Recent Advances of PtCu Alloy in Electrocatalysis: Innovations and Applications
Abstract
1. Introduction
2. Synthesis of PtCu Alloy Electrocatalysts
2.1. Modification of Innovative Supports
2.1.1. Modified Carbon Supports
2.1.2. Metal Oxide Support Modification
2.2. Reactant Selection and Synthesis Strategies
2.2.1. Surfactant-Free Synthesis
2.2.2. Special Intermediate-Assisted Synthesis
2.2.3. Advanced Template Strategy
2.3. Post-Processing: Advanced High-Temperature Processing Strategies
2.3.1. Exploration of Annealing Mechanisms and Surface Overcoating
2.3.2. Novel High-Temperature Post-Processing Strategy

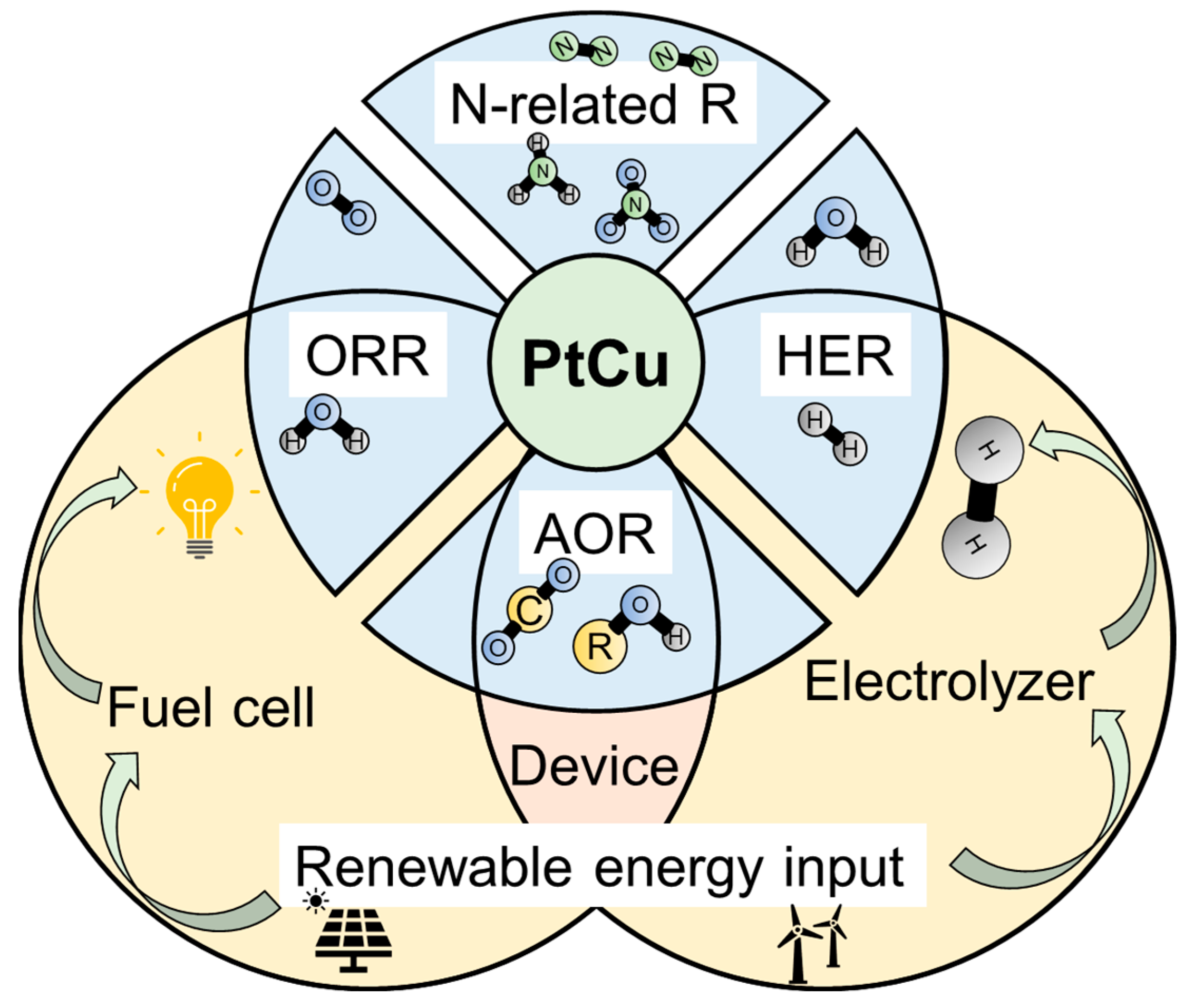
3. Application of PtCu Alloy Electrocatalysis
3.1. Fundamental Level Application
3.1.1. Hydrogen Evolution Reaction (HER)
3.1.2. Alcohol Oxidation Reaction (AOR)
Methanol (C1)
Ethanol (C2)

C3 Alcohols
3.1.3. Nitrogen-Related Reaction
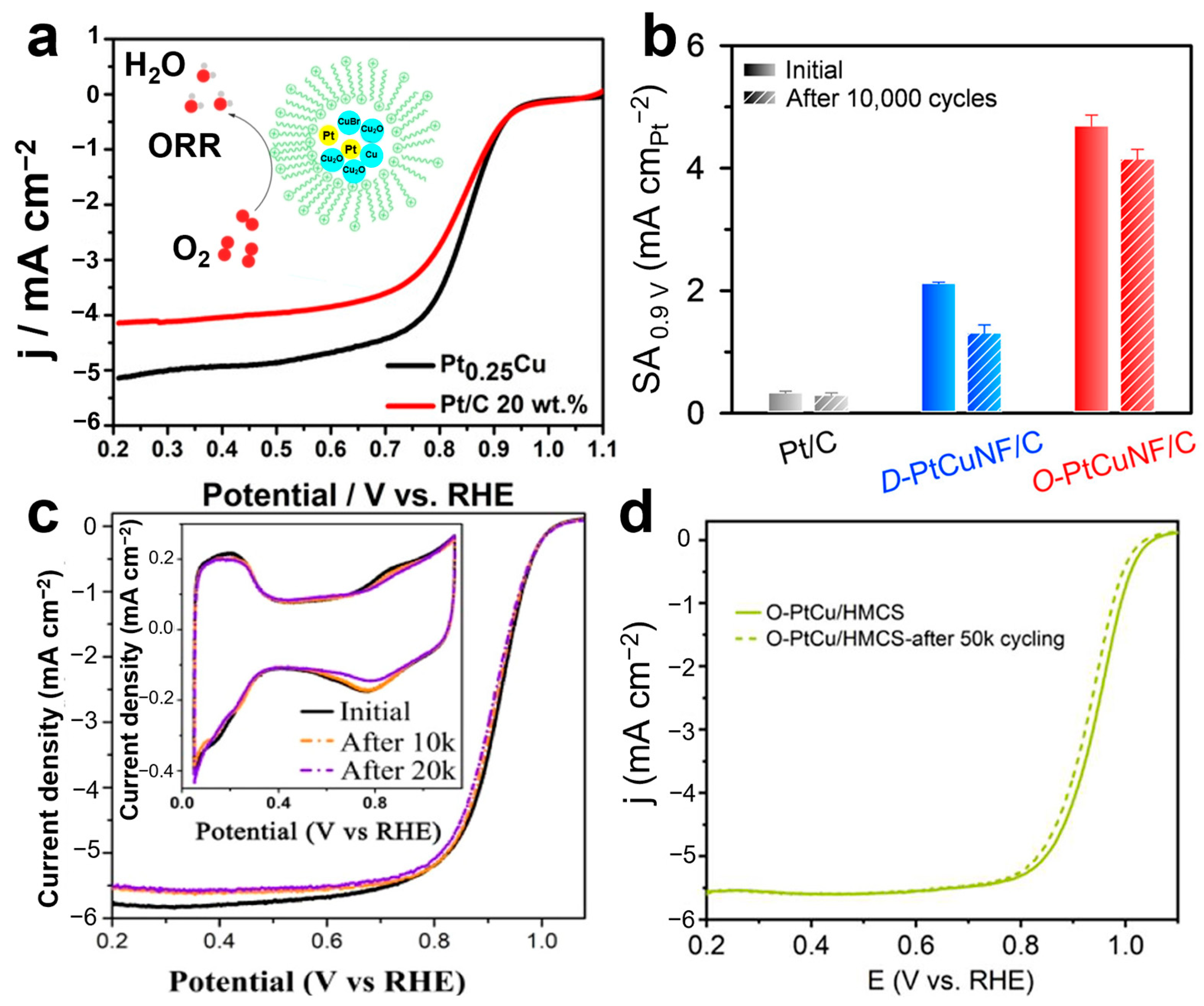
3.1.4. Oxygen Reduction Reaction (ORR)
3.2. Device Level Application
3.2.1. Electrolyzer
3.2.2. Fuel Cell
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández, P.S.; Fernandes Gomes, J.; Angelucci, C.A.; Tereshchuk, P.; Martins, C.A.; Camara, G.A.; Martins, M.a.E.; Da Silva, J.L.F.; Tremiliosi-Filho, G. Establishing a Link between Well-Ordered Pt(100) Surfaces and Real Systems: How Do Random Superficial Defects Influence the Electro-oxidation of Glycerol? ACS Catal. 2015, 5, 4227–4236. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Eid, K.; Lebechi, A.K.; Abdullah, A.M.; Ozoemena, K.I. Porous multi-metallic Pt-based nanostructures as efficient electrocatalysts for ethanol oxidation: A mini-review. Electrochem. Commun. 2022, 140, 107330. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Shen, J.; Yan, W.; He, L.; Thapa, P.S.; Ren, S.; Subramaniam, B.; Chaudhari, R.V. Exceptional performance of bimetallic Pt1Cu3/TiO2 nanocatalysts for oxidation of gluconic acid and glucose with O2 to glucaric acid. J. Catal. 2015, 330, 323–329. [Google Scholar] [CrossRef]
- Xu, H.; Wang, A.-L.; Tong, Y.-X.; Li, G.-R. Enhanced Catalytic Activity and Stability of Pt/CeO2/PANI Hybrid Hollow Nanorod Arrays for Methanol Electro-oxidation. ACS Catal. 2016, 6, 5198–5206. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.-W.; Liang, H.; Ge, H.; Gu, X.; Chen, S.; Yang, H.; Qin, Y. Tailoring Pt–Fe2O3 Interfaces for Selective Reductive Coupling Reaction To Synthesize Imine. ACS Catal. 2016, 6, 6560–6566. [Google Scholar] [CrossRef]
- Jiang, B.; Li, C.; Malgras, V.; Imura, M.; Tominaka, S.; Yamauchi, Y. Mesoporous Pt nanospheres with designed pore surface as highly active electrocatalyst. Chem. Sci. 2016, 7, 1575–1581. [Google Scholar] [CrossRef]
- Li, S.; Tang, X.; Jia, H.; Li, H.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.-J. Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 2020, 383, 164–171. [Google Scholar] [CrossRef]
- Menshchikov, V.; Alekseenko, A.; Guterman, V.; Nechitailov, A.; Glebova, N.; Tomasov, A.; Spiridonova, O.; Belenov, S.; Zelenina, N.; Safronenko, O. Effective Platinum-Copper Catalysts for Methanol Oxidation and Oxygen Reduction in Proton-Exchange Membrane Fuel Cell. Nanomaterials 2020, 10, 742. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, D.; Zhang, Q.; Sun, Y.; Zhang, S.; Du, F.; Jin, X. Synthesis of PtCu–based nanocatalysts: Fundamentals and emerging challenges in energy conversion. J. Energy Chem. 2022, 64, 583–606. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Pan, X.; Liu, M.; Wu, G.; Xie, Z. PtCu anchored on N,S-codoped electrospinning porous carbon nanofibers for oxygen reduction reaction. Int. J. Hydrogen Energy 2023, 48, 34794–34803. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Wu, L.; Wang, Y.; Du, X.; Li, J. Charge transfer and spillover effect enabled high-performance titanium dioxide supported PtCu catalyst towards acidic oxygen reduction. Chem. Eng. J. 2024, 481, 148097. [Google Scholar] [CrossRef]
- Su, Z.; Chen, T. Porous Noble Metal Electrocatalysts: Synthesis, Performance, and Development. Small 2021, 17, e2005354. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Huang, J.; Zhou, H.; Liu, X.; Tan, P.; Chen, H.; Liang, Y.; Pan, J. Microbial synthesis of N, P co-doped carbon supported PtCu catalysts for oxygen reduction reaction. J. Energy Chem. 2023, 84, 486–495. [Google Scholar] [CrossRef]
- Qi, Z.; Xiao, C.; Liu, C.; Goh, T.W.; Zhou, L.; Maligal-Ganesh, R.; Pei, Y.; Li, X.; Curtiss, L.A.; Huang, W. Sub-4 nm PtZn Intermetallic Nanoparticles for Enhanced Mass and Specific Activities in Catalytic Electrooxidation Reaction. J. Am. Chem. Soc. 2017, 139, 4762–4768. [Google Scholar] [CrossRef]
- Nie, M.; Xu, Z.; Wang, Y.; You, H.; Luo, L.; Li, B.; Mutahir, S.; Gan, W.; Yuan, Q. Ultrafast synthesis of efficient TS-PtCoCu/CNTs composite with high feed-to-product conversion rate by Joule heating for electrocatalytic oxidation of ethanol. J. Colloid. Interface Sci. 2024, 660, 334–344. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Guan, J. Optimization strategies of covalent organic frameworks and their derivatives for electrocatalytic applications. Adv. Funct. Mater. 2024, 34, 2310195. [Google Scholar] [CrossRef]
- Xiao, L.; Qi, L.; Sun, J.; Husile, A.; Zhang, S.; Wang, Z.; Guan, J. Structural regulation of covalent organic frameworks for advanced electrocatalysis. Nano Energy 2023, 120, 109155. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, N.; Lai, F.; Zhang, L.; Chen, S.; Li, H.; Jiang, K.; Zhu, T.; Xu, F.; Liu, T. Surface-regulated platinum–copper nanoframes in electrochemical reforming of ethanol for efficient hydrogen production. ACS Catal. 2022, 12, 11402–11411. [Google Scholar] [CrossRef]
- Gao, J.; Tang, X.; Du, P.; Li, H.; Yuan, Q.; Xie, G.; Qiu, H.-J. Synergistically coupling ultrasmall PtCu nanoalloys with highly porous CoP nanosheets as an enhanced electrocatalyst for electrochemical hydrogen evolution. Sustain. Energy Fuels 2020, 4, 2551–2558. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Hou, J.; Liu, S.; Qin, Y.; Liu, Q.; Cai, X.; Sun, Z.; Yang, M.; Luo, J. Cu2O-derived PtCu nanoalloy toward energy-efficient hydrogen production via hydrazine electrolysis under large current density. ACS Appl. Energy Mater. 2022, 5, 9487–9494. [Google Scholar] [CrossRef]
- Kaya, D.; Demiroglu, I.; Isik, I.B.; Isik, H.H.; Çetin, S.K.; Sevik, C.; Ekicibil, A.; Karadag, F. Highly active bimetallic Pt–Cu nanoparticles for the electrocatalysis of hydrogen evolution reactions: Experimental and theoretical insight. Int. J. Hydrogen Energy 2023, 48, 37209–37223. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H. Ultrahigh Pt-mass-activity hydrogen evolution catalyst electrodeposited from bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, Y.; Zhao, X.; Yin, P.F.; Dong, C.K.; Liu, H.; Du, X.W.; Yang, J. Dislocation-Activated Low Platinum-Loaded PtCu Nanoparticles Welded onto the Substrate for Practical Acidic Hydrogen Generation. ACS Appl. Energy Mater. 2024, 7, 3848–3857. [Google Scholar] [CrossRef]
- Tuo, Y.; Lu, Q.; Chen, C.; Liu, T.; Pan, Y.; Zhou, Y.; Zhang, J. The facile synthesis of core–shell PtCu nanoparticles with superior electrocatalytic activity and stability in the hydrogen evolution reaction. RSC Adv. 2021, 11, 26326–26335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Q.; Wang, P.; Zhu, J.; Chen, D.; Yang, Y.; Zhao, Y.; Pu, Z.; Mu, S. Molybdenum Carbide-PtCu Nanoalloy Heterostructures on MOF-Derived Carbon toward Efficient Hydrogen Evolution. Small 2021, 17, 2104241. [Google Scholar] [CrossRef]
- Ye, K.; Liu, Y.; Wang, X.; Wang, P.; Cao, K.; Liang, J.; Zuo, Y. Strain-activated porous helical-spiny-like PtCu with exposed high-index facets for efficient alkaline hydrogen evolution. Mater. Today Chem. 2023, 30, 101581. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, X.; Liu, W.; Wu, D.; Cao, D.; Cheng, D. Regulating surface composition of platinum-copper nanotubes for enhanced hydrogen evolution reaction in all pH values. J. Colloid. Interface Sci. 2023, 629, 53–62. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Son, J.I.; Jang, S.; Chung, D.Y. Mixed Potential Driven Self-Cleaning Strategy in Direct Isopropanol Fuel Cells. ACS Catal. 2024, 14, 8480–8487. [Google Scholar] [CrossRef]
- Wang, J.; Yan, H.; Li, X. Synthesis of PtCu rhombic dodecahedral nanoframes decorated with six nanobranches for efficient methanol electrooxidation. Energy Fuels 2022, 36, 3947–3953. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Sun, S.; Wu, L.; Tian, J.; Wu, Y.; Chen, Y.; Liu, X. Cu Tailoring Pt Enables Branched-Structured Electrocatalysts with High Concave Surface Curvature Toward Efficient Methanol Oxidation. Small 2023, 20, 2307970. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, Z.; Zhao, L.; Zhang, D.; Wang, Z.; Dai, Y.; Li, B.; Starostenko, O.; Lai, J.; Wang, L. Polysulfide modified PtCu intermetallic nanocatalyst with enrichment realizes efficient electrooxidation ethanol to CO2. Nano Res. 2024, 17, 2320–2327. [Google Scholar] [CrossRef]
- Bayat, R.; El Attar, A.; Akin, M.; Bekmezci, M.; El Rhazi, M.; Sen, F. Copper nanoparticle modified chain-like platinum nanocomposites for electro-oxidation of C1-, C2-, and C3-type alcohols. Int. J. Hydrogen Energy 2024, 51, 1577–1586. [Google Scholar] [CrossRef]
- Castagna, R.M.; Sieben, J.M.; Alvarez, A.E.; Duarte, M.M. Electrooxidation of ethanol and glycerol on carbon supported PtCu nanoparticles. Int. J. Hydrogen Energy 2019, 44, 5970–5982. [Google Scholar] [CrossRef]
- Pu, H.; Zhang, T.; Dong, K.; Dai, H.; Zhou, L.; Wang, K.; Bai, S.; Wang, Y.; Deng, Y. Evolution of PtCu tripod nanocrystals to dendritic triangular nanocrystals and study of the electrochemical performance to alcohol electrooxidation. Nanoscale 2021, 13, 20592–20600. [Google Scholar] [CrossRef] [PubMed]
- Sieben, J.M.; Alvarez, A.E.; Sanchez, M.D. Platinum nanoparticles deposited on Cu-doped NiO/C hybrid supports as high-performance catalysts for ethanol and glycerol electrooxidation in alkaline medium. J. Alloys Compd. 2022, 921, 166112. [Google Scholar] [CrossRef]
- Fang, C.; Hu, J.; Jiang, X.; Cui, Z.; Xu, X.; Bi, T. Bifunctional PtCu electrocatalysts for the N 2 reduction reaction under ambient conditions and methanol oxidation. Inorg. Chem. Front. 2020, 7, 1411–1419. [Google Scholar] [CrossRef]
- Prabowo Rahardjo, S.S.; Shih, Y.-J. Electrocatalytic Ammonia Oxidation Mediated by Nickel and Copper Crystallites Decorated with Platinum Nanoparticle (PtM/G, M = Cu, Ni). ACS Sustain. Chem. Eng. 2022, 10, 5043–5054. [Google Scholar] [CrossRef]
- Islam, M.N.; Ahsan, M.; Aoki, K.; Nagao, Y.; Alsafrani, A.E.; Marwani, H.M.; Almahri, A.; Rahman, M.M.; Hasnat, M.A. Development of CuNi immobilized Pt surface to minimize nitrite evolution during electrocatalytic nitrate reduction in neutral medium. J. Environ. Chem. Eng. 2023, 11, 111149. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Fajardo, A.S.; Sánchez-Sánchez, C.M.; Garcia-Segura, S. Highly reactive Cu-Pt bimetallic 3D-electrocatalyst for selective nitrate reduction to ammonia. Appl. Catal. B 2022, 302, 120844. [Google Scholar] [CrossRef]
- Ding, J.; Li, W.; Chen, Q.; Liu, J.; Tang, S.; Wang, Z.; Chen, L.; Zhang, H. Sustainable ammonia synthesis from air by the integration of plasma and electrocatalysis techniques. Inorg. Chem. Front. 2023, 10, 5762–5771. [Google Scholar] [CrossRef]
- Parkash, A.; Jia, Z.; Tian, T.; Ge, Z.; Yu, C.; Chunli, X. A New Generation of Platinum-Copper Electrocatalysts with Ultra-Low Concentrations of Platinum for Oxygen-Reduction Reactions in Alkaline Media. ChemistrySelect 2020, 5, 3391–3397. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, T.; Ha, Y.; Jun, M.; Baik, H.; Jeong, H.Y.; Kim, H.; Lee, K.; Joo, S.H. Intermetallic PtCu nanoframes as efficient oxygen reduction electrocatalysts. Nano Lett. 2020, 20, 7413–7421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, H.; Song, L.; Han, L.; Zhang, R.; Kwon, G.; Ma, L.; Ehrlich, S.N.; Frenkel, A.I.; Yang, J. Rhombohedral ordered intermetallic nanocatalyst boosts the oxygen reduction reaction. ACS Catal. 2020, 11, 184–192. [Google Scholar] [CrossRef]
- Deng, Z.; Gong, Z.; Gong, M.; Wang, X. Multiscale Regulation of Ordered PtCu Intermetallic Electrocatalyst for Highly Durable Oxygen Reduction Reaction. Nano Lett. 2024, 24, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Macauley, N.; Papadias, D.D.; Fairweather, J.; Spernjak, D.; Langlois, D.; Ahluwalia, R.; More, K.L.; Mukundan, R.; Borup, R.L. Carbon Corrosion in PEM Fuel Cells and the Development of Accelerated Stress Tests. J. Electrochem. Soc. 2018, 165, F3148–F3160. [Google Scholar] [CrossRef]
- Wang, C.; Ricketts, M.; Soleymani, A.P.; Jankovic, J.; Waldecker, J.; Chen, J. Effect of Carbon Support Characteristics on Fuel Cell Durability in Accelerated Stress Testing. J. Electrochem. Soc. 2021, 168, 044507. [Google Scholar] [CrossRef]
- Yan, Q.-Q.; Yin, P.; Liang, H.-W. Engineering the Electronic Interaction between Metals and Carbon Supports for Oxygen/Hydrogen Electrocatalysis. ACS Mater. Lett. 2021, 3, 1197–1212. [Google Scholar] [CrossRef]
- Nie, Y.; Wei, Z. Surface-confined Pt-based catalysts for strengthening oxygen reduction performance. Prog. Nat. Sci. Mater. Int. 2020, 30, 796–806. [Google Scholar] [CrossRef]
- Yue, R.; Yao, Z.; Geng, J.; Du, Y.; Xu, J.; Yang, P. Facile electrochemical synthesis of a conducting copolymer from 5-aminoindole and EDOT and its use as Pt catalyst support for formic acid electrooxidation. J. Solid State Electrochem. 2012, 17, 751–760. [Google Scholar] [CrossRef]
- Dao, V.-D.; Larina, L.L.; Tran, Q.C.; Bui, V.-T.; Nguyen, V.-T.; Pham, T.-D.; Mohamed, I.M.A.; Barakat, N.A.M.; Huy, B.T.; Choi, H.-S. Evaluation of Pt-based alloy/graphene nanohybrid electrocatalysts for triiodide reduction in photovoltaics. Carbon 2017, 116, 294–302. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Dao, V.-D.; Larina, L.L.; Lee, J.-K.; Choi, H.-S. Optimum strategy for designing PtCo alloy/reduced graphene oxide nanohybrid counter electrode for dye-sensitized solar cells. Carbon 2016, 96, 229–236. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, H.; Lu, X.; Guo, L.; Du, D.; Shan, H.; Geng, L.; Akdim, O.; Huang, X.; Park, G.-S.; et al. Enhanced redox catalysis of electrochemical alcohol oxidation in alkaline medium by using Pt-Cu/C catalyst. J. Alloys Compd. 2022, 926, 166994. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Y.; Dai, Y.; Jin, Z.; Wang, Z.; Wang, F.; Zhu, H. Preparation and properties of polypyrrole-modified carbon black supported Pt3Cu alloy catalyst. New J. Chem. 2023, 47, 17999–18009. [Google Scholar] [CrossRef]
- Liang, X.; Huang, M.; Huang, F.; Peng, X.; Rani, K.K.; Wang, L.; Yang, Z.; Fan, Y.; Chen, D.-H. Composition-adjustable PtCoCu alloy nanoparticles for promoting methanol oxidation reaction. Mol. Catal. 2023, 545, 113225. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Sharifi Khangheshlaghi, L.; Jadali, S. Efficient methanol electrooxidation on activated pencil graphite electrode modified with PtCu catalyst. J. Appl. Electrochem. 2022, 53, 919–933. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Fernandes, C.G.; Hood, Z.D.; Peng, R.; Wu, Z.; Dourado, A.H.B.; Parreira, L.S.; de Oliveira, D.C.; Camargo, P.H.C.; de Torresi, S.I.C. PdPt-TiO2 nanowires: Correlating composition, electronic effects and O-vacancies with activities towards water splitting and oxygen reduction. Appl. Catal. B Environ. 2020, 277, 119177. [Google Scholar] [CrossRef]
- Noh, K.-J.; Im, H.; Lim, C.; Jang, M.G.; Nam, I.; Han, J.W. Tunable nano-distribution of Pt on TiO2 nanotubes by atomic compression control for high-efficient oxygen reduction reaction. Chem. Eng. J. 2022, 427, 131568. [Google Scholar] [CrossRef]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hubner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef]
- Tao, L.; Shi, Y.; Huang, Y.-C.; Chen, R.; Zhang, Y.; Huo, J.; Zou, Y.; Yu, G.; Luo, J.; Dong, C.-L.; et al. Interface engineering of Pt and CeO2 nanorods with unique interaction for methanol oxidation. Nano Energy 2018, 53, 604–612. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Chang, Y.; Yang, Z.; Song, X.; Zhou, W.; Wang, J.; Li, H. Pt3Cu alloy anchored on nanoporous WO3 with high activity and stability in methanol oxidation. Int. J. Hydrogen Energy 2024, 50, 1441–1449. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhi, H.; Yang, L.; Xu, F. The heat-promoted metal–support interaction of a PtCu/SiO2 carbon-free catalyst for the methanol oxidation and oxygen reduction reactions. RSC Sustain. 2023, 1, 1989–1994. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhen, S.; Sun, G.; Pei, C.; Zhao, Z.J.; Gong, J. Support stabilized PtCu single-atom alloys for propane dehydrogenation. Chem. Sci. 2022, 13, 9537–9543. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Wang, Y.; Xu, F. Defect-Engineered Charge Transfer in a PtCu/Pr(x)Ce(1-x)O(2) Carbon-Free Catalyst for Promoting the Methanol Oxidation and Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2023, 15, 58296–58308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Yang, D.; Lv, H.; Zhang, C. Preparation optimization and single cell application of PtNi/C octahedral catalyst with enhanced ORR performance. Electrochim. Acta 2018, 288, 126–133. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yang, C.; Lu, J.; Cao, L.; Liang, H.-P. Surfactant-assisted implantation strategy for facile construction of Pt-based hybrid electrocatalyst to accelerate oxygen reduction reaction. Mater. Today Energy 2022, 24, 100919. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Y.; Cao, L.; Yang, L.; Fang, D.; Yi, B.; Shao, Z. Enhanced electrocatalytic performance of ultrathin PtNi alloy nanowires for oxygen reduction reaction. Front. Energy 2017, 11, 260–267. [Google Scholar] [CrossRef]
- He, W.; Han, X.; Jia, H.; Cai, J.; Zhou, Y.; Zheng, Z. AuPt Alloy Nanostructures with Tunable Composition and Enzyme-like Activities for Colorimetric Detection of Bisulfide. Sci. Rep. 2017, 7, 40103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Kuang, Q.; Xie, S.; Jiang, Z.; Xie, Z.; Zheng, L. Cu(2+)-assisted synthesis of hexoctahedral Au-Pd alloy nanocrystals with high-index facets. J. Am. Chem. Soc. 2011, 133, 17114–17117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Shi, Y.; Wu, F.; Xu, Y.; Zhou, T.; Niu, W.; Zhang, J.; Xu, G. Copper and iron mediated growth of surfactant-free PtCu and PtFe advanced electrocatalysts for water oxidation and oxygen reduction. Electrochem. Sci. Adv. 2021, 2, e2100033. [Google Scholar] [CrossRef]
- Xiang, H.; Zheng, Y.; Sun, Y.; Guo, T.; Zhang, P.; Li, W.; Kong, S.; Ouzounian, M.; Chen, H.; Li, H.; et al. Bimetallic and postsynthetically alloyed PtCu nanostructures with tunable reactivity for the methanol oxidation reaction. Nanoscale Adv. 2020, 2, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Pavlets, A.; Alekseenko, A.; Kozhokar, E.; Pankov, I.; Alekseenko, D.; Guterman, V. Efficient Pt-based nanostructured electrocatalysts for fuel cells: One-pot preparation, gradient structure, effect of alloying, electrochemical performance. Int. J. Hydrogen Energy 2023, 48, 22379–22388. [Google Scholar] [CrossRef]
- Nie, M.; Xu, Z.; Luo, L.; Wang, Y.; Gan, W.; Yuan, Q. One-pot synthesis of ultrafine trimetallic PtPdCu alloy nanoparticles decorated on carbon nanotubes for bifunctional catalysis of ethanol oxidation and oxygen reduction. J. Colloid. Interface Sci. 2023, 643, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, W.; He, D.; Wan, J.; Pei, J.; Dong, J.; Wang, Y.; An, P.; Jin, Z.; Xing, W. Design of ultrathin Pt-Mo-Ni nanowire catalysts for ethanol electrooxidation. Sci. Adv. 2017, 3, e1603068. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Wang, W.; Guo, R.; Bi, W.; Guo, Y.; Jin, M. H(2)-Induced coalescence of Pt nanoparticles for the preparation of ultrathin Pt nanowires with high-density planar defects. Nanoscale 2019, 11, 14828–14835. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, W.; Shan, H.; Chen, W.; Shang, W.; Tao, P.; Song, C.; Addiego, C.; Deng, T.; Pan, X.; et al. Platinum-Based Nanowires as Active Catalysts toward Oxygen Reduction Reaction: In Situ Observation of Surface-Diffusion-Assisted, Solid-State Oriented Attachment. Adv. Mater. 2017, 29, 1703460. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-L.; Yin, P.; Zuo, L.-J.; Yin, S.-Y.; Lin, Y.; Zhang, W.; Chu, S.-Q.; Liang, H.-W.; Fu, X.-Z. Scalable Synthesis of Low-Pt PtCu3 Intermetallic Oxygen Reduction Electrocatalysts via Sulfur-Containing Inorganic Salt-Assisted Strategy. ACS Sustain. Chem. Eng. 2023, 11, 12093–12101. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, S.; Wu, M.; Wang, S.; Wang, Y.; Yang, H.; Chen, J.; Hao, X.; Zhi, C.; Wang, Y.; et al. Controllable Synthesis of PtIrCu Ternary Alloy Ultrathin Nanowires for Enhanced Ethanol Electrooxidation. ACS Appl. Mater. Interfaces 2023, 15, 3934–3940. [Google Scholar] [CrossRef]
- Chen, G.; Shan, H.; Li, Y.; Bao, H.; Hu, T.; Zhang, L.; Liu, S.; Ma, F. Hollow PtCu nanoparticles encapsulated into a carbon shellviamild annealing of Cu metal–organic frameworks. J. Mater. Chem. A 2020, 8, 10337–10345. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, Y.; Di, S.; Tian, L.; Wang, F.; Wang, Z.; Lu, Y. Bimetallic ZIF-Based PtCuCo/NC Electrocatalyst Pt Supported with an N-Doped Porous Carbon for Oxygen Reduction Reaction in PEM Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 1575–1584. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Lou, X.W. Formation of Asymmetric Bowl-Like Mesoporous Particles via Emulsion-Induced Interface Anisotropic Assembly. J. Am. Chem. Soc. 2016, 138, 11306–11311. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.C. Hollowing Sn-doped TiO2 nanospheres via Ostwald ripening. J. Am. Chem. Soc. 2007, 129, 15839–15847. [Google Scholar] [CrossRef]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow Micro-/Nanostructures: Synthesis and Applications. Adv. Mater. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Luo, X.; Fu, C.; Shen, S.; Luo, L.; Zhang, J. Free–templated synthesis of N–doped PtCu porous hollow nanospheres for efficient ethanol oxidation and oxygen reduction reactions. Appl. Catal. B Environ. 2023, 330, 122602. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Liu, S.; Zhou, X.; Xu, L.; Tian, X.; Yang, J.; Tang, Y. Self-Templating-Oriented Manipulation of Ultrafine Pt(3) Cu Alloyed Nanoparticles into Asymmetric Porous Bowl-Shaped Configuration for High-Efficiency Methanol Electrooxidation. Small 2022, 18, e2202782. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cai, S.; Lin, B.; Yang, L.; Le, H.; Mu, S. Geometric Engineering of Porous PtCu Nanotubes with Ultrahigh Methanol Oxidation and Oxygen Reduction Capability. Small 2022, 18, e2107387. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Recnik, A.; Bele, M.; Hocevar, S.; Gaberscek, M.; Mayrhofer, K.J. Effect of ordering of PtCu(3) nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [PubMed]
- Gatalo, M.; Ruiz-Zepeda, F.; Hodnik, N.; Dražić, G.; Bele, M.; Gaberšček, M. Insights into thermal annealing of highly-active PtCu3/C Oxygen Reduction Reaction electrocatalyst: An in-situ heating transmission Electron microscopy study. Nano Energy 2019, 63, 103892. [Google Scholar] [CrossRef]
- Yang, P.; Devasenathipathy, R.; Xu, W.; Wang, Z.; Chen, D.-H.; Zhang, X.; Fan, Y.; Chen, W. Pt1(CeO2)0.5 Nanoparticles Supported on Multiwalled Carbon Nanotubes for Methanol Electro-oxidation. ACS Appl. Nano Mater. 2021, 4, 10584–10591. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Cui, C.; Strasser, P. Thermal Facet Healing of Concave Octahedral Pt–Ni Nanoparticles Imaged in Situ at the Atomic Scale: Implications for the Rational Synthesis of Durable High-Performance ORR Electrocatalysts. ACS Catal. 2015, 6, 692–695. [Google Scholar] [CrossRef]
- Liu, Q.; Tripp, J.; Mitchell, C.; Rzepka, P.; Sadykov, I.I.; Beck, A.; Krumeich, F.; Nundy, S.; Artiglia, L.; Ranocchiari, M.; et al. Influence of alloying and surface overcoating engineering on the electrochemical properties of carbon-supported PtCu nanocrystals. J. Alloys Compd. 2023, 968, 172128. [Google Scholar] [CrossRef]
- Ye, X.; Shao, R.Y.; Yin, P.; Liang, H.W.; Chen, Y.X. Ordered Intermetallic PtCu Catalysts Made from Pt@Cu Core/Shell Structures for Oxygen Reduction Reaction. Inorg. Chem. 2022, 61, 15239–15246. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yao, Y.; Ko, B.H.; Huang, Z.; Dong, Q.; Gao, J.; Chen, W.; Li, J.; Li, S.; Wang, X.; et al. Carbon-Supported High-Entropy Oxide Nanoparticles as Stable Electrocatalysts for Oxygen Reduction Reactions. Adv. Funct. Mater. 2021, 31, 2010561. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, X.; Dong, Q.; Zheng, H.; Chen, G.; Hong, M.; Yang, C.-P.; Wu, M.; He, K.; Hu, L. A high-entropy phosphate catalyst for oxygen evolution reaction. Nano Energy 2021, 86, 106029. [Google Scholar] [CrossRef]
- Cui, S.-K.; Guo, D.-J. Microwave-assisted preparation of PtCu/C nanoalloys and their catalytic properties for oxygen reduction reaction. J. Alloys Compd. 2021, 874, 159869. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water splitting: From electrode to green energy system. Nano Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Atomically dispersed catalysts for hydrogen/oxygen evolution reactions and overall water splitting. J. Power Sources 2020, 471, 228446. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, T.; Han, J.; Qi, H.; Jiao, S.; Hou, C.; Guan, J. Interface engineering of Fe-Sn-Co sulfide/oxyhydroxide heterostructural electrocatalyst for synergistic water splitting. Nano Res. Energy 2024, 3, e9120106. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.-X.; Gou, W.; Zhang, M.; Xia, Z.; Zhang, S.; Chang, C.-R.; Ma, Y.; Qu, Y. Ethylene-glycol ligand environment facilitates highly efficient hydrogen evolution of Pt/CoP through proton concentration and hydrogen spillover. Energy Environ. Sci. 2019, 12, 2298–2304. [Google Scholar] [CrossRef]
- Li, W.; Hu, Z.-Y.; Zhang, Z.; Wei, P.; Zhang, J.; Pu, Z.; Zhu, J.; He, D.; Mu, S.; Van Tendeloo, G. Nano-single crystal coalesced PtCu nanospheres as robust bifunctional catalyst for hydrogen evolution and oxygen reduction reactions. J. Catal. 2019, 375, 164–170. [Google Scholar] [CrossRef]
- Shao, M.; Adzic, R. Electrooxidation of ethanol on a Pt electrode in acid solutions: In situ ATR-SEIRAS study. Electrochim. Acta 2005, 50, 2415–2422. [Google Scholar] [CrossRef]
- Dos Reis, R.G.; Colmati, F. Electrochemical alcohol oxidation: A comparative study of the behavior of methanol, ethanol, propanol, and butanol on carbon-supported PtSn, PtCu, and Pt nanoparticles. J. Solid. State Electrochem. 2016, 20, 2559–2567. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, G.; Zhang, Y.; Guo, T.; Chang, F.; Zhong, C.-J. Composition-tunable PtCu alloy nanowires and electrocatalytic synergy for methanol oxidation reaction. J. Phys. Chem. C 2016, 120, 10476–10484. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Carrera-Cerritos, R.; Sebastián, D.; Ledesma-García, J.; Arriaga, L.; Aricò, A.; Baglio, V. PtCu catalyst for the electro-oxidation of ethanol in an alkaline direct alcohol fuel cell. Int. J. Hydrogen Energy 2017, 42, 27919–27928. [Google Scholar] [CrossRef]
- Wang, K.; Sriphathoorat, R.; Luo, S.; Tang, M.; Du, H.; Shen, P.K. Ultrathin PtCu hexapod nanocrystals with enhanced catalytic performance for electro-oxidation reactions. J. Mater. Chem. A 2016, 4, 13425–13430. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Zhang, W.; Hu, H.; Wang, C.; Huang, C.; Wang, Y. Tailoring the morphology of Pt 3 Cu 1 nanocrystals supported on graphene nanoplates for ethanol oxidation. Nanoscale 2016, 8, 3075–3084. [Google Scholar] [CrossRef]
- Liu, T.; Wang, K.; Yuan, Q.; Shen, Z.; Wang, Y.; Zhang, Q.; Wang, X. Monodispersed sub-5.0 nm PtCu nanoalloys as enhanced bifunctional electrocatalysts for oxygen reduction reaction and ethanol oxidation reaction. Nanoscale 2017, 9, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Joghee, P.; Malik, J.N.; Pylypenko, S.; O’Hayre, R. A review on direct methanol fuel cells–In the perspective of energy and sustainability. MRS Energy Sustain. 2015, 2, E3. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.Z.; Ying, J.; Tian, G.; Liu, Y.; Geng, W.; Hu, J.; Lu, Y.; Chang, G.G.; Ozoemena, K.I. Ultimate Corrosion to Pt-Cu Electrocatalysts for Enhancing Methanol Oxidation Activity and Stability in Acidic Media. Chem.—Eur. J. 2021, 27, 9124–9128. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Rao, P.; Deng, P.; Li, J.; Chen, Q.; Shen, Y.; Tian, X. Three-dimensional porous PtCu as highly efficient electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2022, 47, 35701–35708. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.-F.; Fu, C.; Zhang, R.-H.; Zhan, W.; Wang, P.; Zhang, X.; Wang, Q.; Zhou, X.-W. In-situ loading synthesis of graphene supported PtCu nanocube and its high activity and stability for methanol oxidation reaction. J. Colloid. Interface Sci. 2021, 595, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, L.; Pan, J.; Li, K.; Zeng, B.; Cui, Z.; Liu, Q. Understanding the key role of the surface structure of L11-ordered PtCu intermetallic electrocatalyst toward methanol oxidation reaction by dealloying methods. Appl. Surf. Sci. 2023, 618, 156573. [Google Scholar] [CrossRef]
- Oberhauser, W.; Poggini, L.; Capozzoli, L.; Bellini, M.; Filippi, J.; Vizza, F. Oxidation of Ethanol to Acetic Acid by Supported PtCu Nanoparticles Stabilized by a Diamine Ligand. Inorg. Chem. 2023, 62, 2848–2858. [Google Scholar] [CrossRef]
- Han, C.; Lyu, Y.; Wang, S.; Liu, B.; Zhang, Y.; Weigand, J.J.; Du, H.; Lu, J. Highly Utilized Active Sites on Pt@ Cu/C for Ethanol Electrocatalytic Oxidation in Alkali Metal Hydroxide Solutions. Adv. Funct. Mater. 2023, 33, 2305436. [Google Scholar] [CrossRef]
- Sieben, J.M.; Alvarez, A.E.; Sanchez, M.D. Glycerol electrooxidation on carbon-supported Pt-CuO and PtCu-CuO catalysts. Electrochim. Acta 2023, 439, 141672. [Google Scholar] [CrossRef]
- Sievi, G.; Geburtig, D.; Skeledzic, T.; Bösmann, A.; Preuster, P.; Brummel, O.; Waidhas, F.; Montero, M.A.; Khanipour, P.; Katsounaros, I. Towards an efficient liquid organic hydrogen carrier fuel cell concept. Energy Environ. Sci. 2019, 12, 2305–2314. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Pishva, P.; Xie, R.; Peng, Z. Aqueous, Rechargeable Liquid Organic Hydrogen Carrier Battery for High-Capacity, Safe Energy Storage. ACS Energy Lett. 2023, 8, 3727–3732. [Google Scholar] [CrossRef]
- Hauenstein, P.; Seeberger, D.; Wasserscheid, P.; Thiele, S. High performance direct organic fuel cell using the acetone/isopropanol liquid organic hydrogen carrier system. Electrochem. Commun. 2020, 118, 106786. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Y.; Zhang, W.; Wang, L.; Yang, S.; Liang, J.; Zhang, Z.; Zhang, X.; Jin, Z. Regulating the alloying degree and electronic structure of Pt–Au nanoparticles for high-efficiency direct C2+ alcohol fuel cells. Chem. Mater. 2021, 33, 3767–3778. [Google Scholar] [CrossRef]
- Alekseenko, A.; Moguchikh, E.; Safronenko, O.; Guterman, V. Durability of de-alloyed PtCu/C electrocatalysts. Int. J. Hydrogen Energy 2018, 43, 22885–22895. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, G.; Lu, W.; Qin, X.; Shao, Z.; Yi, B. Preparation of hollow PtCu nanoparticles as high-performance electrocatalysts for oxygen reduction reaction in the absence of a surfactant. RSC Adv. 2016, 6, 39993–40001. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W.D. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Sarkar, A.; Manthiram, A. Synthesis of Pt@ Cu core− shell nanoparticles by galvanic displacement of Cu by Pt4+ ions and their application as electrocatalysts for oxygen reduction reaction in fuel cells. J. Phys. Chem. C 2010, 114, 4725–4732. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Cheng, T.; Guo, H.; Sun, B.; Wang, Y. Preparation and application in assembling high-performance fuel cell catalysts of colloidal PtCu alloy nanoclusters. J. Power Sources 2018, 395, 66–76. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, H.; Liu, S.; Zhang, B.; Zhong, H.; Su, D.S. Facile synthesis of supported Pt–Cu nanoparticles with surface enriched Pt as highly active cathode catalyst for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 17978–17983. [Google Scholar] [CrossRef]
- Johansson, T.P.; Ulrikkeholm, E.T.; Hernandez-Fernandez, P.; Escudero-Escribano, M.; Malacrida, P.; Stephens, I.; Chorkendorff, I. Towards the elucidation of the high oxygen electroreduction activity of Pt x Y: Surface science and electrochemical studies of Y/Pt (111). Phys. Chem. Chem. Phys. 2014, 16, 13718–13725. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Brault, P.; Coutanceau, C.; Caillard, A.; Baranton, S. Pt3MeAu (Me= Ni, Cu) fuel cell nanocatalyst growth, shapes, and efficiency: A molecular dynamics simulation approach. J. Phys. Chem. C 2019, 123, 29656–29664. [Google Scholar] [CrossRef]
- Asano, M.; Kawamura, R.; Sasakawa, R.; Todoroki, N.; Wadayama, T. Oxygen reduction reaction activity for strain-controlled Pt-based model alloy catalysts: Surface strains and direct electronic effects induced by alloying elements. ACS Catal. 2016, 6, 5285–5289. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Liu, J.; Wang, F. Dependent relationship between quantitative lattice contraction and enhanced oxygen reduction activity over Pt–Cu alloy catalysts. ACS Appl. Mater. Interfaces 2017, 9, 35740–35748. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Zhao, Y.; Hu, Y.; Zhao, F.; Chen, N.; Qu, L. A dually spontaneous reduction and assembly strategy for hybrid capsules of graphene quantum dots with platinum–copper nanoparticles for enhanced oxygen reduction reaction. Carbon 2014, 74, 170–179. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, D.; Deng, Z.; Zhang, R.; Xia, W.; Zhao, T.; Liu, X.; Shen, T.; Hu, Y.; Lu, Y. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction. Appl. Catal. B 2021, 282, 119617. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, K.; Shen, L.; Ding, C.; Lu, Z.; Tan, H.; Guo, C.; Yan, C. Understanding the in-plane electron transport in low noble metal proton exchange membrane water electrolyser. J. Power Sources 2022, 549, 232130. [Google Scholar] [CrossRef]
- Grandi, M.; Gatalo, M.; Kamšek, A.R.; Kapun, G.; Mayer, K.; Ruiz-Zepeda, F.; Šala, M.; Marius, B.; Bele, M.; Hodnik, N. Mechanistic Study of Fast Performance Decay of PtCu Alloy-based Catalyst Layers for Polymer Electrolyte Fuel Cells through Electrochemical Impedance Spectroscopy. Materials 2023, 16, 3544. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, L.; Yuan, Q.; Zhang, Q.; Sheng, T.; Yang, X.; Gu, L.; Wang, X. PtCu subnanoclusters epitaxial on octahedral PtCu/Pt skin matrix as ultrahigh stable cathode electrocatalysts for room-temperature hydrogen fuel cells. Nano Res. 2023, 16, 2252–2258. [Google Scholar] [CrossRef]
- Tang, J.; Xie, R.; Pishva, P.; Shen, X.; Zhu, Y.; Peng, Z. Recent progress and perspectives of liquid organic hydrogen carrier electrochemistry for energy applications. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, J.; Li, L.; Hu, T.; Qiu, M.; Lützenkirchen-Hecht, D.; Yuan, K.; Chen, Y. Arranging Electronic Localization of PtCu Nanoalloys to Stimulate Improved Oxygen Electroreduction for High-Performance Fuel Cells. CCS Chem. 2023, 5, 2545–2556. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; He, Y.; Du, S. Catalyst electrodes with PtCu nanowire arrays in situ grown on gas diffusion layers for direct formic acid fuel cells. ACS Appl. Mater. Interfaces 2022, 14, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Falina, I.; Pavlets, A.; Alekseenko, A.; Titskaya, E.; Kononenko, N. Influence of PtCu/C catalysts composition on electrochemical characteristics of polymer electrolyte fuel cell and properties of proton exchange membrane. Catalysts 2021, 11, 1063. [Google Scholar] [CrossRef]
- Zhang, X.; An, Z.; Xia, Z.; Li, H.; Xu, X.; Yu, S.; Wang, S.; Sun, G. Phosphoric acid resistance PtCu/C oxygen reduction reaction electrocatalyst for HT-PEMFCs: A theoretical and experimental study. Appl. Surf. Sci. 2023, 619, 156663. [Google Scholar] [CrossRef]
- Alekseenko, A.; Pavlets, A.; Mikheykin, A.; Belenov, S.; Guterman, E. The integrated approach to studying the microstructure of de-alloyed PtCu/C electrocatalysts for PEMFCs. Appl. Surf. Sci. 2023, 631, 157539. [Google Scholar] [CrossRef]
- Matsui, H.; Shoji, A.; Chen, C.; Zhao, X.; Uruga, T.; Tada, M. Local structures and robust oxygen reduction performances of TiN-supported bimetallic Pt–Cu electrocatalysts for fuel cells. Catal. Sci. Technol. 2024, 14, 1501–1511. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Yan, H.; Sun, L.; Chen, S.; Zhang, S.; Zhang, J. Manipulating Pt-skin of porous network Pt-Cu alloy nanospheres toward efficient oxygen reduction. J. Colloid. Interface Sci. 2023, 652, 1006–1015. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, Q. PtCu/Pt core/atomic-layer shell hollow octahedra for oxygen reduction and methanol oxidation electrocatalysis. Chem. Commun. 2024, 60, 2918–2921. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Z.; Liang, J.; Li, S.; Lu, G.; Priest, C.; Wang, T.; Han, J.; Wu, G.; Wang, X. Inducing Covalent Atomic Interaction in Intermetallic Pt Alloy Nanocatalysts for High-Performance Fuel Cells. Angew. Chem. Int. Ed. 2023, 62, e202302134. [Google Scholar] [CrossRef]
- An, Z.; Li, H.; Zhang, X.; Xia, Z.; Zhang, H.; Chu, W.; Yu, S.; Wang, S.; Sun, G. Ultrastable and Phosphoric Acid-Resistant PtRhCu@ Pt Oxygen Reduction Electrocatalyst for High-Temperature Polymer Electrolyte Fuel Cells. ACS Catal. 2024, 14, 2572–2581. [Google Scholar] [CrossRef]
- Ju, B.; Song, H.J.; Yoon, H.; Kim, D.K.; Jin, H.W.; Lim, T.; Kim, D.-W. Understanding the Metal Carbonyl-Assisted Synthesis of PtMoCu/C Nanocatalysts for Proton-Exchange Membrane Fuel Cells. ACS Sustain. Chem. Eng. 2023, 11, 16108–16116. [Google Scholar] [CrossRef]










| PtCu Synthesis | Categorizations | Examples | Pros and Cons |
|---|---|---|---|
| Pre-Synthesis: Modification of Innovative Supports | Modified Carbon Supports | HNO3 and H2O2 modification on pristine carbon [53] |
|
| C-PPy [54] | |||
| Graphene [55] | |||
| NS-PCNF [11] | |||
| APGE [56] | |||
| Metal Oxide Supports | WO3 [61] |
| |
| SiO2 [62] | |||
| CuSiO3 [63] | |||
| TiO2−xN [12] | |||
| Pr0.15Ce0.85O2 [64] | |||
| Reactant Selection and Synthesis Strategies | Surfactant-Free Synthesis | Using ascorbic acid in water-based solvent at 40 °C [70] |
|
| Cu nanowires reacted with ascorbic acid [71] | |||
| Stepwise one-pot synthesis using NaBH4 reduction [72] | |||
| One-pot solvothermal method using ascorbic acid [73] | |||
| Special intermediate-assisted synthesis | DMF decomposes to produce H2 intermediates [78] |
| |
| CuS2 intermediates [77] | |||
| MOFs intermediates [79] | |||
| Escherichia coli [14] | |||
| CuCo-ZIF intermediate [80] | |||
| Advanced template strategy | Reverse diffusion, template-free method [85] |
| |
| Self-templated with the assistance of MBAA [86] | |||
| Self-template using Cu nanowires [87] | |||
| Post-treatment: Advanced High-Temperature Processing Strategies | Surface overcoating | Carbon nano-shells [93] |
|
| Core/shell structures [94] | |||
| Novel High-temperature processing strategy | Thermal shock irradiation approach [16] |
| |
| Microwave-assist [98] |
| PtCu Catalyzed Reaction | Catalyst | Structure | Performance | Durability | Pros and Cons |
|---|---|---|---|---|---|
| HER | PtCu/WO3@CF [23] | hollow nanospheres | 1.35 A mg−1Pt at overpotentials of 20 mV | 2000 cycles |
|
| HER | PtCu-Mo2C [26] | MOF | 1 A mgPt−1 at −0.04 V | 5000 cycles | |
| HER | phs-PtCu [27] | porous helical-spiny-like nanowires | 85 mA/cm2 at 200 mV | 1000 cycles | |
| HER | PtCu-NA [21] | nanoalloys | overpotentials of 224 mV at 100 mA cm–2 | 70 h | |
| HER | Pt1Cu3 NPs [25] | core–shell structure with a PtCu core and Pt-rich shell | 10 mV (acid) and 17 mV (alkaline) overpotentials at 10 mA cm−2 | 24 h (acid) and 9 h (alkaline) | |
| HER | PtCu NCs [93] | nanoporous and nanodendritic structure | 6.4 A/mgPt at 50 mV overpotential | 500 cycles | |
| HER | Pt5Cu2 NTs [28] | nanotubes | 34 (basic), 32 (acidic), and 284 (neutral) mV at 10 mA cm−2 | >50 h (basic), 10,000 cycles (acidic), and 30 h (neutral) | |
| HER/EOR | PtCu NF [19] | nanoframes with high-index facets and multi-channels | 0.58 V to reach 10 mA cm–2 | 7200 s | |
| HER | PtCu/CoP [20] | PtCu nanocluster decorated CoP nanosheet | overpotential of 20 mV at 10 mA cm−2 | 100 h in both acid and alkaline media | |
| MOR & ORR | PtCu/Pr0.15Ce0.85O2 [64] | one-dimensional PtCu | 1.05 (MOR) & 0.12 (ORR) A·mg–1 | 5000 cycles |
|
| MOR | PtCu RDFs [30] | rhombic dodecahedral nanoframes | 3.65 mA cm−2 | 1400 s | |
| MOR | PtCu BNCs-S [31] | branched nanocrystals with long and sharp arms | 1.59 A mg−1 | 500 cycles | |
| MOR | H-PNTs, A-PNTs and S-PNTs [87] | hollow nanospheres (H-PNTs), solid alloy (A-PNTs), and Pt-rich skinned nanoparticles (S-PNTs) | 1.33 (H-PNTs), 2.56 (A-PNTs), and 0.63 (S-PNTs) A mgPt–1 | 5000 cycles | |
| MOR | Pt-Cu [113] | 3D architecture with uniform interconnected pores | 302 mA/mg, 1.72 mA/cm2 | 5000 cycles | |
| MOR | P-PtCu [114] | porous 3D nanocubes | 2.3 A·mg−1Pt, 11.9 mA cm−2Pt | 1000 cycles | |
| MOR | PtCu-NCb [115] | nanocubes | 0.67 mA cm−2 | 500 cycles | |
| MOR | PtCu/C-700-ED, PtCu/C-700-CD [116] | L11-ordered with rough and smooth Pt shells | 1625.2 mA mgPt−1 | 2000 cycles | |
| MOR & EOR & IOR | PtCu [33] | chain-like nanoparticles | 253.1 (MOR), 187.7 (EOR) and 37.2 (IOR) mA cm−2 | 1000 s | |
| EOR & GOR | Pt1Cu1−x/C [34] | core–shell | 39.4 (EOR) & 24.5 (GOR)mA mg−1 | 5 h | |
| EOR and EGOR | PtCu TRNs [35] | dendritic triangular nanocrystals | 2079 (EOR) and 767 (EGOR) mA mg−1 | 1200 s | |
| EOR | PtCu [117] | nanoparticles | 3.0 mA/μg(Pt) | - | |
| EOR | Pt@Cu/C [118] | nanoparticles | 8184 mA mgPt−1 | 100 cycles | |
| EOR & ORR | N-doped Pt7Cu [85] | porous hollow nanospheres | 2.14 (EOR) and 1.42 (ORR) A mgPt–1 | - | |
| GOR | Pt0.85Cu0.15-CuO(3)/C [119] | nanoparticles | 270 mA mgPt−1 | 3500 s | |
| NH3OR | PtCu/G [38] | copper crystallites decorated with platinum nanoparticle | >90% efficiency | - |
|
| NRR & MOR | Pt6Cu [37] | networked nanocrystals | 6.15% FE (NRR) & 21.3 mA cm−2 (MOR) | 10 (NRR) and 500 (MOR) cycles | |
| NiRR | Cu-Pt [40] | nanocomposite foam | 84% selectivity toward ammonia | - | |
| NiRR | PtCuNi [39] | - | 99.6% selectivity to NH4+ | - | |
| ORR | Pt0.25Cu [42] | nanoparticles | Onset potential 0.98 V vs. RHE | 1000 cycles |
|
| ORR | O-PtCuNF/C [43] | nanoframes with an atomically ordered intermetallic structure | 2.5 A mgPt−1 | 10,000 cycles | |
| ORR | Int-PtCuN/KB [44] | N-doped rhombohedral ordered | 1.15 A mgPt–1 | 20,000 cycles | |
| ORR | O-PtCu/HMCS [45] | monodisperse nanosized intermetallics | 2.73 A cm–2Pt | 50,000 cycles | |
| MOR & ORR | PtCux−y/C [8] | - | 997 (MOR) and 200 (ORR) A/g(Pt) | 5000 cycles | |
| ORR | L11-PtCu [137] | intermetallics with an L11 structure | 0.82 A mg−1Pt, 1.24 mA cm−2 | 30,000 cycles | |
| ORR | PtCu–H2-600 [94] | ordered nanoparticles | 1.85 mA cm–2 | 3000 cycles |
| Catalyst | PtCu Catalyzed Reaction in Fuel Cell | Max Current Density (A cm−2) | Max Power Density (mW cm−2) | ECSA (m2 gPt−1) | Reference |
|---|---|---|---|---|---|
| PtCu/KB_0.8 | ORR | 1 | ~350 | 57.9 | [139] |
| PtCu1.60/C | ORR | ~1.5 | 318.8 | 48.1 | [140] |
| PtCuNC-700 | ORR | ~2.3 | 929.7 | 41.6 | [142] |
| Pt3Cu1 NW | Formic acid oxidation reaction | ~0.5 | 116.3 | 15.8 | [143] |
| PtCu0.3/C | ORR | ~0.06 | - | 32 | [144] |
| Pt2Cu/C | ORR | 1 | 383.4 | - | [145] |
| PtCu/C-N | ORR | ~2300 (A gPt−1) | ~275 | 45 | [146] |
| PtCu-1.0/TiN | ORR | 4 A (mg−1) | 1500 (mW mg−1) | 60.3 | [147] |
| PtCuNSs/C | ORR | ~4.2 | 1200 | 41.96 | [148] |
| H-PtCu/PtL OHs/C | ORR & MOR | ~130 | 55.7 | 82.3 | [149] |
| PtCuCo/NC | ORR | 1.7 | 642.86 | 124.4 | [80] |
| L10−Pt2CuGa/C | ORR | ~6.5 | 2600 | 48.6 | [150] |
| PtRhCu@Pt/C | ORR | ~1.5 | 977 | 91.4 | [151] |
| PtMoCu/C | ORR | ~4 | 1300 | 32.4 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Tang, J.; Shen, X. Recent Advances of PtCu Alloy in Electrocatalysis: Innovations and Applications. Catalysts 2024, 14, 373. https://doi.org/10.3390/catal14060373
Shen Z, Tang J, Shen X. Recent Advances of PtCu Alloy in Electrocatalysis: Innovations and Applications. Catalysts. 2024; 14(6):373. https://doi.org/10.3390/catal14060373
Chicago/Turabian StyleShen, Ziyang, Jinyao Tang, and Xiaochen Shen. 2024. "Recent Advances of PtCu Alloy in Electrocatalysis: Innovations and Applications" Catalysts 14, no. 6: 373. https://doi.org/10.3390/catal14060373
APA StyleShen, Z., Tang, J., & Shen, X. (2024). Recent Advances of PtCu Alloy in Electrocatalysis: Innovations and Applications. Catalysts, 14(6), 373. https://doi.org/10.3390/catal14060373









