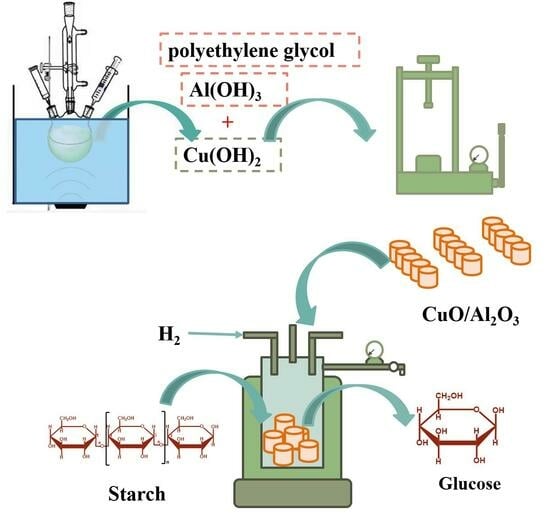
Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystallinity and Textural Properties
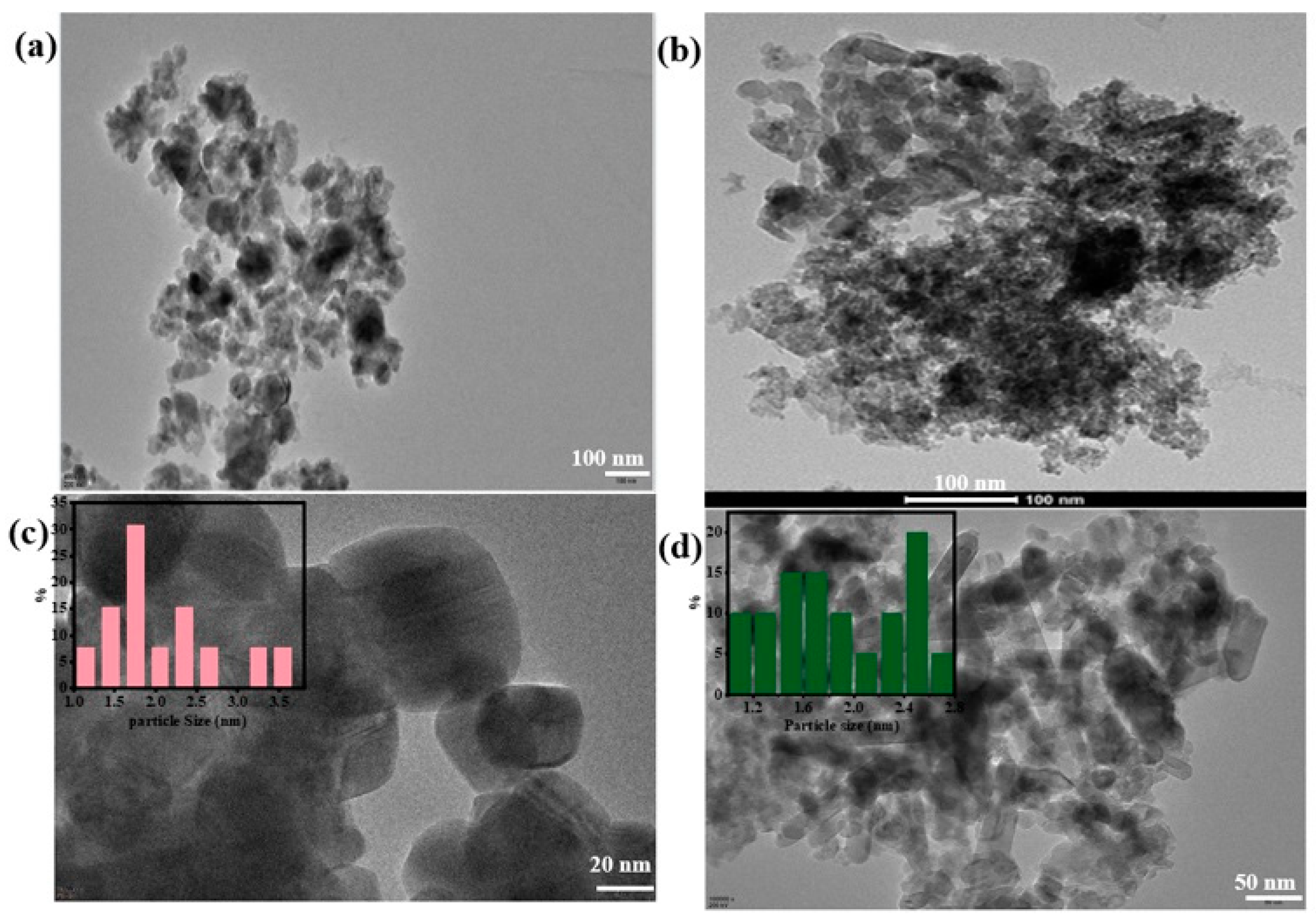
2.2. Morphology and Microstructure
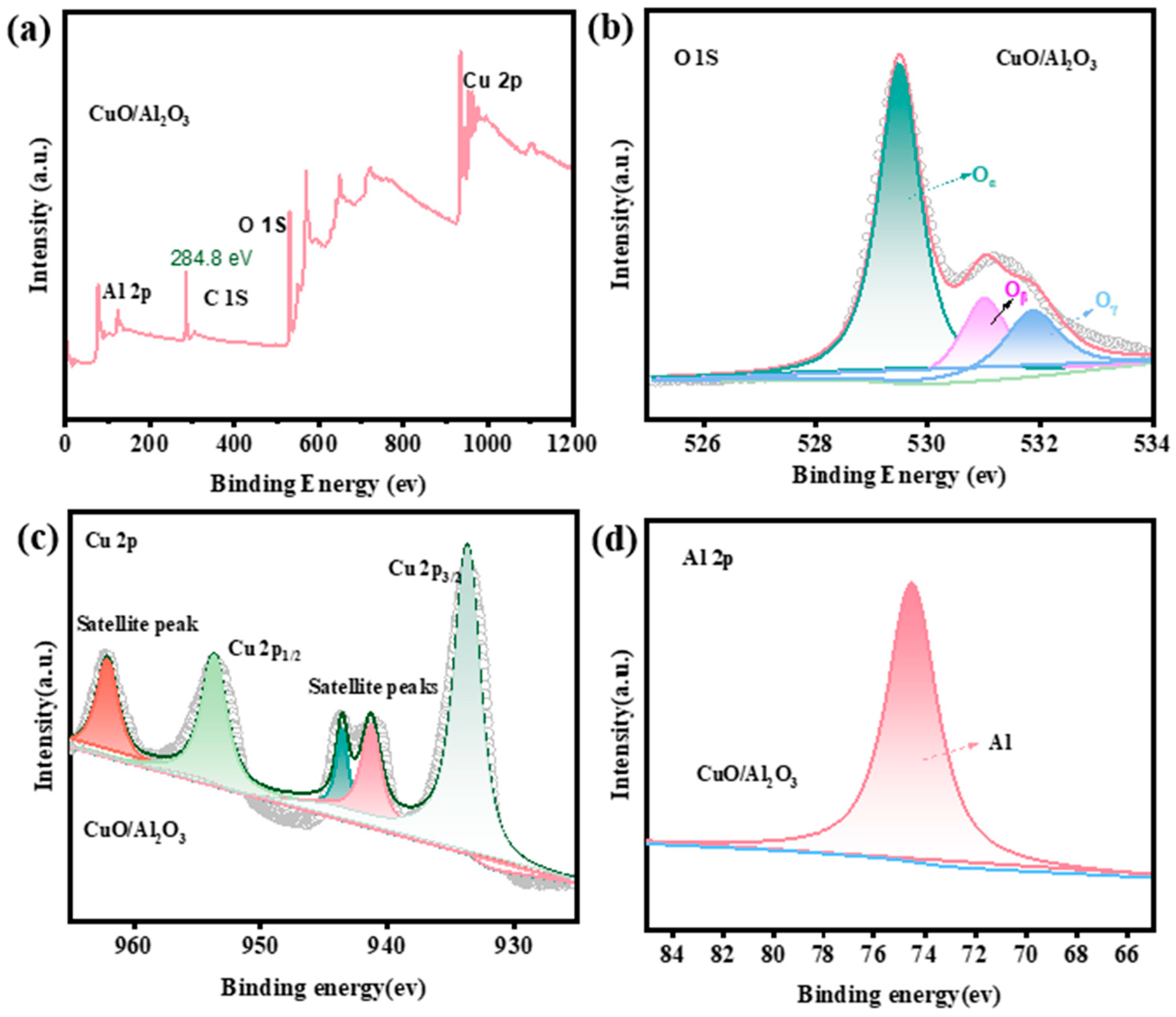
2.3. Surface Characterization of the Two Materials
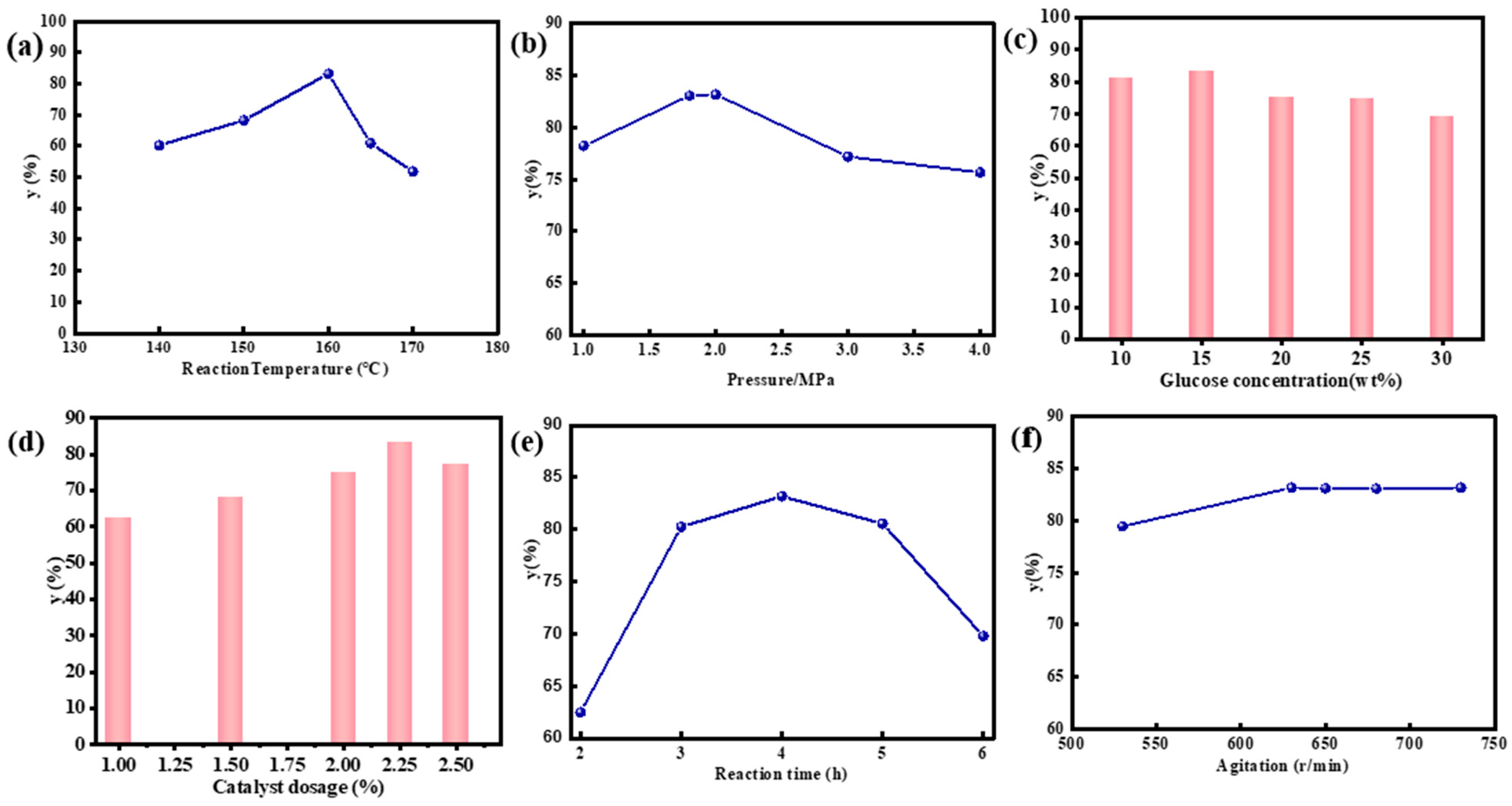
2.4. Catalytic Activity
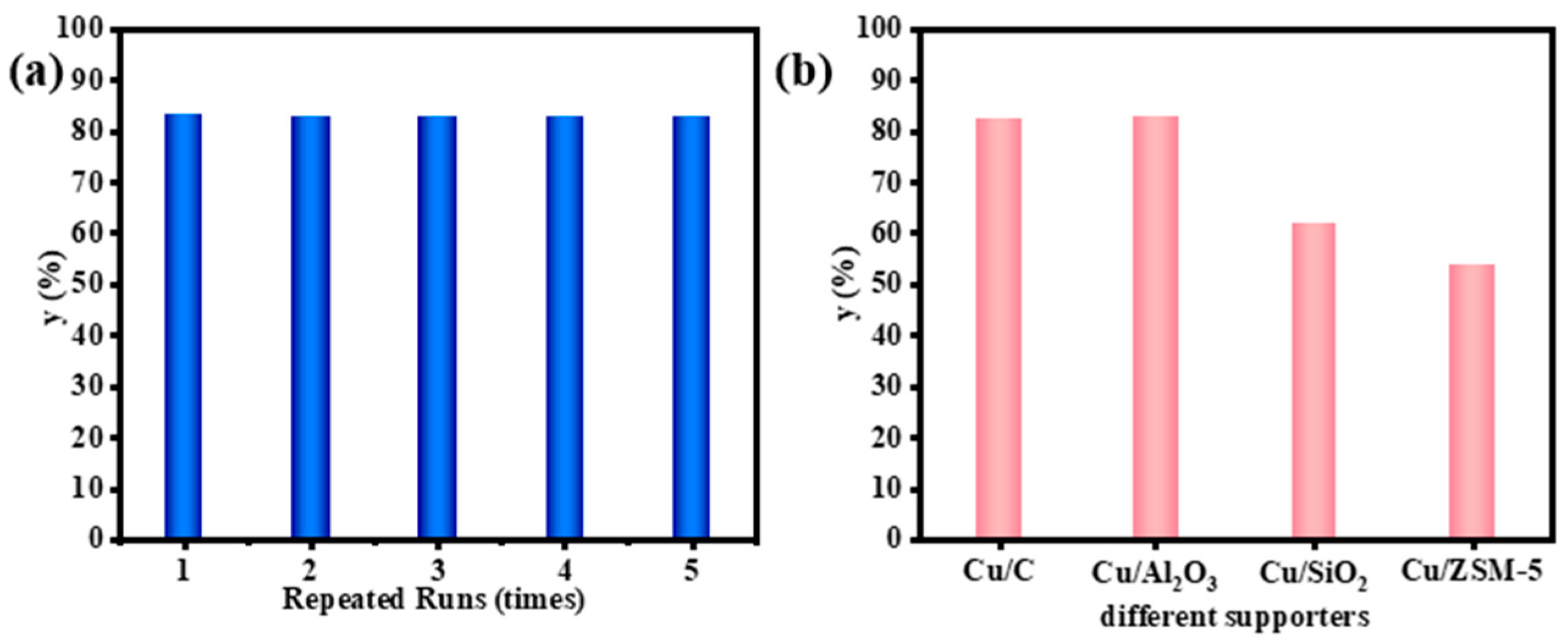
2.5. Reusability and Supporter Comparison
3. Catalytic Mechanism Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Catalytic Hydrogenation of Starch to Glucose
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rasheed, T.; Anwar, M.; Ahmad, N.; Sher, F.; Khan, S.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and Emerging Perspective of Biomass-Based Waste-to-Energy Technologies and their Socio-Environmental Impact: A Review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, L.; Liu, C. Biomass Derived Bifunctional Catalyst for the Conversion of Cassava Dreg into Sorbitol. Ind. Crop. Prod. 2023, 197, 116493. [Google Scholar] [CrossRef]
- Zelwicka, A.; Kolanowska, A.; Latos, P.; Jurczyk, S.; Boncel, S.; Chrobok, A. Carbon Nanotube/PTFE as a Hybrid Platform for Lipase B from Candida Antarctica in Transformation of A-Angelica Lactone into Alkyl-levulinates. Catal. Sci. Technol. 2020, 10, 3255–3264. [Google Scholar] [CrossRef]
- Cheng, X.L.; Liu, Y.; Wang, K.; Yu, H.L.; Yu, S.T.; Liu, S.W. High-efficient Conversion of Cellulose to Levulinic Acid Catalyzed via Functional Brønsted–Lewis Acidic Ionic Liquids. Catal. Lett. 2022, 152, 1064–1075. [Google Scholar] [CrossRef]
- Yepez, A.; Garcia, A.; Climent, M.S.; Romero, A.A.; Luque, R. Catalytic Conversion of Starch into Valuable Furan Derivatives Using Supported Metal Nanoparticles on Mesoporous Aluminosilicate Materials. Catal. Sci. Technol. 2014, 4, 428–434. [Google Scholar] [CrossRef]
- Uchegbu, N.N.; Ude, C.F.; Nwadi, O.M.M. Optimisation of Enzymatic Fermented Glucose Production of Wild Cocoyam Starch Using Response Surface Methodology. Glob. NEST J. 2022, 24, 351–361. [Google Scholar]
- Kang, S.; Fu, J.; Zhang, G. From Lignocellulosic Biomass to Levulinic Acid: A Review on Acid-Catalyzed Hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Guo, Z.; Mao, J.; Zhang, Q.; Zhang, Q.L.; Xu, F. Integrated Biorefinery of Bamboo for Fermentable Sugars, Native-like Lignin, and Furfural Production by Novel Deep Eutectic Solvents Treatment. Ind. Crop. Prod. 2022, 188, 115453. [Google Scholar] [CrossRef]
- Ba lachandran Kirali, A.; Sreekantan, S.; Marimuthu, B. Ce Promoted Cu/γ-Al2O3 Catalysts for the Enhanced Selectivity of 1,2-Propanediol from Catalytic Hydrogenolysis of Glucose. Catal. Commun. 2022, 165, 106447. [Google Scholar] [CrossRef]
- Gallezot, P. Alternative Value Chains for Biomass Conversion to Chemicals. Top. Catal. 2010, 53, 1209–1213. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Santek, M.I.; Komes, D.; Novak, S.; Santek, B. Bioethanol Production from Renewable Raw Materials and its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Muhammad, S.; Qurratulann, A.; Ahmad, S.; Akhtar, M.W. Structural Engineering and Truncation of A-Amylase from the Hyperthermophilic Archaeon. Methanocaldococcus Jannaschii. Int. J. Biol. Macromol. 2024, 256, 128387. [Google Scholar]
- Li, Z.; Cai, L.; Gu, Z.; Shi, Y. Effects of Granule Swelling on Starch Saccharification by Granular Starch Hydrolyzing Enzyme. J. Agricul. Food Chem. 2014, 62, 8114–8119. [Google Scholar] [CrossRef] [PubMed]
- Intaramas, K.; Jonglertjunya, W.; Laosiripojana, N.; Sakdaronnarong, C. Selective Conversion of Cassava Mash to Glucose Using Solid Acid Catalysts by Sequential Solid State Mixed-Milling Reaction and Thermo-Hydrolysis. Energy 2018, 149, 837–847. [Google Scholar] [CrossRef]
- Stummann, M.; Høj, M.; Gabrielsen, J.; Clausen, L.R.; Jensen, P.A.; Jensen, A.D. A Perspective on Catalytic Hydropyrolysis of Biomass. Renew. Sustain. Energy Rev. 2021, 143, 110960. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Wang, W.; Liang, J.H.; Orooji, Y.; Dai, C.; Fu, X.M.; Yang, Y.; Xu, W.L.; Zhu, J. Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-Co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3. Catalysts 2020, 10, 1068. [Google Scholar] [CrossRef]
- Fu, X.; Ren, X.; Shen, J.; Jiang, Y.; Wang, Y.H.; Orooji, Y.; Xu, W.L.; Liang, J.H. Synergistic Catalytic Hydrogenation of Furfural to 1,2-Pentanediol and 1,5-Pentanediol with LDO Derived from Cumgal Hydrotalcite. Mol. Catal. 2021, 499, 111298–111305. [Google Scholar] [CrossRef]
- Christina, Å.; Guido, Z.; Nelson, T.; Gorton, L. A Kinetic Model for Enzymatic Wheat Starch Saccharification. Chem. Technol. Biotechnol. 2000, 75, 306–314. [Google Scholar]
- He, Z.; Zhang, L.; Gu, J.; Gu, J.; Pan, Q.; Zhou, S.; Gao, B.; Wei, D. Cloning of a Novel Thermostable Glucoamylase from Thermo Ph Ilic Fungus Rhizomucor Pusillus and High-Level Co-Expression with A-Amylase in Pichia Pastoris. BMC Biotechnol. 2014, 14, 114. [Google Scholar] [CrossRef]
- Wang, D.; Hou, F.; Ma, X.; Chen, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Study on the Mechanism of Ultrasound-Accelerated Enzymatic Hydrolysis of Starch: Analysis of Ultrasound Effect on Different Objects. Int. J. Biol. Macromol. 2020, 148, 493–500. [Google Scholar] [CrossRef]
- Kumar, V.; Pulidindi, I.; Gedanken, A. Selective Conversion of Starch to Glucose Using Carbon Based Solid Acid Catalyst. Renew. Energy 2015, 78, 141–145. [Google Scholar] [CrossRef]
- Manna, S.; Antonchick, A. Catalytic Transfer Hydrogenation Using Biomass as Hydrogen Source. Chemsuschem 2019, 12, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, R. Hydrothermal Conversion of Xylose, Glucose, and Cellulose under the Catalysis of Transition Mental Sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef]
- Xiang, Y.; Chitry, V.; Liddicoat, P.; Felfer, P.; Cairney, J.; Ringer, S.; Kruse, N. Long-Chain Terminal Alcohols through Catalytic CO Hydrogenation. J. Am. Chem. Soc. 2013, 135, 7114–7117. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, S.; Liu, R.; Zhai, X.H.; Wang, X.K.; Wang, L.; Guo, H.C.; Yi, Y.H. Plasma-Catalytic CO2 Hydrogenation to Methanol over Cuo-Mgo/Beta Catalyst with High Selectivity. Appl. Catal. B Environ. 2024, 342, 123422. [Google Scholar] [CrossRef]
- Mondal, S.; Malviya, H.; Biswas, P. Kinetic Modelling for the Hydrogenolysis of Bio-Glycerol in the Presence of a Highly Selective Cu-Ni-Al2O3 Catalyst in a Slurry Reactor. React. Chem. Eng. 2019, 4, 595–609. [Google Scholar] [CrossRef]
- Oberhauser, W.; Evangelisti, C.; Jumde, R.; Psaro, R.; Vizza, F.; Bevilacqua, M.; Filippi, J.; Machado, B.F.; Serp, P. Platinum on Carbonaceous Supports for Glycerol Hydrogenolysis: Support Effect. J. Catal. 2015, 325, 111–117. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, J.; Wang, J.; Jia, D.S.; Zheng, H.Y.; Li, Z. Effect of Carbon Support on the Catalytic Performance of Cu-Based Nanoparticles for Oxidative Carbonylation of Methanol. Appl. Surf. Sci. 2018, 455, 696–704. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B. Recent Progress on Catalytic Pyrolysis of Lignocellulosic Biomass to Highgrade Bio-Oil and Bio-Chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Liang, J.; Zhu, J. Efficient and Selective Catalytic Conversion of Hemicellulose in Rice Straw by Metal Catalyst under Mild Conditions. Sustainability 2020, 12, 10601. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Chen, L.; Liu, J.; Zhang, Q.; Ma, L. A Cu-ZnO-Al2O3 Catalyst with Oxygen Vacancy for Efficient Hydrodeoxygenation of Lignin-Derived Guaiacol to Hydrocarbons. Chem. Eng. Sci. 2024, 285, 119616. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, X.; Liu, B.Q.; Xu, S.; Zhang, H.; Gu, Y.; Xu, Y.; Lu, L.; Xu, W.; Liang, J. Ultrahigh Selectivity Self-Condensation of Cyclohexanone over TiO2/Al2O3. Catal. Kinet. Study 2023, 136, 2071–2087. [Google Scholar] [CrossRef]
- Yang, X.; Erickson, L.; Hohn, K.; Jeevanandam, P.; Klabunde, K.J. Sol-Gel Cu-Al2O3 Adsorbents for Selective Adsorption of Thiophene out of Hydrocarbon. Ind. Eng. Chem. Res. 2006, 45, 6169–6174. [Google Scholar] [CrossRef]
- Ostovar, S.; Moussavi, G.; Mohammadi, S.; Marin, M.L.; Bosca, F.; Diego-Lopez, A.; Giannakis, S. Rapid Degradation of Omeprazole and Highly Effective Inactivation of E. coli in the UVA-light Photocatalytic Process with Cu-doped in Spinel-Structured ɣ-Al2O3 as a Stable Catalyst. Chem. Eng. J. 2024, 479, 147536. [Google Scholar] [CrossRef]
- Wang, X.; Chong, J.; Liang, S.; Guo, M.; Zhang, M. Contributions of Mn-Doping in Cuo/Al2O3 Sorbent for Enhancement of H2S Removal at Low and Wide Temperature Range. Fuel 2023, 334, 126546. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, J.; Lin, B.; Zhou, Y. A Highly Atom-Efficient and Stable Copper Catalyst Loaded on Amorphous Uio-66-NH2 for Hcl Oxidation to Chlorine. Appl. Catal. A Gen. 2022, 634, 118532. [Google Scholar] [CrossRef]
- Wu, X.; Meng, H.; Du, Y.; Liu, J.; Hou, B.; Xie, X. Insight into Cu2O/CuO Collaboration in the Selective Catalytic Reduction of NO with NH3: Enhanced Activity and Synergistic Mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Singh, R.; Kundu, K.; Pant, K.K. CO2 Hydrogenation to Methanol over Cu-ZnO-CeO2 Catalyst: Reaction Structure-Activity Relationship, Optimizing Ce and Zn Ratio, and Kinetic Study. Chem. Eng. J. 2024, 479, 147783. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Peng, J.; Zhang, H.; Zhang, Y.; Lai, B. Efficient Degradation of Sulfamethoxazole by the Cuo@Al2O3 (EPC) Coupled PMS System: Optimization, Degradation Pathways and Toxicity Evaluation. Chem. Eng. J. 2019, 359, 1097–1110. [Google Scholar] [CrossRef]
- Zhou, C.; Lv, G.; Zou, X.; Wang, J.; Chen, Y.; Shen, J.; Su, S.; Xing, W.; Fan, D.; Shen, Y. Construction of Core-Shell Coordination Sponge-Fe0@Cu-Pd Trimetal for High Efficient Activation of Room-Temperature Dissolved Ambient Oxygen toward Synergistic Catalytic Degradation of Tetracycline and p-Nitrophenol. Sep. Purif. Technol. 2024, 329, 125195. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, J.; Peng, F.; Chen, S.; Wang, Q.; Liu, Z. A Non-Noble Monometallic Catalyst Derived from Cu–MOFs for Highly Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemistrySelect 2019, 4, 13517–13524. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol Hydrogenolysis on Heterogeneous Catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Tajvidi, K.; Pupovac, K.; Kükrek, M.; Palkovits, R. Copper-Based Catalysts for Efficient Valorization of Cellulose. ChemSusChem 2012, 5, 2139–2142. [Google Scholar] [CrossRef] [PubMed]



| Samples | SBET/(m2/g) | VT/(cm3/g) | Dpore/nm |
|---|---|---|---|
| Cu/Al2O3 | 24.7 | 0.26 | 7.9 |
| CuO/Al2O3 | 51.2 | 0.32 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Li, J.; Cheng, F.; Liang, J. Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose. Catalysts 2024, 14, 132. https://doi.org/10.3390/catal14020132
Zhu S, Li J, Cheng F, Liang J. Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose. Catalysts. 2024; 14(2):132. https://doi.org/10.3390/catal14020132
Chicago/Turabian StyleZhu, Shenghua, Jue Li, Fuchang Cheng, and Jinghua Liang. 2024. "Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose" Catalysts 14, no. 2: 132. https://doi.org/10.3390/catal14020132
APA StyleZhu, S., Li, J., Cheng, F., & Liang, J. (2024). Forming a Cu-Based Catalyst for Efficient Hydrogenation Conversion of Starch into Glucose. Catalysts, 14(2), 132. https://doi.org/10.3390/catal14020132