Synthesis of Hydroxylammonium Nitrate and Its Decomposition over Metal Oxide/Honeycomb Catalysts
Abstract
1. Introduction
2. Results and Discussion
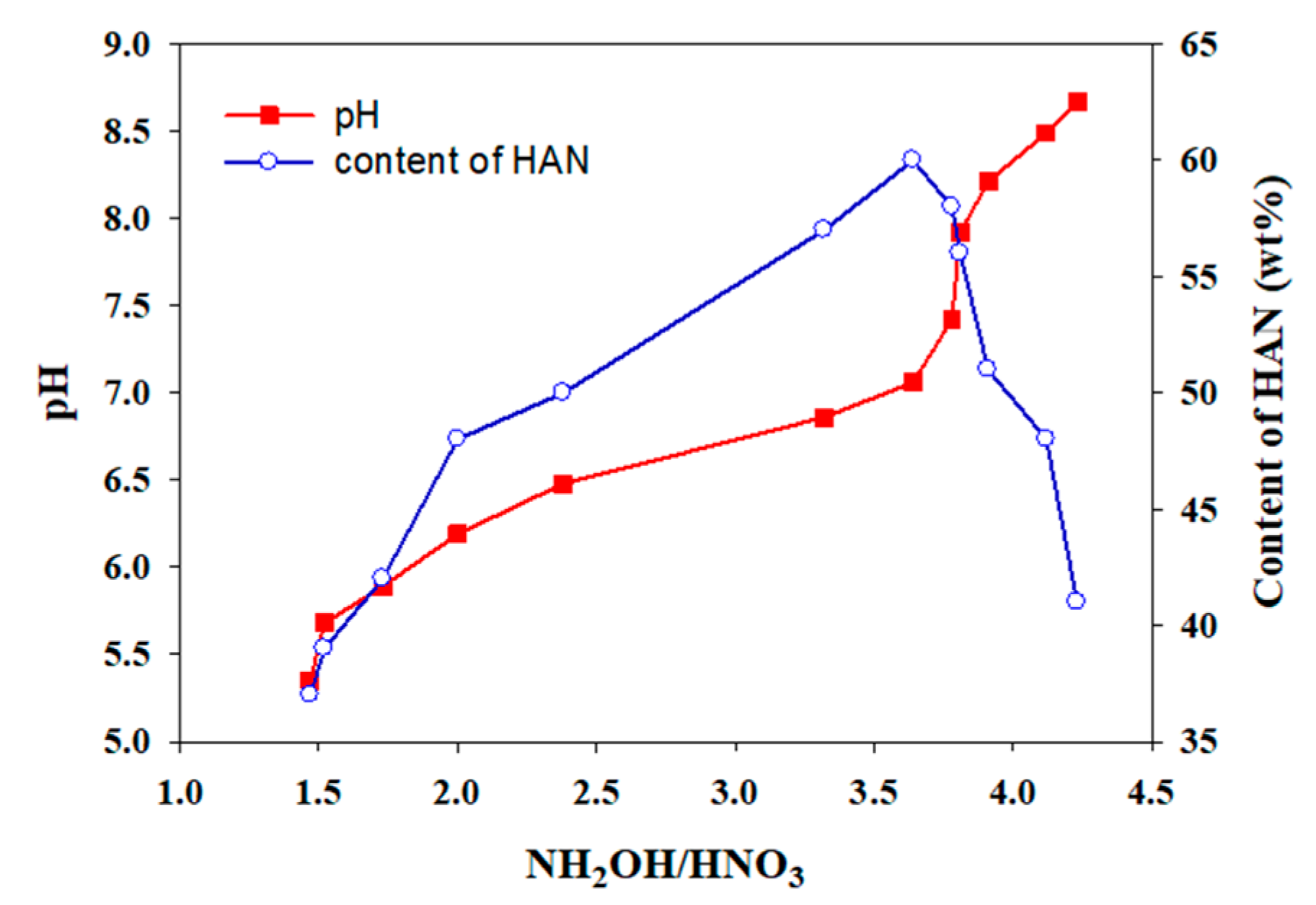
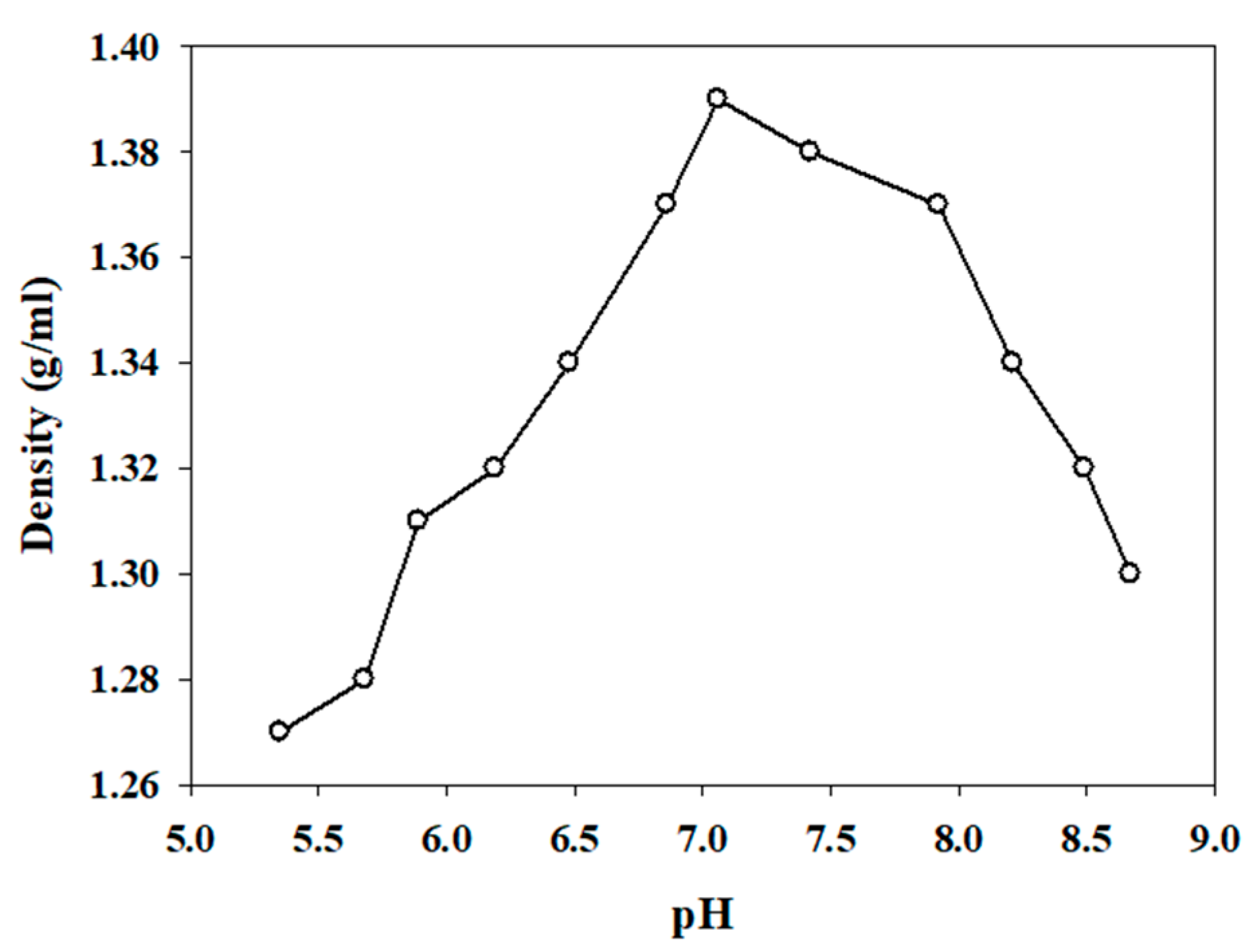
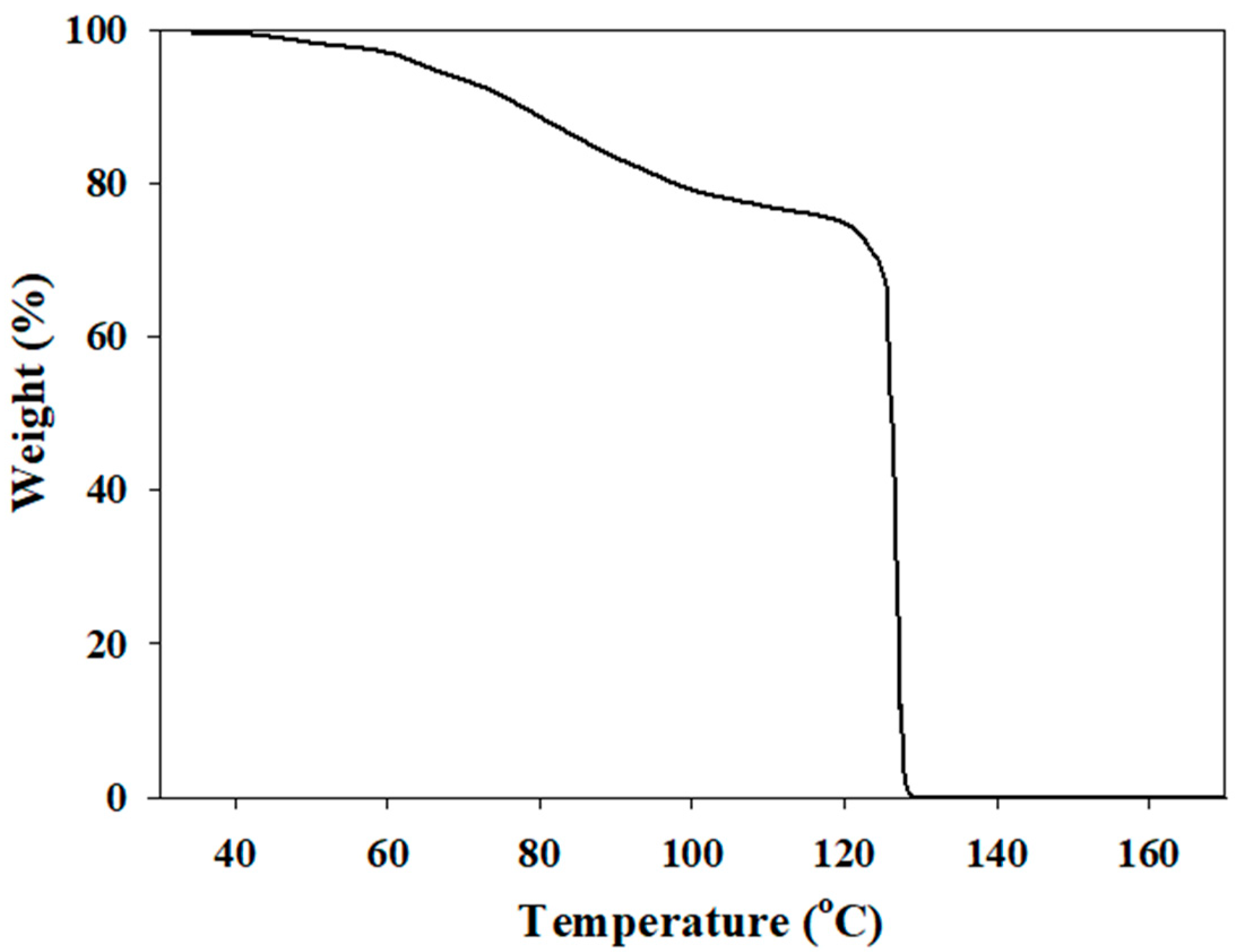
2.1. Synthesis of Hydroxylammonium Nitrate
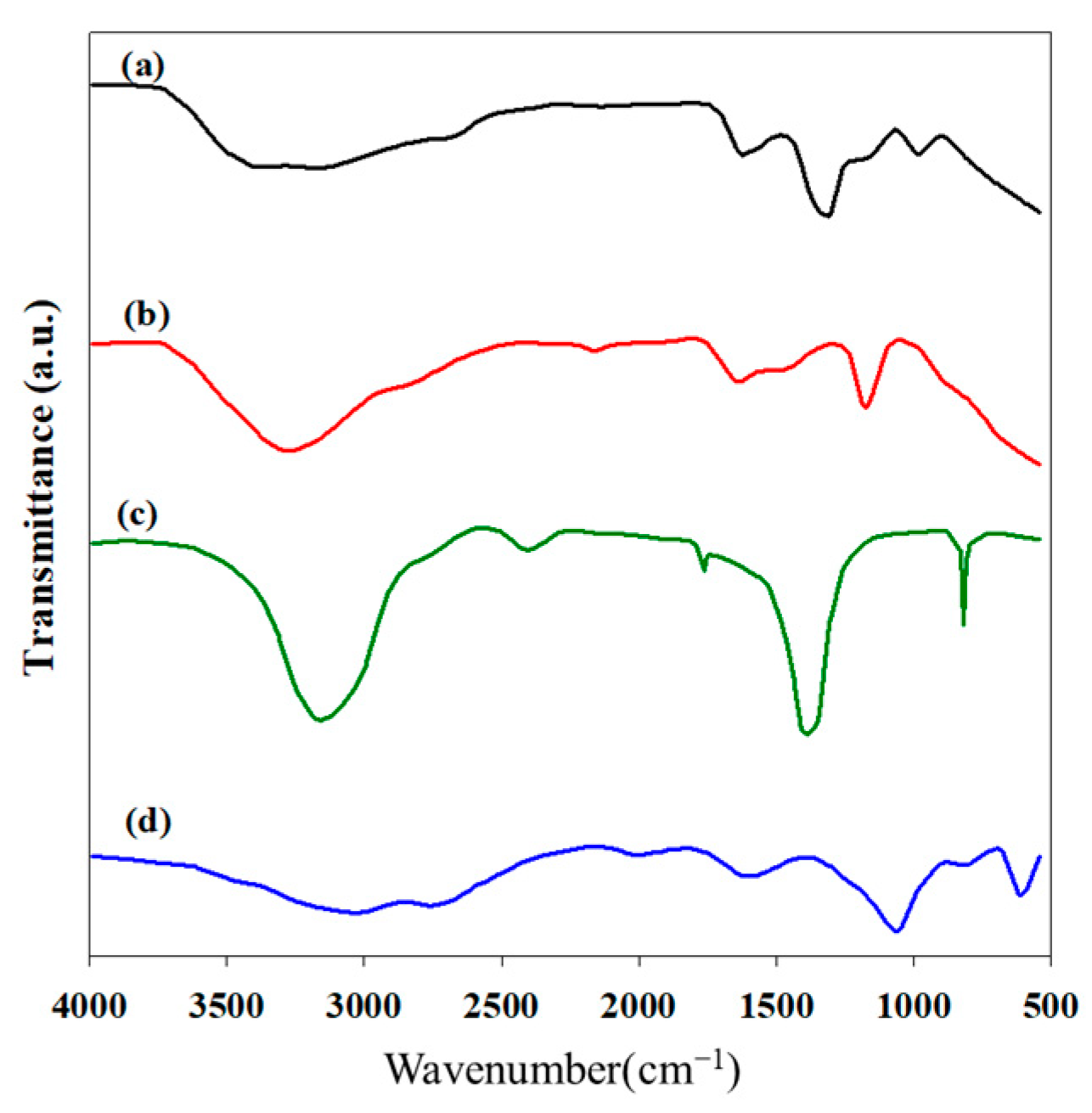
2.2. FT-IR Analysis of Hydroxylammonium Nitrate Solution
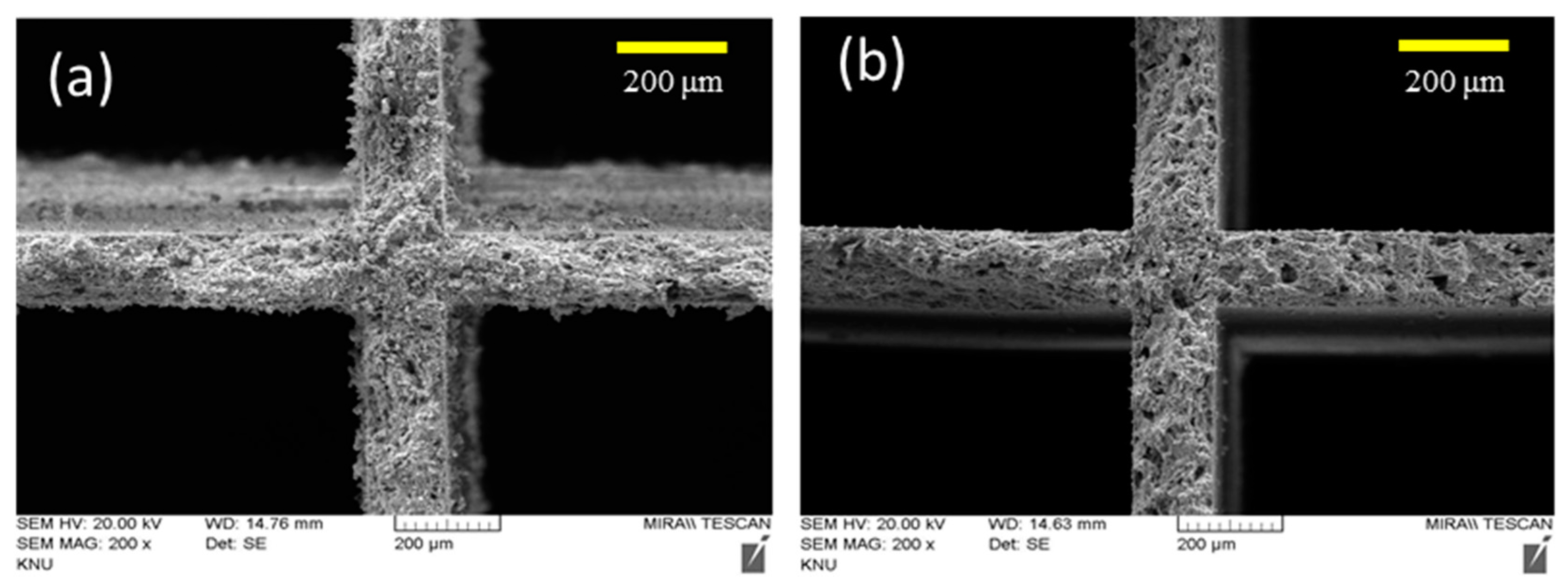
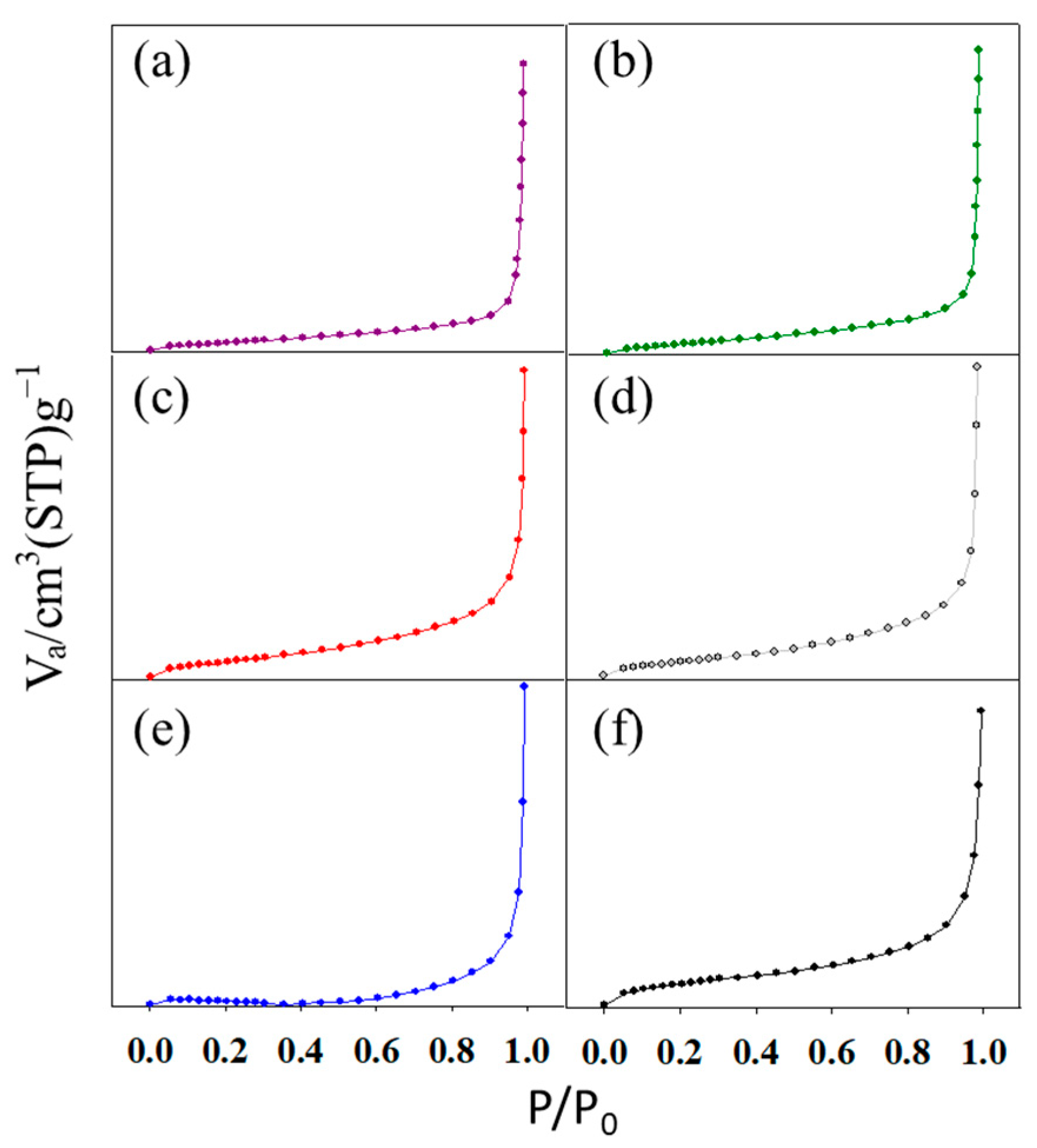
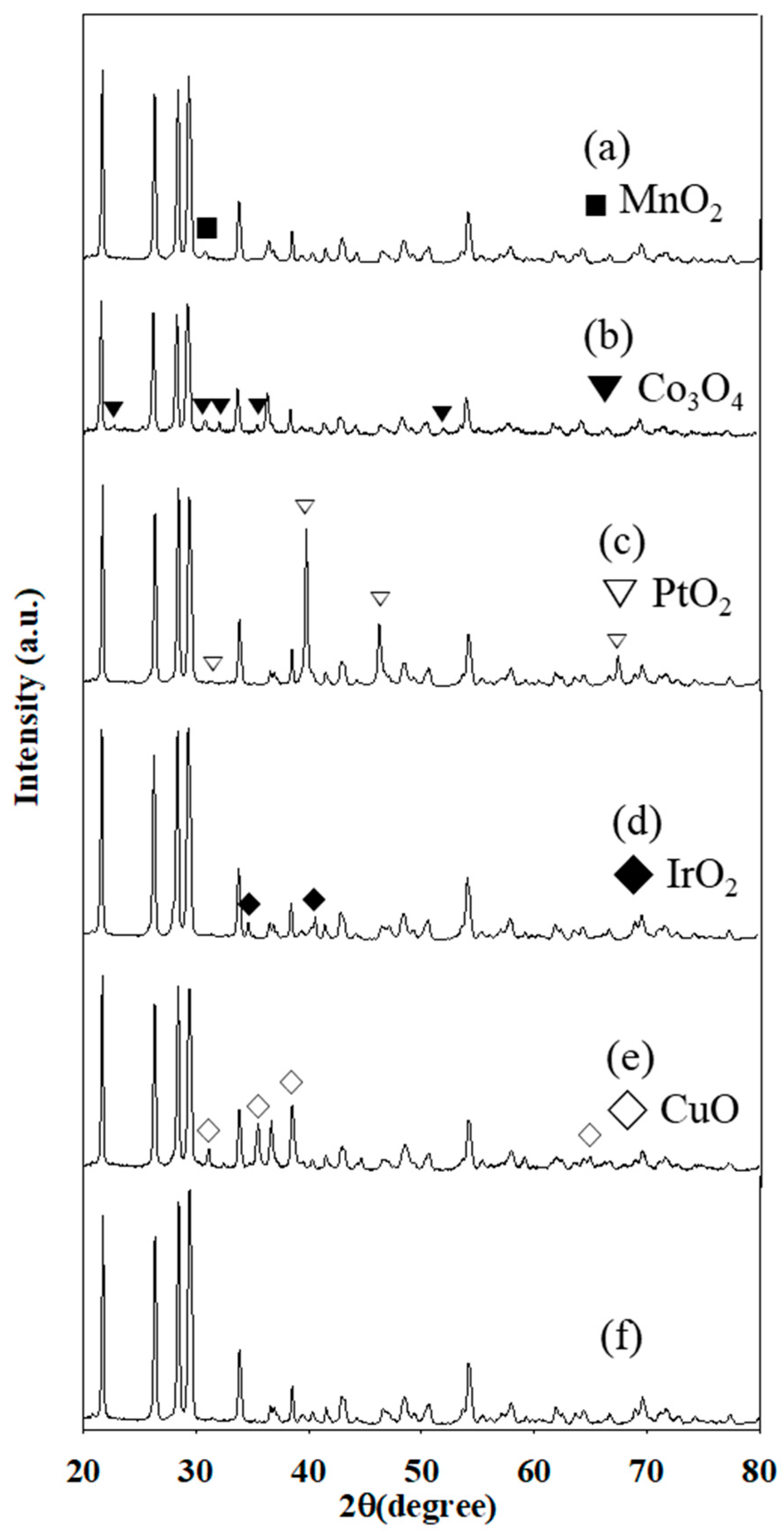
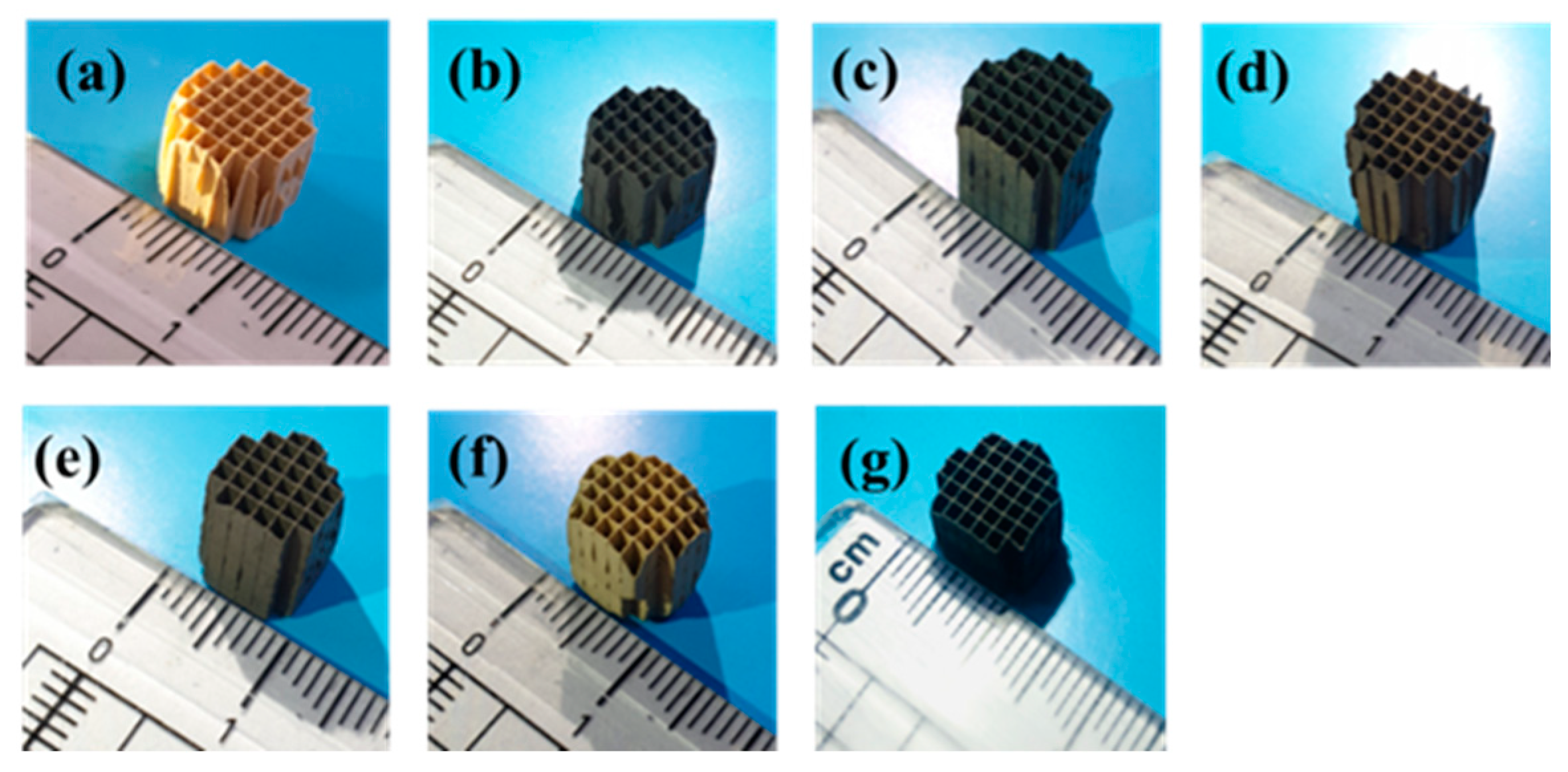
2.3. Characterization of Metal Oxide/Honeycomb Catalysts
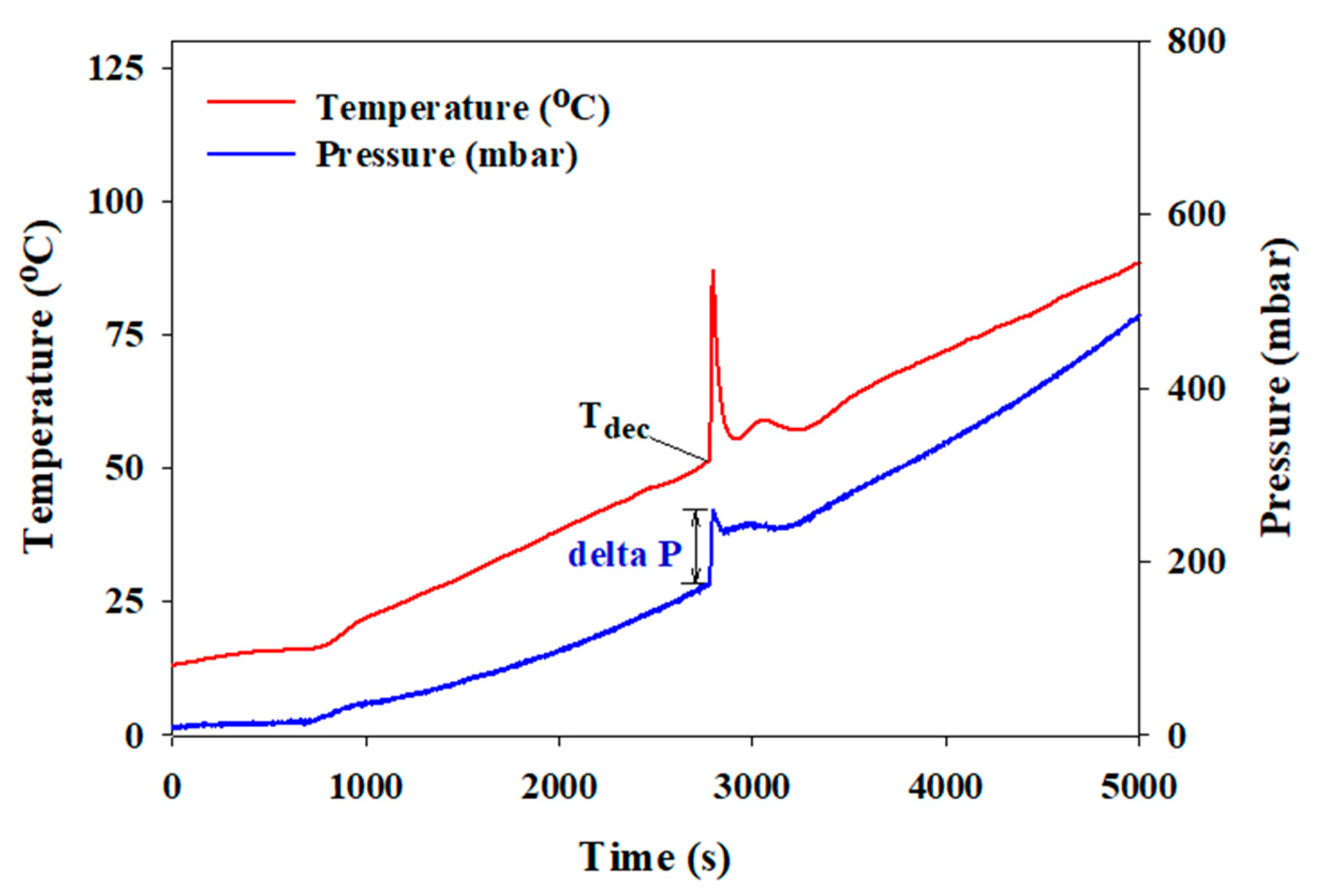
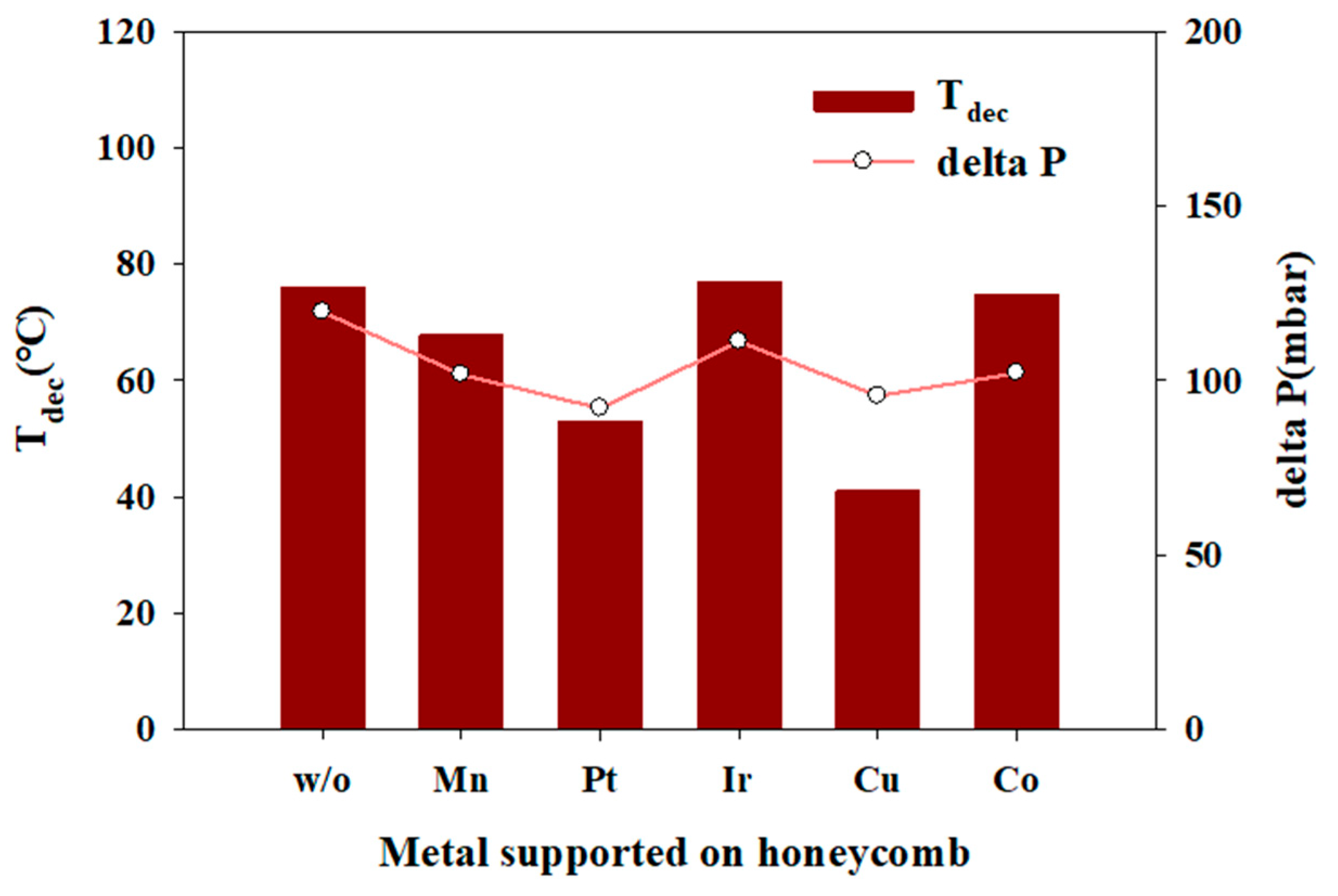
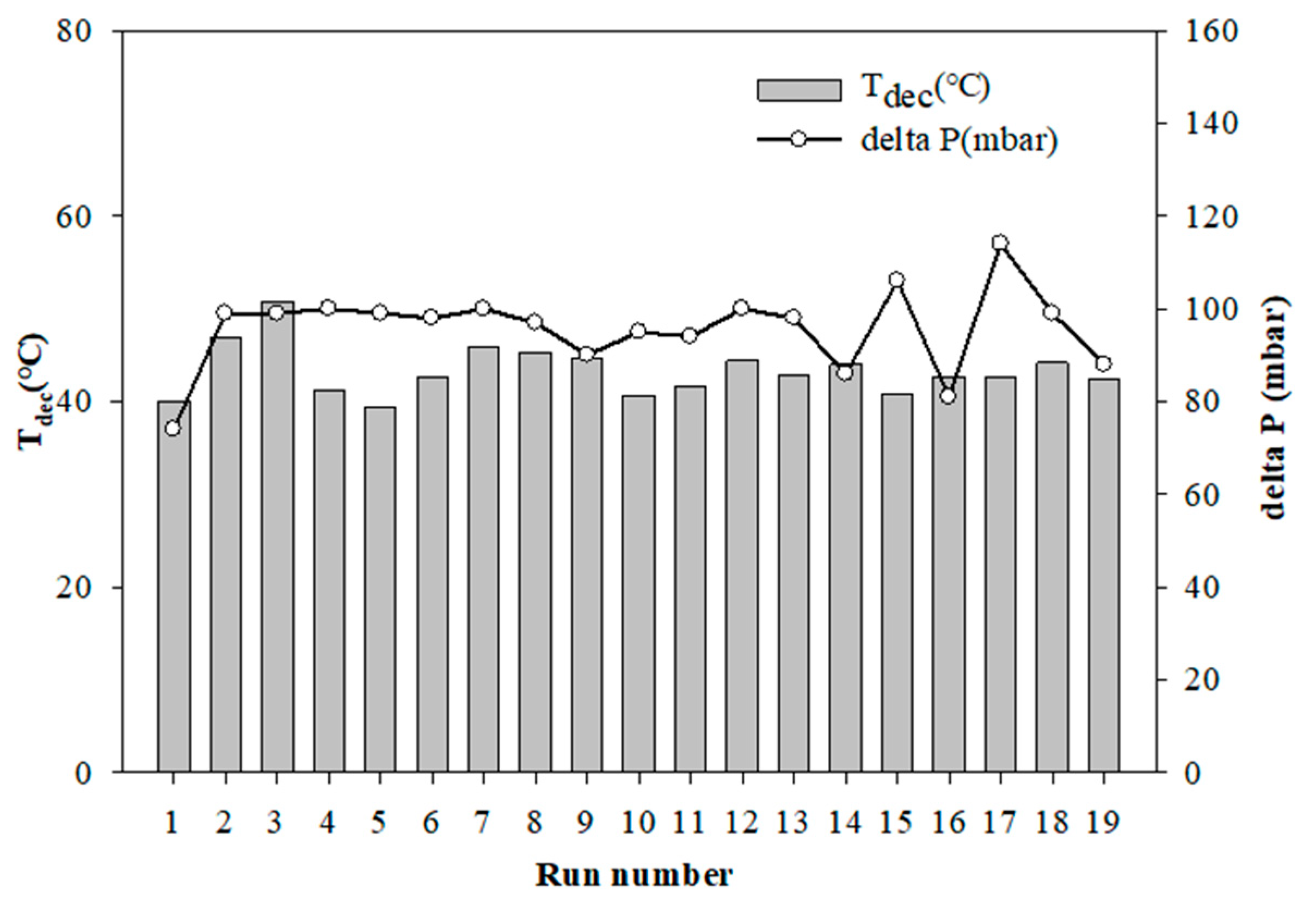
2.4. Thermal and Catalytic Decomposition of the Aqueous HAN Solution
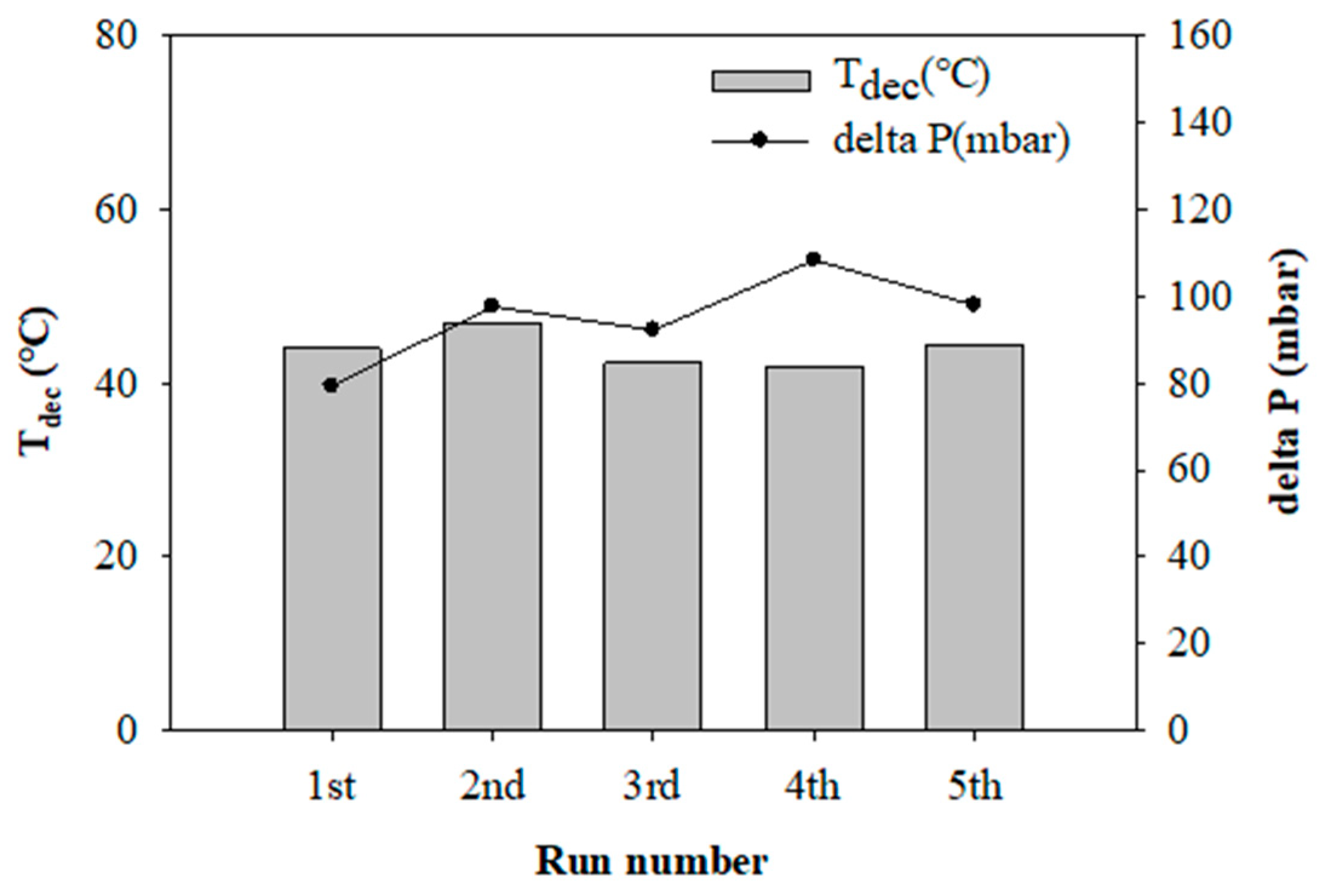
2.5. Catalyst Reusability Test
3. Experimental Details
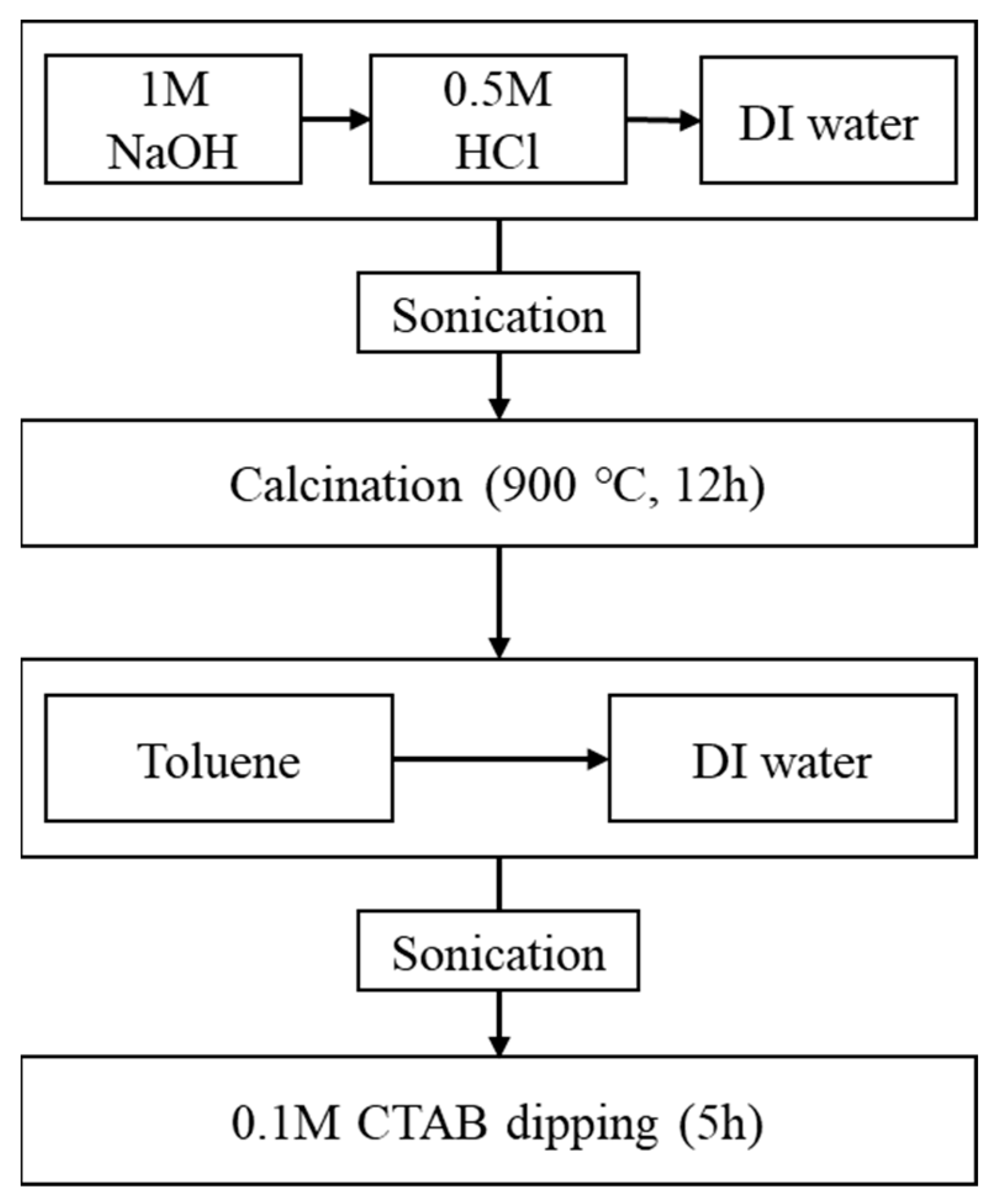
3.1. Synthesis of Hydroxylammonium Nitrate
3.2. Analysis of Hydroxylammonium Nitrate
3.3. Preparation of the Catalysts
3.4. Characterization of the Catalysts
3.5. Catalytic Decomposition of the HAN Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McKechnie, T.; Shchetkovskiy, A. Development of Metallic Foams monolithic Catalysts for Green Monopropellants Propulsion. In Proceedings of the Space Propulsion Conference, Rome, Italy, 2–6 May 2016; p. 232. [Google Scholar]
- Amrousse, R.; Katsumi, T.; Azuma, N.; Hori, K. Hydroxylammonium nitrate (HAN)-based green propellant as alternative energy resource for potential hydrazine substitution: From lab scale to pilot plant scale-up. Combust. Flame 2017, 176, 334–348. [Google Scholar] [CrossRef]
- Courtheoux, L.; Gautron, E.; Rossignol, S.; Kappenstein, C. Transformation of platinum supported on silicon-doped alumina during the catalytic decomposition of energetic ionic liquid. J. Catal. 2005, 232, 10–18. [Google Scholar] [CrossRef]
- Oommen, C.; Rajaraman, S.; Chandru, R.A.; Rafeev, R. Catalytic Decomposition of Hydroxylammonium Nitrate Monopopellant. In Proceedings of the International Conference on Chemistry and Chemical Process, Bangkok, Thailand, 7–9 May 2011; p. 10. [Google Scholar]
- Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; Batonneau, Y.; Kappenstein, C.; Schwentenwein, M.; Koopmans, R.J.; Schuh, S.; Scharlemann, C. Development of catalytic materials for decomposition of ADN-based monopropellants. Acta Astronaut. 2019, 158, 407–415. [Google Scholar] [CrossRef]
- Neto, T.G.S.; Ferreira, J.G.; Cobo, A.J.G.; Cruz, G.M. Ir-Ru/Al2O3 catalysts used in satellite propulsion. Brazlhan J. Chem. Eng. 2003, 20, 273–282. [Google Scholar] [CrossRef]
- Masse, R.; Spores, A.R.; Kimbrel, S.; Allen, M.; Lorimor, E.; Myers, P. GPIM AF-M315E Propulsion System. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014; pp. 2015–3753. [Google Scholar]
- Jang, I.J.; Jang, Y.B.; Shin, H.S.; Shin, N.R.; Kim, S.K.; Yu, M.J.; Cho, S.J. Preparation and characterization of lanthanum hexaaluminate granule for catalytic application in aerospace technology. In Proceedings of the 18th International Conference on Composite Materials, Jeju, Korea, 21–26 August 2011. [Google Scholar]
- Amrousse, R.; Hori, K.; Fetimi, W.; Farhat, K. HAN and ADN as liquid ionic monopropellants: Thermal and catalytic ă decomposition processes. Appl. Catal. B Environ. 2012, 127, 426–428. [Google Scholar] [CrossRef]
- Hong, S.; Heo, S.; Kim, W.; Jo, Y.M.; Park, Y.K.; Jeon, J.K. Catalytic decomposition of an energetic ionic liquid solution over hexaaluminate catalysts. Catalysts 2019, 9, 80. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheah, K.H.; Koh, K.S.; Chin, J.; Chik, T.F.W.K. Parametric studies of electrolytic decomposition of hydroxylammonium nitrate (HAN) energetic ionic liquid in microreactor using image processing technique. Chem. Eng. J. 2016, 296, 19–27. [Google Scholar] [CrossRef]
- Shobaky, G.A.E.; Turky, A.E.M.M. Catalytic decomposition of H2O2 on Co3O4 doped with MgO and V2O5. Colloids Surfaces 2000, 170, 161–172. [Google Scholar] [CrossRef]
- Xiaoguang, R.; Minghui, L.; Aiqin, W.; Lin, L.; Xiaodong, W.; Zhang, T. Catalytic decomposition of hydroxyl ammonium nitrate at room temperature. Chin. J. Catal. 2007, 28, 1–2. [Google Scholar]
- Kang, S.J. Evaluation of Catalytic Decomposition Performance for 1 n Class Ionic Liquid Monopropellant Thruster. Master′s Thesis, Korea Advanced Institute of Science and Technology, Daejeon, Korea, 2012. [Google Scholar]
- Sam, I.I.; Gayathri, S.; Santhosh, G.; Cyriac, J.; Reshmi, S. Exploring the possibilities of energetic ionic liquids as non-toxic hypergolic bipropellants in liquid rocket engines. J. Mol. Liq. 2022, 350, 118–217. [Google Scholar]
- Kang, L.; Liu, J.; Yao, Y.; Wu, X.; Zhang, J.; Zhu, C.G.; Xu, F.; Xu, S. Enhancing risk/safety management of HAN-based liquid propellant as a green space propulsion fuel: A study of its hazardous characteristics. Process Saf. Environ. Prot. 2023, 177, 921–931. [Google Scholar] [CrossRef]
- Nosseir, A.E.S.; Cervone, A.; Pasini, A. Review of state-of-the-art green monopropellants: For propulsion systems analysts and designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Masse, R.K.; Allen, M.; Spores, R.A.; Driscoll, E. AF-M315E Propulsion System Advances & Improvements. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016. [Google Scholar]
- Neff, G.A. The Decomposition of Hydroylammonium Nitrate under Vacuum Conditions. Master’s Thesis, University of West Michigan, Kalamazoo, MI, USA, 2016. [Google Scholar]
- Liggett, T. Process for Producing Concentrated Solutions of Hydroxylammonium Nitrate and Hydroxylammonium Perchlorate. U.S. Patent 4066736A, 3 January 1978. [Google Scholar]
- Wagaman, K.L. Synthesis of Hydroxylamine Salts. U.S. Patent 4956168A, 11 September 1990. [Google Scholar]
- Liggett, T. Hydroxylammonium Nitrate Process. U.S. Patent 5182092A, 26 January 1993. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2000; pp. 10815–10837. [Google Scholar]
- Cawlfield, D.W. Process for the Production of High Purity Hydroxylammonium Nitrate. U.S. Patents 5213784A, 25 May 1993. [Google Scholar]
- Hong, S.; Heo, S.; Jo, Y.M.; Kim, T.; Jeon, J.K. A Study on Nanoporous Catalysts for Decomposition of AND-Based Liquid Monopropellant. Korean Soc. Propuls. Eng. 2016, 47, 1319–1322. [Google Scholar]
- Lee, H.S.; Litzinger, T.A. Chemical kinetic study of HAN decomposotion. Combust. Flame 2003, 135, 151–169. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, S.; Wang, X. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]
- Kotani, W.; Hamaguchi, K.; Kasai, Y. Ceramic Honeycomb Structure with Grooves and Outer Coating, Process of Producing the Same, and Coating Material Used in the Honeycomb Structure. U.S. Patent 5629067A, 13 May 1997. [Google Scholar]
- Shamshuddin, S.Z.M.; Sundar, M.S.; Thimmaraju, N.; Vatsalya, V.G.; Senthilkumar, M. Synthesis, characterization and catalytic activity studies on cordierite honeycomb coated with ZrO2 based solid super acids. Comptes Rendus Chim. 2012, 15, 799–807. [Google Scholar] [CrossRef]
- Agnihotri, R.; Oommen, C. Cerium oxide based active catalyst for hydroxylammonium nitrate (HAN) fueled monopropellant thrusters. RSC Adv. 2018, 8, 22293–22302. [Google Scholar] [CrossRef]
- Chai, W.S. Characterization & Analysis on Electrolytic Decomposition of Hydroxylammoniu Natrate (HAN) Ternary Mixtures in Microreactors. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Kuo, B.H. A Study on the Electrolytic Decomposition of HAN-Based Propellants for Microthruster Applications. Master’s Thesis, Pennsylvania state University, University Park, PA, USA, 2010. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Reinoso, F.R.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Heo, S.; Kim, M.; Lee, J.; Park, Y.C.; Jeon, J.K. Decomposition of ammonium dinitramide-based liquid propellant over Cu/hexaaluminate pellet catalysts. Korean J. Chem. Eng. 2019, 36, 660–668. [Google Scholar] [CrossRef]
- Pipa, A.V.; Röpcke, J. Analysis of the Mid-Infrared Spectrum of the Exhaust Gas From an Atmospheric Pressure Plasma Jet (APPJ) Working With an Argon–Air Mixture. IEEE Trans. Plasma Sci. 2009, 37, 1000–1003. [Google Scholar] [CrossRef]
- Dorhout, J.M.; Anderson, A.S.; Batista, E.; Carlson, R.K.; Currier, R.P.; Martinez, R.K.; Clegg, S.M.; Wilkerson, M.P.; Nowak-Lovato, K. NOx speciation from copper dissolution in nitric acid/water solutions using FTIR spectroscopy. J. Mol. Spec. 2020, 372, 111334. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C10024972&Type=IR-SPEC&Index=1 (accessed on 25 January 2024).
- Bengtsson, G.; Fronæus, S.; Kloo, L.B. The kinetics and mechanism of oxidation of hydroxylamine by iron (III). J. Chem. Soc., Dalton Trans. 2002, 12, 2548–2552. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, E.; Lee, B.; Ko, T.; Oh, J. The microstructure of Magnetite Coated on Honeycomb and Characteristics of CO2 Decomposition. J. Korean Ceram. Soc. 2004, 41, 410–416. [Google Scholar]
- Yoo, D.; Woo, J.; Oh, S.; Jeon, J.K. Performance of Pt and Ir Supported on Mesoporous Materials for Decomposition of Hydroxylammonium Nitrate Solution. J. Nanosci. Nanotechnol. 2020, 20, 4461–4465. [Google Scholar] [CrossRef]




| Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Honeycomb | 1.3 | 0.005 |
| Cu/honeycomb | 0.3 | 0.004 |
| Ir/honeycomb | 0.9 | 0.010 |
| Pt/honeycomb | 0.6 | 0.010 |
| Co/honeycomb | 1.0 | 0.010 |
| Mn/honeycomb | 0.8 | 0.010 |
| Catalyst | Metal Oxide Loading (wt%) |
|---|---|
| Cu/honeycomb | 13.9 |
| Ir/honeycomb | 13.5 |
| Pt/honeycomb | 14.2 |
| Co/honeycomb | 17.8 |
| Mn/honeycomb | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Kim, M.; Oh, S.K.; Hwang, S.; Kim, S.; Kim, W.; Kwon, Y.; Jo, Y.; Jeon, J.-K. Synthesis of Hydroxylammonium Nitrate and Its Decomposition over Metal Oxide/Honeycomb Catalysts. Catalysts 2024, 14, 116. https://doi.org/10.3390/catal14020116
Yoo D, Kim M, Oh SK, Hwang S, Kim S, Kim W, Kwon Y, Jo Y, Jeon J-K. Synthesis of Hydroxylammonium Nitrate and Its Decomposition over Metal Oxide/Honeycomb Catalysts. Catalysts. 2024; 14(2):116. https://doi.org/10.3390/catal14020116
Chicago/Turabian StyleYoo, Dalsan, Munjeong Kim, Seung Kyo Oh, Seoyeon Hwang, Sohee Kim, Wooram Kim, Yoonja Kwon, Youngmin Jo, and Jong-Ki Jeon. 2024. "Synthesis of Hydroxylammonium Nitrate and Its Decomposition over Metal Oxide/Honeycomb Catalysts" Catalysts 14, no. 2: 116. https://doi.org/10.3390/catal14020116
APA StyleYoo, D., Kim, M., Oh, S. K., Hwang, S., Kim, S., Kim, W., Kwon, Y., Jo, Y., & Jeon, J.-K. (2024). Synthesis of Hydroxylammonium Nitrate and Its Decomposition over Metal Oxide/Honeycomb Catalysts. Catalysts, 14(2), 116. https://doi.org/10.3390/catal14020116









