Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines at Room Temperature
Abstract
1. Introduction
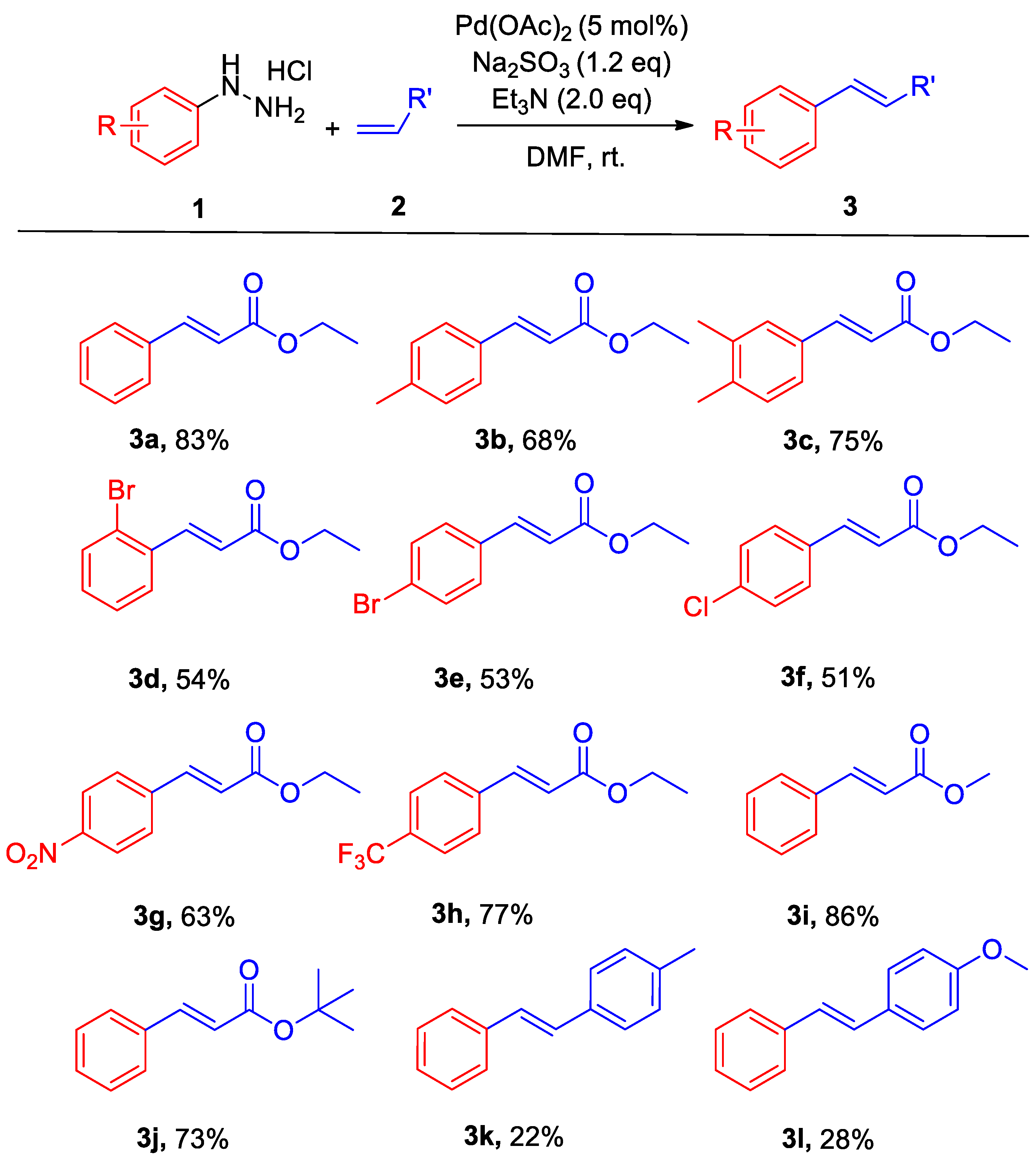
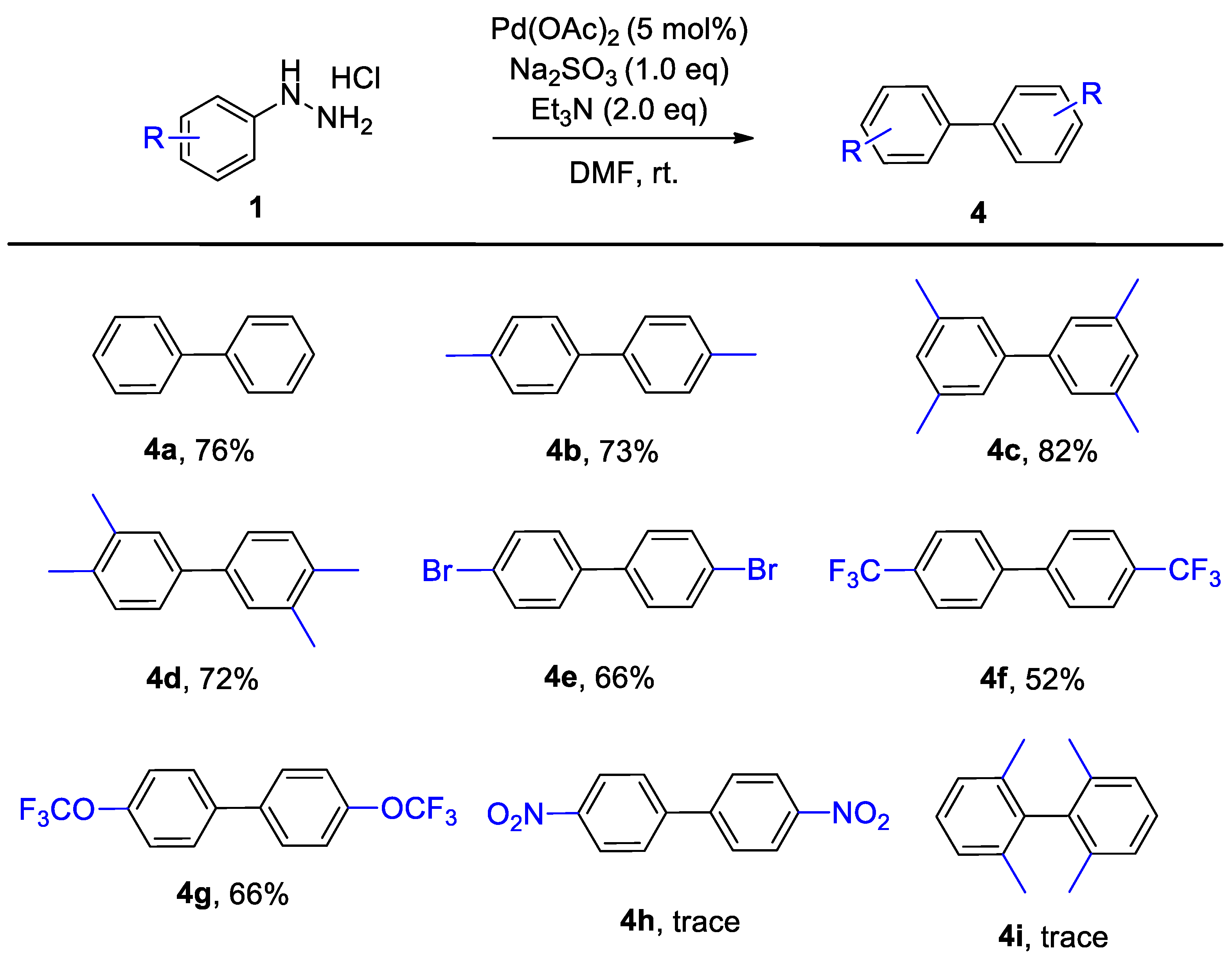
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Method for the Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines
3.3. Characterization Data for Products 3a–4g
- Ethyl cinnamate (3a) [24]. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 16.0 Hz, 1H), 7.56–7.48 (m, 2H), 7.41–7.34 (m, 3H), 6.44 (d, J = 16.0 Hz, 1H), 4.26 (q, J = 7.1 Hz, 2H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.03, 144.62, 134.45, 130.26, 128.90, 128.08, 118.26, 60.53, 14.36.
- Ethyl (E)-3-(p-tolyl)acrylate (3b) [25]. 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 16.0 Hz, 1H), 7.42 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 6.39 (d, J = 16.0 Hz, 1H), 4.25 (q, J = 7.1 Hz, 2H), 2.36 (s, 3H), 1.35–1.32 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 167.26, 144.64, 140.66, 131.72, 129.63, 128.07, 117.15, 60.45, 21.49, 14.37.
- Ethyl (E)-3-(3,4-dimethylphenyl)acrylate (3c) [26]. 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 16.0 Hz, 1H), 7.29–7.23 (m, 2H), 7.12 (d, J = 7.8 Hz, 1H), 6.38 (d, J = 16.0 Hz, 1H), 4.25 (q, J = 7.1 Hz, 2H), 2.25 (s, 6H), 1.32 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.30, 144.83, 139.41, 137.11, 132.14, 130.17, 129.30, 125.71, 116.95, 60.39, 19.83, 19.77, 14.38.
- Ethyl (E)-3-(2-bromophenyl)acrylate (3d) [25]. 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J = 15.9, 3.4 Hz, 1H), 7.66–7.54 (m, 2H), 7.26 (ddd, J = 17.9, 7.5, 2.0 Hz, 2H), 6.46–6.32 (m, 1H), 4.29 (qd, J = 7.1, 3.6 Hz, 2H), 1.37–1.33 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 166.42, 142.92, 134.51, 133.41, 131.15, 127.72 127.61, 125.30, 121.10, 60.72, 14.30.
- Ethyl 3-(4-bromophenyl)acrylate (3e) [24]. 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 16.0 Hz, 1H), 7.51 (d, J = 8.5 Hz, 2H), 7.38 (d, J = 8.5 Hz, 2H), 6.42 (d, J = 16.0 Hz, 1H), 4.26 (q, J = 7.1 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H).13C NMR (101 MHz, CDCl3) δ 166.77, 143.22, 133.36, 132.14, 129.44, 124.48, 118.96, 60.68, 14.33.
- Ethyl (E)-3-(4-chlorophenyl)acrylate (3f) [24]. 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 16.0 Hz, 1H), 7.47–7.42 (m, 2H), 7.39–7.31 (m, 2H), 6.41 (d, J = 16.0 Hz, 1H), 4.27 (q, J = 7.1 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.79, 143.16, 136.12, 132.93, 129.22, 129.17, 118.84, 60.66, 14.32.
- Ethyl (E)-3-(4-nitrophenyl)acrylate (3g) [24]. 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 8.8 Hz, 2H), 7.70 (dd, J = 15.9, 8.7 Hz, 3H), 6.57 (d, J = 16.0 Hz, 1H), 4.30 (q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1 Hz, 3H).13C NMR (101 MHz, CDCl3) δ 166.06, 148.44, 141.64, 140.58, 128.64, 124.18, 122.57, 61.04, 14.27.
- Ethyl (E)-3-(4-(trifluoromethyl)phenyl)acrylate (3h) [24]. 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 16.0 Hz, 1H), 7.63 (d, J = 1.9 Hz, 4H), 6.51 (d, J = 16.0 Hz, 1H), 4.29 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.45, 142.71, 137.82, 131.68, 131.68, 128.16. 125.83, 120.82, 60.82, 14.26. 19F NMR (377 MHz, CDCl3) δ −62.88.
- Methyl cinnamate (3i) [22]. 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 16.0 Hz, 1H), 7.56–7.46 (m, 2H), 7.43–7.36 (m, 3H), 6.44 (d, J = 16.0 Hz, 1H), 3.80 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.50, 144.93, 134.36, 130.3, 128.92, 128.11, 117.78, 51.76.
- Tert-butyl cinnamate (3J) [22]. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 16.0 Hz, 1H), 7.50 (dd, J = 6.5, 3.1 Hz, 2H), 7.36 (dd, J = 5.0, 1.9 Hz, 3H), 6.37 (d, J = 16.0 Hz, 1H), 1.54 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 166.38, 143.59, 129.99, 128.85, 128.42, 127.98, 120.18, 80.53, 28.22.
- (E)-1-Methyl-4-styrylbenzene (3k) [22]. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 7.4 Hz, 2H), 7.40 (d, J = 8.1 Hz, 2H), 7.33 (t, J = 7.6 Hz, 2H), 7.23 (s, 1H), 7.15 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 2.6 Hz, 2H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 137.60, 137.57, 134.60, 129.49, 128.75, 128.68, 127.76, 127.50, 126.52, 126.49, 21.36.
- (E)-1-Methoxy-4-styrylbenzene (3l) [27]. 1H NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.7 Hz, 4H), 7.34 (t, J = 7.6 Hz, 2H), 7.23 (dd, J = 9.1, 5.5 Hz, 1H), 7.07 (d, J = 16.3 Hz, 1H), 6.97 (d, J = 16.3 Hz, 1H), 6.90 (d, J = 8.8 Hz, 2H), 3.82 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.31, 137.66, 130.15, 128.69, 128.22, 127.76, 127.26, 126.62, 126.29, 114.15, 55.37.
- 1,1′-Biphenyl (4a) [28]. 1H NMR (400 MHz, CDCl3) δ 7.69–7.63 (m, 4H), 7.50 (t, J = 7.5 Hz, 4H), 7.40 (t, J = 7.3 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 141.30, 128.85, 127.30.
- 4,4′-Dimethyl-1,1′-biphenyl (4b) [28]. 1H NMR (400 MHz, CDCl3) δ 7.25–7.21 (m, 4H), 7.10 (d, J = 7.9 Hz, 4H), 2.32 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 131.06, 129.94, 129.82, 128.52, 21.11.
- 3,3′,5,5′-Tetramethyl-1,1′-biphenyl (4c) [28]. 1H NMR (400 MHz, CDCl3) δ 7.13 (s, 4H), 6.86 (s, 2H), 2.29 (s, 12H). 13C NMR (101 MHz, CDCl3) δ 138.77, 136.80, 129.03, 125.09, 21.31.
- 3,3′,4,4′-Tetramethyl-1,1′-biphenyl (4d) [28]. 1H NMR (400 MHz, CDCl3) δ 7.31 (s, 2H), 7.27 (d, J = 7.8 Hz, 2H), 7.13 (d, J = 6.8 Hz, 2H), 2.27 (s, 6H), 2.24 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 138.99, 136.88, 135.39, 130.08, 128.36, 124.45, 20.06, 19.54.
- 4,4′-Dibromo-1,1′-biphenyl (4e) [28]. 1H NMR (400 MHz, CDCl3) δ 7.59–7.54 (m, 4H), 7.45–7.38 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 138.92, 132.05, 128.54, 121.97.
- 4-(Trifluoromethyl)-biphenyl (4f) [28]. 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.5 Hz, 4H), 7.69 (d, J = 8.5 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 143.25, 130.28 (q, J = 32.7 Hz), 127.65, 125.97 (q, J = 3.8 Hz). 124.13 (q, J = 272.1 Hz). 19F NMR (377 MHz, CDCl3) δ −62.56.
- 4,4′-Bis(trifluoromethyl)-1,1′-biphenyl (4g) [23]. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 8.4 Hz, 4H), 7.29 (d, J = 8.5 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 148.95, 138.59, 128.49, 121.38, 120.52 (q, J = 257.4 Hz). 19F NMR (377 MHz, CDCl3) δ −57.85.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Overman, L.E.; Ricca, D.J.; Tran, V.D. First total synthesis of scopadulcic acid B. J. Am. Chem. Soc. 1993, 115, 2042–2044. [Google Scholar] [CrossRef]
- Bader, R.R.; Baumeister, P.; Blaser, H.-U. Catalysis at ciba-geigy. Chimia 1996, 50, 99–105. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1, 3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Farina, V. Catalysis, High-turnover palladium catalysts in cross-coupling and Heck chemistry: A critical overview. Adv. Synth. Catal. 2004, 346, 1553–1582. [Google Scholar] [CrossRef]
- El-Bendary, M.M.; Saleh, T.S.; Al-Bogami, A.S. Synthesis and structural characterization of a palladium complex as an anticancer agent, and a highly efficient and reusable catalyst for the Heck coupling reaction under ultrasound irradiation: A convenient sustainable green protocol. Polyhedron 2021, 194, 114924. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The palladium-catalyzed Ullmann cross-coupling reaction: A modern variant on a time-honored process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Ayogu, J.I.; Onoabedje, E.A. Technology, Recent advances in transition metal-catalysed cross-coupling of (hetero) aryl halides and analogues under ligand-free conditions. Catal. Sci. Technol. 2019, 9, 5233–5255. [Google Scholar] [CrossRef]
- Kang, K.; Huang, L.; Weix, D.J. Sulfonate versus sulfonate: Nickel and palladium multimetallic cross-electrophile coupling of aryl triflates with aryl tosylates. J. Am. Chem. Soc. 2020, 142, 10634–10640. [Google Scholar] [CrossRef]
- Volla, C.M.R.; Vogel, P. Iron-catalyzed desulfinylative CC cross-coupling reactions of sulfonyl chlorides with Grignard reagents. Angew. Chem. Int. Ed. 2008, 47, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Dong, G.; Zhang, Y.; Wang, J. Recent applications of arene diazonium salts in organic synthesis. Org. Biomol. Chem. 2013, 11, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhao, J. Recent Advances in the Pd-Catalyzed Coupling of Arylhydrazines and Ammonium Salts via C−N Bond Cleavage. Chem. Rec. 2021, 21, 3442–3457. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R. Recent advances in indole syntheses: New routes for a classic target. Org. Biomol. Chem. 2011, 9, 6469–6480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, S.; Song, X.; Qiu, G. Recent Advances in the Coupling Reactions of Arylhydrazine via C–N Bond Cleavage. Chin. J. Org. Chem. 2016, 36, 1790. [Google Scholar] [CrossRef]
- Zhu, M.K.; Zhao, J.F.; Loh, T.P. Palladium-catalyzed C-C bond formation of arylhydrazines with olefins via carbon-nitrogen bond cleavage. Org. Lett. 2011, 13, 6308–6311. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hu, G.; Qiao, H.; Xu, P.; Gao, Y.; Zhao, Y. Palladium-catalyzed suzuki cross-coupling of arylhydrazines via C–N bond cleavage. J. Org. Chem. 2014, 79, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, C.; Li, Z.; Wang, Z. Palladium-catalyzed denitrogenative Hiyama cross-coupling with arylhydrazines under air. Tetrahedron Lett. 2015, 56, 5371–5376. [Google Scholar] [CrossRef]
- Liu, J.-B.; Yan, H.; Chen, H.-X.; Luo, Y.; Weng, J.; Lu, G. Palladium-catalyzed Suzuki cross-coupling of N′-tosyl arylhydrazines. Chem. Commun. 2013, 49, 5268–5270. [Google Scholar] [CrossRef]
- Zhou, H.-P.; Liu, J.-B.; Yuan, J.-J.; Peng, Y.-Y. Palladium-catalyzed Suzuki cross-couplings of N′-mesyl arylhydrazines via C–N bond cleavage. RSC Adv. 2014, 4, 25576–25579. [Google Scholar] [CrossRef]
- Liu, J.-B.; Zhou, H.-P.; Peng, Y.-Y. Palladium-catalyzed Suzuki cross-coupling of arylhydrazines via CNHNH2 bond activation in water. Tetrahedron Lett. 2014, 55, 2872–2875. [Google Scholar] [CrossRef]
- Liu, J.-B.; Chen, F.-J.; Liu, N.; Hu, J. Palladium-catalyzed Heck coupling of arylhydrazines via C–NHNH2 bond activation. RSC Adv. 2015, 5, 45843–45846. [Google Scholar] [CrossRef]
- Liu, J.-B.; Nie, L.; Yan, H.; Jiang, L.-H.; Weng, J.; Lu, G. Palladium-catalyzed reductive homocoupling of N′-tosyl arylhydrazines. Org. Biomol. Chem. 2013, 11, 8014–8017. [Google Scholar] [CrossRef] [PubMed]
- França, S.B.; de Lima, L.C.B.; da Silva Cunha, C.R.; Anunciação, D.S.; da Silva-Júnior, E.F.; de Sá Barreto Barros, M.E.; da Paz Lima, D.J. Larvicidal activity and in silico studies of cinnamic acid derivatives against Aedes aegypti (Diptera: Culicidae). Bioorg. Med. Chem. 2021, 44, 116299. [Google Scholar]
- Suárez-Escobedo, L.; Gotor-Fernández, V. Solvent role in the lipase-catalysed esterification of cinnamic acid and derivatives. Optimisation of the biotransformation conditions. Tetrahedron 2021, 81, 131873. [Google Scholar] [CrossRef]
- Meiß, R.; Kumar, K.; Waldmann, H. Divergent gold (I)-catalyzed skeletal rearrangements of 1, 7-enynes. Chem. Eur. J. 2015, 21, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kinoshita, H.; Shinokubo, H.; Oshima, K. Biaryl synthesis from two different aryl halides with tri (2-furyl) germane. Org. Lett. 2002, 4, 3165–3167. [Google Scholar] [CrossRef]
- Carson, C.; Hassing, J.; Olguin, T.; Peterson, K.P.; Haley, R.A. Reviews, Reactivity trends for mechanochemical reductive coupling of aryl iodides. Green Chem. Lett. 2023, 16, 2153628. [Google Scholar] [CrossRef]

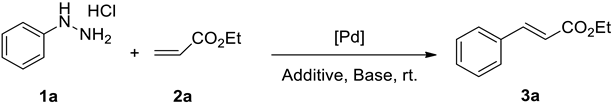 | |||||
|---|---|---|---|---|---|
| Entry | [Pd] | Base (Equiv.) | Additive | Solvent | Yield of 3a b (%) |
| 1 | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 64 |
| 2 | Pd(OAc)2 | Et3N | — | DMF | 36 |
| 3 b | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 60 |
| 4 c | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 83 |
| 5 d | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 80 |
| 6 | Pd(OAc)2 | Et3N | Na2SO3 | THF | 53 |
| 7 | Pd(OAc)2 | Et3N | Na2SO3 | EtOH | 48 |
| 8 | Pd(OAc)2 | Et3N | Na2SO3 | CH3CN | 64 |
| 9 | Pd(OAc)2 | Et3N | Na2SO3 | H2O | Trace |
| 10 | Pd(OAc)2 | Et3N | Na2SO3 | DMSO | 70 |
| 11 | Pd(OAc)2 | Et3N | Na2SO3 | Acetone | 35 |
| 12 | Pd(OAc)2 | DMAP | Na2SO3 | DMF | 70 |
| 13 | Pd(OAc)2 | K2CO3 | Na2SO3 | DMF | 51 |
| 14 | Pd(OAc)2 | Cs2CO3 | Na2SO3 | DMF | 31 |
| 15 | Pd(OAc)2 | Et3N | Na2S2O8 | DMF | 48 |
| 16 | Pd(OAc)2 | Et3N | NaHSO3 | DMF | 73 |
| 17 | Pd(OAc)2 | Et3N | Na2S2O3 | DMF | Trace |
| 18 | Pd(OAc)2 | Et3N | Na2S | DMF | Trace |
| 19 | Pd(TFA)2 | Et3N | Na2SO3 | DMF | 46 |
| 20 | PdCl2 | Et3N | Na2SO3 | DMF | 59 |
| 21 | — | Et3N | Na2SO3 | DMF | Trace |
| 22 e | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 66 |
| 23 f | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 75 |
| 24 g | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 79 |
| 25 h | Pd(OAc)2 | Et3N | Na2SO3 | DMF | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wang, W.; Liu, J.-B.; Luo, N. Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines at Room Temperature. Catalysts 2024, 14, 338. https://doi.org/10.3390/catal14060338
Du J, Wang W, Liu J-B, Luo N. Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines at Room Temperature. Catalysts. 2024; 14(6):338. https://doi.org/10.3390/catal14060338
Chicago/Turabian StyleDu, Jianxiong, Wanhe Wang, Jin-Biao Liu, and Nianhua Luo. 2024. "Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines at Room Temperature" Catalysts 14, no. 6: 338. https://doi.org/10.3390/catal14060338
APA StyleDu, J., Wang, W., Liu, J.-B., & Luo, N. (2024). Na2SO3-Promoted Heck Coupling and Homo-Coupling of Arylhydrazines at Room Temperature. Catalysts, 14(6), 338. https://doi.org/10.3390/catal14060338








