Simple Fabrication of Hydrophobicity-Controlled Fe-ZSM-5 for Aqueous-Phase Partial Oxidation of Methane with Hydrogen Peroxide
Abstract
1. Introduction
2. Results and Discussion
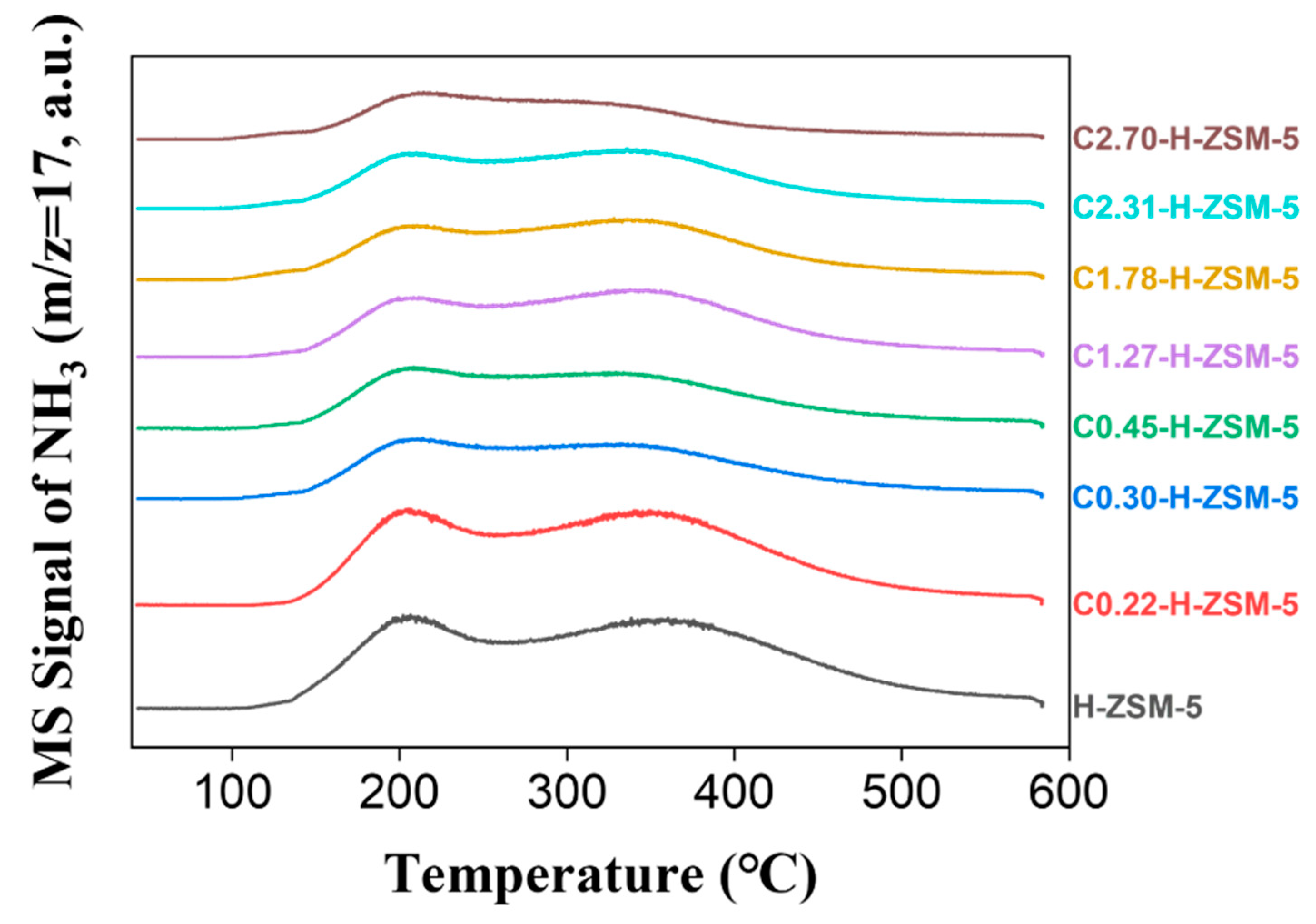
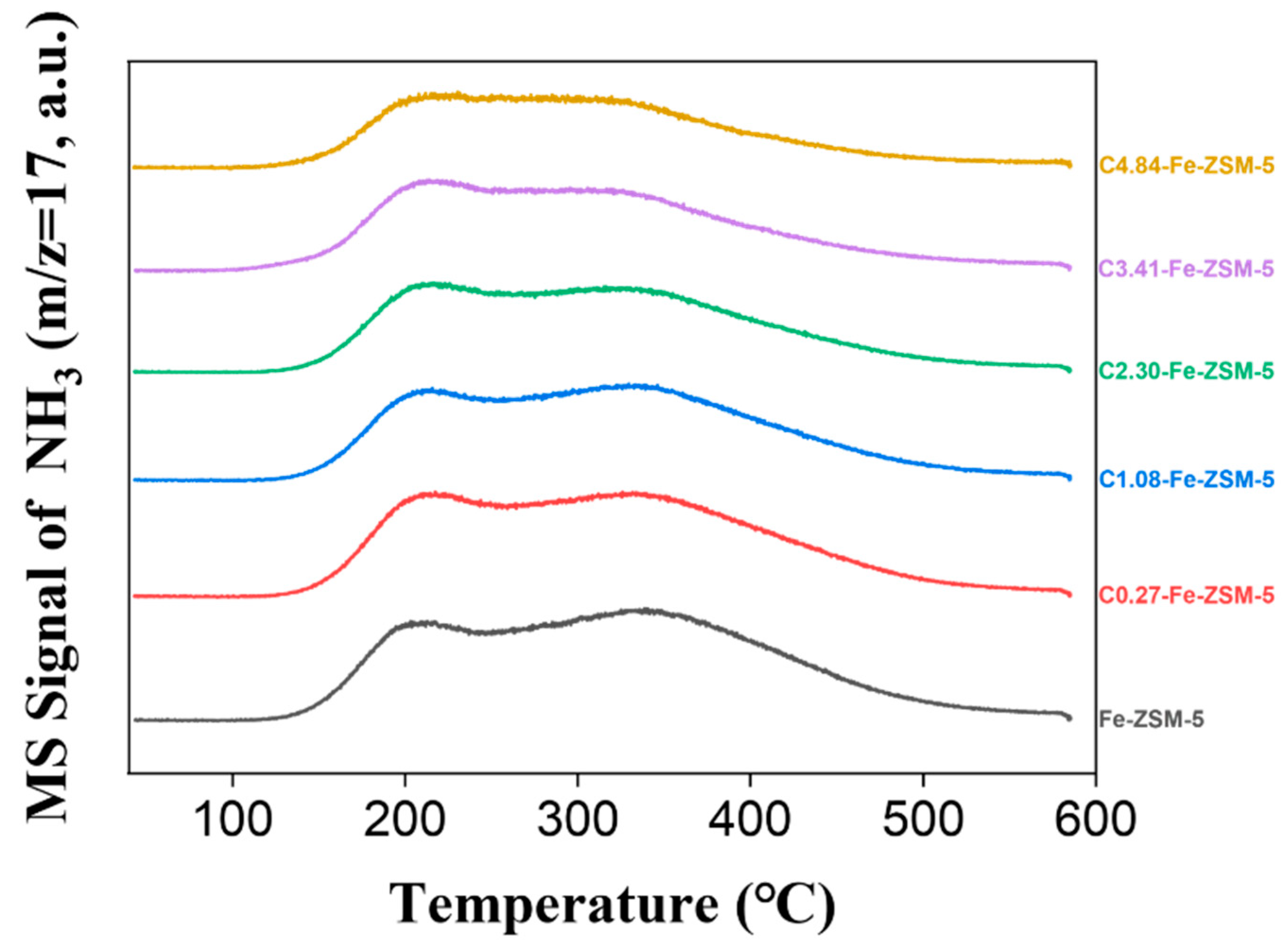
2.1. Characterization of Catalysts
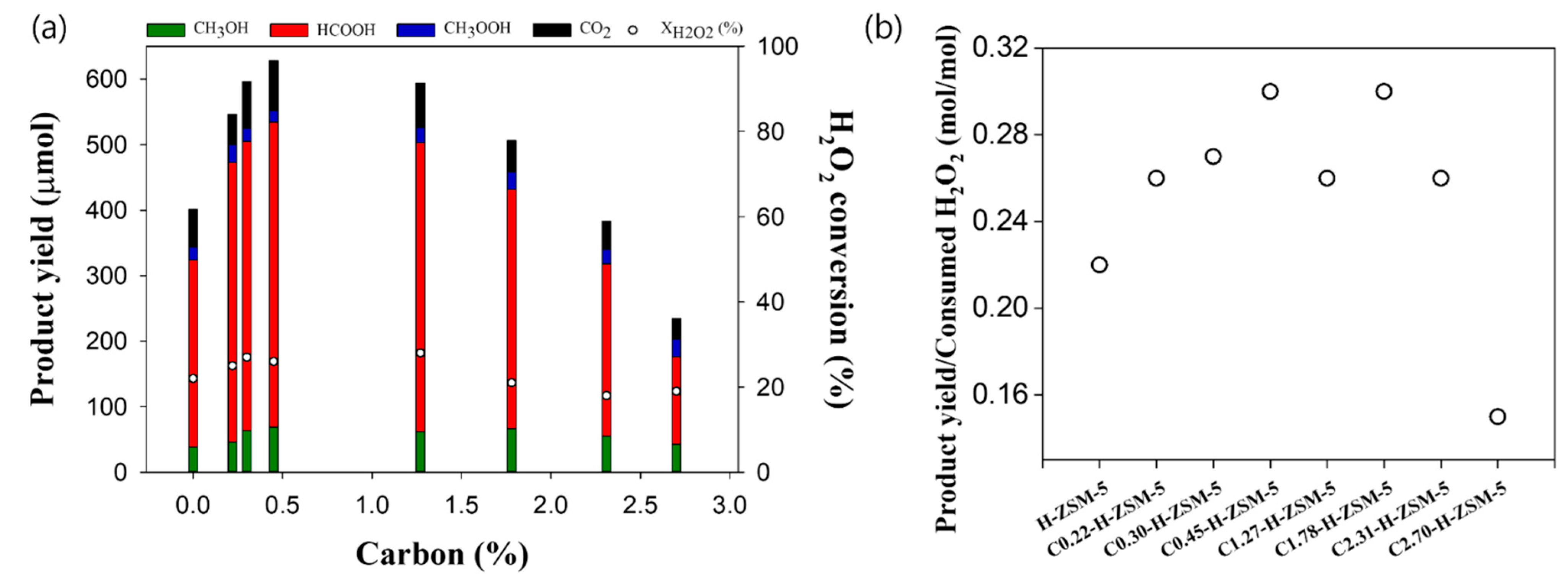
2.2. Catalytic Performance of Catalysts
3. Experiment
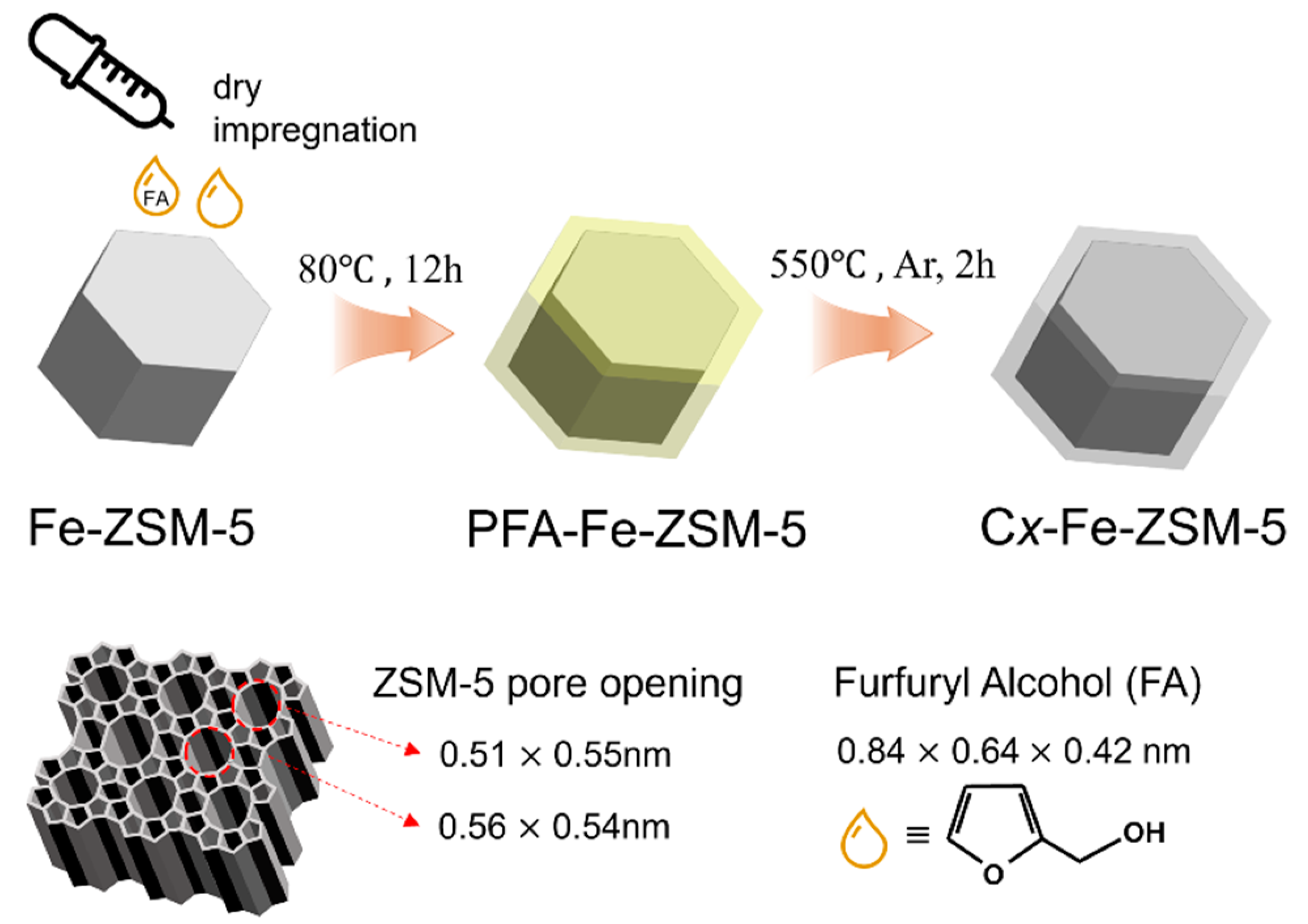
3.1. Catalyst Preparation
3.2. Catalytic Performance
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, D.M.; Kim, Y.H.; Park, E.D.; Yie, J.E. Role of Surface Hydrophilicity of Alumina in Methanol Dehydration. Catal. Commun. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Vanoye, L.; Favre-Réguillon, A.; Munno, P.; Rodríguez, J.F.; Dupuy, S.; Pallier, S.; Pitault, I.; De Bellefon, C. Methanol Dehydration over Commercially Available Zeolites: Effect of Hydrophobicity. Catal. Today 2013, 215, 239–242. [Google Scholar] [CrossRef]
- Li, D.; Wu, Z.; Zhou, D.; Xia, Y.; Lu, X.; He, H.; Xia, Q. One-Step Synthesis of Hybrid Zeolite with Exceptional Hydrophobicity to Accelerate the Interfacial Reaction at Low Temperature. Microporous Mesoporous Mater. 2019, 280, 195–202. [Google Scholar] [CrossRef]
- Inagaki, S.; Takeyama, M.; Asanuma, K.; Yokose, Y.; Zhang, S.; Okuda, T.; Nakamura, K.; Nishi, Y.; Kubota, Y. Preparation of Hydrophobic Ti-Beta Catalyst Derived from Al-Rich Zeolite Beta Synthesized without Using Organic Structure-Directing Agent and Its Enhanced Catalytic Performance for Phenol Oxidation. Microporous Mesoporous Mater. 2022, 346, 112323. [Google Scholar] [CrossRef]
- Wei, M.; Kuang, Y.; Duan, Z.; Li, H. The Crucial Role of Catalyst Wettability for Hydrogenation of Biomass and Carbon Dioxide over Heterogeneous Catalysts. Cell Rep. Phys. Sci. 2023, 4, 101340. [Google Scholar] [CrossRef]
- Freakley, S.J.; Dimitratos, N.; Willock, D.J.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Methane Oxidation to Methanol in Water. Acc. Chem. Res. 2021, 54, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Dummer, N.F.; Willock, D.J.; He, Q.; Howard, M.J.; Lewis, R.J.; Qi, G.; Taylor, S.H.; Xu, J.; Bethell, D.; Kiely, C.J.; et al. Methane Oxidation to Methanol. Chem. Rev. 2023, 123, 6359–6411. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, E.D. Liquid-Phase Selective Oxidation of Methane to Methane Oxygenates. Catalysts 2024, 14, 167. [Google Scholar] [CrossRef]
- Wang, V.C.C.; Maji, S.; Chen, P.P.Y.; Lee, H.K.; Yu, S.S.F.; Chan, S.I. Alkane Oxidation: Methane Monooxygenases, Related Enzymes, and Their Biomimetics. Chem. Rev. 2017, 117, 8574–8621. [Google Scholar] [CrossRef]
- Hammond, C.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Murphy, D.M.; Hagen, H.; Stangland, E.E.; et al. Catalytic and Mechanistic Insights of the Low-Temperature Selective Oxidation of Methane over Cu-Promoted Fe-ZSM-5. Chem.—Eur. J. 2012, 18, 15735–15745. [Google Scholar] [CrossRef]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by Using Copper-Promoted Fe-ZSM-5. Angew. Chem.—Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Jenkins, R.L.; Lopez-Sanchez, J.A.; Kondrat, S.A.; Hasbi Ab Rahim, M.; Forde, M.M.; Thetford, A.; Taylor, S.H.; Hagen, H.; et al. Elucidation and Evolution of the Active Component within Cu/Fe/ZSM-5 for Catalytic Methane Oxidation: From Synthesis to Catalysis. ACS Catal. 2013, 3, 689–699. [Google Scholar] [CrossRef]
- Hutchings, G.J. Methane Activation by Selective Oxidation. Top. Catal. 2016, 59, 658–662. [Google Scholar] [CrossRef]
- Peneau, V.; Armstrong, R.D.; Shaw, G.; Xu, J.; Jenkins, R.L.; Morgan, D.J.; Dimitratos, N.; Taylor, S.H.; Zanthoff, H.W.; Peitz, S.; et al. The Low-Temperature Oxidation of Propane by Using H2O2 and Fe/ZSM-5 Catalysts: Insights into the Active Site and Enhancement of Catalytic Turnover Frequencies. ChemCatChem 2017, 9, 642–650. [Google Scholar] [CrossRef]
- Hammond, C.; Hermans, I.; Dimitratos, N. Biomimetic Oxidation with Fe-ZSM-5 and H2O2? Identification of an Active, Extra-Framework Binuclear Core and an FeIII-OOH Intermediate with Resonance-Enhanced Raman Spectroscopy. ChemCatChem 2015, 7, 434–440. [Google Scholar] [CrossRef]
- Kalamaras, C.; Palomas, D.; Bos, R.; Horton, A.; Crimmin, M.; Hellgardt, K. Selective Oxidation of Methane to Methanol over Cu- And Fe-Exchanged Zeolites: The Effect of Si/Al Molar Ratio. Catal. Lett. 2016, 146, 483–492. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; Van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and Beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Al-Shihri, S.; Richard, C.J.; Chadwick, D. Selective Oxidation of Methane to Methanol over ZSM-5 Catalysts in Aqueous Hydrogen Peroxide: Role of Formaldehyde. ChemCatChem 2017, 9, 1276–1283. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, K.H.; Cho, S.J.; Park, E.D. Partial Oxidation of Methane with Hydrogen Peroxide over Fe-ZSM-5 Catalyst. Catal. Today 2021, 376, 113–118. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane with H2O2 over Fe-ZSM-5 Catalysts Prepared from Different Iron Precursors. Microporous Mesoporous Mater. 2021, 324, 111278. [Google Scholar] [CrossRef]
- Zhu, K.; Liang, S.; Cui, X.; Huang, R.; Wan, N.; Hua, L.; Li, H.; Chen, H.; Zhao, Z.; Hou, G.; et al. Highly Efficient Conversion of Methane to Formic Acid under Mild Conditions at ZSM-5-Confined Fe-Sites. Nano Energy 2021, 82, 105718. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Lin, L.; Chu, S.; Su, Y.; Song, W.; Wang, A.; Weckhuysen, B.M.; Luo, W. Highly Selective Oxidation of Methane into Methanol over Cu-Promoted Monomeric Fe/ZSM-5. ACS Catal. 2021, 11, 6684–6691. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, C.; Keum, C.; Kim, H.E.; Lee, H.; Han, B.; Lee, S.Y. Methane Partial Oxidation by Monomeric Cu Active Center Confined on ZIF-7. Chem. Eng. J. 2022, 450, 138472. [Google Scholar] [CrossRef]
- Kim, M.S.; Yang, G.S.; Park, E.D. Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts. Catalysts 2022, 12, 1224. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, C.; Vikneshvaran, S.; Lee, S.; Lee, S.Y. Partial Oxidation of Methane to Methyl Oxygenates with Enhanced Selectivity Using a Single-Atom Copper Catalyst on Amorphous Carbon Support. Appl. Surf. Sci. 2023, 639, 158289. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.Y. High Metal Loaded Cu(i)N3 Single-Atom Catalysts: Superior Methane Conversion Activity and Selectivity under Mild Conditions. J. Mater. Chem. A Mater. 2023, 11, 15691–15701. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Aqueous-Phase Selective Oxidation of Methane with Oxygen over Iron Salts and Pd/C in the Presence of Hydrogen. ChemCatChem 2019, 11, 4247–4251. [Google Scholar] [CrossRef]
- Kang, J.; Puthiaraj, P.; Ahn, W.S.; Park, E.D. Direct Synthesis of Oxygenates via Partial Oxidation of Methane in the Presence of O2 and H2 over a Combination of Fe-ZSM-5 and Pd Supported on an Acid-Functionalized Porous Polymer. Appl. Catal. A Gen. 2020, 602, 117711. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2. Catalysts 2020, 10, 299. [Google Scholar] [CrossRef]
- Wu, B.; Lin, T.; Huang, M.; Li, S.; Li, J.; Yu, X.; Yang, R.; Sun, F.; Jiang, Z.; Sun, Y.; et al. Tandem Catalysis for Selective Oxidation of Methane to Oxygenates Using Oxygen over PdCu/Zeolite. Angew. Chem.—Int. Ed. 2022, 61, e202204116. [Google Scholar] [CrossRef]
- Yang, G.S.; Kang, J.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane over Pd−Fe/ZSM-5 with O2 in the Presence of H2. ChemCatChem 2023, 15, e202201630. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Partial Oxidation of Methane over Fe/ZSM-5 with Hydrogen Peroxide Generated in Situ over Pd/C in the Presence of Halide Ions. Catal. Today 2024, 426, 114367. [Google Scholar] [CrossRef]
- Ab Rahim, M.H.; Forde, M.M.; Jenkins, R.L.; Hammond, C.; He, Q.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Taylor, S.H.; Willock, D.J.; et al. Oxidation of Methane to Methanol with Hydrogen Peroxide Using Supported Gold-Palladium Alloy Nanoparticles. Angew. Chem.—Int. Ed. 2013, 52, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahim, M.H.; Armstrong, R.D.; Hammond, C.; Dimitratos, N.; Freakley, S.J.; Forde, M.M.; Morgan, D.J.; Lalev, G.; Jenkins, R.L.; Lopez-Sanchez, J.A.; et al. Low Temperature Selective Oxidation of Methane to Methanol Using Titania Supported Gold Palladium Copper Catalysts. Catal. Sci. Technol. 2016, 6, 3410–3418. [Google Scholar] [CrossRef]
- He, Y.; Luan, C.; Fang, Y.; Feng, X.; Peng, X.; Yang, G.; Tsubaki, N. Low-Temperature Direct Conversion of Methane to Methanol over Carbon Materials Supported Pd-Au Nanoparticles. Catal. Today 2020, 339, 48–53. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Imai, Y.; Ueda, K.; Li, H.; Guo, X.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Highly Selective Synthesis of Methanol from Methane over Carbon Materials Supported Pd-Au Nanoparticles under Mild Conditions. Catal. Today 2020, 352, 104–110. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, C.; Zou, S.; Liu, J.; Xiao, L.; Fan, J. High H2O2 Utilization Promotes Selective Oxidation of Methane to Methanol at Low Temperature. Front. Chem. 2020, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Huang, M.; Yu, X.; Liu, J.; Lin, T.; Zhong, L. Selective Oxidation of Methane to Oxygenates Using Oxygen via Tandem Catalysis. Chem.—Eur. J. 2023, 29, e202203057. [Google Scholar] [CrossRef]
- Ni, F.; Richards, T.; Smith, L.R.; Morgan, D.J.; Davies, T.E.; Lewis, R.J.; Hutchings, G.J. Selective Oxidation of Methane to Methanol via In Situ H2O2 Synthesis. ACS Org. Inorg. Au 2023, 3, 177–183. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic Zeolite Modification for in Situ Peroxide Formation in Methane Oxidation to Methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Perathoner, S.; Pino, F.; Arrigo, R.; Giordano, G.; Katovic, A.; Pedulà, V. Performances of Fe-[Al, B]MFI Catalysts in Benzene Hydroxylation with N2O: The Role of Zeolite Defects as Host Sites for Highly Active Iron Species. Catal. Today 2005, 110, 211–220. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; McVicker, R.; Wells, P.P.; Dimitratos, N.; He, Q.; Lu, L.; Jenkins, R.L.; Hammond, C.; Lopez-Sanchez, J.A.; et al. Light Alkane Oxidation Using Catalysts Prepared by Chemical Vapour Impregnation: Tuning Alcohol Selectivity through Catalyst Pre-Treatment. Chem. Sci. 2014, 5, 3603–3616. [Google Scholar] [CrossRef]
- Joo, S.; Choi, S.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaither, A.S.; De Lasa, H. HZSM-5 Zeolites with Different SiO2/Al2O3 Ratios. Characterization and NH3 Desorption Kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, X.; Xie, S.; Wang, Q.; Xu, L. The Effect of Acidity on Olefin Aromatization over Potassium Modified ZSM-5 Catalysts. Catal. Lett. 2004, 97, 31–36. [Google Scholar] [CrossRef]
- Han, Y.; Lu, C.; Xu, D.; Zhang, Y.; Hu, Y.; Huang, H. Molybdenum Oxide Modified HZSM-5 Catalyst: Surface Acidity and Catalytic Performance for the Dehydration of Aqueous Ethanol. Appl. Catal. A Gen. 2011, 396, 8–13. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Fehrmann, R.S.N. Selective Catalytic Reduction of NOx with NH3 on Cu-, Fe-, and Mn-Zeolites Prepared by Impregnation: Comparison of Activity and Hydrothermal Stability. J. Chem. 2018, 2018, 8614747. [Google Scholar] [CrossRef]
- Bagnasco, G. Improving the Selectivity of NH3 TPD Measurements. J. Catal. 1996, 159, 249–252. [Google Scholar] [CrossRef]
- Katada, N.; Niwa, M. Analysis of Acidic Properties of Zeolitic and Non-Zeolitic Solid Acid Catalysts Using Temperature-Programmed Desorption of Ammonia. Catal. Surv. Asia 2004, 8, 161–170. [Google Scholar] [CrossRef]
- Xu, S.; Sheng, H.; Ye, T.; Hu, D.; Liao, S. Hydrophobic Aluminosilicate Zeolites as Highly Efficient Catalysts for the Dehydration of Alcohols. Catal. Commun. 2016, 78, 75–79. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Li, J.; Zhan, X.; Chen, J.; Yang, J. Tuning the Hydrophobicity of ZSM-5 Zeolites by Surface Silanization Using Alkyltrichlorosilane. Appl. Surf. Sci. 2011, 257, 9525–9531. [Google Scholar] [CrossRef]
- Catuzo, G.L.; Santilli, C.V.; Martins, L. Hydrophobic-Hydrophilic Balance of ZSM-5 Zeolites on the Two-Phase Ketalization of Glycerol with Acetone. Catal. Today 2021, 381, 215–223. [Google Scholar] [CrossRef]
- Cavuoto, D.; Zaccheria, F.; Ravasio, N. Some Critical Insights into the Synthesis and Applications of Hydrophobic Solid Catalysts. Catalysts 2020, 10, 1337. [Google Scholar] [CrossRef]
- Wang, H.; Xin, W.; Wang, Q.; Zheng, X.; Lu, Z.; Pei, R.; He, P.; Dong, X. Direct Methane Conversion with Oxygen and CO over Hydrophobic DB-ZSM-5 Supported Rh Single-Atom Catalyst. Catal. Commun. 2022, 162, 106374. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of Zeolites to C1 Chemistry: Recent Advances, Challenges, and Opportunities. Adv. Mater. 2020, 32, e2002927. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic Zeolites for Biofuel Upgrading Reactions at the Liquid-Liquid Interface in Water/Oil Emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, T.; Kwon, Y.; Seo, Y.; Song, J.; Park, J.K.; Lee, H.; Park, J.Y.; Ihee, H.; Cho, S.J.; et al. Lanthanum-Catalysed Synthesis of Microporous 3D Graphene-like Carbons in a Zeolite Template. Nature 2016, 535, 131–135. [Google Scholar] [CrossRef]
- Wang, Y.; Guerra, P.; Zaker, A.; Maag, A.R.; Tompsett, G.A.; Smith, L.J.; Huang, X.; Bond, J.Q.; Timko, M.T. Strategies for Extending Zeolite Stability in Supercritical Water Using Thermally Stable Coatings. ACS Catal. 2020, 10, 6623–6634. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Wang, J.; Wang, Q.; Chen, Y.; Liu, X.; Zhao, Y. Microporous Carbon Coated Zeolite Particles for Efficient Carbon Capture from Wet Flue Gas. Sep. Purif. Technol. 2023, 317, 123762. [Google Scholar] [CrossRef]
- Asghari, A.; Khorrami, M.K.; Kazemi, S.H. Hierarchical H-ZSM5 zeolites based on natural kaolinite as a high-performance catalyst for methanol to aromatic hydrocarbons conversion. Sci. Rep. 2019, 9, 17526. [Google Scholar] [CrossRef] [PubMed]
- Setyaningrum, D.L.; Riyanto, S.; Rohman, A. Analysis of corn and soybean oils in red fruit oil using FTIR spectroscopy in combination with partial least square. Int. Food Res. J. 2013, 20, 1977–1981. [Google Scholar]

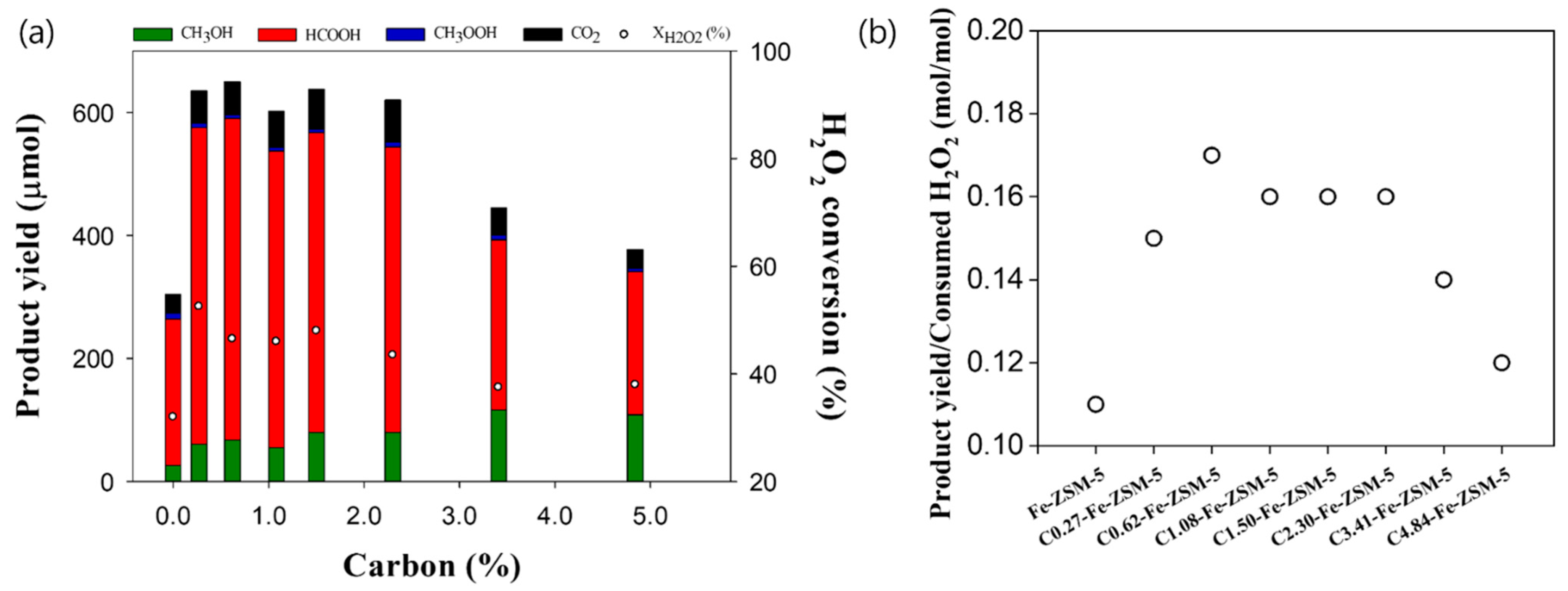
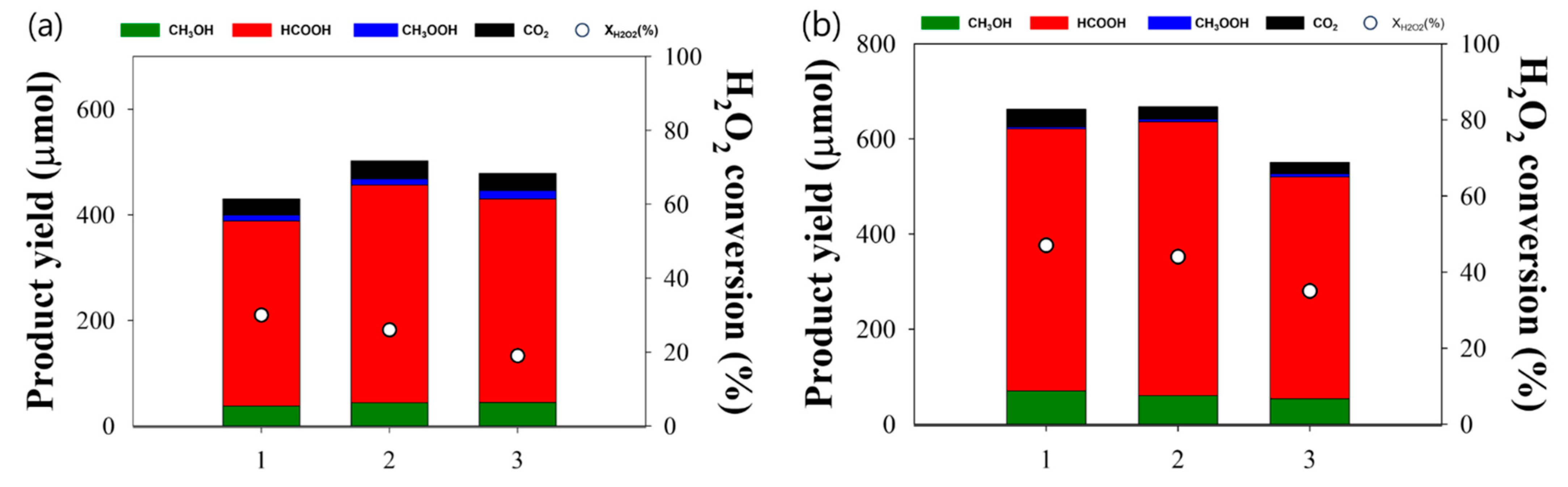
| Catalyst a | BET Surface Area (m2/g) | Micropore Surface Area (m2/g) | Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|---|
| H-ZSM-5 | 355 | 280 | 0.20 | 0.127 |
| C0.22-H-ZSM-5 | 315 | 210 | 0.22 | 0.095 |
| C0.30-H-ZSM-5 | 310 | 199 | 0.23 | 0.092 |
| C0.45-H-ZSM-5 | 308 | 204 | 0.23 | 0.094 |
| C1.27-H-ZSM-5 | 277 | 204 | 0.20 | 0.093 |
| C1.78-H-ZSM-5 | 281 | 211 | 0.20 | 0.097 |
| C2.31-H-ZSM-5 | 244 | 183 | 0.18 | 0.083 |
| C2.70-H-ZSM-5 | 186 | 142 | 0.16 | 0.065 |
| Fe-ZSM-5 | 267 | 187 | 0.18 | 0.085 |
| C0.27-Fe-ZSM-5 | 250 | 201 | 0.17 | 0.092 |
| C0.62-Fe-ZSM-5 | 252 | 186 | 0.18 | 0.085 |
| C1.08-Fe-ZSM-5 | 254 | 194 | 0.17 | 0.089 |
| C1.50-Fe-ZSM-5 | 251 | 195 | 0.18 | 0.089 |
| C2.30-Fe-ZSM-5 | 253 | 189 | 0.18 | 0.087 |
| C3.41-Fe-ZSM-5 | 222 | 177 | 0.16 | 0.081 |
| C4.84-Fe-ZSM-5 | 188 | 150 | 0.14 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.Y.; Kwon, M.; Hwang, J.; Park, E.D. Simple Fabrication of Hydrophobicity-Controlled Fe-ZSM-5 for Aqueous-Phase Partial Oxidation of Methane with Hydrogen Peroxide. Catalysts 2024, 14, 280. https://doi.org/10.3390/catal14040280
Hwang SY, Kwon M, Hwang J, Park ED. Simple Fabrication of Hydrophobicity-Controlled Fe-ZSM-5 for Aqueous-Phase Partial Oxidation of Methane with Hydrogen Peroxide. Catalysts. 2024; 14(4):280. https://doi.org/10.3390/catal14040280
Chicago/Turabian StyleHwang, Seok Young, Minjae Kwon, Jongkook Hwang, and Eun Duck Park. 2024. "Simple Fabrication of Hydrophobicity-Controlled Fe-ZSM-5 for Aqueous-Phase Partial Oxidation of Methane with Hydrogen Peroxide" Catalysts 14, no. 4: 280. https://doi.org/10.3390/catal14040280
APA StyleHwang, S. Y., Kwon, M., Hwang, J., & Park, E. D. (2024). Simple Fabrication of Hydrophobicity-Controlled Fe-ZSM-5 for Aqueous-Phase Partial Oxidation of Methane with Hydrogen Peroxide. Catalysts, 14(4), 280. https://doi.org/10.3390/catal14040280








