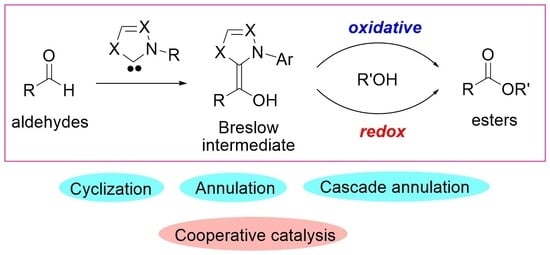
NHC-Catalyzed Reaction of Aldehydes for C(sp2)–O Bond Formation
Abstract
1. Introduction
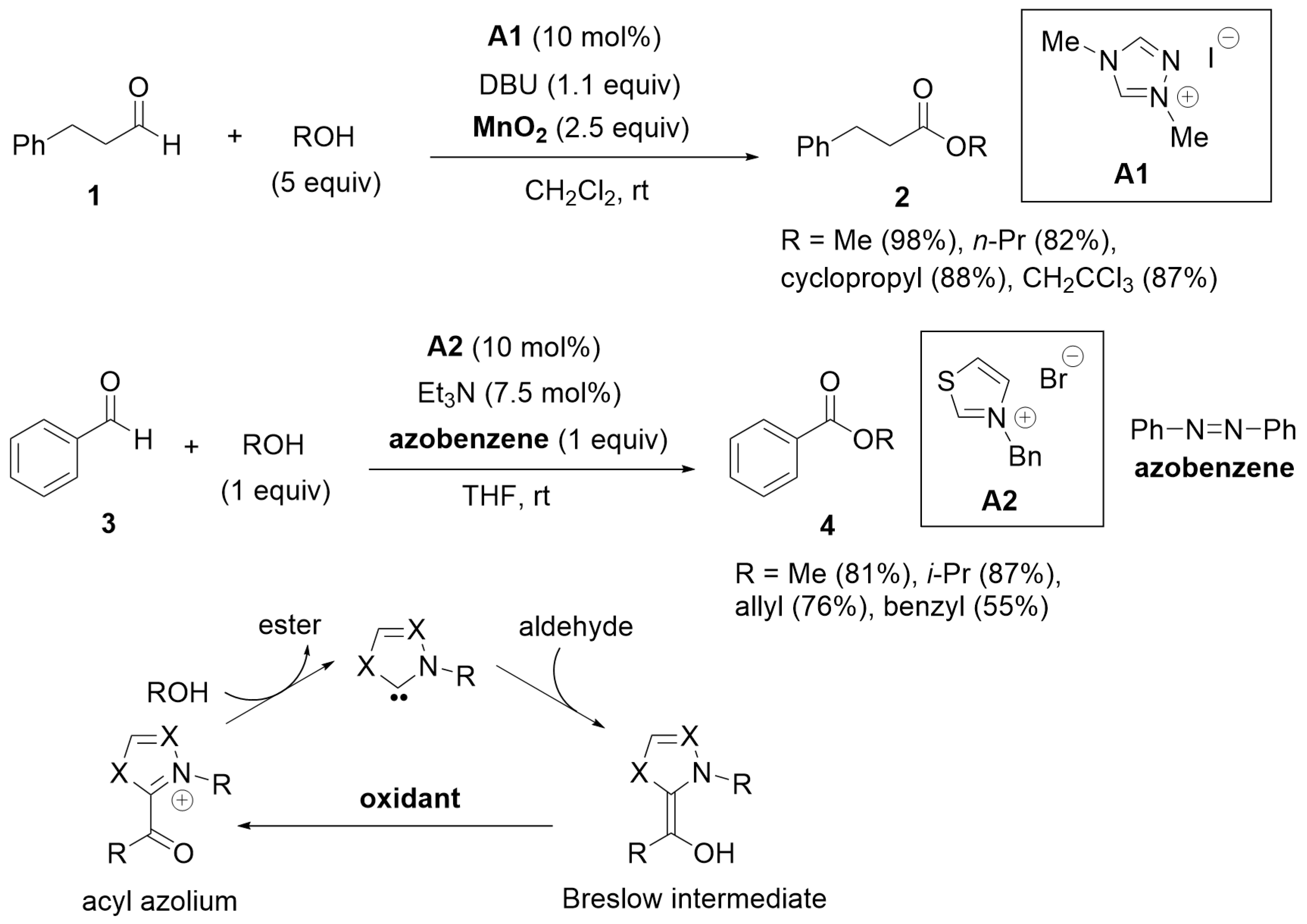
2. Oxidative Esterification of Aldehydes
2.1. Esterification of Aldehydes under Oxidation Conditions
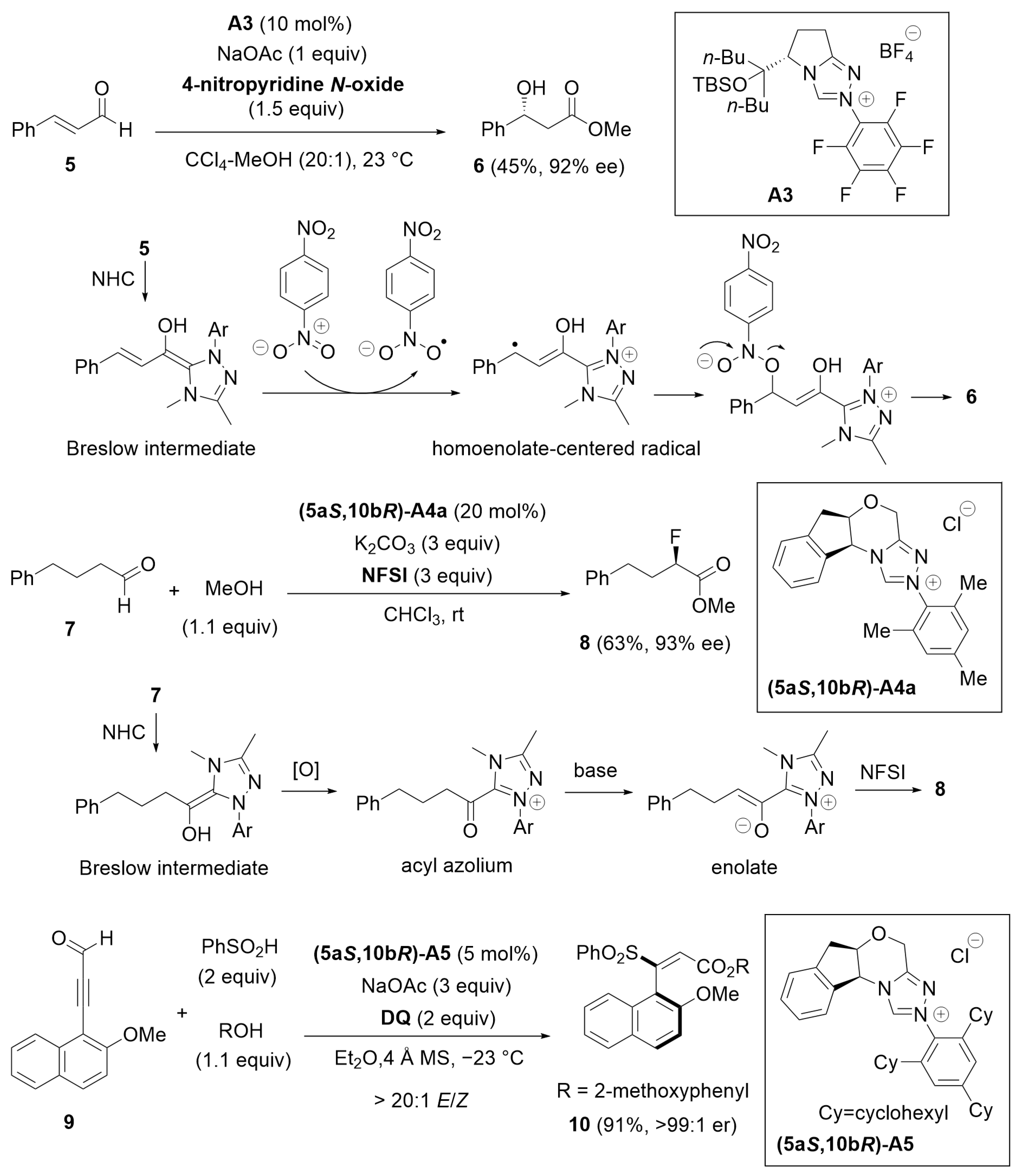
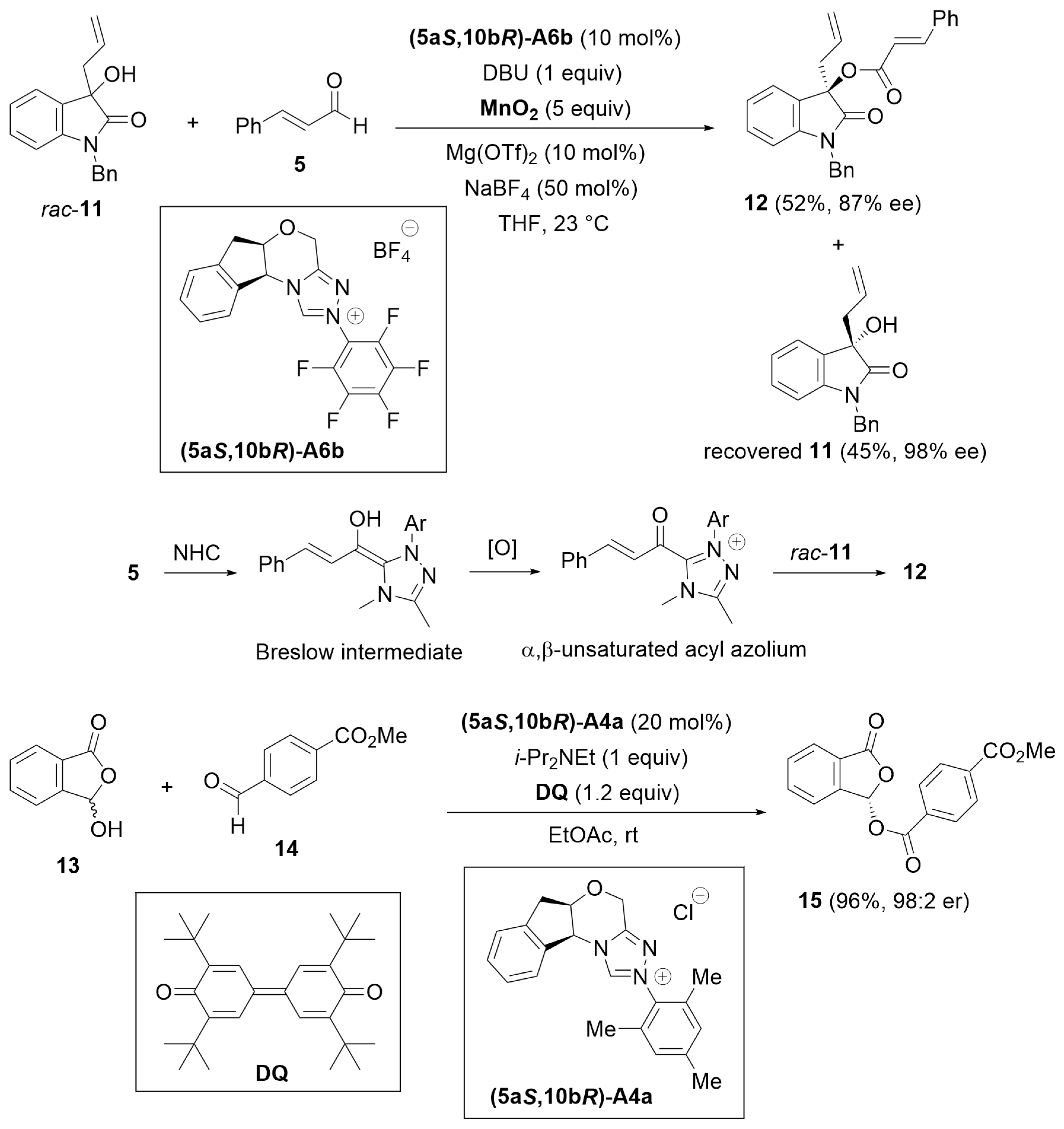
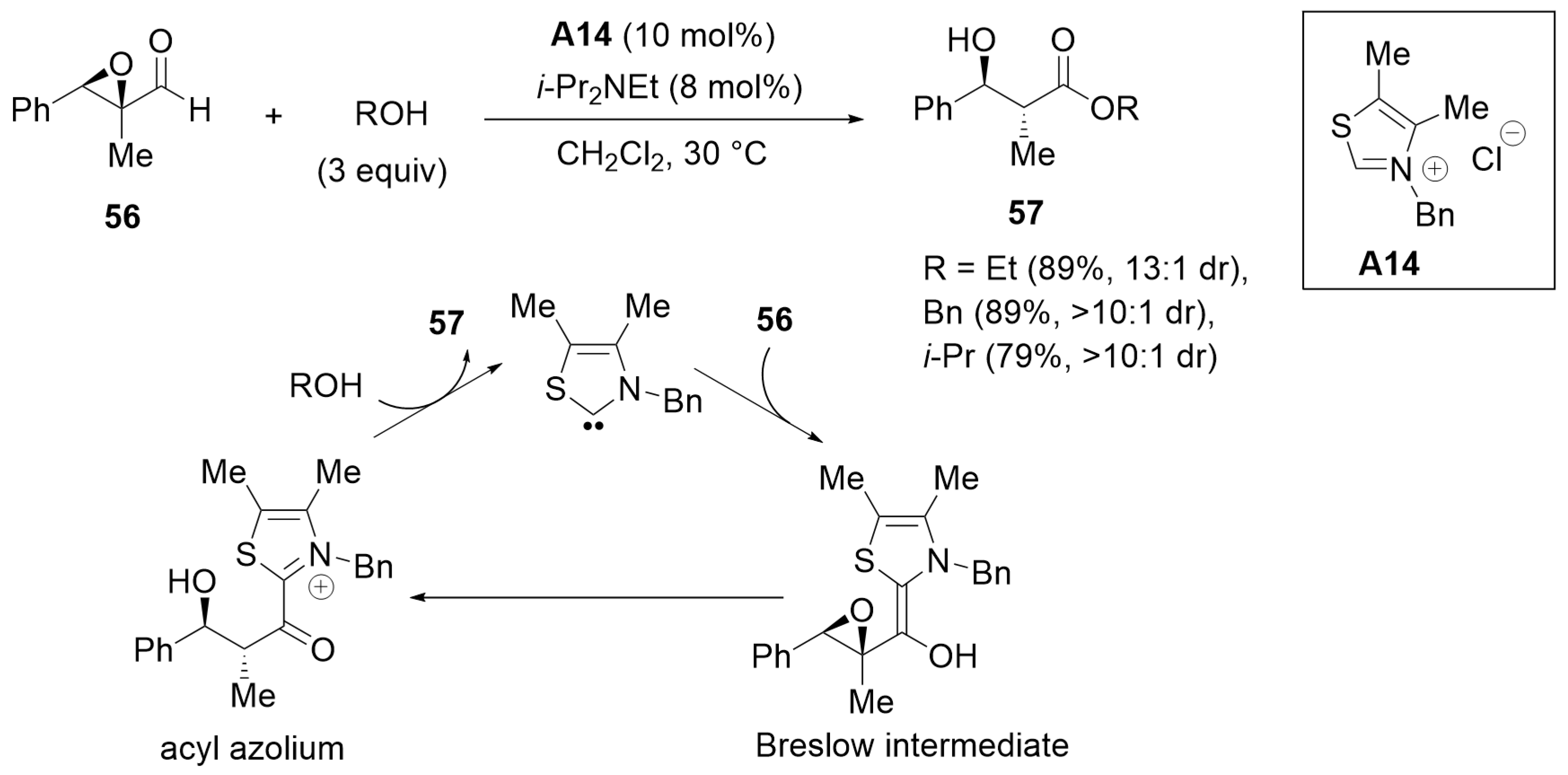
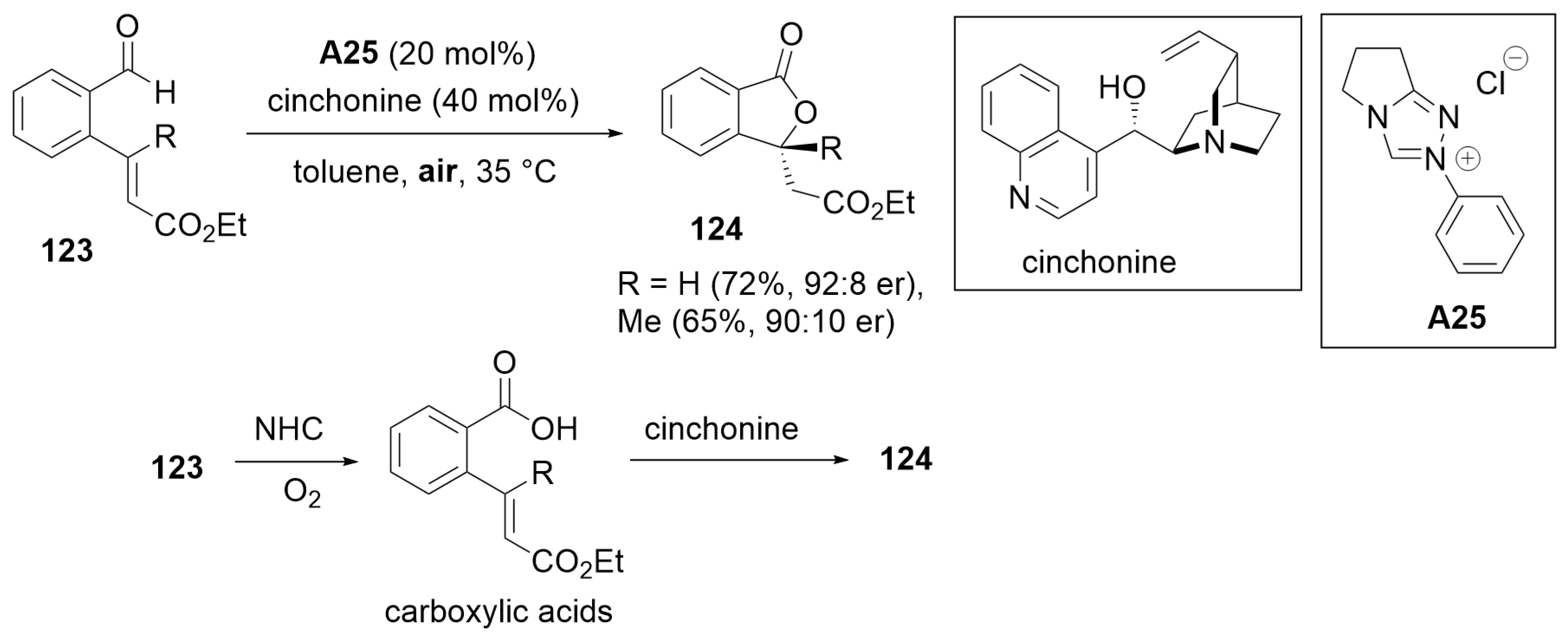
2.2. Kinetic Resolution
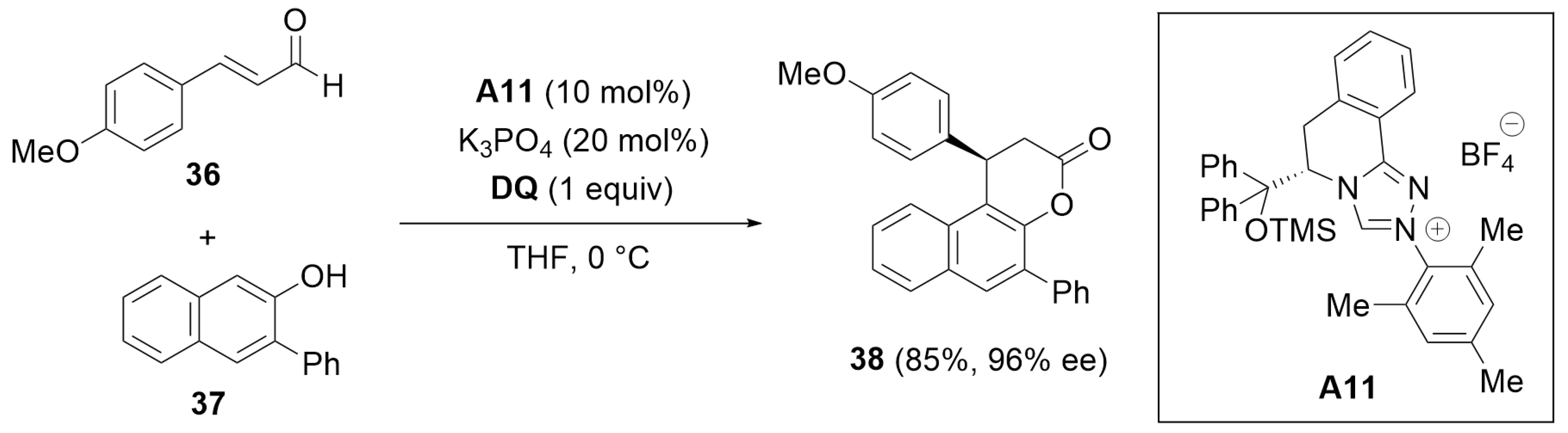
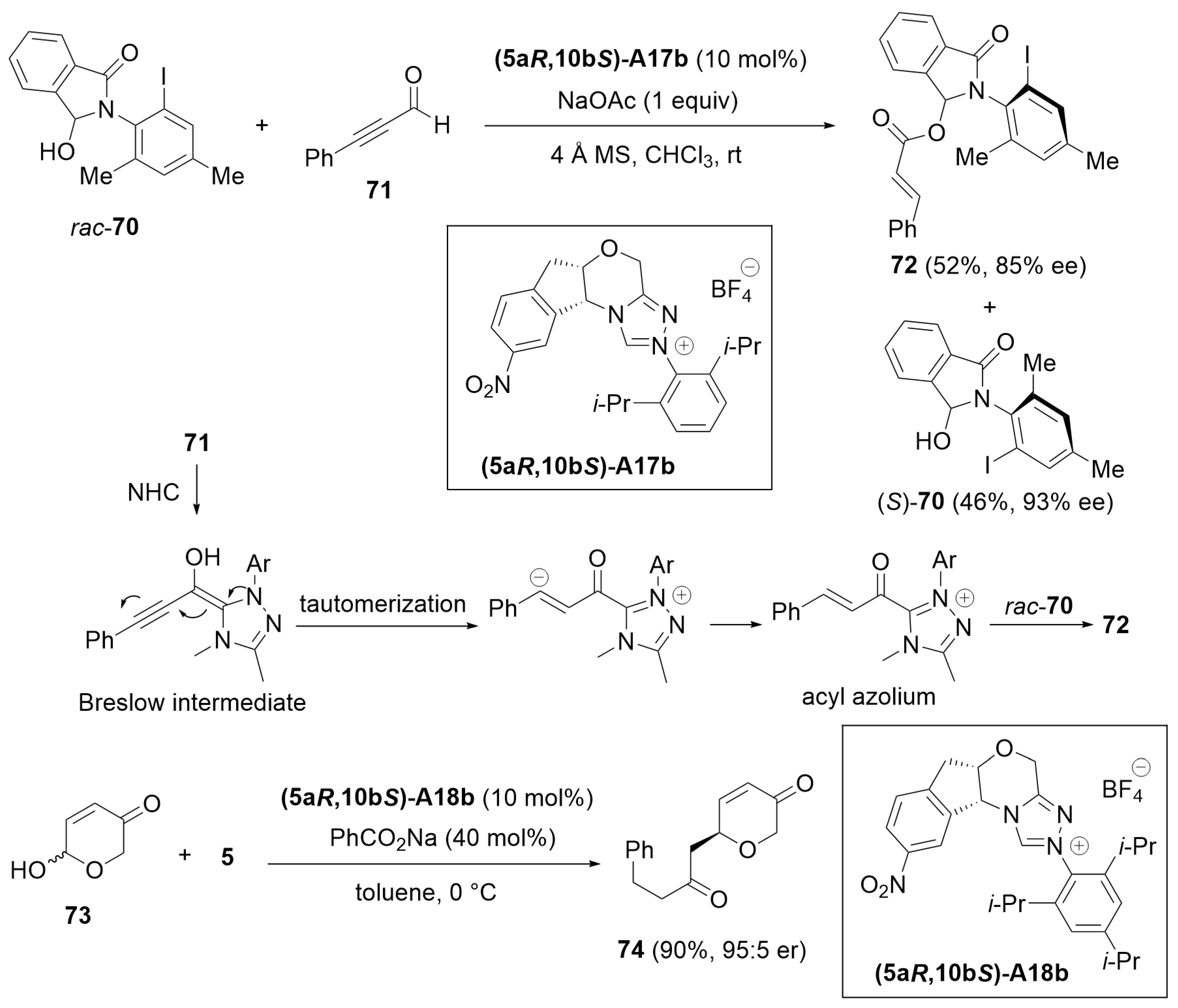
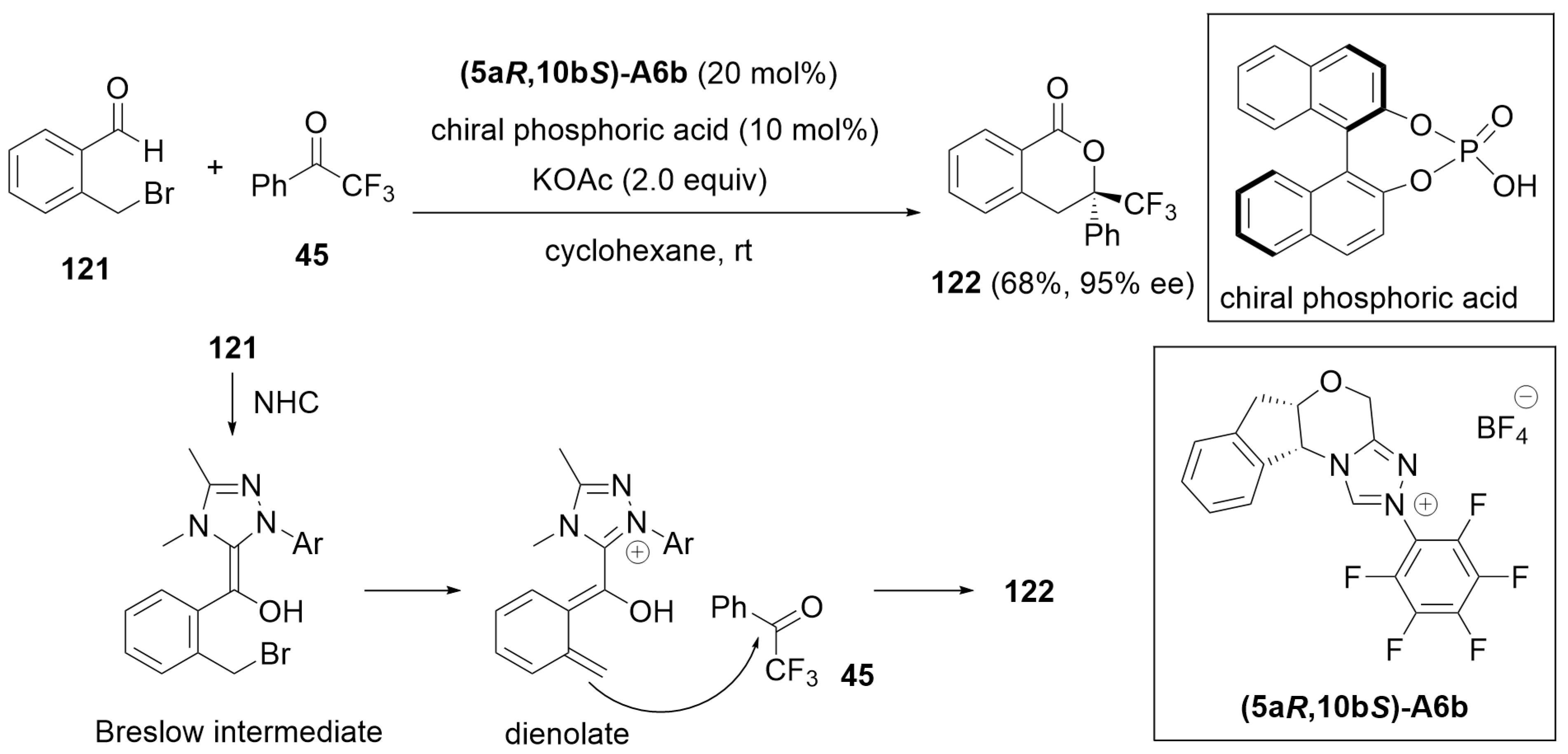
2.3. Desymmetrization
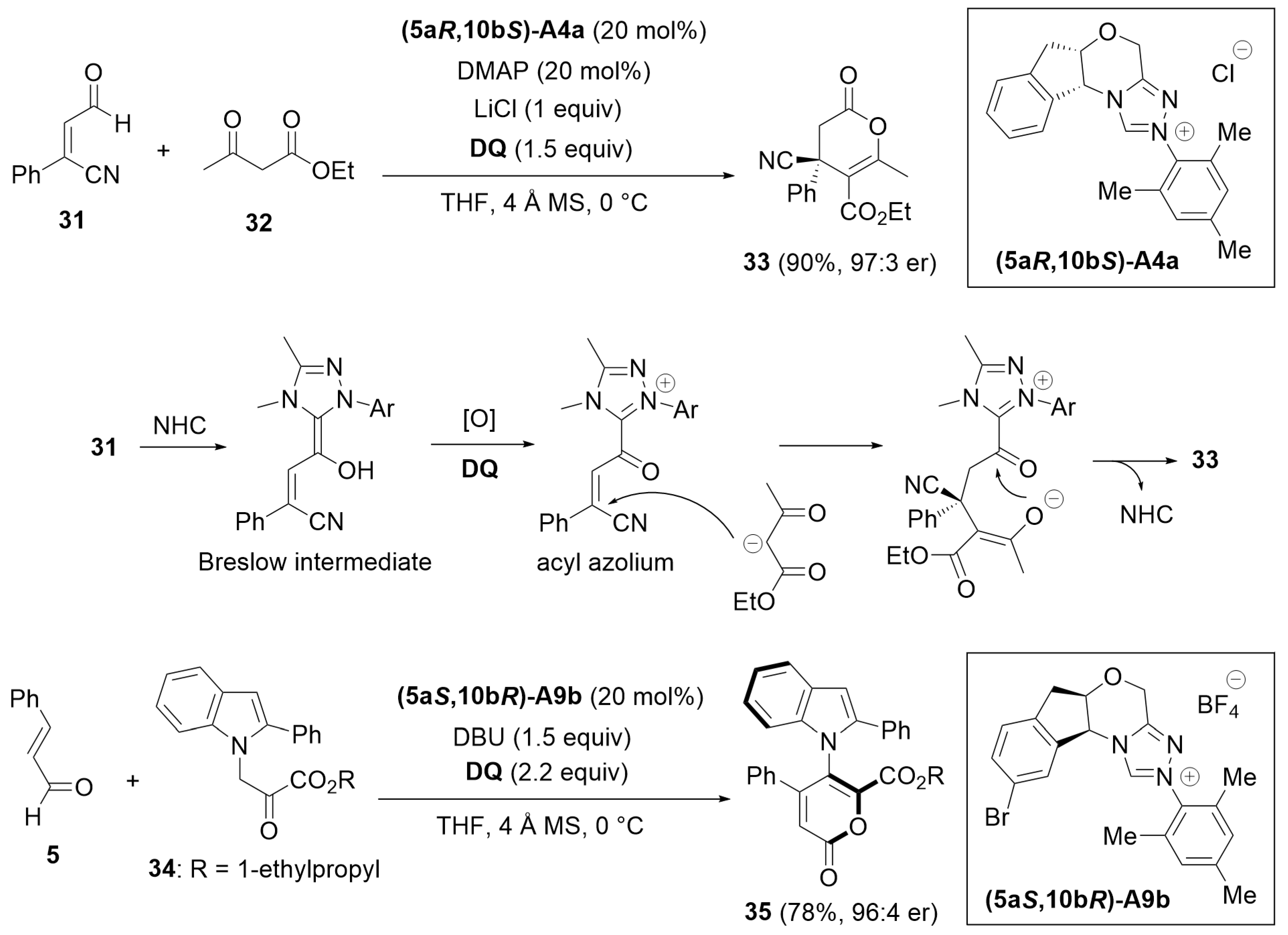
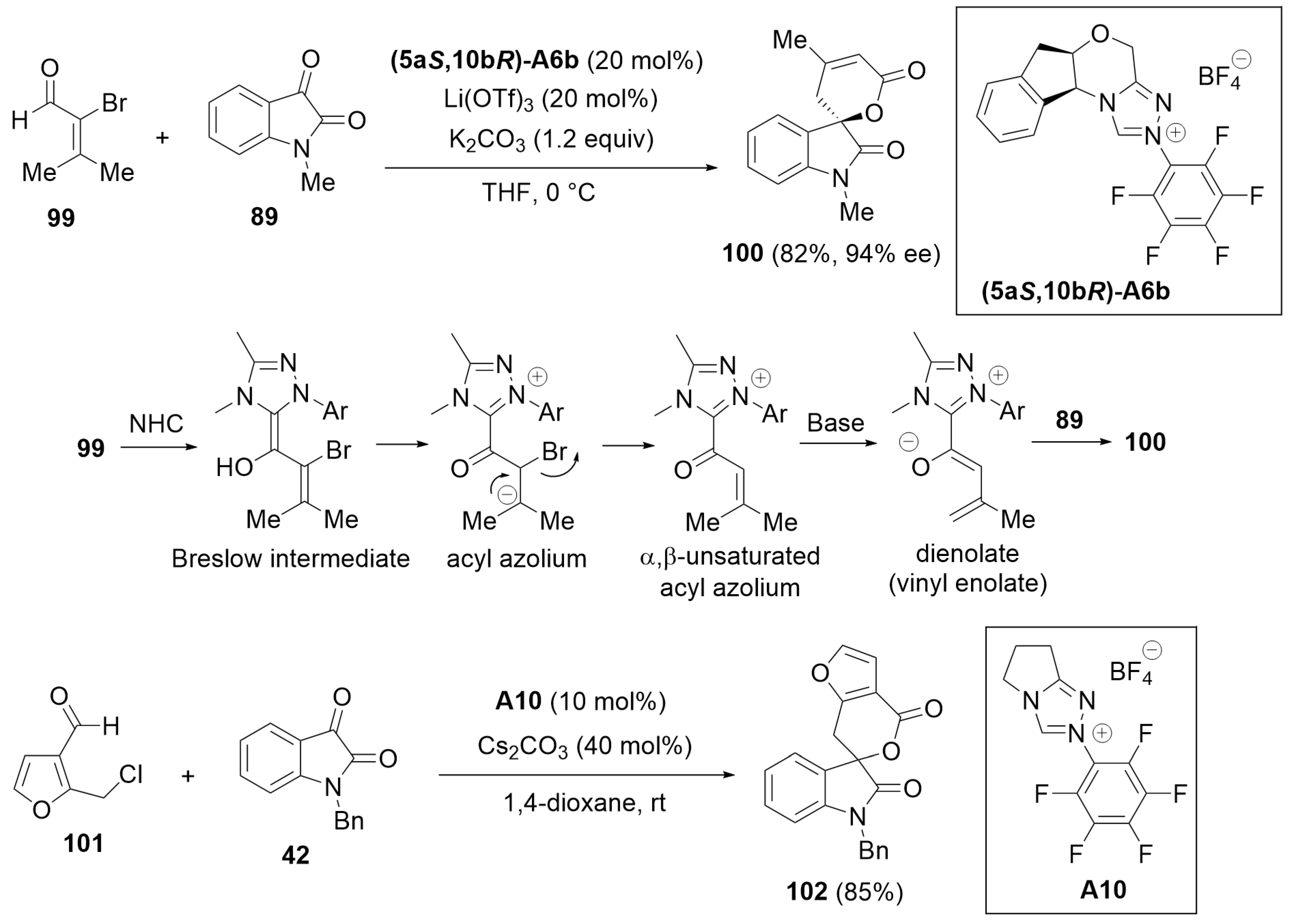
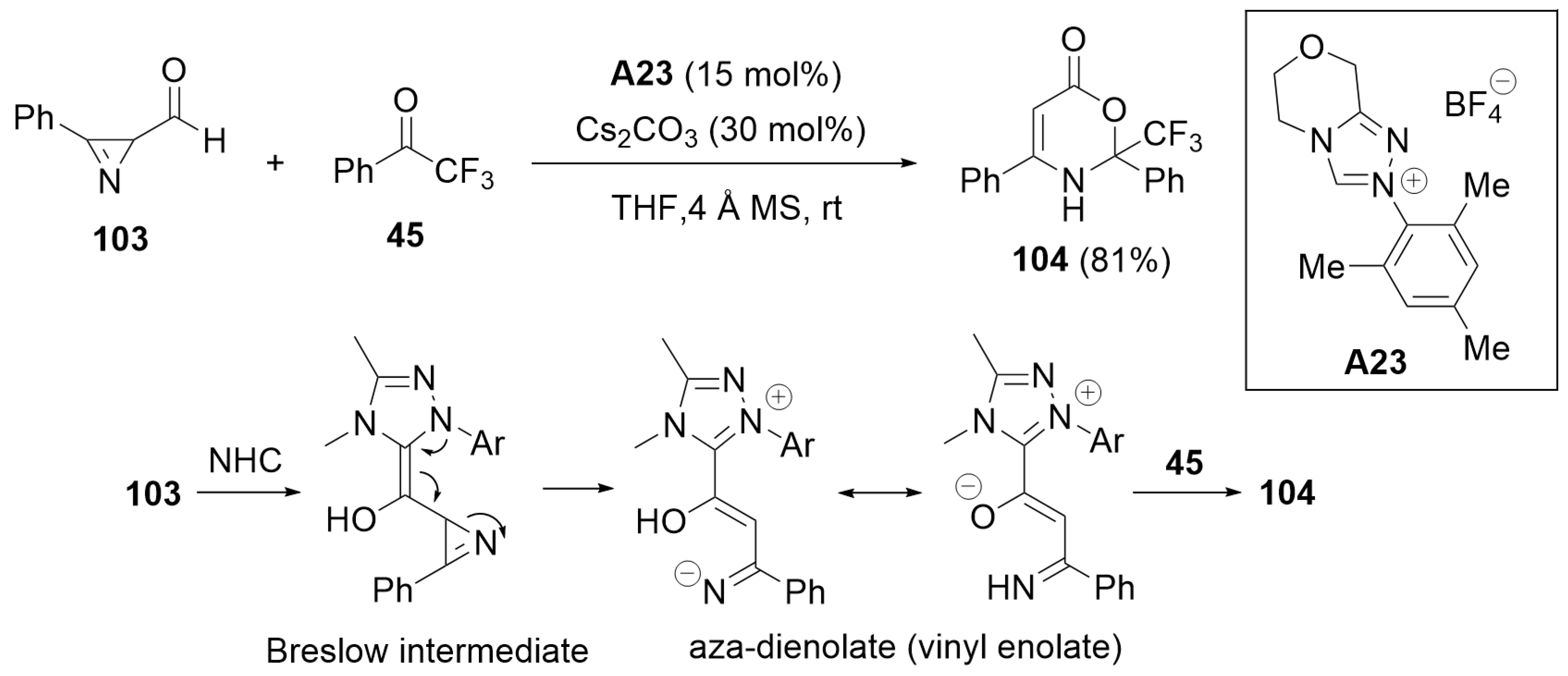
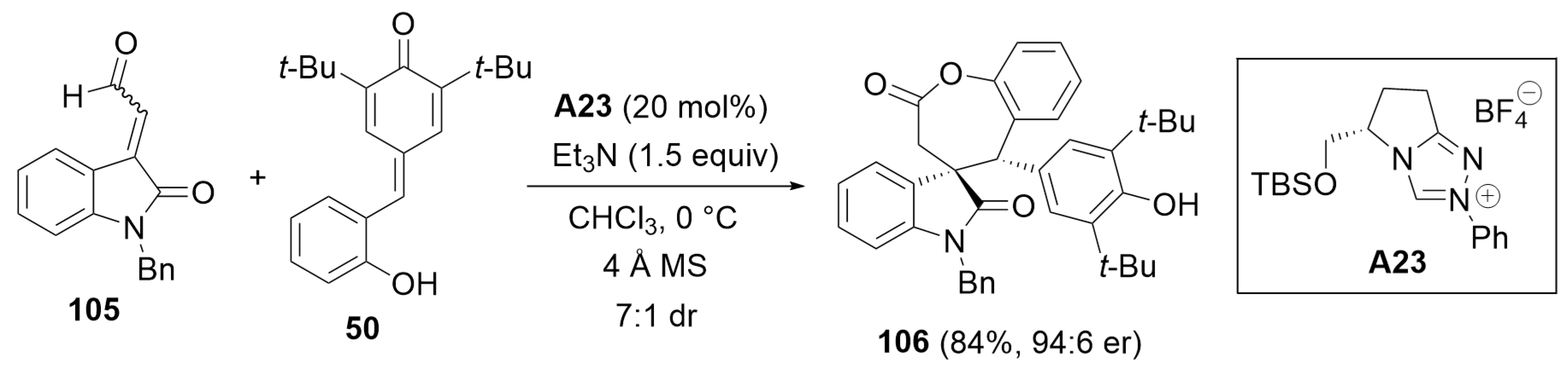
3. Oxidative Cyclization and Annulation
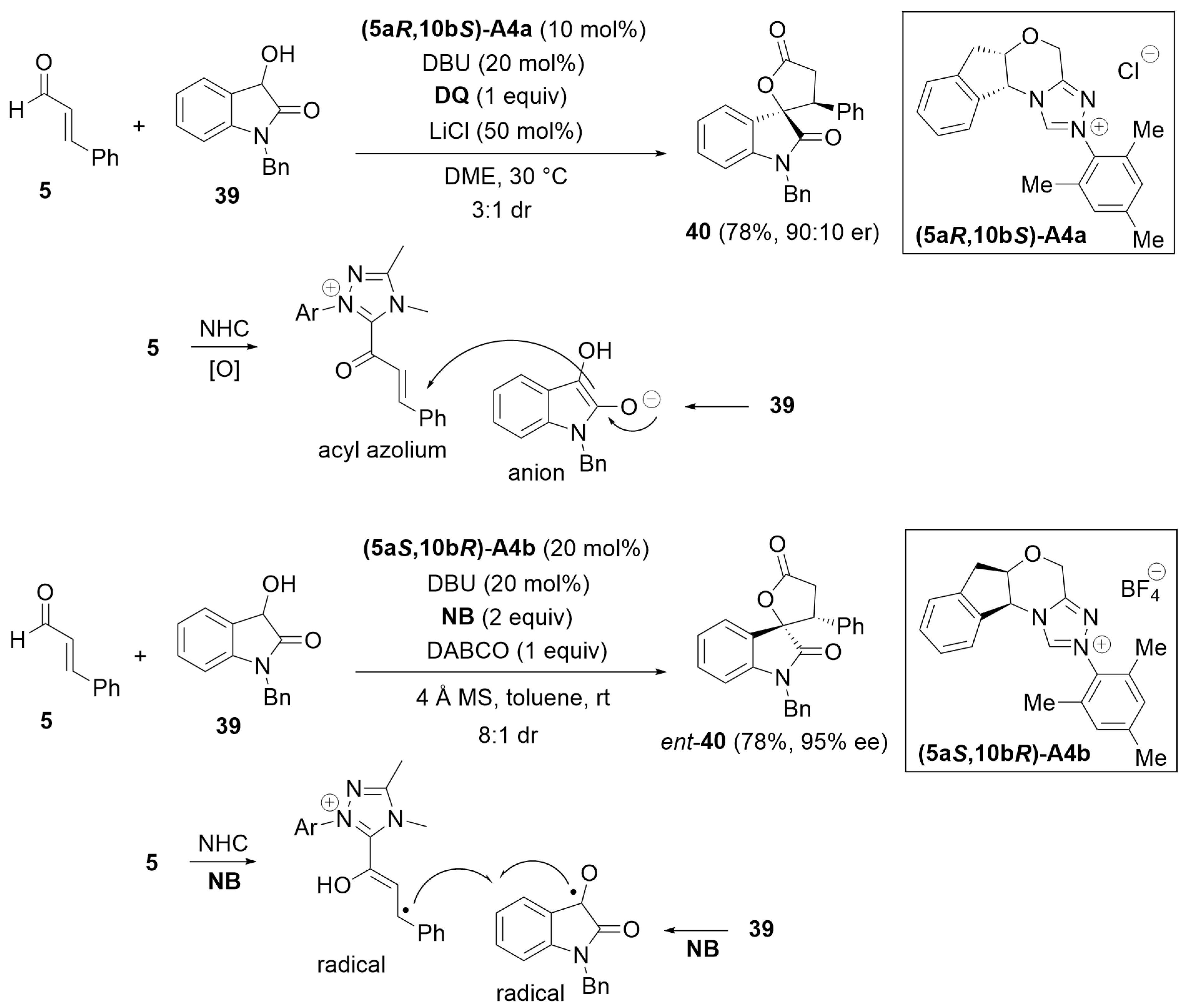
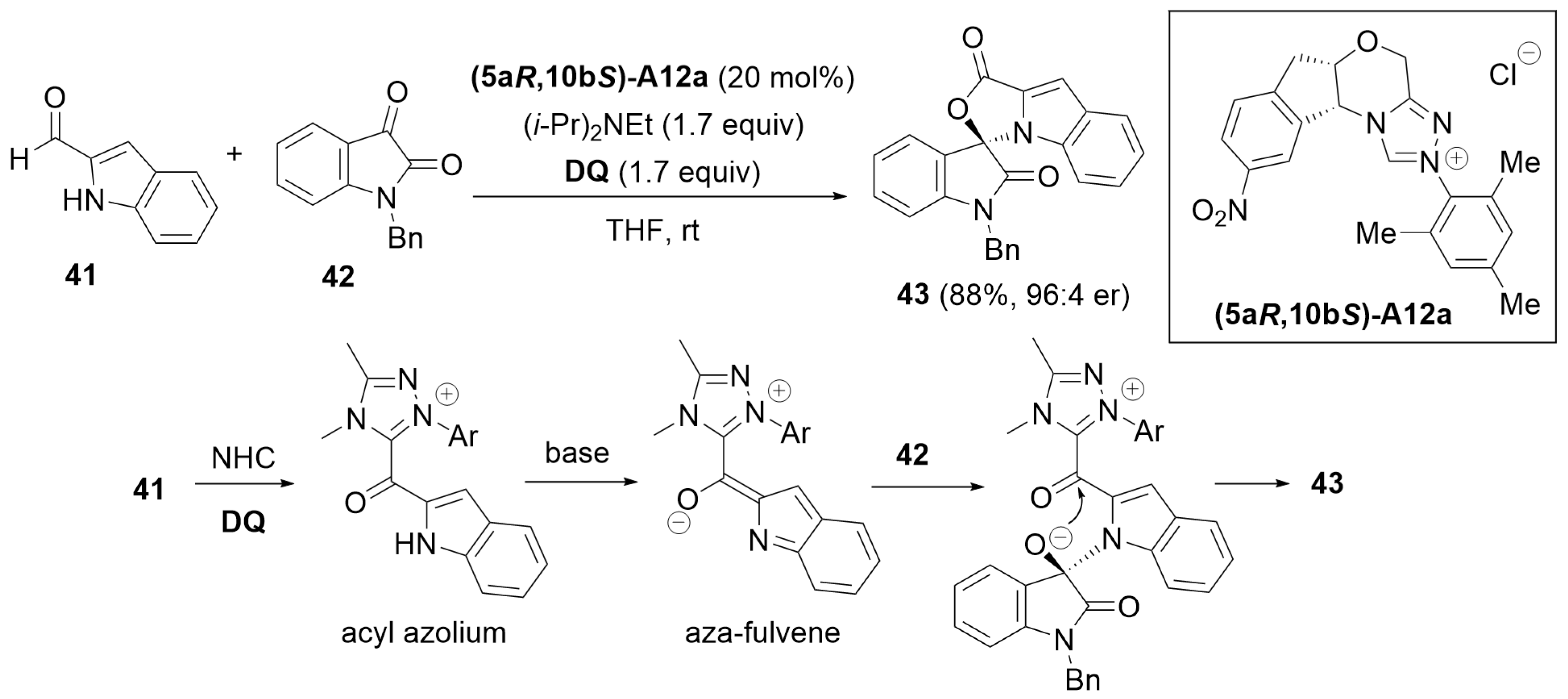
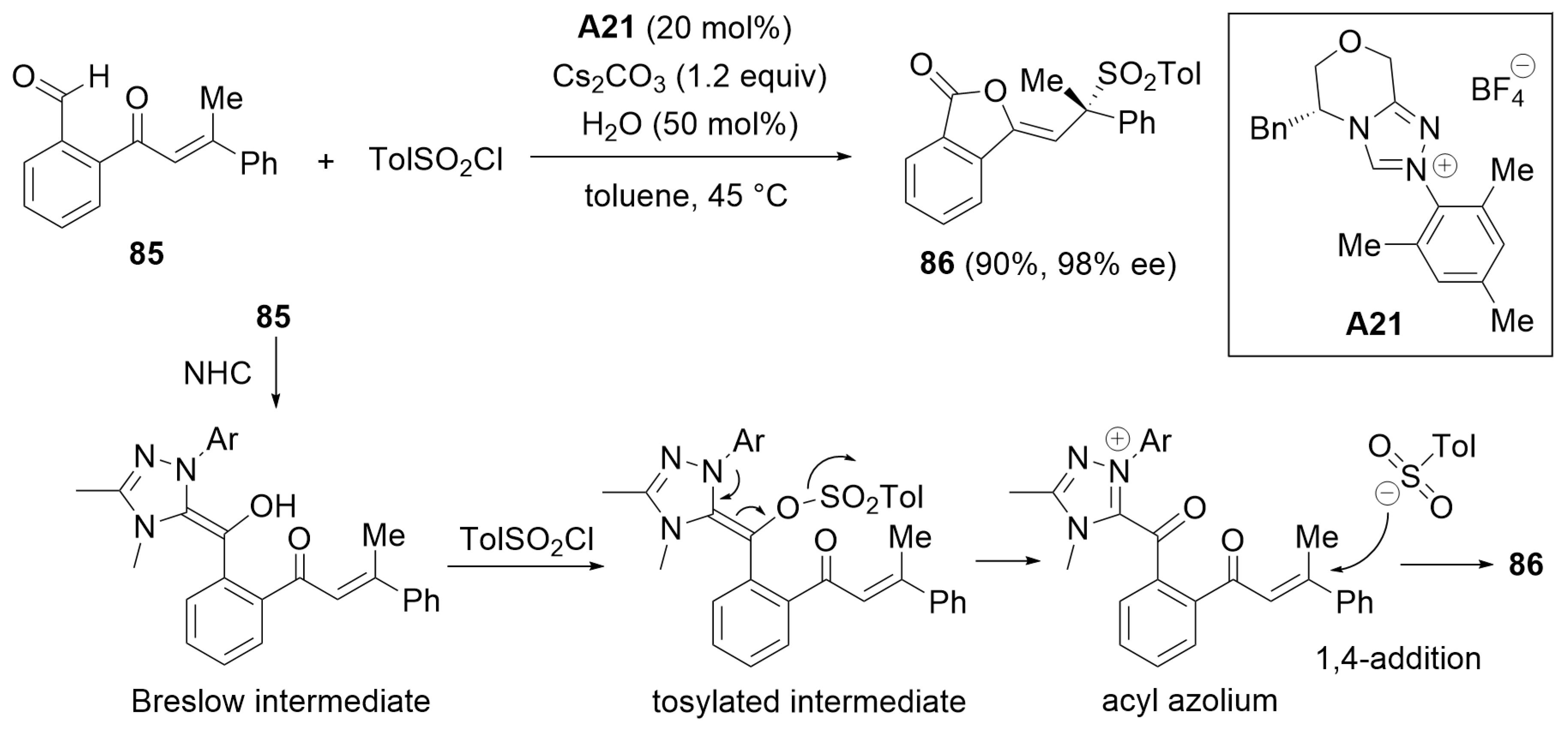
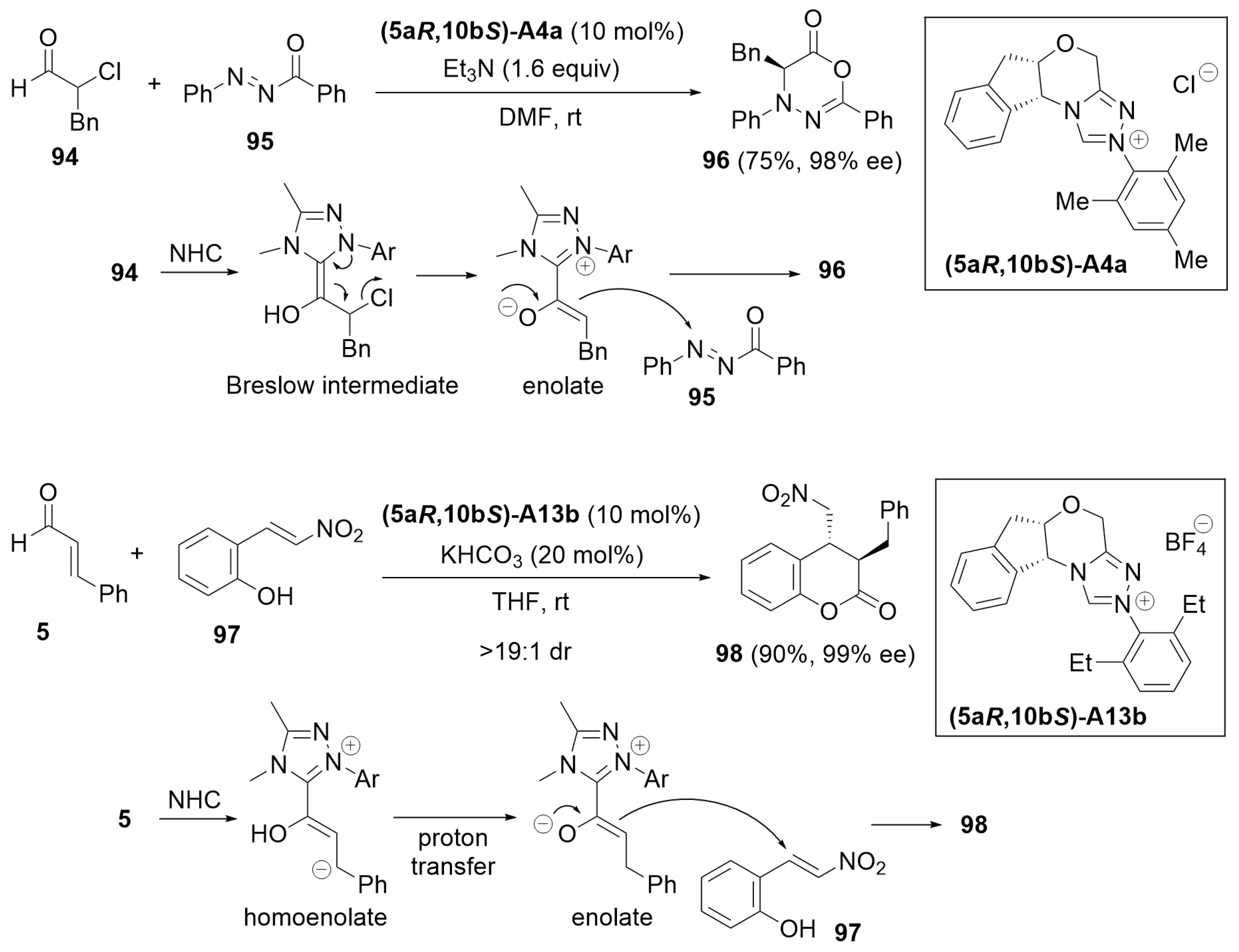
3.1. Cyclization
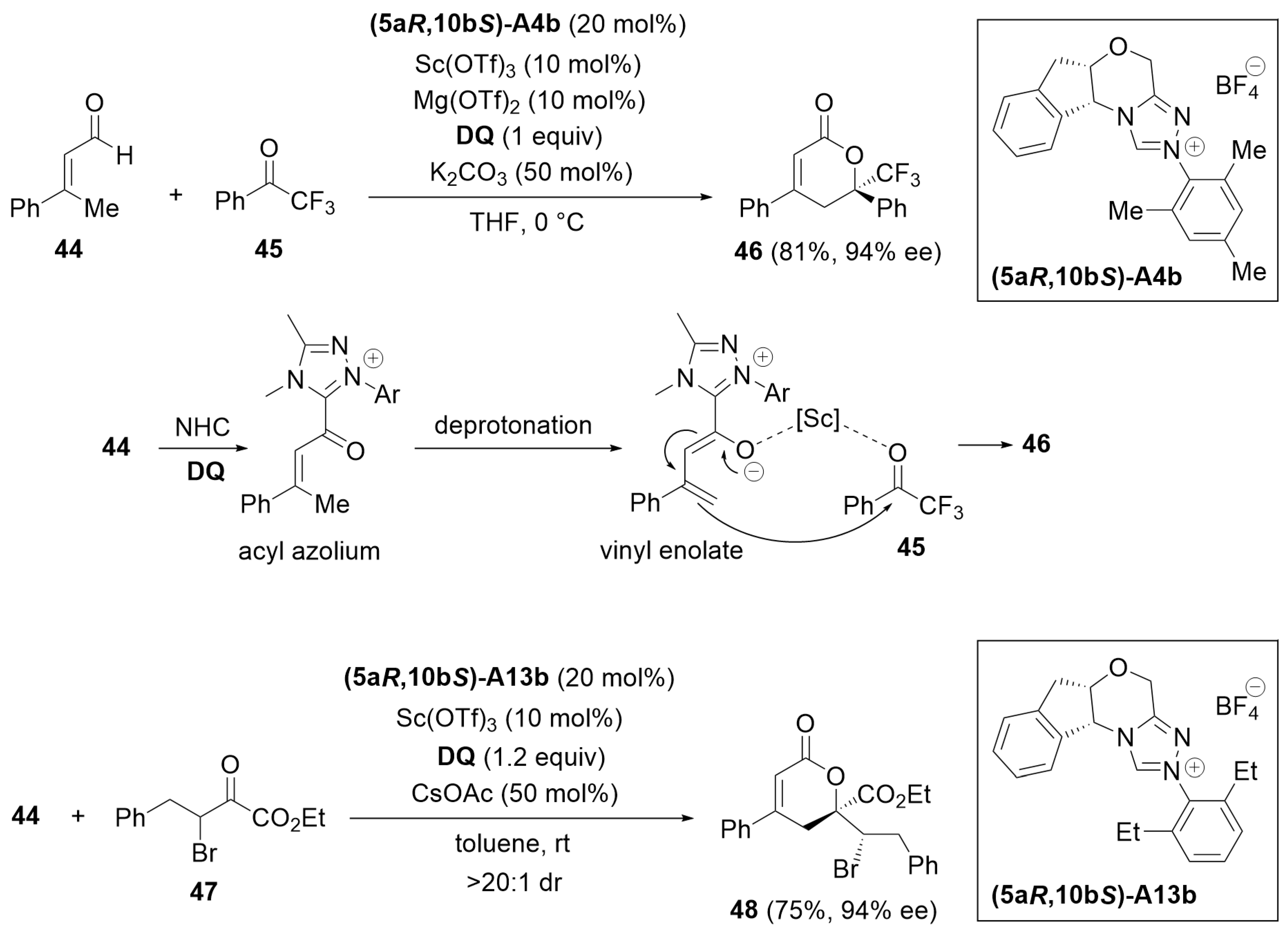
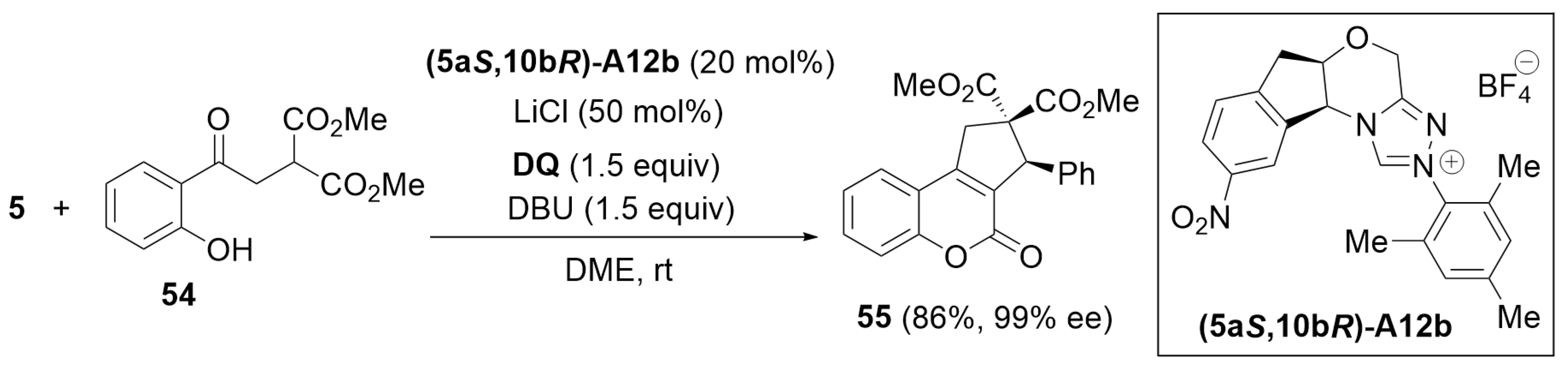
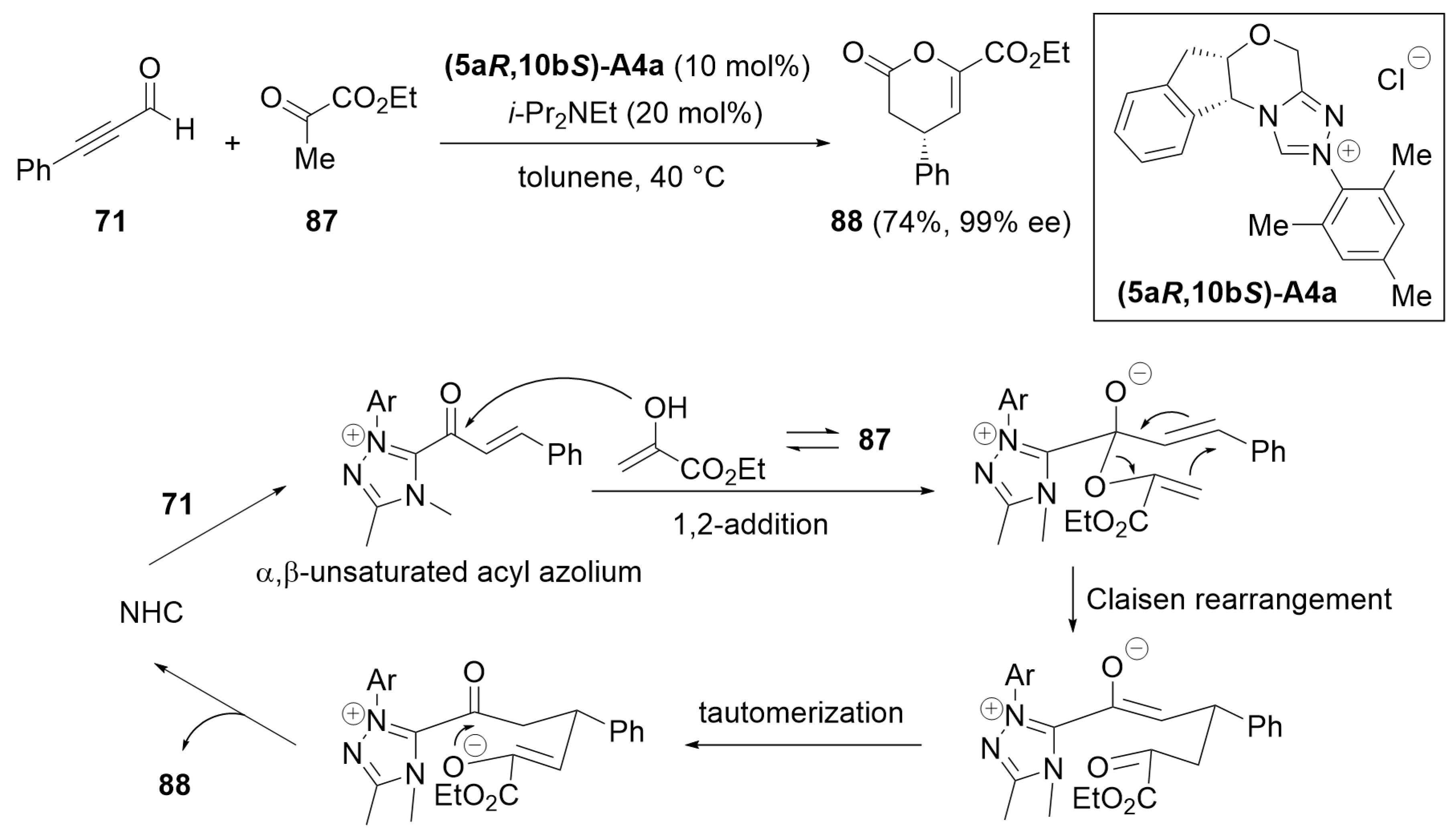
3.2. [3 + 3] Annulation
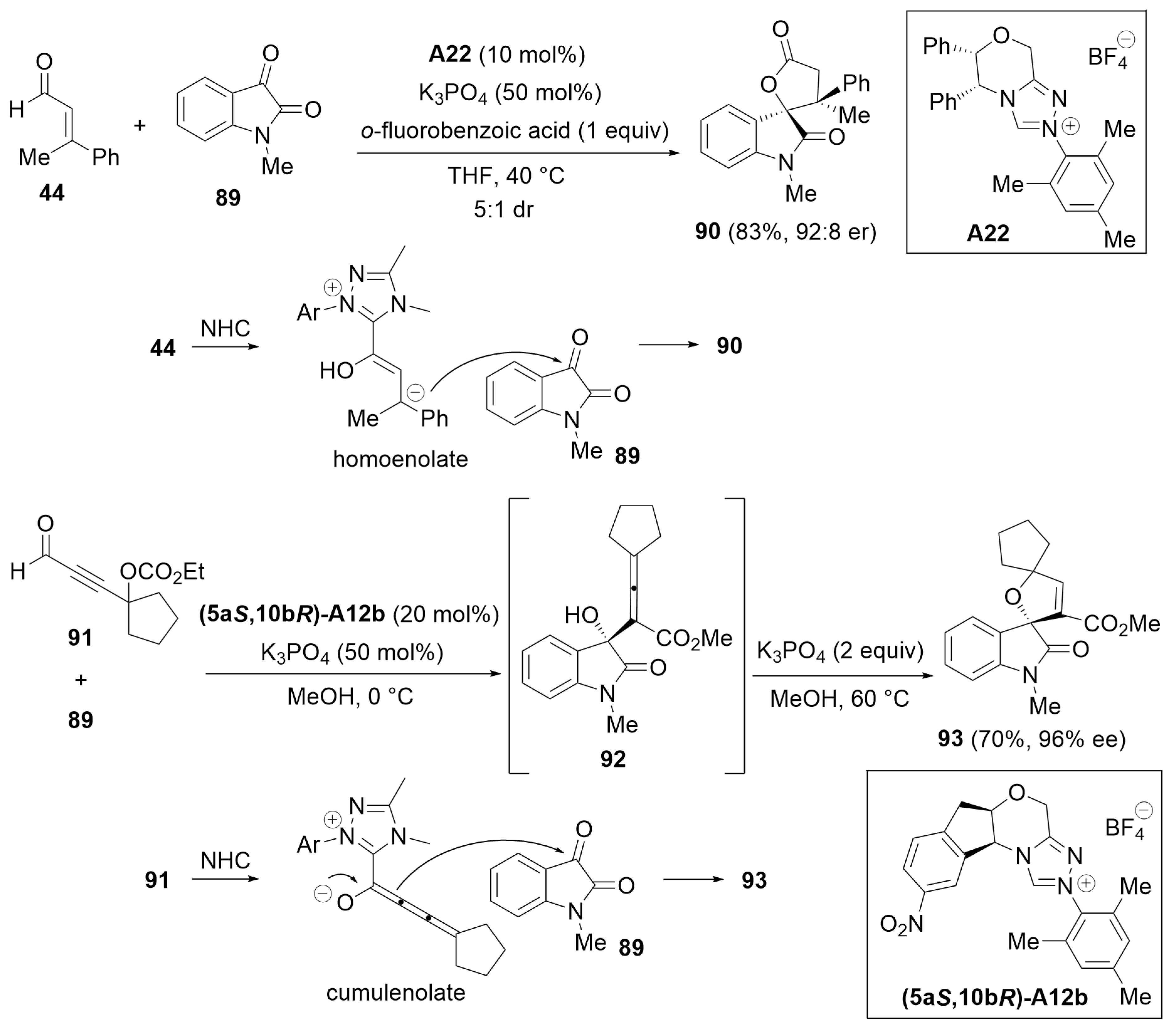
3.3. [3 + 2] Annulation
3.4. [4 + 2] Annulation
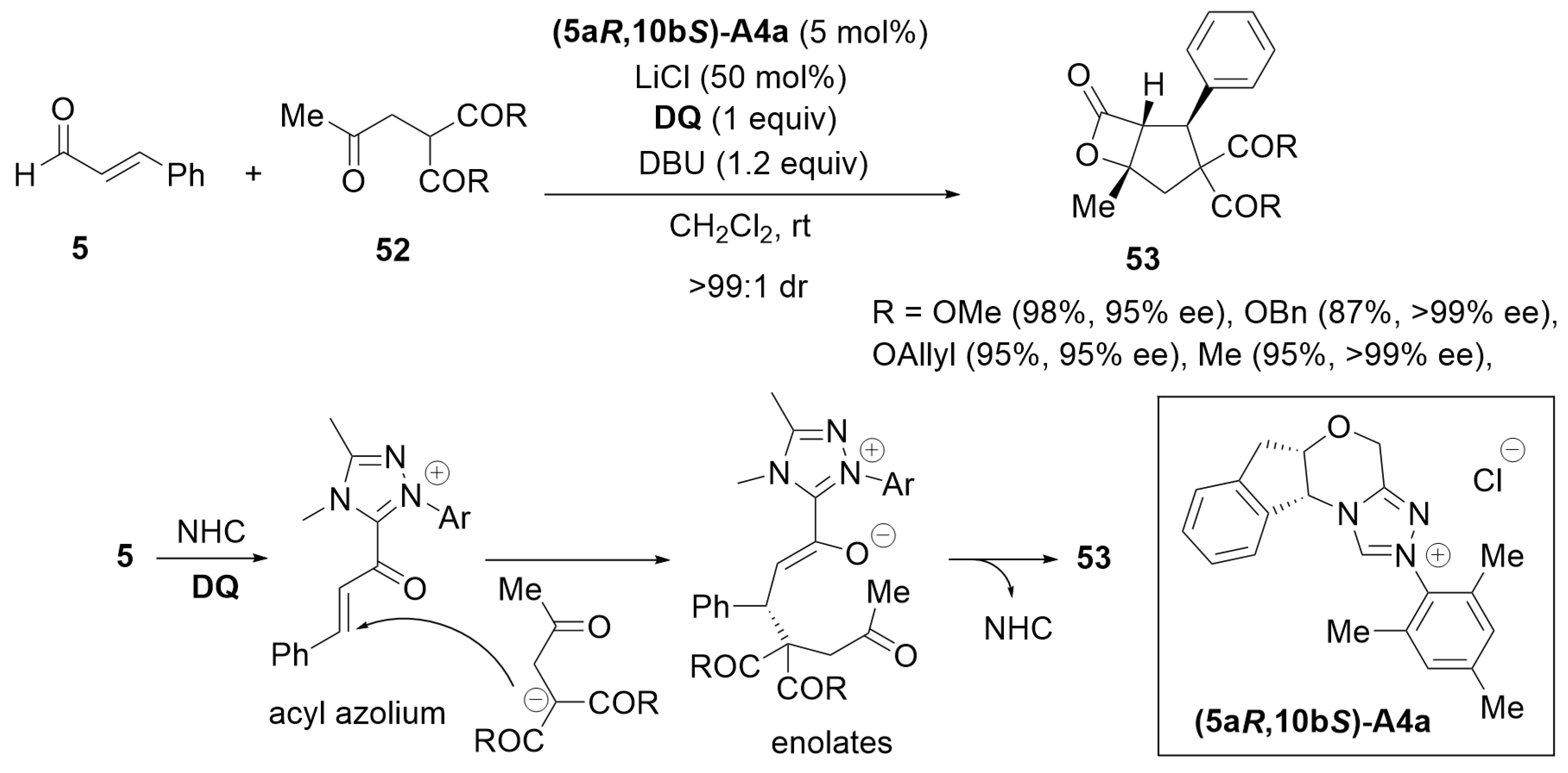
3.5. [4 + 3] Annulation
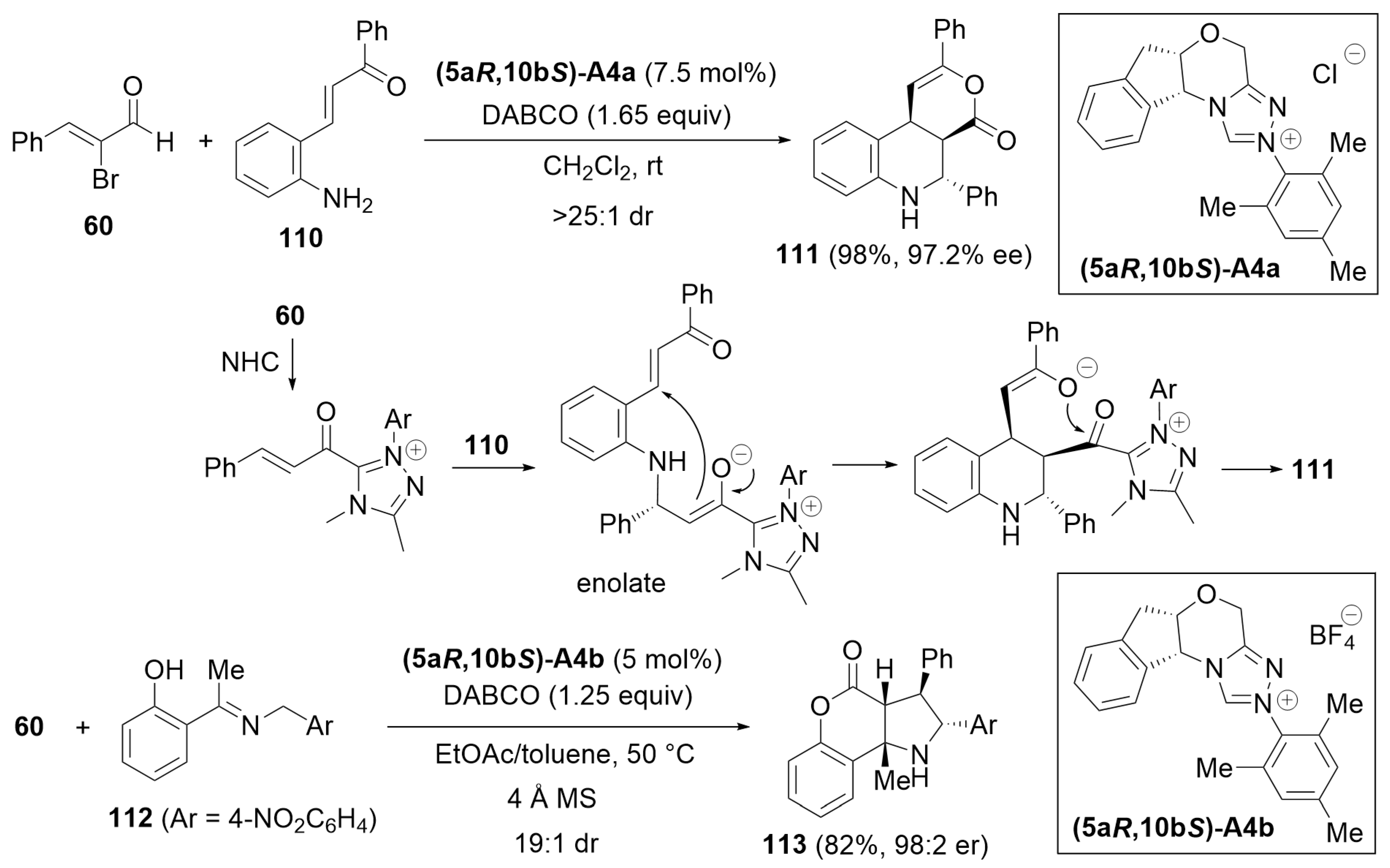
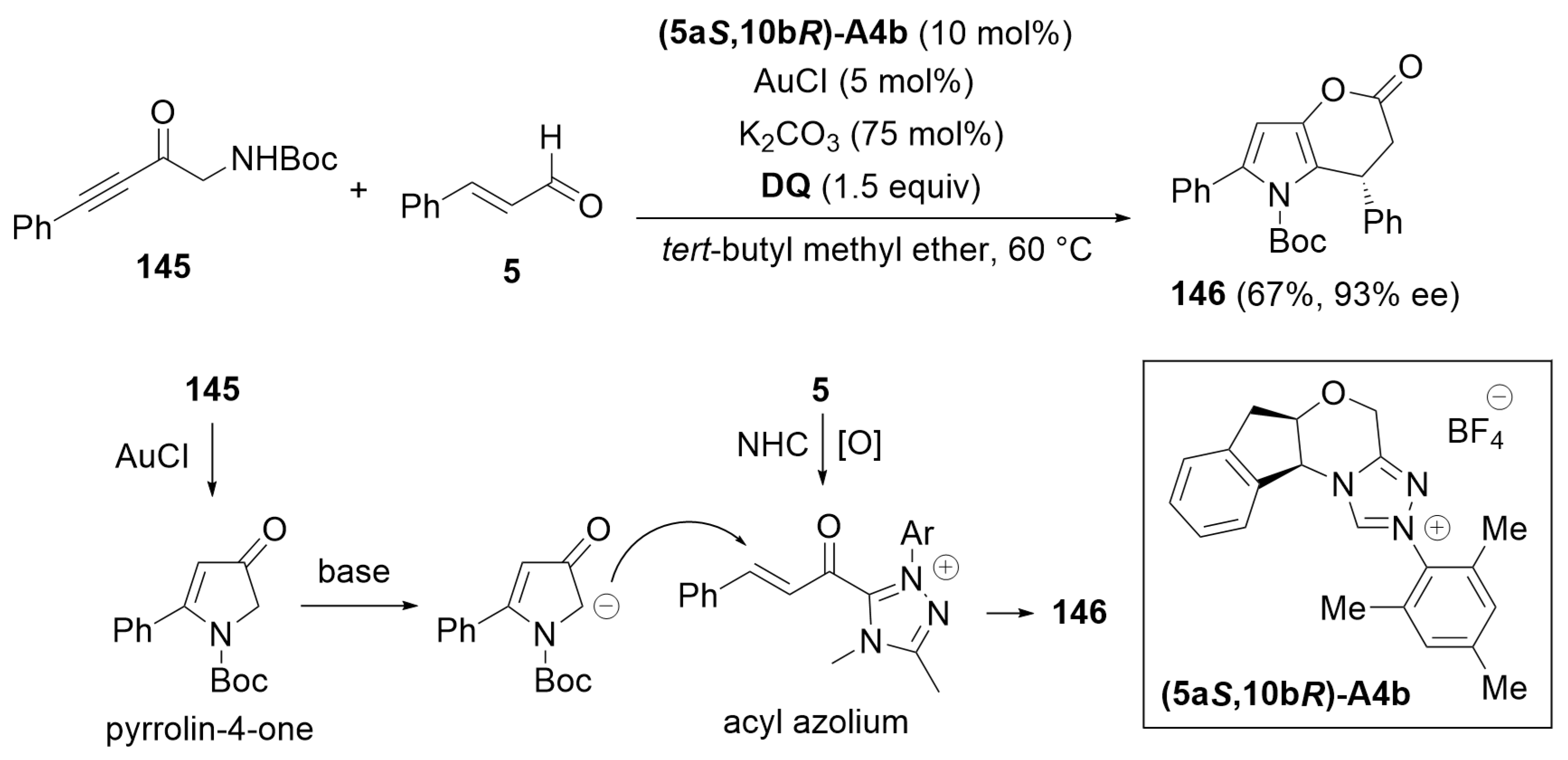
3.6. Cascade Annulation
4. External Oxidant-Free Redox Esterification
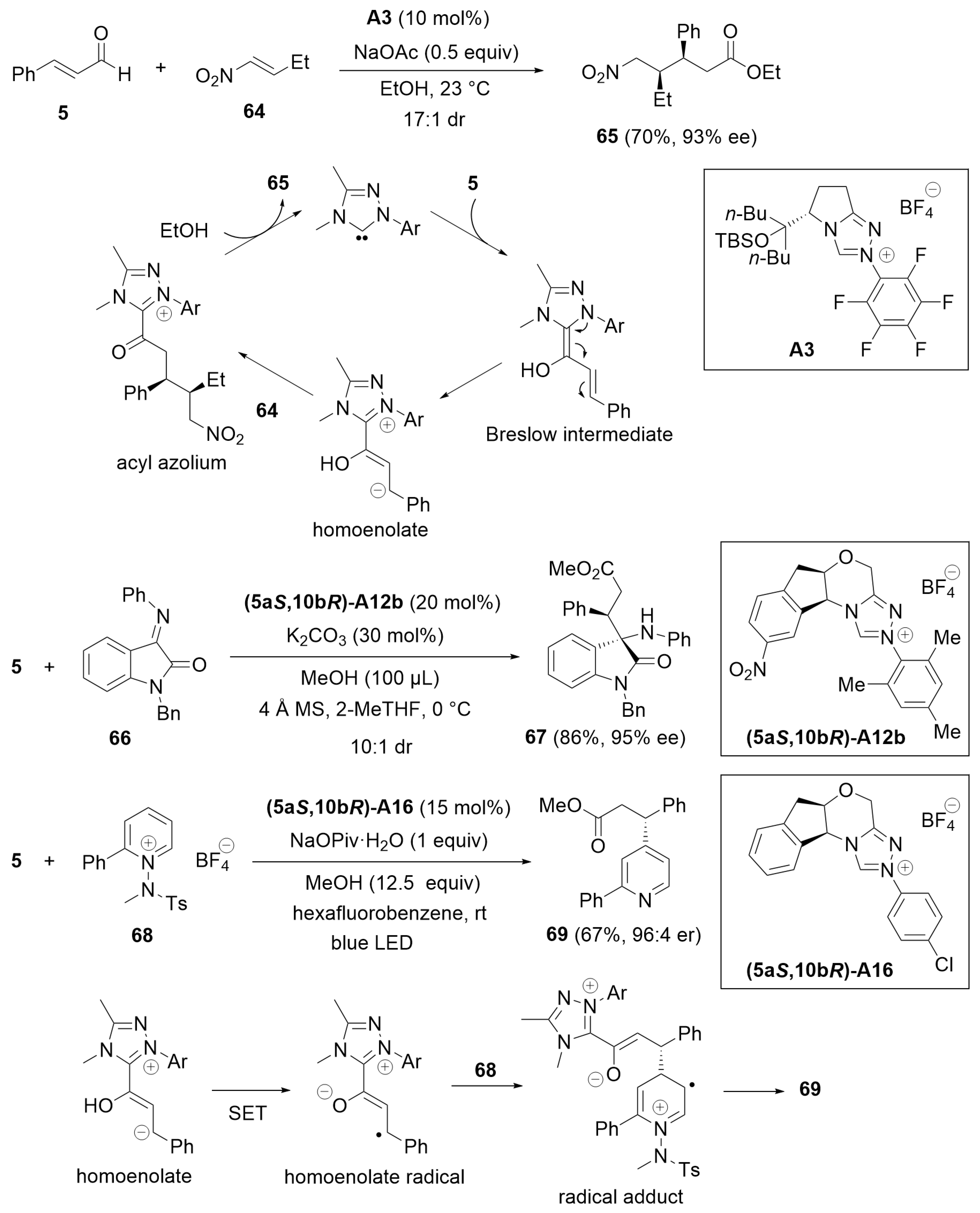
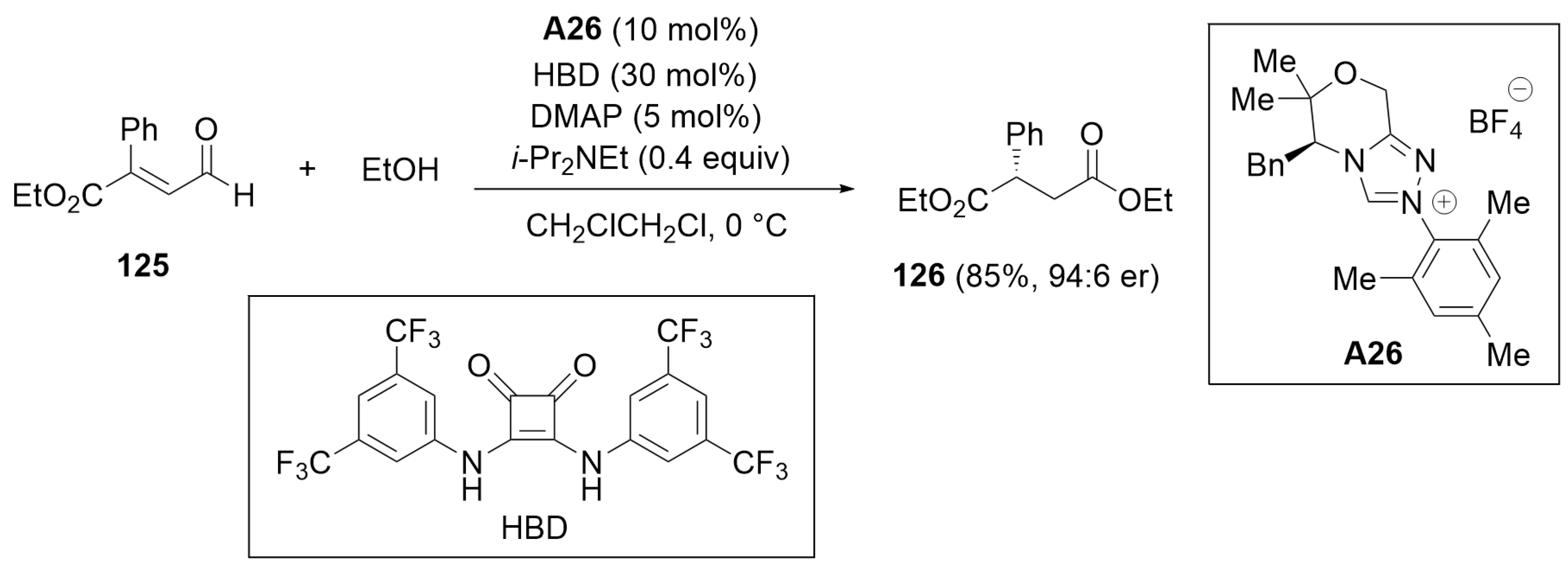
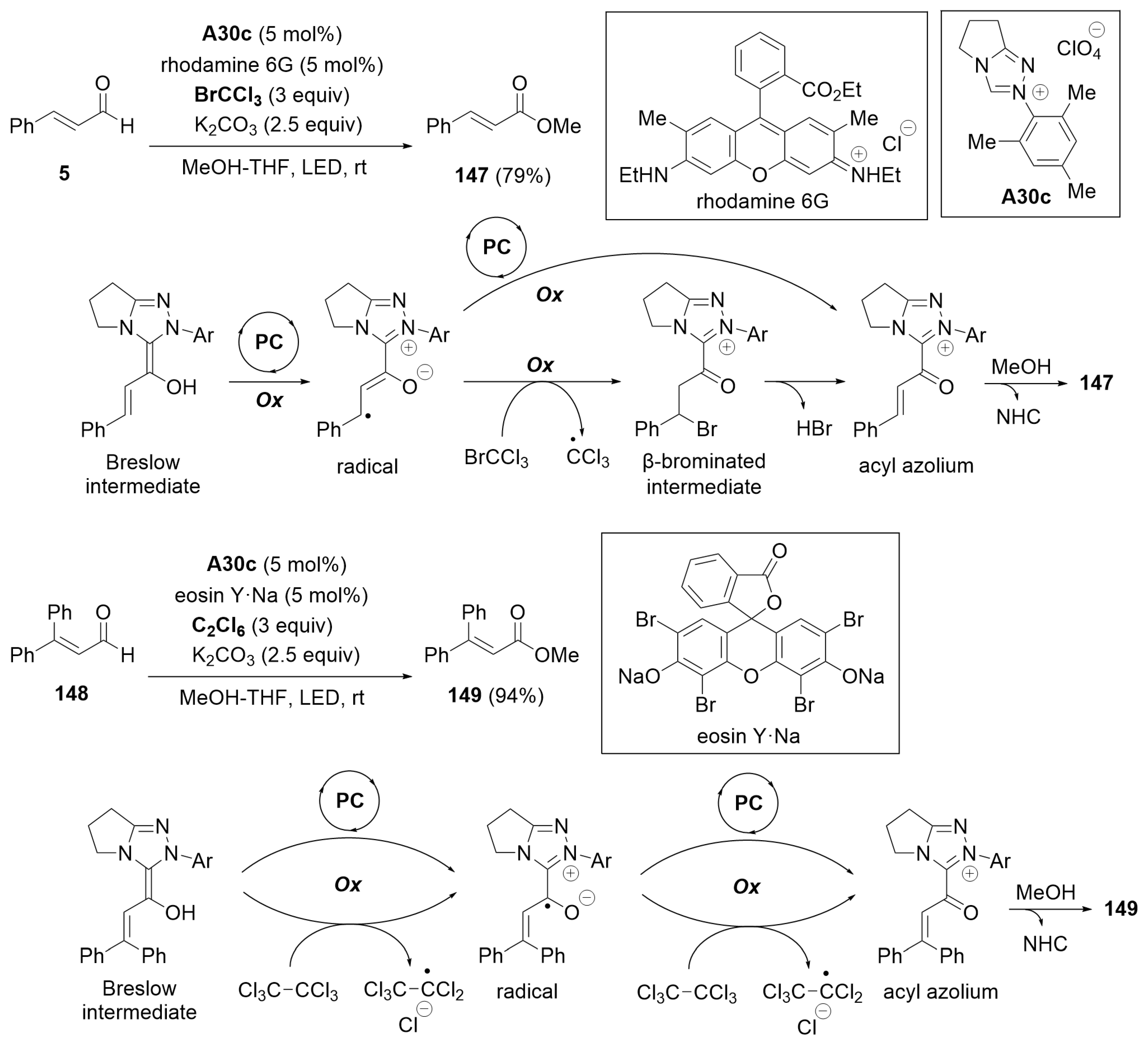
4.1. Esterification of Aldehydes under Redox Conditions
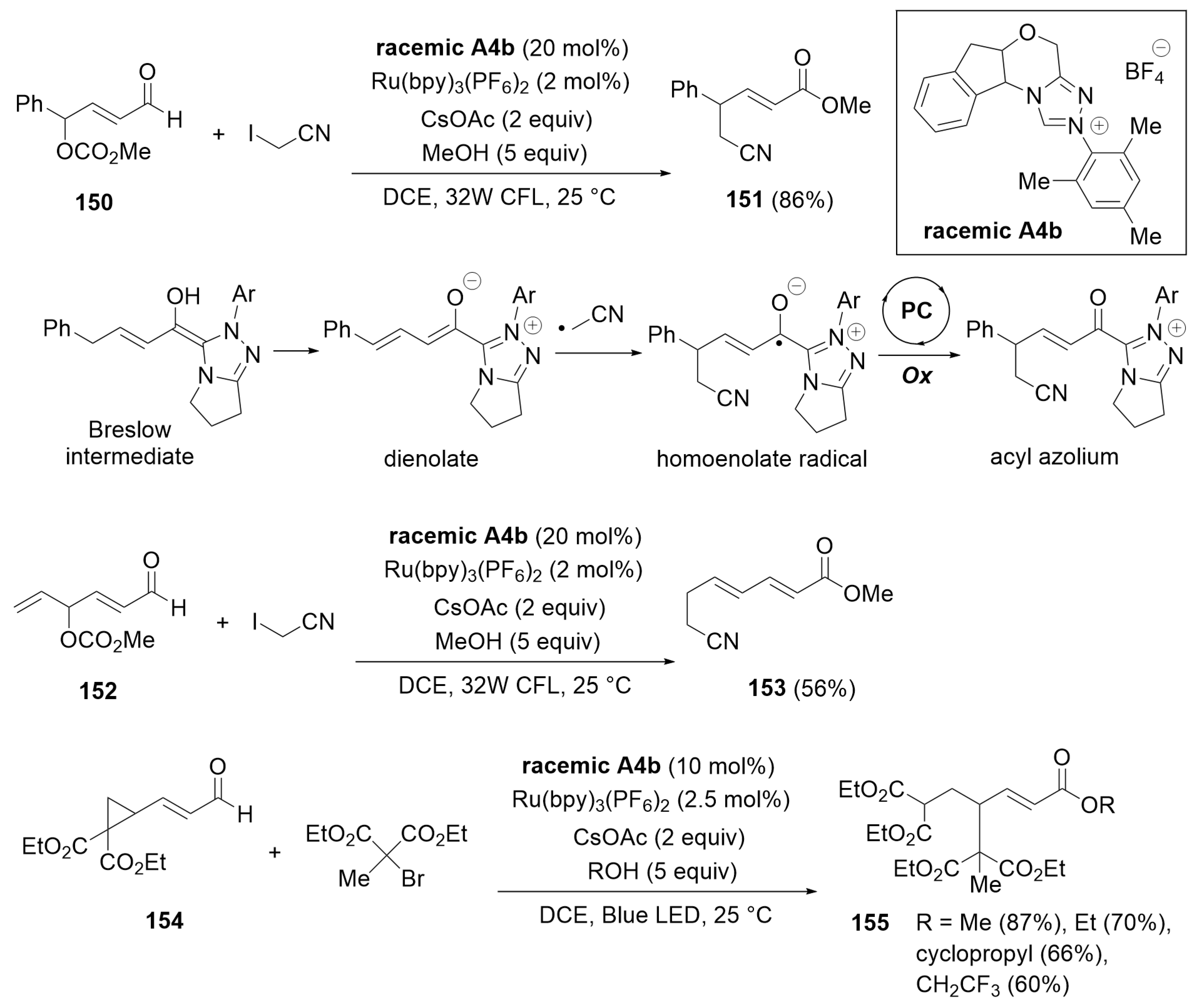
4.2. Cascade Redox Esterification of Aldehydes
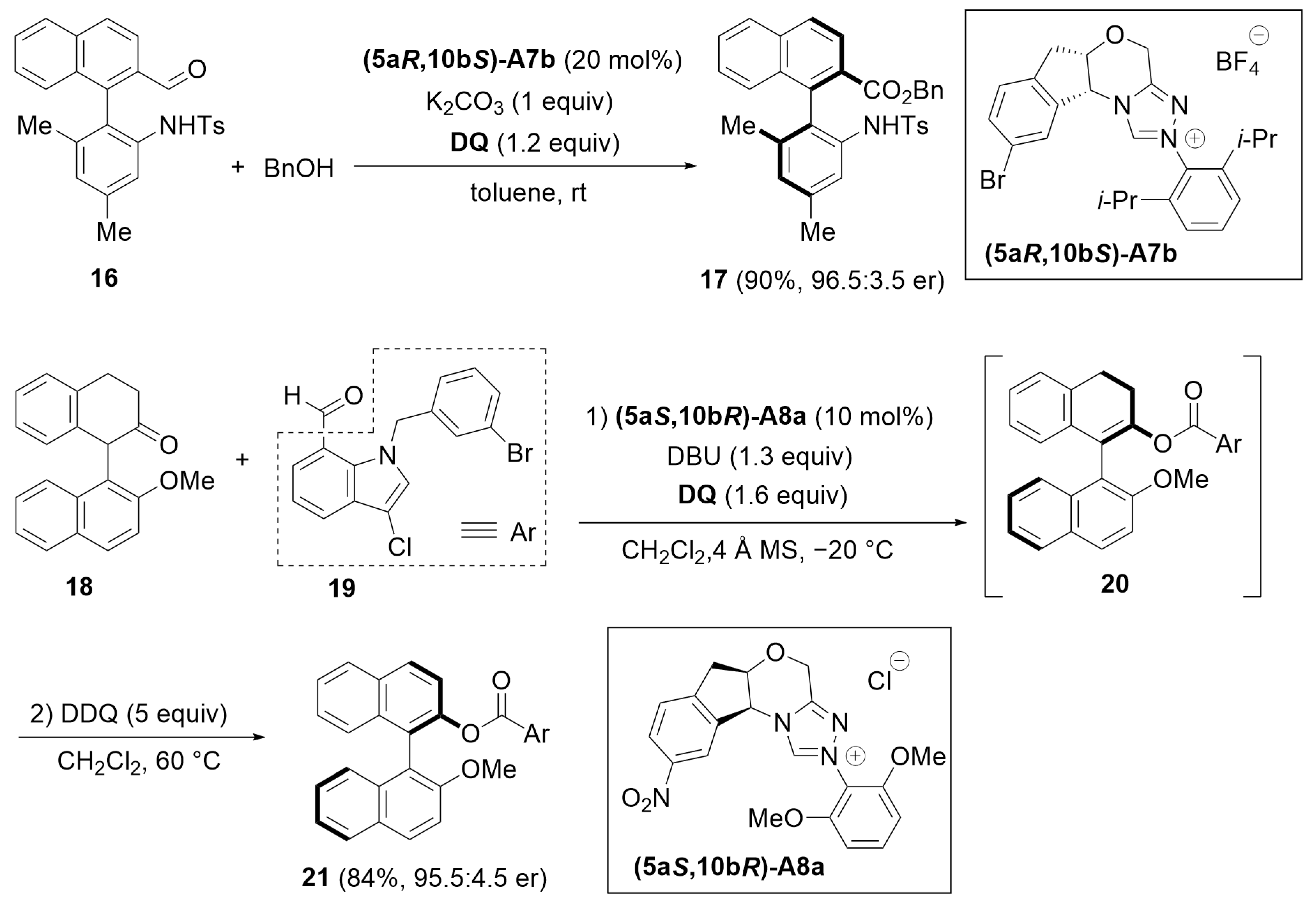
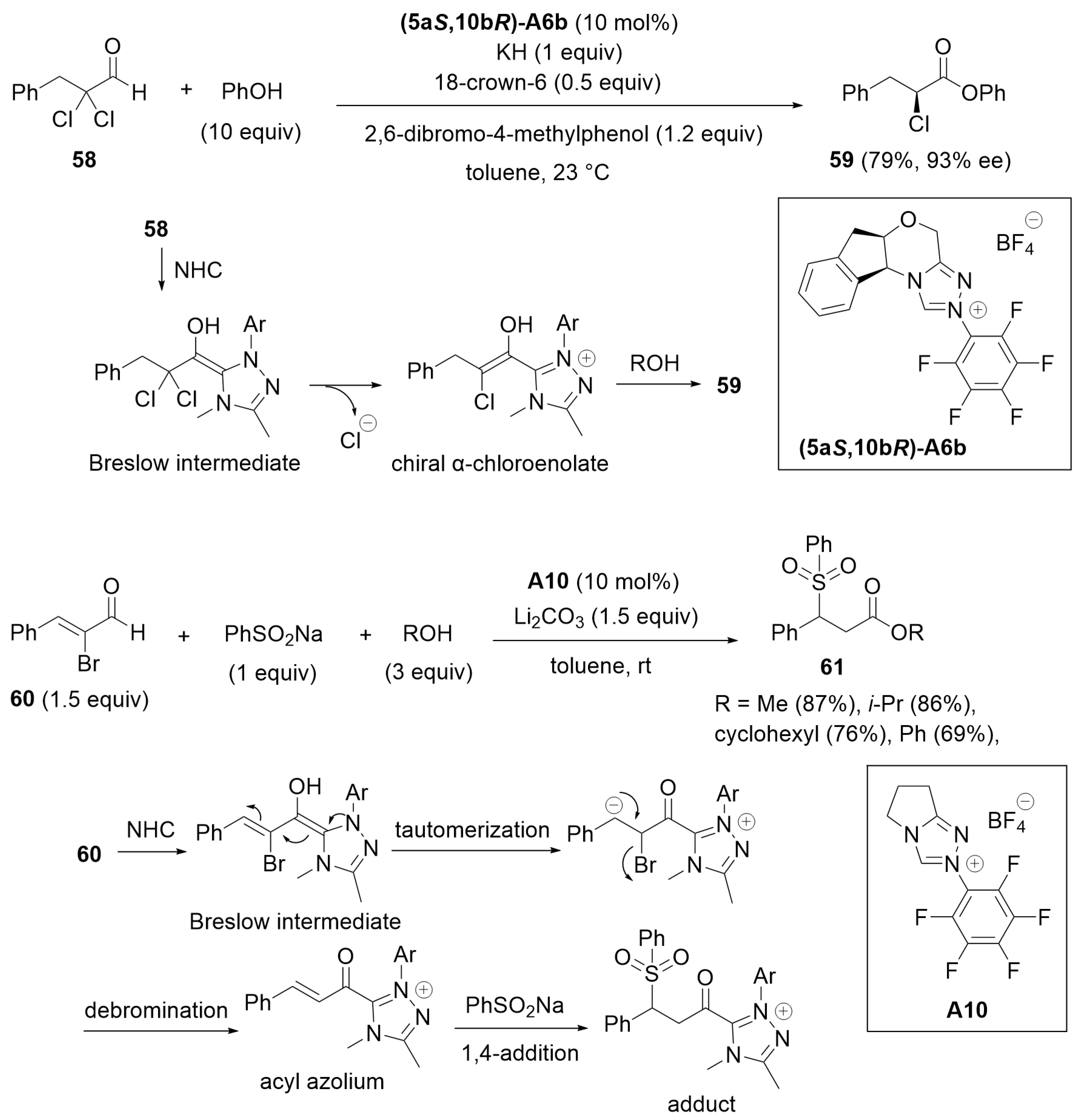
4.3. Kinetic Resolution
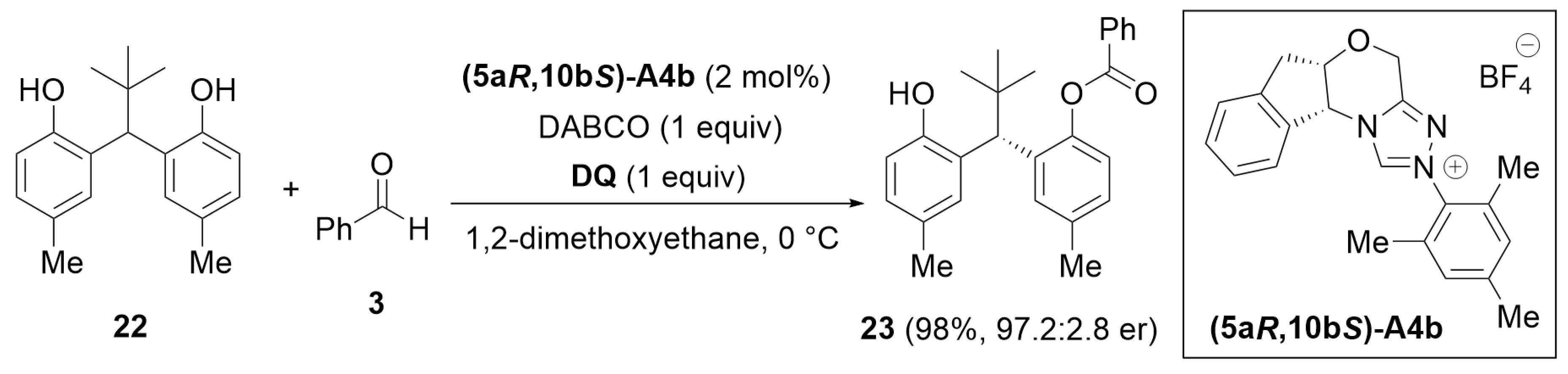
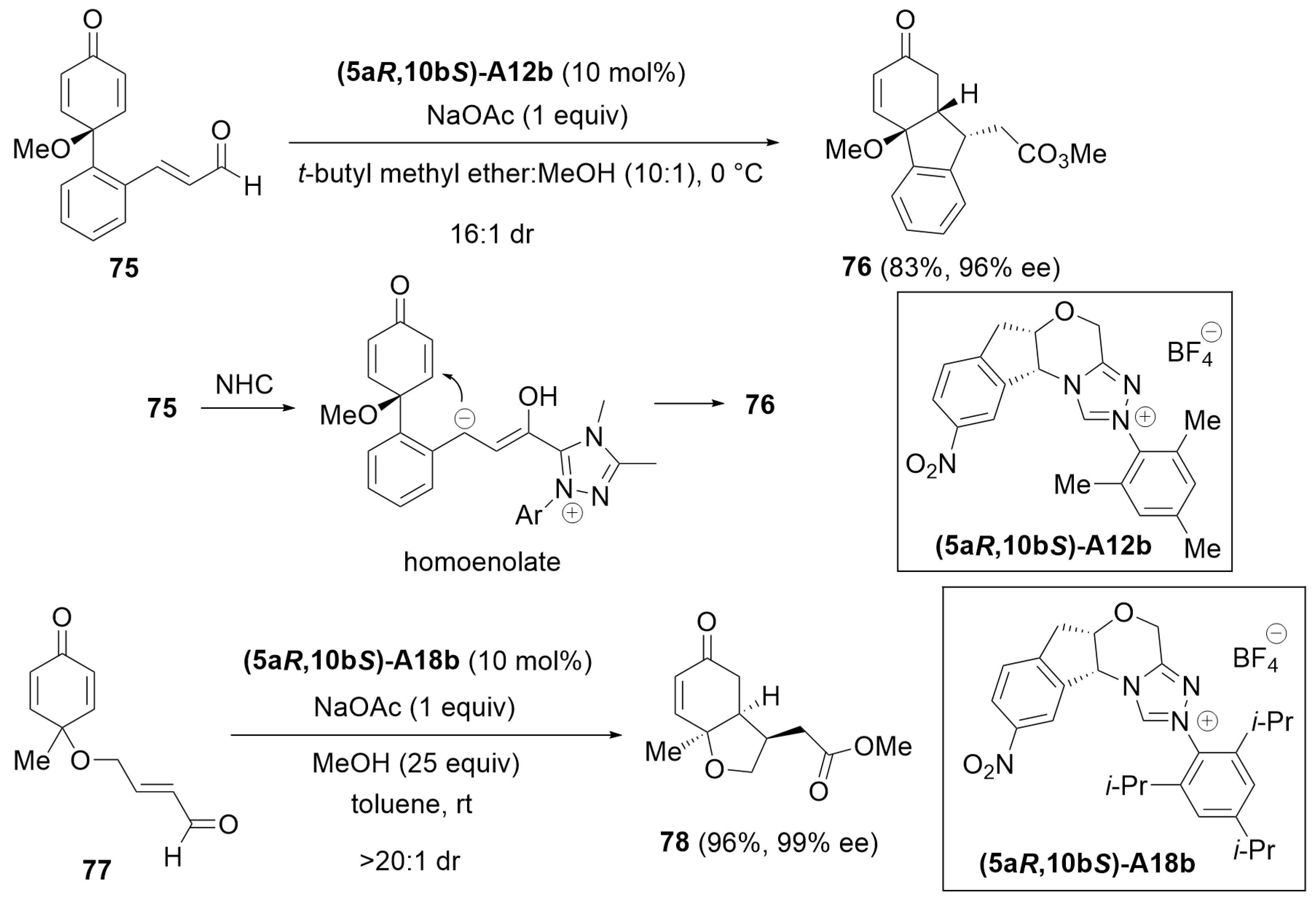
4.4. Desymmetrization
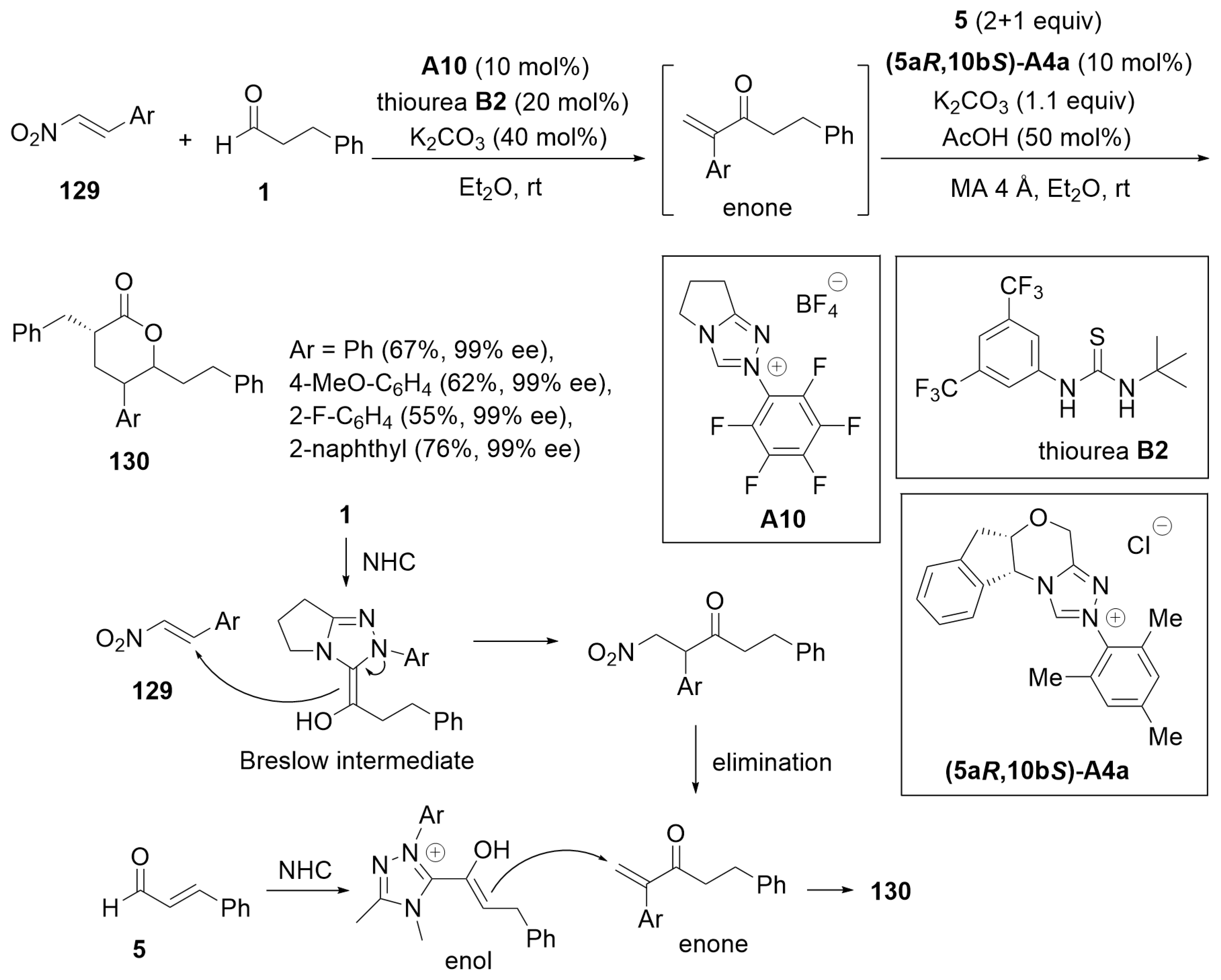
4.5. Dearomatization
5. Redox Cyclization and Annulation
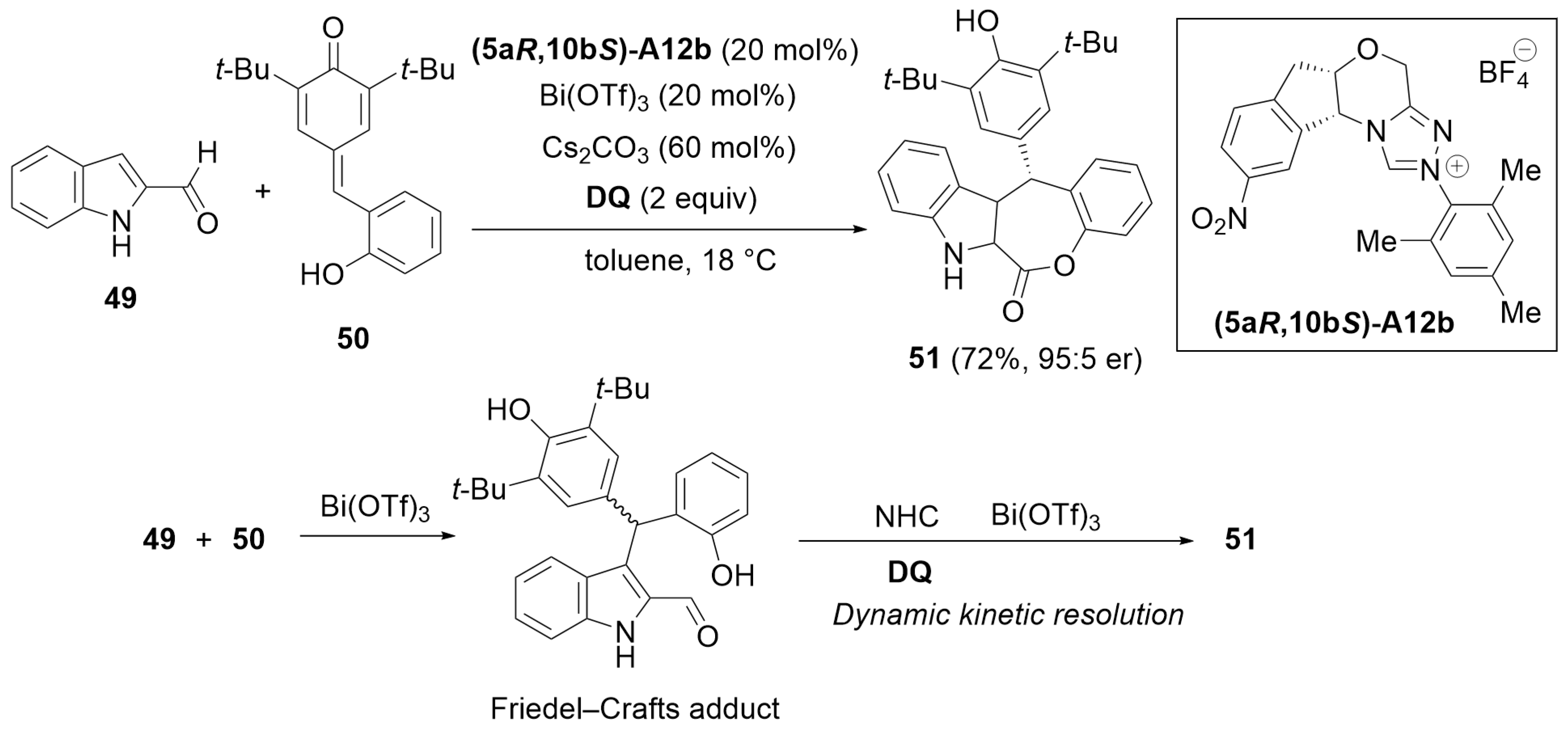
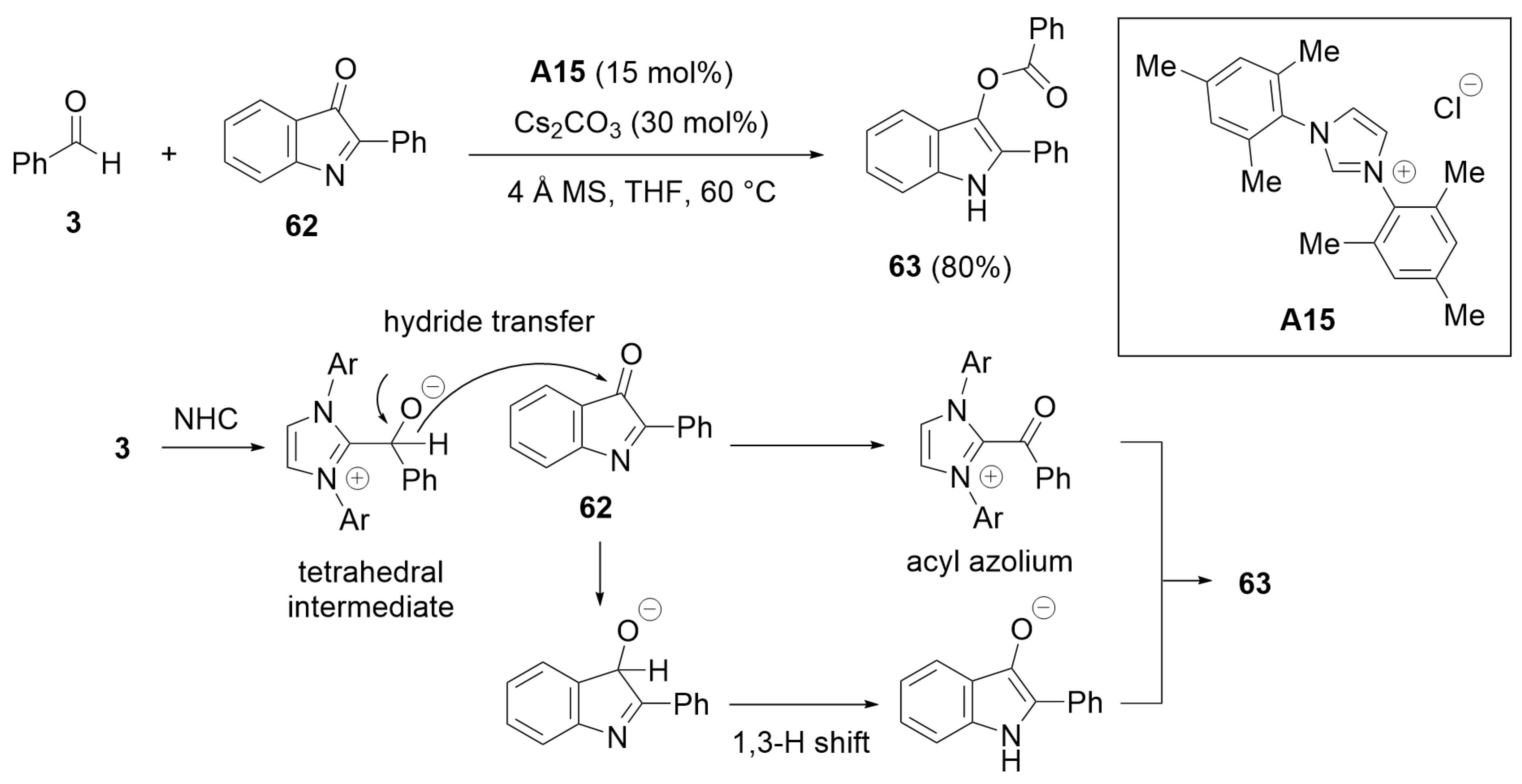
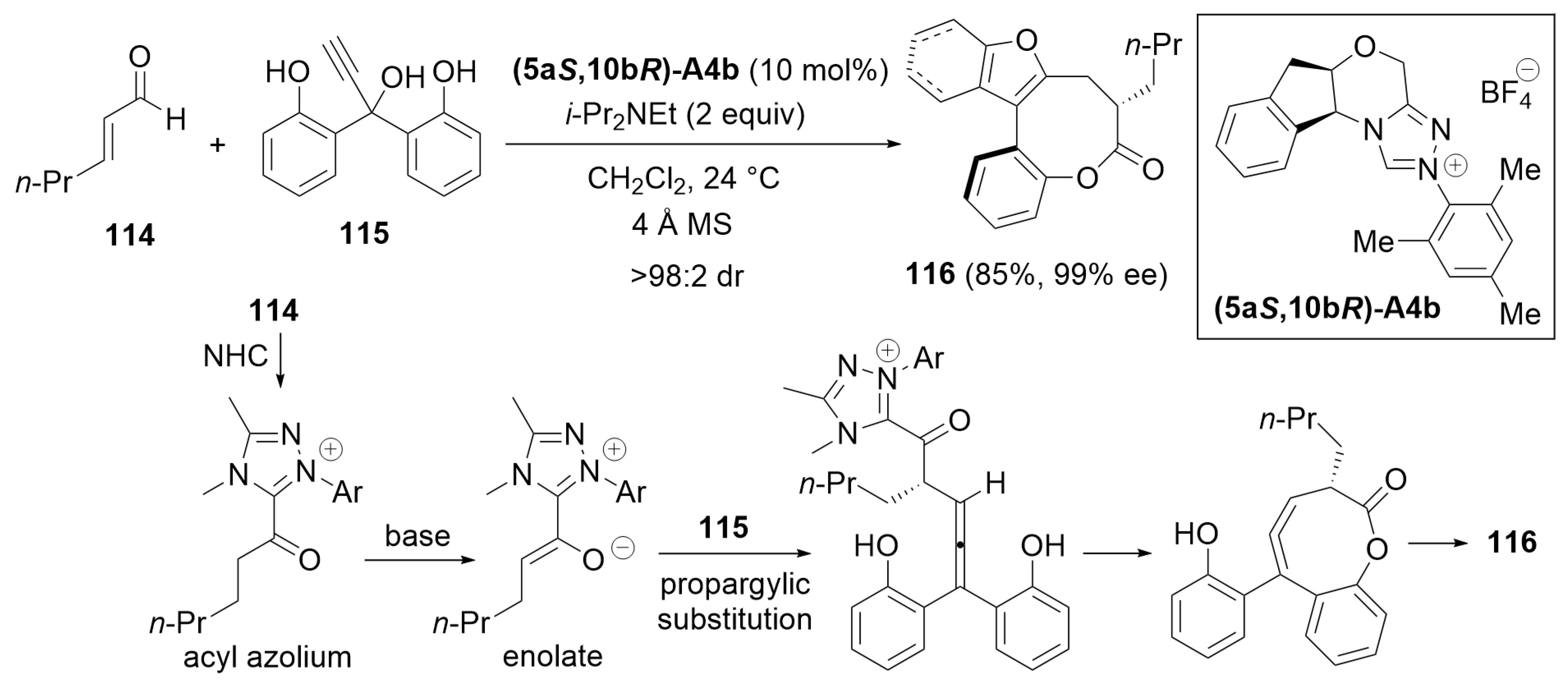
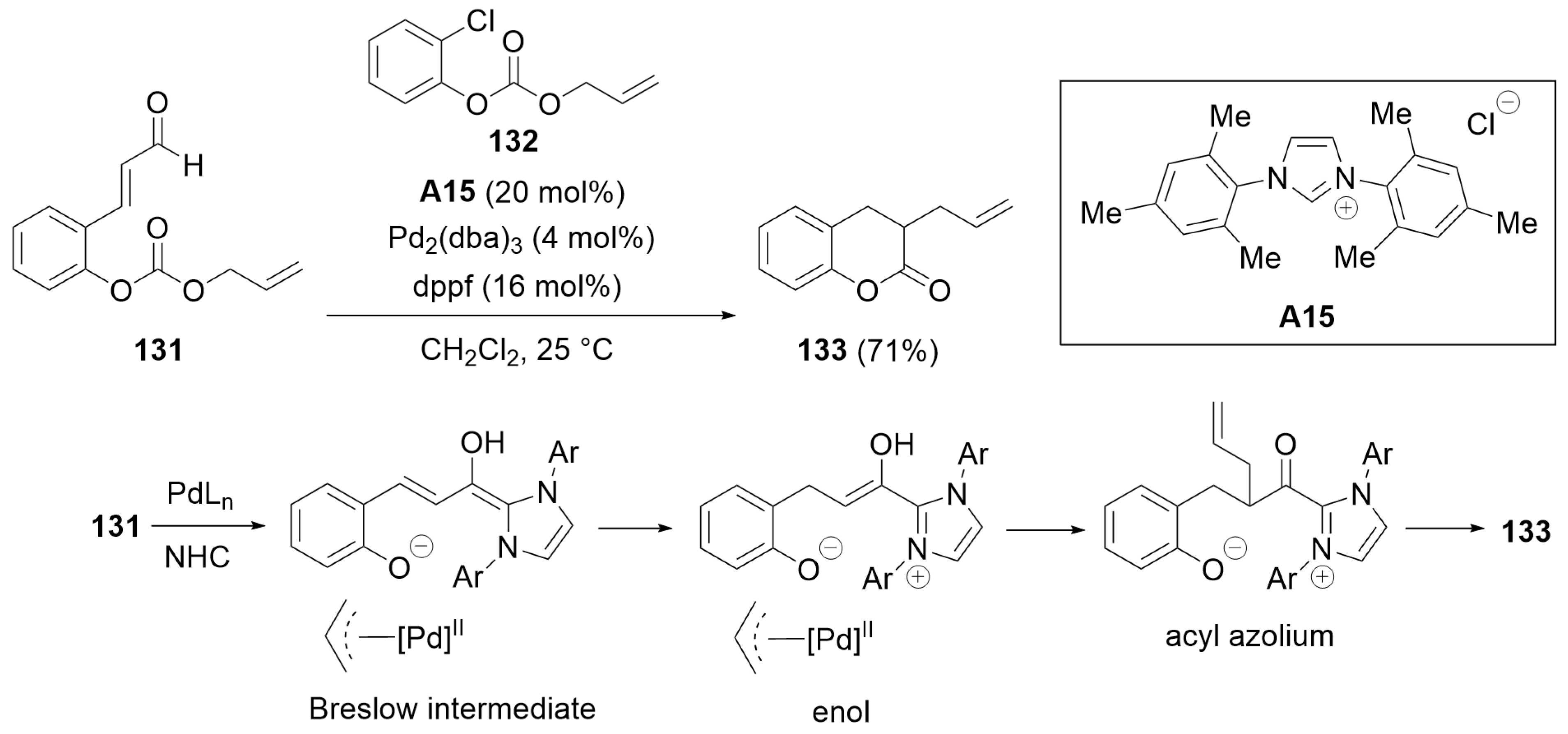
5.1. Cyclization
5.2. [3 + 3] Annulation
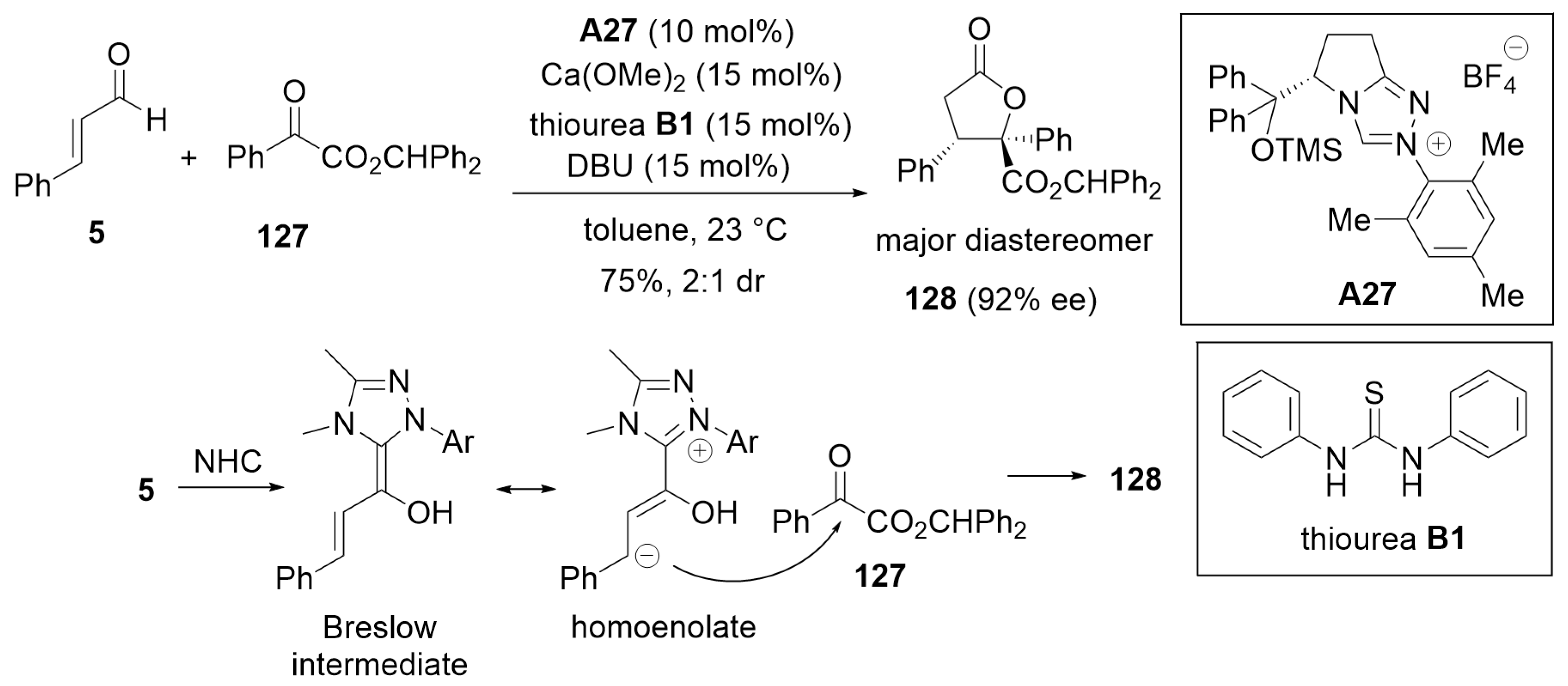
5.3. [3 + 2] Annulation
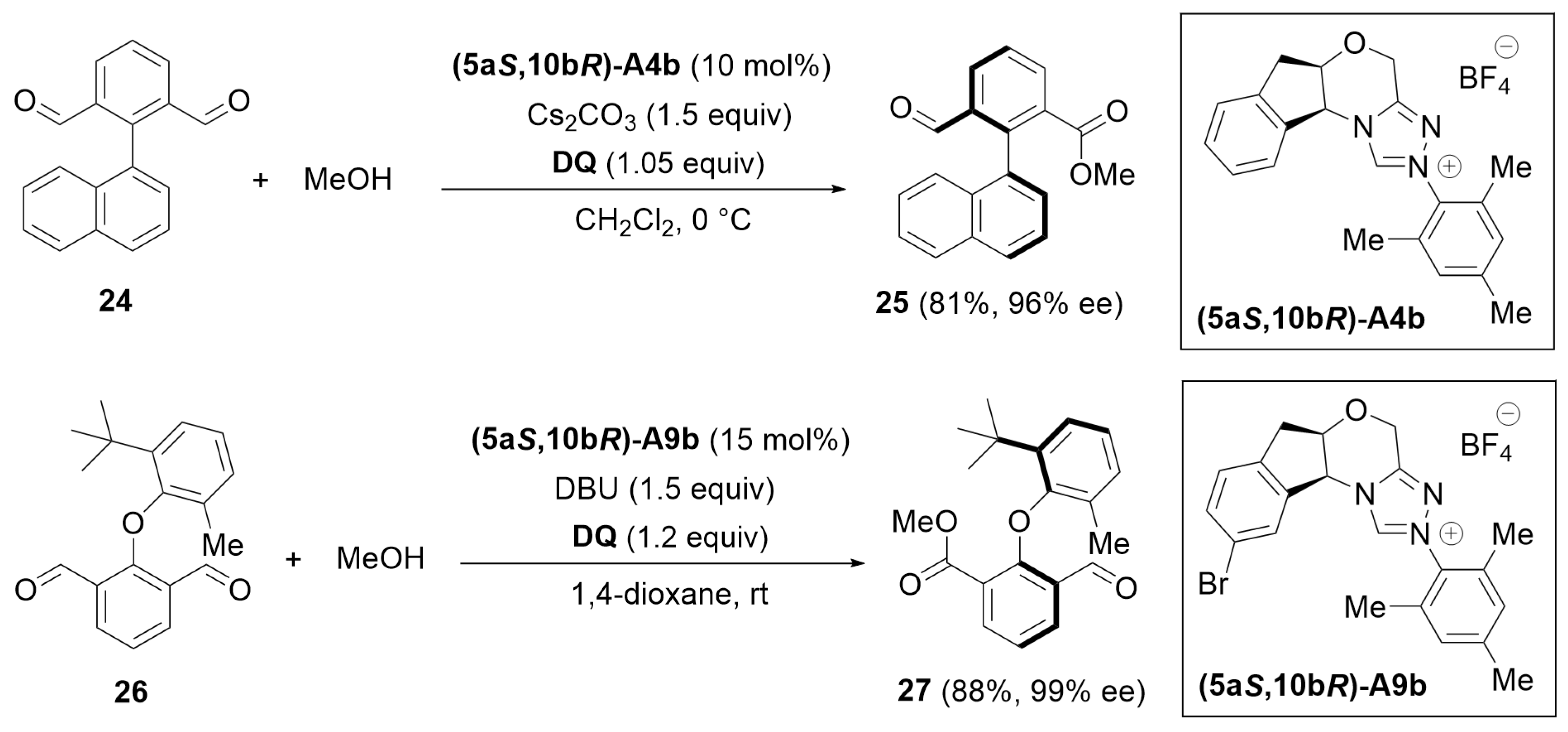
5.4. [4 + 2] Annulation
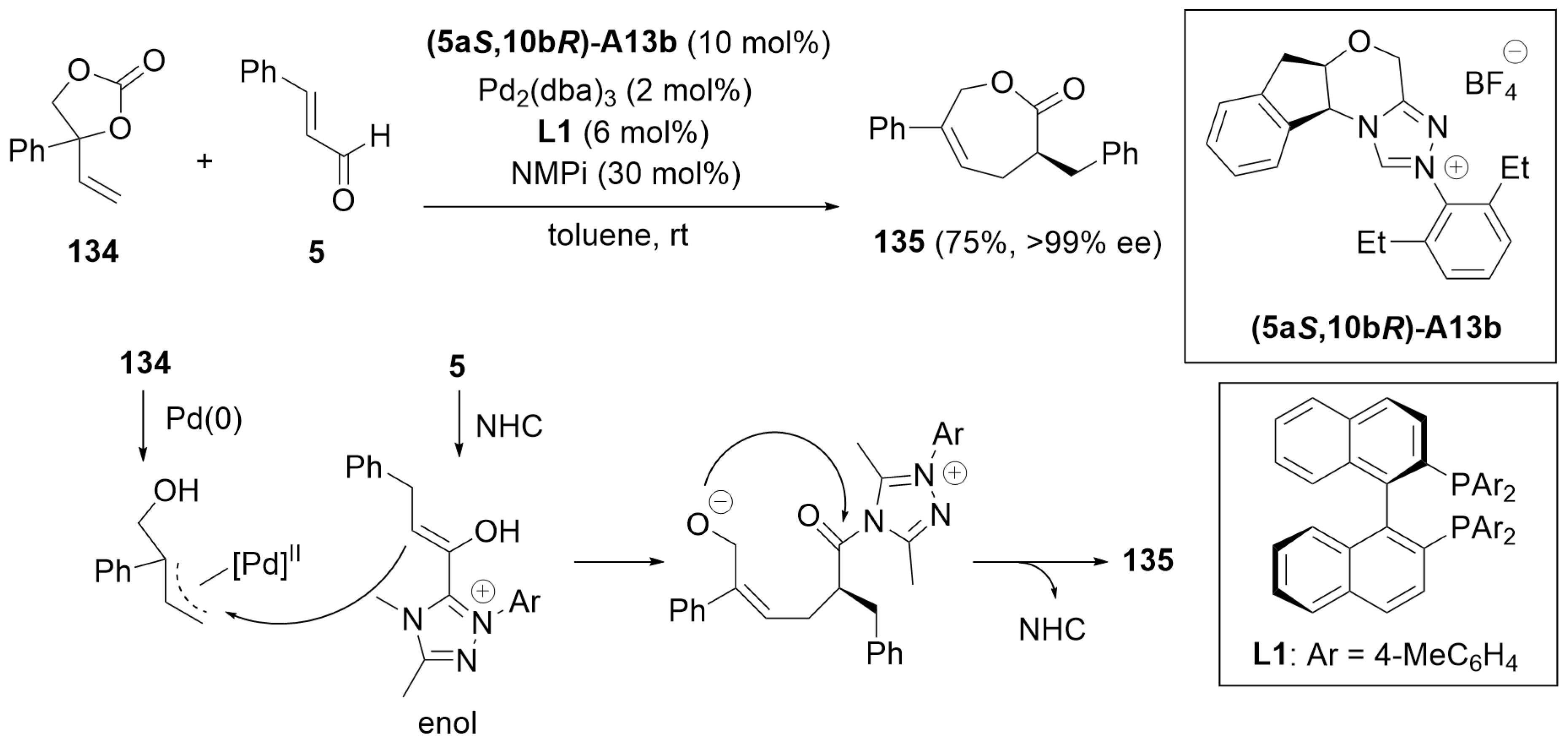
5.5. [4 + 3] Annulation
5.6. [2 + 2] Annulation
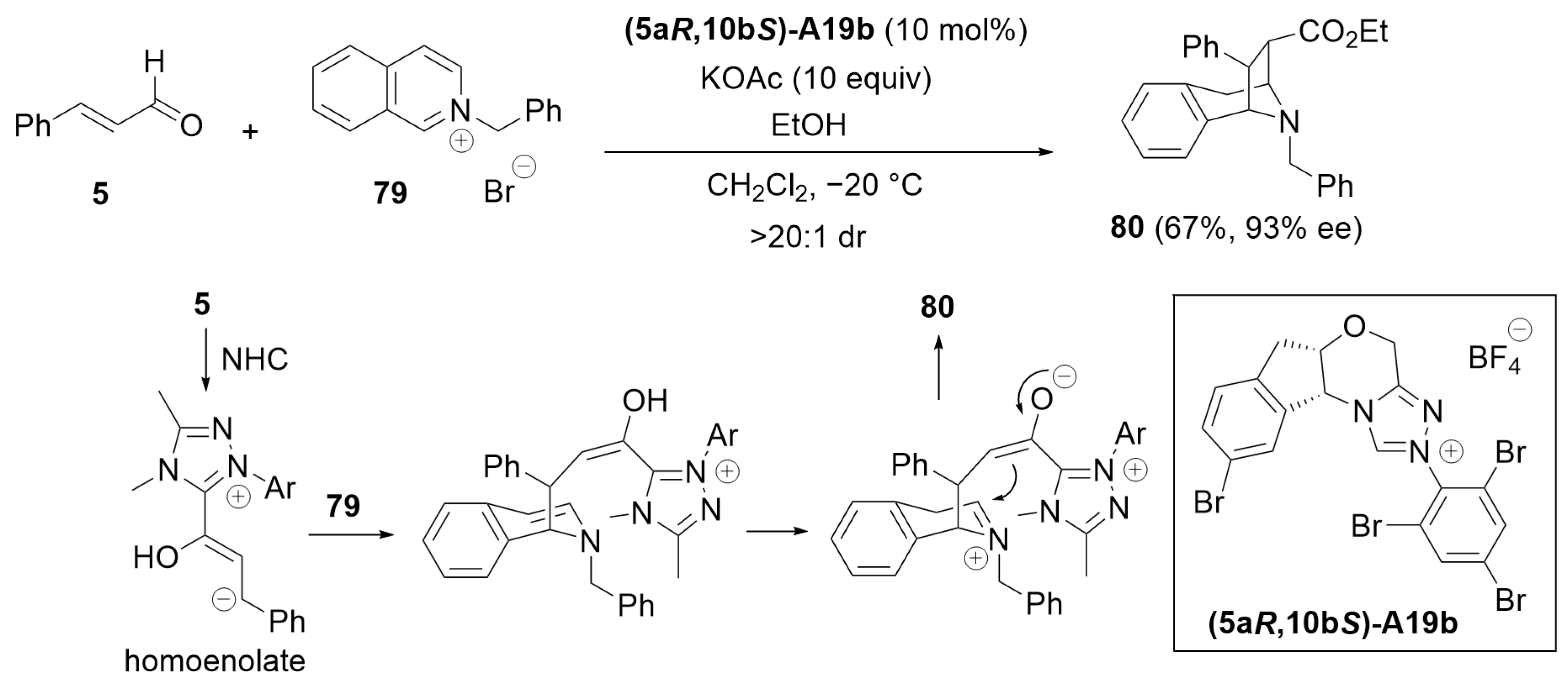
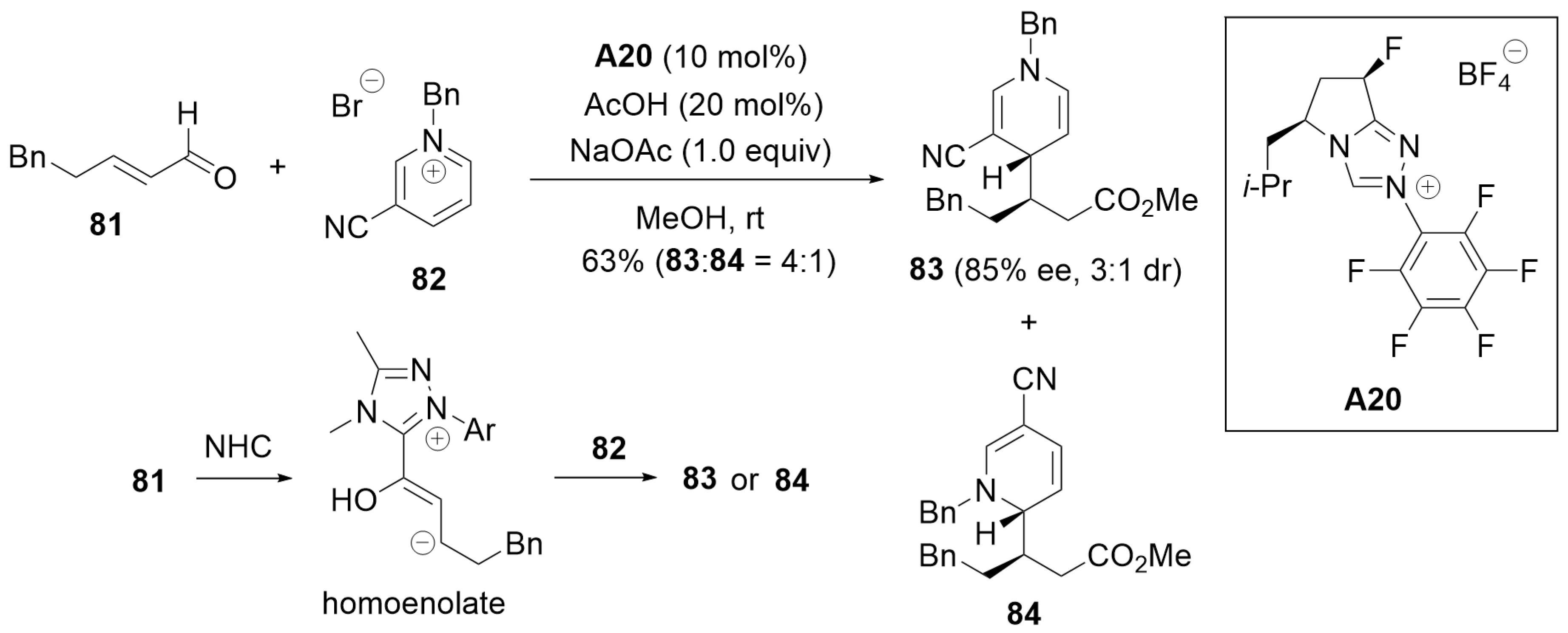
5.7. Cascade Annulation
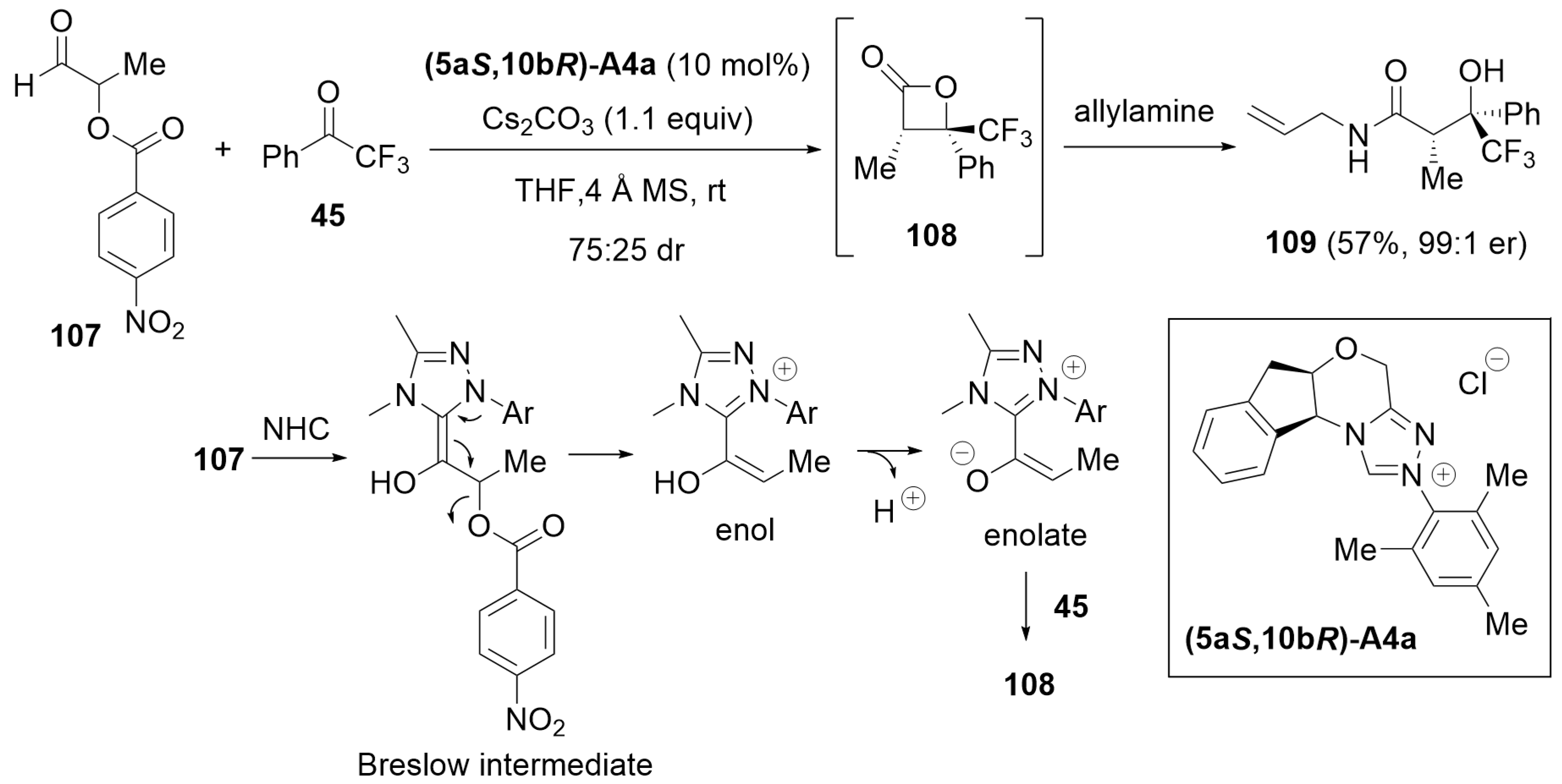
6. Cooperative Catalysis with Brønsted Acid and a Hydrogen-Bonding Catalyst
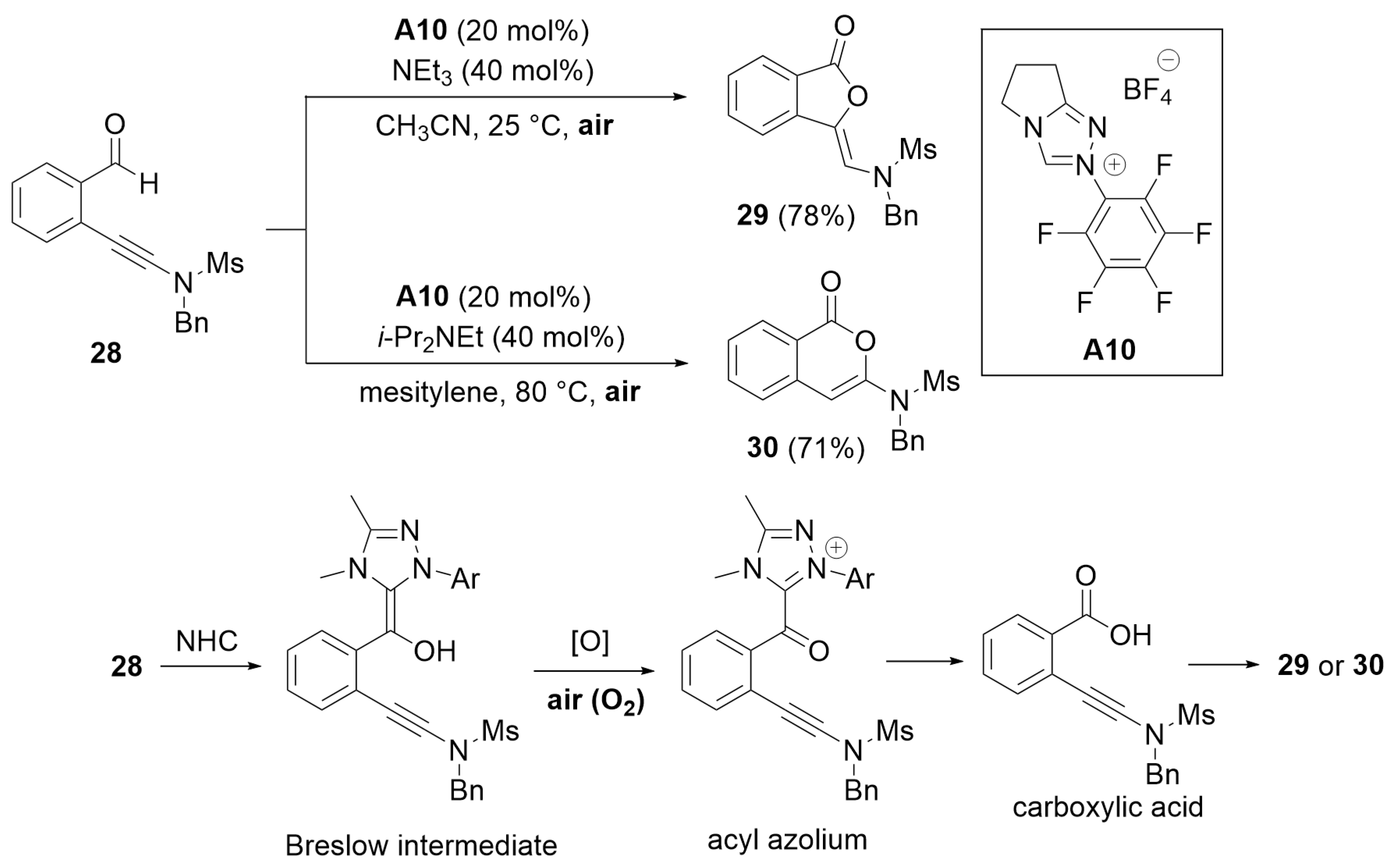
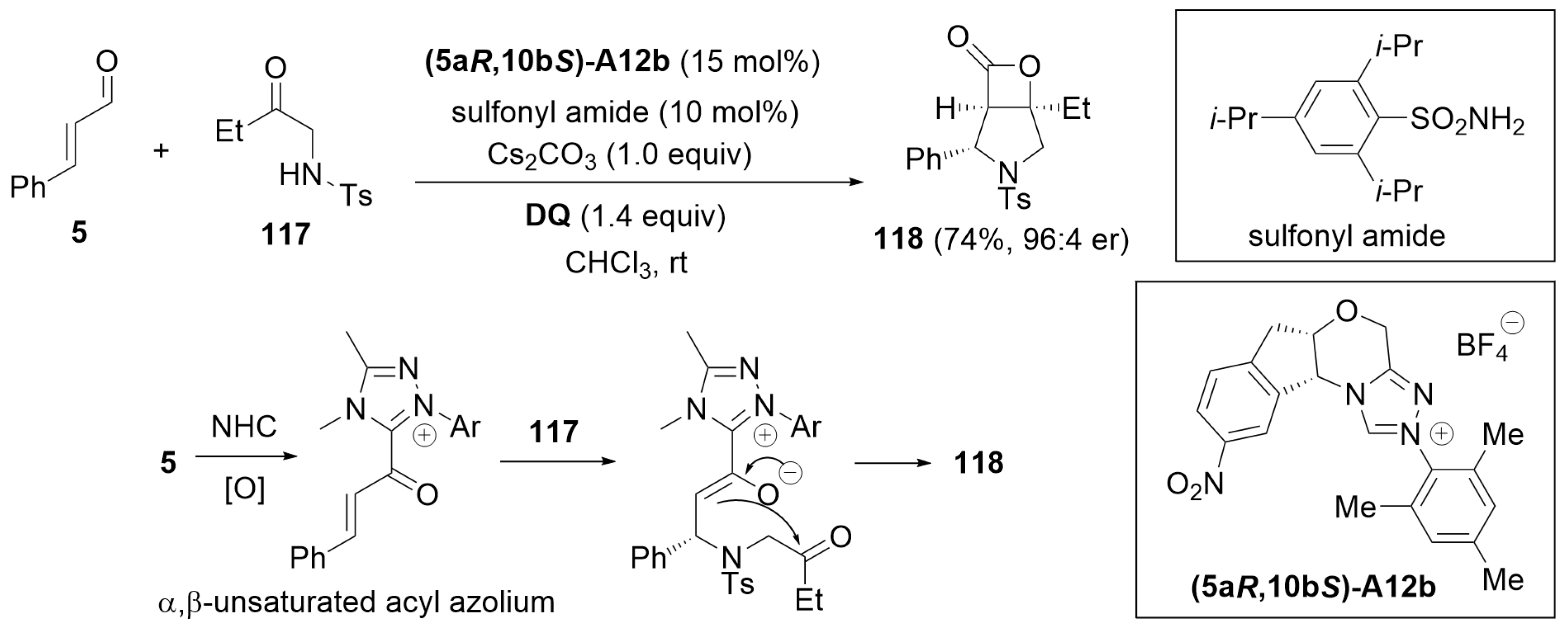
6.1. Cooperative Catalysis Using Brønsted Acid
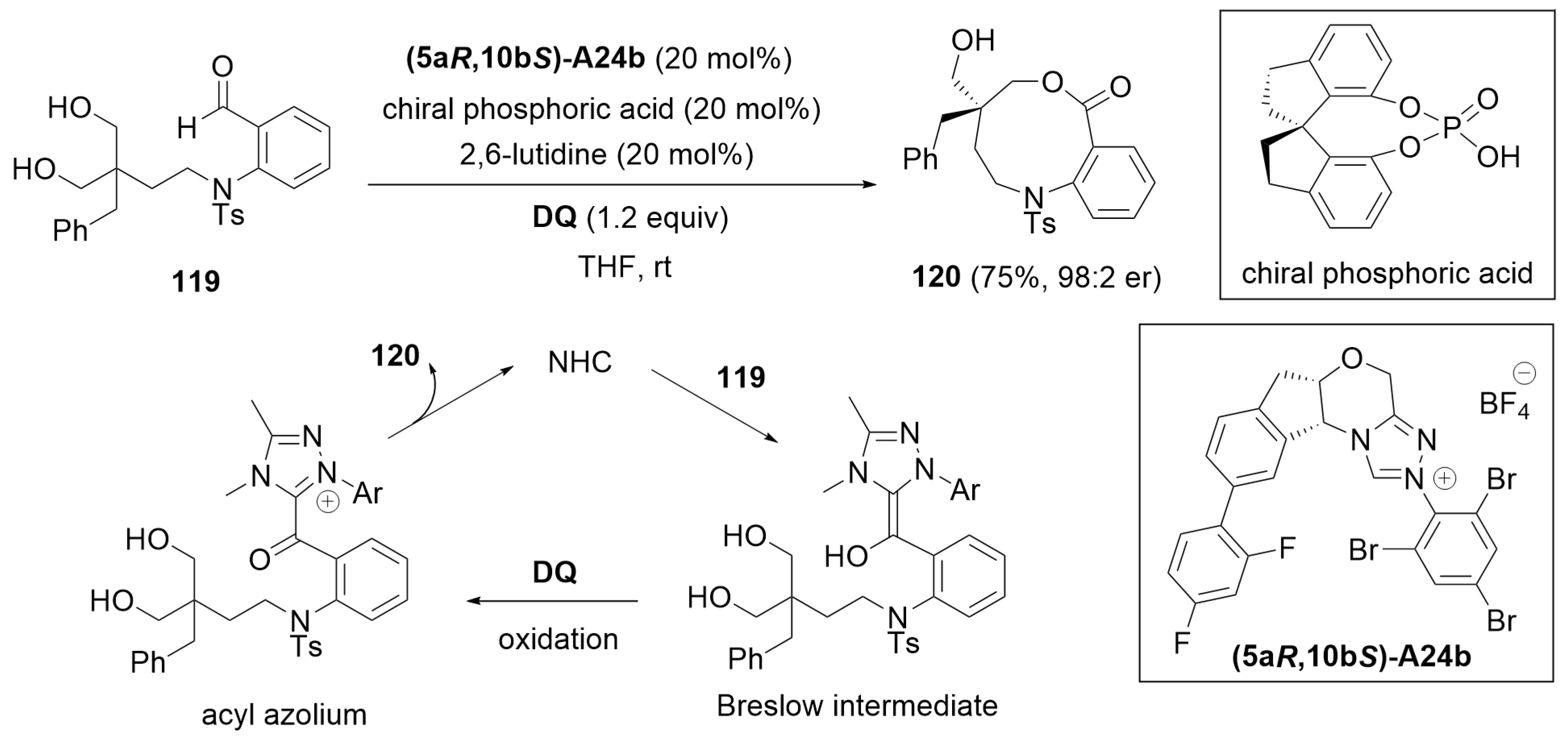
6.2. Cooperative Catalysis Using a Hydrogen-Bonding Catalyst
7. Cooperative Catalysis with Transition-Metal Catalyst
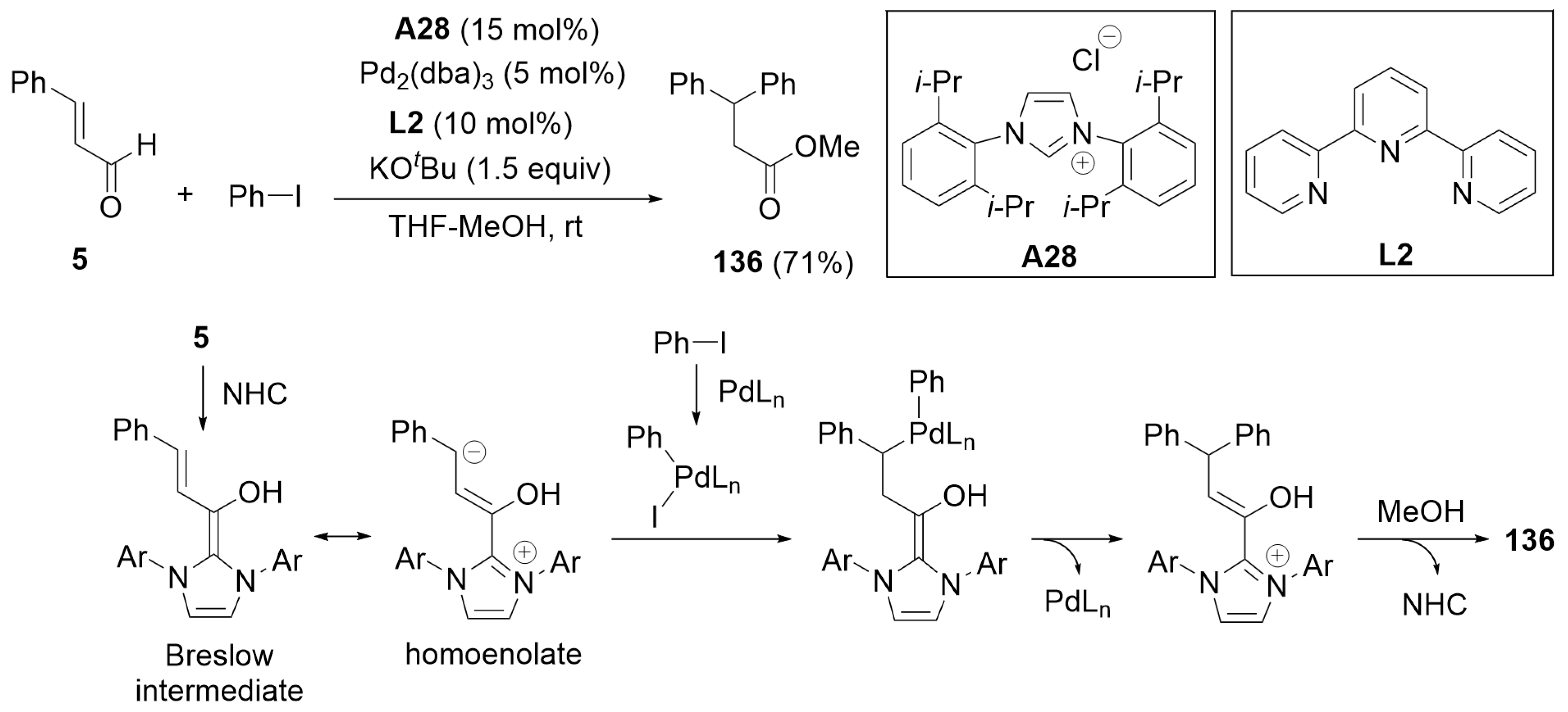
7.1. Cooperative Catalysis Using a Palladium Catalyst
7.2. Cooperative Catalysis Using a Copper Catalyst
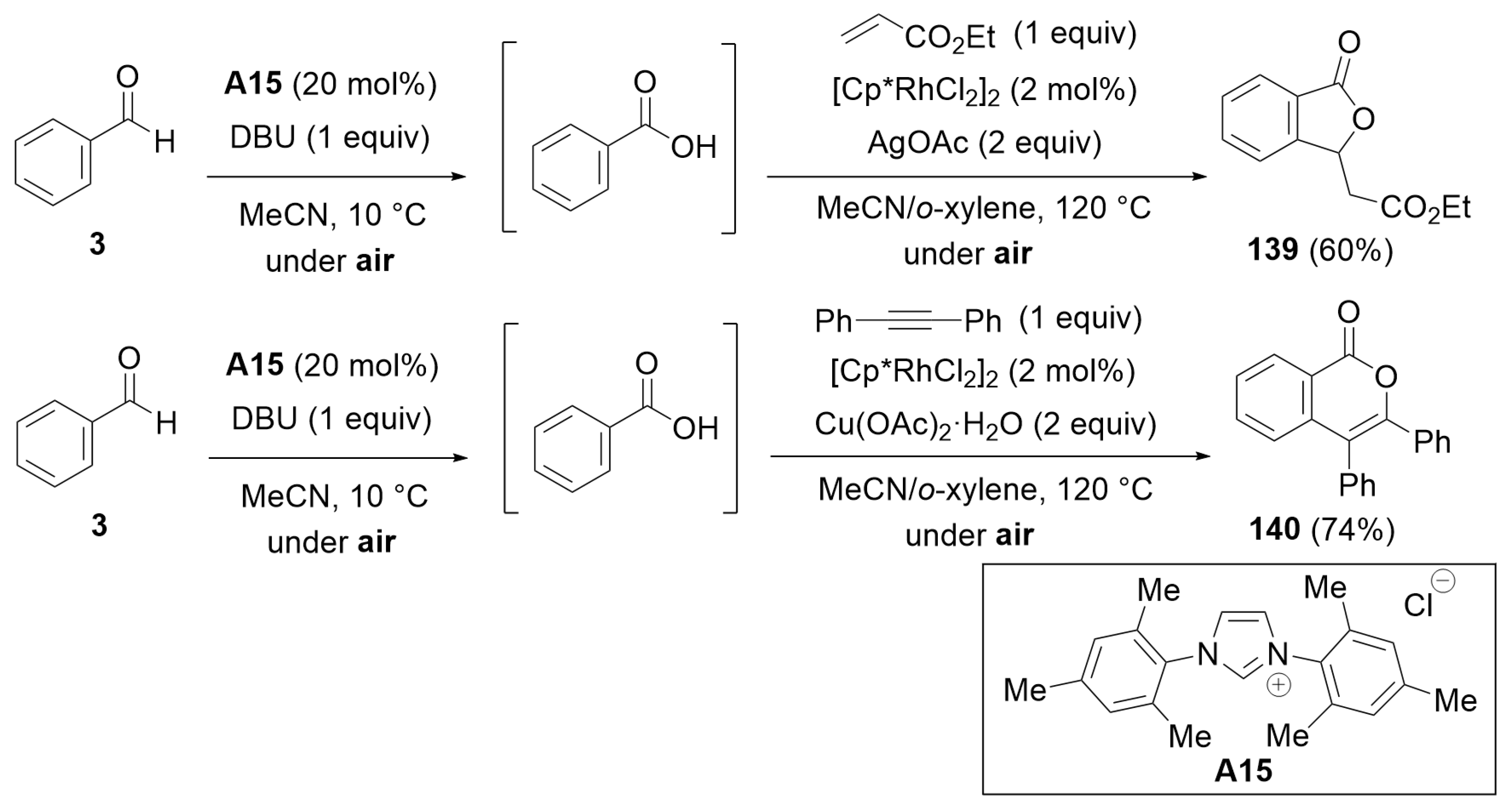
7.3. Cooperative Catalysis Using a Rhodium Catalyst
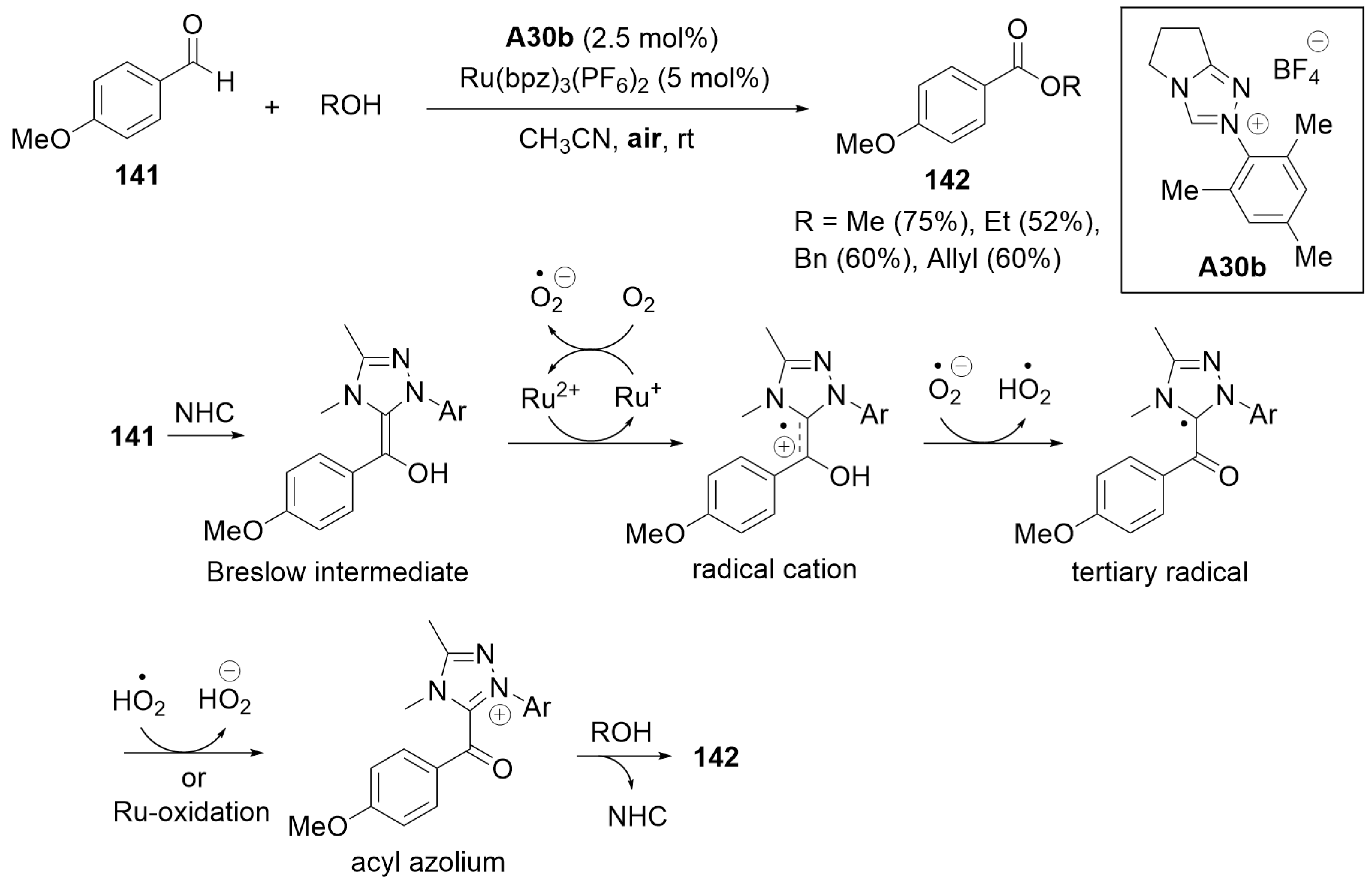
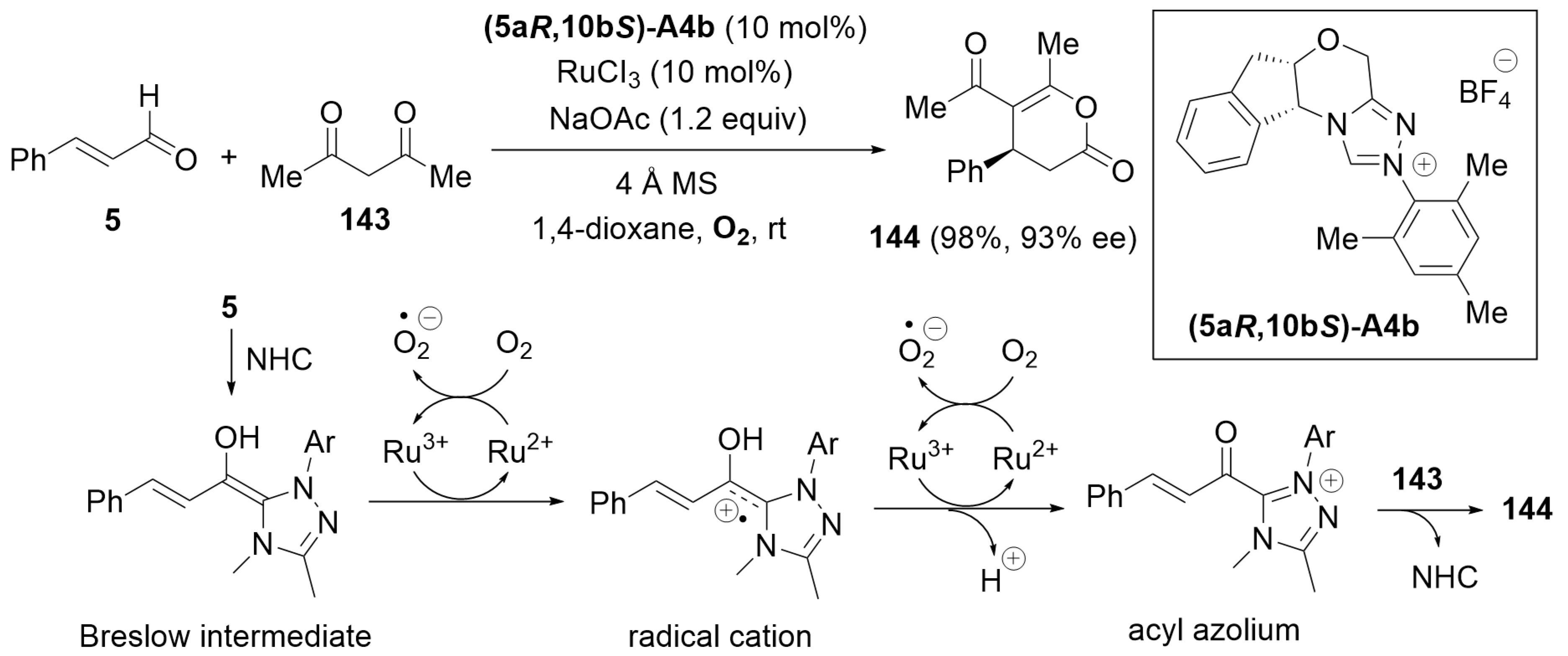
7.4. Cooperative Catalysis Using a Ruthenium Catalyst
7.5. Cooperative Catalysis Using a Gold Catalyst
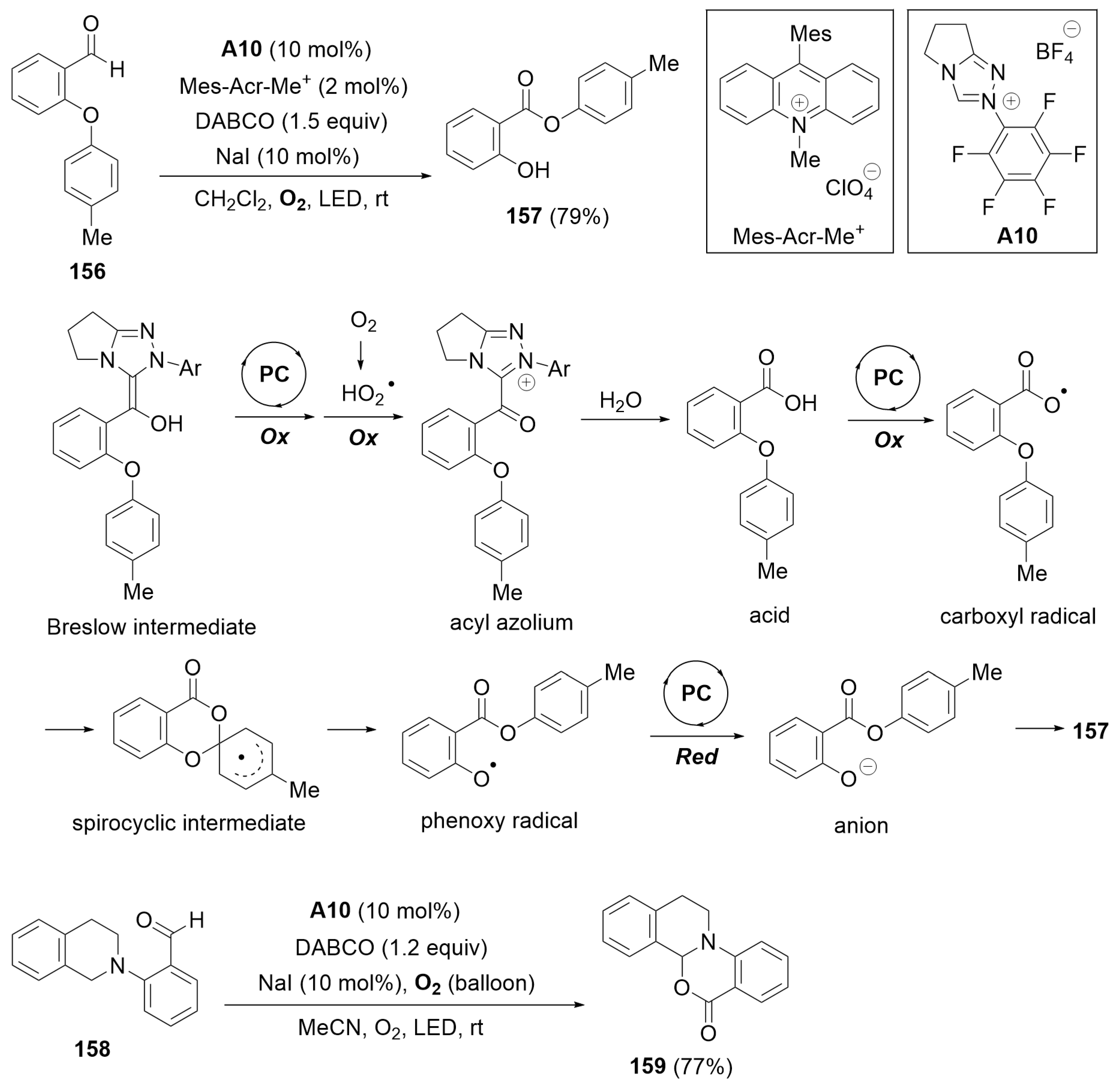
8. Cooperative Catalysis with Photocatalysts
9. Conclusion and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef]
- Biju, A.T.; Kuhl, N.; Glorius, F. Extending NHC-Catalysis: Coupling Aldehydes with Unconventional Reaction Partners. Acc. Chem. Soc. 2011, 44, 1182–1195. [Google Scholar] [CrossRef]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef]
- Vora, H.U.; Wheeler, P.; Rovis, T. Exploiting Acyl and Enol Azolium Intermediates via N-Heterocyclic Carbene-Catalyzed Reactions of α-Reducible Aldehydes. Adv. Synth. Catal. 2012, 354, 1617–1639. [Google Scholar] [CrossRef]
- Knappke, C.E.I.; Imami, A.; Jacobi von Wangelin, A. Oxidative N-Heterocyclic Carbene Catalysis. ChemCatChem 2012, 4, 937–941. [Google Scholar] [CrossRef]
- De Sarkar, D.; Biswas, A.; Samanta, R.C.; Studer, A. Catalysis with N-Heterocyclic Carbenes under Oxidative Conditions. Chem. Eur. J. 2013, 19, 4664–4678. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Kafshdarzadeh, K.; Amiri, Z. Recent Advances in Stetter Reaction and Related Chemistry: An update. Asian J. Org. Chem. 2020, 9, 1999–2034. [Google Scholar] [CrossRef]
- Barik, S.; Biju, A.T. N-Heterocyclic carbene (NHC) organocatalysis using aliphatic aldehydes. Chem. Commun. 2020, 56, 15484–15495. [Google Scholar] [CrossRef]
- Mahatthananchai, J.; Bode, J.W. On the Mechanism of N-Heterocyclic Carbene-Catalyzed Reactions Involving Acyl Azoliums. Acc. Chem. Res. 2014, 47, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Zhang, C.; Hooper, J.F.; Lupton, D.W. N-Heterocyclic Carbene Catalysis via the α,β-Unsaturated Acyl Azolium. ACS Catal. 2017, 7, 2583–2596. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Liu, Q.; Chauhan, P.; Enders, D. N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations. Angew. Chem. Int. Ed. 2018, 57, 3862–3873. [Google Scholar] [CrossRef] [PubMed]
- Dzieszkowski, K.; Rafiński, Z. N-Heterocyclic Carbene Catalysis under Oxidizing Conditions. Catalysts 2018, 8, 549. [Google Scholar] [CrossRef]
- Mondal, S.; Yetra, S.R.; Mukherjee, S.; Biju, A.T. NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles. Acc. Chem. Res. 2019, 52, 425–436. [Google Scholar] [CrossRef]
- Das, T.K.; Biju, A.T. Imines as acceptors and donors in N-heterocyclic carbene (NHC) organocatalysis. Chem. Commun. 2020, 56, 8537–8552. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Jin, Z.; Chi, Y.R. N-Heterocyclic Carbene Organocatalysis: Activation Modes and Typical Reactive Intermediates. Chin. J. Chem. 2020, 38, 1167–1202. [Google Scholar] [CrossRef]
- Ghosh, A.; Biju, A.T. Revealing the Similarities of α,β-Unsaturated Iminiums and Acylazoliums in Organocatalysis. Angew. Chem. Int. Ed. 2021, 60, 13712–13724. [Google Scholar] [CrossRef]
- Gao, J.; Feng, J.; Du, D. Generation of azolium dienolates as versatile nucleophilic synthons via N-heterocyclic carbene catalysis. Org. Chem. Front. 2021, 8, 6138–6166. [Google Scholar] [CrossRef]
- Pavithra, T.; Devi, E.S.; Maheswari, C.U. Recent Advances in N-Heterocyclic Carbene Catalyzed Oxidative Cyclization for the Formation of Heterocycles. Asian J. Org. Chem. 2021, 10, 1861–1883. [Google Scholar] [CrossRef]
- Nie, G.; Li, T. NHC-Catalyzed Cascade Reactions for the Construction of Fused Cycles via LUMO Activation of α,β-Unsaturated Carbonyls. Asian J. Org. Chem. 2023, 12, e202200680. [Google Scholar] [CrossRef]
- De Risi, C.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A.; Bortolini, O. Oxidative N-Heterocyclic Carbene Catalysis. Chem. Eur. J. 2023, 29, e202202467. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.T.; Scheidt, K.A. Cooperative Lewis acid/N-heterocyclic carbene catalysis. Chem. Sci. 2012, 3, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Scheidt, K.A. Cooperative catalysis and activation with N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2016, 55, 14912–14922. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Gao, Z.-H.; Ye, S. Bifunctional N-Heterocyclic Carbenes Derived from L-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis. Acc. Chem. Res. 2020, 53, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, H. N-Heterocyclic Carbene-Based Catalysis Enabling Cross-Coupling Reactions. ACS Catal. 2020, 10, 6862–6869. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, Y.; Wu, L.; Li, E.-Q. Recent advances in annulations enabled by nucleophilic Lewis base/metal dual catalysis. Chin. Chem. Lett. 2023, 34, 108544. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.-Y. Dual N-heterocyclic carbene/photocatalysis: A new strategy for radical processes. Org. Chem. Front. 2020, 7, 2082–2087. [Google Scholar] [CrossRef]
- Liu, J.; Xing, X.-N.; Huang, J.-H.; Lu, L.-Q.; Xiao, W.-J. Light opens a new window for N-heterocyclic carbene catalysis. Chem. Sci. 2020, 11, 10605–10613. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, R.; Song, H.; Liu, Y.; Wang, Q. Recent advances in combining photo- and N-heterocyclic carbene catalysis. Chem. Sci. 2023, 14, 13367–13383. [Google Scholar] [CrossRef]
- Xu, G.-Q.; Wang, W.D.; Xu, P.-F. Photocatalyzed Enantioselective Functionalization of C(sp3)−H Bonds. J. Am. Chem. Soc. 2024, 146, 1209–1223. [Google Scholar] [CrossRef]
- Ishii, T.; Nagao, K.; Ohmiya, H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 2020, 11, 5630–5636. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Zeng, R.; Han, B.; Li, J.-L. Single-Electron Transfer Reactions Enabled by N-Heterocyclic Carbene Organocatalysis. Chem. Eur. J. 2021, 27, 3238–3250. [Google Scholar] [CrossRef]
- Maki, B.E.; Scheidt, K.A. N-Heterocyclic Carbene-Catalyzed Oxidation of Unactivated Aldehydes to Esters. Org. Lett. 2008, 10, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.; Baragwanath, L.; Connon, S.J. Nucleophilic carbene-catalysed oxidative esterification reactions. Tetrahedron Lett. 2008, 49, 4003–4006. [Google Scholar] [CrossRef]
- De Sarkar, S.; Grimme, S.; Studer, A. NHC Catalyzed Oxidations of Aldehydes to Esters: Chemoselective Acylation of Alcohols in Presence of Amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef]
- Samanta, R.C.; Studer, A. N-heterocyclic carbene catalysed oxidative esterification of aliphatic aldehydes. Org. Chem. Front. 2014, 1, 936–939. [Google Scholar] [CrossRef]
- Berry, M.T.; Castrejon, D.; Hein, J.E. Oxidative Esterification of Aldehydes Using Mesoionic 1,2,3-Triazolyl Carbene Organocatalysts. Org. Lett. 2014, 16, 3676–3679. [Google Scholar] [CrossRef]
- Li, W.; Ajitha, M.J.; Lang, M.; Huang, K.-W.; Wang, J. Catalytic Intermolecular Cross-Couplings of Azides and LUMO Activated Unsaturated Acyl Azoliums. ACS Catal. 2017, 7, 2139–2144. [Google Scholar] [CrossRef]
- Chun, S.; Chung, Y.K. Transition-Metal-Free Poly(thiazolium) Iodide/1,8-Diazabicyclo [5.4.0]undec-7-ene/Phenazine-Catalyzed Esterification of Aldehydes with Alcohols. Org. Lett. 2017, 19, 3787–3790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, D.; Wang, J. Carbene-catalyzed oxidative acylation promoted by an unprecedented oxidant CCl3CN. Org. Chem. Front. 2019, 6, 688–693. [Google Scholar] [CrossRef]
- Di Carmine, G.; Ragno, D.; Massi, A.; D’Agostino, C. Oxidative Coupling of Aldehydes with Alcohol for the Synthesis of Esters Promoted by Polystyrene-Supported N-Heterocyclic Carbene: Unraveling the Solvent Effect on the Catalyst Behavior Using NMR Relaxation. Org. Lett. 2020, 22, 4927–4931. [Google Scholar] [CrossRef]
- Harnying, W.; Sudkaow, P.; Biswas, A.; Berkessel, A. N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading. Angew. Chem. Int. Ed. 2021, 60, 19631–19636. [Google Scholar] [CrossRef]
- Sun, C.; Nong, Y.; Pang, C.; Zhang, S.; Li, T. Carbene-Catalyzed Regioselective Addition of Oxindoles to Ynals for Quick Access to Allenes. Synlett 2023, 34, 1997–2000. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Song, R.; Xu, J.; Tian, W.; Li, S.; Jin, Z.; Chi, Y.R. Carbene-Catalyzed α,γ-Deuteration of Enals under Oxidative Conditions. ACS Catal. 2020, 10, 5475–5482. [Google Scholar] [CrossRef]
- Singh, A.; Narula, A.K. N-Heterocyclic carbene (NHC) catalyzed amidation of aldehydes with amines via the tandem N-hydroxysuccinimide ester formation. New J. Chem. 2021, 45, 7486–7490. [Google Scholar] [CrossRef]
- Reddy, R.S.; Rosa, J.N.; Veiros, L.F.; Caddick, S.; Gois, P.M.P. NHC/Iron cooperative catalysis: Aerobic oxidative esterification of aldehydes with phenols. Org. Biomol. Chem. 2011, 9, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Delany, E.G.; Fagan, C.-L.; Gundala, S.; Mari, A.; Broja, T.; Zeitler, K.; Connon, S.J. NHC-catalysed aerobic aldehyde-esterifications with alcohols: No additives or cocatalysts required. Chem. Commun. 2013, 49, 6510–6512. [Google Scholar] [CrossRef] [PubMed]
- Delany, E.G.; Fagan, C.-L.; Gundala, S.; Zeitler, K.; Connon, S.J. Aerobic oxidation of NHC-catalysed aldehyde esterifications with alcohols: Benzoin, not the Breslow intermediate, undergoes oxidation. Chem. Commun. 2013, 49, 6513–6515. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.; Axelsson, A.; Sundén, H. Attractive aerobic access to the α,β-unsaturated acyl azolium intermediate: Oxidative NHC catalysis via multistep electron transfer. Green Chem. 2016, 18, 686–690. [Google Scholar] [CrossRef]
- Luo, X.-L.; Ge, D.; Yu, Z.-L.; Chu, X.-Q.; Xu, P. Vitamin B1-catalyzed aerobic oxidative esterification of aromatic aldehydes with alcohols. RSC Adv. 2021, 11, 30937–30942. [Google Scholar] [CrossRef]
- Finney, E.E.; Ogawa, K.A.; Boydston, A.J. Organocatalyzed Anodic Oxidation of Aldehydes. J. Am. Chem. Soc. 2012, 134, 12374–12377. [Google Scholar] [CrossRef]
- Arde, P.; Ramanjaneyulu, B.T.; Reddy, V.; Saxena, A.; Anand, R.V. N-Heterocyclic carbene catalysed aerobic oxidation of aromatic aldehydes to aryl esters using boronic acids. Org. Biomol. Chem. 2012, 10, 848–851. [Google Scholar] [CrossRef]
- Meng, J.-J.; Gao, M.; Wei, Y.-P.; Zhang, W.-Q. N-Heterocyclic Carbene-Catalyzed Aerobic Oxidative Direct Esterification of Aldehydes with Organoboronic Acids. Chem. Asian J. 2012, 7, 872–875. [Google Scholar] [CrossRef]
- Guin, J.; De Sarkar, S.; Grimme, S.; Studer, A. Biomimetic Carbene-Catalyzed Oxidations of Aldehydes Using TEMPO. Angew. Chem. Int. Ed. 2008, 47, 8727–8730. [Google Scholar] [CrossRef]
- Xin, Y.-C.; Shi, S.-H.; Xie, D.-D.; Hui, X.-P.; Xu, P.-F. N-Heterocyclic Carbene-Catalyzed Oxidative Esterification Reaction of Aldehydes with Alkyl Halides under Aerobic Conditions. Eur. J. Org. Chem. 2011, 2011, 6527–6531. [Google Scholar] [CrossRef]
- White, N.A.; Rovis, T. Enantioselective N-Heterocyclic Carbene-Catalyzed β-Hydroxylation of Enals Using Nitroarenes: An Atom Transfer Reaction That Proceeds via Single Electron Transfer. J. Am. Chem. Soc. 2014, 136, 14674–14677. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Y.; Huang, Z.; Xu, J.; Wu, X.; Wang, Y.; Wang, M.; Yang, S.; Webster, R.D.; Chi, Y.R. N-Heterocyclic Carbene-Catalyzed Radical Reactions for Highly Enantioselective β-Hydroxylation of Enals. J. Am. Chem. Soc. 2015, 137, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, W.; Hu, W.; Sun, J. N-Heterocyclic Carbene Catalyzed Enantioselective α-Fluorination of Aliphatic Aldehydes and α-Chloro Aldehydes: Synthesis of α-Fluoro Esters, Amides, and Thioesters. Angew. Chem. Int. Ed. 2015, 54, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-L.; Maiti, R.; Ren, S.-C.; Tian, W.; Li, T.; Xu, J.; Mondal, B.; Jin, Z.; Chi, Y.R. Carbene-catalyzed atroposelective synthesis of axially chiral styrenes. Nat. Commun. 2022, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Y.; Gao, Z.-H.; Li, C.-B.; Ye, S. Enantioselective N-Heterocyclic Carbene Catalyzed α-Oxidative Coupling of Enals with Carboxylic Acids Using an Iodine(III) Reagent. Angew. Chem. Int. Ed. 2023, 62, e202218362. [Google Scholar] [CrossRef]
- Cramer, D.L.; Bera, S.; Studer, A. Exploring Cooperative Effects in Oxidative NHC Catalysis: Regioselective Acylation of Carbohydrates. Chem. Eur. J. 2016, 22, 7403–7407. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zou, J.; Nong, Y.; Song, J.; Shen, T.; Cai, H.; Mou, C.; Lyu, W.; Jin, Z.; Chi, Y.R. Catalytic Regioselective Acylation of Unprotected Nucleosides for Quick Access to COVID and Other Nucleoside Prodrugs. ACS Catal. 2023, 13, 9567–9576. [Google Scholar] [CrossRef]
- Lu, S.; Poh, S.B.; Siau, W.-Y.; Zhao, Y. Kinetic Resolution of Tertiary Alcohols: Highly Enantioselective Access to 3-Hydroxy-3-Substituted Oxindoles. Angew. Chem. Int. Ed. 2013, 52, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, X.; Li, B.; Mou, C.; Yang, S.; Song, B.A.; Chi, Y.R. Access to P-Stereogenic Phosphinates via N-Heterocyclic Carbene-Catalyzed Desymmetrization of Bisphenols. J. Am. Chem. Soc. 2016, 138, 7524–7527. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, J.; Huang, R.; Wang, W.; Jin, Z.; Zanoni, G.; Zheng, P.; Yang, S.; Chi, Y.R. Kinetic Resolution of 1,2-Diols via NHC-Catalyzed Site-Selective Esterification. Org. Lett. 2018, 20, 3447–3450. [Google Scholar] [CrossRef]
- Liu, Y.; Majhi, P.K.; Song, R.; Mou, C.; Hao, L.; Chai, H.; Jin, Z.; Chi, Y.R. Carbene-Catalyzed Dynamic Kinetic Resolution and Asymmetric Acylation of Hydroxyphthalides and Related Natural Products. Angew. Chem. Int. Ed. 2020, 59, 3859–3863. [Google Scholar] [CrossRef]
- Porey, A.; Mondal, B.D.; Guin, J. Hydrogen-Bonding Assisted Catalytic Kinetic Resolution of Acyclic β-Hydroxy Amides. Angew. Chem. Int. Ed. 2021, 60, 8786–8791. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Zhang, C.-L.; Dai, L.; Han, Y.-F.; Ye, S. Dynamic Kinetic Resolution of α-Trifluoromethyl Hemiaminals without α-Hydrogen via NHC-Catalyzed O-Acylation. Org. Lett. 2021, 23, 1361–1366. [Google Scholar] [CrossRef]
- Guo, D.; Peng, Q.; Zhang, B.; Wang, J. Atroposelective Dynamic Kinetic Resolution via In Situ Hemiaminals Catalyzed by N-Heterocyclic Carbene. Org. Lett. 2021, 23, 7765–7770. [Google Scholar] [CrossRef]
- Mondal, B.; Chen, H.; Maiti, R.; Wang, H.; Cai, H.; Mou, C.; Hao, L.; Chai, H.; Chi, Y.R. Carbene-Catalyzed Direct O-Functionalization of Ketone: Atroposelective Access to Non-C2-Symmetric Binaphthyls. Org. Lett. 2023, 25, 8252–8257. [Google Scholar] [CrossRef]
- Hu, D.; Poh, S.B.; Liu, F.; Tu, Z.; Wang, X.; Lu, S.; Zhao, Y. Anion effect on enantioselective oxidative NHC catalysis: Highly efficient kinetic resolution of tertiary alcohols and beyond. Org. Chem. Front. 2023, 10, 416–421. [Google Scholar] [CrossRef]
- Li, B.-S.; Wang, Y.; Proctor, R.S.J.; Jin, Z.; Chi, Y.R. Carbene-catalyzed desymmetrization of 1,3-diols: Access to optically enriched tertiary alkyl chlorides. Chem. Commun. 2016, 52, 8313–8316. [Google Scholar] [CrossRef]
- Li, S.; Liu, B.; Chen, L.; Li, X.; Cheng, J.-P. N-Heterocyclic carbene promoted enantioselective desymmetrization reaction of diarylalkane-bisphenols. Org. Chem. Front. 2018, 5, 1101–1107. [Google Scholar] [CrossRef]
- Dutta, S.; Porey, A.; Guin, J. N-Heterocyclic carbene catalyzed desymmetrization of diols: Access to enantioenriched oxindoles having a C3-quaternary stereocenter. Chem. Commun. 2023, 59, 5771–5774. [Google Scholar] [CrossRef] [PubMed]
- Di Carmine, G.; Ragno, D.; Brandolese, A.; Bortolini, O.; Pecorari, D.; Sabuzi, F.; Mazzanti, A.; Massi, A. Enantioselective Desymmetrization of 1,4-Dihydropyridines by Oxidative NHC Catalysis. Chem. Eur. J. 2019, 25, 7469–7474. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, M.; Sun, J.; Zheng, G.; Zhang, Q. Synthesis of Axially Chiral Aldehydes by N-Heterocyclic-Carbene-Catalyzed Desymmetrization Followed by Kinetic Resolution. Angew. Chem. Int. Ed. 2022, 61, e202117340. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Chen, X.; Xu, J. N-Heterocyclic Carbene-Catalyzed Desymmetrization of Siladials to Access Silicon-Stereogenic Organosilanes. J. Org. Chem. 2022, 87, 16127–16137. [Google Scholar] [CrossRef]
- Zhou, B.-A.; Li, X.-N.; Zhang, C.-L.; Wang, Z.-X.; Ye, S. Enantioselective Synthesis of Axially Chiral Diaryl Ethers via NHC Catalyzed Desymmetrization and Following Resolution. Angew. Chem. Int. Ed. 2023, 62, e202314228. [Google Scholar] [CrossRef]
- Shee, S.; Ranganathappa, S.S.; Gadhave, M.S.; Gogoi, R.; Biju, A.T. Enantioselective Synthesis of C−O Axially Chiral Diaryl Ethers by NHC-Catalyzed Atroposelective Desymmetrization. Angew. Chem. Int. Ed. 2023, 62, e202311709. [Google Scholar] [CrossRef]
- Rose, C.A.; Zeitler, K. Efficient Catalytic, Oxidative Lactonization for the Synthesis of Benzodioxepinones Using Thiazolium-Derived Carbene Catalysts. Org. Lett. 2010, 12, 4552–4555. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Barik, S.; Barik, S.; Shee, S.; Biju, A.T. Oxidative N-heterocyclic carbene (NHC) catalysis for the rapid access to functionalized pyrrolo-oxazinones. Tetrahedron 2021, 94, 132330. [Google Scholar] [CrossRef]
- Deng, Q.; Mu, F.; Qiao, Y.; Wei, D. N-Heterocyclic Carbene-Catalyzed Asymmetric C−O Bond Construction between Benzoic Acid and o-Phthalaldehyde: Mechanism and Origin of Stereoselectivity. Chem Asian J. 2021, 16, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-S.; Kim, P.-S.; Ha, W.; Kim, Y.H.; Yoo, H.J.; Lee, J.; Youn, S.W. Harnessing NHC/Base-Catalyzed Regiodivergent Oxidative Cyclization for Versatile Aminolactone Synthesis. ACS Catal. 2023, 13, 15939–15947. [Google Scholar] [CrossRef]
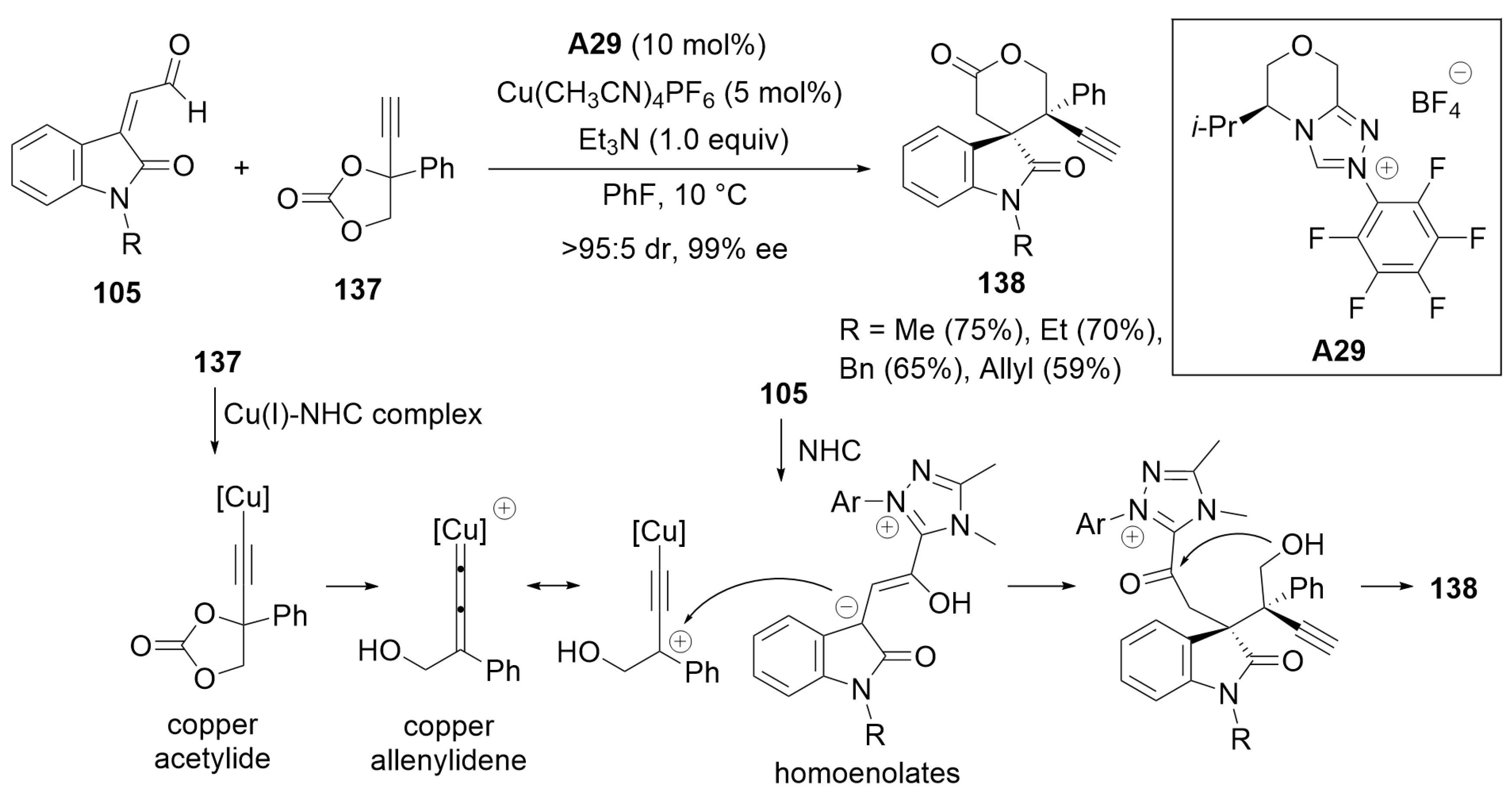
- Yang, G.; He, Y.; Wang, T.; Li, Z.; Wang, J. Atroposelective Synthesis of Planar-Chiral Indoles via Carbene Catalyzed Macrocyclization. Angew. Chem. Int. Ed. 2024, 63, e202316739. [Google Scholar] [CrossRef]
- De Sarkar, S.; Studer, A. NHC-Catalyzed Michael Addition to α,β-Unsaturated Aldehydes by Redox Activation. Angew. Chem. Int. Ed. 2010, 49, 9266–9269. [Google Scholar] [CrossRef]
- Rong, Z.-Q.; Jia, M.-Q.; You, S.-L. Enantioselective N-Heterocyclic Carbene-Catalyzed Michael Addition to α,β-Unsaturated Aldehydes by Redox Oxidation. Org. Lett. 2011, 13, 4080–4083. [Google Scholar] [CrossRef]
- Mo, J.; Shen, L.; Chi, Y.R. Direct β-Activation of Saturated Aldehydes to Formal Michael Acceptors through Oxidative NHC Catalysis. Angew. Chem. Int. Ed. 2013, 52, 8588–8591. [Google Scholar] [CrossRef]
- Axelsson, A.; Hammarvid, E.; Ta, L.; Sundeń, H. Asymmetric aerobic oxidative NHC-catalysed synthesis of dihydropyranones utilising a system of electron transfer mediators. Chem. Commun. 2016, 52, 11571–11574. [Google Scholar] [CrossRef]
- Wu, Q.; Li, C.; Wang, W.; Wang, H.; Pan, D.; Zheng, P. NHC-catalyzed enantioselective synthesis of dihydropyran-4-carbonitriles bearing all-carbon quaternary centers. Org. Chem. Front. 2017, 4, 2323–2326. [Google Scholar] [CrossRef]
- Zheng, P.; Li, C.; Mou, C.; Pan, D.; Wu, S.; Xue, W.; Jin, Z.; Chi, Y.R. Efficient Access to 2-Pyrones via Carbene-Catalyzed Oxidative [3 + 3] Reactions between Enals and Nitrogen Ylides. Asian J. Org. Chem. 2019, 8, 1067–1070. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Zhang, R.; Duan, X.-Y.; Yu, H.-F.; Sun, B.-Y.; Qi, J. Access to dihydropyrano[3,2-b]pyrrol-5-ones skeletons by N-heterocyclic carbene-catalyzed [3+3] annulations. Chem. Commun. 2020, 56, 9854–9857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Yang, T.; Wang, K.-K.; Chen, R.; Liu, M.; Liu, H. Oxidative N-heterocyclic carbene-catalyzed [3 + 3] annulation reaction of enals with benzofuran-3-ones: Efficient access to benzofuran-fused δ-lactones. Org. Chem. Front. 2020, 7, 1011–1015. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Liu, Q.; Wang, K.-K.; Liu, M.; Han, Y.; Sun, A.; Ma, X. NHC-Catalyzed Oxidative Annulation of α,β-unsaturated Aldehydes with Benzyl Ketones: Direct Access to 4,5,6-Trisubstituted Dihydropyranones. Asian J. Org. Chem. 2021, 10, 766–770. [Google Scholar] [CrossRef]
- Axelsson, A.; Westerlund, M.; Zacharias, S.C.; Runemark, A.; Haukka, M.; Sundén, H. Asymmetric Synthesis of Dihydropyranones with Three Contiguous Stereocenters by an NHC-Catalyzed Kinetic Resolution. Eur. J. Org. Chem. 2021, 25, 3657–3661. [Google Scholar] [CrossRef]
- Jiang, C.; Dong, Z.; Wang, J.; Zhao, C. N-Heterocyclic Carbene-Catalyzed [3 + 3] Annulation of Alkynyl Acyl Azolium with β-Keto Ester for the Synthesis of Tri-Substituted α-Pyranones. Asian J. Org. Chem. 2023, 12, e202300371. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Q.; Ren, M.; Zhang, H.; Zhang, X.; Liu, J.; Fu, Z. N-Heterocyclic Carbene-Catalyzed Atroposelective Synthesis of 5-Indo-1-yl Pyran-2-ones with an N−C axis from Enals. Adv. Synth. Catal. 2023, 365, 3467–3472. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Liu, S.; Wang, X.; Wang, S.-J.; Yang, H.; Li, L.; Yang, B.; Wong, M.W.; Zhao, Y.; Lu, S. Enantioselective Access to Triaryl-2-pyrones with Monoaxial or Contiguous C−C Diaxes via Oxidative NHC Catalysis. ACS Catal. 2023, 13, 2565–2575. [Google Scholar] [CrossRef]
- Li, G.-T.; Gu, Q.; You, S.-L. Enantioselective annulation of enals with 2-naphthols by triazolium salts derived from L-phenylalanine. Chem. Sci. 2015, 6, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Joseph, S.; Bhunia, A.; Gonnade, R.G.; Yetra, S.R.; Biju, A.T. Enantioselective synthesis of spiro γ-butyrolactones by N-heterocyclic carbene (NHC)-catalyzed formal [3 + 2] annulation of enals with 3-hydroxy oxindoles. Org. Biomol. Chem. 2017, 15, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Chen, K.-Q.; Sun, D.-Q.; Ye, S. N-Heterocyclic carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: Cross coupling of homoenolate and enolate. Chem. Sci. 2017, 8, 1936–1941. [Google Scholar] [CrossRef]
- Song, Z.-Y.; Chen, K.-Q.; Chen, X.-Y.; Ye, S. Diastereo- and Enantioselective Synthesis of Spirooxindoles with Contiguous Tetrasubstituted Stereocenters via Catalytic Coupling of Two Tertiary Radicals. J. Org. Chem. 2018, 83, 2966–2970. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Yang, X.; Jiang, S.; Xue, W.; Chi, Y.R.; Jin, Z. Carbene-Catalyzed Enantioselective Aromatic N-Nucleophilic Addition of Heteroarenes to Ketones. Angew. Chem. Int. Ed. 2020, 59, 442–448. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhang, J.; Hui, X.-P. Asymmetric N-alkylation of indoles with isatins catalyzed by N-heterocyclic carbene: Efficient synthesis of functionalized cyclic N,O-aminal indole derivatives. Org. Chem. Front. 2020, 7, 1647–1652. [Google Scholar] [CrossRef]
- Balanna, K.; Madica, K.; Mukherjee, S.; Ghosh, A.; Poisson, T.; Besset, T.; Jindal, G.; Biju, A.T. N-Heterocyclic Carbene-Catalyzed Formal [6 + 2] Annulation Reaction via Cross-Conjugated Aza-Trienolate Intermediates. Chem. Eur. J. 2020, 26, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Chen, X.; Chi, Y.R. Oxidative γ-Addition of Enals to Trifluoromethyl Ketones: Enantioselectivity Control via Lewis Acid/N-Heterocyclic Carbene Cooperative Catalysis. J. Am. Chem. Soc. 2012, 134, 8810–8813. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, F.; Wang, J. Intermolecular Dynamic Kinetic Resolution Cooperatively Catalyzed by an N-Heterocyclic Carbene and a Lewis Acid. Angew. Chem. Int. Ed. 2015, 54, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, C.; Xiao, Z.; Li, T.; Wang, X.; Xie, Y.; Yao, C. NHC-catalyzed oxidative γ-addition of α,β-unsaturated aldehydes to isatins: A high-efficiency synthesis of spirocyclic oxindole-dihydropyranones. Org. Biomol. Chem. 2014, 12, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, W.; Lan, J.; Zhu, T. Electroredox carbene organocatalysis with iodide as promoter. Nat. Commun. 2022, 13, 3827. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Barik, S.; Shee, S.; Gonnade, R.G.; Biju, A.T. NHC-Catalyzed Enantioselective Synthesis of Tetracyclic δ-Lactones by (4 + 2) Annulation of ortho-Quinodimethanes with Activated Ketones. Org. Lett. 2023, 25, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Doitomi, K.; Ooi, C.Y.; Zheng, P.; Liu, W.; Guo, H.; Yang, S.; Song, B.-A.; Hirao, H.; et al. A reaction mode of carbene-catalysed aryl aldehyde activation and induced phenol OH functionalization. Nat. Commun. 2017, 8, 15598. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, G.; Zhou, L.; Liu, B.; Zhang, X.; Gao, H.; Jin, Z.; Chi, Y.R. Enantioselective Indole N−H Functionalization Enabled by Addition of Carbene Catalyst to Indole Aldehyde at Remote Site. ACS Catal. 2019, 9, 10971–10976. [Google Scholar] [CrossRef]
- Singh, A.; Narula, A.K. Substituted, Bicyclic 3-Benzoyl Flavanones Synthesis by Highly Efficient N-Heterocyclic Carbene (NHC) Catalysis. ChemistrySelect 2021, 6, 7794–7798. [Google Scholar] [CrossRef]
- Ji, H.; Zou, J.; Mou, C.; Liu, Y.; Ren, S.-C.; Chi, Y.R. NHC-catalyzed [12 + 2] reaction of polycyclic arylaldehydes for access to indole derivatives. Chem. Commun. 2023, 59, 6351–6354. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Yang, R.; Chen, X.; Tiwari, B.; Chi, Y.R. Direct α-Functionalization of Simple Aldehydes via Oxidative N-Heterocyclic Carbene Catalysis. Org. Lett. 2013, 15, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Teng, K. Facile Approach for the Oxidative Enolate Activation of Aliphatic Aldehydes. J. Org. Chem. 2023, 88, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Balanna, K.; Barik, S.; Shee, S.; Gonnade, R.G.; Biju, A.T. Dynamic kinetic resolution of γ,γ-disubstituted indole 2-carboxaldehydes via NHC-Lewis acid cooperative catalysis for the synthesis of tetracyclic ɛ-lactones. Chem. Sci. 2022, 13, 11513–11518. [Google Scholar] [CrossRef]
- Bera, S.; Samanta, R.C.; Daniliuc, C.G.; Studer, A. Asymmetric Synthesis of Highly Substituted β-Lactones through Oxidative Carbene Catalysis with LiCl as Cooperative Lewis Acid. Angew. Chem. Int. Ed. 2014, 53, 9622–9626. [Google Scholar] [CrossRef]
- Bera, S.; Daniliuc, C.G.; Studer, A. Enantioselective Synthesis of Substituted δ-Lactones by Cooperative Oxidative N-Heterocyclic Carbene and Lewis Acid Catalysis. Org. Lett. 2015, 17, 4940–4943. [Google Scholar] [CrossRef]
- Liang, Z.-Q.; Wang, D.-L.; Zhang, H.-M.; Ye, S. Enantioselective Synthesis of Bicyclic δ-Lactones via N-Heterocyclic Carbene-Catalyzed Cascade Reaction. Org. Lett. 2015, 17, 5140–5143. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.-Y.; Puttreddy, R.; Rissanen, K.; Enders, D. N-Heterocyclic Carbene Catalyzed Quadruple Domino Reactions: Asymmetric Synthesis of Cyclopenta[c]chromenones. Angew. Chem. Int. Ed. 2018, 57, 17100–17103. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Barik, S.; Shee, S.; Biju, A.T. Enantioselective synthesis of tetra-substituted tetralines and tetrahydro-indolizines by NHC-catalyzed azolium–enolate cascade. Chem. Commun. 2021, 57, 7794–7797. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wang, Q.; Xu, J.; Zheng, P.; Chi, Y.R. Carbene-catalyzed chemoselective reaction of unsymmetric enedials for access to Furo[2,3-b]pyrroles. Nat. Commun. 2023, 14, 4243. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.Y.-K.; Bode, J.W. Catalytic Generation of Activated Carboxylates: Direct, Stereoselective Synthesis of β-Hydroxyesters from Epoxyaldehydes. J. Am. Chem. Soc. 2004, 126, 8126–8127. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.T.; Read de Alaniz, J.; Rovis, T. Conversion of α-Haloaldehydes into Acylating Agents by an Internal Redox Reaction Catalyzed by Nucleophilic Carbenes. J. Am. Chem. Soc. 2004, 126, 9518–9519. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.T.; Rovis, T. Enantioselective Protonation of Catalytically Generated Chiral Enolates as an Approach to the Synthesis of α-Chloroesters. J. Am. Chem. Soc. 2005, 127, 16406–16407. [Google Scholar] [CrossRef]
- Vora, H.U.; Rovis, T. N-Heterocyclic Carbene Catalyzed Asymmetric Hydration: Direct Synthesis of α-Protio and α-Deuterio α-Chloro and α-Fluoro Carboxylic Acids. J. Am. Chem. Soc. 2010, 132, 2860–2861. [Google Scholar] [CrossRef]
- Gelat, F.; Patra, A.; Pannecoucke, X.; Biju, A.T.; Poisson, T.; Besset, T. N-Heterocyclic Carbene-Catalyzed Synthesis of α-Trifluoromethyl Esters. Org. Lett. 2018, 20, 3897–3901. [Google Scholar] [CrossRef]
- Jin, S.; Fang, S.; Ma, R.; Liang, Z.; Xu, Y.; Lu, T.; Du, D. β-Sulfonylation of α-bromoenals enabled by N-heterocyclic carbene catalysis. Org. Chem. Front. 2019, 6, 3392–3396. [Google Scholar] [CrossRef]
- Barik, S.; Shee, S.; Ghosh, A.; Biju, A.T. Catalytic, enantioselective C2-functionalization of 3-aminobenzofurans using N-heterocyclic carbenes. Org. Lett. 2020, 22, 3865–3869. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Dai, L.; Gao, Z.-H.; Ye, S. Distal p-benzylic deuteration via N-heterocyclic carbene catalyzed ring opening of p-cyclopropylbenzaldehydes. Org. Biomol. Chem. 2023, 21, 4750–4754. [Google Scholar] [CrossRef] [PubMed]
- Soeta, T.; Kaneta, K.; Hatanaka, Y.; Ida, T.; Ukaji, Y. N-Heterocyclic Carbene-Catalyzed Chemoselective Monoacylation of 1,n-Linear Diols. Org. Lett. 2021, 23, 8138–8142. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Y.; Zhang, C.-L.; Jin, M.-L.; Gao, Z.-H.; Ye, S. Bifunctional NHC-Catalyzed Remote Enantioselective Mannich-type Reaction of 5-(Chloromethyl) furfural via Trienolate Intermediates. Angew. Chem. Int. Ed. 2023, 62, e202301126. [Google Scholar] [CrossRef]
- Chan, A.; Scheidt, K.A. Conversion of α,β-Unsaturated Aldehydes into Saturated Esters: An Umpolung Reaction Catalyzed by Nucleophilic Carbenes. Org. Lett. 2005, 7, 905–908. [Google Scholar] [CrossRef]
- Sohn, S.S.; Bode, J.W. Catalytic Generation of Activated Carboxylates from Enals: A Product-Determining Role for the Base. Org. Lett. 2005, 7, 3873–3876. [Google Scholar] [CrossRef]
- Zeitler, K. Stereoselective Synthesis of (E)-α‚β-Unsaturated Esters via Carbene-Catalyzed Redox Esterification. Org. Lett. 2006, 8, 637–640. [Google Scholar] [CrossRef]
- Feroci, M.; Chiarotto, I.; Orsini, M.; Pelagalli, R.; Inesi, A. Umpolung reactions in an ionic liquid catalyzed by electrogenerated N-heterocyclic carbenes. Synthesis of saturated esters from activated α,β-unsaturated aldehydes. Chem. Commun. 2012, 48, 5361–5363. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Grossmann, A.; Craen, D.V. N-Heterocyclic carbene catalyzed synthesis of oxime esters. Org. Biomol. Chem. 2013, 11, 138–141. [Google Scholar] [CrossRef]
- Wang, M.H.; Barsoum, D.; Schwamb, C.B.; Cohen, D.T.; Goess, B.C.; Riedrich, M.; Chan, A.; Maki, B.E.; Mishra, R.K.; Scheidt, K.A. Catalytic, Enantioselective β-Protonation through a Cooperative Activation Strategy. J. Org. Chem. 2017, 82, 4689–4702. [Google Scholar] [CrossRef]
- Yatham, V.R.; Harnying, W.; Kootz, D.; Neudörfl, J.-M.; Schlörer, N.E.; Berkessel, A. 1,4-Bis-Dipp/Mes-1,2,4-Triazolylidenes: Carbene Catalysts That Efficiently Overcome Steric Hindrance in the Redox Esterification of α- and β-Substituted α,β-Enals. J. Am. Chem. Soc. 2016, 138, 2670–2677. [Google Scholar] [CrossRef]
- Zhu, J.; Fang, S.; Sun, K.; Fang, C.; Lu, T.; Du, D. N-Heterocyclic Carbene-Catalyzed Formal Conjugate Hydroacylation: An Atom-Economic Synthesis of 1H-Indol-3-yl Esters. J. Org. Chem. 2018, 83, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Sinu, C.R.; Babu, B.P.; Varghese, V.; Jose, A.; Suresh, E. Novel Nucleophilic Heterocyclic Carbene Mediated Stereoselective Conjugate Addition of Enals to Nitrostyrenes via Homoenolate. Org. Lett. 2009, 11, 5570–5573. [Google Scholar] [CrossRef] [PubMed]
- White, N.A.; DiRocco, D.A.; Rovis, T. Asymmetric N-Heterocyclic Carbene Catalyzed Addition of Enals to Nitroalkenes: Controlling Stereochemistry via the Homoenolate Reactivity Pathway to Access δ-Lactams. J. Am. Chem. Soc. 2013, 135, 8504–8507. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Xiong, J.-W.; Liu, Q.; Li, S.; Sheng, H.; von Essen, C.; Rissanen, K.; Enders, D. Control of N-Heterocyclic Carbene Catalyzed Reactions of Enals: Asymmetric Synthesis of Oxindole-γ-Amino Acid Derivatives. Angew. Chem. Int. Ed. 2018, 57, 300–304. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Han, Y.-F.; Ye, S. N-Heterocyclic carbene-catalyzed β-addition of enals to 3-alkylenyloxindoles: Synthesis of oxindoles with all-carbon quaternary stereocenters. Chem. Commun. 2019, 55, 7966–7969. [Google Scholar] [CrossRef]
- Dzieszkowski, K.; Słotwiński, M.; Rafińska, K.; Muzioł, T.M.; Rafiński, Z. NHC-catalyzed enantioselective C2-functionalization of 3-hydroxychromenones via α,β-unsaturated acyl azoliums. Chem. Commun. 2021, 57, 9999–10002. [Google Scholar] [CrossRef] [PubMed]
- Dyguda, M.; Skrzyńska, A.; Sieroń, L.; Albrecht, Ł. Dearomative Michael addition involving enals and 2-nitrobenzofurans realized under NHC-catalysis. Chem. Commun. 2022, 58, 5367–5370. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.M.; Pratap, A.; Maji, B. N-Heterocyclic carbene-catalysed homoenolate addition reaction to 3-cyano-2-imino-2H-chromenes: Synthesis of C4-functionalized 2-amino-3-cyano-4H-chromene. Org. Biomol. Chem. 2022, 20, 8203–8208. [Google Scholar] [CrossRef]
- Li, E.; Tang, K.; Ren, Z.; Liao, X.; Liu, Q.; Huang, Y.; Chen, J. Enantioselective SN2 Alkylation of Homoenolates by N-Heterocyclic Carbene Catalysis. Adv. Sci. 2023, 10, 2303517. [Google Scholar] [CrossRef]
- Li, Z.; Huang, M.; Zhang, X.; Chen, J.; Huang, Y. N-Heterocyclic Carbene-Catalyzed Four-Component Reaction: Chemoselective Cradical-Cradical Relay Coupling Involving the Homoenolate Intermediate. ACS Catal. 2021, 11, 10123–10130. [Google Scholar] [CrossRef]
- Choi, H.; Mathi, G.R.; Hong, S.; Hong, S. Enantioselective functionalization at the C4 position of pyridinium salts through NHC catalysis. Nat. Commun. 2022, 13, 1776. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sun, J. Catalytic Asymmetric α-Aldol Reaction of Vinylogous N-Heterocyclic Carbene Enolates: Formation of Quaternary and Labile Tertiary Stereocenters. Org. Lett. 2014, 16, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, X.; Wang, M.; Zheng, P.; Song, B.-A.; Chi, Y.R. Aminomethylation of Enals through Carbene and Acid Cooperative Catalysis: Concise Access to β2-Amino Acids. Angew. Chem. Int. Ed. 2015, 54, 5161–5165. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ye, S. NHC-Catalyzed ε-Umpolung via p-Quinodimethanes and Its Nucleophilic Addition to Ketones. ACS Catal. 2020, 10, 994–998. [Google Scholar] [CrossRef]
- Bie, J.; Lang, M.; Wang, J. Enantioselective N-Heterocyclic Carbene-Catalyzed Kinetic Resolution of Anilides. Org. Lett. 2018, 20, 5866–5871. [Google Scholar] [CrossRef]
- Zhao, C.; Li, F.; Wang, J. N-Heterocyclic Carbene Catalyzed Dynamic Kinetic Resolution of Pyranones. Angew. Chem. Int. Ed. 2016, 55, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, J. Divergent Synthesis of Dihydropyranone Stereoisomers via N-Heterocyclic Carbene Catalysis. Adv. Synth. Catal. 2019, 361, 1668–1672. [Google Scholar] [CrossRef]
- Wang, Y.; Yamauchi, A.; Hashimoto, K.; Fujiwara, T.; Inokuma, T.; Mitani, Y.; Ute, K.; Kuwano, S.; Yamaoka, Y.; Takasu, K.; et al. Enhanced Molecular Recognition through Substrate−Additive Complex Formation in N-Heterocyclic-Carbene-Catalyzed Kinetic Resolution of α-Hydroxythioamides. ACS Catal. 2022, 12, 6100–6107. [Google Scholar] [CrossRef]
- Yamada, K.; Yamauchi, A.; Fujiwara, T.; Hashimoto, K.; Wang, Y.; Kuwano, S.; Inokuma, T. Kinetic Resolution of α-Hydroxyamide via N-Heterocyclic Carbene-Catalyzed Acylation. Asian J. Org. Chem. 2022, 11, e202200452. [Google Scholar] [CrossRef]
- An, H.; Liu, S.; Wang, S.-J.; Yu, X.; Shi, C.; Lin, H.; Poh, S.B.; Yang, H.; Wong, M.W.; Zhao, Y.; et al. Kinetic Resolution of Acyclic Tertiary Propargylic Alcohols by NHC-Catalyzed Enantioselective Acylation. Org. Lett. 2024, 26, 702–707. [Google Scholar] [CrossRef]
- Shu, T.; Li, S.; Chen, X.-Y.; Liu, Q.; von Essen, C.; Rissanen, K.; Enders, D. Asymmetric synthesis of functionalized tetrahydrofluorenones via an NHC-catalyzed homoenolate Michael addition. Chem. Commun. 2018, 54, 7661–7664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Wang, Y.-F.; Kang, W.-Y.; Lu, W.-Y.; Wang, Y.-H.; Tian, P. A Highly Enantioselective Homoenolate Michael Addition/Esterification Sequence of Cyclohexadienone-Tethered Enals via NHC Catalysis. Org. Lett. 2023, 25, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Zheng, S.-C.; Zhang, J.-W.; Liu, X.-Y.; Tan, B. Construction of Tropane Derivatives by the Organocatalytic Asymmetric Dearomatization of Isoquinolines. Angew. Chem. Int. Ed. 2016, 55, 11834–11839. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Rovis, T. Enantioselective N-heterocyclic carbene-catalyzed nucleophilic dearomatization of alkyl pyridiniums. Chem. Sci. 2017, 8, 6566–6569. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wu, S.; Mou, C.; Liu, J.; Zheng, P.; Zhang, X.; Chi, Y.R. Carbene-Catalyzed Enantioselective Sulfonylation of Enone Aryl Aldehydes: A New Mode of Breslow Intermediate Oxidation. J. Am. Chem. Soc. 2022, 144, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Mahatthananchai, J.; Kaeobamrung, J.; Bode, J.W. Chiral N-Heterocyclic Carbene-Catalyzed Annulations of Enals and Ynals with Stable Enols: A Highly Enantioselective Coates−Claisen Rearrangement. ACS Catal. 2012, 2, 494–503. [Google Scholar] [CrossRef]
- Yetra, S.R.; Roy, T.; Bhunia, A.; Porwal, D.; Biju, A.T. Synthesis of Functionalized Coumarins and Quinolinones by NHC-Catalyzed Annulation of Modified Enals with Heterocyclic C−H Acids. J. Org. Chem. 2014, 79, 4245–4251. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Liu, Y.; Zhu, S.; Liu, M.; Hua, X.; Chen, S.; Lu, T.; Du, D. Formal [3 + 3] annulation of isatin-derived 2-bromoenals with 1,3-dicarbonyl compounds enabled by Lewis acid/N-heterocyclic carbene cooperative catalysis. RSC Adv. 2016, 6, 18601–18606. [Google Scholar] [CrossRef]
- Luo, C.; Xu, X.; Xu, J.; Chen, X. Oxidant free synthesis of α-pyrones via an NHC catalyzed [3 + 3] annulation of bromoenals with 2-chloro-1,3-diketones. Org. Biomol. Chem. 2022, 20, 9298–9301. [Google Scholar] [CrossRef]
- Li, J.-L.; Sahoo, B.; Daniliuc, C.-G.; Glorius, F. Conjugate Umpolung of β,β-Disubstituted Enals by Dual Catalysis with an N-Heterocyclic Carbene and a Brønsted Acid: Facile Construction of Contiguous Quaternary Stereocenters. Angew. Chem. Int. Ed. 2014, 53, 10515–10519. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Tang, W.; Lu, T.; Du, D. Cooperative N-heterocyclic carbene (NHC)–Lewis acid-mediated regioselective umpolung formal [3 + 2] annulations of alkynyl aldehydes with isatins. Org. Biomol. Chem. 2014, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Huang, X.; Wang, Z.; Pan, D.; Zhang, J.; Chi, Y.R. Umpolung of donor–acceptor cyclopropanes via N-heterocyclic carbene organic catalysis. Org. Chem. Front. 2021, 8, 5105–5111. [Google Scholar] [CrossRef]
- Kyan, R.; Kitagawa, Y.; Ide, R.; Sato, K.; Mase, N.; Narumi, T. β,γ-trans-selective γ-butyrolactone formation via homoenolate cross-annulation of enals and aldehydes catalyzed by sterically hindered N-heterocyclic carbene. Tetrahedron 2021, 91, 132191. [Google Scholar] [CrossRef]
- Wang, G.; Wu, J.; Cheng, H.; Zhong, C.; He, Z.-L. N-Heterocyclic Carbene Catalyzed [3 + 2] Annulations of β-Halocycloenals with Isatins and Mechanism Study. Eur. J. Org. Chem. 2021, 6, 983–989. [Google Scholar] [CrossRef]
- Gil-Ordóñez, M.; Maestro, A.; Ortega, P.; Jambrina, P.G.; Andrés, J.M. NHC-catalysed [3 + 2]-asymmetric annulation between pyrazolin-4,5-diones and enals: Synthesis of novel spirocyclic pyrazolone γ-butyrolactones and computational study of mechanism and stereoselectivity. Org. Chem. Front. 2022, 9, 420–427. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Liao, X.; Chen, J.; Huang, Y. An NHC-catalyzed [3 + 2] cyclization of β-disubstituted enals with benzoyl cyanides. Chem. Commun. 2022, 58, 9742–9745. [Google Scholar] [CrossRef]
- Gil-Ordóñez, M.; Maestro, A.; Andrés, J.M. Access to Spiropyrazolone-butenolides through NHC-Catalyzed [3 + 2]-Asymmetric Annulation of 3-Bromoenals and 1H-Pyrazol-4,5-diones. J. Org. Chem. 2023, 88, 6890–6900. [Google Scholar] [CrossRef]
- Liang, Z.; Li, J.; Liu, C.; Zhu, Y.; Du, D. N-heterocyclic carbene-catalyzed enantioselective synthesis of spirocyclic ketones bearing gemdifluoromethylenes. Org. Chem. Front. 2023, 10, 3027–3032. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, X.; Xu, J.; Chai, H.; Liu, H.; Zhang, J.; Song, J.; Gao, Y.; Jin, Z.; Chi, Y.R. Access to Allene-Containing Molecules via Enantioselective Reactions of Azolium Cumulenolate Intermediates. Angew. Chem. Int. Ed. 2021, 60, 14817–14823. [Google Scholar] [CrossRef]
- Viveki, A.B.; Pol, M.D.; Halder, P.; Sonavane, S.R.; Mhaske, S.B. Annulation of Enals with Carbamoylpropiolates via NHC-Catalyzed Enolate Pathway: Access to Functionalized Maleimides/Isomaleimides and Synthesis of Aspergillus FH-X-213. J. Org. Chem. 2021, 86, 9466–9477. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Lee, R.; Lv, Y.; Huang, K.-W.; Zhong, G. Asymmetric NHC-Catalyzed Aza-Diels−Alder Reactions: Highly Enantioselective Route to α-Amino Acid Derivatives and DFT Calculations. Org. Lett. 2014, 16, 3872–3875. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, X.; Li, F.; Wu, J.; Wang, J. Chemoselective N-Heterocyclic Carbene-Catalyzed Cascade of Enals with Nitroalkenes. Org. Lett. 2015, 17, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, R.; Liu, Y.; Ooi, C.Y.; Jin, Z.; Zhu, T.; Wang, H.; Hao, L.; Chi, Y.R. Carbene and Acid Cooperative Catalytic Reactions of Aldehydes and o-Hydroxybenzhydryl Amines for Highly Enantioselective Access to Dihydrocoumarins. Org. Lett. 2017, 19, 5892–5895. [Google Scholar] [CrossRef]
- Prieto, L.; Sánchez-Díez, E.; Uria, U.; Reyes, E.; Carrillo, L.; Vicario, J.L. Catalytic Generation of Donor-Acceptor Cyclopropanes under N-Heterocyclic Carbene Activation and their Stereoselective Reaction with Alkylideneoxindoles. Adv. Synth. Catal. 2017, 359, 1678–1683. [Google Scholar] [CrossRef]
- Verma, R.S.; Khatana, A.K.; Mishra, M.; Kumar, S.; Tiwari, B. Access to enantioenriched 4-phosphorylated δ-lactones from β-phosphorylenones and enals via carbene organocatalysis. Chem. Commun. 2020, 56, 7155–7158. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, J.; Li, T.; Chi, Y.R.; Jin, Z. Chemo-selective cross reaction of two enals via carbene-catalyzed dual activation. Chem. Sci. 2020, 11, 12533–12539. [Google Scholar] [CrossRef]
- Liu, L.; Guo, D.; Wang, J. NHC-Catalyzed Asymmetric α-Regioselective [4 + 2] Annulation to Construct α-Alkylidene-δ-lactones. Org. Lett. 2020, 22, 7025–7029. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, Y.; Lan, Y.; Wei, D. Predicting the origin of selectivity in NHC catalyzed ring opening of formylcyclopropane: A theoretical investigation. Catal. Sci. Technol. 2021, 11, 332–337. [Google Scholar] [CrossRef]
- Khatana, A.K.; Singh, V.; Gupta, M.K.; Tiwari, B. Carbene Catalyzed Access to 3,6-Disubstituted α-Pyrones via Michael Addition/Lactonization/Elimination Cascade. Adv. Synth. Catal. 2021, 363, 4862–4866. [Google Scholar] [CrossRef]
- Verma, R.S.; Talukdar, R.; Azaz, T.; Tiwari, B. Carbene Catalyzed Asymmetric Synthesis of Selenylated δ-Lactones via [4 + 2] Annulation of Selenyl Vinyl Ketones and Enals. Adv. Synth. Catal. 2022, 364, 4031–4035. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Huang, X.; Jin, Z. Asymmetric Synthesis of Structurally Sophisticated Spirocyclic Pyrano[2,3-c]pyrazole Derivatives Bearing a Chiral Quaternary Carbon Center. Org. Lett. 2022, 24, 5474–5479. [Google Scholar] [CrossRef] [PubMed]
- Nong, Y.; Pang, C.; Teng, K.; Zhang, S.; Liu, Q. NHC-Catalyzed Chemoselective Reactions of Enals and Cyclopropylcarbaldehydes for Access to Chiral Dihydropyranone Derivatives. J. Org. Chem. 2023, 88, 13535–13543. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Tam, A.T.; Miller, E.R.; Scheidt, K.A. A Platform for the Synthesis of Corynantheine-Type Corynanthe Alkaloids. J. Am. Chem. Soc. 2024, 146, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yu, C.; Li, T.; Wang, X.-S.; Yao, C. N-Heterocyclic Carbene/Lewis Acid Strategy for the Stereoselective Synthesis of Spirocyclic Oxindole−Dihydropyranones. Org. Lett. 2014, 16, 3632–3635. [Google Scholar] [CrossRef] [PubMed]
- Przydacz, A.; Topolska, A.; Skrzyńska, A.; Albrecht, Ł. NHC-Catalyzed 1,4-Elimination in the Dearomative Activation of 3-Furaldehydes towards (4 + 2)-Cycloadditions. Adv. Synth. Catal. 2022, 364, 1434–1439. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, B.; Xie, Y.; Wang, J. Carbene-Catalyzed [4 + 2] Annulation of 2H-Azirine-2-carboxaldehydes with Ketones via Azolium Aza-Dienolate Intermediate. Org. Lett. 2018, 20, 7641–7644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z. A DFT study on NHC-catalyzed [4 + 2] annulation of 2H-azirines with ketones: Mechanism and selectivity. Int J Quantum Chem. 2021, 121, e26557. [Google Scholar] [CrossRef]
- Izquierdo, J.; Orue, A.; Scheidt, K.A. A Dual Lewis Base Activation Strategy for Enantioselective Carbene-Catalyzed Annulations. J. Am. Chem. Soc. 2013, 135, 10634–10637. [Google Scholar] [CrossRef]
- Wang, M.; Rong, Z.-Q.; Zhao, Y. Stereoselective synthesis of ε-lactones or spiro-heterocycles through NHC-catalyzed annulation: Divergent reactivity by catalyst control. Chem. Commun. 2014, 50, 15309–15312. [Google Scholar] [CrossRef]
- Liang, Z.-Q.; Gao, Z.-H.; Jia, W.-Q.; Ye, S. Bifunctional N-Heterocyclic Carbene Catalyzed [3 + 4] Annulation of Enals and Aurones. Chem. Eur. J. 2015, 21, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-Q.; Yi, L.; Chen, K.-Q.; Ye, S. N-Heterocyclic Carbene-Catalyzed [3 + 4] Annulation of Enals and Alkenyl Thiazolones: Enantioselective Synthesis of Thiazole-Fusedε-Lactones. J. Org. Chem. 2016, 81, 4841–4846. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Chen, X.-Y.; Rissanen, K.; Enders, D. Asymmetric Synthesis of Spiro-oxindole-ε-lactones through N-Heterocyclic Carbene Catalysis. Org. Lett. 2018, 20, 3622–3626. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Z.; Zhang, Z.; Cheng, Y.; Li, P. NHC-Catalyzed Enantioselective [4 + 3] Cycloaddition of Ortho-Hydroxyphenyl Substituted Para-Quinone Methides with Isatin-Derived Enals. Adv. Synth. Catal. 2018, 360, 2460–2464. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Zhang, Z. Mechanistic study on the NHC-catalyzed [3 + 4] annulation of enals and thiazolones. New J. Chem. 2021, 45, 12129–12137. [Google Scholar] [CrossRef]
- Davies, A.T.; Greenhalgh, M.D.; Slawin, A.M.Z.; Smith, A.D. NHC-catalyzed enantioselective synthesis of β-trifluoromethyl-β-hydroxyamides. Beilstein J. Org. Chem. 2020, 16, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Hu, Z.; Jin, J.; Lu, Y.; Tang, W.; Wang, B.; Lu, T. N-Heterocyclic Carbene-Catalyzed Three-Component Domino Reaction of Alkynyl Aldehydes with Oxindoles. Org. Lett. 2012, 14, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-R.; Dong, Z.-W.; Yang, Y.-J.; Wang, P.-L.; Hui, X.-P. N-Heterocyclic Carbene-Catalyzed Stereoselective Cascade Reaction: Synthesis of Functionalized Tetrahydroquinolines. Org. Lett. 2013, 15, 4750–4753. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Xu, J.; Jin, J.; Chi, Y.R.; Jin, Z. Enantio- and Diastereoselective Synthesis of Chromeno[4,3-b]pyrrole Derivatives Bearing Tetrasubstituted Chirality Centers through Carbene Catalyzed Cascade Reactions. Org. Lett. 2020, 22, 326–330. [Google Scholar] [CrossRef]
- Shee, S.; Mukherjee, S.; Gonnade, R.G.; Biju, A.T. Enantioselective Synthesis of Tricyclic β-Lactones by NHC-Catalyzed Desymmetrization of Cyclic 1,3-Diketones. Org. Lett. 2020, 22, 5407–5411. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Shen, F.; Yang, T.; Zhang, J.-K.; Chen, R.; Wang, K.-K.; Liu, H. Carbene-Catalyzed Three-Component Cascade Reaction of Benzofuran-2-ones and Enals: Construction of Spirobenzofuranone-δ-lactones. Asian J. Org. Chem. 2021, 10, 3293–3296. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yang, T.; Liu, D.; Chen, R.; Wang, N.; Liu, H.; Li, J.; Wang, K.-K.; Liu, H. Catalyst-Controlled Selectivity Switch in Three-Component Reaction: An NHC-Catalyzed Strategy for the Synthesis of δ-Lactone-Fused Spirobenzofuran-3-ones. Molecules 2022, 27, 5952. [Google Scholar] [CrossRef]
- Lu, S.; Ong, J.-Y.; Yang, H.; Poh, S.B.; Liew, X.; Seow, C.S.D.; Wong, M.W.; Zhao, Y. Diastereo- and Atroposelective Synthesis of Bridged Biaryls Bearing an Eight-Membered Lactone through an Organocatalytic Cascade. J. Am. Chem. Soc. 2019, 141, 17062–17067. [Google Scholar] [CrossRef]
- Bhunia, A.; Patra, A.; Puranik, V.G.; Biju, A.T. NHC-Catalyzed Reaction of Enals with Hydroxy Chalcones: Diastereoselective Synthesis of Functionalized Coumarins. Org. Lett. 2013, 15, 1756–1759. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.-Y.; Niu, S.-S.; Rao, Y.; Cheng, Y. The Reaction of 2-Aroylvinylcinnamaldehydes with Aromatic Aldehydes by Dual Catalysis with a Chiral N-Heterocyclic Carbene and a Lewis Acid: Enantioselective Construction of Tetrahydroindeno[1,2-c]furan-1-ones. J. Org. Chem. 2016, 81, 8276–8286. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Ding, Y.-L.; Li, S.-N.; Cheng, Y. N-Heterocyclic Carbene/Lewis Acid Dual Catalysis for the Divergent Construction of Enantiopure Bridged Lactones and Fused Indenes. J. Org. Chem. 2016, 81, 11871–11881. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yao, L.; Zhao, H.; Tang, X.; Zhao, Q.; Wu, Y.; Han, B.; Huang, W.; Zhan, G. Construction of Cyclopentanes Consisting of Five Stereocenters via NHC-Catalyzed Cascade Reactions of Enals with Oxindole-Dienones. Org. Lett. 2023, 25, 8445–8450. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.-Y.; Wang, Z.-T.; Cheng, Y. Changing Reaction Pathways of the Dimerization of 2-Formylcinnamates by N-Heterocyclic Carbene/Lewis Acid Cooperative Catalysis: An Unusual Cleavage of the Carbon−Carbon Bond. Org. Lett. 2014, 16, 5520–5523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; DiRocco, D.A.; Rovis, T. N-Heterocyclic Carbene and Brønsted Acid Cooperative Catalysis: Asymmetric Synthesis of trans-γ-Lactams. J. Am. Chem. Soc. 2011, 133, 12466–12469. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, L.; Deng, Y.; Zhong, G. Cooperative catalysis of N-heterocyclic carbene and Brønsted acid for a highly enantioselective route to unprotected spiro-indoline-pyrans. Chem. Commun. 2015, 51, 8330–8333. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hao, L.; Zhang, Y.; Rakesh, M.; Reddi, R.N.; Yang, S.; Song, B.-A.; Chi, Y.R. Construction of Fused Pyrrolidines and β-Lactones by Carbene-Catalyzed C−N, C−C, and C−O Bond Formations. Angew. Chem. Int. Ed. 2017, 56, 4201–4205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J. Enantioselective Medium-Ring Lactone Synthesis through an NHC-Catalyzed Intramolecular Desymmetrization of Prochiral 1,3-Diols. ACS Catal. 2017, 7, 7647–7652. [Google Scholar] [CrossRef]
- Janssen-Meller, D.; Singha, S.; Olyschläger, T.; Daniliuc, C.G.; Glorius, F. Annulation of o-Quinodimethanes through N-Heterocyclic Carbene Catalysis for the Synthesis of 1-Isochromanones. Org. Lett. 2016, 18, 4444–4447. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-F.; Rovis, T. N-Heterocyclic Carbene and Chiral Brønsted Acid Cooperative Catalysis for a Highly Enantioselective [4 + 2] Annulation. Synthesis 2017, 49, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chi, Y.R.; Huang, X. Enantioselective Dual Catalysis of N-Heterocyclic Carbene and Hydrogen-Bond Donor Organocatalysts. Eur. J. Org. Chem. 2022, 27, e202200548. [Google Scholar] [CrossRef]
- Youn, S.W.; Song, H.S.; Park, J.H. Asymmetric Domino Multicatalysis for the Synthesis of 3-Substituted Phthalides: Cinchonine/NHC Cooperative System. Org. Lett. 2014, 16, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Cohen, D.T.; Schwamb, C.B.; Mishra, R.K.; Scheidt, K.A. Enantioselective β-Protonation by a Cooperative Catalysis Strategy. J. Am. Chem. Soc. 2015, 137, 5891–5894. [Google Scholar] [CrossRef] [PubMed]
- Murauski, K.J.R.; Walden, D.M.; Cheong, P.H.-Y.; Scheidt, K.A. A Cooperative Ternary Catalysis System for Asymmetric Lactonizations of α-Ketoesters. Adv. Synth. Catal. 2017, 359, 3713–3719. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.J.W.; Zeitler, K. An N-Heterocyclic Carbene-Mediated, Enantioselective and Multicatalytic Strategy to Access Dihydropyranones in a Sequential Three-Component One-Pot Reaction. Org. Lett. 2017, 19, 6076–6079. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.J.W.; Zeitler, K. Nitroalkenes as Latent 1,2-Biselectrophiles—A Multicatalytic Approach for the Synthesis of 1,4-Diketones and Their Application in a Four-Step One-Pot Reaction to Polysubstituted Pyrroles. J.Org. Chem. 2017, 82, 7796–7805. [Google Scholar] [CrossRef]
- Shee, S.; Barik, S.; Ghosh, A.; Biju, A.T. Synthesis of Functionalized Dihydrocoumarins by NHC-Catalyzed [3 + 3] Annulation of Enals with 2-Substituted Naphthoquinones. Org. Lett. 2021, 23, 8039–8044. [Google Scholar] [CrossRef]
- Liu, K.; Hovey, M.T.; Scheidt, K.A. A cooperative N-heterocyclic carbene/palladium catalysis system. Chem. Sci. 2014, 5, 4026–4031. [Google Scholar] [CrossRef]
- Guo, C.; Fleige, M.; Janssen-Müller, D.; Daniliuc, C.G.; Glorius, F. Cooperative N-Heterocyclic Carbene/Palladium-Catalyzed Enantioselective Umpolung Annulations. J. Am. Chem. Soc. 2016, 138, 7840–7843. [Google Scholar] [CrossRef]
- Guo, C.; Janssen-Müller, D.; Fleige, M.; Lerchen, A.; Daniliuc, C.G.; Glorius, F. Mechanistic Studies on a Cooperative NHC Organocatalysis/Palladium Catalysis System: Uncovering Significant Lessons for Mixed Chiral Pd(NHC)(PR3) Catalyst Design. J. Am. Chem. Soc. 2017, 139, 4443–4451. [Google Scholar] [CrossRef]
- Singha, S.; Patra, T.; Daniliuc, C.G.; Glorius, F. Highly Enantioselective [5 + 2] Annulations through Cooperative N-Heterocyclic Carbene (NHC) Organocatalysis and Palladium Catalysis. J. Am. Chem. Soc. 2018, 140, 3551–3554. [Google Scholar] [CrossRef]
- Singha, S.; Serrano, E.; Mondal, S.; Daniliuc, C.G.; Glorius, F. Diastereodivergent synthesis of enantioenriched α,β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catalysis 2020, 3, 48–54. [Google Scholar] [CrossRef]
- Bhaskararao, B.; Rotella, M.E.; Kim, D.Y.; Kee, J.-M.; Kim, K.S.; Kozlowski, M.C. Ir and NHC Dual Chiral Synergetic Catalysis: Mechanism and Stereoselectivity in γ-Butyrolactone Formation. J. Am. Chem. Soc. 2022, 144, 16171–16183. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Song, J.; Gong, L.-Z. Asymmetric Redox Allylic Alkylation to Access 3,3′-Disubstituted Oxindoles Enabled by Ni/NHC Cooperative Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202201678. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, M.; Huang, J.; Guo, C.; Gong, L.-Z.; Song, J. Enantio- and Diastereodivergent N-Heterocyclic Carbene/Nickel Dual-Catalyzed Umpolung Propargylic Substitutions of Enals. J. Am. Chem. Soc. 2023, 145, 28085–28095. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ling, B.; Hu, B.; Yin, H.; Mao, J.; Walsh, P.J. Synergistic N-Heterocyclic Carbene/Palladium-Catalyzed Umpolung 1,4-Addition of Aryl Iodides to Enals. Angew. Chem. Int. Ed. 2020, 59, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Yang, W.; Wang, Y.-E.; Mao, J. Cooperative N-Heterocyclic Carbene/Palladium-Catalyzed Umpolung 1,4-Addition of Vinyl Bromides to Enals. Org. Lett. 2020, 22, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
- Namitharan, K.; Zhu, T.; Cheng, J.; Zheng, P.; Li, X.; Yang, S.; Song, B.-A.; Chi, Y.R. Metal and carbene organocatalytic relay activation of alkynes for stereoselective reactions. Nat. Commun. 2014, 5, 3982. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhang, L.; Geng, R.-L.; Song, J.; Chen, X.-H.; Gong, L.-Z. N-Heterocyclic Carbene/Copper Cooperative Catalysis for the Asymmetric Synthesis of Spirooxindoles. Angew. Chem. Int. Ed. 2019, 58, 12190–12194. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Yoo, H.J. One-Pot Sequential N-Heterocyclic Carbene/Rhodium(III) Catalysis: Synthesis of Fused Polycyclic Isocoumarins. Adv. Synth. Catal. 2017, 359, 2176–2183. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Yang, F.; Li, S.; Yao, X.; Song, J.; Gong, L.-Z. Diastereodivergent Desymmetric Annulation to Access Spirooxindoles: Chemical Probes for Mitosis. J. Am. Chem. Soc. 2023, 145, 4199–4207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mück-Lichtenfeld, C.; Studer, A. Cooperative N-Heterocyclic Carbene (NHC) and Ruthenium Redox Catalysis: Oxidative Esterification of Aldehydes with Air as the Terminal Oxidant. Adv. Synth. Catal. 2013, 355, 1098–1106. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Huang, Y. Aerobic Oxidation/Annulation Cascades through Synergistic Catalysis of RuCl3 and N-Heterocyclic Carbenes. Chem. Eur. J. 2018, 24, 12806–12810. [Google Scholar] [CrossRef]
- Li, S.; Wen, Y.-H.; Song, J.; Gong, L.-Z. Asymmetric redox benzylation of enals enabled by NHC/Ru cooperative catalysis. Sci. Adv. 2023, 9, eadf5606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Liu, S.; Zhang, S.; Yang, B.; Zhao, Y.; Lu, S. Enantioselective Cascade Annulation of α-Amino-ynones and Enals Enabled by Gold and Oxidative NHC Relay Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202115464. [Google Scholar] [CrossRef]
- DiRocco, D.A.; Rovis, T. Catalytic Asymmetric α-Acylation of Tertiary Amines Mediated by a Dual Catalysis Mode: N-Heterocyclic Carbene and Photoredox Catalysis. J. Am. Chem. Soc. 2012, 134, 8094–8097. [Google Scholar] [CrossRef]
- Yoshioka, E.; Inoue, M.; Nagoshi, Y.; Kobayashi, A.; Mizobuchi, R.; Kawashima, A.; Kohtani, S.; Miyabe, H. Oxidative Functionalization of Cinnamaldehyde Derivatives: Control of Chemoselectivity by Organophotocatalysis and Dual Organocatalysis. J. Org. Chem. 2018, 83, 8962–8970. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Takahashi, H.; Kubo, A.; Ohno, M.; Watanabe, F.; Shiono, R.; Miyazaki, Y.; Miyabe, H. N-Heterocyclic Carbene Catalyzed Cross Dehydrogenative Coupling of Aldehydes with Methanol: Combined Use of Eosin Y and Hexachloroethane. Synthesis 2022, 54, 5520–5528. [Google Scholar] [CrossRef]
- Dai, L.; Xia, Z.-H.; Gao, Y.-Y.; Gao, Z.-H.; Ye, S. Visible-Light-Driven N-Heterocyclic Carbene Catalyzed γ- and ε-Alkylation with Alkyl Radicals. Angew. Chem. Int. Ed. 2019, 58, 18124–18130. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ye, S. Photo/N-Heterocyclic Carbene Co-catalyzed Ring Opening and γ-Alkylation of Cyclopropane Enal. Org. Lett. 2020, 22, 986–990. [Google Scholar] [CrossRef]
- Dai, L.; Xu, Y.-Y.; Xia, Z.-H.; Ye, S. γ-Difluoroalkylation: Synthesis of γ-Difluoroalkyl-α,β-Unsaturated Esters via Photoredox NHC-Catalyzed Radical Reaction. Org. Lett. 2020, 22, 8173–8177. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Dai, L.; Gao, Z.-H.; Ye, S. ε-Benzylation via Cooperative Photoredox and N-Heterocyclic Carbene Catalysis. J. Org. Chem. 2022, 87, 14970–14974. [Google Scholar] [CrossRef]
- Xia, Z.-H.; Dai, L.; Gao, Z.-H.; Ye, S. N-Heterocyclic carbene/photo-cocatalyzed oxidative smiles rearrangement: Synthesis of aryl salicylates from O-aryl salicylaldehydes. Chem. Commun. 2020, 56, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-H.; Xia, Z.-H.; Dai, L.; Ye, S. N-Heterocyclic Carbene Catalyzed Photooxidation: Intramolecular Cross Dehydrogenative Coupling of Tetrahydroisoquinoline-Tethered Aldehydes. Adv. Synth. Catal. 2020, 362, 1819–1824. [Google Scholar] [CrossRef]
- Krylov, I.B.; Vil’, V.A.; Terent’ev, A.O. Cross-dehydrogenative coupling for the intermolecular C–O bond formation. Beilstein J. Org. Chem. 2015, 11, 92–146. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, L.; Luo, S. Organocatalysis in Inert C−H Bond Functionalization. Chem. Rev. 2017, 117, 9433–9520. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.; Antoine-Michard, A.; Sundén, H. Organocatalytic valorisation of glycerol via a dual NHC-catalysed telescoped reaction. Green Chem. 2017, 19, 2477–2481. [Google Scholar] [CrossRef]
- Ragno, D.; Brandolese, A.; Urbani, D.; Di Carmine, G.; De Risi, C.; Bortolini, O.; Giovannini, P.P.; Massi, A. Esterification of glycerol and solketal by oxidative NHC-catalysis under heterogeneous batch and flow conditions. React. Chem. Eng. 2018, 3, 816–825. [Google Scholar] [CrossRef]
- Brandolese, A.; Ragno, D.; Di Carmine, G.; Bernardi, T.; Bortolini, O.; Giovannini, P.P.; Pandoli, O.G.; Altomare, A.; Massi, A. Aerobic oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid and its derivatives by heterogeneous NHC-catalysis. Org. Biomol. Chem. 2018, 16, 8955–8964. [Google Scholar] [CrossRef] [PubMed]
- Ragno, D.; Di Carmine, G.; Vannini, M.; Bortolini, O.; Perrone, D.; Buoso, S.; Bertoldo, M.; Massi, A. Organocatalytic synthesis of poly (hydroxymethylfuroate) via ring-opening polymerization of 5-hydroxymethylfurfural-based cyclic oligoesters. Polym. Chem. 2022, 13, 1350–1358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaoka, Y.; Miyabe, H. NHC-Catalyzed Reaction of Aldehydes for C(sp2)–O Bond Formation. Catalysts 2024, 14, 219. https://doi.org/10.3390/catal14040219
Yamaoka Y, Miyabe H. NHC-Catalyzed Reaction of Aldehydes for C(sp2)–O Bond Formation. Catalysts. 2024; 14(4):219. https://doi.org/10.3390/catal14040219
Chicago/Turabian StyleYamaoka, Yousuke, and Hideto Miyabe. 2024. "NHC-Catalyzed Reaction of Aldehydes for C(sp2)–O Bond Formation" Catalysts 14, no. 4: 219. https://doi.org/10.3390/catal14040219
APA StyleYamaoka, Y., & Miyabe, H. (2024). NHC-Catalyzed Reaction of Aldehydes for C(sp2)–O Bond Formation. Catalysts, 14(4), 219. https://doi.org/10.3390/catal14040219