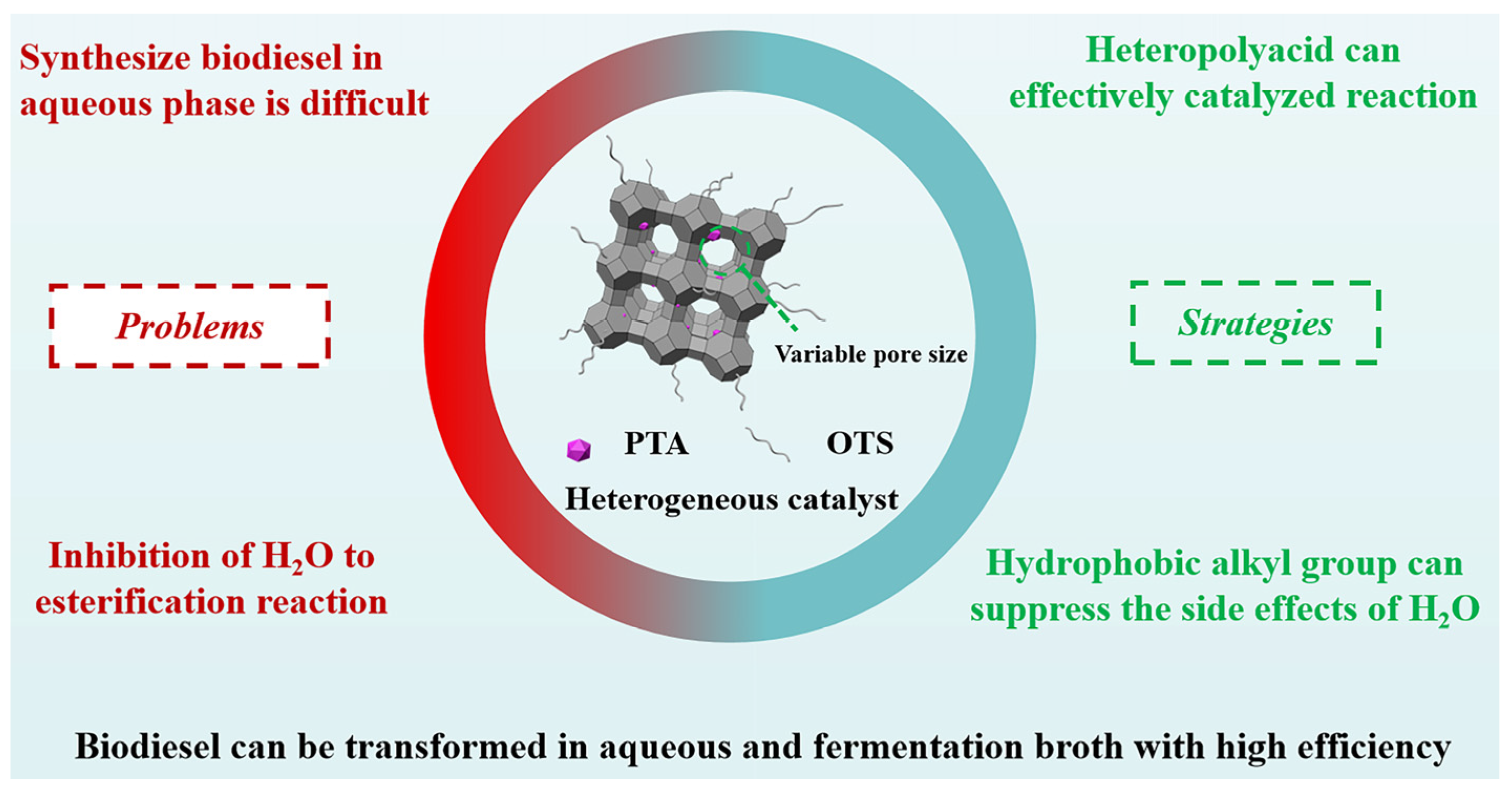
The Green Synthesis of Biodiesel via Esterification in Water Catalyzed by the Phosphotungstic Acid–Functionalized Hydrophobic MCM–41 Catalyst
Abstract
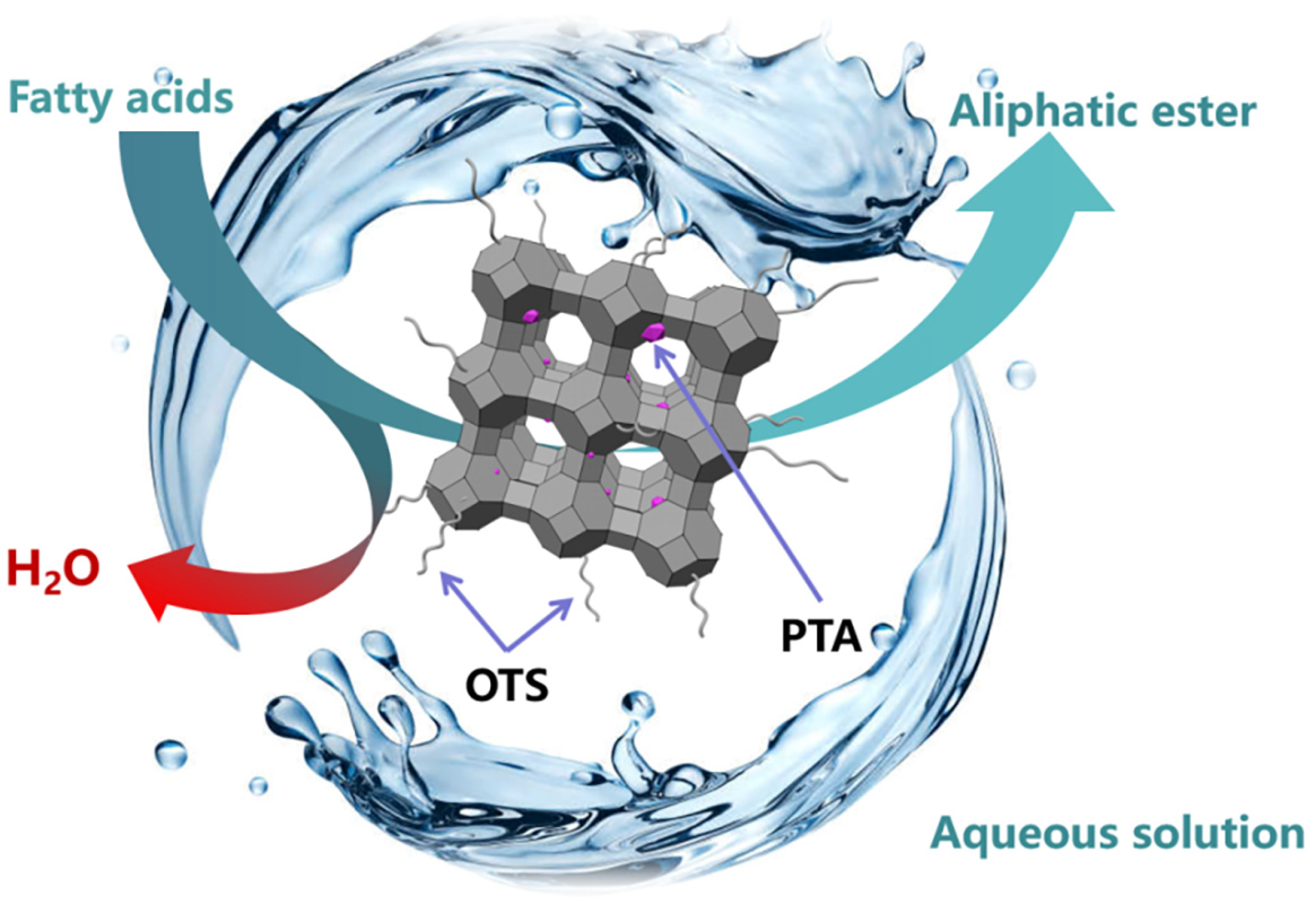
1. Introduction
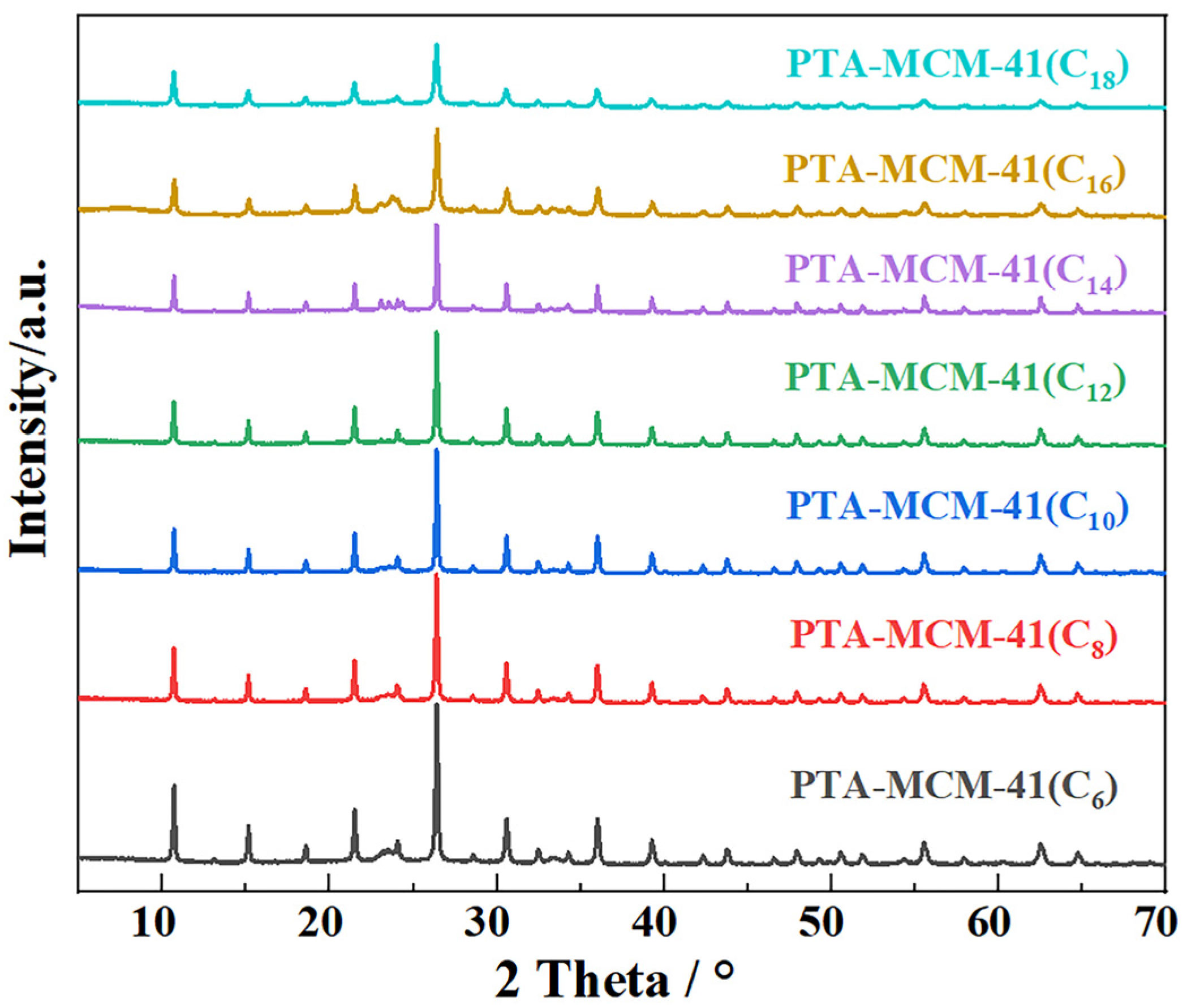
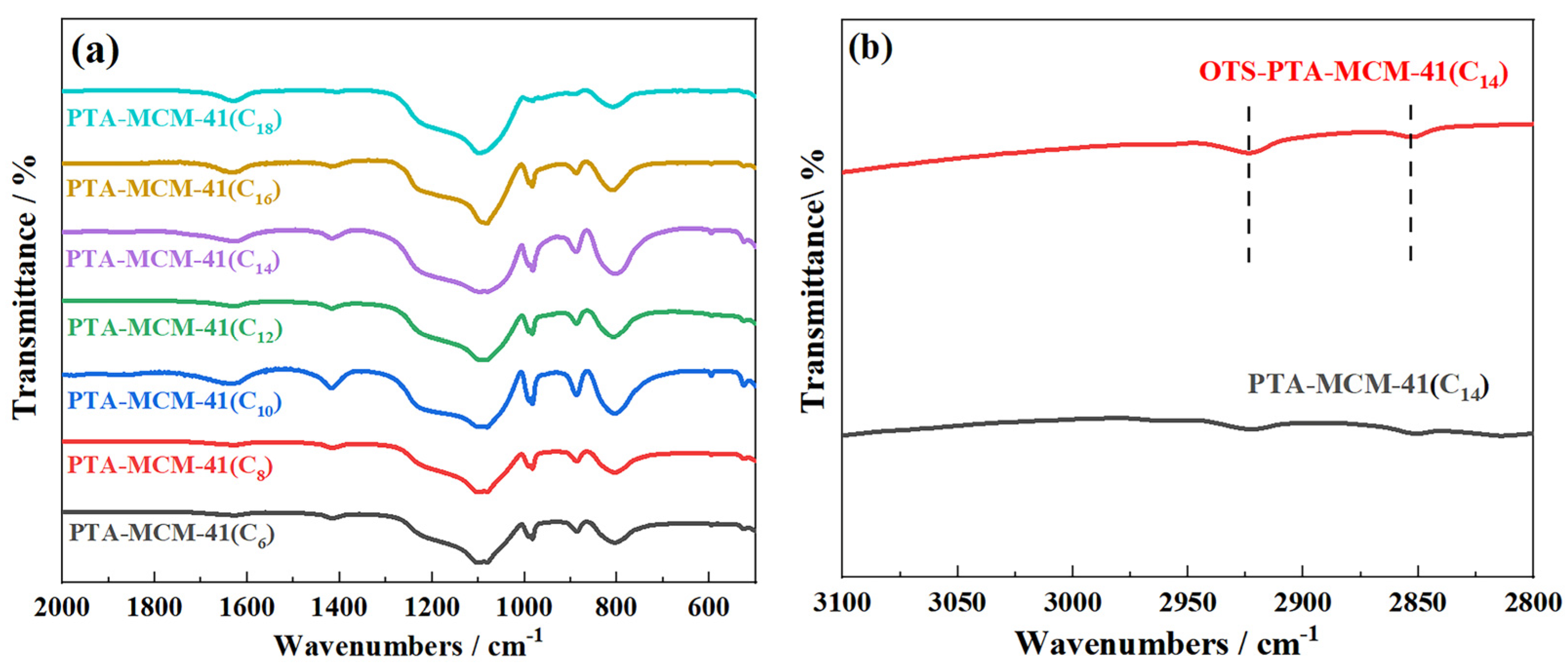
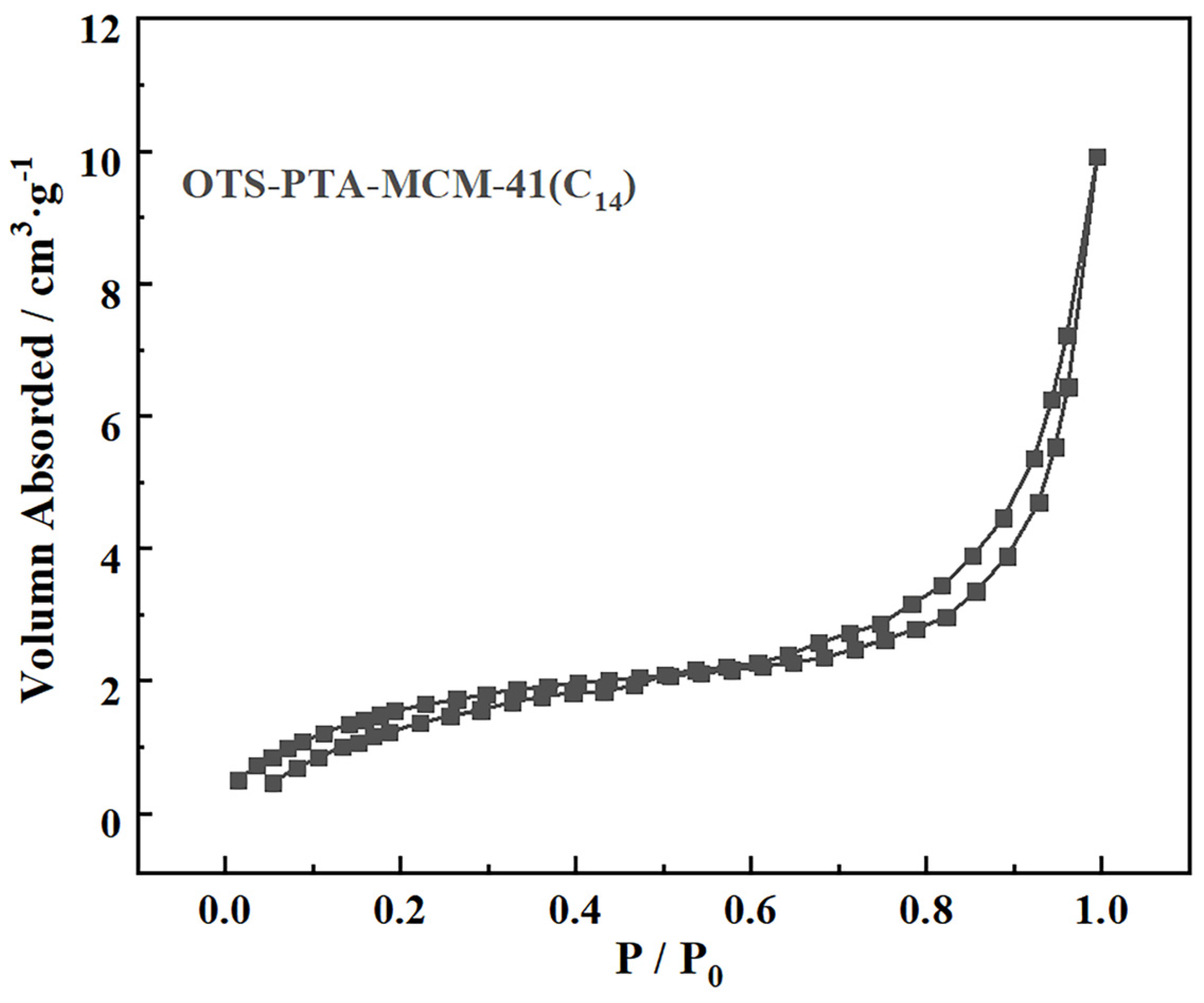
2. Results
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Characterization Details
3.3. General Catalytic Procedure
3.3.1. Esterification of Free Fatty Acids in the Water
3.3.2. In Situ Esterification of Fatty Acids in Fermentation Broth
3.4. Cyclic Catalytic Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashedi, A.; Khanam, T.; Jonkman, M. On Reduced Consumption of Fossil Fuels in 2020 and Its Consequences in Global Environment and Exergy Demand. Energies 2020, 13, 6048. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Mohamed, I.M.A.; Chen, Z.; Chen, L.; Ihara, I.; Yap, P.S.; Rooney, D.W. Strategies to Save Energy in the Context of the Energy Crisis: A Review. Environ. Chem. Lett. 2023, 21, 2003–2039. [Google Scholar] [CrossRef] [PubMed]
- Debeni Devi, N.; Chaudhuri, A.; Goud, V.V. Algae Biofilm as a Renewable Resource for Production of Biofuel and Value-added Products: A Review. Sustain. Energy Techn. 2022, 53, 102479. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and other Applications: A Review. Renew. Sust. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A Comprehensive study on Prospects of Economy, Environment, and Efficiency of Palm Oil Biodiesel as a Renewable Fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Kang, X.; Guo, X.; You, H. Biodiesel Development in the Global Scene. Energy Source. Part B 2014, 10, 155–161. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Shaah, M.A.; Hossain, M.S.; Allafi, F.; Ab Kadir, M.O.; Ahmad, M.I. Biodiesel Production from Candlenut Oil using a Non-catalytic Supercritical Methanol Transesterification Process: Optimization, Kinetics, and Thermodynamic Studies. RSC Adv. 2022, 12, 9845–9861. [Google Scholar] [CrossRef] [PubMed]
- Zulqarnain; Yusoff, M.H.M.; Ayoub, M.; Hamza Nazir, M.; Zahid, I.; Ameen, M.; Abbas, W.; Shoparwe, N.F.; Abbas, N. Comprehensive Review on Biodiesel Production from Palm Oil Mill Effluent. ChemBioEng Rev. 2021, 8, 439–462. [Google Scholar] [CrossRef]
- Khalaji, M. Evaluation of Fatty Acid Profiles of Chlorella Vulgaris Microalgae Grown in Dairy Wastewater for Producing Biofuel. J. Environ. Health Sci. 2022, 20, 691–697. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Kuroki, A.; Dai, Y.; Tong, Y.W. Microbial Biodiesel Production from Industrial Organic Wastes by Oleaginous Microorganisms: Current Status and Prospects. J. Hazard. Mater. 2021, 402, 123543. [Google Scholar] [CrossRef]
- Chaowamalee, S.; Yan, N.; Ngamcharussrivichai, C. Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis. Nanomaterials 2022, 12, 604. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Recent Developments in Heterogeneous Catalysis for the Sustainable Production of Biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Yang, S.; Guo, Z.; Zhang, T.; Song, H.; Shao, Q. Esterification Synthesis of Ethyl Oleate Catalyzed by Brønsted acid–surfactant-combined Ionic Liquid. Green Chem. Lett. Rev. 2017, 10, 202–209. [Google Scholar] [CrossRef]
- Björk, E.M.; Militello, M.P.; Tamborini, L.H.; Coneo Rodriguez, R.; Planes, G.A.; Acevedo, D.F.; Moreno, M.S.; Odén, M.; Barbero, C.A. Mesoporous Silica and Carbon Based Catalysts for Esterification and Biodiesel Fabrication—The Effect of Matrix Surface Composition and Porosity. Appl. Catal. A-Gen. 2017, 533, 49–58. [Google Scholar] [CrossRef]
- Shi, S.; Valle-Rodriguez, J.O.; Khoomrung, S.; Siewers, V.; Nielsen, J. Functional Expression and Characterization of Five Wax Ester Synthases in Saccharomyces Cerevisiae and Their Utility for Biodiesel Production. Biotechnol. Biofuels 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, J.L.; Zhao, X.R.; Liu, S.C.; Liu, Z.J.; Wei, L.J.; Hua, Q. Yarrowia Lipolytica as a Metabolic Engineering Platform for the Production of Very-Long-Chain Wax Esters. J. Agric. Food Chem. 2020, 68, 10730–10740. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, C.; Feng, D.; Cheng, T.; Meng, X.; Liu, W.; Zou, H.; Xian, M. Production of Extracellular Fatty Acid using Engineered Escherichia coli. Microb. Cell Fact. 2012, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis. Catalysts 2021, 11, 384. [Google Scholar] [CrossRef]
- Hsiao, M.; Kuo, J.; Hsieh, S.; Hsieh, P.; Hou, S. Optimized Conversion of Waste Cooking oil to Biodiesel Using Modified Calcium Oxide as Catalyst via a Microwave Heating System. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Hassan, S.Z.; Vinjamur, M. Concentration-independent Rate Constant for Biodiesel Synthesis from Homogeneous-catalytic Esterification of Free Fatty Acid. Chem. Eng. Sci. 2014, 107, 290–301. [Google Scholar] [CrossRef]
- Li, M.; Lu, P.; Ye, C.; Chen, J.; Qiu, T. Amphiphilic Heteropolyacid-based Sulfonic Acid-functionalised Ionic Liquids as Efficient Catalysts for Biodiesel Production. Fuel 2023, 354, 129269. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Q.; Wu, Y.; Yu, R.; Cheng, J.; Zhang, Y. Construction of a Keggin Heteropolyacid/Ni-MOF Catalyst for Esterification of Fatty Acids. RSC Adv. 2021, 11, 33416–33424. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xie, W.; Gao, C. Heterogeneous H6PV3MoW8O40/AC-Ag Catalyst for Biodiesel Production: Preparation, Characterization and Catalytic Performance. Fuel 2022, 316, 123352. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.; Wu, Y.; Zhao, P. Sulfonated Organic Heteropolyacid Salts: Recyclable Green Solid Catalysts for Esterifications. J. Mol. Catal. A-Chem. 2009, 313, 1–6. [Google Scholar] [CrossRef]
- Mohan, V.; Pramod, C.V.; Suresh, M.; Prasad Reddy, K.H.; Raju, B.D.; Rama Rao, K.S. Advantage of Ni/SBA-15 Catalyst over Ni/MgO Catalyst in terms of Catalyst Stability due to Release of Water during Nitrobenzene Hydrogenation to Aniline. Catal. Commun. 2012, 18, 89–92. [Google Scholar] [CrossRef]
- Ruan, S.; Chen, S.; Lu, J.; Zeng, Q.; Liu, Y.; Yan, D. Waterproof Geopolymer Composites Modified by Hydrophobic Particles and Polydimethylsiloxane. Compos. Part B-Eng. 2022, 237, 109865. [Google Scholar] [CrossRef]
- Li, B.; Ma, W.; Liu, J.; Zuo, S.; Li, X. Preparation of MCM-41 Incorporated with Lacunary Keggin Polyoxometalate and its Catalytic Performance in Esterification. J. Colloid Interf. Sci. 2011, 362, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Brasil, H.; Carvalho, A.L.G.d.; Costa, F.F.; Nascimento, L.A.S.d.; Mhadmhan, S.; Pineda, A.; Luque, R.; Valença, G.P. Preparation of Novel Mesoporous Ca/P MCM-41-based Materials for Mechanochemical Diphenyl Sulfide Oxidation. Micropor. Mesopor. Mat. 2020, 297, 110017. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Tobbala, D.E.; El-Bahy, S.M.; Nassan, M.A.; Salama, R.S. The Role of Phosphotungstic Acid in Enhancing the Catalytic Performance of UiO-66 (Zr) and its Applications as an Efficient Solid Acid Catalyst for Coumarins and Dihydropyrimidinones Synthesis. Catal. Commun. 2022, 169, 106479. [Google Scholar] [CrossRef]
- Zhu, W. Phosphotungstic Acid Passivated Enhanced Photocatalytic Performance of ZnS Nanoparticles under Solar Light. Solid State Sci. 2021, 118, 106406. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Pandurangan, A. Heteropolyacid (H3PW12O40) Supported MCM-41: An Efficient Solid Acid Catalyst for the Green Synthesis of Xanthenedione Derivatives. J. Mol. Catal. A-Chem. 2009, 311, 36–45. [Google Scholar] [CrossRef]
- Andas, J.; Ekhbal, S.H.; Ali, T.H. MCM-41 Modified Heterogeneous Catalysts from Rice Husk for Selective Oxidation of Styrene into Benzaldehyde. Environ. Technol. Inno. 2021, 21, 101308. [Google Scholar] [CrossRef]
- Wang, R.; Wan, J.; Li, Y.; Sun, H. An Improvement of MCM-41 Supported Phosphoric Acid Catalyst for Alkylation Desulfurization of Fluid Catalytic Cracking Gasoline. Fuel 2015, 143, 504–511. [Google Scholar] [CrossRef]
- Ma, T.; Yun, Z.; Xu, W.; Chen, L.; Li, L.; Ding, J.; Shao, R. Pd-H3PW12O40/Zr-MCM-41: An Efficient Catalyst for the Sustainable Dehydration of Glycerol to Acrolein. Chem. Eng. J. 2016, 294, 343–352. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, D.; Wang, S.; Xiao, M.; Sun, L.; Meng, Y. Heteropolyacid Salt Catalysts for Methanol Conversion to Hydrocarbons and Dimethyl Ether: Effect of Reaction Temperature. Catalysts 2019, 9, 320. [Google Scholar] [CrossRef]
- Zapata, P.A.; Faria, J.; Ruiz, M.P.; Jentoft, R.E.; Resasco, D.E. Hydrophobic Zeolites for Biofuel Upgrading Reactions at the Liquid-liquid Interface in Water/oil Emulsions. J. Am. Chem. Soc. 2012, 134, 8570–8578. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dutta, P.K. Use of Surface-modified Zeolite Y for Extraction of Metal Ions from Aqueous to Organic Phase. Micropor. Mesopor. Mat. 1999, 32, 29–35. [Google Scholar] [CrossRef]
- Adam, F.; Sek, K.L. Heterogenization of Indium for the Friedel-Craft Benzoylation of Toluene. Chinese J. Catal. 2012, 33, 1802–1808. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, T.; Lin, W.; Lo, H.; Lo, K.; Chen, C.; Lin, K. Molecular Sieve Material from Liquid–crystal-display Waste Glass and Silicon Carbide Sludge via Hydrothermal Process with Alkali Fusion Pretreatment. J. Mater. Cycles Waste 2021, 23, 1081–1089. [Google Scholar] [CrossRef]
- Bharathi, M.; Mathivathani, S.; Indira, S.; Vinoth, G.; Christopher Leslee, D.B.; Shanmuga Bharathi, K. Anchoring of a Nickel Schiff Base Complex with Mixed Ligands on MCM-41 as a Heterogeneous Catalyst for the Synthesis of Quinoxaline Derivatives by Various Energies. Polyhedron 2023, 229, 116188. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Balogi, Z.; Kónya, Z.; Toba, M.; Lentz, P.; Niwa, S.I.; Mizukami, F.; Molnar, A.; Nagy, J.B.; Kiricsi, I. Synthesis, Characterisation and Catalytic Applications of Sol-gel Derived Silica-phosphotungstic Acid Composites. Appl. Catal. A-Gen. 2002, 228, 83–94. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Y.; Li, J.; Fu, W.; Zhang, J. Preparation of a Liquefied Banana Pseudo-stem Based PVAc-nanosilica Hybrid Membrane and its Modification by Octadecyltrichlorosilane. RSC Adv. 2016, 6, 94170–94176. [Google Scholar]
- Senoymak Tarakcı, M.I.; Ilgen, O. Esterification of Oleic Acid with Methanol Using Zr(SO4)2 as a Heterogeneous Catalyst. Chem. Eng. Technol. 2018, 41, 845–852. [Google Scholar] [CrossRef]
- Patel, A.; Singh, S. Undecatungstophosphate Anchored to MCM-41: An Ecofriendly and Efficient Bifunctional Solid Catalyst for Non-solvent Liquid-phase Oxidation as well as Esterification of Benzyl alcohol. Micropor. Mesopor. Mat. 2014, 195, 240–249. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M. Toluene Hydrogenation over Nickel-containing MCM-41 and Ti-MCM-41 Materials. J. Porous Mat. 2009, 17, 663–668. [Google Scholar] [CrossRef]
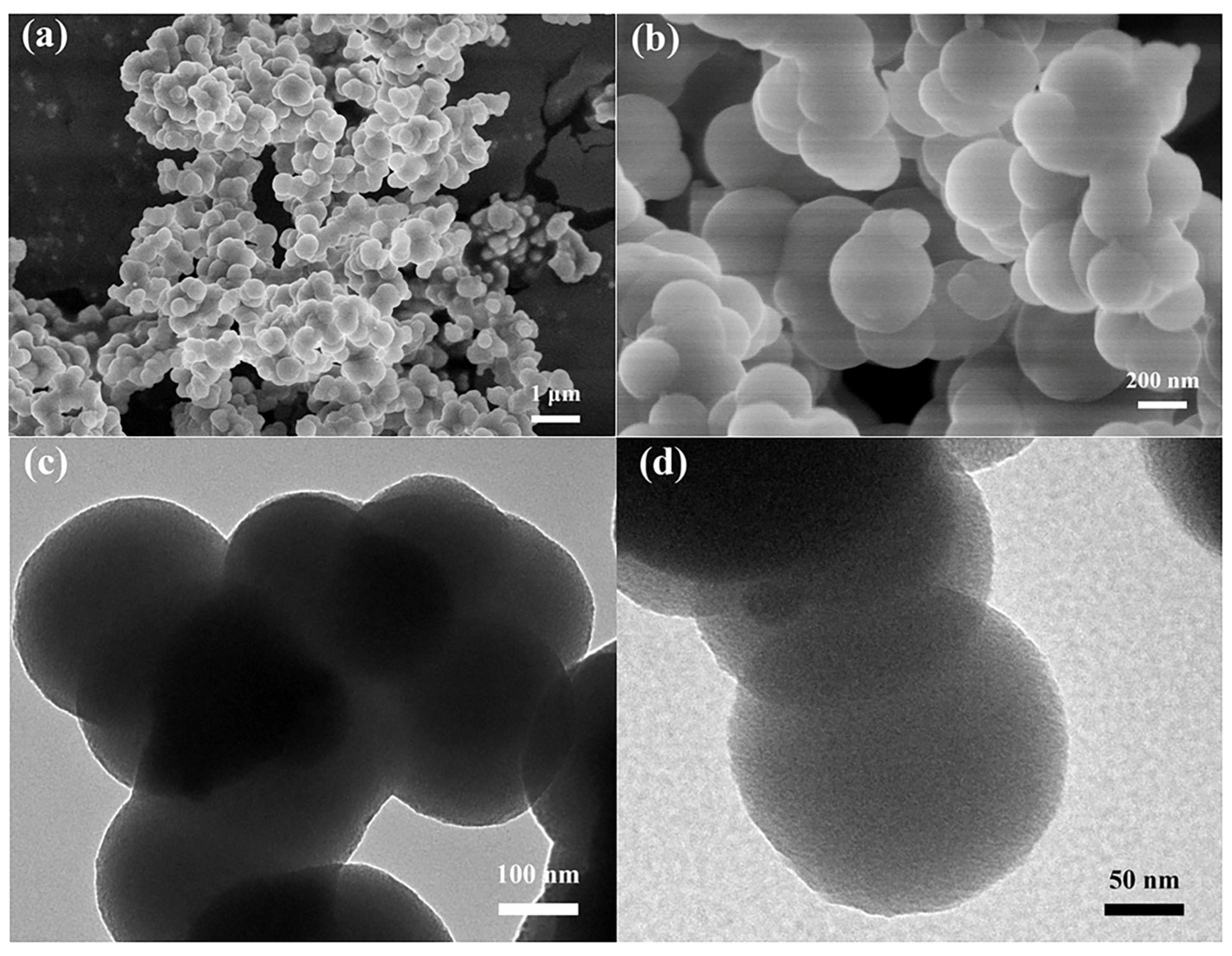
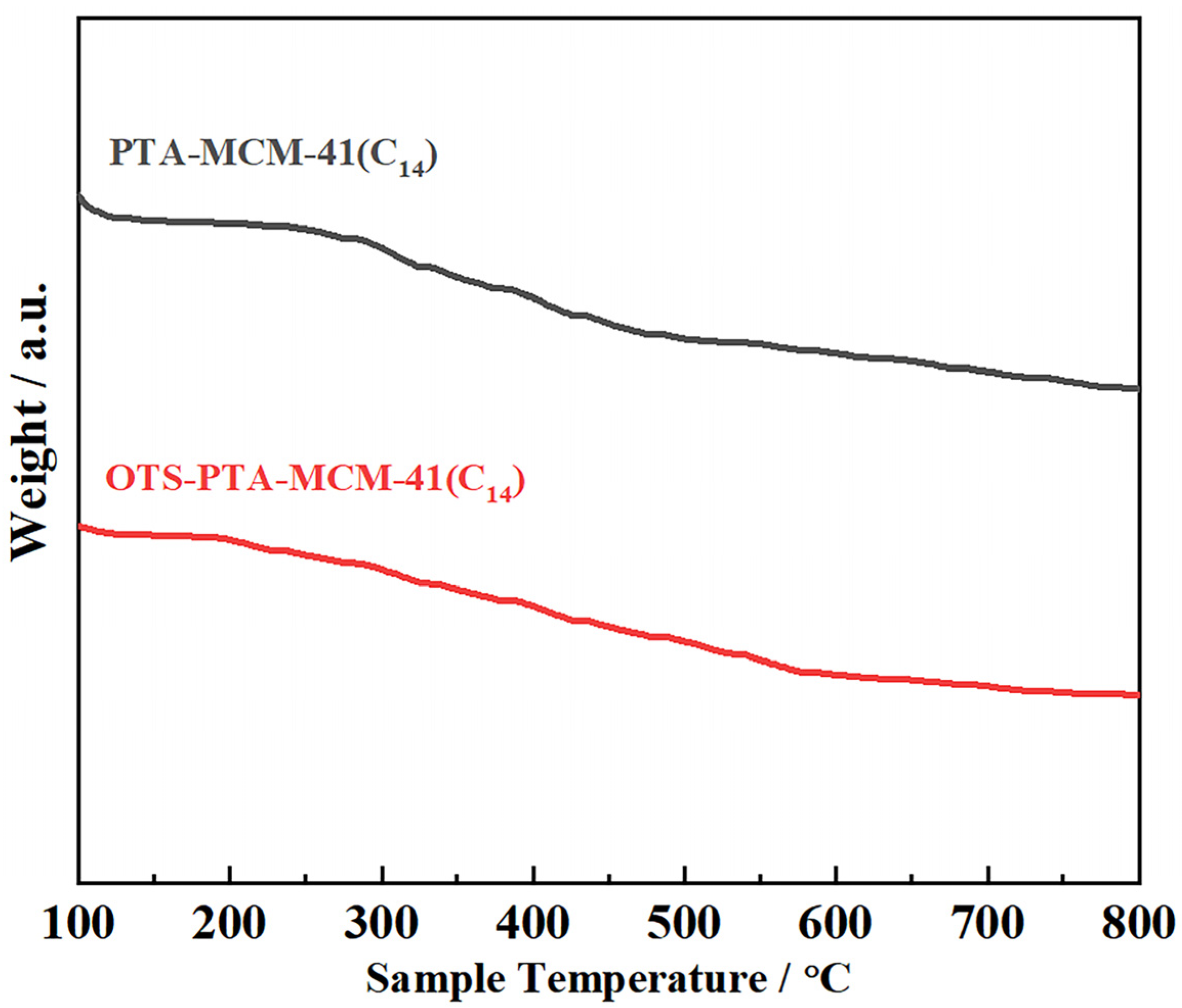
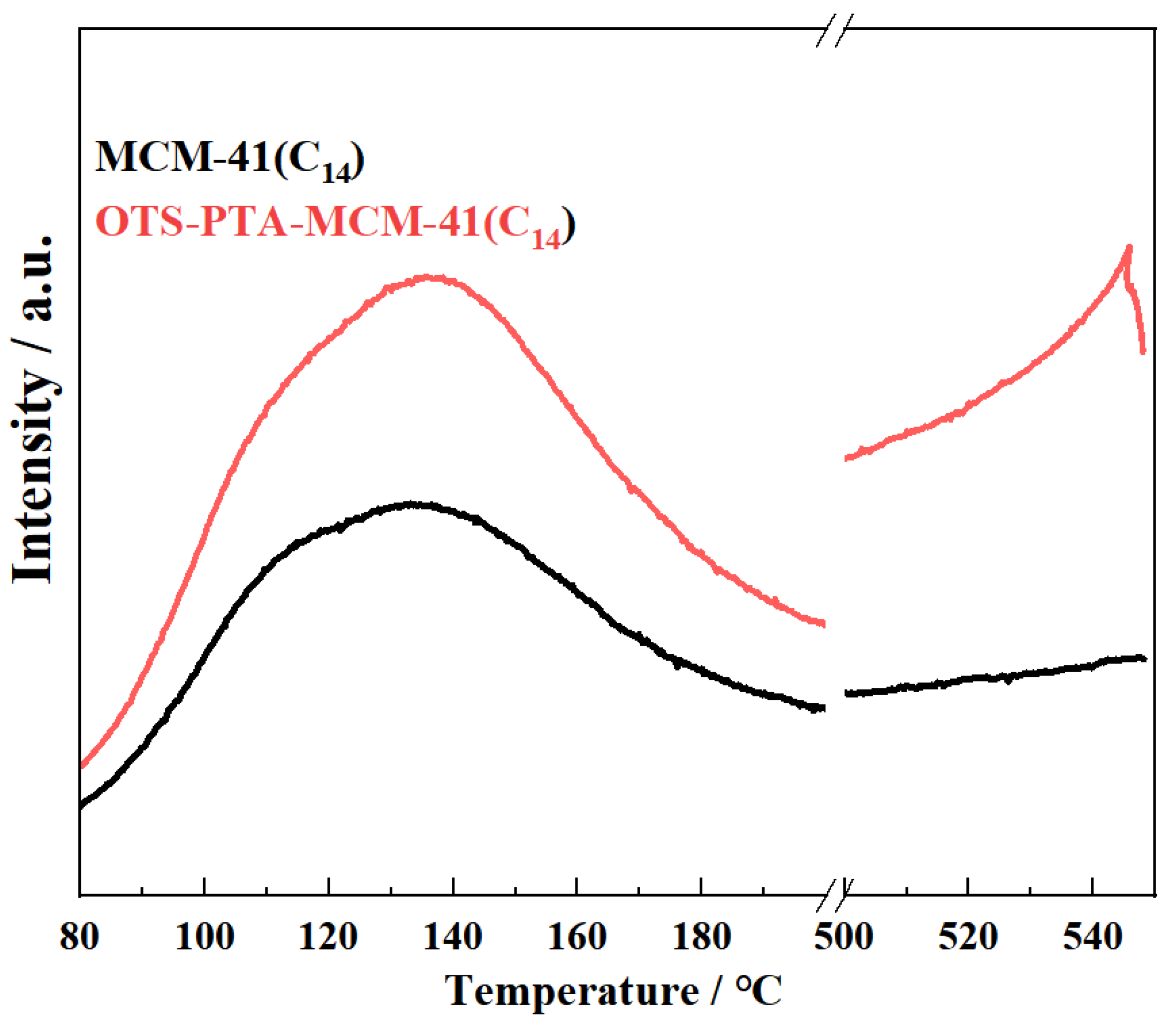
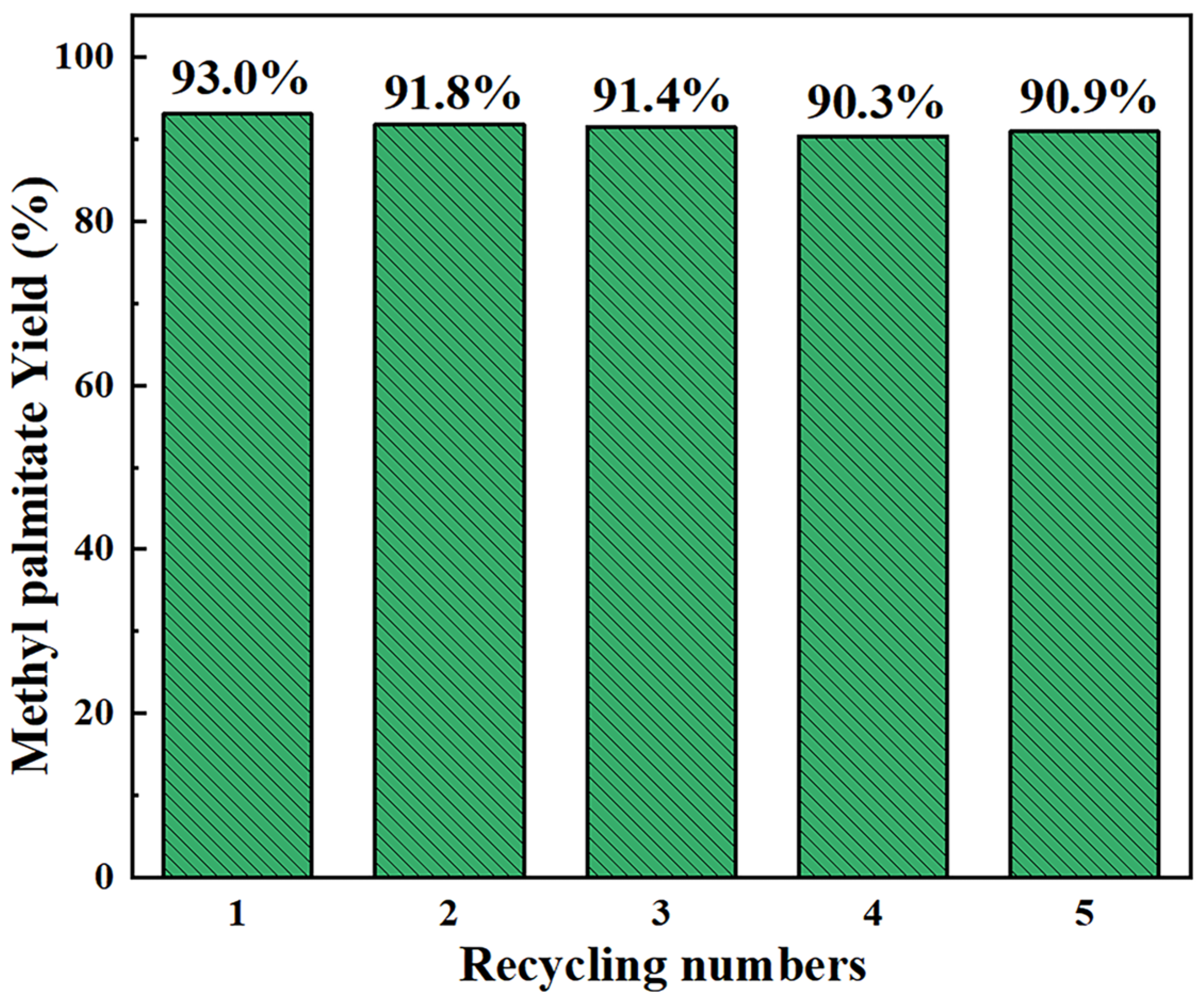
| Entry | Catalyst | Solvent | Conditions 1 | Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | C 2 (mg) | L 3 | M 4 | P 5 | |||
| 1 | PTA–MCM–41(C6) | water | 70 | 3 | 100 | 63.2 | 64.8 | 60.1 |
| 2 | PTA–MCM–41(C8) | water | 70 | 3 | 100 | 64.8 | 62.7 | 64.2 |
| 3 | PTA–MCM–41(C10) | water | 70 | 3 | 100 | 67.1 | 66.9 | 67.4 |
| 4 | PTA–MCM–41(C12) | water | 70 | 3 | 100 | 66.9 | 74.3 | 68.7 |
| 5 | PTA–MCM–41(C14) | water | 70 | 3 | 100 | 67.0 | 74.4 | 71.8 |
| 6 | PTA–MCM–41(C16) | water | 70 | 3 | 100 | 65.2 | 72.8 | 70.4 |
| 7 | PTA–MCM–41(C18) | water | 70 | 3 | 100 | 65.2 | 73.0 | 71.1 |
| 8 | OTS–PTA–MCM–41(C6) | water | 70 | 3 | 100 | 65.4 | 67.7 | 63.2 |
| 9 | OTS–PTA–MCM–41(C8) | water | 70 | 3 | 100 | 68.2 | 68.3 | 67.8 |
| 10 | OTS–PTA–MCM–41(C10) | water | 70 | 3 | 100 | 72.1 | 69.9 | 67.7 |
| 11 | OTS–PTA–MCM–41(C12) | water | 70 | 3 | 100 | 71.2 | 76.5 | 70.2 |
| 12 | OTS–PTA–MCM–41(C14) | water | 70 | 3 | 100 | 72.6 | 76.6 | 74.4 |
| 13 | OTS–PTA–MCM–41(C16) | water | 70 | 3 | 100 | 71.1 | 74.3 | 71.1 |
| 14 | OTS–PTA–MCM–41(C18) | water | 70 | 3 | 100 | 71.2 | 74.8 | 72.3 |
| Entry | Catalyst | Solvent | Conditions 1 | Yield (%) | ||
|---|---|---|---|---|---|---|
| T (°C) | t (h) | C 2 (mg) | Methyl Palmitate | |||
| 1 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | / | 35.6 |
| 2 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 50 | 78.2 |
| 3 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 100 | 88.0 |
| 4 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 150 | 93.0 |
| 5 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 200 | 92.7 |
| 6 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 250 | 91.9 |
| 7 | OTS–PTA–MCM–41(C14) | water | 70 | 3 | 150 | 74.4 |
| 8 | OTS–PTA–MCM–41(C14) | water | 90 | 3 | 150 | 75.9 |
| 9 | OTS–PTA–MCM–41(C14) | water | 110 | 3 | 150 | 86.1 |
| 10 | OTS–PTA–MCM–41(C14) | water | 130 | 3 | 150 | 89.8 |
| 11 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 150 | 93.0 |
| 12 | OTS–PTA–MCM–41(C14) | water | 170 | 3 | 150 | 91.2 |
| 13 | OTS–PTA–MCM–41(C14) | water | 190 | 3 | 150 | 90.5 |
| 14 | OTS–PTA–MCM–41(C14) | water | 210 | 3 | 150 | 91.1 |
| 15 | OTS–PTA–MCM–41(C14) | water | 150 | 1 | 150 | 55.7 |
| 16 | OTS–PTA–MCM–41(C14) | water | 150 | 3 | 150 | 93.0 |
| 17 | OTS–PTA–MCM–41(C14) | water | 150 | 6 | 150 | 82.0 |
| 18 | OTS–PTA–MCM–41(C14) | water | 150 | 9 | 150 | 82.5 |
| 19 | OTS–PTA–MCM–41(C14) | water | 150 | 12 | 150 | 90.9 |
| 20 | OTS–PTA–MCM–41(C14) | water | 150 | 24 | 150 | 84.2 |
| Entry | Catalyst | Solvent | Conditions 1 | Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|
| T (℃) | T (h) | C 2 (mg) | L 3 | M 4 | P 5 | |||
| 1 | PTA–MCM–41(C6) | water | 70 | 3 | 100 | 71.4 | 74.1 | 59.1 |
| 2 | PTA–MCM–41(C8) | water | 70 | 3 | 100 | 76.4 | 73.2 | 70.3 |
| 3 | PTA–MCM–41(C10) | water | 70 | 3 | 100 | 85.9 | 79.8 | 73.6 |
| 4 | PTA–MCM–41(C12) | water | 70 | 3 | 100 | 84.7 | 84.8 | 80.5 |
| 5 | PTA–MCM–41(C14) | water | 70 | 3 | 100 | 83.8 | 84.1 | 86.4 |
| 6 | PTA–MCM–41(C16) | water | 70 | 3 | 100 | 83.5 | 84.3 | 81.9 |
| 7 | PTA–MCM–41(C18) | water | 70 | 3 | 100 | 83.0 | 83.7 | 82.3 |
| Entry | Catalyst and Dosage | Solvent | Conditions 1 | Yield (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ca 2 | Cb 3 | Cc 4 | T (°C) | t (h) | L 5 | M 6 | P 7 | ||
| 1 | 150 | / | / | water | 150 | 3 | 92.9 | 83.8 | 86.4 |
| 2 | / | 150 | / | water | 150 | 3 | 90.6 | 92.1 | 89.4 |
| 3 | / | / | 150 | water | 150 | 3 | 91.7 | 86.6 | 92.1 |
| 4 | 33.75 | 90.75 | 25.5 | water | 150 | 3 | 91.9 | 89.7 | 91.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Shi, Q.; Liang, F.; Feng, D. The Green Synthesis of Biodiesel via Esterification in Water Catalyzed by the Phosphotungstic Acid–Functionalized Hydrophobic MCM–41 Catalyst. Catalysts 2024, 14, 142. https://doi.org/10.3390/catal14020142
Li D, Shi Q, Liang F, Feng D. The Green Synthesis of Biodiesel via Esterification in Water Catalyzed by the Phosphotungstic Acid–Functionalized Hydrophobic MCM–41 Catalyst. Catalysts. 2024; 14(2):142. https://doi.org/10.3390/catal14020142
Chicago/Turabian StyleLi, Dengke, Qinghao Shi, Fengbing Liang, and Dexin Feng. 2024. "The Green Synthesis of Biodiesel via Esterification in Water Catalyzed by the Phosphotungstic Acid–Functionalized Hydrophobic MCM–41 Catalyst" Catalysts 14, no. 2: 142. https://doi.org/10.3390/catal14020142
APA StyleLi, D., Shi, Q., Liang, F., & Feng, D. (2024). The Green Synthesis of Biodiesel via Esterification in Water Catalyzed by the Phosphotungstic Acid–Functionalized Hydrophobic MCM–41 Catalyst. Catalysts, 14(2), 142. https://doi.org/10.3390/catal14020142





