Abstract
The development of sustainable and high-performance oxygen reduction reaction (ORR) electrocatalysts is fundamental to fuel cell implementation. Non-precious transition metal oxides present interesting electrocatalytic behavior, and their incorporation into N-doped carbon supports leads to excellent ORR performance. Herein, we prepared a shrimp shell-derived biochar (CC), which was doped with nitrogen via a ball milling approach (N-CC), and then used as support for Co3O4 nanoparticles growth (N-CC@Co3O4). Co3O4 loading was optimized using three different amounts of cobalt precursor: 1.56, 2.33 and 3.11 mmol in N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3, respectively. Interestingly, all prepared electrocatalysts, including the initial biochar CC, presented electrocatalytic activity towards ORR. Both N-doping and the introduction of Co3O4 NPs had a significant positive effect on ORR performance. Meanwhile, the three composites showed distinct ORR behavior, demonstrating that it is possible to tune their electrocatalytic performance by changing the Co3O4 loading. Overall, N-CC@Co3O4_2 achieved the most promising ORR results, displaying an Eonset of 0.84 V vs. RHE, jL of −3.45 mA cm−2 and excellent selectivity for the 4-electron reduction (n = 3.50), besides good long-term stability. These results were explained by a combination of high content of pyridinic-N and graphitic-N, high ratio of pyridinic-N/graphitic-N, and optimized Co3O4 loading interacting synergistically with the porous N-CC support.
1. Introduction
Fuel cells (FCs) are electrochemical devices that directly convert chemical energy into electrical energy [1]. FCs constitute a promising alternative to internal combustion engines, as they present higher energy efficiency and lower environmental impact compared to conventional energy conversion devices [2,3]. Nevertheless, most of the environmental impact of and economic barriers to FCs results from the conventional precious metal electrocatalysts required by several electrochemical reactions behind FC operation [2].
The oxygen reduction reaction occurs at the FC’ cathode, where O2 molecules are reduced to water. Two ORR mechanisms are possible, involving the direct transfer of four electrons in a single step or via two steps, each involving two-electron transfer [4]. The 4-electron reduction is the most economically viable for FC applications [5]. ORR is a challenging reaction, presenting large overpotentials and sluggish kinetics, which must be overcome by an efficient electrocatalyst [6,7].
While Pt-based materials are currently the state-of-the-art electrocatalysts for ORR, their industrial application is economically and environmentally problematic [8]. Alternatively, non-precious transition metals and their oxides display interesting electrocatalytic properties arising from the variable oxidation state, besides lower cost and higher availability than platinum [9,10]. In particular, spinel Co3O4 has attracted attention due to its intrinsic electrocatalytic activity towards ORR, with Co2+ sites favoring O2 adsorption and electron transfer [10,11]. However, these oxides have low electrical conductivity, low stability and a tendency to agglomerate, reducing the exposure of active sites to reactants [12,13]. These limitations can be mitigated by employing a conductive support, such as carbon materials [14]. Moreover, heteroatom doping of carbon materials confers additional active sites for ORR [15,16].
Reportedly, N-doping enhances ORR performance of carbon materials by changing its charge distribution, as nitrogen atoms have higher electronegativity than carbon [17]. However, this enhancement is not always related to nitrogen content present in the doped material, but to the position in which nitrogen atoms are introduced into the graphitic structure [18,19]. The role played by each type of nitrogen in ORR is still not fully disclosed [20,21], but pyridinic and graphitic nitrogen are often considered responsible for the improvement of ORR activity by reducing the O2 adsorption energy in adjacent carbon atoms [22,23,24]. Rocha et al. reported that the interaction between these two species displays an important effect on ORR performance of N-doped MWCNTs, with higher pyridinic/graphitic ratios leading to better electrocatalytic activity [18].
Therefore, the combination of N-doped carbon materials and Co3O4 particles is a popular strategy to obtain high performance ORR electrocatalysts [25,26,27,28,29]. While graphene and MWCNTs are regularly used, it should be considered that they are obtained from petrochemical sources, through energy-intensive procedures. To lower the production cost and improve the sustainability of the final electrocatalyst, without compromising its effectiveness, biomass-derived carbons (biochars) may be used [30,31]. Additionally, the numerous oxygen-containing functional groups usually present on biochar’s surface may help to anchor the metal oxide particles, facilitating the growth/incorporation of these particles under mild conditions [30].
The composition of shrimp shells, rich in nitrogen and containing significant amounts of sulfur and phosphorus, makes them a promising biomass source for biochar production [32,33]. Moreover, they also contain CaCO3, which promotes porosity formation without requiring any activation procedure by acting as a self-template, thus eliminating time-consuming and energy-demanding steps and the use of expensive (e.g., gaseous CO2) or corrosive (e.g., NaOH, FeCl3, etc.) activation agents [34,35]. Crustacean shells are a common waste, available globally, with 6 to 8 million tons being produced annually [36]. As the shells are currently returned to oceans, incinerated or discarded in landfills [37], their utilization in biochar production also contributes to mitigating environmental problems generated by this disposal.
Herein, we prepared composites containing N-doped biochar and Co3O4 NPs, with different Co3O4 loadings (N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3), through simple procedures: the initial biochar (CC), obtained from pyrolysis of shrimp shells, was doped via a ball milling approach, followed by in situ co-precipitation of Co3O4. Both N-doping and Co3O4 incorporation led to significant improvements in ORR performance. While all prepared composites showed good ORR activity, the Co3O4 loading had a considerable effect on their results, with N-CC@Co3O4_2, containing an intermediary loading, achieving the best performance. The synergistic effect between Co3O4 NPs and our N-doped biochar, containing high amounts of pyridinic and graphitic nitrogen, was responsible for N-CC@Co3O4_2’s excellent ORR performance.
2. Results and Discussion
2.1. Electrocatalysts Characterization
The morphology of the prepared electrocatalysts was assessed by SEM. Figure 1 shows the micrographs of CC, N-CC, bare Co3O4 and the three prepared N-CC@Co3O4 composites. The CC micrograph shows several large pores in thin carbonaceous sheets. After doping, the biochar developed visibly smaller and interconnected pores, and an overall rougher surface. The Co3O4 micrograph revealed a large network of small needle-like particles. The original morphology of both N-CC and the metal oxide particles were maintained on the composites. The elemental distribution maps (Figures S1–S3) revealed that the oxide particles are uniformly dispersed into the biochar support. EDS analyses were also performed for all prepared catalysts (Figure S4), showing the presence of C, O, P, S, Cl, Ca and a small amount of K in CC and N-CC, all remaining from shrimp shell composition (the N signal was significantly overlapped with the high intensity C peak). The same elements were found in the composite’s spectra, with additional Co peaks appearing. It should be noticed that Pd and Au signals in all EDS spectra should be attributed to the thin AuPd film coating the samples, necessary to ensure enough conductivity to acquire good quality SEM images.

Figure 1.
SEM micrographs of (a) CC, (b) N-CC, (c) Co3O4, (d) N-CC@Co3O4_1, (e) N-CC@Co3O4_2 and (f) N-CC@Co3O4_3, obtained at magnifications of 1000, 25,000, 50,000, 25,000, 25,000 and 25,000×, respectively.
The content of C, H, N and S was ascertained by elemental analysis. CC contained mostly carbon (71.00%), but also a reasonable amount of nitrogen (3.12%) and sulfur (1.13%), besides a residual content of hydrogen (0.85%). As expected, the content of nitrogen increased significantly to 6.64% after doping, while the amount of sulfur decreased to 0.11% and the contents of carbon and hydrogen remained similar to the original biochar (69.54% and 0.84%, respectively).
Co contents in the prepared composites were determined by ICP-OES. Co content increased from 3.35 wt% in N-CC@Co3O4_1 to 18.35 wt% in N-CC@Co3O4_3. N-CC@Co3O4_2 obtained an intermediary value of 14.02 wt%.
The success of the doping approach and of the incorporation of metal oxide particles into N-CC was evaluated by Raman spectroscopy. Figure 2 displays the Raman spectra of the CC, N-CC and the prepared composites, as well as bare Co3O4 NPs for comparison. CC and N-CC spectra exhibit the characteristic profile of carbon materials [38,39], presenting the D and G bands in the range of 1365–1368 cm−1 and 1580–1590 cm−1, respectively (Table 1). Both bands are also detected in the spectra of all composites, around the same Raman shifts. As the D band occurs due to phonon scattering at defective sites and the G band is related to the conjugated structure of sp2 carbon, the ratio between the intensity of these two bands (ID/IG) gives a measure of the disorder level of the material [38,40]. ID/IG values can also be consulted in Table 1. CC presented a disordered carbon structure, as suggested by the high ID/IG ratio (1.25). This structure is mainly preserved during N-doping and the growth of metal oxide NPs, with ID/IG values of N-CC and the three composites varying from 1.12 to 1.26. The Raman spectrum of Co3O4 NPs presents the characteristic bands of inverted spinel Co3O4 structure at 469.89, 515.21, 611.67 and 675.48 cm−1 [41]. The most intense band, around 675 cm−1, is also observed on N-CC@Co3O4 spectra, indicating the successful preparation of the three composites.
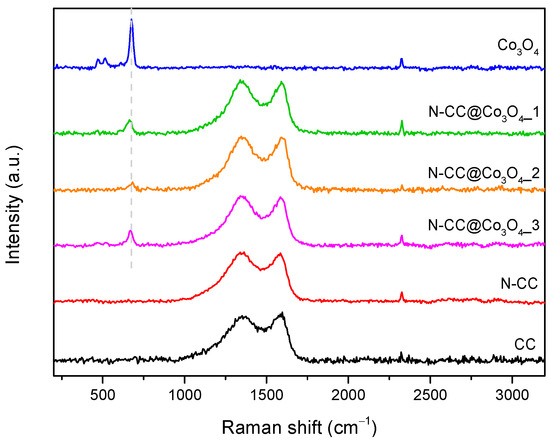
Figure 2.
Raman spectra of CC, N-CC and N-CC@Co3O4 composites.

Table 1.
Raman shift of D and G bands, and ID/IG values for CC, N-CC and the prepared composites.
The crystallinity of the prepared materials was evaluated by XRD (Figure 3). CC and N-CC diffractograms display a broad peak around 25°, corresponding to plane (002) of graphite [42,43], besides several peaks over 30–50° attributed to mineral phases remaining from the shrimp shells’ original composition [44,45,46]. All these diffraction peaks are observed in the composites’ diffractograms, in addition to extra peaks around 31.6, 36.8, 39.4, 44.8, 59.4 and 65.3°, associated to planes (220), (311), (222), (400), (511) and (440) of Co3O4 [47,48]. The crystallite size (D) of bare Co3O4 NPs, as well as Co3O4 NPs on the three composites, was estimated by the Debye–Scherrer equation (Equation (1)).
where K stands for the Scherrer constant, λ is the X-ray wavelength, β is the FWHM of the selected peak and θ is the diffraction angle, applied to the most intense plane (311) [49,50]. Bare Co3O4 NPs showed a crystallite size of 7.9 nm, while the particles present on the N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3 displayed sizes of 12.1, 9.2 and 5.0 nm, respectively.
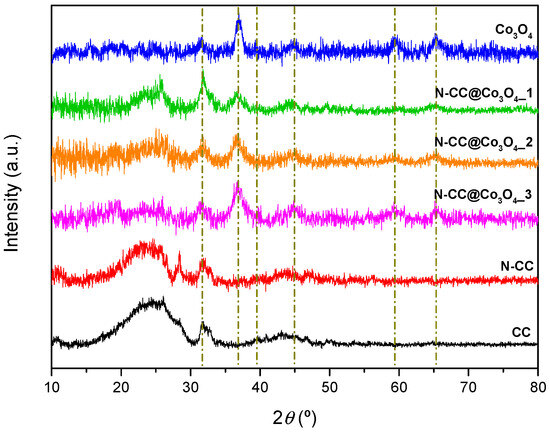
Figure 3.
Diffraction patterns of CC, N-CC, the prepared N-CC@Co3O4 composites and bare Co3O4 NPs.
The surface composition of the prepared materials was assessed by XPS (Table 2). CC and N-CC contain a large amount of carbon (90.55 and 87.24 at %, respectively), which progressively decreased in the composites to 69.45 (N-CC@Co3O4_1), 66.77 (N-CC@Co3O4_2) and 47.17 at % (N-CC@Co3O4_3). As expected, and in accordance with CHNS results, the N content increased significantly after doping, from 2.99 at% in CC to 6.87 at % in N-CC. In contrast, N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3 presented N contents of 5.72, 5.58 and 3.48 at %, respectively. The amount of O remained constant during the biochar doping (4.95 at % in CC vs. 4.59 at % in N-CC) but increased progressively in the composites according to the loading of Co3O4: N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3 obtained values of 17.90, 19.56 and 34.56 at %, respectively. Co content in the composites increased, according to the amount of Co precursor used, to 5.33, 6.36 and 14.79 at % in N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3, respectively. Residual amounts of S 2p were detected only on CC and N-CC (1.01 and 0.15 at %, respectively), while P 2p was identified on all materials, except N-CC@Co3O4_3, in amounts of 0.50–1.12 at %. Ca 2p was found in small amounts (0.38–0.54 at %) on N-CC, N-CC@Co3O4_1 and N-CC@Co3O4_2, but not on CC, suggesting that the doping procedure, especially the ball milling approach, may expose residual amounts of calcium remaining in the bulk of CC after acid leaching.

Table 2.
Surface composition of the prepared materials.
C 1s spectra of all prepared materials present an intense peak at 284.60 eV, corresponding to sp2 C domains, as well as five less intense peaks related to sp3 C, C–N, C–O–C, C=O and O–C=O groups at 285.20, 285.90, 286.90, 288.20 and 289.30 eV, respectively, and a satellite peak attributed to π–π* transitions around 290.54–291.17 eV (Figure S5) [29,51]. Additionally, contributions from C–O–P at 284–285 eV should be taken into account in all spectra except N-CC@Co3O4_3 [52], and C–S/C=S may contribute to CC and N-CC’ spectra around 286–287 eV [53].
N 1s spectra of CC, N-CC and the composites were deconvoluted in four peaks: pyridinic-N, pyrrolic-N, graphitic-N and oxidized-N, around 398.07–398.44, 399.66–400.28, 400.99–401.87 and 403.18–404.81 eV, respectively (Figure 4) [19,54]. Our doping strategy led to a significant increase in pyridinic-N content and decrease in pyrrolic-N compared to the undoped biochar (52.33% vs. 33.11% and 22.38% vs. 32.44%, respectively), while the contents of graphitic-N and oxidized-N did not change considerably (Table S1). Nitrogen in the composites was found majorly under pyridinic-N (46.87, 43.17 and 37.36% in N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3, respectively), followed by pyrrolic-N (33.96–36.36%), graphitic-N (10.84–22.70%) and oxidized-N (5.46–7.17%) (Table S1).
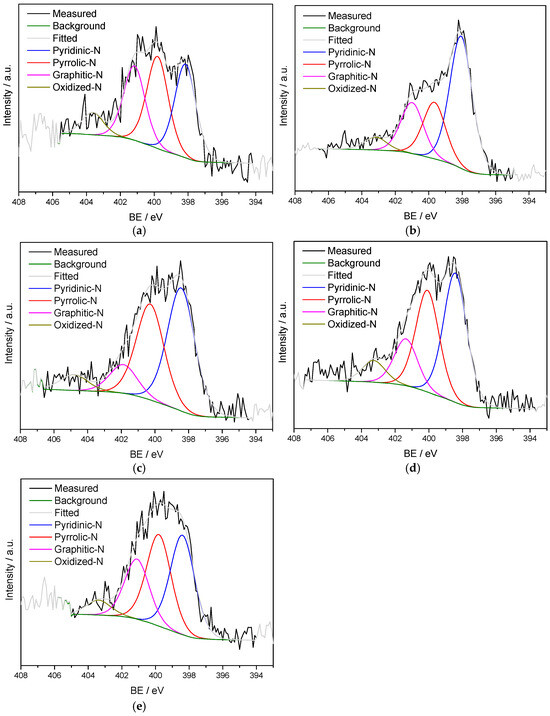
Figure 4.
High resolution XPS spectra of N 1s region of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1, (d) N-CC@Co3O4_2 and (e) N-CC@Co3O4_3.
The deconvolution of O 1s spectra in all prepared materials is represented in Figure S6. Both CC and N-CC displayed three peaks at 530.88–530.90, 532.13–532.33 and 533.60–533.61 eV, corresponding to C=O, C–O and O–C=O groups, respectively [55,56]. As expected, the co-precipitation of Co3O4 NPs drastically affected the O 1s region, with the composite’s spectra presenting peaks related to O2− in Co3O4 lattice (529.94–530.05 eV), adsorbed hydroxyl groups into Co3O4 structure (531.13–531.28 eV), C–O and C=O groups from N-CC (531.97–532.32 eV), hydroxyl groups from MIPA (532.97–533.48 eV) and adsorbed water molecules (533.97–534.95 eV) [27,29].
The composite’s XPS spectra of the region Co 2p showed two main peaks around 781 and 797 eV corresponding to Co 2p3/2 and Co 2p1/2 spin orbital splitting (Figure 5). All spectra presented a doublet at 779.88–779.95 and 795.02–795.11 eV corresponding to Co(III) 2p3/2 and Co(III) 2p1/2 contributions, and a Co(II) doublet at 781.36–781.43 and 796.03–797.93 eV. Two shake-up satellite peaks were fitted on the Co 2p3/2 main peak, at 782.86–783.41 and 788.35–788.70 eV, while a single satellite of Co 2p1/2 was located at 803.90–804.31 eV [27,29]. The same peaks were observed in the bare Co3O4 spectrum, at similar binding energies (Figure 5).

Figure 5.
High-resolution XPS spectra of region Co 2p of (a) N-CC@Co3O4_1, (b) N-CC@Co3O4_2 and (c) N-CC@Co3O4_3.
S 2p was detected only in CC and N-CC (Figure S7). While CC presented a well-resolved spectrum, showing S 2p3/2 and S 2p1/2 contributions of C–S–C (163.81 and 164.94 eV, respectively), C–SH (165.39 and 166.52 eV, respectively), C–SOx–C (167.92 and 169.05 eV, respectively) and a single contribution for C–SO3 groups (170.11 eV) [55], the low amount of S at the N-CC surface only allowed to deconvolute its spectrum in S 2p3/2 and S 2p1/2 at 164.48 and 165.66 eV, respectively. Ca 2p was found in N-CC, N-CC@Co3O4_1 and N-CC@Co3O4_2 (Figure S8), showing the Ca 2p3/2 and Ca 2p1/2 contributions of Ca(II) in CaCO3, around 346.93–347.33 and 350.43–350.82 eV, respectively [57,58]. P 2p spectra of CC, N-CC, N-CC@Co3O4_1 and N-CC@Co3O4_2 are represented in Figure S9. The CC spectrum was fitted into three doublets corresponding to P–C (131.35 and 132.22 eV), [P2O7]4− (133.04 and 133.91 eV) and [PO3]− (135.37 and 136.24 eV) [59]; the remaining materials showed only the last two doublets at similar binding energies.
2.2. ORR Performance
The electrochemical behavior of the prepared catalysts was initially evaluated by cyclic voltammetry, in N2 and O2-saturated electrolyte. As displayed in Figure S10, CC, N-CC and the three composites showed an irreversible cathodic peak around 0.75–0.80 V vs. RHE in presence of O2 that is not visible in N2-saturated electrolyte, corresponding to oxygen reduction; this peak was also observed in bare Co3O4 around 0.50 V vs. RHE. The CVs of the composites and Co3O4 also displayed additional cathodic and anodic peaks between 1.1 and 1.5 V vs. RHE, both in N2 and O2-saturated electrolyte, associated with redox processes occurring in the cobalt oxide [60,61].
ORR polarization curves of the prepared materials at 1600 rpm are shown in Figure 6a. Through this technique, onset potential (Eonset) and diffusion-limited current density (jL) values were calculated, and the results can be found in Table 3. Some catalysts, such as CC, N-CC and Co3O4, did not reach a plateau region at the evaluated potential window. In these cases, jL was estimated as the current density obtained at the last screened potential (E = 0.26 V vs. RHE), although these values may not correspond to diffusion-limited current density by definition, as kinetics may still be controlling the ORR process along with diffusion. CC displayed decent jL and Eonset of −3.11 mA cm−2 and 0.79 V vs. RHE, respectively, which were further enhanced after the N-doping process: N-CC obtained a significantly more positive Eonset (0.88 V vs. RHE) and higher jL (−3.31 mA cm−2). This improvement is explained by increasing contents of pyridinic-N and decreasing amounts of pyrrolic-N after doping, while the graphitic-N was mostly maintained. Indeed, N-CC presented higher pyridinic-N/pyrrolic-N and pyridinic-N/graphitic-N ratios (Table S2), which are reportedly related to higher ORR activity [18,62].
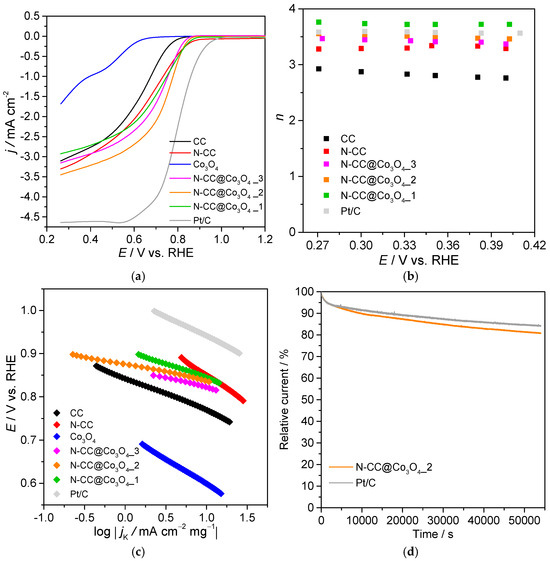
Figure 6.
(a) ORR polarization curves of the prepared electrocatalysts, obtained in O2-saturated 0.1 mol dm−3 KOH solution, at 1600 rpm and 0.005 V s−1, (b) influence of applied potential in n, (c) Tafel plots and (d) chronoamperograms of N-CC@Co3O4_2 and Pt/C at E = 0.47 V vs. RHE and 1600 rpm, in O2-saturated 0.1 mol dm−3 KOH solution.

Table 3.
ORR results obtained in O2-saturated 0.1 mol dm−3 KOH electrolyte, including the onset potentials (Eonset), diffusion-limited current densities (jL, determined at 1600 rpm and 0.26 V), numbers of electrons transferred by O2 molecule (n) and Tafel slopes (TS).
While Co3O4 NPs showed poor electrocatalytic behavior, with Eonset of 0.63 V vs. RHE and jL of only −1.69 mA cm−2, their incorporation into N-CC resulted in a considerable improvement of the composites’ catalytic performance. The composites achieved Eonset between 0.83 and 0.86 V vs. RHE, with decreasing loading of Co3O4 leading to more positive values (Table 3). Although N-CC@Co3O4_3 and N-CC@Co3O4_1 presented lower jL in absolute value than N-CC (−3.16 and −2.92 mA cm−2, respectively), N-CC@Co3O4_2 exhibited the highest value of the prepared materials (−3.45 mA cm−2), evidencing the importance of loading optimization. Therefore, the composite with an intermediary amount of Co3O4 showed the most promising balance between Eonset and jL. Nevertheless, it is still slightly outperformed by Pt/C in terms of these parameters (Eonset = 0.93 V vs. RHE and jL = −4.62 mA cm−2). On the other hand, the obtained values are similar to the ones reported in the literature for composites containing carbon materials and Co3O4, as can be confirmed in Table 4. For instance, our composites presented more positive Eonset and higher jL than Co3O4@GF_O3 and Co3O4@GF_KMnO4, prepared using oxidized graphene flakes [27], and very close values to the ones reported to Co/N3S3-GF, containing N,S-doped graphene [29].
To further evaluate the electrocatalytic performance of the prepared materials, LSV was also performed at different rotation speeds (400, 800, 1200, 1600, 2000 and 3000 rpm, Figure S11), allowing the analysis of K–L plots (Figure S12) and the determination of the number of electrons transferred per O2 molecule (n); as the LSV of Co3O4 did not present a well-defined diffusion-controlled region, it was not possible to apply the K–L equation and, consequently, determine its n value. All K–L plots showed good linearity, indicating that the prepared materials (except Co3O4) followed first order kinetics regarding the dissolved O2 concentration [63]. It is important to mention that there is some controversy about the correct way to estimate n values, with recent reports indicating that the K–L method may not apply fully to porous carbon materials with high surface area (SBET > 550 m2 g−1) [64]. However, as our catalysts showed low surface areas (see N2 adsorption–desorption results below), the K–L method could be successfully applied herein.
The variation of n to the applied potential was plotted in Figure 6b for all the catalysts plus Pt/C, added for comparison purposes. N-CC and the composites showed practically constant n values in the potential window of 0.27–0.42 V vs. RHE, while CC displayed slightly decreasing n values at higher potentials. Therefore, all materials but CC followed a mechanism independent of applied potential. CC achieved an average n of 2.83, suggesting a mixed process between the 2-electron reduction and the 4-electron pathway. N-doping led to an n closer to 4, with N-CC obtaining a value of 3.30: high pyridinic-N/graphitic-N ratios on carbon materials are correlated with higher n [18,62]. The incorporation of Co3O4 NPs into N-CC further improved the selectivity for the direct reduction. Interestingly, the composite prepared with a lower amount of cobalt precursor obtained a value of n closer to 4 (n = 3.72), following a predominantly 4-electron reduction. In contrast, N-CC@Co3O4_3, with the highest loading of Co3O4, obtained n of 3.42, while N-CC@Co3O4_2 achieved an intermediate value of 3.50. Therefore, while the presence of Co3O4 was important for improving the selectivity for the 4-electron pathway, its loading should be carefully optimized. Once again, the obtained n values are in accordance with the ones reported in the literature for heteroatom doped carbon/Co3O4 composites (Table 4).
Tafel plots were constructed to evaluate the kinetics of all prepared materials (Figure 6c). CC and N-CC obtained the highest Tafel slopes (TS), with values of 74 and 125 mV dec−1. On the other hand, the composites displayed considerably lower values, with N-CC@Co3O4_2 showing the lowest TS (35 mV dec−1), followed by N-CC@Co3O4_3 (44 mV dec−1) and N-CC@Co3O4_1 (64 mV dec−1). Pt/C obtained a TS of 91 mV dec−1. The significantly lower TS values presented by the composites compared to Pt/C indicates that O2 is easily adsorbed at the catalyst surface [63]. Comparing all these values, it is possible to infer that the rate-limiting step was different for the evaluated catalysts: CC, N-CC and Pt/C kinetics were likely limited by Equation (2), while the mechanism rate of the three composites was dominated by Equations (3) and (4), where M is an empty site on the catalyst surface [65]. Therefore, the introduction of Co3O4 led to changes in the rate-limiting step.
Overall, N-doping led to enhanced electrocatalytic activity, which was further improved by incorporation of Co3O4 NPs into the biochar support. Herein, N-CC@Co3O4_2 achieved the most promising ORR performance, showing the best balance between Eonset, jL, n and TS. To help clarify these results, the textural properties of N-CC@Co3O4_2 (and CC and N-CC for comparison purposes) were further investigated by N2 adsorption–desorption isotherms at 77 K. CC, N-CC and N-CC@Co3O4_2 displayed similar specific surface areas (SBET of 265.76, 286.24 and 277.11 m2 g−1, respectively), indicating that this parameter was not determining their ORR performance. So, we also investigated the pore distribution on these catalysts (Figure S13). CC, N-CC and N-CC@Co3O4_2 showed broad peak width distribution along 0 to 50 nm (in the first two catalysts) or up to 60 nm (in the composite). CC presented predominantly pores with width of 2.73 and 4.75 nm, containing therefore mostly mesopores, according to IUPAC classification. Similarly, pores of 2.41 and 4.72 nm are predominant in N-CC, indicating that the doping procedure did not cause significant changes in the biochar’s porosity. In contrast, most of N-CC@Co3O4_2 pores presented width equal or inferior to 1.75 nm, indicating the presence of abundant micropores along with a smaller amount of mesopores. Considering these results, the composite’s microporosity could be attributed to the incorporation of Co3O4 into the N-CC support. Although mesopores promote fast transport of reactants and products towards or from the catalytic sites during the ORR process [66], some works also associated them with high selectivity for H2O2 production [67,68]. Micropores are reported to provide active sites for ORR [69,70]. Therefore, the combination of microporosity and mesoporosity may help explain the enhanced ORR performance of N-CC@Co3O4_2 relative to CC and N-CC [71].
Moreover, while the amount of nitrogen is not directly related to a better ORR performance [18,19], our doping procedure allowed us to increase significantly the content of pyridinic-N, while also obtaining a considerable amount of graphitic-N. Although the exact nature of active sites in N-doped carbon materials is still controversial [21], most studies revealed that pyridinic-N and graphitic-N play an important role in the electrocatalytic process [72,73,74,75]. On the other hand, the introduction of transition metal oxides, including Co3O4, into N-doped carbons is a well-known strategy to improve its ORR performance, as it synergistically combines Co3O4 electrocatalytic activity and the support’s conductivity and active sites [12,13]. Nevertheless, it should be noted that our support presented a defective structure with a low graphitization degree, which could negatively affect the composite’s performance.
The long-term stability of our most promising material was assessed by chronoamperometry at E = 0.47 V vs. RHE for 15 h (Figure 6d). N-CC@Co3O4_2 displayed good stability, with current retention of 80.80%, a similar value to the one obtained with Pt/C (84.08%).

Table 4.
ORR performance of carbon/Co3O4-based composites reported in the literature, in 0.1 mol dm−3 KOH electrolyte.
Table 4.
ORR performance of carbon/Co3O4-based composites reported in the literature, in 0.1 mol dm−3 KOH electrolyte.
| Catalyst | Eonset/V vs. RHE | jL/mA cm−2 | n | Reference |
|---|---|---|---|---|
| N-CC@Co3O4_1 | 0.85 | −2.92 | 3.73 | This work |
| N-CC@Co3O4_2 | 0.84 | −3.45 | 3.50 | This work |
| N-CC@Co3O4_3 | 0.82 | −3.16 | 3.42 | This work |
| Co3O4@bio-C | 0.86 | −5.20 | 3.86 | [25] |
| Co/Co3O4@NC | 0.94 | −4.78 | 3.82 | [13] |
| Co3O4/OPAC | 0.76 | −2.49 | 2.46 | [76] |
| Co3O4/NCMT-800 | 0.91 | −4.50 | 3.95 | [26] |
| Co3O4/NHPC | 0.96 | −6.0 | 3.91 | [12] |
| Co/N3S3-GF | 0.87 | −3.98 | ~ 4.0 | [29] |
| Co3O4@GF_O3 | 0.82 | −2.70 | 3.5 | [27] |
| Co3O4@GF_KMnO4 | 0.79 | −2.50 | 3.1 | [27] |
| N-rGO/CNT/Co3O4 | 0.87 | ~−4.0 | ~4.0 | [28] |
| Co3O4/CW | 0.89 | −4.79 | ~4 | [77] |
| Co3O4/N-rGO | --- | ~−2.5 | 2.9–3.4 | [78] |
3. Experimental
3.1. Materials and Chemicals
Shrimp shells were obtained from Mar Cabo—Produtos Congelados Lda and ground to a fine powder before utilization. Melamine (99%, Alfa Aesar, Kandel, Germany), hydrochloric acid (37%, Fluka, Porto Salvo, Portugal), 1-amine-2-propanol (MIPA, 93%, Sigma-Aldrich, Algés, Portugal), cobalt(II) chloride hexahydrate (98–102%, Thermo Scientific, Porto Salvo, Portugal), N,N-dimethylformamide (99.5%, Sigma-Aldrich, Algés, Portugal), Nafion 117 (5 wt% in lower aliphatic alcohols, Aldrich, Algés, Portugal) and Pt/C 20 wt% HiSPEC® 3000 (Alfa Aesar, Kandel, Germany) were used as received. Ultrapure water (resistivity 18.2 MΩ cm at 25 °C, Interlab, Portugal) was used in all electrochemical analysis and material preparations, except for the initial biochar preparation in which deionized water was used.
3.2. Electrocatalysts Preparation
3.2.1. Preparation of the Biochar (CC)
To obtain the initial biochar, denoted as CC, powdered shrimp shells were carbonized at 900 °C for 2 h, with a heating rate of 10 °C min−1, under a constant N2 flow (100 mL min−1). Then, the resulting material was treated with a 2.0 mol dm−3 HCl solution under reflux at 80 °C for 4 h, collected by filtration and washed with deionized water until reaching neutral pH. The material was dried at 80 °C under vacuum overnight.
3.2.2. N-Doping of CC (N-CC)
N-CC was obtained by doping CC with nitrogen, following a procedure described elsewhere [54]. Briefly, 0.60 g of CC and 0.39 g of melamine were milled together in a Retsch ball mill for 5 h at a frequency of 15 s−1, using a zirconium oxide ball with diameter of 1.5 cm. The obtained material was pyrolyzed at 600 °C for 1 h, under N2 flow (100 mL min−1), with a heating rate of 10 °C min−1.
3.2.3. Preparation of N-CC@Co3O4 Composites
N-CC@Co3O4 composites were prepared through in situ co-precipitation of Co3O4 particles according to the procedure described by Fernandes et al. [29]. A quantity of 250 mg of N-CC was dispersed in 50 mL ultrapure water by ultrasonication during 10 min. A certain amount of CoCl2∙6H2O was added to this dispersion under magnetic stirring. A 3.0 mol dm−3 aqueous solution of MIPA was added drop-by-drop (addition rate of 50 mL h−1), under vigorous magnetic stirring, until pH 10 was reached; the stirring was maintained during 24 h at room temperature. The precipitated solid was collected by filtration, washed with ultrapure water until neutral pH was obtained and then with ethanol, and dried at 60 °C in a glass vacuum oven. Finally, the material was calcinated under a constant flow of air (100 mL min−1) at 250 °C for 3 h with a heating rate of 5 °C min−1. Composites with tunable loading of Co3O4 were prepared by changing the amount of CoCl2∙6H2O added: 3.11, 2.33 and 1.56 mmol of Co(II) precursor were used in the synthesis of N-CC@Co3O4_3, N-CC@Co3O4_2 and N-CC@Co3O4_1, respectively. For comparison, Co3O4 nanoparticles were also prepared through the procedure described without the addition of the biochar support.
3.3. Characterization Techniques
All the prepared materials were extensively characterized by several techniques, namely micro-Raman, powder XRD, XPS and SEM. Additionally, CC and N-CC were analyzed by elemental analysis (CHNS mode), while the cobalt content of N-CC@Co3O4_1, N-CC@Co3O4_2 and N-CC@Co3O4_3 was determined by ICP-OES. The textural properties of CC, N-CC and N-CC@Co3O4_2 were evaluated by N2 adsorption-desorption isotherms. More details of the characterization techniques employed can be found in the Supplementary Materials.
3.4. Electrochemical Measurements
The electrochemical tests were carried out in a conventional three-electrode compartment cell, in 0.1 M KOH electrolyte, using an Ag/AgCl (3 mol dm−3 KCl, Metrohm, Lisboa, Portugal) reference electrode, a glassy carbon rod (diameter 2.0 mm, Metrohm, Lisboa, Portugal) auxiliary electrode and a rotating disk electrode (RDE) of glassy carbon (diameter of 3.0 mm, Metrohm, Lisboa, Portugal) as working electrode. The measurements were obtained on an AutoLab PGSTAT 302N potentiostat/galvanostat controlled by Nova 2.1 software. The RDE was extensively polished, before being modified by drop-cast of 5.0 μL of the catalyst ink (containing 1.0 mg of catalyst, dispersed in 250 μL DMF and 20 μL Nafion 117) and dried under an air flow. The ORR performance of the prepared materials was assessed in 0.1 mol dm−3 KOH solution via cyclic voltammetry (CV), linear sweep voltammetry (LSV) and chronoamperometry [29,56,79,80]. More detailed information can be found in the Supplementary Materials.
4. Conclusions
Composites containing Co3O4 NPs and N-doped biochars were prepared through the carbonization of shrimp shells, followed by doping via a solvent-free approach and in situ co-precipitation of Co3O4 under mild conditions. The loading of Co3O4 was optimized by preparing three composites using different amounts of cobalt precursor.
All the prepared materials were extensively characterized by several techniques. SEM micrographs revealed that CC and N-CC presented porous structures, which were modified by Co3O4 growth. Elemental analysis and XPS confirmed the successful N-doping of CC, with N content increasing from ~3 to ~7 wt%. Raman, XRD and XPS verified the correct preparation of spinel Co3O4 into N-CC structure.
N-doping and Co3O4 incorporation improve CC’s electrocatalytic performance. N-CC@Co3O4_2 achieved the most promising activity, obtaining Eonset and jL of 0.84 V vs. RHE and −3.45 mA cm−2, respectively, and nO2 and stability comparable to Pt/C. The high content of pyridinic-N and graphitic-N, besides an optimized Co3O4 loading that allowed an enhanced synergistic effect between the NPs and N-CC support, contributed to N-CC@Co3O4_2’s excellent performance. Moreover, our catalyst, derived from shrimp shell waste, displayed comparable performance to other composites reported in the literature containing conventional carbon nanomaterials obtained from petrochemical resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14120951/s1, Experimental—Characterization techniques and Electrochemical measurements [29,56,79,80]. Figure S1. Elemental mapping analysis of N-CC@Co3O4_3: (a) SEM micrograph and respective (b) C, (c) O and (d) Co distribution; Figure S2. Elemental mapping analysis of N-CC@Co3O4_2: (a) SEM micrograph and respective (b) C, (c) O and (d) Co distribution; Figure S3. Elemental mapping analysis of N-CC@Co3O4_1: (a) SEM micrograph and respective (b) C, (c) O and (d) Co distribution; Figure S4. EDS spectra of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1, (d) N-CC@Co3O4_2 and (e) N-CC@Co3O4_3; Figure S5. High-resolution XPS spectra of the region C 1s of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1, (d) N-CC@Co3O4_2 and (e) N-CC@Co3O4_3; Figure S6. High-resolution XPS spectra of O 1s region of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1, (d) N-CC@Co3O4_2 and (e) N-CC@Co3O4_3; Figure S7. High-resolution XPS spectra of region S 2p of (a) CC and (b) N-CC; Figure S8. High-resolution XPS spectra of region Ca 2p of (a) N-CC, (b) N-CC@Co3O4_1 and (c) N-CC@Co3O4_2; Figure S9. High-resolution XPS spectra of region P 2p of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1 and (d) N-CC@Co3O4_2; Figure S10. Cyclic voltammograms of (a) CC, (b) N-CC, (c) Co3O4, (d) N-CC@Co3O4_3, (e) N-CC@Co3O4_2, (f) N-CC@Co3O4_1 and (g) Pt/C, obtained in N2 and O2-saturated electrolyte at 0.005 V s−1; Figure S11. LSVs obtained in O2-saturated 0.1 mol dm−3 KOH solution, at different rotation speeds (400, 800, 1200, 1600, 2000 and 3000 rpm) and 5 mV s−1, of (a) CC, (b) N-CC, (c) N-CC@Co3O4_1, (d) N-CC@Co3O4_2, (e) N-CC@Co3O4_3, and (f) Pt/C; Figure S12. K–L plots of (a) CC, (b) N-CC, (c) N-CC@Co3O4_3, (d) N-CC@Co3O4_2, (e) N-CC@Co3O4_3 and (f) Pt/C; Figure S13. Pore distribution in (a) CC, (b) N-CC and (c) N-CC@Co3O4_2; Table S1. N functionalities on the prepared materials, determined by XPS; Table S2. Relationships between N functionalities in CC and N-CC.
Author Contributions
The synthesis of the prepared materials was performed by R.M. and J.V.M., and their characterization was performed by R.M.; A.J.S.F. was responsible for Raman spectra acquisition. V.K.A.-F. acquired XRD results for CC and N-CC. ORR experiments were conducted by R.M. The research was supervised by D.M.F. and A.F.P.; R.M. was responsible for writing up the manuscript, which was proofread by D.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT/MCTES) in the framework of the project EXPL/BII-BIO/0436/2021.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Thanks are also due to FCT for Laboratório Associado para a Química Verde—Tecnologias e Processos Limpos funding: 10.54499/LA/P/0008/2020, 10.54499/UIDP/50006/2020 and 10.54499/UIDB/50006/2020. D.M.F. and A.F.P. thank FCT for funding through the Individual Call to Scientific Employment Stimulus (Refs. 2021.00771.CEECIND/CP1662/CT0007 (10.54499/2021.00771.CEECIND/CP1662/CT0007) and 2020.01614.CEECIND/CP1596/CT0007 (10.54499/2020.01614.CEECIND/CP1596/CT0007), respectively). R.M. thanks FCT for the PhD fellowship 2020.05342.BD (10.54499/2020.05342.BD). V.K.A.F thanks the Junta de Andalucía (Spanish regional government) for his current Postdoc contract (PAIDI 2020, Young Doctors Program).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Qasem, N.A.A.; Abdulrahman, G.A.Q. A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications. Int. J. Energy Res. 2024, 2024, 36. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 16. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.X.; Tu, Z.K.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Yao-Lin, A.; Du, Z.Y.; Ze, H.J.; Wang, X.T.; Zhang, Y.; Zhang, H.; Zheng, Q.N.; Dong, J.C.; Tian, J.H.; Li, J.F. Understanding the molecular mechanism of oxygen reduction reaction using in-situ Raman spectroscopy. Curr. Opin. Electrochem. 2023, 42, 12. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Ma, R.G.; Lin, G.X.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.C.; Yang, M.H.; Wang, J.C. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 15. [Google Scholar] [CrossRef]
- Raj, C.R.; Samanta, A.; Noh, S.H.; Mondal, S.; Okajima, T.; Ohsaka, T. Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 11156–11178. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, Z.J.; Qu, Y.T.; Yuan, T.W.; Wang, W.Y.; Wu, Y.; Li, Y.D. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Mladenovic, D.; Mladenovic, A.; Santos, D.M.F.; Yurtcan, A.B.; Miljanic, S.; Mentus, S.; Sljukic, B. Transition metal oxides for bifunctional ORR/OER electrocatalysis in unitized regenerative fuel cells. J. Electroanal. Chem. 2023, 946, 17. [Google Scholar] [CrossRef]
- Xue, Y.J.; Sun, S.S.; Wang, Q.; Dong, Z.H.; Liu, Z.P. Transition metal oxide-based oxygen reduction reaction electrocatalysts for energy conversion systems with aqueous electrolytes. J. Mater. Chem. A 2018, 6, 10595–10626. [Google Scholar] [CrossRef]
- Xiao, J.W.; Kuang, Q.; Yang, S.H.; Xiao, F.; Wang, S.; Guo, L. Surface Structure Dependent Electrocatalytic Activity of Co3O4 Anchored on Graphene Sheets toward Oxygen Reduction Reaction. Sci. Rep. 2013, 3, 8. [Google Scholar] [CrossRef]
- Guan, J.L.; Zhang, Z.P.; Ji, J.; Dou, M.L.; Wang, F. Hydrothermal Synthesis of Highly Dispersed Co3O4 Nanoparticles on Biomass-Derived Nitrogen-Doped Hierarchically Porous Carbon Networks as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2017, 9, 30662–30669. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Wang, Y.M.; Xiao, Z.X.; Li, M.T.; Ding, Y.L.; Qi, M.L. Electrocatalytic oxygen reduction by a Co/Co3O4@N-doped carbon composite material derived from the pyrolysis of ZIF-67/poplar flowers. RSC Adv. 2021, 11, 2693–2700. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Choi, B.; Kim, Y.B. Development of Highly Active Bifunctional Electrocatalyst Using Co3O4 on Carbon Nanotubes for Oxygen Reduction and Oxygen Evolution. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lim, J.S.; Kim, J.H.; Joo, S.H. Heteroatom-doped carbon-based oxygen reduction electrocatalysts with tailored four-electron and two-electron selectivity. Chem. Commun. 2021, 57, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bai, Y.; Liu, M.Q.; Li, Y.; Yang, H.Y.; Wang, X.R.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Wu, B.; Meng, H.B.; Morales, D.M.; Zeng, F.; Zhu, J.J.; Wang, B.; Risch, M.; Xu, Z.C.J.; Petit, T. Nitrogen-Rich Carbonaceous Materials for Advanced Oxygen Electrocatalysis: Synthesis, Characterization, and Activity of Nitrogen Sites. Adv. Funct. Mater. 2022, 32, 36. [Google Scholar] [CrossRef]
- Rocha, I.M.; Soares, O.; Fernandes, D.M.; Freire, C.; Figueiredo, J.L.; Pereira, M.F.R. N-doped Carbon Nanotubes for the Oxygen Reduction Reaction in Alkaline Medium: Synergistic Relationship between Pyridinic and Quaternary Nitrogen. ChemistrySelect 2016, 1, 2522–2530. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Wang, D.W.; Su, D.S.; Gentle, I.R. A Discussion on the Activity Origin in Metal-Free Nitrogen-Doped Carbons For Oxygen Reduction Reaction and their Mechanisms. ChemSusChem 2015, 8, 2772–2788. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Guo, D.H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Wang, D.; Ge, L.P.; Xiao, L.H.; Zhang, H.Y.; Liu, L.L.; Zhang, J.Q.; An, M.Z.; Yang, P.X. Enriched graphitic N in nitrogen-doped graphene as a superior metal-free electrocatalyst for the oxygen reduction reaction. New J. Chem. 2018, 42, 19665–19670. [Google Scholar] [CrossRef]
- Wang, N.; Lu, B.Z.; Li, L.G.; Niu, W.H.; Tang, Z.H.; Kang, X.W.; Chen, S.W. Graphitic Nitrogen Is Responsible for Oxygen Electroreduction on Nitrogen-Doped Carbons in Alkaline Electrolytes: Insights from Activity Attenuation Studies and Theoretical Calculations. ACS Catal. 2018, 8, 6827–6836. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, Y.L.; Wang, H.Q.; Wang, C.M.; Wang, A.S.; Zhao, S.; Li, X.Y.; Sun, D.F.; Jiang, J.Z. Efficient ORR electrocatalytic activity of peanut shell-based graphitic carbon microstructures. J. Mater. Chem. A 2018, 6, 12018–12028. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.P.; Zhang, P.F.; Zhu, W.S.; Xia, J.X.; Li, H.M. Biomass willow catkin-derived Co3O4/N-doped hollow hierarchical porous carbon microtubes as an effective tri-functional electrocatalyst. J. Mater. Chem. A 2017, 5, 20170–20179. [Google Scholar] [CrossRef]
- Araújo, M.P.; Nunes, M.; Rocha, I.M.; Pereira, M.F.R.; Freire, C. Co3O4 Nanoparticles Anchored on Selectively Oxidized Graphene Flakes as Bifunctional Electrocatalysts for Oxygen Reactions. ChemistrySelect 2018, 3, 10064–10076. [Google Scholar] [CrossRef]
- Lu, H.Y.; Huang, Y.P.; Yan, J.J.; Fan, W.; Liu, T.X. Nitrogen-doped graphene/carbon nanotube/Co3O4 hybrids: One-step synthesis and superior electrocatalytic activity for the oxygen reduction reaction. RSC Adv. 2015, 5, 94615–94622. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Mathumba, P.; Fernandes, A.J.S.; Iwuoha, E.I.; Freire, C. Towards efficient oxygen reduction reaction electrocatalysts through graphene doping. Electrochim. Acta 2019, 319, 72–81. [Google Scholar] [CrossRef]
- Ramos, R.; Abdelkader-Fernandez, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Y.; Li, Y.; Han, X.; Xing, B.; Wang, S. Nitrogen and Sulfur Co-doped Hierarchical Porous Biochar Derived from the Pyrolysis of Mantis Shrimp Shell for Supercapacitor Electrodes. Energy Fuels 2021, 35, 1557–1566. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Salama, M.F.; El-Banna, H.A. Shrimp’s waste: Chemical composition, nutritional value and utilization. Food Nahr. 1999, 43, 418–423. [Google Scholar] [CrossRef]
- Gao, F.; Xie, Y.Q.; Zang, Y.H.; Zhou, G.; Qu, J.Y.; Wu, M.B. A sustainable strategy to prepare porous carbons with tailored pores from shrimp shell for use as supercapacitor electrode materials. New Carbon Mater. 2022, 37, 750–751. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.Y.; Geng, C.; Shao, G.H.; Wu, M.B. Self-templating synthesis of nitrogen-decorated hierarchical porous carbon from shrimp shell for supercapacitors. J. Mater. Chem. A 2016, 4, 7445–7452. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 13. [Google Scholar] [CrossRef]
- Mathew, G.M.; Mathew, D.C.; Sukumaran, R.K.; Sindhu, R.; Huang, C.C.; Binod, P.; Sirohi, R.; Kim, S.H.; Pandey, A. Sustainable and eco-friendly strategies for shrimp shell valorization. Environ. Pollut. 2020, 267, 15. [Google Scholar] [CrossRef]
- Li, Z.L.; Deng, L.B.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 58. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.W.; Ling, P.; Zhang, X.; Xu, K.; He, L.M.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 10. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D Graphene-Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Yang, J.G.; Bai, X.J.; Wang, Y.L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.Y.; Ikeya, K.; Watanabe, A. Size distribution of carbon layer planes in biochar from different plant type of feedstock with different heating temperatures. Chemosphere 2016, 163, 252–258. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.Y.; Liu, H.H.; Jia, X.P.; Bao, Y.C. Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: Co-pyrolysis characteristics and its adsorption capability. Chemosphere 2021, 282, 13. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.C.; Sousa, M.F.B.; Alobaid, F.; Bertran, C.A.; Wang, Y. A two-fluid model for calcium carbonate precipitation in highly supersaturated solutions. Adv. Powder Technol. 2018, 29, 1571–1581. [Google Scholar] [CrossRef]
- Mujtaba, J.; Sun, H.Y.; Huang, G.Y.; Molhave, K.; Liu, Y.G.; Zhao, Y.Y.; Wang, X.; Xu, S.M.; Zhu, J. Nanoparticle Decorated Ultrathin Porous Nanosheets as Hierarchical Co3O4 Nanostructures for Lithium Ion Battery Anode Materials. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Ahmad, N.; Ali, N.; Munawar, K.S. Synthesis of carbon nanotubes anchored with mesoporous Co3O4 nanoparticles as anode material for lithium-ion batteries. Electrochim. Acta 2013, 105, 481–488. [Google Scholar] [CrossRef]
- Leng, X.N.; Wei, S.F.; Jiang, Z.H.; Lian, J.S.; Wang, G.Y.; Jiang, Q. Carbon-Encapsulated Co3O4 Nanoparticles as Anode Materials with Super Lithium Storage Performance. Sci. Rep. 2015, 5, 11. [Google Scholar] [CrossRef]
- Prabaharan, D.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S. Precipitation method and characterization of cobalt oxide nanoparticles. Appl. Phys. A-Mater. Sci. Process. 2017, 123, 6. [Google Scholar] [CrossRef]
- Marques, I.S.; Jarrais, B.; Ramos, R.; Abdelkader-Fernandez, V.K.; Yaremchenko, A.; Freire, C.; Fernandes, D.M.; Peixoto, A.F. Nitrogen-doped biochar-supported metal catalysts: High efficiency in both catalytic transfer hydrogenation of furfural and electrocatalytic oxygen reactions. Catal. Today 2023, 418, 13. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Matos, R.; Nunes, M.S.; Kuzniarska-Biernacka, I.; Rocha, M.; Guedes, A.; Estrada, A.C.; Lopes, J.L.; Trindade, T.; Freire, C. Graphene@Metal Sulfide/Oxide Nanocomposites as Novel Photo-Fenton-like Catalysts for 4-Nitrophenol Degradation. Eur. J. Inorg. Chem. 2021, 2021, 4915–4928. [Google Scholar] [CrossRef]
- Jarrais, B.; Guedes, A.; Freire, C. Heteroatom-Doped Carbon Nanomaterials as Metal-Free Catalysts for the Reduction of 4-Nitrophenol. ChemistrySelect 2018, 3, 1737–1748. [Google Scholar] [CrossRef]
- Braga, R.; Fernandes, D.M.; Adán-Más, A.; Silva, T.M.; Montemor, M.F. The Role of the Precursor on the Electrochemical Performance of N,S Co-Doped Graphene Electrodes in Aqueous Electrolytes. Batteries 2023, 9, 168. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Novais, H.C.; Bacsa, R.; Serp, P.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Polyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) Electrocatalysts. Langmuir 2018, 34, 6376–6387. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Chastain, J. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Gopinath, C.S.; Hegde, S.G.; Ramaswamy, A.V.; Mahapatra, S. Photoernission studies of polymorphic CaCO3 materials. Mater. Res. Bull. 2002, 37, 1323–1332. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Wang, B.; Huang, C.C.; Wang, L.Z.; Hulicova-Jurcakova, D. Synthesis of Phosphorus-Doped Graphene and its Wide Potential Window in Aqueous Supercapacitors. Chem. Eur. J. 2015, 21, 80–85. [Google Scholar] [CrossRef]
- Luo, Y.; Gu, K.K.; Yang, T.Y.; Zhang, M.Z. Synthesis of porous nanosheet Co3O4 with two pairs of redox peaks for high performance as a battery electrode. J. Energy Storage 2019, 21, 362–369. [Google Scholar] [CrossRef]
- Fan, X.; Ohlckers, P.; Chen, X.Y. Tunable Synthesis of Hollow Co3O4 Nanoboxes and Their Application in Supercapacitors. Appl. Sci. 2020, 10, 1208. [Google Scholar] [CrossRef]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Glucose-derived carbon materials with tailored properties as electrocatalysts for the oxygen reduction reaction. Beilstein J. Nanotechnol. 2019, 10, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.; Fernandes, D.M.; Morales, M.V.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Cu and Pd nanoparticles supported on a graphitic carbon material as bifunctional HER/ORR electrocatalysts. Catal. Today 2020, 357, 279–290. [Google Scholar] [CrossRef]
- Bouleau, L.; Pérez-Rodríguez, S.; Quílez-Bermejo, J.; Izquierdo, M.T.; Xu, F.; Fierro, V.; Celzard, A. Best practices for ORR performance evaluation of metal-free porous carbon electrocatalysts. Carbon 2022, 189, 349–361. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 21. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Fuertes, A.B.; Sevilla, M.; Titirici, M.M. The influence of pore size distribution on the oxygen reduction reaction performance in nitrogen doped carbon microspheres. J. Mater. Chem. A 2016, 4, 2581–2589. [Google Scholar] [CrossRef]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.A. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Albashir, A.I.M.; Lu, X.Y.; Dai, X.Y.; Qi, W. Effects of porous structure and oxygen functionalities on electrochemical synthesis of hydrogen peroxide on ordered mesoporous carbon. Comm. Chem. 2024, 7, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.X.; Ge, B.C.; Pu, L.T.; Liu, Z.Q. Influence of Micropore and Mesoporous in Activated Carbon Air-cathode Catalysts on Oxygen Reduction Reaction in Microbial Fuel Cells. Electrochim. Acta 2016, 214, 110–118. [Google Scholar] [CrossRef]
- Liu, X.J.; Culhane, C.; Li, W.Y.; Zou, S.Z. Spinach-Derived Porous Carbon Nanosheets as High-Performance Catalysts for Oxygen Reduction Reaction. ACS Omega 2020, 5, 24367–24378. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, X.L.; Ye, G.H.; Gao, X.H.; Zhuang, Z.H.; Li, P.; Xiang, D.; Li, X.K.; Clayborne, A.Z.; Zhou, X.G.; et al. Balancing the Micro-Mesoporosity for Activity Maximization of N-Doped Carbonaceous Electrocatalysts for the Oxygen Reduction Reaction. ChemSusChem 2019, 12, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Wang, W.; Asif, M.; Yu, Y.; Wang, Z.Y.; Wang, J.L.; Liu, H.F.; Xiao, J.W. Cobalt ion-coordinated self-assembly synthesis of nitrogen-doped ordered mesoporous carbon nanosheets for efficiently catalyzing oxygen reduction. Nanoscale 2017, 9, 15534–15541. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.M.; Li, Y.H.; Ming, J.Y.; Wang, Q.; Wang, H.J.; Cao, Y.H.; Peng, F.; Yang, Y.H.; Yu, H. Electronic synergism of pyridinic- and graphitic-nitrogen on N-doped carbons for the oxygen reduction reaction. Chem. Sci. 2019, 10, 1589–1596. [Google Scholar] [CrossRef]
- Faisal, S.N.; Haque, E.; Noorbehesht, N.; Zhang, W.M.; Harris, A.T.; Church, T.L.; Minett, A.I. Pyridinic and graphitic nitrogen-rich graphene for high-performance supercapacitors and metal-free bifunctional electrocatalysts for ORR and OER. RSC Adv. 2017, 7, 17950–17958. [Google Scholar] [CrossRef]
- Liang, J.Y.; Yuan, Q.H. Effects of graphitic- and pyridinic-N co-doping on structure regulation and ORR activity of N-doped graphene. Appl. Surf. Sci. 2024, 648, 8. [Google Scholar] [CrossRef]
- Vazhayil, A.; Ashok, S.; Majumder, S.; Jeffery, A.A.; Altaf, M.; Ahn, Y.H.; Thomas, N. Orange peel derived activated carbon supported Ni-Co3O4 as an efficient electrocatalyst for oxygen evolution and reduction reaction in alkaline media. Mater. Chem. Phys. 2024, 328, 15. [Google Scholar] [CrossRef]
- Qian, C.; Guo, X.M.; Zhang, W.; Yang, H.X.; Qian, Y.; Xu, F.; Qian, S.L.; Lin, S.L.; Fan, T.X. Co3O4 nanoparticles on porous bio-carbon substrate as catalyst for oxygen reduction reaction. Microporous Mesoporous Mat. 2019, 277, 45–51. [Google Scholar] [CrossRef]
- Zhang, T.T.; He, C.S.; Sun, F.Z.; Ding, Y.Q.; Wang, M.C.; Peng, L.; Wang, J.H.; Lin, Y.Q. Co3O4 nanoparticles anchored on nitrogen-doped reduced graphene oxide as a multifunctional catalyst for H2O2 reduction, oxygen reduction and evolution reaction. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef]
- Nunes, M.; Fernandes, D.M.; Morales, M.V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Cu-based N-doped/undoped graphene nanocomposites as electrocatalysts for the oxygen reduction. J. Appl. Electrochem. 2019, 49, 693–703. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, S.W.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Visible Light Driven Photoelectrochemical Water Oxidation on Nitrogen-Modified TiO2 Nanowires. Nano Lett. 2012, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).