Design of Experiments for Process Optimization of the Direct Wacker-Type Oxidation of 1-Decene to n-Decanal
Abstract
1. Introduction
Reaction System
2. Results and Calculations
2.1. Original Conditions
2.2. Seven-Factor DoE Results
2.3. Results of Optimization
2.4. Validation Experiments
3. Methodology
3.1. Materials
3.2. Experimental Setup and Analysis for the Reaction of 1-Decene to n-Decanal
3.3. Initial DoE
3.4. Optimization
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| p-value | The p-value is a measure that helps determine the statistical significance of the observed results in a hypothesis test. It represents the probability of obtaining results as extreme as the observed results, assuming that the null hypothesis is true. In statistical analysis, if the p-value is less than a predetermined significance level (commonly 0.05), the null hypothesis is rejected, indicating that the results are statistically significant. A lower p-value suggests stronger evidence against the null hypothesis and greater statistical significance. |
| VIF (Variance Inflation Factor) | The VIF is a measure used to assess multicollinearity among independent variables in a regression analysis. Multicollinearity occurs when independent variables in a regression model are highly correlated with each other. The VIF quantifies how much the variance of an estimated regression coefficient is inflated due to multicollinearity. A VIF value of 1 indicates no multicollinearity, while values greater than 5 or 10 are often considered indicative of multicollinearity. High VIF values suggest that the corresponding independent variable may be redundant or highly correlated with other variables in the model, which can affect the interpretation of regression coefficients and the overall reliability of the regression analysis. |
| Effect | The effect, also known as the effect size, represents the estimated impact of an independent variable (factor) on the dependent variable (response) in a statistical model. It indicates the magnitude of the change in the dependent variable for a one-unit change in the independent variable, while holding all other variables constant. In the context of regression analysis, the effect size is typically measured by the coefficient (Coef) associated with each independent variable. |
| Coef | The coefficient, also known as the estimated coefficient or parameter estimate, represents the estimated effect size of an independent variable on the dependent variable. It indicates the change in the dependent variable for a one-unit change in the independent variable, while holding other variables constant. |
| SE Coef | The standard error of the coefficient measures the variability or uncertainty in the estimated coefficient. It represents the standard deviation of the coefficient’s sampling distribution. A smaller standard error suggests more precise estimates. |
| t-value | The t-value is the ratio of the estimated coefficient to its standard error. It indicates the number of standard deviations that the coefficient estimate is away from zero. A larger t-value suggests a more statistically significant coefficient. Typically, t-values are used to assess whether a coefficient is significantly different from zero. |
References
- Murray, P.M.; Bellany, F.; Benhamou, L.; Bučar, D.-K.; Tabor, A.B.; Sheppard, T.D. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org. Biomol. Chem. 2016, 14, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.E. Design of Experiments in Pharmaceutical Process Research and Development. In Chemical Process Research; Abdel-Magid, A.F., Ragan, J.A., Eds.; American Chemical Society: Washington, DC, USA, 2003; pp. 87–109. ISBN 9780841238244. [Google Scholar]
- Mullin, R. Statistical Method Makes a Comeback. American Chemical Society [Online], 20201022. Available online: https://cen.acs.org/articles/91/i13/Design-Experiments-Makes-Comeback.html (accessed on 25 January 2024).
- Nadin, A.; Hattotuwagama, C.; Churcher, I. Lead-oriented synthesis: A new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 2012, 51, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.A.; Anderson, N.G. Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Tye, H. Application of statistical ‘design of experiments’ methods in drug discovery. Drug Discov. Today 2004, 9, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; Wiley: Hoboken, NJ, USA, 2017; ISBN 9781119113478. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; 1. paperback; Oxford University Press: Oxford, UK, 2000; ISBN 0198502346. [Google Scholar]
- Lendrem, D.; Owen, M.; Godbert, S. DOE (Design of Experiments) in Development Chemistry: Potential Obstacles. Org. Process Res. Dev. 2001, 5, 324–327. [Google Scholar] [CrossRef]
- Weissman, S.A. Application of Design of Experiments to Process Development. Org. Process Res. Dev. 2015, 19, 1604. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The Design Of Optimum Multifactorial Experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Sanchez, S.M.; Sanchez, P.J. Very large fractional factorial and central composite designs. ACM Trans. Model. Comput. Simul. 2005, 15, 362–377. [Google Scholar] [CrossRef]
- Zhou, Y.-D.; Xu, H. Composite Designs Based on Orthogonal Arrays and Definitive Screening Designs. J. Am. Stat. Assoc. 2017, 112, 1675–1683. [Google Scholar] [CrossRef]
- Berger, M.; Wong, W.K. (Eds.) Applied Optimal Designs; Wiley: Hoboken, NJ, USA, 2005; ISBN 0470856971. [Google Scholar]
- Iordache, O. Evolvable Designs of Experiments: Applications for Circuits; Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-32424-8. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building; Wiley: New York, NY, USA, 1978; ISBN 0471093157. [Google Scholar]
- Hedayat, A.S.; Sloane, N.J.A.; Stufken, J. Orthogonal Arrays; Springer: New York, NY, USA, 1999; ISBN 978-1-4612-7158-1. [Google Scholar]
- Lucas, S.; Vidal, E.; Amiri, A.; Hanlon, S.; Amengual, J.C. A comparison of syntactic and statistical techniques for off-line OCR. In Grammatical Inference and Applications; Carbonell, J.G., Siekmann, J., Goos, G., Hartmanis, J., Leeuwen, J., Carrasco, R.C., Oncina, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 168–179. ISBN 978-3-540-58473-5. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 0471718130. [Google Scholar]
- Carlson, R.; Carlson, J.E. Design and Optimization in Organic Synthesis, 2nd ed.; Elsevier: Amsterdam, The Netherlands; San Diego, CA, USA, 2005; ISBN 9780080455273. [Google Scholar]
- Lazić, Ž.R. Design of Experiments in Chemical Engineering: A Practical Guide; Wiley-VCH: Weinheim, Germany, 2004; ISBN 9783527604166. [Google Scholar]
- Owen, M.R.; Luscombe, C.; Lai; Godbert, S.; Crookes, D.L.; Emiabata-Smith, D. Efficiency by Design: Optimisation in Process Research. Org. Process Res. Dev. 2001, 5, 308–323. [Google Scholar] [CrossRef]
- Owen, S.C.; Shoichet, M.S. Design of three-dimensional biomimetic scaffolds. J. Biomedical. Mater. Res. 2010, 94A, 1321–1331. [Google Scholar] [CrossRef]
- Rekab, K.; Shaikh, M. Statistical Design of Experiments with Engineering Applications; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-57444-625-8. [Google Scholar]
- Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; release 2015; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527306732.
- Fugmann, B.; Lang-Fugmann, S.; Steglich, W. (Eds.) RÖMPP Encyclopedia Natural Products, 1st ed.; 1. Auflage; Thieme: Stuttgart, Germany, 2014; ISBN 9783131795519. [Google Scholar]
- Keim, W. Oligomerization of ethylene to α-olefins: Discovery and development of the shell higher olefin process (SHOP). Angew. Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Walker, K.L.; Dornan, L.M.; Zare, R.N.; Waymouth, R.M.; Muldoon, M.J. Mechanism of Catalytic Oxidation of Styrenes with Hydrogen Peroxide in the Presence of Cationic Palladium(II) Complexes. J. Am. Chem. Soc. 2017, 139, 12495–12503. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J.; Nagashima, H.; Hori, K. A new preparative method for 1,3-dicarbonyl compounds by the regioselective oxidation of α,β-unsaturated carbonyl compounds, catalyzed by PdCl2 using hydroperoxides as the reoxidant of Pd0. Chem. Lett. 1980, 9, 257–260. [Google Scholar] [CrossRef]
- ICIS Explore. INTERVIEW: German Biofuels Producer Verbio Develops Ethenolysis-Based Renewable Chemicals Project|ICIS. Available online: https://www.icis.com/explore/resources/news/2024/03/13/10980232/interview-german-biofuels-producer-verbio-develops-ethenolysis-based-renewable-chemicals-project/ (accessed on 21 March 2024).
- Muzart, J. Aldehydes from Pd-catalysed oxidation of terminal olefins. Tetrahedron 2007, 63, 7505–7521. [Google Scholar] [CrossRef]
- Mann, S.; Benhamou, L.; Sheppard, T. Palladium(II)-Catalysed Oxidation of Alkenes. Synthesis 2015, 47, S19–S22. [Google Scholar] [CrossRef][Green Version]
- Cornils, B.; Herrmann, W.A.; Xu, J.-H.; Zanthoff, H.-W. (Eds.) Catalysis from A to Z: A Concise Encyclopedia; 5. aktualis. u. erw. Auflage; Wiley-VCH: Weinheim, Germany, 2019; ISBN 978-3-527-34311-9. [Google Scholar]
- Wenzel, T.T. Oxidation of olefins to aldehydes using a palladium–copper catalyst. J. Chem. Soc. Chem. Commun. 1993, 10, 862–864. [Google Scholar] [CrossRef]
- Usama, W.O.; Markewich, D.J.; East, A. Regioselectivity in Wacker oxidations of internal alkenes: Antiperiplanar effects? Can. J. Chem. 2023, 101, 579–584. [Google Scholar] [CrossRef]
- Michel, B.W.; Steffens, L.D.; Sigman, M.S. On the mechanism of the palladium-catalyzed tert-butylhydroperoxide-mediated Wacker-type oxidation of alkenes using quinoline-2-oxazoline ligands. J. Am. Chem. Soc. 2011, 133, 8317–8325. [Google Scholar] [CrossRef]
- Wright, J.A.; Gaunt, M.J.; Spencer, J.B. Novel anti-Markovnikov regioselectivity in the Wacker reaction of styrenes. Chemistry 2006, 12, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, G.; Jira, R.; Bolt, H.M.; Golka, K. Acetaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts: New Perspectives for the 21st Century, 2nd ed.; J. Wiley: Hoboken, NJ, USA, 2004; ISBN 0470850329. [Google Scholar]
- Dong, G.; Teo, P.; Wickens, Z.K.; Grubbs, R.H. Primary alcohols from terminal olefins: Formal anti-Markovnikov hydration via triple relay catalysis. Science 2011, 333, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.J.; Browne, W.R.; Feringa, B.L. Palladium-catalyzed anti-Markovnikov oxidation of terminal alkenes. Angew. Chem. Int. Ed. Engl. 2015, 54, 734–744. [Google Scholar] [CrossRef]
- Keith, J.A.; Henry, P.M. The mechanism of the Wacker reaction: A tale of two hydroxypalladations. Angew. Chem. Int. Ed. Engl. 2009, 48, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.; Wickens, Z.K.; Dong, G.; Grubbs, R.H. Efficient and highly aldehyde selective Wacker oxidation. Org. Lett. 2012, 14, 3237–3239. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L. Catalytic oxidation of alk-1-enes to aldehydes. J. Chem. Soc. Chem. Commun. 1986, 12, 909. [Google Scholar] [CrossRef]
- Carretero, J.C.; Arrayás, R.G. Dichloro Bis(acetonitrile) Palladium. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L.A., Ed.; Wiley: Chichester, UK, 1995; ISBN 0471936235. [Google Scholar]
- Ganesamoorthy, S.; Shanmugasundaram, K.; Karvembu, R. Remarkable catalytic activity of [PdCl2(CH3CN)2] in Suzuki–Miyaura cross-coupling reaction in aqueous media under mild conditions. J. Mol. Catal. A Chem. 2013, 371, 118–124. [Google Scholar] [CrossRef]
- Minitab, L. Minitab® Statistical Software; Minitab, L.L.C.: State College, PA, USA, 2022. [Google Scholar]




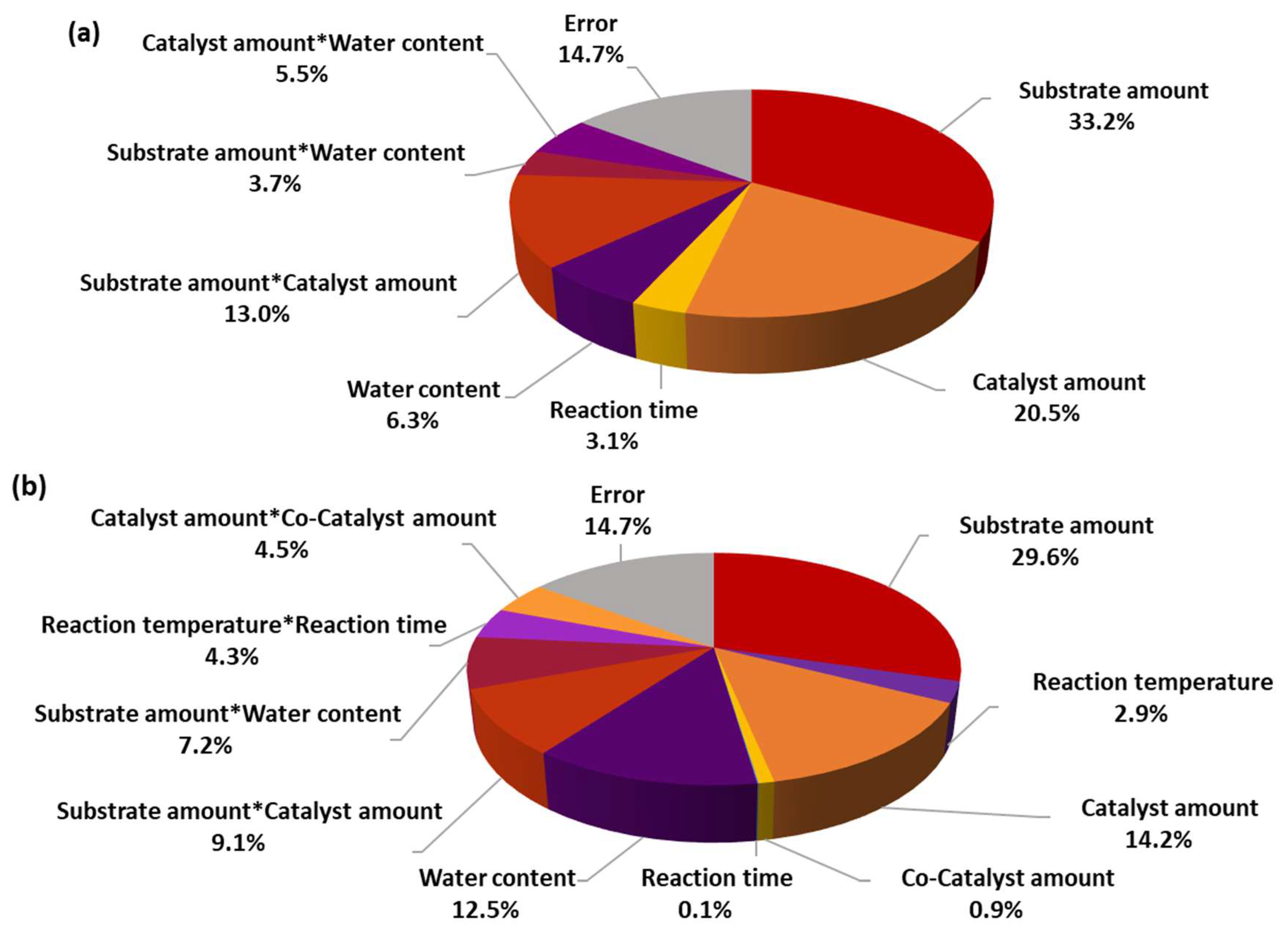
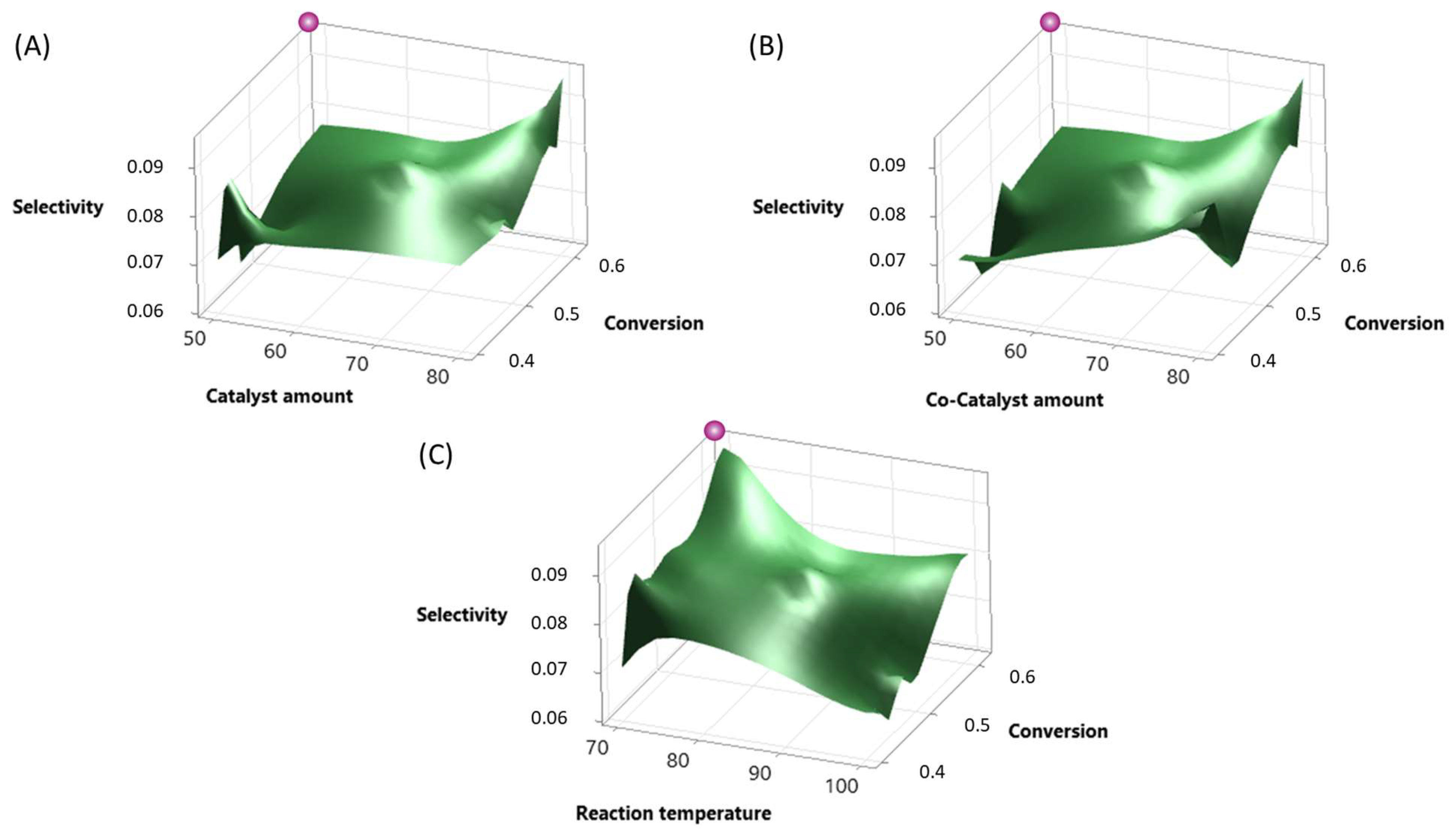
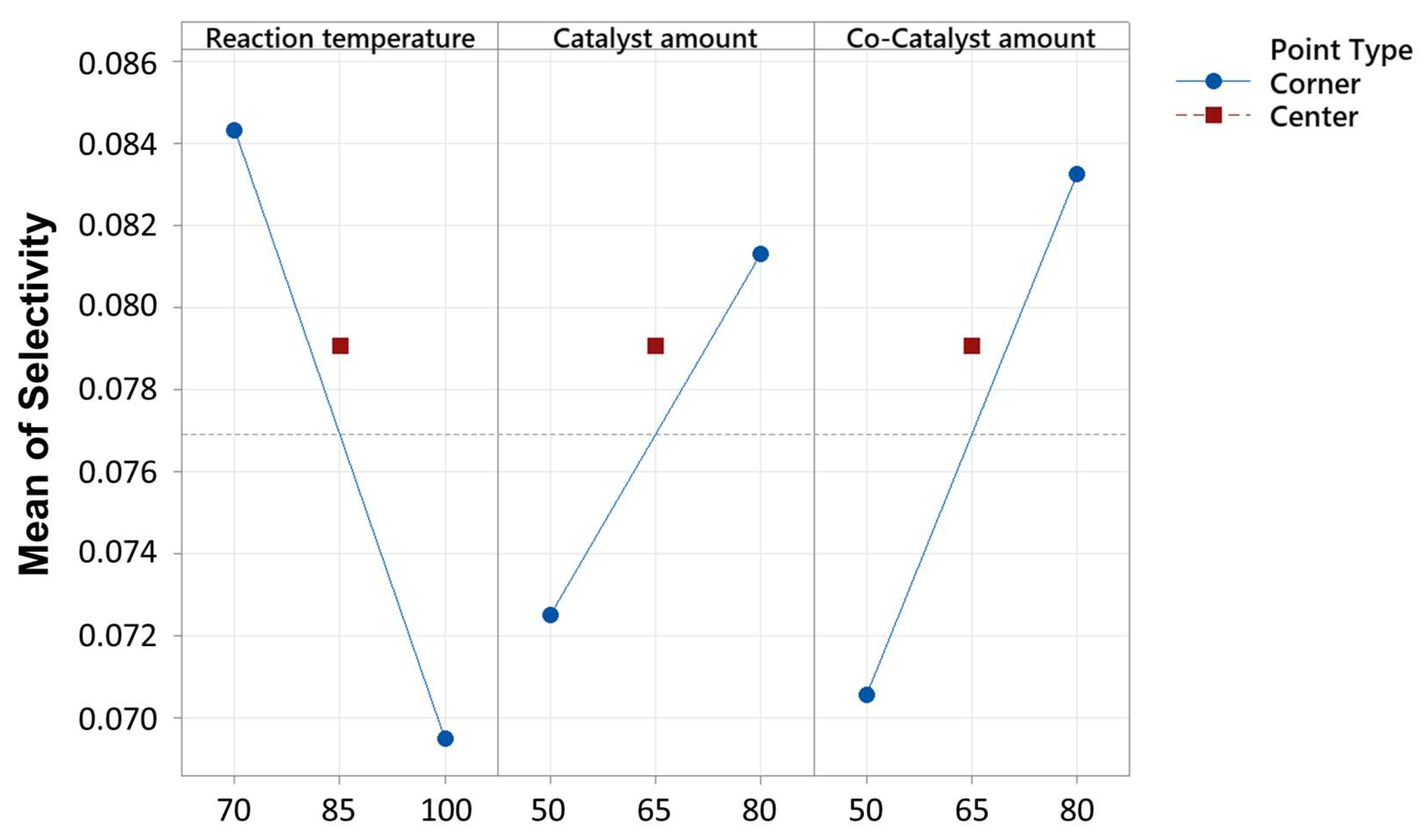
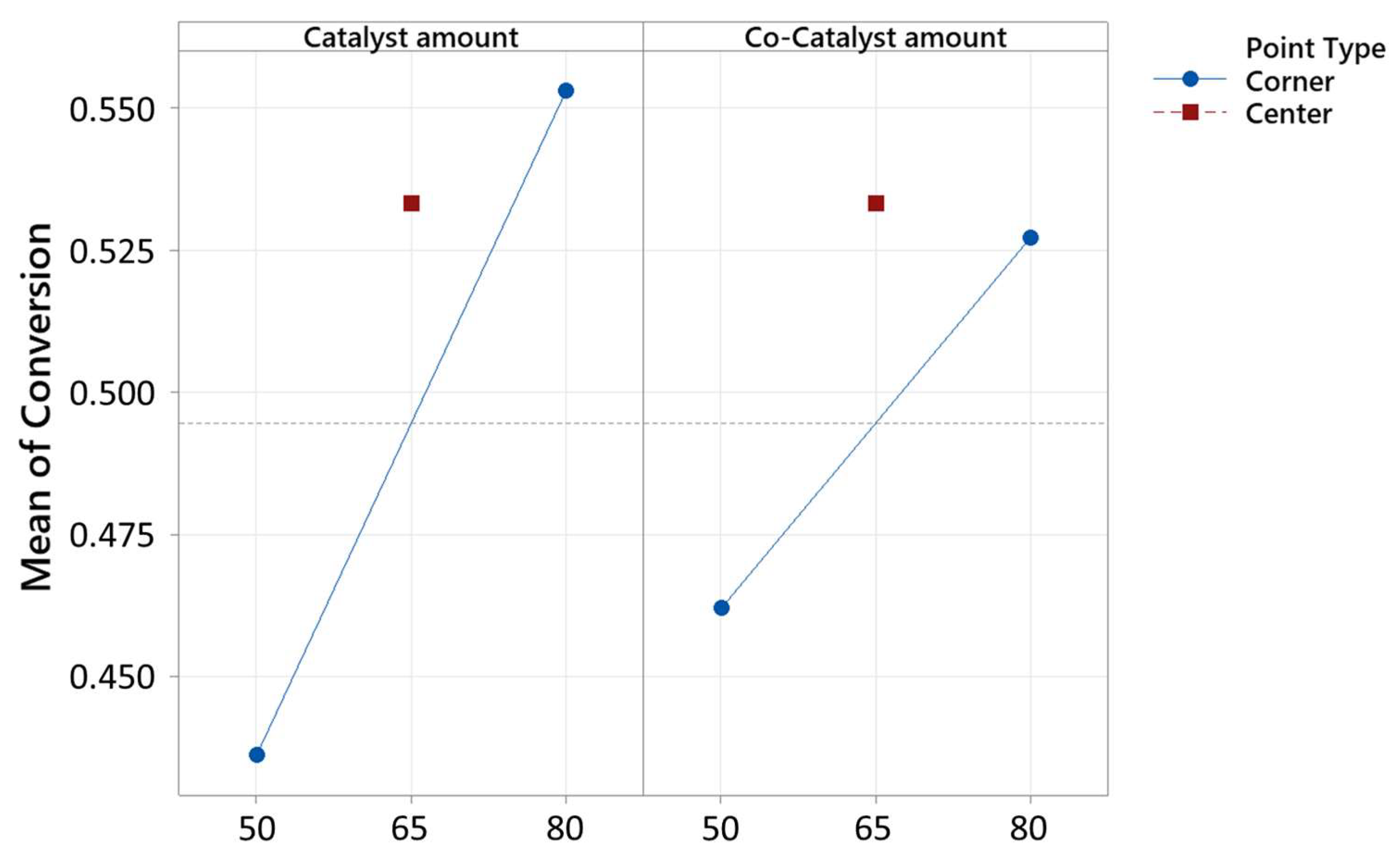

| Term | Effect | Coef. | SE Coef. | t-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 0.01786 | 0.00210 | 8.49 | 0.000 | ||
| Substrate amount | −0.02920 | −0.01460 | 0.00210 | −6.94 | 0.000 | 1.00 |
| Reaction temperature | 0.00914 | 0.00457 | 0.00210 | 2.17 | 0.040 | 1.00 |
| Catalyst amount | 0.02024 | 0.01012 | 0.00210 | 4.81 | 0.000 | 1.00 |
| Co-catalyst amount | 0.00498 | 0.00249 | 0.00210 | 1.18 | 0.249 | 1.00 |
| Reaction time | 0.00141 | 0.00070 | 0.00210 | 0.33 | 0.741 | 1.00 |
| Water content | −0.01899 | −0.00950 | 0.00210 | −4.51 | 0.000 | 1.00 |
| Substrate amount*Catalyst amount | −0.01621 | −0.00811 | 0.00210 | −3.85 | 0.001 | 1.00 |
| Substrate amount*Water content | 0.01439 | 0.00720 | 0.00210 | 3.42 | 0.002 | 1.00 |
| Reaction temperature*Reaction time | −0.01108 | −0.00554 | 0.00210 | −2.63 | 0.015 | 1.00 |
| Catalyst amount*Co-catalyst amount | 0.01136 | 0.00568 | 0.00210 | 2.70 | 0.013 | 1.00 |
| Central points | −0.00137 | 0.00631 | −0.22 | 0.829 | 1.00 |
| Term | Effect | Coef. | SE Coef. | t-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 0.39393 | 0.00885 | 44.49 | 0.000 | ||
| Substrate amount | −0.13843 | −0.06921 | 0.00885 | −7.82 | 0.000 | 1.00 |
| Catalyst amount | 0.10872 | 0.05436 | 0.00885 | 6.14 | 0.000 | 1.00 |
| Reaction time | 0.04246 | 0.02123 | 0.00885 | 2.40 | 0.024 | 1.00 |
| Water content | 0.06036 | 0.03018 | 0.00885 | 3.41 | 0.002 | 1.00 |
| Substrate amount*Catalyst amount | −0.08659 | −0.04330 | 0.00885 | −4.89 | 0.000 | 1.00 |
| Substrate amount*Water content | −0.04622 | −0.02311 | 0.00885 | −2.61 | 0.015 | 1.00 |
| Catalyst amount*Water content | 0.05650 | 0.02825 | 0.00885 | 3.19 | 0.004 | 1.00 |
| Central points | 0.0012 | 0.0266 | 0.05 | 0.963 | 1.00 |
| Term | Effect | Coef. | SE Coef. | t-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 0.07691 | 0.00103 | 72.07 | 0.000 | ||
| Reaction temperature | −0.01486 | −0.00743 | 0.00103 | −6.96 | 0.000 | 1.00 |
| Catalyst amount | 0.00884 | 0.00442 | 0.00103 | 4.14 | 0.004 | 1.00 |
| Co-catalyst amount | 0.01273 | 0.00637 | 0.00103 | 5.97 | 0.000 | 1.00 |
| Central points | 0.00217 | 0.00178 | 1.17 | 0.262 | 1.00 |
| Term | Effect | Coef. | SE Coef. | t-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 0.4946 | 0.0127 | 39.03 | 0.000 | ||
| Catalyst amount | 0.1168 | 0.0584 | 0.0127 | 4.61 | 0.002 | 1.00 |
| Co-catalyst amount | 0.0652 | 0.0326 | 0.0127 | 2.57 | 0.033 | 1.00 |
| Central points | 0.0385 | 0.0220 | 1.75 | 0.118 | 1.00 |
| Factors: | 7 | Basic experimental plan: | 7; 32 | Resolution: | IV |
| Runs: | 36 | Replications: | 1 | Fraction: | ¼ |
| Blocks: | 1 | Central points (total): | 4 |
| Factors | Steps | ||
|---|---|---|---|
| (−) | Origin | (+) | |
| (A) Substrate amount (mL) | 0.5 | 2.25 | 4 |
| (B) Reaction temperature (°C) | 30 | 55 | 80 |
| (C) Catalyst amount i (mg) | 10 | 30 | 50 |
| (D) Co-catalyst amount i (mg) | 10 | 35 | 60 |
| (E) Homogenization temperature (°C) | 30 | 55 | 80 |
| (F) Reaction time (h) | 0.5 | 12.25 | 24 |
| (G) Water content (mL) | 0.001 | 0.25 | 0.5 |
| Factors: | 3 | Basic experimental plan: | 3; 8 |
| Runs: | 12 | Replications: | 1 |
| Blocks: | 1 | Central points (total): | 4 |
| Resolution | IV | Fraction | ¼ |
| Factors | Steps | ||
|---|---|---|---|
| (−) | Origin | (+) | |
| (B) Reaction temperature (°C) | 70 | 85 | 100 |
| (C) Catalyst amount (mg) | 50 (4.9 eq) | 65 (6.4 eq) | 80 (8 eq) |
| (D) Co-catalyst amount (mg) | 50 (14 eq) | 65 (18 eq) | 80 (23 eq) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouveyron, T.; Bratenberg, P.; Bell, P.; Eisenacher, M. Design of Experiments for Process Optimization of the Direct Wacker-Type Oxidation of 1-Decene to n-Decanal. Catalysts 2024, 14, 360. https://doi.org/10.3390/catal14060360
Bouveyron T, Bratenberg P, Bell P, Eisenacher M. Design of Experiments for Process Optimization of the Direct Wacker-Type Oxidation of 1-Decene to n-Decanal. Catalysts. 2024; 14(6):360. https://doi.org/10.3390/catal14060360
Chicago/Turabian StyleBouveyron, Thomas, Patricia Bratenberg, Peter Bell, and Matthias Eisenacher. 2024. "Design of Experiments for Process Optimization of the Direct Wacker-Type Oxidation of 1-Decene to n-Decanal" Catalysts 14, no. 6: 360. https://doi.org/10.3390/catal14060360
APA StyleBouveyron, T., Bratenberg, P., Bell, P., & Eisenacher, M. (2024). Design of Experiments for Process Optimization of the Direct Wacker-Type Oxidation of 1-Decene to n-Decanal. Catalysts, 14(6), 360. https://doi.org/10.3390/catal14060360






