Abstract
Ni-based catalysts play a fundamental role in catalytic CO2 methanation. In this study, the possibility of using siderite ore as a catalyst or catalytic support material for nickel-based catalysts was investigated, aiming at the exploitation of an abundant natural resource. The catalytic performance of Ni-based catalysts with reduced siderite ore as a support was evaluated and compared to MgO as a support material. MgO is known as an effective support material, as it provides access to bifunctional catalysts because of its basicity and high CO2 adsorption capacity. It was shown that undoped and Ni-doped reduced siderite ore have comparable catalytic activity for CO2 hydrogenation (20−23%) at 648 K, but show limited selectivity toward methane (<20% for sideritereduced and 60.2% for Ni/sideritereduced). When MgO was added to the support material (Ni/sideritereduced/MgO), both the CO2 conversion and the selectivity toward methane increased significantly. CO2 conversions were close to the thermodynamic equilibrium, and methane selectivities of ≥99% were achieved.
1. Introduction
Carbon dioxide (CO2) is one of the major contributors to global climate change. To mitigate CO2 emissions, increasing attention is directed to carbon capture and utilization (CCU) and carbon utilization (CU) technologies. These technologies aim at utilizing CO2 from industrial processes as raw material for the production of value-added chemicals and fuels, for instance, methane (CH4) [1,2,3], methanol (CH3OH), and gasoline [4].
Methane synthesis by CO2 hydrogenation has gained wide attention from researchers all over the world [5,6,7]. Methanation, referring to the conversion of carbon monoxide (CO) and/or carbon dioxide with hydrogen (H2) into methane, is thermodynamically favorable (Equations (1) and (2)). Both reactions are highly exothermic; thus, high temperatures are unfavorable for the carbon oxide conversion [3,8,9].
CO2 + 4 H2 ⇌ CH4 + 2 H2O ∆RH0 = −165.0 kJ mol−1, ∆RG0 = −113.61 kJ mol−1
CO + 3 H2 ⇌ CH4 + H2O ∆RH0 = −206.2 kJ mol−1, ∆RG0 = −142.25 kJ mol−1
Hydrogenation of CO2 may also result in CO formation via the reverse water-gas shift reaction (Equation (3)).
CO2 + H2 ⇌ CO + H2O ∆RH0 = −41.2 kJ mol−1, ∆RG0 = −28.64 kJ mol−1
To catalyze CO2 methanation, ruthenium (Ru)- and rhodium (Rh)-based catalysts have shown promising catalytic activity and selectivity [10,11]. However, their high costs are disadvantageous for industrial application. Currently, nickel (Ni)-based catalysts are the most widely used catalysts for CO2 methanation because of their high catalytic activity, selectivity toward methane, and long-term stability. They help overcome the activation energy barrier, allowing the reaction to occur under mild conditions [12,13]. In addition, nickel is an abundant, relatively low-cost metal that helps extend the operational lifetime of the catalyst and thus reduces costs for catalyst replacement, which is crucial for industrial application. It is evident that the support material for the catalytically active nickel species has a pronounced effect on the activity and selectivity of the catalyst [14,15,16,17,18]. A series of different support materials for Ni-based catalysts is presented in the literature. Aluminum oxide (Al2O3), silicon dioxide (SiO2), titanium dioxide (TiO2), cerium oxide (CeO2), and zirconium dioxide (ZrO2), along with magnesium oxide (MgO), have been reported as support materials for Ni-based methanation catalysts [19,20,21,22,23,24,25,26,27]. Rahmani et al. produced a range of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3. The catalysts possessed large surface areas, with the 20 wt% Ni/Al2O3 catalyst showing the highest activity and stability between 473 K and 623 K [28]. Xu et al. investigated CO2 methanation using Ni/SiO2 catalysts that were prepared through a combustion-impregnation method and obtained a CO2 conversion of 66.9% and a methane selectivity of 94.1% at 593 K [29]. However, the challenges encountered when using alumina and silica as support materials were carbon deposition and poor catalyst stability at the reaction temperatures [27,30]. Perkas et al. [31] developed Ni catalysts supported on mesoporous ZrO2 modified with Ce and Sm cations, featuring 30 mol% Ni loading. These catalysts exhibited elevated catalytic activity for CO2 methanation with a turnover frequency of 1.5 s−1 at 573 K. Ni/ZrO2 catalysts with various amounts of tetragonal polymorph ZrO2 were prepared from an amorphous Ni-Zr alloy by Yamasaki et al. [32]. The tetragonal zirconia-supported nickel nanoparticles showed an even higher turnover frequency (TOF = 5.43 s−1 at 473 K). Nevertheless, ZrO2 and CeO2 are expensive compared with other widely used support materials. Therefore, MgO has become an attractive alternative support material. Apart from being cost-effective, MgO has the significant advantages of exhibiting increased basicity of the surface, preventing catalyst deactivation, and mitigating issues of sintering and the formation of carbon deposits. Several studies have shown the efficiency and potential of MgO as a support material [33,34,35]. Ho et al. examined the CO2 adsorption capability of MgO at various temperatures from 303 to 623 K [36]. As expected, with increasing temperatures, the CO2 uptake capacity decreased. Takezawa et al. studied 13% Ni/MgO catalysts for CO2 methanation. The catalysts were prepared by impregnation and calcination at temperatures of 673−973 K. The study revealed that with increasing calcination temperatures, the activity and selectivity of the Ni/MgO catalysts decreased. A methane selectivity of 98% was presented for catalyst calcination at 773 K followed by reduction at 873 K, a reaction temperature of 480 K, a pressure of 1 atm, and a feed gas flow rate of 100 mL min−1 (CO2:H2 = 5:95) [37]. Varun et al. prepared NiO/MgO nanocomposite catalysts for CO2 hydrogenation via sonochemical treatment and achieved a CO2 conversion of 85%, with 98% selectivity toward methane at 673 K [38]. Baldauf-Sommerbauer et al. investigated two catalysts with 11 and 17 wt% Ni on MgO. The CO2 conversion and methane selectivity approached the thermodynamic equilibrium at a moderate reaction temperature of 598 K and a feed composition of H2:CO2:N2 = 4:1:5 at a feed gas flow rate of 250 mLSTP min−1 [39]. Loder et al. investigated the effect of the Ni loading (0−27 wt%) on MgO and the MgO quality on the rate of CO2 methanation in a temperature range of 533–648 K. They reported CO2 conversions of 87% and a methane selectivity of ≥99% [9]. In a current review, the role of carbonate formation during CO2 hydrogenation over MgO-supported catalysts was discussed, explicitly stating the beneficial effect of bifunctional Ni/MgO catalysts toward methane synthesis [40]. In the case of Ni/MgO catalysts, nickel provides the adsorbent capacity for hydrogen and is highly selective for methane, whereas the basic support material, MgO, activates CO2 through chemisorption, giving access to a highly active bifunctional catalyst.
In addition, Pandey et al. showed that adsorbed carbonate species on iron oxide sites on Ni-Fe catalysts serve as additional factors that enhance the efficiency of catalysts for CO2 hydrogenation. The presence of a Ni-Fe alloy and a number of metal sites were reported to enhance CO2 conversion and methane yield [41]. In general, iron-based catalysts were suggested as a cost-effective alternative for methane synthesis from CO2, providing long-term stability when appropriately combined and modified with promoters [42]. Sehested et al. confirmed that a Ni-Fe alloy catalyst gave higher CO2 conversion and CH4 selectivity compared with a pure nickel catalyst at 603 K with excess hydrogen [43]. Mutz et al. studied the potential of Ni3Fe on γ-Al2O3 as a methanation catalyst in a microchannel packed bed reactor. With a 17% Ni3Fe catalyst, a CO2 conversion of 71% and a selectivity toward methane > 98% were achieved at 631 K and 6 bar in long-term experiments (45 h). Thus, the Ni3Fe catalyst showed outstanding performance and stability at mid-temperatures when combining Ni and Fe [44]. In addition, Serrer et al. investigated bimetallic Ni3.2Fe/Al2O3 catalysts by using an advanced combination of operando XAS and XRD with quantitative on-line product analysis. The results showed that Fe addition to Ni/Al2O3 catalysts protected active Ni0 species from oxidation and preserved the catalytic activity under dynamic reaction conditions [45]. Mebrahtu et al. studied Ni-Fe bimetallic catalysts on a (Mg,Al)Ox support, which were synthesized by co-precipitation. Ni-Fe alloy nanoparticles were favorable for the methanation of CO2. The activity and selectivity were remarkably affected by iron, attributable to its small particle size, facilitated CO dissociation, and tailored surface basicity. With the best catalyst (Fe/Ni = 0.1), the CO2 conversion rate was 6.96 mmol CO2 molFe+Ni−1 s−1 at 608 K, with a consistent selectivity of 99.3% toward CH4 over 24 h on stream [46]. In contrast, Wang et al. used calcined olivine ((Mg, Fe)2SiO4) as support for Ni catalysts that were prepared by the incipient wetness method, and applied them for CO2 methanation. The results showed that a FeOx phase was formed on the surface of the calcined olivine and that the unreduced FeOx between the active Ni-Fe alloy phase and the olivine support played a crucial role in CO2 methanation. A 98% CO2 conversion and a selectivity of 99% toward CH4 were achieved at a temperature of 673 K, a H2/CO2 molar ratio of 6, and an hourly space velocity of 11,000 h−1 [47].
Table 1 gives a list of experimental studies with Ni- and Fe-based catalysts on different support materials for CO2 methanation.

Table 1.
Experimental studies with Ni- and Fe-based catalysts on different support materials for CO2 methanation.
In several studies, iron-bearing materials, for instance, iron-bearing minerals such as siderite ore (FeCO3), were used for catalyst preparation for a variety of applications and processes [56,57,58,59]. For instance, Hadjltaief et al. studied two natural samples, natural Tunisian hematite and siderite, as catalysts for the photocatalytic degradation of 4-chlorophenol (4-CP) in aqueous solution. Siderite exhibited higher photocatalytic oxidation activity than hematite at pH 3. Use of the siderite catalyst gave 100% conversion of 4-CP and 54% TOC removal. In terms of the removal of several organic compounds in an aqueous condition, the work confirmed that natural materials can be used as catalysts [60]. Wei et al. used siderite (doped with Mn and Ce) for the selective catalytic reduction (SCR) of NOx by NH3. The siderite catalysts showed high efficiency for the removal of NOx (NOx conversions were higher than 90% at T = 513–573 K and Tcalcined = 723 K). A 3% Mn/1% Ce-siderite catalyst also showed high resistance against sulfur poisoning (the NOx conversion remained above 75% after introducing 0.01% SO2 in the feed for 7.5 h) [61]. Furthermore, Görmez et al. studied the use of rhombohedral FeCO3 that was synthesized hydrothermally as a catalyst in the electro-fenton oxidation of p-benzoquinone. 95% of the total organic carbon was removed at 400 mA current. Increasing catalyst dosage had a beneficial effect on the mineralization of p-benzoquinone [62].
These studies show the beneficial catalytic effect of iron in various aspects and highlight its use by exploiting natural iron resources as abundant catalysts or catalyst support materials. Siderite ore, for instance, is an important source for iron and steel production in Austria [63] and China [64]. In general, siderite ore is converted to blast furnace-grade hematite through roasting in air in the sinter plant (Equation (4)).
FeCO3 + 0.25 O2 ⇌ 0.5 Fe2O3 + CO2
In the context of decarbonizing iron and steel production, a novel direct reduction process (Equation (5)) for siderite ore with hydrogen was developed, which can be combined with subsequent catalytic CO2 hydrogenation [65,66,67].
FeCO3 + H2 ⇌ Fe + H2O + CO2
It was shown that during the direct reduction process, not only is CO2 released from the carbonaceous ore, but also CO and methane are formed [65]. Moreover, Bock et al. suggested the application of this inexpensive and abundant natural siderite ore for energy storage with combined hydrogen and heat release [68].
The above-stated publications clearly show the beneficial effect of iron during CO2 methanation and the potential of using natural iron-bearing minerals with regard to catalysis. Methane formation during the direct reduction of siderite ore suggests that this iron ore has a certain catalytic effect on CO2 methanation. However, its potential as a catalyst for CO2 methanation has not been evaluated yet. This raises the question of whether siderite ore can be considered a catalyst or catalyst support material for Ni-based catalysts for CO2 methanation. For this purpose, Ni-based catalysts on MgO as a support material may act as benchmark catalysts in this study. The advantageous effect of MgO as a support material for nickel catalysts in CO2 methanation has already been described. This in turn raises the question of whether the catalytic performance of Ni/MgO catalysts can be increased by adding siderite ore, which could create synergies and provide iron species acting as catalyst promoters.
Thus, the aim of this study was to investigate the potential of abundantly available siderite ore as a cheap raw material source for catalyst production for CO2 methanation. Both its sole catalytic effect and a possible synergistic effect with MgO as a support material for Ni-based catalysts were considered. For this purpose, in a hydrogen atmosphere reduced siderite ore with and without nickel doping was used. Furthermore, the interplay of mixed reduced siderite ore/MgO support materials was examined, possibly opening a path to abundant, inexpensive bifunctional catalysts for CO2 methanation.
2. Results and Discussion
The catalytic performance of hydrogen-reduced siderite ore for CO2 hydrogenation to methane was investigated. The use of unreduced siderite ore is not reasonable, as otherwise CO2 would constantly be released from the ore during the hydrogenation reaction, which would change the catalyst composition and its properties during the process.
In addition, the catalytic effect of Ni-based catalysts on support materials of MgO and siderite ore reduced with hydrogen prior to their application was evaluated. The process conditions were chosen based on the work of Sommerbauer et al. [39] and Loder et al. [9]. The molar feed gas ratio of CO2:H2 was 4:1. Inert nitrogen was added to the feed gas stream for balancing purposes (H2:CO2:N2 = 56:14:30). From the literature it can be deduced that the availability of adsorbed hydrogen is a limiting factor for the rate of reaction of CO2 methanation. Loder et al. investigated this effect and varied the H2:CO2 ratio in the feed gas from 3:1 to 5:1 [9]. As expected, the CO2 conversion rose with rising hydrogen concentrations in the feed gas stream, yielding a maximum CO2 conversion of 98% (equilibrium conversion: 99.8%) for the H2:CO2 ratio of 5:1. However, as most of the experiments in the study of Loder et al. were performed with a stoichiometric feed gas ratio of CO2:H2 = 4:1, for reasons of comparability, this ratio was also chosen in this study.
The performance of the different catalysts for CO2 methanation was investigated at temperatures of 548 K, 598 K, and 648 K, and feed gas flow rates of 8.02, 11.32, and 14.66 m3 kg−1 h−1 (STP), referring to the flow rate of the feed gas stream per mass of catalyst.
To provide a baseline reference, the catalytic effect of the support material MgO was also tested and compared to the undoped, reduced siderite ore. Then, both—in hydrogen-reduced siderite ore and MgO—were used as support materials and were doped with Ni in various amounts (Ni loading from 22 to 31 wt%).
2.1. Catalytic Effect of Reduced Siderite Ore and MgO
As a baseline reference, the catalytic effect of hydrogen-reduced siderite ore was studied and compared to the catalytic performance of undoped MgO for CO2 hydrogenation at 548 K, 598 K, and 648 K, respectively.
For the reduced siderite ore, siderite ore was reduced in a hydrogen atmosphere at two different reduction temperatures (Tred): 773 K and 973 K, respectively. The reduced ore was then removed from the reactor and kept at atmospheric conditions so that it was partially reoxidized and reached a stable state at atmospheric conditions. After that, the reduced ore was filled back into the reactor to perform the CO2 hydrogenation experiments. The effect of the reduction temperature during siderite ore reduction was then evaluated regarding the characteristics of the support material during CO2 hydrogenation.
For the undoped MgO catalyst, undoped MgCO3 was prepared as described in Section 3.1, with the MgCO3 being calcined in a muffle furnace with air at 723 K for 2 h and at 823 K for a further 5 h.
The CO2 hydrogenation was carried out with the help of the undoped materials at different feed gas flow rates (8.02, 11.32, and 14.66 m3 kg−1 h−1) and a constant feed gas ratio of H2:CO2:N2 = 56:14:30, and at reaction temperatures of 548 K, 598 K, and 648 K, respectively. The reaction temperatures were chosen because temperatures below 700 K are known to promote CO2 methanation over CO formation from CO2 [9].
2.1.1. Reduced Siderite Ore as a Catalyst
First, the catalytic effect of reduced siderite ore was proven for methane formation from CO2. As depicted in Figure 1, the reduction temperature of siderite ore influences its performance during CO2 hydrogenation, both regarding CO2 conversion (a) and selectivity toward CH4 (b). When the CO2 hydrogenation was carried out at temperatures of 548 K and 598 K, the CO2 conversion was below 5%, and the two siderite ore samples, that were reduced at different temperatures, showed no clear difference regarding their catalytic effect. At a reaction temperature of 648 K, the siderite ore sample that was reduced at Tred = 973 K showed a pronounced catalytic effect, yielding CO2 conversions of 12−15%. At all reaction temperatures, the siderite ore samples that were reduced at Tred = 973 K gave higher CH4 selectivities: 19.0% at 548 K and 5% at 598 K and 648 K, respectively. Compared with siderite ore reduction at Tred = 773 K, the higher reduction temperature of Tred = 973 K enhanced both the CO2 conversion and the selectivity toward CH4. At Tred = 973 K, at the lowest feed gas flow rate (8.02 m3 kg−1 h−1), which meant the highest residence time in the reactor, the highest CO2 conversion (19.9%) was obtained, while the highest CH4 selectivity (19.0%) was obtained at the higher feed gas flow rates (11.32–14.66 m3 kg−1 h−1). Since the effect of increasing methane selectivity with increasing feed gas flow rate and thus lower residence time was only observed at the lowest reaction temperature of 548 K and only the methane selectivity at a feed gas flow rate of 8.02 m3 kg−1 h−1 differed from the one at 11.32 and 14.66 m3 kg−1 h−1, respectively, it is assumed that this value should rather be regarded as an outlier.
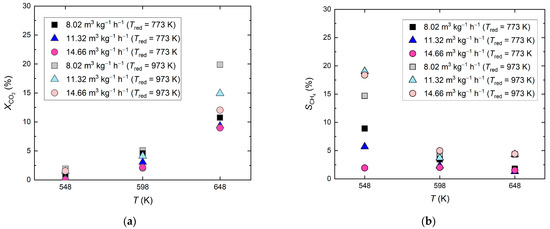
Figure 1.
Catalytic effect of reduced siderite ore during CO2 hydrogenation; reduction of siderite ore in hydrogen atmosphere at Tred = 773 K and 973 K; molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02–14.66 m3 kg−1 h−1 (STP), reaction temperatures 548–648 K; (a) CO2 conversion and (b) CH4 selectivity.
As the reaction temperature increased, the CH4 selectivity decreased. CO was formed instead, showing that the reverse water-gas shift reaction was the dominant reaction. This is consistent with the findings of Lux et al., who investigated the direct reduction process of siderite ore with hydrogen, called reductive calcination in the study, with the aim of optimizing the process parameters to maximize the methane yield. Since it can be assumed that CO2 is released from iron carbonate during direct reduction with hydrogen in a first step, and is further reduced to methane in a subsequent catalytic step, the results are directly transferable. As expected, in their study, it was proven that methane formation is favored at low temperatures and increased pressure, whereas the formation of CO is favored at high temperatures and low pressure [67].
The direct reduction of siderite ore with hydrogen has been extensively investigated for iron production. Loder et al., for instance, investigated the reaction kinetics and gave a detailed report on the degree of metallization (=mass of elemental iron per total mass of iron in the reduced ore) for different reduction temperatures under atmospheric conditions [66]. The direct reduction experiments were carried out with the same original siderite ore from the Styrian Erzberg, with a feed gas ratio of H2:CO2:N2 = 56:14:30 and a feed gas flow rate of 0.05 m3 h−1 (STP) (=0.0083 m3 kg−1 min−1 (STP)) at ambient pressure. The effect of the reduction temperature was investigated in a temperature range of 773−1023 K. After the reduction experiments, the reduced ore was removed from the reactor under inert nitrogen conditions and analyzed for its degree of metallization. At a reduction temperature of 773 K, the degree of metallization of the reduced ore was 40%. At a reduction temperature of 973 K, a degree of metallization of 89% was obtained.
As the handling of catalysts under ambient conditions in air is easier and the impregnation of the reduced ore requires handling under ambient conditions and exposure to the solution anyway, the behavior of the reduced siderite ore when exposed to air was evaluated in this study. For this purpose, the same direct reduction experiments were carried out, but the removal of the reduced ore from the reactor was done under ambient conditions in air as compared to emptying the reactor and keeping the reduced siderite ore under an inert nitrogen atmosphere. For a reduction temperature of 973 K, this resulted in a partial reoxidation of the reduced iron species and thus a reduced degree of metallization of 58.2%, as compared with 89% under inert nitrogen conditions. The remaining iron fraction was split up into 32.6% Fe2+ and 9.1% Fe3+ for the partially reoxidized reduced siderite ore sample. This means that the majority of iron was present as metallic iron Fe0 (58.2%) together with smaller fractions of Fe2+ (32.6%) and Fe3+ (9.1%) when siderite ore was reduced in a hydrogen atmosphere at 973 K and finally kept under ambient conditions in air. The determination of the chemical composition of the reduced (and partially oxidized) siderite ore samples is described in Section 3.4.3.
To conclude, two different reduction temperatures (Tred = 773 and 973 K) were studied for the preparation of the reduced siderite ore via direct reduction with hydrogen in a tubular reactor under ambient pressure. The reduction temperature was chosen according to suggestions in the literature for direct reduction of siderite ore [65,66]. Via this procedure, CO2 was released from the ore, and the carbonaceous iron ore was reduced to elemental iron with minor remaining fractions of Fe2+ and Fe3+. When exposed to air under ambient conditions, the reduced siderite ore was partially oxidized, and for the siderite ore reduced at 973 K, more than half of the iron was still present as metallic iron Fe0 (58.2%), together with smaller fractions of Fe2+ (32.6%) and Fe3+ (9.1%). The degree of metallization strongly depends on the reduction temperature. Furthermore, reduction at higher temperatures results in a reduced iron ore that is more chemically stable against reoxidation. When reduced siderite ore was used as the sole catalyst for CO2 hydrogenation/methanation, higher CO2 conversions were obtained with the siderite ore that was reduced at the higher reduction temperature of 973 K. This may be attributed to the higher fraction of metallic iron in the reduced siderite ore. However, CO2 conversions were comparably low, as the maximum CO2 conversion was around 20%, and the selectivity toward methane even remained below 20%.
2.1.2. Undoped MgO
Second, undoped MgO was used for CO2 hydrogenation in the same reaction conditions as with the reduced siderite ore. In this case, CO2 was hardly converted. CO2 conversions were below 0.7%, as depicted in Figure 2. Methane was not detected in the product gas.
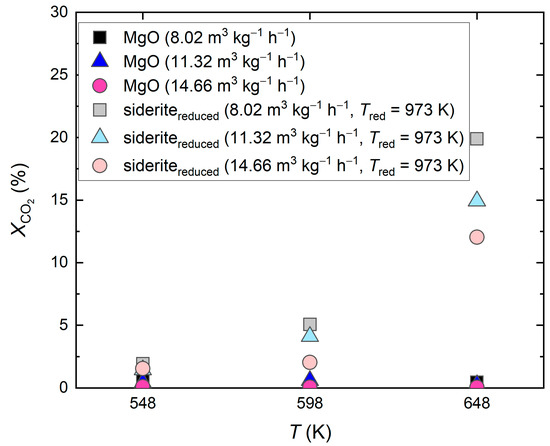
Figure 2.
Catalytic effect of undoped MgO and reduced siderite ore (sideritereduced) during CO2 hydrogenation; reduction of siderite ore in hydrogen atmosphere at Tred = 973 K; molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02–14.66 m3 kg−1 h−1 (STP), reaction temperatures 548–648 K.
The catalytic effect of MgO during CO2 hydrogenation was already reported by Loder et al. [9], who postulated that MgO shows minor catalytic activity for the reverse water-gas shift reaction but does not promote methane formation [9]. This was confirmed by the findings in this work.
2.2. Ni-Based Catalysts on Reduced Siderite Ore as Support Material
Next, siderite ore reduced in a hydrogen atmosphere was studied as a support material for Ni-based catalysts (Figure 3). As undoped siderite ore that was reduced at Tred = 973 K had shown a pronounced catalytic effect during CO2 hydrogenation as compared with the siderite ore that was reduced at Tred = 773 K, a reduction temperature of Tred of 973 K was chosen for siderite ore reduction in hydrogen prior to loading with nickel.


Figure 3.
Catalytic performance of Ni-based catalytsts on reduced siderite ore as support material during CO2 hydrogenation; molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02–14.66 m3 kg−1 h−1 (STP), reaction temperature 548 K, 598 K, and 648 K; (a) 22 wt% Ni/sideritereduced, (b) 24 wt% Ni/sideritereduced, (c) 25 wt% Ni/sideritereduced, and (d) 27 wt% Ni/sideritereduced.
The effect of Ni loading was investigated at different reaction temperatures and feed gas flow rates at ambient pressure. Ni loading was varied from 22 wt% Ni (Figure 3a) to 24 wt% Ni (Figure 3b), 25 wt% Ni (Figure 3c), and 27 wt% Ni (Figure 3d) for feed gas flow rates of 8.02, 11.32, and 14.66 m3 kg−1 h−1, and reaction temperatures of 548 K, 598 K, and 648 K.
For all Ni loadings of the Ni/sideritereduced catalysts, the CO2 conversion increased with increasing reaction temperature and decreasing feed gas flow rate (increasing residence time). The highest CO2 conversion was 23.8% and was obtained with the 27 wt% Ni/sideritereduced catalyst at a reaction temperature of 648 K. The effect of Ni loading on the CO2 conversion and the CH4 selectivity at a constant feed gas flow rate of 8.02 m3 kg−1 h−1 is depicted in Figure 4. Both the CO2 conversion and the CH4 selectivity increased with increasing Ni loading. At reaction temperatures of 548 K and 598 K, the CO2 conversion increased significantly with increasing Ni loading from 22 wt%/24 wt% to 25 wt%, but only slightly increased further for a Ni loading of 27 wt%. At a reaction temperature of 648 K, only a minor effect of Ni loading on the CO2 conversion was visible. As opposed to the findings with undoped support material, with increasing reaction temperatures, the selectivity toward CH4 increased.
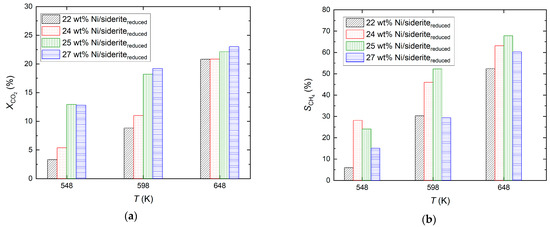
Figure 4.
Effect of Ni loading of Ni/sideritereduced catalysts on CO2 conversion (a) and selectivity toward CH4 (b) during CO2 hydrogenation; molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02 m3 kg−1 h−1 (STP), reaction temperatures 548 K, 598 K, and 648 K.
The catalytic activity of the 27 wt% Ni/sideritereduced catalyst adopts an exceptional position in the range of Ni/sideritereduced catalysts. Its catalytic activity in terms of CO2 conversion is only marginally higher than that of the 25 wt% Ni/sideritereduced catalyst. At all three reaction temperatures, however, it shows a significantly reduced selectivity for methane. Furthermore, the 27 wt% Ni/sideritereduced catalyst shows poor catalytic activity when the feed gas flow rate is high and, thus, the residence time is low. This suggests that in this range, a maximum Ni load is present, which is advantageous in terms of methane selectivity. Furthermore, it must be noted that the production of catalysts with such a high Ni loading on reduced siderite ore is more difficult to reproduce, which could also be reflected in the experimental results. However, at low feed gas flow rates (Figure 4), the catalyst with the highest Ni loading (27 wt% Ni/sideritereduced) gave the highest CO2 conversion (23.0%) and high CH4 selectivity (60.2%). Thus, a high Ni loading (26–28 wt% Ni) was chosen in the subsequent study to investigate the catalytic performance of Ni-based catalysts on mixed reduced siderite ore/MgO support material.
2.3. Mixed Reduced Siderite Ore/Magnesium Oxide as Support Material for Ni-Based Catalysts
In previous studies, MgO has already been proven to be an effective support material for Ni-based catalysts, giving access to bifunctional Ni/MgO catalysts [9,39]. In this study, a 31 wt% Ni/MgO catalyst was prepared and used as a benchmark catalyst.
In order to test the catalytic performance of mixed MgO and reduced siderite ore as support materials for Ni-based catalysts, various ratios of reduced siderite ore and MgO were tested at constant Ni loading of 28 wt%; sideritereduced/MgO (w/w) = 30:70, 50:50, and 70:30, respectively.
As depicted in Figure 5, Ni/sideritereduced/MgO catalysts efficiently catalyze CO2 hydrogenation. As expected, for all three catalysts—sideritereduced/MgO = 30:70, 50:50, 70:30—the CO2 conversion increases with increasing reaction temperature and decreasing feed gas flow rate. The highest CO2 conversions were obtained with the catalysts with a higher fraction of reduced siderite (sideritereduced/MgO = 50:50, 70:30), with CO2 conversions of 74.2−78.0%, 67.0–69.95%, and 63.5−65.8% at a reaction temperature of 648 K and feed gas flow rates of 8.02, 11.32, and 14.66 m3 kg−1 h−1, respectively.
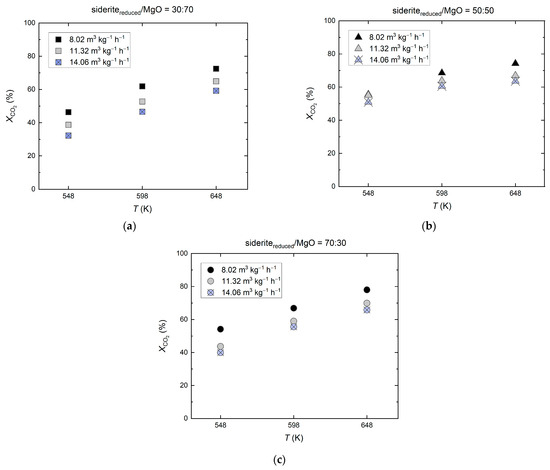
Figure 5.
Catalytic performance of Ni-based catalysts (28 wt% Ni) on mixed sideritereduced/MgO support material in various compositions for CO2 hydrogenation; molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02−14.66 m3 kg−1 h−1 (STP), reaction temperatures 548–648 K, reduction of siderite ore in hydrogen at Tred = 973 K; (a) sideritereduced/MgO = 30:70, (b) sideritereduced/MgO = 50:50, and (c) sideritereduced/MgO = 30:70.
Figure 6 depicts the catalytic performance of the 28 wt% Ni/sideritereduced/MgO catalysts compared with Ni-based catalysts on MgO (31 wt% Ni/MgO) or reduced siderite ore (28 wt% Ni/sideritereduced) only. It is evident that the Ni/sideritereduced catalyst performed the worst. When MgO was added to the support material, there was a clear improvement in catalytic performance. At lower reaction temperatures (548 K), the catalytic performance seemed to be worse with an excess of MgO (sideritereduced/MgO = 30:70) in the support material compared with the catalysts with lower MgO fractions (sideritereduced/MgO = 50:50, 70:30). However, the difference was small and seemed to be canceled out at higher temperatures (648 K). No difference was visible for the Ni/sideritereduced/MgO catalysts with reduced siderite ore to MgO mass ratios of 50:50 and 70:30. CH4 selectivities were >95% with all Ni/sideritereduced/MgO catalysts.
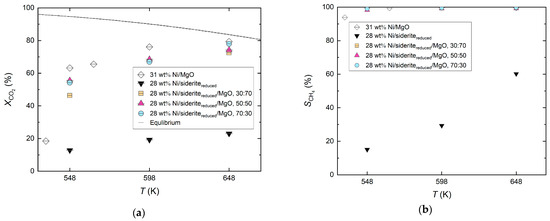
Figure 6.
Comparison of the catalytic performance of Ni-based catalysts on reduced siderite ore, MgO, and mixed reduced siderite ore/MgO support material (27–30 wt% Ni) for various reaction temperatures (548–648 K); molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rate 8.02 m3 kg−1 h−1 (STP), reduction of siderite ore in hydrogen at Tred = 973 K; (a) CO2 conversion, and (b) CH4 selectivity.
In this series, the Ni/MgO catalyst turned out to be the best catalyst. However, it must be noted that this catalyst had a higher Ni loading of 31 wt% as compared with the 28 wt% of the Ni/sideritereduced/MgO catalysts. This may be dedicated to the production of the Ni-doped catalysts. The doping of MgO according to the procedure described in Section 3.1 is easier than the doping of (mixed) reduced siderite ore. As a result, a higher loading was achieved, which could not be achieved at all with the mixed catalysts.
To conclude, the study revealed that reduced siderite ore acts as an efficient support material for a Ni-based catalyst for CO2 methanation when combined with MgO. As expected, the CO2 conversion increased with increasing Ni loading of the respective catalyst, both for Ni/sideritereduced and Ni/sideritereduced/MgO catalysts, as well as with increasing temperature from 548 K to 648 K, and decreasing feed gas flow rate. When reduced siderite ore was used as sole support material (Ni/sideritereduced catalysts), the catalytic performance (XCO2, 648 K = 23.0% for 27 wt% Ni/sideritereduced) was only marginally higher than the catalytic performance of undoped reduced siderite ore (XCO2, 648 K = 19.9%). This leads to the conclusion that both the iron species in and the nickel on the reduced siderite ore show a comparable catalytic effect toward CO2 hydrogenation. However, the selectivity toward CH4 is significantly higher when the reduced siderite ore is doped with Ni, specifically, 60.2% compared with 19.0% for undoped reduced siderite ore. This shows the high catalytic activity of the nickel species for CH4 formation.
With XRD, a NiFe23+O4 (trevorite) peak was identified in all Ni/sideritereduced catalysts, as well as the Ni-Fe alloy and FeNi (tetrataenite) in the catalysts after CO2 hydrogenation (see Section 2.4.2).
However, adding MgO to the support material of the Ni-based catalysts significantly enhanced CO2 methanation. The ratio of reduced siderite ore and MgO seemed to play a subordinate role, with higher proportions of reduced siderite ore causing slightly higher CO2 conversions. With ≥50% reduced siderite ore in the mixed sideritereduced/MgO support material, no difference in the catalytic performance was visible anymore. Most importantly, adding MgO to the reduced siderite ore support drastically enhanced the selectivity toward CH4. Even if only 30% of MgO was present, the selectivity toward methane approached 100%. It can be concluded that with identical Ni loading, Ni/sideritereduced/MgO (≥30% MgO) and Ni/MgO catalysts show comparable catalytic performance, with CO2 conversions close to the thermodynamic equilibrium and high CH4 selectivity ≥ 99.9%. This supports the findings of Loder et al., highlighting the fundamental role of MgO as a basic support material for Ni-based catalysts for CO2 methanation [9].
2.4. Catalyst Characterization
2.4.1. X-ray Fluorescence Spectrometry
The freshly prepared 24 wt% Ni/sideritereduced catalyst (after calcination, before reduction of NiO to Ni with hydrogen) was analyzed by X-ray fluorescence (XRF) spectrometry for its Ni loading. The Ni loading was compared with the value obtained by AAS analysis. The XRF result showed that NiO was present, and the percentage of Ni (24.81%) was in good agreement with the AAS analysis (24.1%). The other constituents were present at 55.0 wt% Fe2O3, 7.45 wt% SiO2, 2.55 wt% MgO, 1.79 wt% MnO, and 2.31 wt% CaO. This shows that during the wet preparation/impregnation procedure, elemental iron and Fe2+ were oxidized to Fe3+.
2.4.2. X-ray Diffraction
Fresh and used Ni/sideritereduced catalysts were analyzed by X-ray diffraction (XRD). The Ni-based catalysts on reduced siderite ore support material that were analyzed were (i) 22 wt% Ni/sideritereduced (fresh catalyst), (ii) 22 wt% Ni/sideritereduced (used catalyst), (iii) 24 wt% Ni/sideritereduced (fresh catalyst), (iv) 24 wt% Ni/sideritereduced (used catalyst), (v) 25 wt% Ni/sideritereduced (fresh catalyst), and (vi) 25 wt% Ni/sideritereduced (used catalyst), as shown in Figure 7.
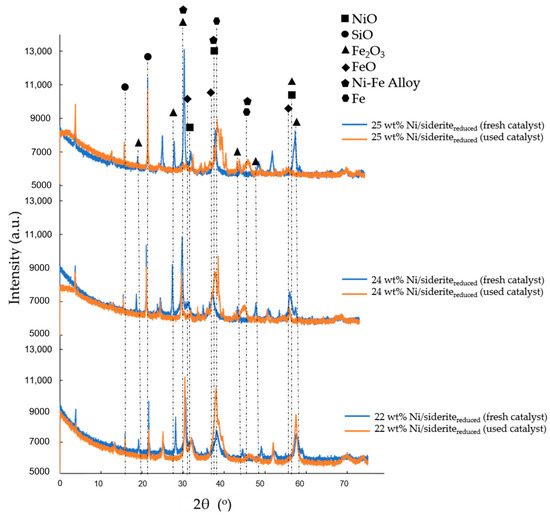
Figure 7.
X-ray diffraction results of the fresh and used Ni/sideritereduced catalysts; 22 wt% Ni/sideritereduced, 24 wt% Ni/sideritereduced, and 25 wt% Ni/sideritereduced.
In the fresh Ni/sideritereduced catalysts with 22 wt%, 24 wt%, and 25 wt% Ni loading, the diffraction peaks of NiO (bunsenite), NiFe23+O4 (trevorite), Fe2O3 (hematite), KAl2(Si3Al)O10(OH2) (muscovite), SiO2 (quartz), and FeO (wüstite) were identified. When used for CO2 hydrogenation, the catalysts changed phases; NiFe23+O4 (trevorite) and Fe2O3 (hematite) did not appear anymore; nonetheless, Fe2+(Fe3+)2O4 (magnetite), Ni-Fe alloy, and FeNi (tetrataenite) were found in all of the used catalysts (after CO2 hydrogenation with a molar feed gas ratio H2:CO2:N2 = 56:14:30, feed gas flow rates of 8.02–14.66 m3 kg−1 h−1 (STP), and reaction temperatures of 548 K, 598 K, and 648 K). The result showed that the Ni-Fe alloys were small crystallites with increasing % of Ni. In addition, another iron alloy was found in the 24 wt% Ni/sideritereduced and 25% wt% Ni/sideritereduced catalysts; (Fe,Ni,Co)3C (cohenite) was present in the catalysts with increased Ni loading. However, the presence of significant quantities of cobalt can be excluded.
2.4.3. Scanning Electron Microscopy (SEM) Analysis
SEM images were taken by Field Emission Scanning Electron Microscopy from the catalyst samples: 21.69 wt% Ni/sideritereduced (Figure 8a), 23.77 wt% Ni/sideritereduced (Figure 8b), 24.80 wt% Ni/sideritereduced (Figure 8c), and 27.71 wt% Ni/sideritereduced/MgO (30:70) (Figure 9a), 28.01 wt% Ni/sideritereduced/MgO (Figure 9b) (50:50), and (c) 28.18 wt% Ni/sideritereduced/MgO (Figure 9c) (70:30).

Figure 8.
Scanning Electron Microscopy (SEM) analysis of different Ni loadings on the reduced siderite ore support material: (a) 21.69 wt% Ni/sideritereduced, (b) 23.77 wt% Ni/sideritereduced, and (c) 24.80 wt% Ni/sideritereduced; fresh catalysts.
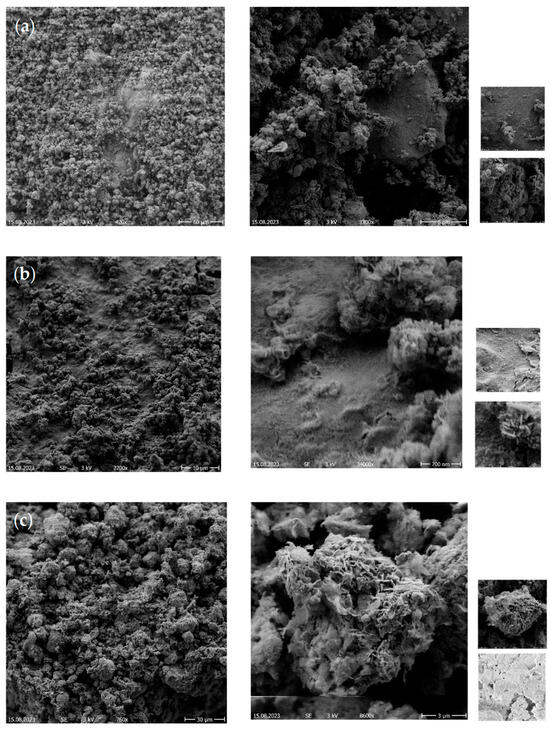
Figure 9.
Scanning Electron Microscopy (SEM) analysis of different proportions of reduced siderite ore and MgO as support material for Ni-based catalysts: (a) 27.71 wt% Ni/sideritereduced/MgO (30:70), (b) 28.01 wt% Ni/sideritereduced/MgO, and (c) 28.18 wt% Ni/sideritereduced/MgO.
The SEM images in Figure 8 show that the Ni/sideritereduced catalysts became slick and homogeneous when the Ni loading increased. For all Ni loadings, the particles had a clear distribution of NiO and FeO on the surface. At 21.69 wt% Ni loading, the catalyst had a more porous structure when compared with 23.77 wt% and 24.80 wt% Ni loading on reduced siderite ore. Thus, it can be said that surface morphology changed with increasing Ni loading.
Furthermore, adding MgO to the reduced siderite ore support (sideritereduced:MgO = 30:70, 50:50, 70:30) resulted in more irregularities when increasing the MgO ratio (Figure 9). At sideritereduced:MgO ratios of 30:70 and 50:50, the surface had a flat and homogeneous structure, while the catalyst with the sideritereduced:MgO = 70:30 support had irregular particles on the surface.
3. Materials and Methods
3.1. Materials and Catalyst Preparation
Ni-based catalysts on two different support materials and combinations of the support materials were tested: (i) Ni/MgO, (ii) Ni/sideritereduced, and (iii) Ni/sideritereduced/MgO. The catalysts were prepared by wet impregnation with nickel nitrate hexahydrate (Ni(NO3)2·6 H2O, 99%, p.a., Lactan). The MgO support was prepared from MagGran© (4MgCO3·Mg(OH)2·4H2O), while the siderite ore originated from the Styrian Erzberg, Austria, and was provided by VA Erzberg GmbH, Eisenerz, Austria (particle size 0.5−1 mm). Its mineral composition is given in Table 2.

Table 2.
Chemical composition of the original siderite ore determined by XRF spectroscopy [65].
This siderite ore consists of the iron-bearing minerals siderite ((Fe0.83Mg0.11 Mn0.05Ca0.01)CO3) with substitutions of magnesium carbonate (MgCO3), calcium carbonate (CaCO3) and manganese carbonate (MnCO3), and ankerite ((Ca0.51Fe0.31Mg0.15Mn0.03)CO3). Furthermore, dolomite ((Ca,Mg)(CO3)2), calcite (CaCO3), quartz (SiO2), and muscovite (KAl2(Al-Si3O10)(OH)2) are present in this carbonaceous ore (Table 3).

Table 3.
Composition of the original siderite ore from the Austrian Erzberg (particle size 0.5−1 mm) [65].
The preparation of the catalysts was based on the work of Loder et al. [9] and extended for reduced siderite ore as further catalyst and catalytic support material. There are four main preparation steps:
- (i)
- calcination of magnesium carbonate for magnesium oxide preparation (Equation (6)) and/or reduction of siderite ore (Equation (7)) [69,70],4 MgCO3∙Mg(OH)2∙4 H2O → 5 MgO + 4 CO2 + 5 H2OFeCO3 + (x + y + 4z) H2 → FeO1−x + (1 − y − z)CO2 + yCO + zCH4 + (x + y + 2z)H2O
- (ii)
- impregnation of the support material with nickel nitrate,
- (iii)
- thermal decomposition of nickel nitrate to nickel oxide (Equations (8)–(10)) [71], andNi(NO3)2∙6 H2O ⇌ NiO +2 NO2 + 0.5 O2 + 6 H2OMgO + Ni(NO3)2 → (Mg1−xNix)(OH)2(Mg1−xNix)(OH)2 → Mg1−xNixO + H2O
- (iv)
- reduction of Ni with hydrogen (Equations (11) and (12)) [39,71].Mg1−xNixO2 + H2 → [Ni]x Mg1−xO] + H2ONiO + H2 → Ni + H2O
The experimental procedure was as follows:
- (i)
- Preparation of the support material:
For magnesium oxide preparation, magnesium carbonate powder (4 MgCO3·Mg(OH)2·4H2O) was calcined in a muffle furnace (Heraeus M 110) with air at 723 K for 2 h and at 823 K for 5 h.
For preparation of the reduced siderite ore support (for details see Section 3.2), siderite ore was reduced in the tubular reactor used for the methanation experiments, in 90% hydrogen (feed gas ratio of H2:N2 = 9:1, at a feed gas flow rate of 0.048 m3 h−1), and at 773 K and 973 K until the exit gas composition equaled the feed gas composition. The reduced siderite ore was then exposed to air at room temperature, where it partially oxidized.
- (ii)
- Impregnation:
The nickel nitrate solution was prepared from Ni(NO3)2·6 H2O that was mixed with ultrapure water at a nickel concentration of 53−60 g dm−3 in a flask. After that, the 70 cm3 nickel solution in the continuously stirred flask was cooled in a water bath (T = 293−298 K). 10 g of calcined MgO and/or reduced (and partially oxidized) siderite ore was added to the nickel solution (adding 1 g per 3 min). The mixed solution (slurry phase) was constantly stirred for 2 h and filtrated with the help of a vacuum pump (separated slurry phase and residual water phase). The filtrated slurry (green) phase was dried overnight at room temperature.
- (iii)
- Thermal deposition:
The freshly prepared and pre-dried catalysts were then dried in a muffle furnace (Heraeus M 110) at 393 K for 2 h and at 673 K for 5 h in air. After the drying process, the catalysts had a light gray color.
- (iv)
- Reduction with hydrogen/activation:
This step was required for the reduction of NiO to Ni. A total of 4 g of the catalyst powder was reduced with hydrogen (feed gas ratio of H2:N2 = 9:1, feed gas flow rate of 0.048 m3 h−1) in the tubular reactor at 773 or 973 K (temperature measurement at T3 thermocouple position, as described in Section 3.4.1, for 4 h.
Then, the catalysts were kept in the tubular reactor and used for the CO2 methanation experiments.
3.2. Siderite Ore Reduction
The original siderite ore was reduced in a hydrogen atmosphere at two different reduction temperatures, 773 K and 973 K, at ambient pressure and a feed gas ratio of H2:N2 = 90:10. This process step is known as the direct reduction of siderite ore with hydrogen. In the literature, it is suggested for the production of elemental iron from siderite ore in a single process step [65]. The course of the direct reduction process is shown in Figure 10 via the product gas composition at the reactor exit and the reduction temperature at thermocouple position T3.
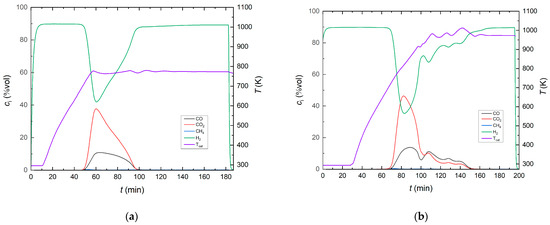
Figure 10.
Product gas composition during direct reduction of siderite ore with hydrogen (feed gas ratio H2:N2 = 90:10, feed gas flow rate 0.048 m3 h−1) for preparation of the support material; (a) Tred = 773 K and (b) Tred = 973 K.
CO and CO2 formation started in a temperature range of 656−683 K (heat-up phase) for both experiments (final reduction temperatures of 773 K and 973 K, respectively). At 773 K, the highest CO concentration in the product gas was 10.8%, and the highest CO2 concentration was 37.7% (at the lowest H2 concentration, 41.9%). At a reduction temperature of 973 K, the highest CO concentration was 13.8%, and the highest CO2 concentration was found to be 46.4% at the lowest H2 concentration of 35.3%. In addition, when kept under an inert nitrogen atmosphere, the degree of metallization was 40% and 89% for the reduction temperatures of 773 K and 973 K, respectively.
The concomitant carbonate species in the siderite ore (magnesium, manganese, and calcium carbonate) were converted to their respective bivalent oxides, as shown in Equation (13).
MeCO3 ⇌ MeO + CO2; Me = Mg, Mn or Ca
3.3. Catalyst Characterization
3.3.1. X-ray Diffraction
The XRD patterns of the catalyst samples were obtained by a Rigaku SmartLab® X-ray diffractometer, Rigaku Corporation, Tokyo, Japan. The samples were collected using a sweep speed of 2.0° min−1 and 2θ = 5.0–80.0° with a scan step-size of 0.01°.
3.3.2. X-ray Fluorescence Spectrometry
The catalyst samples were dried in a furnace at 378 K for 2 h to determine the loss on ignition (LOI). After that, the samples were mixed with lithium tetraborat (Li2B4O7) and lithium metaborat (LiBO2) and melted in a melting furnace at 1273 K for 1 h. An S8 TIGER Series 2 XRF wavelength dispersive (WDX) spectrometer from Bruker AXS GmbH, Karlsruhe, Germany, was used to determine the catalyst composition using the Best Detection-Vac34mm measurement method.
3.3.3. Scanning Electron Microscopy
The DSM 982 Gemini Field Emission Scanning Electron Microscope (FE-SEM) from Carl Zeiss Microscopy Deutschland GmbH, Oberkochen, Germany, was used to study the surface of the Ni/sideritereduced and Ni/sideritereduced/MgO catalysts. The specimen stage was from −15° to +90°, and the working resolution was between 1 and 4 nm. The samples were coated by a single Leica EM ACE600 sputter coater, Leica Mikrosysteme GmbH, Austria.
3.3.4. AAS Analysis
The nickel loading of the catalysts was primarily analyzed by atomic absorption spectrometry (AAS). During the catalyst preparation (impregnation with nickel nitrate), process samples of the nickel solution (before and after impregnation of the support material) were taken and mixed with a solution of 10 cm3 HNO3 (Carl Roth GmbH, Karlsruhe, Germany) and 990 cm3 deionized water. An AAnalyst 400 atomic absorption spectrometer (Perkin Elmer Instruments LLC, Shelton, United States of America) was used, equipped with a nickel hollow cathode lamp set to 25 mA current at a wavelength of 232 nm, and applying a compressed air/ethylene flame, to determine the respective nickel concentrations. From the AAS results, the amount of nickel loading was determined.
3.4. Experimental Setup and Experimental Procedure of the Methanation Experiments
Hydrogen (99.999%), carbon dioxide (99.998%), and nitrogen (99.999%) supplied by Air Liquide were used for the CO2 hydrogenation experiments. Nitrogen was used as an inert gas for heat transport and balancing purposes.
3.4.1. Experimental Setup
The experimental setup is shown in Figure 11. The feed gases (H2, CO2, and N2) were controlled by three mass flow controllers (MFC1-3). They passed the fixed-bed stainless steel tubular reactor (Parr Instrument GmbH, Illinois, United States of America), which was 0.82 m in length and had an inner diameter of 25 mm. The feed gas stream was preheated by a pre-heating coil (PHC) at the top of the reactor tube before entering the catalyst bed. The tubular reactor was heated by an electric furnace with three heating zones (HT1–HT3) at the outer wall of the reactor tube. The temperature in the reactor was measured by six thermocouples (T1–T6). They were placed at two positions per heating zone. Stainless steel spacers and a sieve were placed inside the reactor to maintain the catalyst’s position. A total of 4 g of the Ni-based catalysts was placed in the middle of the reactor with the thermocouple string (thermocouple diameter = 6 mm) at the lower end of the catalyst bed. When the gas stream had passed the catalyst bed, the gaseous product stream was cooled (i) in the cooler at the outlet of the reactor (HE-1) where the condensate was collected in the condensate trap (CT-1), and (ii) by the gas analyzer cooler (HE-2) with a condensate trap (CT-2) installed to prevent moisture or condensation in the gas analyzer. The dried product gas was sent to the online gas analyzer (GA) consisting of a Caldos27 thermal conductivity analyzer for measuring the hydrogen concentration (measurement ranges: 0–0.5 vol% and 0–100 vol%; output error (2σ): ≤0.5% of smallest measurement range span) and an Uras26 infrared photometer for measuring the CO2, CO, and CH4 concentration (measurement ranges: 0–10 vol% and 0–100 vol%; output error (2σ): ≤0.2% of span).
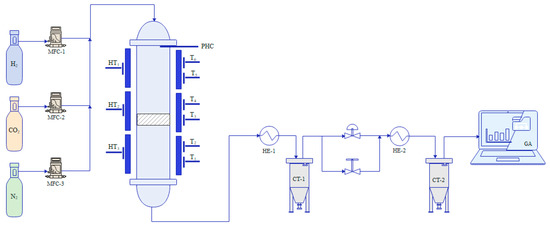
Figure 11.
Experimental setup of the tubular reactor used for the CO2 methanation experiments (MFC: mass flow controller, T1–T6: thermocouple inside the reactor tube, HT1–HT3, temperature measurement position in the middle of a heating zone, PHC: pre-heating coil, HE: heat exchanger, CT: condensate tank, BPR: back pressure regulator, GA: gas analyzer).
3.4.2. Experimental Procedure
CO2 methanation experiments were performed at ambient pressure, feed gas flow rates of 8.02−14.66 m3 kg−1 h−1 (STP), and a constant volumetric feed gas ratio (H2:CO2:N2 = 56:14:30). According to Sommerbauer et al. [72], preliminary temperature scanning tests were conducted for each catalyst to evaluate the characteristic temperature effects of the respective catalyst regarding CO2 conversion and methane selectivity before proceeding to steady-state experiments. Therefore, the reaction temperature range was set to 548–623 K. 4 g of the catalyst sample was used for each experiment. It was placed in the middle of the reactor tube. Pure nitrogen was used for purging the system at ambient temperature and pressure, and the composition was checked by the online gas analyzer. Next, the feed gas stream (H2:CO2:N2 = 56:14:30) with the designed feed gas flow rate was fed to the reactor while the heater was heated to the target temperature, which was measured at the end of the catalyst bed (Tcat = T3). The gaseous products in the product gas stream were analyzed by the online gas analyzer after passing two heat exchangers for condensation of water. The dry gaseous product stream consisted of N2, CO, CO2, CH4, and H2 only. There were no other constituents present in the dry product gas (at concentrations exceeding 0.1 vol%).
The performance of the catalysts was evaluated with regard to CO2 conversion (, Equation (14)) and CH4 selectivity (, Equation (15)), with being the initial concentration of CO2 and ci being the concentration of any species i at the outlet of the reactor. The volumetric expansion coefficient was calculated by Equation (16), with being the molar feed fraction of CO2 and the stoichiometric coefficients of the methanation reactions a, b, c, and d (CO2: a = −1, H2: b = −4, CH4: c = 1, and H2O: d = 2). The concentrations of the species (, and the product concentration for the products CH4 and CO, respectively) were calculated with Equations (17)−(19).
3.4.3. Determination of the Chemical Composition of the Reduced Siderite Ore
The chemical composition of the reduced siderite ore was characterized in a five step analysis procedure at the Chair of Mineral Processing, Montanuniverstät Leoben:
- (i)
- Measurement of the weight increase in air (equaling the reactivity of the sample in air) and determination of the oxidation state by loss on ignition (LOI, fully oxidized and in a neutral atmosphere);
- (ii)
- Combustion analysis via the Leco method to determine the total (residual) carbon content;
- (iii)
- Selective dissolution of metallic iron from iron oxides in bromine/methanol to determine elemental iron and for the determination of dissolved iron as FeII via the Zimmermann–Reinhardt method;
- (iv)
- Digestion of the filter cake in boiling hydrochloric acid (HCl) to determine bivalent FeII and trivalent FeIII iron using the Zimmermann–Reinhardt method;
- (v)
- X-ray diffraction of the residual elements.
The degree of metallization (wmet) is defined as the mass of elemental iron (mFe0) with respect to the total mass of iron-bearing components in the reduced iron ore (mFe,tot).
4. Conclusions
In this study, the catalytic potential of hydrogen-reduced siderite ore, and Ni-based catalysts on reduced siderite ore and mixed reduced siderite ore/MgO support material was evaluated. It was shown that siderite ore that was reduced in a hydrogen atmosphere can act as a CO2 hydrogenation catalyst but only causes low selectivity toward CH4. Ni-based catalysts on reduced siderite ore only showed marginally higher catalytic activity than undoped reduced siderite ore. However, it was proven that MgO plays a fundamental role as a basic support material for Ni-based CO2 methanation catalysts by drastically enhancing both CO2 conversion and selectivity toward CH4. When MgO was present in the support material, even fractions as low as 30% resulted in CO2 conversions close to the thermodynamic equilibrium and CH4 selectivities of at least 95% (mainly ≥ 99.9%). It could be shown that reduced siderite ore itself shows minor catalytic activity for CO2 methanation but, in combination with MgO, has a clear synergistic effect as a support material for Ni-based catalysts giving access to highly efficient CO2 methanation catalysts
Author Contributions
Conceptualization, S.L. and K.S.; methodology, S.L. and K.S.; validation, K.S.; formal analysis, K.S.; investigation, K.S.; resources, S.L.; data curation, K.S. and S.S.; writing—original draft preparation, K.S. and S.L.; writing—review and editing, S.L. and C.A.H.; visualization, K.S.; supervision, S.L.; project administration, S.L.; funding acquisition, K.S. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zukunftsfonds Steiermark, grant number 1481. Open Access Funding by the Graz University of Technology.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge financial support from NAWI Graz. The analytical equipment data was supported by Michael Gostencnik, Department of Earth Sciences—NAWI Graz Geocenter, University of Graz. Special thanks go to Alfred Stadtschnitzer from VA Erzberg GmbH for providing the siderite ore. Publication was supported by TU Graz Open Access Publishing Fund.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wei, W.; Jinlong, G. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Hao, W.; Vall, M.; Shi, Y.; Wang, Q.; Strømme, M.; Yan, X.; Cheung, O.; Li, R. Novel Ni/MgO Catalysts from Mesoporous MgCO3 for Highly Efficient CO Methanation: Effects of Al and Si Stabilization. ChemRxiv, 2020; preprint. [Google Scholar]
- Park, S.-E.; Yoo, J.S. New CO2 chemistry–Recent advances in utilizing CO2 as an oxidant and current understanding on its role. Carbon Dioxide Utilization for Global Sustainability. In Proceedings of the 7th International Conference on Carbon Dioxide Utilization, Seoul, Republic of Korea, 12–16 October 2004; Elsevier: Amsterdam, The Netherlands, 2004; pp. 303–314. [Google Scholar]
- Downing, C.A.; Sokol, A.A.; Catlow, C.R.A. The reactivity of CO2 on the MgO(100) surface. Phys. Chem. Chem. Phys. 2014, 16, 184–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandey, D.; Ray, K.; Bhardwaj, R.; Bojja, S.; Chary, K.; Deo, G. Promotion of unsupported nickel catalyst using iron for CO2 methanation. Int. J. Hydrog. Energy 2018, 43, 4987–5000. [Google Scholar] [CrossRef]
- Yin, L.; Chen, X.; Sun, M.; Zhao, B.; Chen, J.; Zhang, Q.; Ning, P. Insight into the role of Fe on catalytic performance over the hydrotalcite-derived Ni-based catalysts for CO2 methanation reaction. Int. J. Hydrog. Energy 2022, 47, 7139–7149. [Google Scholar] [CrossRef]
- Huynh, H.L.; Zhu, J.; Zhang, G.; Shen, Y.; Tucho, W.M.; Ding, Y.; Yu, Z. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J. Catal. 2020, 392, 266–277. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Lux, S. The reaction kinetics of CO2 methanation on a bifunctional Ni/MgO catalyst. J. Ind. Eng. Chem. 2020, 85, 196–207. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Aldana, P.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, J.; Du, X.; Wang, P.; Chen, Y.; Zhuang, W.; Song, M.; Sun, L.; Tao, X.; Li, J.; et al. Constructing the highly efficient Ni/ZrO2/SiO2 catalyst by a combustion-impregnation method for low-temperature CO2 methanation. J. Environ. Chem. Eng. 2022, 10, 108476. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, H.; Du, X.; Yao, B.; Wei, S.; Chen, Y.; Zhuang, W.; Yang, H.; Sun, L.; Tao, X.; et al. Highly active Ni/CeO2/SiO2 catalyst for low-temperature CO2 methanation: Synergistic effect of small Ni particles and optimal amount of CeO2. Fuel Process. Technol. 2022, 236, 107418. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Xing, Y.; Xu, S.; Xie, H.; Xiong, K. CO2 hydrogenation to methane over mesoporous Co/SiO2 catalysts: Effect of structure. J. CO2 Util. 2018, 26, 221–229. [Google Scholar] [CrossRef]
- Da Silva, D.C.; Letichevsky, S.; Borges, L.E.; Appel, L.G. The Ni/ZrO2 catalyst and the methanation of CO and CO2. Int. J. Hydrog. Energy 2012, 37, 8923–8928. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Park, J.-N. Bifunctional mechanism of CO2 methanation on Pd-MgO/SiO2 catalyst: Independent roles of MgO and Pd on CO2 methanation. J. Phys. Chem. C 2010, 114, 7128–7131. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Q. Mesostructured cellular foam silica supported bimetallic LaNi1-xCoxO3 catalyst for CO2 methanation. Int. J. Hydrog. Energy 2020, 45, 4417–4426. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, X.; Han, J.; Wang, H.; Ge, Q. Rhenium-promoted selective CO2 methanation on Ni-based catalyst. J. CO2 Util. 2018, 26, 8–18. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Song, M.; Shi, L.; Xu, X.; Du, X.; Chen, Y.; Zhuang, W.; Tao, X.; Sun, L.; Xu, Y. Ni/M/SiO2 catalyst (M=La, Ce or Mg) for CO2 methanation: Importance of the Ni active sites. J. CO2 Util. 2022, 64, 102150. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Wang, X.; Fang, X.; Wang, H.; Xu, X. New insights into CO2 methanation mechanisms on Ni/MgO catalysts by DFT calculations: Elucidating Ni and MgO roles and support effects. J. CO2 Util. 2019, 33, 55–63. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline γ-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Li, J.; Wei, S.; Gao, X.; Wang, P. Combustion-impregnation preparation of Ni/SiO2 catalyst with improved low-temperature activity for CO2 methanation. Int. J. Hydrog. Energy 2021, 46, 20919–20929. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Zhong, Z.; Teo, J.; Gofer, Y.; Gedanken, A. Methanation of Carbon Dioxide on Ni Catalysts on Mesoporous ZrO2 Doped with Rare Earth Oxides. Catal. Lett. 2009, 130, 455–462. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/MgO catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Bette, N.; Thielemann, J.; Schreiner, M.; Mertens, F. Methanation of CO2 over a (Mg,Al)Ox Supported Nickel Catalyst Derived from a (Ni,Mg,Al)-Hydrotalcite-like Precursor. ChemCatChem 2016, 8, 2903–2906. [Google Scholar] [CrossRef]
- Fedoročková, A.; Raschman, P. Effects of pH and acid anions on the dissolution kinetics of MgO. Chem. Eng. J. 2008, 143, 265–272. [Google Scholar] [CrossRef]
- Ho, K.; Jin, S.; Zhong, M.; Vu, A.-T.; Lee, C.-H. Sorption capacity and stability of mesoporous magnesium oxide in post-combustion CO2 capture. Mater. Chem. Phys. 2017, 198, 154–161. [Google Scholar] [CrossRef]
- Takezawa, N.; Terunuma, H.; Shimokawabe, M.; Kobayashib, H. Methanation of carbon dioxide: Preparation of Ni/MgO catalysts and their performance. Appl. Catal. 1986, 23, 291–298. [Google Scholar] [CrossRef]
- Varun, Y.; Sreedhar, I.; Singh, S.A. Highly stable M/NiO–MgO (M = Co, Cu and Fe) catalysts towards CO2 methanation. Int. J. Hydrog. Energy 2020, 45, 28716–28731. [Google Scholar] [CrossRef]
- Baldauf-Sommerbauer, G.; Lux, S.; Aniser, W.; Bitschnau, B.; Letofsky-Papst, I.; Siebenhofer, M. Steady-state and controlled heating rate methanation of CO2 on Ni/MgO in a bench-scale fixed bed tubular reactor. J. CO2 Util. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Suksumrit, K.; Kleiber, S.; Lux, S. The Role of Carbonate Formation during CO2 Hydrogenation over MgO-Supported Catalysts: A Review on Methane and Methanol Synthesis. Energies 2023, 16, 2973. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Promotional effects in alumina and silica supported bimetallic Ni–Fe catalysts during CO2 hydrogenation. J. Mol. Catal. A Chem. 2014, 382, 23–30. [Google Scholar] [CrossRef]
- Franken, T.; Heel, A. Are Fe based catalysts an upcoming alternative to Ni in CO2 methanation at elevated pressure? J. CO2 Util. 2020, 39, 101175. [Google Scholar] [CrossRef]
- Sehested, J.; Larsen, K.E.; Kustov, A.L.; Frey, A.M.; Johannessen, T.; Bligaard, T.; Andersson, M.P.; Nørskov, J.K.; Christensen, C.H. Discovery of technical methanation catalysts based on computational screening. Top. Catal. 2007, 45, 9–13. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.-A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.-D. Potential of an Alumina-Supported Ni3Fe Catalyst in the Methanation of CO2: Impact of Alloy Formation on Activity and Stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Serrer, M.-A.; Kalz, K.F.; Saraҫi, E.; Lichtenberg, H.; Grunwaldt, J.-D. Role of Iron on the Structure and Stability of Ni3.2Fe/Al2O3 during Dynamic CO2 Methanation for P2X Applications. ChemCatChem 2019, 11, 5018–5021. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni–Fe/(Mg,Al)Ox catalysts for CO2 methanation–Tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Jiang, L.; Wang, C. Nickel supported on iron-bearing olivine for CO2 methanation. Int. J. Hydrog. Energy 2016, 41, 12910–12919. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Da Diniz Costa, J.C. Influence of water on high-temperature CO2 capture using layered double hydroxide derivatives. Ind. Eng. Chem. Res. 2008, 47, 2630–2635. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Synthesis of nanocrystalline mesoporous Ni/Al2O3 single bond SiO2 catalysts for CO2 methanation reaction. Int. J. Hydrog. Energy 2018, 43, 19038–19046. [Google Scholar] [CrossRef]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Seo, J.G.; Baik, J.H.; Koh, D.J.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Al2O3 catalysts prepared by a coprecipitation method: Effect of precipitation agent. J. Ind. Eng. Chem. 2013, 19, 2016–2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Liu, Q. Green synthesis of MCM-41 derived from renewable biomass and construction of VOx-modified nickel phyllosilicate catalyst for CO2 methanation. Int. J. Hydrog. Energy 2021, 46, 32003–32016. [Google Scholar] [CrossRef]
- Gao, W.; Meng, X.; Jin, D.; Xu, B.; Dai, W.; Zhao, R.; Xin, Z. Polyol-pretreated SBA-16 supported Ni-Fe bimetallic catalyst applied in CO methanation at low temperature. Mol. Catal. 2021, 512, 111769. [Google Scholar] [CrossRef]
- González-Castaño, M.; Navarro de Miguel, J.C.; Boelte, J.-H.; Centeno, M.A.; Klepel, O.; Arellano-García, H. Assessing the impact of textural properties in Ni–Fe catalysts for CO2 methanation performance. Microporous Mesoporous Mater. 2021, 327, 111405. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Kumar Prabhakar, J.; Apte, P.A.; Deo, G. The kinetics of Ni/Al2O3 and Ni-Fe/Al2O3 catalysts for the CO2 methanation reaction and the reasons for promotion. Chem. Eng. J. 2023, 471, 144252. [Google Scholar] [CrossRef]
- Lan, P.-W.; Wang, C.-C.; Chen, C.-Y. Effect of Ni/Fe ratio in Ni–Fe catalysts prepared under external magnetic field on CO2 methanation. J. Taiwan Inst. Chem. Eng. 2021, 127, 166–174. [Google Scholar] [CrossRef]
- Tsuji, M.; Kodama, T.; Yoshida, T.; Kitayama, Y.; Tamaura, Y. Preparation and CO2 methanation activity of an ultrafine Ni(II) ferrite catalyst. J. Catal. 1996, 164, 315–321. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Sdiri, A.; Gálvez, M.; Zidi, H.; Da Costa, P.; Ben Zina, M. Natural Hematite and Siderite as Heterogeneous Catalysts for an Effective Degradation of 4-Chlorophenol via Photo-Fenton Process. ChemEngineering 2018, 2, 29. [Google Scholar] [CrossRef]
- Wei, Y.; Gui, K.; Liu, X.; Liang, H.; Gu, S.; Ren, D. Performance of Mn-Ce co-doped siderite catalysts in the selective catalytic reduction of NOx by NH3. J. Fuel Chem. Teshimachnol. 2019, 47, 1495–1503. [Google Scholar] [CrossRef]
- Görmez, Ö.; Saçlı, B.; Çağlayan, U.; Kalderis, D.; Gözmen, B. Hydrothermal Synthesis of Siderite and Application as Catalyst in the Electro-Fenton Oxidation of p-Benzoquinone. Molecules 2022, 27, 8056. [Google Scholar] [CrossRef]
- Boehm, A.; Boehm, M.; Kogelbauer, A. Neutrons for Mineral Processing–Thermo Diffractometry to Investigate Mineral Selective Magnetizing Flash Roasting. Chem. Ing. Tech. 2014, 86, 883–890. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, L.; Peng, J.; Hu, T.; Yang, L. Microwave roasting of siderite and the catalytic combustion effects on anthracite. Appl. Therm. Eng. 2017, 117, 668–674. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Böhm, A.; Lux, S. Clean iron production through direct reduction of mineral iron carbonate with low-grade hydrogen sources; the effect of reduction feed gas composition on product and exit gas composition. Clean. Eng. Technol. 2021, 5, 100345. [Google Scholar] [CrossRef]
- Loder, A.; Santner, S.; Siebenhofer, M.; Böhm, A.; Lux, S. Reaction kinetics of direct reduction of mineral iron carbonate with hydrogen: Determination of the kinetic triplet. Chem. Eng. Res. Des. 2022, 188, 575–589. [Google Scholar] [CrossRef]
- Lux, S.; Baldauf-Sommerbauer, G.; Ottitsch, B.; Loder, A.; Siebenhofer, M. Iron Carbonate Beneficiation Through Reductive Calcination-Parameter Optimization to Maximize Methane Formation. Eur. J. Inorg. Chem. 2019, 2019, 1748–1758. [Google Scholar] [CrossRef]
- Bock, S.; Pauritsch, M.; Lux, S.; Hacker, V. Natural iron ores for large-scale thermochemical hydrogen and energy storage. Energy Convers. Manag. 2022, 267, 115834. [Google Scholar] [CrossRef]
- Lux, S.; Baldauf-Sommerbauer, G.; Siebenhofer, M. Hydrogenation of Inorganic Metal Carbonates: A Review on Its Potential for Carbon Dioxide Utilization and Emission Reduction. ChemSusChem 2018, 11, 3357–3375. [Google Scholar] [CrossRef]
- Reller, A.; Emmenegger, R.; Padeste, C.; Oswald, H.-R. Thermochemical Reactivity of Metal Carbonates. Chimia 1991, 45, 262. [Google Scholar] [CrossRef]
- Kleiber, S.; Loder, A.; Siebenhofer, M.; Böhm, A.; Lux, S. Direct reduction of siderite ore combined with catalytic CO/CO2 hydrogenation to methane and methanol: A technology concept. Chem. Ing. Tech. 2022, 94, 701–711. [Google Scholar] [CrossRef]
- Baldauf-Sommerbauer, G.; Lux, S.; Wagner, J.; Siebenhofer, M. Determination of the kinetic triplet by an isoconversional and a regression method applied to the decomposition of mineral iron carbonate in nitrogen. Thermochim. Acta 2017, 649, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).