Parwaz Khan, A.A.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Djaballah, M.L.; Belghit, A.; Dehane, A.; Merouani, S.; Hamdaoui, O.; Ashokkumar, M. Radicals (OH, Cl, ClO and Cl2−) Concentration Profiles in the Intensified Degradation of Reactive Green 12 by UV/Chlorine Process: Chemical Kinetic Analysis Using a Validated Model. J. Photochem. Photobiol. A Chem. 2023, 439, 114557. [Google Scholar] [CrossRef]
- Jawad, A.; Lang, J.; Liao, Z.; Khan, A.; Ifthikar, J.; Lv, Z.; Long, S.; Chen, Z.; Chen, Z. Activation of Persulfate by CuOx@Co-LDH: A Novel Heterogeneous System for Contaminant Degradation with Broad PH Window and Controlled Leaching. Chem. Eng. J. 2018, 335, 548–559. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Zhou, C.; Bai, J.; Chen, S.; Li, J.; Wang, J.; Guan, X.; Rahim, M.; Zhou, B. Exhaustive Denitrification via Chlorine Oxide Radical Reactions for Urea Based on a Novel Photoelectrochemical Cell. Water Res. 2020, 170, 115357. [Google Scholar] [CrossRef]
- Repousi, V.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photocatalytic Degradation of Bisphenol A over Rh/TiO2 Suspensions in Different Water Matrices. Catal. Today 2017, 284, 59–66. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and Characterization of CoOx/BiVO4 Photocatalysts for the Degradation of Propyl Paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized Degradation of Amoxicillin in Natural Organic Matter Isolate Solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Enriquez, R.; Pichat, P. Interactions of Humic Acid, Quinoline, and TiO2 in Water in Relation to Quinoline Photocatalytic Removal. Langmuir 2001, 17, 6132–6137. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary Magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 Nano-Junction for Visible Light and Solar Powered Degradation of Sulfamethoxazole from Aqueous Environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Wang, K.; Yu, X.; Liu, Z.; Zhang, T.; Ma, Y.; Niu, J.; Yao, B. Interface Engineering of 0D/2D Cu2O/BiOBr Z-Scheme Heterojunction for Efficient Degradation of Sulfamethoxazole: Mechanism, Degradation Pathway, and DFT Calculation. Chemosphere 2024, 346, 140596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Z.; Zhao, T.; He, N.; Tan, Q.; Song, Y.; Chen, Y. The Degradation of Sulfamethoxazole Using a Heterojunction Photocatalytic Membrane Composed of Z-Scheme LaFeO3/Ag3PO4@GO: Assessment of Activity and Degradation Mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 705, 135733. [Google Scholar] [CrossRef]
- Shen, T.; Zeng, D.; Liu, Z.; Hu, Y.; Tian, Y.; Song, J.; Yang, T.; Guan, R.; Zhou, C. S-Scheme Bi/Bi2WO6/TiO2 Nanofiber Photocatalyst for Efficient Degradation of Sulfamethoxazole. Sep. Purif. Technol. 2024, 351, 128130. [Google Scholar] [CrossRef]
- Reddy, C.V.; Kakarla, R.R.; Cheolho, B.; Shim, J.; Aminabhavi, T.M. Heterostructured 2D/2D ZnIn2S4/g-C3N4 Nanohybrids for Photocatalytic Degradation of Antibiotic Sulfamethoxazole and Photoelectrochemical Properties. Environ. Res. 2023, 225, 115585. [Google Scholar] [CrossRef]
- Le-Duy, N.; Hoang, L.-A.T.; Nguyen, T.D.; Lee, T. Pd Nanoparticles Decorated BiVO4 Pine Architectures for Photocatalytic Degradation of Sulfamethoxazole. Chemosphere 2023, 321, 138118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, C.; Ning, J.; Dai, R.; Liu, Y.; Wu, Q.; Zhang, F.; Zhang, W.; Dou, S.; Liu, X. Unusual Aliovalent Cd Doped γ-Bi2MoO6 Nanomaterial for Efficient Photocatalytic Degradation of Sulfamethoxazole and Rhodamine B under Visible Light Irradiation. Carbon Neutralization 2023, 2, 646–660. [Google Scholar] [CrossRef]
- Zhang, J.; Gou, S.; Yang, Z.; Li, C.; Wang, W. Photocatalytic Degradation of Sulfamethoxazole over S-Scheme Fe2O3/g-C3N4 Photocatalyst under Visible Light. Water Cycle 2024, 5, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, F.; Guan, Z.; Luo, Y.; Zhou, L.; Lu, Y.; Tian, B.; Zhang, J. S-Scheme BiOCl/MoSe2 Heterostructure with Enhanced Photocatalytic Activity for Dyes and Antibiotics Degradation under Sunlight Irradiation. Sensors 2022, 22, 3344. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, Z.; Yan, S.; Zhou, P.; Miao, F.; Liang, S.; Wang, J.; Cui, X. Bi2WO6–BiOCl Heterostructure with Enhanced Photocatalytic Activity for Efficient Degradation of Oxytetracycline. Sci. Rep. 2020, 10, 18401. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, Q.; Du, C.; Liu, Z. Enhanced Visible Light Photocatalytic Activity in BiOCl/SnO2: Heterojunction of Two Wide Band-Gap Semiconductors. RSC Adv. 2015, 5, 22740–22752. [Google Scholar] [CrossRef]
- Grilla, E.; Kagialari, M.N.; Petala, A.; Frontistis, Z.; Mantzavinos, D. Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions. Catalysts 2021, 11, 650. [Google Scholar] [CrossRef]
- Ding, J.; Su, G.; Zhou, Y.; Yin, H.; Wang, S.; Wang, J.; Zhang, W. Construction of Bi/BiOI/BiOCl Z-Scheme Photocatalyst with Enhanced Tetracycline Removal under Visible Light. Environ. Pollut. 2024, 341, 122942. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Tang, Y.; Zhang, X.; Ji, S.; Luo, L.; Jiang, F. Photoinduced RhB-Sensitized Effect on a Novel AgI/BiOCl/Biochar Photocatalyst to Boost Its Photocatalytic Performance for 17α-Ethinyl Estradiol Degradation. Sep. Purif. Technol. 2024, 332, 125774. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, H.; Yao, C.; Song, W.; Liu, C.; Song, W.; Zhang, Z. 2D/2D Ni-MOF/BiOCl S-Scheme Heterojunction with Boosted Charge Transfer and Photocatalytic Degradation of Tetracycline. Sustain. Mater. Technol. 2024, 39, e00825. [Google Scholar] [CrossRef]
- Wang, R.; You, H.; Li, Z.; Zhao, J.; Li, M.; Zhu, J.; Zhang, G.; Wang, X.; Leng, H.; Qi, S.; et al. Non-Radical Mediated Reduced Graphene Oxide/Polypyrrole Catalytic Ceramic Membrane-PDS System for Source Control of SMX. Chem. Eng. J. 2024, 479, 147769. [Google Scholar] [CrossRef]
- Mahdi Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate Radical Anion Oxidation of Diclofenac and Sulfamethoxazole for Water Decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Yin, R.-L.; Zhou, X.-J.; Du, J.-S.; Cao, H.-O.; Yang, S.-S.; Ren, N.-Q. Sulfamethoxazole Degradation by Ultrasound/Ozone Oxidation Process in Water: Kinetics, Mechanisms, and Pathways. Ultrason. Sonochem. 2015, 22, 182–187. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo Activated Persulfate Oxidation of Antibiotic Sulfamethoxazole and Structurally Related Compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar Photocatalytic Degradation of Sulfamethoxazole over Tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Antonopoulou, Μ.; Konstantinou, I. Photocatalytic Degradation and Mineralization of Tramadol Pharmaceutical in Aqueous TiO2 Suspensions: Evaluation of Kinetics, Mechanisms and Ecotoxicity. Appl. Catal. A Gen. 2016, 515, 136–143. [Google Scholar] [CrossRef]
- Kouvelis, K.; Ioannidi, A.A.; Petala, A.; Souliotis, M.; Frontistis, Z. Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration. Catalysts 2023, 13, 1175. [Google Scholar] [CrossRef]




| Parameter | Wastewater (WW) | Bottled Water (BW) |
|---|---|---|
| pH | 8.5 | 7.4 |
| Conductivity (20 °C), [μS/cm] | 934 | 513 |
| Total dissolved solids (TDS), [mg/L] | 654 | 312 |
| Total suspended solids (TSS), [mg/L] | 22 | - |
| Total hardness (CaCO3), [mg/L] | 287 | 260 |
| Chemical oxygen demand, [mg/L] | 48.5 | - |
| Total organic carbon, [mg/L] | 4.7 | - |
| Chlorides (Cl−), [mg/L] | 262 | 9.9 |
| Bicarbonates (HCO3−), [mg/L] | 278 | 263.1 |
| Sulfates (SO42−), [mg/L] | 62.3 | 29 |
| Phosphates (PO4−) [mg/L] | 14.9 | - |
| Nitrates (NO3−), [mg/L] | 2.3 | 10.1 |
| Bromides (Br−), [mg/L] | 165.6 | - |
| Ca+2, [mg/L] | 112 | 92.7 |
| K+, [mg/L] | 15.4 | 0.65 |
| Na+, [mg/L] | 76.3 | 5.5 |
| Mg2+, [mg/L] | - | 7.1 |
| Photocatalysts | [Catalyst], mg/L | [SMX], μg/L | Type of Irradiation | Removal | Ref. |
|---|---|---|---|---|---|
| BiOCl/g-C3N4/Cu2O/Fe3O4 | 200 | 25,325 | Visible light (800 W Xe lamp) | 100% at 60 min in UPW | [81] |
| Cu2O/BiOBr | 1000 | 20,000 | Solar light (250 W Xe lamp) | 90.7% at 30 min in UPW | [82] |
| LaFeO3/Ag3PO4@GO | - | 20,000 | 300 W Xe lamp | 80.4% at 60 min in UPW | [83] |
| Bi/Bi2WO6/TiO2 | 300 | 20,000 | 300 W Xe lamp (λ > 300 nm) | 96% at 60 min in UPW 86% at 60 min in 10 mg/L HA | [84] |
| ZnIn2S4/g-C3N4 | 200 | 15,000 | Visible light | 89.4% at 120 min in UPW | [85] |
| 2.4% wt. Pd/BiVO4 | 500 | 10,000 | Visible light (300 W Xe lamp) | 100% at 200 min in UPW | [86] |
| 8% Cd doped γ-Bi2MoO6 | 1000 | 5000 | 500 W long-arc xenon lamp | 100% at 210 min in UPW | [87] |
| 11% wt. Fe2O3/g-C3N4 | 300 | 10,000 | Visible light (350 W Xe lamp) | 40% at 40 min in UPW at pH 9 | [88] |
| 0.5% wt. MoTe2/BiOCl | 500 | 500 | Solar light (100 W Xe lamp) | 96% at 90 min in UPW and 100% at 60 min in WW | This study |
| Photocatalysts | [Photocatalysts], mg/L | Compound, μg/L | Type of Irradiation | Removal | Ref. |
|---|---|---|---|---|---|
| BiOCl/MoSe2−30% wt. | 1000 | 1 SD, 20,000 | Solar light (300 W Xe lamp) | 100% at 120 min in UPW | [89] |
| 1% wt. Bi2WO6–BiOCl | 1000 | Phenol, 20,000 | Solar light (500 W Xe lamp) | 93% at 5 h in UPW | [90] |
| 0.5% wt. BiOCl/SnO2 | 500 | 2 RhB, 4790 | Visible light (500 W Xe lamp) | 80% at 10 h in UPW | [91] |
| MoB/BiOCl | 500 | 3 LOS 500 | Solar light (100 W xe lamp) | 100% at 7.5 min in UPW 20% at 90 min in WW | [45] |
| 0.25% wt. MoS2/BiOCl | 1000 | 4 VLS, 500 | Solar light (100 W Xe lamp) | 10% at 120 min in WW | [92] |
| Bi/BiOI/BiOCl | 1000 | 5 TC, 30,000 | Visible light (300 W Xe lamp) | 80% at 60 min in UPW | [93] |
| AgI/BiOCl/biochar | 500 | 6 EE2, 3000 | Visible light (500 W Xe lamp) | 98.6% at 12 min in UPW | [94] |
| 10% wt. Ni-MOF/BiOCl | 2500 | 5 TC, 10,000 | 32 W UV lamp | 60% at 180 min in UPW | [95] |
| 0.5 MoTe2/BiOCl | 500 | SMX, 500 | Solar light (100 W Xe lamp) | 96% at 90 min in UPW and 100% at 60 min in WW | This study |
| SMX/TBP Code | Ion Molecular Formula | m/z [Μ-H]− | Δ (ppm) | RDBE |
|---|---|---|---|---|
| SMX | C10H10N3O3S− | 252.0455 | −2.6 | 7.5 |
| TBP 1 | C10H8N3O5S− | 282.0187 | 1.2 | 8.5 |
| TBP 2 | C10H8N3O4S− | 266.0241 | −0.6 | 8.5 |
| TBP 3 | C7H6N3O2S− | 196.0184 | 1.4 | 6.5 |
| TBP 4 | C7H4N3O4S− | 225.9931 | −1.4 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidi, A.A.; Kouvelis, K.; Ntourmous, G.; Petala, A.; Mantzavinos, D.; Antonopoulou, M.; Frontistis, Z. Molybdenum Telluride-Promoted BiOCl Photocatalysts for the Degradation of Sulfamethoxazole Under Solar Irradiation: Kinetics, Mechanism, and Transformation Products. Catalysts 2025, 15, 59. https://doi.org/10.3390/catal15010059
Ioannidi AA, Kouvelis K, Ntourmous G, Petala A, Mantzavinos D, Antonopoulou M, Frontistis Z. Molybdenum Telluride-Promoted BiOCl Photocatalysts for the Degradation of Sulfamethoxazole Under Solar Irradiation: Kinetics, Mechanism, and Transformation Products. Catalysts. 2025; 15(1):59. https://doi.org/10.3390/catal15010059
Chicago/Turabian StyleIoannidi, Alexandra A., Konstantinos Kouvelis, Gkizem Ntourmous, Athanasia Petala, Dionissios Mantzavinos, Maria Antonopoulou, and Zacharias Frontistis. 2025. "Molybdenum Telluride-Promoted BiOCl Photocatalysts for the Degradation of Sulfamethoxazole Under Solar Irradiation: Kinetics, Mechanism, and Transformation Products" Catalysts 15, no. 1: 59. https://doi.org/10.3390/catal15010059
APA StyleIoannidi, A. A., Kouvelis, K., Ntourmous, G., Petala, A., Mantzavinos, D., Antonopoulou, M., & Frontistis, Z. (2025). Molybdenum Telluride-Promoted BiOCl Photocatalysts for the Degradation of Sulfamethoxazole Under Solar Irradiation: Kinetics, Mechanism, and Transformation Products. Catalysts, 15(1), 59. https://doi.org/10.3390/catal15010059











