Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery
Abstract
1. Introduction
2. Results and Discussion
2.1. Compositional Analysis and Surface Morphology by SEM
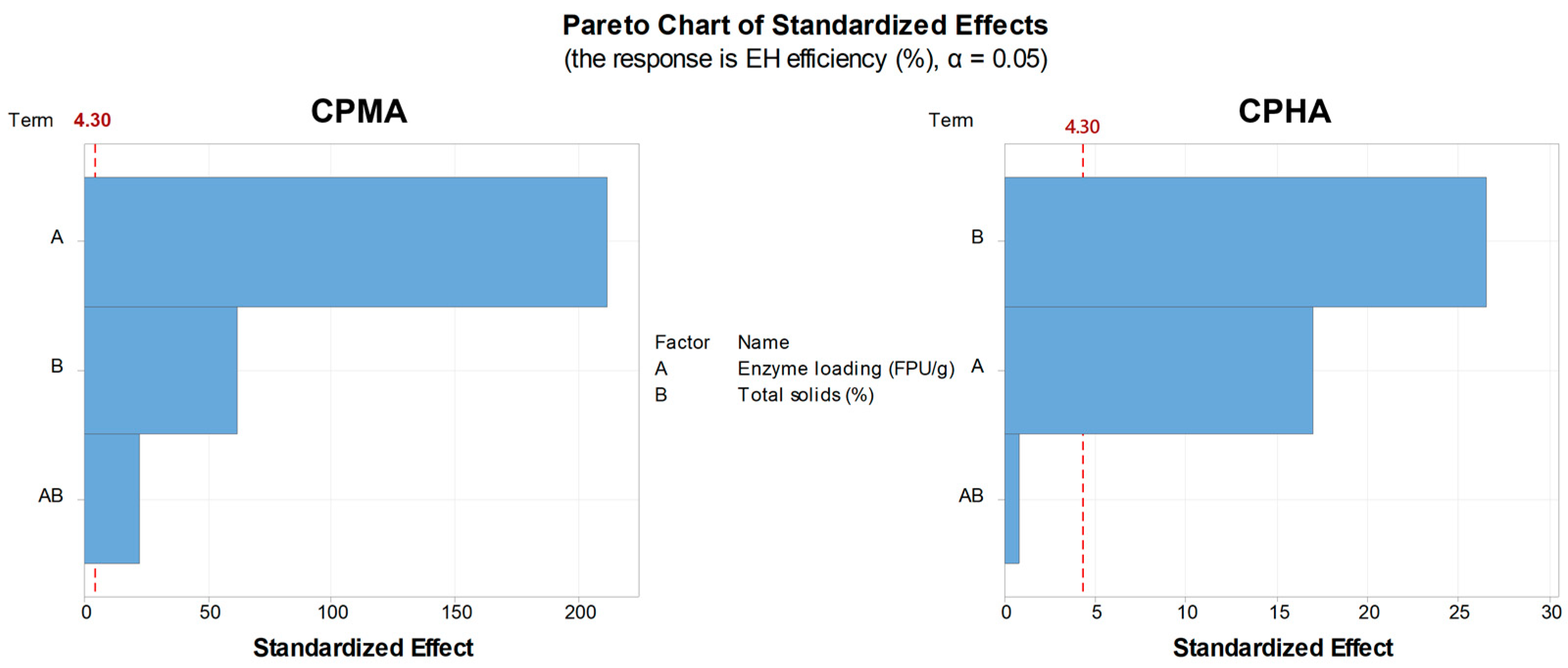
2.2. Effect of Enzyme Loading and Total Solids on Enzymatic Hydrolysis
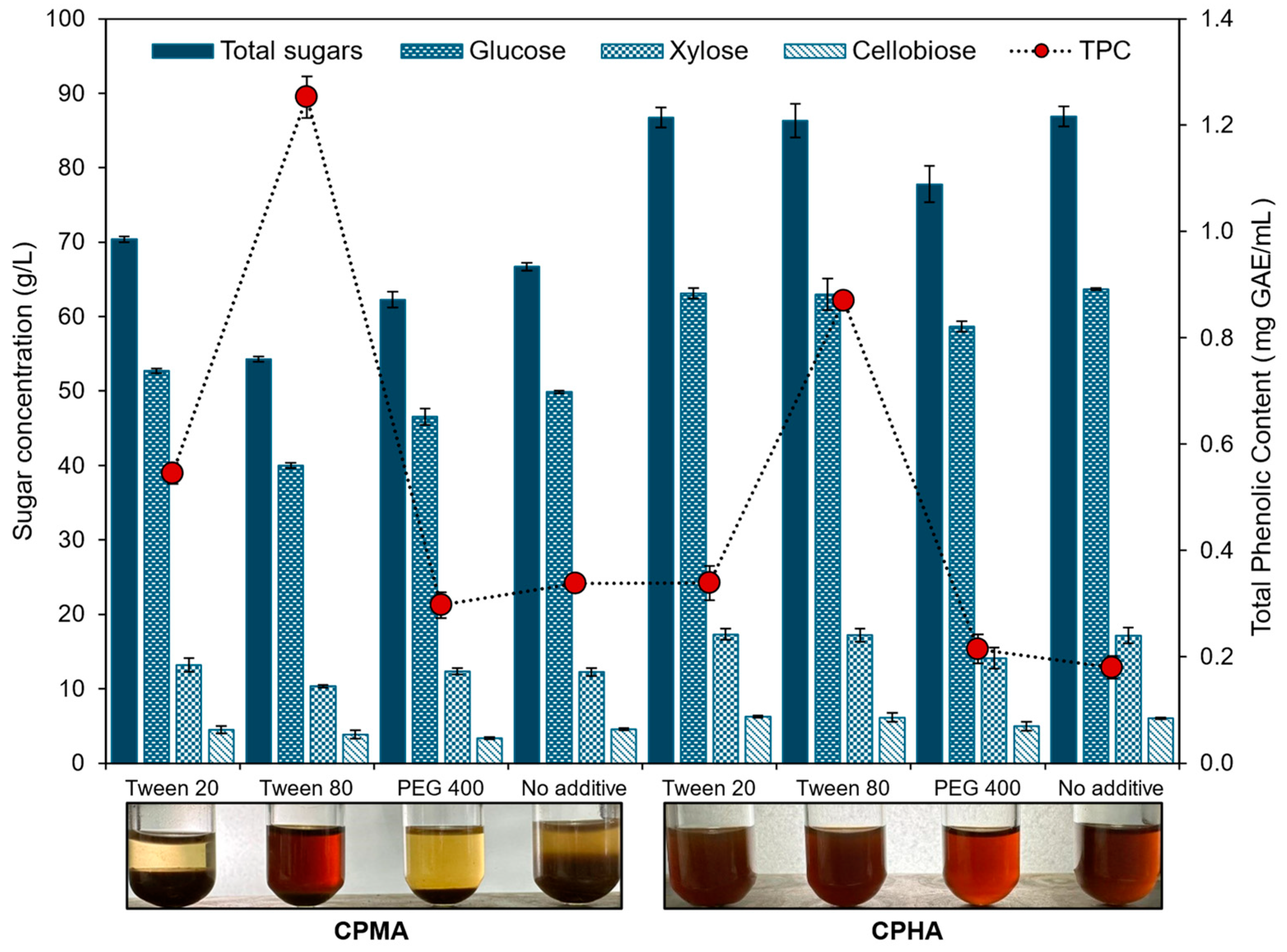
2.3. Effect of Surfactants on Enzymatic Hydrolysis of Cellulose Pulps
2.4. Profile of Sugars and Inhibitors in Cellulosic Hydrolysates with Surfactant Addition
3. Materials and Methods
3.1. Biomass Pretreatment and Chemicals
3.1.1. Compositional Analysis of Biomass (Native and Pretreated)
3.1.2. Scanning Electron Microscopy (SEM) Analysis
3.2. The 22 Full-Factorial Design
3.3. Enzymatic Hydrolysis of Cellulose Pulp and Surfactant Supplementation
3.4. Other Analytical Procedures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, V.Z.; Wu, T.Y. An application of ultrasonication in lignocellulosic biomass valorisation into bio-energy and bio-based products. Renew. Sustain. Energy Rev. 2020, 132, 109924. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, J.J.; Chandel, A.K.; Philippini, R.R.; da Silva, S.S. Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Convers. Biorefinery 2020, 10, 813–822. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Jeevan Kumar, S.; Hans, M.; Singh, A.K.; Kumar, S. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefining 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wei, Y.; Zhang, Y.; Huang, Y.; Zhang, C.; Li, K.; Lu, Q. Tailoring properties of lignin through deep eutectic solvent for phenols production. Ind. Crops Prod. 2024, 222, 119690. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Penín, L.; López, M.; Santos, V.; Alonso, J.L.; Parajó, J.C. Technologies for Eucalyptus wood processing in the scope of biorefineries: A comprehensive review. Bioresour. Technol. 2020, 311, 123528. [Google Scholar] [CrossRef]
- Hu, Y.; Priya, A.; Chen, C.; Liang, C.; Wang, W.; Wang, Q.; Lin, C.S.K.; Qi, W. Recent advances in substrate-enzyme interactions facilitating efficient biodegradation of lignocellulosic biomass: A review. Int. Biodeterior. Biodegrad. 2023, 180, 105594. [Google Scholar] [CrossRef]
- Siqueira, G.; Arantes, V.; Saddler, J.N.; Ferraz, A.; Milagres, A.M. Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Yaverino-Gutierrez, M.A.; Ramos, L.; Ascencio, J.J.; Chandel, A.K. Enhanced Production of Clean Fermentable Sugars by Acid Pretreatment and Enzymatic Saccharification of Sugarcane Bagasse. Processes 2024, 12, 978. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Cebreiros, F.; Risso, F.; Cagno, M.; Cabrera, M.N.; Rochón, E.; Jauregui, G.; Boix, E.; Böthig, S.; Ferrari, M.D.; Lareo, C. Enhanced production of butanol and xylosaccharides from Eucalyptus grandis wood using steam explosion in a semi-continuous pre-pilot reactor. Fuel 2021, 290, 119818. [Google Scholar] [CrossRef]
- Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus wood by glycerol-organosolv pretreatment within the biorefinery concept: An integrated and intensified approach. Renew. Energy 2016, 95, 1–9. [Google Scholar] [CrossRef]
- Prinsen, P.; Rencoret, J.; Gutiérrez, A.; Liitiä, T.; Tamminen, T.; Colodette, J.L.; Berbis, M.A.l.; Jiménez-Barbero, J.s.; Martínez, A.n.T.; del Río, J.C. Modification of the lignin structure during alkaline delignification of eucalyptus wood by kraft, soda-AQ, and soda-O2 cooking. Ind. Eng. Chem. Res. 2013, 52, 15702–15712. [Google Scholar] [CrossRef]
- Mendes, I.S.; Prates, A.; Evtuguin, D.V. New insights into kinetics and mechanisms of acid sulphite delignification of Eucalyptus globulus wood. Chem. Eng. J. 2024, 496, 153959. [Google Scholar] [CrossRef]
- Baptista, C.; Robert, D.; Duarte, A.P. Effect of pulping conditions on lignin structure from maritime pine kraft pulps. Chem. Eng. J. 2006, 121, 153–158. [Google Scholar] [CrossRef]
- Rebola, S.M.; Azevedo, C.A.; Evtuguin, D.V. Effect of cooking and bleaching conditions on the properties of eucalyptus kraft fluff pulps. Cellulose 2021, 28, 4411–4426. [Google Scholar] [CrossRef]
- Reina, L.; Galetta, A.; Vinciguerra, V.; Resquin, F.; Menéndez, P. The relationship between Eucalyptus grandis lignin structure and kraft pulping parameters. J. Anal. Appl. Pyrolysis 2014, 107, 284–288. [Google Scholar] [CrossRef]
- Ribeiro, R.a.A.; Júnior, S.V.; Jameel, H.; Chang, H.-M.; Narron, R.; Jiang, X.; Colodette, J.L. Chemical study of kraft lignin during alkaline delignification of E. urophylla x E. grandis hybrid in low and high residual effective alkali. ACS Sustain. Chem. Eng. 2019, 7, 10274–10282. [Google Scholar] [CrossRef]
- Agarwal, C.; Pandey, A.K. Remediation and recycling of inorganic acids and their green alternatives for sustainable industrial chemical processes. Environ. Sci. Adv. 2023, 2, 1306–1339. [Google Scholar] [CrossRef]
- Brown, J.; Lindstrom, J.K.; Ghosh, A.; Rollag, S.A.; Brown, R.C. Production of sugars from lignocellulosic biomass via biochemical and thermochemical routes. Front. Energy Res. 2024, 12, 1347373. [Google Scholar] [CrossRef]
- Ding, K.; Liu, D.; Chen, X.; Zhang, H.; Shi, S.; Guo, X.; Zhou, L.; Han, L.; Xiao, W. Scalable lignocellulosic biorefineries: Technoeconomic review for efficient fermentable sugars production. Renew. Sustain. Energy Rev. 2024, 202, 114692. [Google Scholar] [CrossRef]
- Guerra, A.; Elissetche, J.P.; Norambuena, M.; Freer, J.; Valenzuela, S.a.; Rodriguez, J.; Balocchi, C. Influence of lignin structural features on Eucalyptus globulus kraft pulping. Ind. Eng. Chem. Res. 2008, 47, 8542–8549. [Google Scholar] [CrossRef]
- Tonoli, G.; Teixeira, E.; Corrêa, A.; Marconcini, J.; Caixeta, L.; Pereira-da-Silva, M.; Mattoso, L. Cellulose micro/nanofibres from Eucalyptus kraft pulp: Preparation and properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef]
- Neiva, D.; Fernandes, L.; Araújo, S.; Lourenco, A.; Gominho, J.; Simões, R.; Pereira, H. Chemical composition and kraft pulping potential of 12 eucalypt species. Ind. Crops Prod. 2015, 66, 89–95. [Google Scholar] [CrossRef]
- Pereira, B.; Marcondes, W.F.; Carvalho, W.; Arantes, V. High yield biorefinery products from sugarcane bagasse: Prebiotic xylooligosaccharides, cellulosic ethanol, cellulose nanofibrils and lignin nanoparticles. Bioresour. Technol. 2021, 342, 125970. [Google Scholar] [CrossRef]
- Pinales-Márquez, C.D.; Rodríguez-Jasso, R.M.; Araújo, R.G.; Loredo-Treviño, A.; Nabarlatz, D.; Gullón, B.; Ruiz, H.A. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: A review. Ind. Crops Prod. 2021, 162, 113274. [Google Scholar] [CrossRef]
- Fockink, D.H.; Urio, M.B.; Sánchez, J.H.; Ramos, L.P. Enzymatic hydrolysis of steam-treated sugarcane bagasse: Effect of enzyme loading and substrate total solids on its fractal kinetic modeling and rheological properties. Energy Fuels 2017, 31, 6211–6220. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Weiss, N.D.; Felby, C.; Thygesen, L.G. Enzymatic hydrolysis is limited by biomass–water interactions at high-solids: Improved performance through substrate modifications. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Gupta, R.P. Understanding the effects of low enzyme dosage and high solid loading on the enzyme inhibition and strategies to improve hydrolysis yields of pilot scale pretreated rice straw. Fuel 2022, 327, 125114. [Google Scholar]
- Amândio, M.S.; Rocha, J.M.; Xavier, A.M. Enzymatic hydrolysis strategies for cellulosic sugars production to obtain bioethanol from eucalyptus globulus bark. Fermentation 2023, 9, 241. [Google Scholar] [CrossRef]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym. Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Seo, D.-J.; Fujita, H.; Sakoda, A. Effects of a non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption 2011, 17, 813–822. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.; Tan, X.; Wang, Q.; Chen, X.; Yu, Q.; Qi, W.; Wang, Z.; Yuan, Z. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuels 2018, 32, 5951–5959. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R.; Wang, D.; Jenkins, B. Non-ionic surfactants and non-catalytic protein treatment on enzymatic hydrolysis of pretreated creeping wild ryegrass. In Proceedings of the Biotechnology for Fuels and Chemicals: Proceedings of the Twenty-Ninth Symposium on Biotechnology for Fuels and Chemicals, Denver, CO, USA, 29 April–2 May 2007; pp. 351–368. [Google Scholar]
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a nonionic surfactant on enzymatic hydrolysis of lignocellulose based on lignocellulosic features and enzyme adsorption. ACS Omega 2020, 5, 15812–15820. [Google Scholar] [CrossRef]
- Chaves, S.F.; Damacena, M.B.; Dias, K.O.G.; de Almada Oliveira, C.V.; Bhering, L.L. Factor analytic selection tools and environmental feature-integration enable holistic decision-making in Eucalyptus breeding. Sci. Rep. 2024, 14, 18429. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Ciesielski, P.; Trass, O.; Park, S.; Tao, L.; Tucker, M.P. Improving sugar yields and reducing enzyme loadings in the deacetylation and mechanical refining (DMR) process through multistage disk and szego refining and corresponding techno-economic analysis. ACS Sustain. Chem. Eng. 2016, 4, 324–333. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Saito, K.; Horikawa, Y.; Sugiyama, J.; Watanabe, T.; Kobayashi, Y.; Takabe, K. Effect of thermochemical pretreatment on lignin alteration and cell wall microstructural degradation in Eucalyptus globulus: Comparison of acid, alkali, and water pretreatments. J. Wood Sci. 2016, 62, 276–284. [Google Scholar] [CrossRef]
- Mo, W.; Li, B.; Liu, J.; Kong, F.; Chen, K. Short-term thermal drying-induced pore expansion effects of cellulosic fibers and its applications. Cellulose 2023, 30, 183–199. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cai, C.M.; Kumar, R.; Wyman, C.E. Overcoming factors limiting high-solids fermentation of lignocellulosic biomass to ethanol. Proc. Natl. Acad. Sci. USA 2017, 114, 11673–11678. [Google Scholar] [CrossRef] [PubMed]
- Ceccherini, S.; Ståhl, M.; Sawada, D.; Hummel, M.; Maloney, T.C. Effect of enzymatic depolymerization of cellulose and hemicelluloses on the direct dissolution of prehydrolysis kraft dissolving pulp. Biomacromolecules 2021, 22, 4805–4813. [Google Scholar] [CrossRef] [PubMed]
- Okino, S.; Ikeo, M.; Ueno, Y.; Taneda, D. Effects of Tween 80 on cellulase stability under agitated conditions. Bioresour. Technol. 2013, 142, 535–539. [Google Scholar] [CrossRef]
- Tu, M.; Saddler, J.N. Potential enzyme cost reduction with the addition of surfactant during the hydrolysis of pretreated softwood. Appl. Biochem. Biotechnol. 2010, 161, 274–287. [Google Scholar] [CrossRef]
- Tejirian, A.; Xu, F. Inhibition of enzymatic cellulolysis by phenolic compounds. Enzym. Microb. Technol. 2011, 48, 239–247. [Google Scholar] [CrossRef]
- Wang, W.; Tan, X.; Yu, Q.; Wang, Q.; Qi, W.; Zhuang, X.; Wang, Z.; Yuan, Z. Effect of stepwise lignin removal on the enzymatic hydrolysis and cellulase adsorption. Ind. Crops Prod. 2018, 122, 16–22. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, R.K.; Bachheti, A.; Milessi, T.S.; Chandel, A.K. Understanding the biochemical changes at molecular level during biomass pretreatment: A comprehensive analysis. Cellulose 2024, 31, 7281–7312. [Google Scholar] [CrossRef]
- Liu, T.; Wang, P.; Tian, J.; Guo, J.; Zhu, W.; Bushra, R.; Huang, C.; Jin, Y.; Xiao, H.; Song, J. Emerging role of additives in lignocellulose enzymatic saccharification: A review. Renew. Sustain. Energy Rev. 2024, 197, 114395. [Google Scholar] [CrossRef]
- Lai, C.; Jia, Y.; Yang, C.; Chen, L.; Shi, H.; Yong, Q. Incorporating lignin into polyethylene glycol enhanced its performance for promoting enzymatic hydrolysis of hardwood. ACS Sustain. Chem. Eng. 2020, 8, 1797–1804. [Google Scholar] [CrossRef]
- Fernandes, C.G.; Sawant, S.C.; Mule, T.A.; Khadye, V.S.; Lali, A.M.; Odaneth, A.A. Enhancing cellulases through synergistic β-glucosidases for intensifying cellulose hydrolysis. Process Biochem. 2022, 120, 202–212. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618; Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 13 November 2024).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. NREL/TP-510-42619; Determination of Extractives in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 13 November 2024).
- Moya, E.B.; Syhler, B.; Manso, J.O.; Dragone, G.; Mussatto, S.I. Enzymatic hydrolysis cocktail optimization for the intensification of sugar extraction from sugarcane bagasse. Int. J. Biol. Macromol. 2023, 242, 125051. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Kallioinen, A.; Sipponen, M.H.; Nyyssölä, A. Modeling and optimization of polyethylene glycol (PEG) addition for cost-efficient enzymatic hydrolysis of lignocellulose. Biochem. Eng. J. 2021, 167, 107894. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
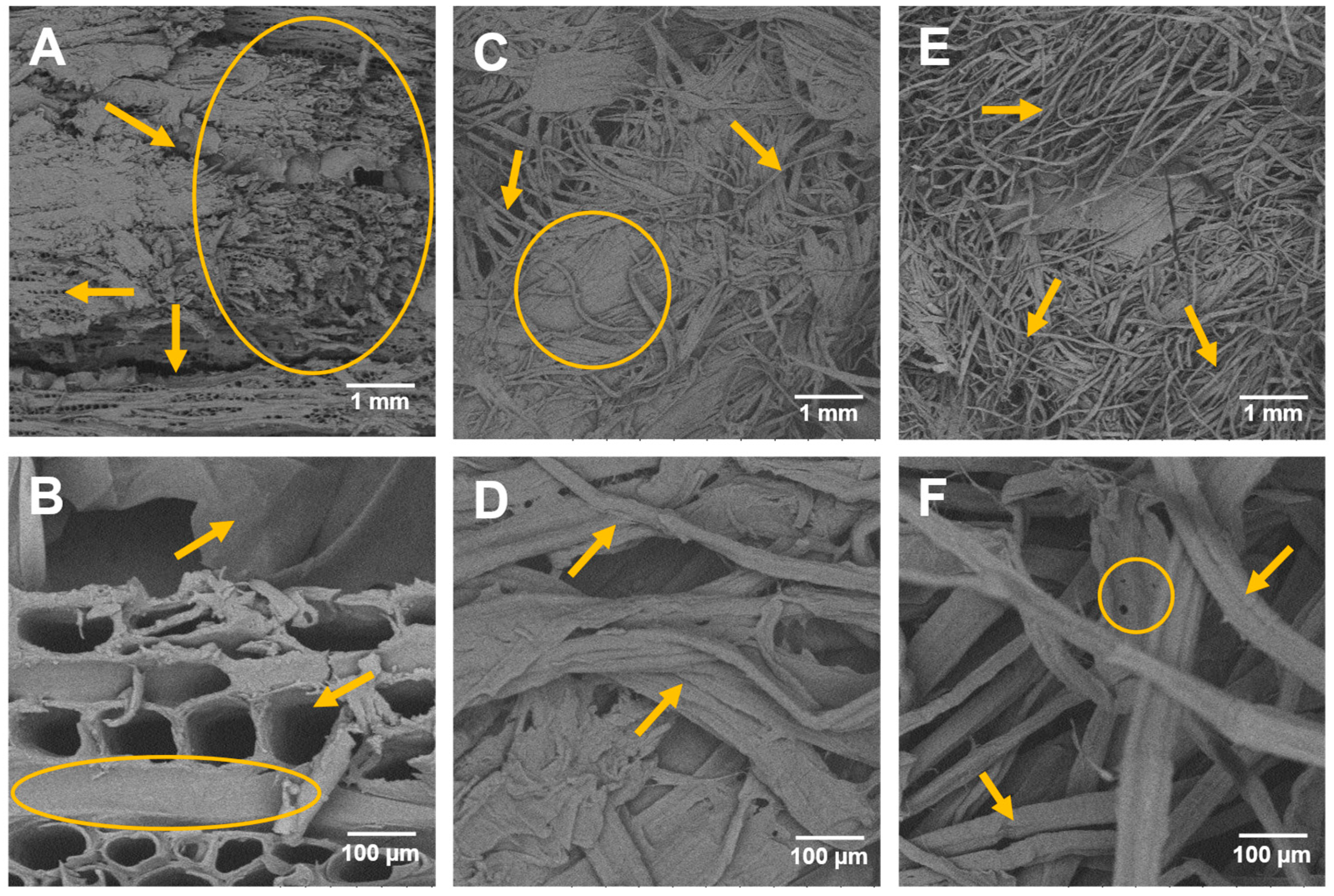


| Component | Untreated Eucalyptus Chips | CPMA 1 | CPHA 2 |
|---|---|---|---|
| Glucan | 40.0 ± 0.4 | 58.7 ± 0.5 | 70.1 ± 0.5 |
| Xylan | 20.8 ± 0.2 | 11.7 ± 0.1 | 15.1 ± 0.2 |
| Lignin | 27.8 ± 1.2 | 19.6 ± 1.8 | 4.7 ± 0.6 |
| Arabinosyl | 1.4 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Acetyl | 2.5 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Ash | 3.8 ± 0.3 | 1.2 ± 0.0 | 1.0 ± 0.0 |
| Extractives | 2.1 ± 0.2 | ND 3 | ND 3 |
| Total | 98.4 ± 1.5 | 91.5 ± 1.9 | 91.3 ± 0.8 |
| Run | Independent Variables | CPMA | CPHA | |||
|---|---|---|---|---|---|---|
| Enzyme Loading (FPU/g Carbohydrate) | Total Solids (%, w/v) | RS (g/L) | EH Efficiency (%) | RS (g/L) | EH Efficiency (%) | |
| 1 | 5 | 5 | 22.6 ± 0.8 | 64.2 | 38.3 ± 0.2 | 89.6 |
| 2 | 15 | 5 | 35.5 ± 0.6 | 100.8 | 43.5 ± 0.7 | 102.0 |
| 3 | 5 | 15 | 61.3 ± 1.8 | 58.0 | 91.9 ± 1.0 | 71.7 |
| 4 | 15 | 15 | 92.6 ± 0.1 | 87.6 | 106.2 ± 0.8 | 82.9 |
| 5 | 10 | 10 | 70.5 ± 0.2 | 100.0 | 84.3 ± 0.4 | 98.7 |
| 6 | 10 | 10 | 70.3 ± 0.9 | 99.7 | 85.0 ± 0.1 | 99.6 |
| 7 | 10 | 10 | 70.3 ± 0.4 | 99.8 | 85.4 ± 0.1 | 100.1 |
| Source | DF 1 | Adj SS 2 | Adj MS 3 | F-Value | p-Value |
|---|---|---|---|---|---|
| CPMA | |||||
| Model | 4 | 2048 | 511.97 | 20895 | 0.0001 |
| Linear | 2 | 1190 | 595.05 | 24286 | 0.0001 |
| Enzyme loading (FPU/g carbohydrate) | 1 | 1096 | 1096.02 | 44732 | 0.0001 |
| Total solids (%, w/v) | 1 | 94 | 94.08 | 3839 | 0.0001 |
| 2-way interactions | 1 | 12 | 12.48 | 509 | 0.0020 |
| Enzyme loading (FPU/g) × Total solids (%) | 1 | 12 | 12.48 | 509 | 0.0020 |
| Curvature | 1 | 845 | 845.31 | 34499 | 0.0001 |
| Error | 2 | 0.05 | 0.02 | ||
| Total | 6 | 204 | |||
| CPHA | |||||
| Model | 4 | 765 | 191 | 397 | 0.0030 |
| Linear | 2 | 480 | 240 | 498 | 0.0020 |
| Enzyme loading (FPU/g carbohydrate) | 1 | 139 | 139 | 289 | 0.0030 |
| Total solids (%, w/v) | 1 | 341 | 341 | 708 | 0.0010 |
| 2-way interactions | 1 | 0.31 | 0.31 | 0.65 | 0.5050 |
| Enzyme loading (FPU/g) × Total solids (%) | 1 | 0.31 | 0.31 | 0.65 | 0.5050 |
| Curvature | 1 | 285 | 284.83 | 591 | 0.0020 |
| Error | 2 | 0.96 | 0.48 | ||
| Total | 6 | 766 | |||
| Variable | Low Level (−1) | Central (0) | High Level (+1) |
|---|---|---|---|
| Enzyme loading (FPU/g carbohydrate) | 0 | 5 | 10 |
| Total solids (%, w/v) | 0 | 5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascencio, J.J.; Magalhães, L.S.; Ferreira, F.B.; Heinz, O.; Ferraz, A.; Chandel, A.K. Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery. Catalysts 2025, 15, 47. https://doi.org/10.3390/catal15010047
Ascencio JJ, Magalhães LS, Ferreira FB, Heinz O, Ferraz A, Chandel AK. Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery. Catalysts. 2025; 15(1):47. https://doi.org/10.3390/catal15010047
Chicago/Turabian StyleAscencio, Jesús J., Leticia S. Magalhães, Fabrício B. Ferreira, Otto Heinz, André Ferraz, and Anuj K. Chandel. 2025. "Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery" Catalysts 15, no. 1: 47. https://doi.org/10.3390/catal15010047
APA StyleAscencio, J. J., Magalhães, L. S., Ferreira, F. B., Heinz, O., Ferraz, A., & Chandel, A. K. (2025). Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery. Catalysts, 15(1), 47. https://doi.org/10.3390/catal15010047








