The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterizations
2.2. Catalytic Performance on Oxidative Desulfurization
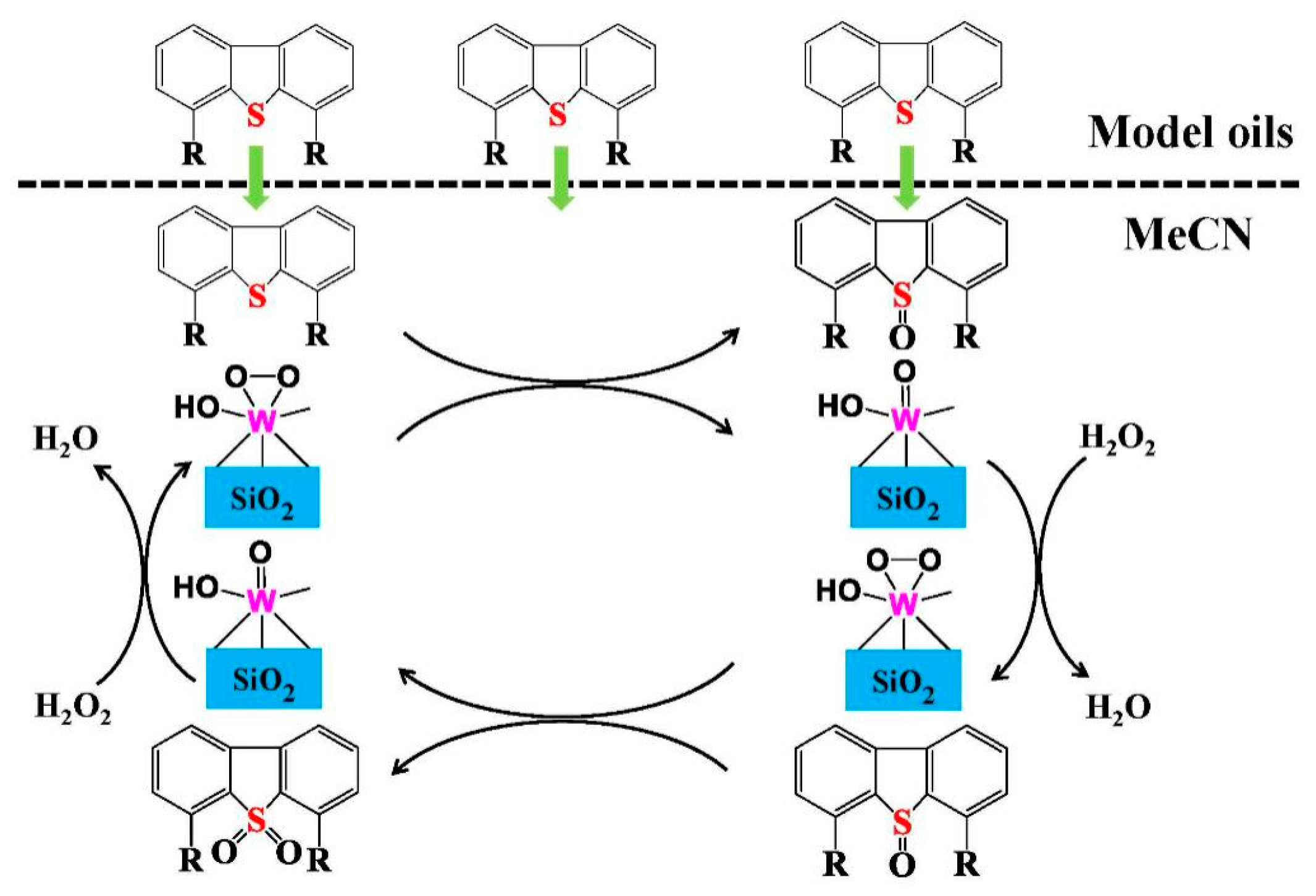
2.3. Reaction Mechanism of ODS Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Pretreatment of RH
3.2.2. Synthesis of WO3/SiO2
3.2.3. Treatment of Samples with Hydrogen Peroxide
3.3. Characterization
3.4. Catalytic Activity and Recycle Tests
3.4.1. Catalytic Activity
3.4.2. Recycle Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Huai, J.L.; Liu, X.X.; Zhang, Y.H.; Yang, Y.; Gou, Y.; Qin, L.; Shan, J.L.; Zheng, Y.C.; Li, H. Synthesis of hierarchical TS-1 and its recycling catalytic property for oxidative desulfurization. J. Renew. Mater. 2022, 10, 1711–1726. [Google Scholar] [CrossRef]
- Qin, Y.J.; Xun, S.H.; Zhan, L.L.; Lu, Q.Q.; He, M.Q.; Jiang, W.; Li, H.P.; Zhang, M.; Zhu, W.S.; Li, H.M. Synthesis of mesoporous WO3/TiO2 catalyst and its excellent catalytic performance for the oxidation of dibenzothiophene. New J. Chem. 2017, 41, 569–578. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, T.; Zhang, S.L.; Fan, Y.C.; Chen, B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep. Purif. Technol. 2020, 231, 115923. [Google Scholar] [CrossRef]
- Song, C.S.; Ma, X.L. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Zhu, W.S.; Zhu, G.P.; Li, H.M.; Chao, Y.H.; Zhang, M.; Du, D.L.; Wang, Q.; Zhao, Z. Catalytic kinetics of oxidative desulfurization with surfactant-type polyoxometalate-based ionic liquids. Fuel Process. Technol. 2013, 106, 70–76. [Google Scholar] [CrossRef]
- Li, B.S.; Liu, Z.X.; Han, C.Y.; Liu, J.J.; Zuo, S.L.; Zhou, Z.Y.; Pang, X.M. Preparation, characterization and catalytic performance of the silica pillared clay incorporated with phosphotungstic acid using different surfactants as template. J. Mol. Catal. A Chem. 2011, 348, 106–113. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, X.X.; Shan, J.L.; Huai, J.L.; Yang, H.; Yan, X.M.; Zheng, Y.C.; Li, H. Synthesis of amorphous mesoporous TiO2-SiO2 and its excellent catalytic performance in oxidative desulfurization. Inorg. Chem. Commun. 2021, 123, 108336. [Google Scholar] [CrossRef]
- Manriquez-Ramirez, M.E.; Valdez, M.T.; Castro, L.V.; Flores, M.E.; Ortiz-Islas, E. Application of CeO2-V2O5 catalysts in the oxidative desulfurization of 4,6-dimethyl dibenzothiophene as a model reaction to remove sulfur from fuels. Mater. Res. Bull. 2022, 153, 111864. [Google Scholar] [CrossRef]
- Wang, C.; Miao, Q.Y.; Huang, X.Q.; Li, J.X.; Duan, Y.S.; Yan, L.J.; Jiang, Y.; Lu, S.X. Fabrication of morphologically various forms of g-C3N4 supported MoO3 catalyst for the oxidative desulfurization of dibenzothiophene. New J. Chem. 2020, 44, 18745–18755. [Google Scholar] [CrossRef]
- Li, X.C.; Huang, S.X.; Xu, Q.R.; Yang, Y.F. Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst. Transit. Met. Chem. 2009, 34, 943–947. [Google Scholar] [CrossRef]
- Zhao, R.X.; Li, X.P.; Su, J.X.; Gao, X.H. Preparation of WO3/g-C3N4 composites and their application in oxidative desulfurization. Appl. Surf. Sci. 2017, 392, 810–816. [Google Scholar] [CrossRef]
- Zhang, H.X.; Ding, X.F.; Chen, X.; Ma, Y.J.; Wang, Z.C.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing D-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73. [Google Scholar] [CrossRef]
- Adam, F.; Appaturi, J.N.; Iqbal, A. The utilization of rice husk silica as a catalyst: Review and recent progress. Catal. Today 2012, 190, 2–14. [Google Scholar] [CrossRef]
- Adam, F.; Iqbal, A. The liquid phase oxidation of styrene with tungsten modified silica as a catalyst. Chem. Eng. J. 2011, 171, 1379–1386. [Google Scholar] [CrossRef]
- Shinde, A.B.; Shrigadi, N.B.; Samant, S.D. Development of Fe-, Sb-, Bi- and Al-impregnated silica catalysts using rice husk silica as a support for Friedel-Crafts benzylation of arenes. J. Chem. Technol. Biotechnol. 2003, 78, 1234–1238. [Google Scholar] [CrossRef]
- Hindryawati, N.; Maniam, G.P.; Karim, M.R.; Chong, K.F. Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. Eng. Sci. Technol. Int. J. 2014, 17, 95–103. [Google Scholar] [CrossRef]
- Priya, A.S.; Devi, K.R.S.; Karthik, K.; Sugunan, S. Modified Rice Husk Silica from Biowaste: An efficient catalyst for transesterification of diethyl malonate and benzyl alcohol. Waste Biomass Valoriz. 2020, 11, 4809–4819. [Google Scholar] [CrossRef]
- Labbé, P. Tungsten oxides, tungsten bronzes and tungsten bronze-type structures. Key Eng. Mater. 1992, 68, 293–339. [Google Scholar] [CrossRef]
- Rajendran, A.; Fan, H.X.; Cui, T.Y.; Feng, J.; Li, W.Y. Enrichment of polymeric WOx species in WOx@SnO2 catalysts for ultra-deep oxidative desulfurization of liquid fuels. Fuel 2021, 290, 120036. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.L.; Cañizares, P.; Rodriguez, L. Partial oxidation of methane to formaldehyde over W/SiO2 catalysts. Appl. Catal. A 1999, 184, 143–152. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.L.; Cañizares, P.; Rodriguez, L. Partial oxidation of methane to formaldehyde over W/HZSM-5 catalysts. Appl. Catal. A 1998, 172, 165–176. [Google Scholar] [CrossRef]
- Luna, M.L.; Alvarez-Amparán, M.A.; Cedeño-Caero, L. Performance of WOx-VOx based catalysts for ODS of dibenzothiophene compounds. J. Taiwan Inst. Chem. Eng. 2019, 95, 175–184. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.R.; Xu, J.F.; Wen, J.; Zhang, H.; Zhang, L.H. Preparation of WO3/CNT catalysts in presence of ionic liquid C(16)mim Cl and catalytic efficiency in oxidative desulfurization. J. Chem. Technol. Biotechnol. 2019, 94, 3403–3412. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.H.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 750–753. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001; pp. 215–217. [Google Scholar]
- Wang, H.X.; Tang, M.X.; Shi, F.L.; Ding, R.M.; Wang, L.C.; Wu, J.B.; Li, X.K.; Liu, Z.; Lv, B.L. Amorphous Cr2WO6-Modified WO3 Nanowires with a Large Specific Surface Area and Rich Lewis Acid Sites: A Highly Efficient Catalyst for Oxidative Desulfurization. ACS. Appl. Mater. Interfaces 2020, 12, 38140–38152. [Google Scholar] [CrossRef]
- Hasannia, S.; Kazemeini, M.; Seif, A.; Rashidi, A. Investigations of the ODS process utilizing CNT- and CNF-based WO3 catalysts for environmental depollution: Experimental and theoretical aspects. Environ. Sci. Pollut. Res. 2023, 30, 26417–26434. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; De los Reyes, J.A.; Torres-García, E. Solvent effect in homogeneous and heterogeneous reactions to remove dibenzothiophene by an oxidation-extraction scheme. Ind. Eng. Chem. Res. 2008, 47, 5353–5361. [Google Scholar] [CrossRef]
- Torres-García, E.; Galano, A.; Rodriguez-Gattorno, G. Oxidative desulfurization (ODS) of organosulfur compounds catalyzed by peroxo-metallate complexes of WOx-ZrO2: Thermochemical, structural, and reactivity indexes analyses. J. Catal. 2011, 282, 201–208. [Google Scholar] [CrossRef]
- Wei, P.P.; Yang, Y.; Li, W.Z.; Li, G.M. Keggin-POM@rht-MOF-1 composite as heterogeneous catalysts towards ultra-deep oxidative fuel desulfurization. Fuel 2020, 274, 117834. [Google Scholar] [CrossRef]
- Chakraverty, A.; Mishra, P.; Banerjee, H.D. Investigation of combustion of raw and acid-leached rice husk for production of pure amorphous white silica. J. Mater. Sci. 1988, 23, 21–24. [Google Scholar] [CrossRef]
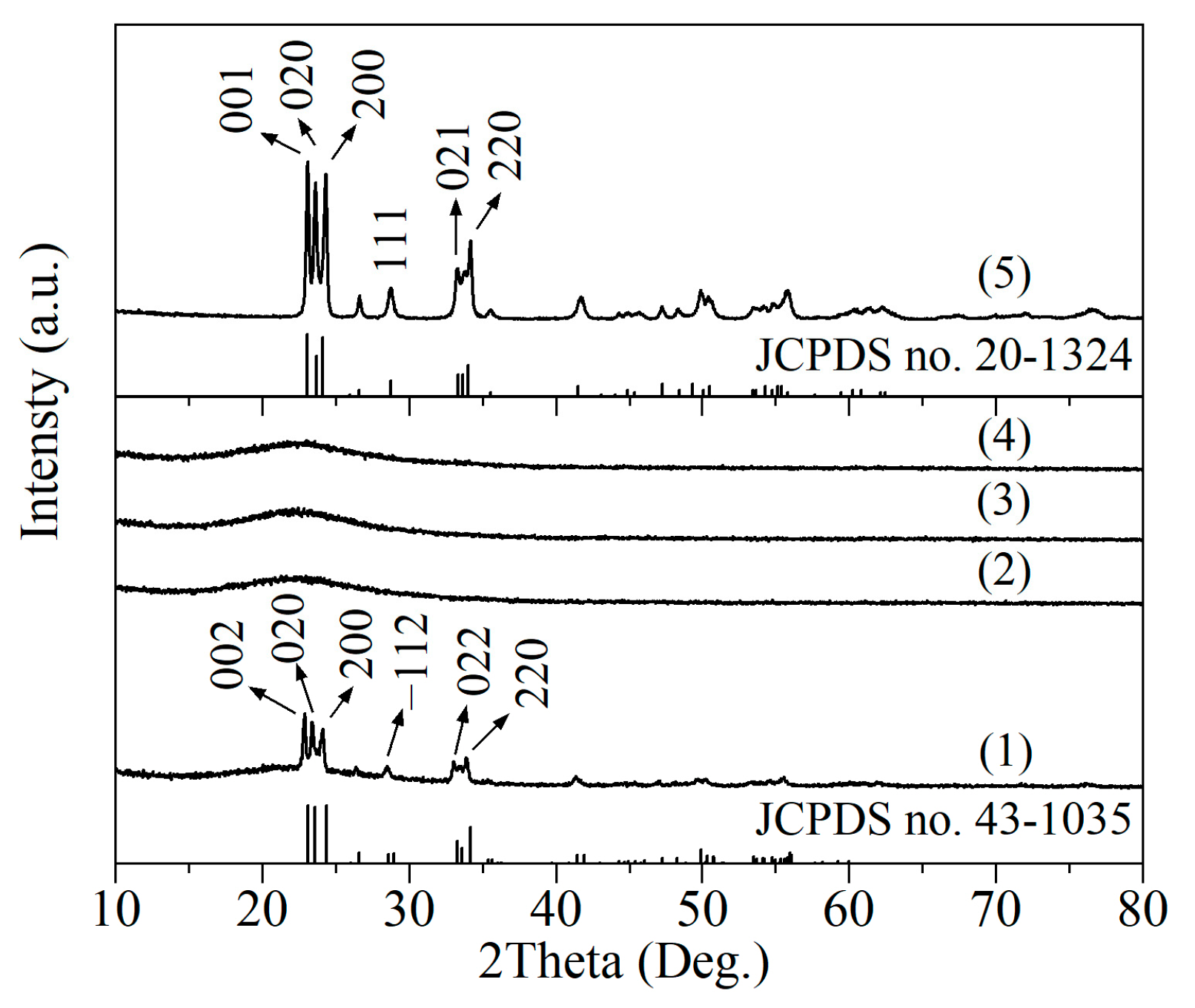
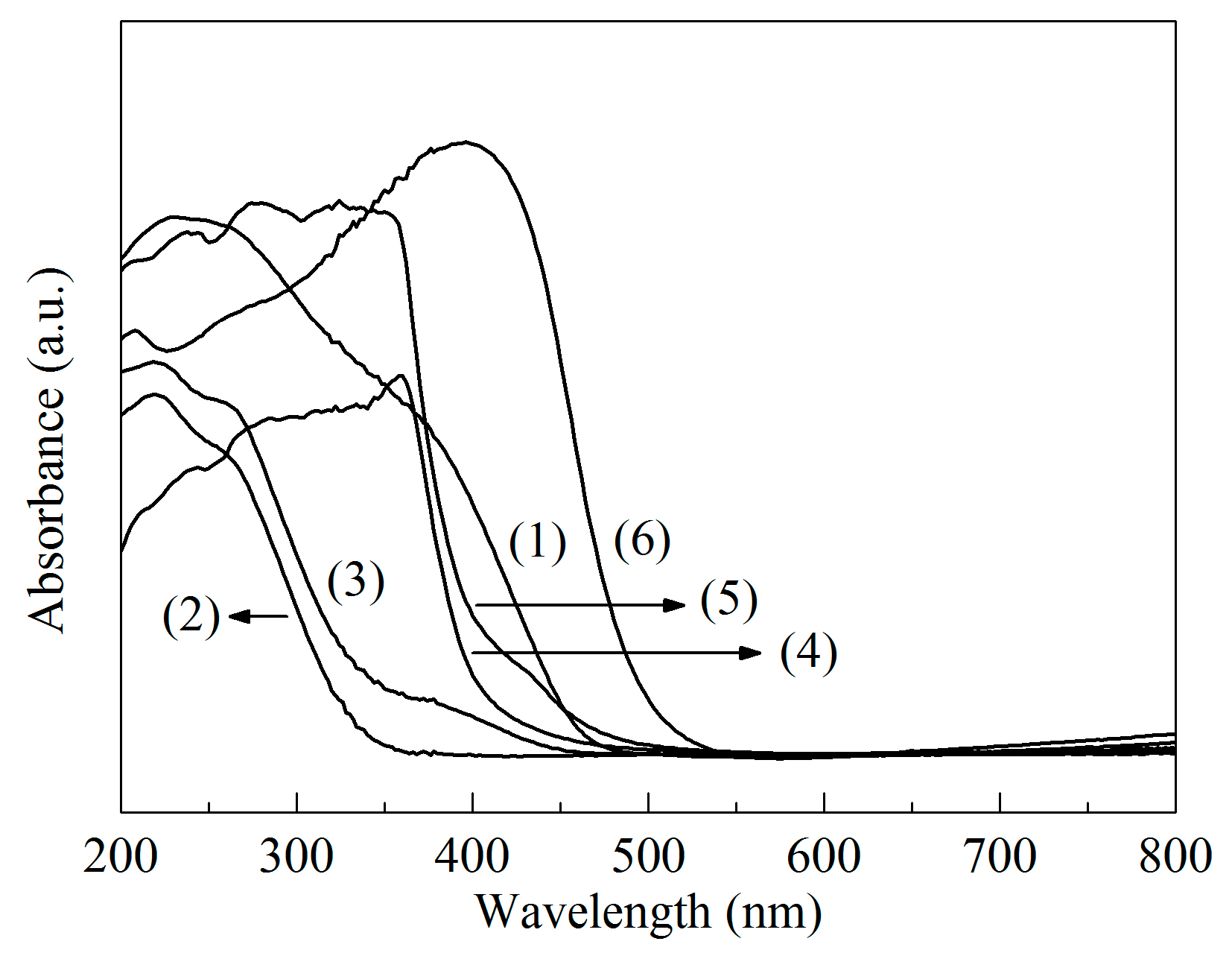

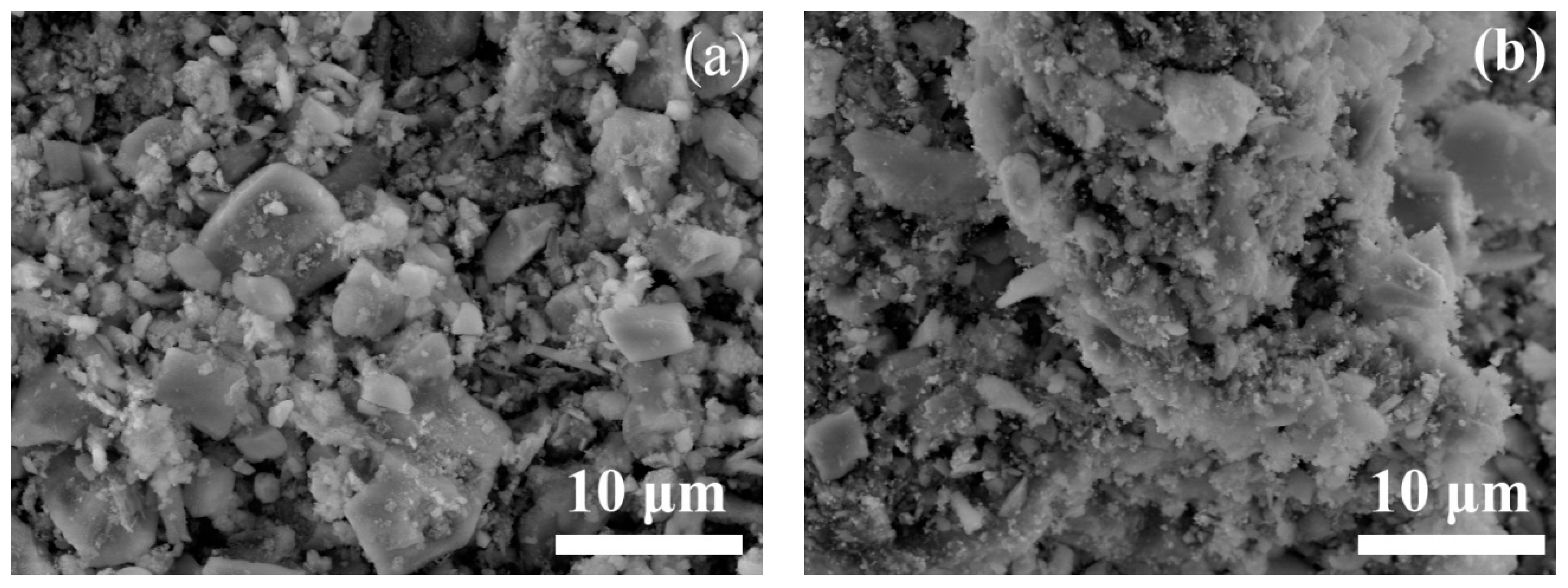

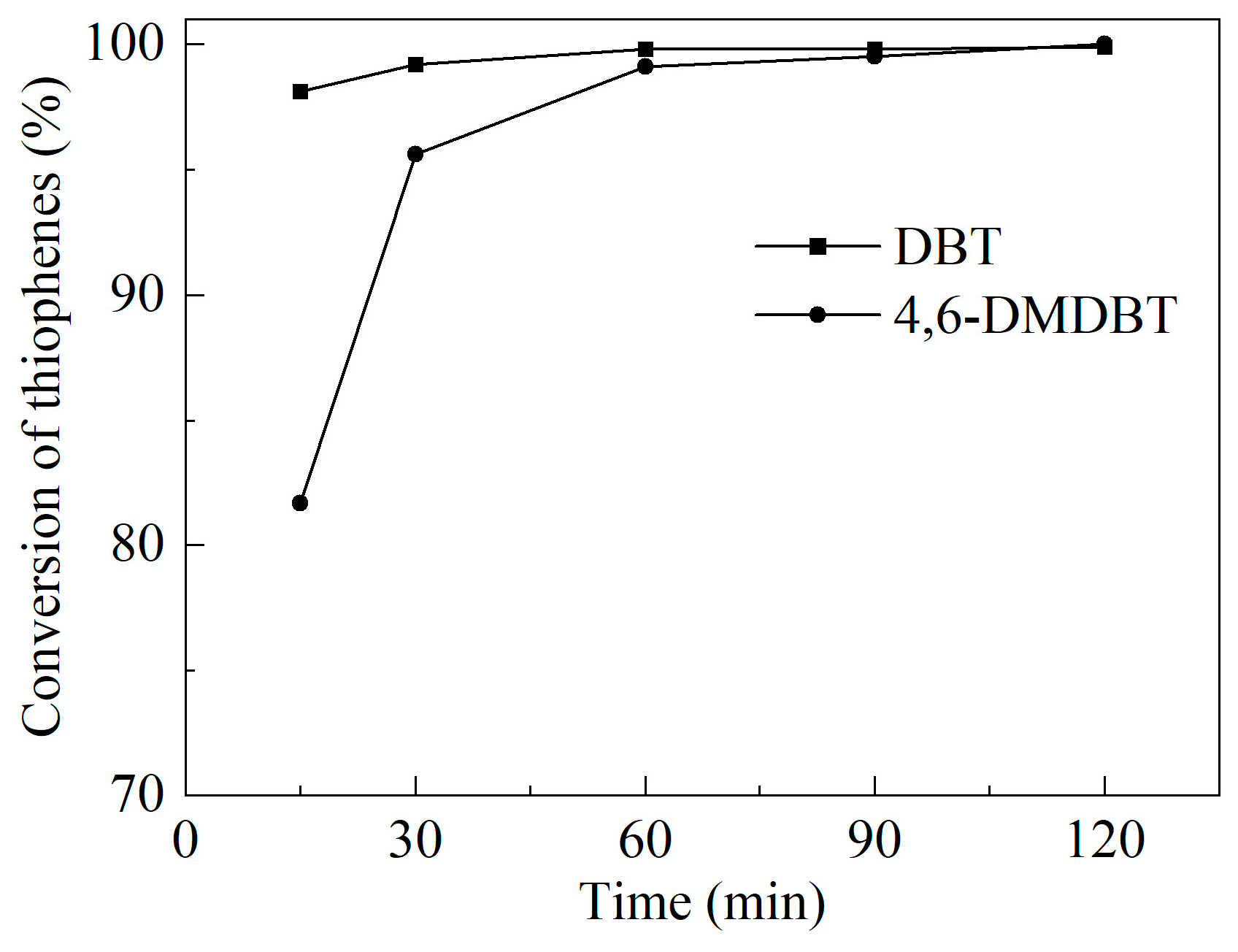
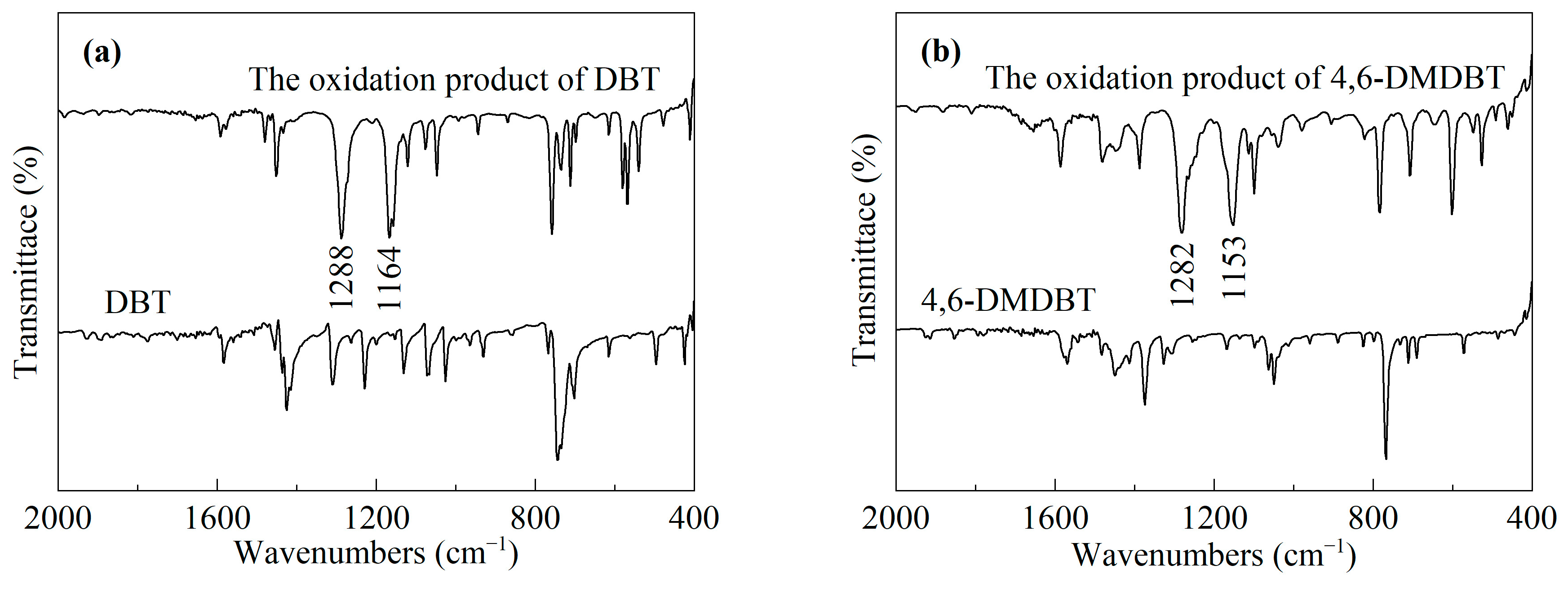

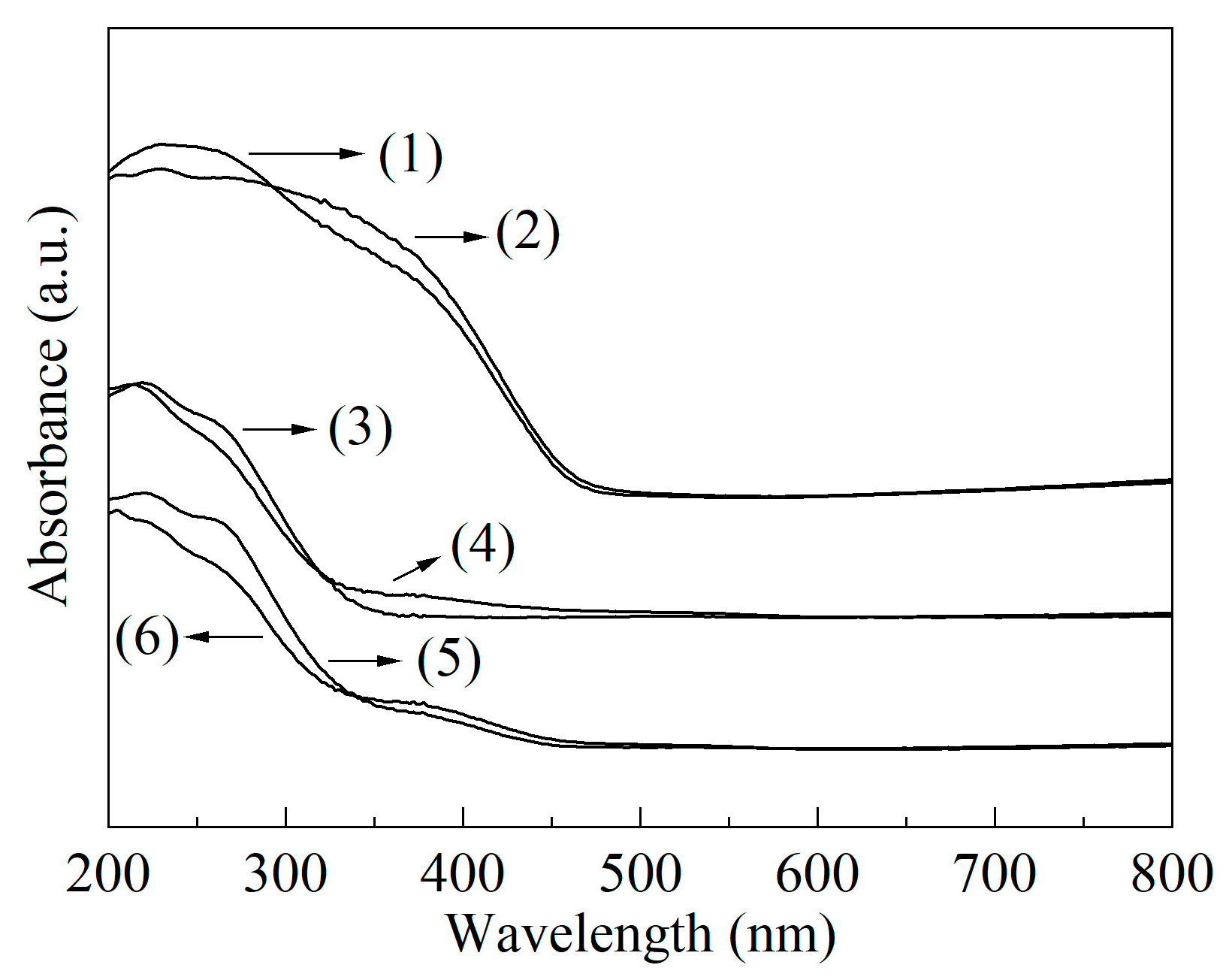
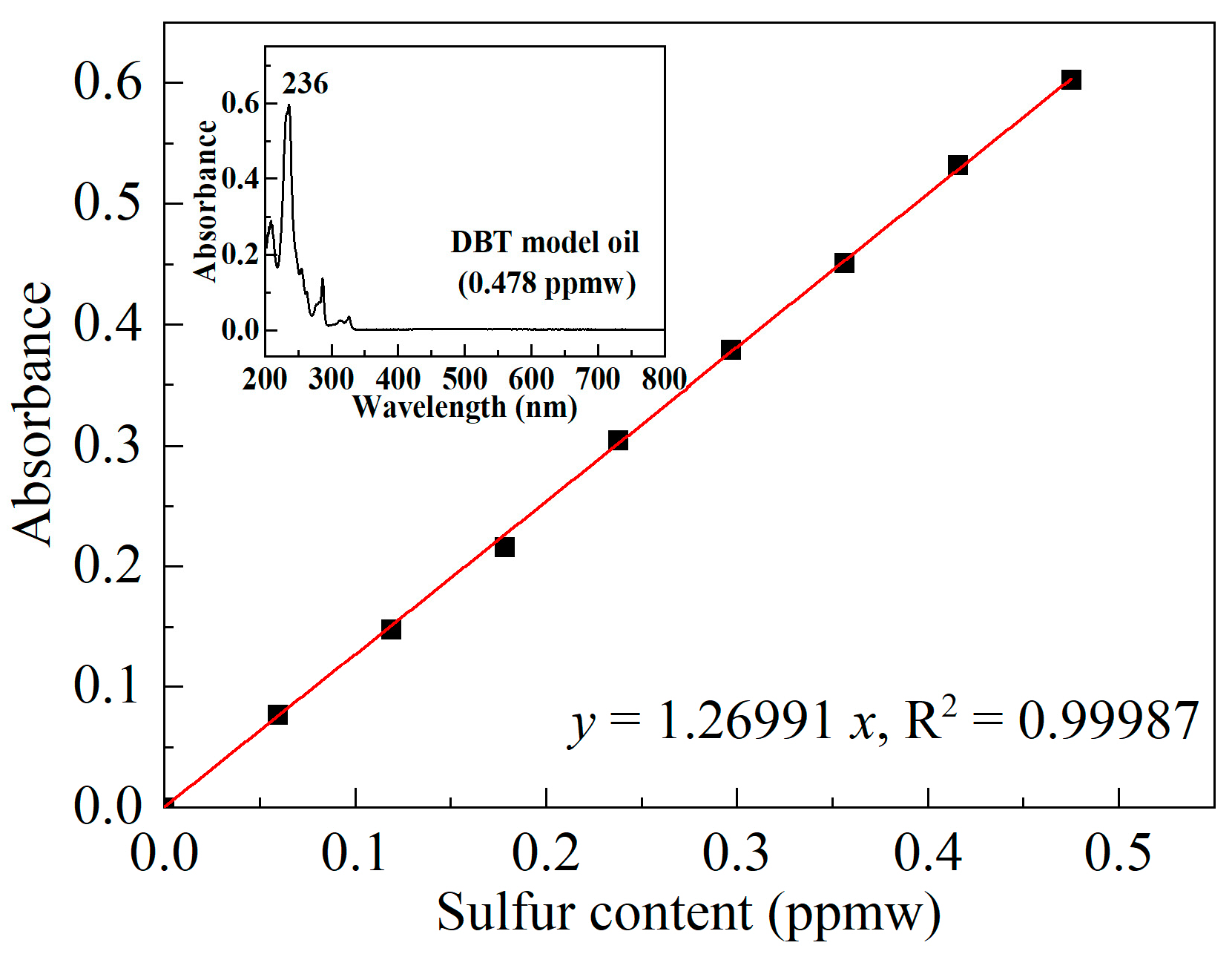
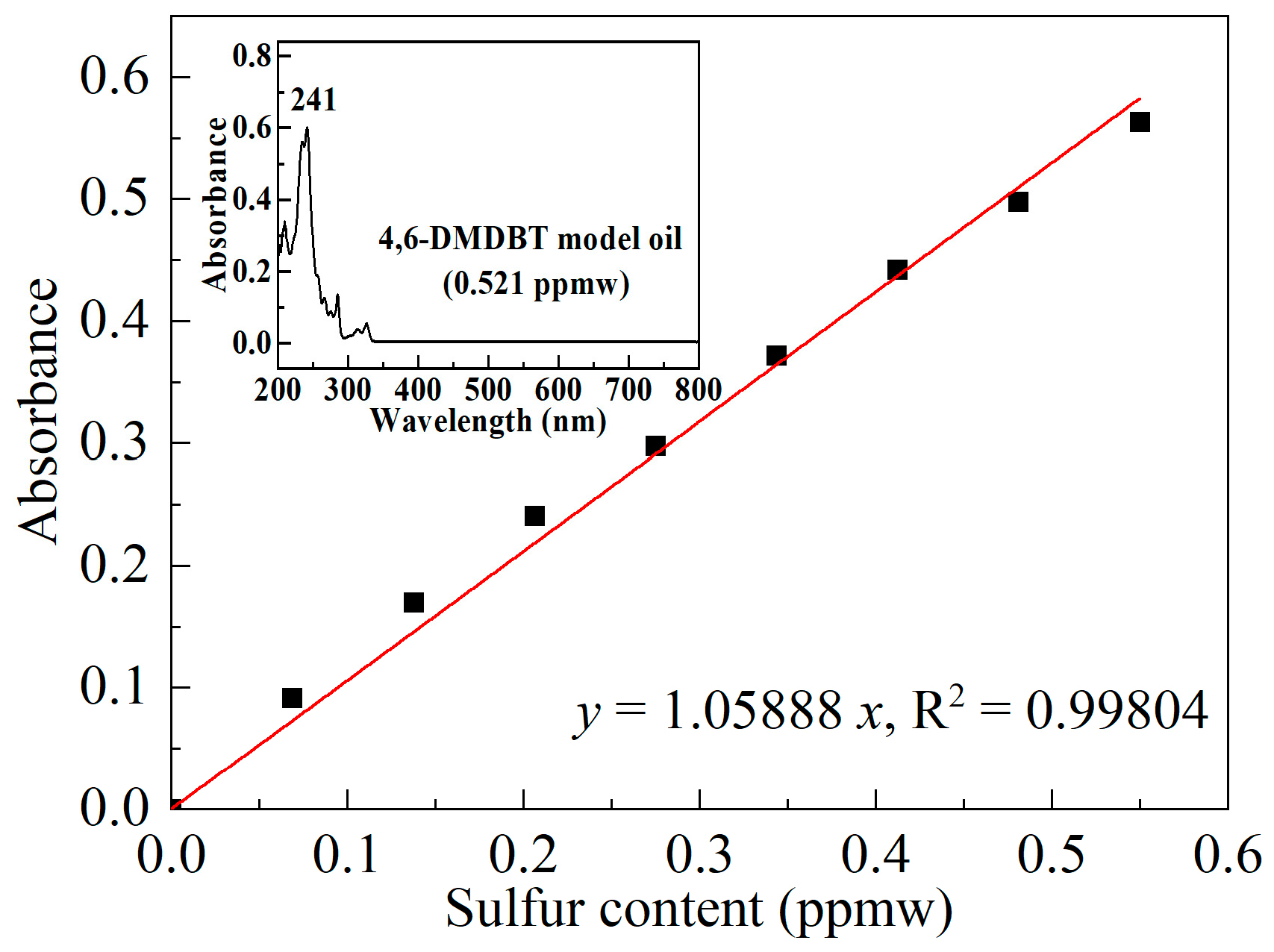
| Sample | SBET/(m2/g) | Vmicro/(cm3/g) | Vmeso/(cm3/g) | XDBT (%) | X4,6-DMDBT (%) |
|---|---|---|---|---|---|
| SiO2 | 294.4 | 0 | 0.39 | - | - |
| Pure WO3 | 0.1 | 0 | 0.01 | - | - |
| Silica gel | 184.0 | 0 | 0.77 | - | - |
| 15%WO3/Silica gel | 157.0 | 0 | 0.65 | - | - |
| WS-823-15 | 246.8 | 0 | 0.33 | 98.4 | 86.8 |
| WS-923-15 | 227.9 | 0 | 0.35 | 99.2 | 95.6 |
| WS-1023-15 | 149.9 | 0 | 0.29 | 97.4 | 86.5 |
| WS-923-5 | 260.9 | 0 | 0.36 | 97.6 | 91.3 |
| WS-923-25 | 196.1 | 0 | 0.30 | 95.7 | 83.7 |
| WS-923-15r a | 249.4 | 0 | 0.38 | - | - |
| WS-923-15r b | 234.7 | 0 | 0.32 | - | - |
| Sample | Oxidant | XDBT (%) | X4,6-DMDBT (%) |
|---|---|---|---|
| - | - | 60.5 | 31.7 |
| - | H2O2 | 58.9 | 30.4 |
| SiO2 | H2O2 | 61.0 | 32.9 |
| Pure WO3 | H2O2 | 68.3 | 46.1 |
| WS-923-15 | - | 62.2 | 32.3 |
| 15%WO3/Silica gel | H2O2 | 84.8 | 81.8 |
| Sample | Temp. (K) | Cyclic Number | XDBT a (%) | X4,6-DMDBT a (%) | Ref. |
|---|---|---|---|---|---|
| WO3-SBA-15 | 333 | 4 | 85 b | - | [12] |
| 550-WO3/TiO2 | 323 | 6 | 96.7 | - | [3] |
| 36%WO3/g-C3N4 | 333 | 5 | 87.5 | - | [13] |
| 15WOx@SnO2 | 333 | 5 | 93.1 | - | [21] |
| WO3/CNT-2 | 323 | 5 | ~80 | - | [25] |
| a-Cr2WO6/WO3 | 343 | 6 | 93.1 | - | [28] |
| CoW (15)/CNT | 348 | 5 | 99.0 | - | [29] |
| WS-923-15 | 333 | 6 | 99.1 | 99.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, X.; Zhao, R.; Zhang, J.; Zhang, L.; Zhang, W.; Hu, J.; Li, H. The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization. Catalysts 2024, 14, 103. https://doi.org/10.3390/catal14020103
Zhang Y, Liu X, Zhao R, Zhang J, Zhang L, Zhang W, Hu J, Li H. The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization. Catalysts. 2024; 14(2):103. https://doi.org/10.3390/catal14020103
Chicago/Turabian StyleZhang, Yinhai, Xiaoxue Liu, Ruyu Zhao, Jingwei Zhang, Lanfen Zhang, Wei Zhang, Jian Hu, and Hao Li. 2024. "The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization" Catalysts 14, no. 2: 103. https://doi.org/10.3390/catal14020103
APA StyleZhang, Y., Liu, X., Zhao, R., Zhang, J., Zhang, L., Zhang, W., Hu, J., & Li, H. (2024). The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization. Catalysts, 14(2), 103. https://doi.org/10.3390/catal14020103







