A Short Review of Layered Double Oxide-Based Catalysts for NH3-SCR: Synthesis and NOx Removal
Abstract
1. Introduction
2. Synthesis of LDO
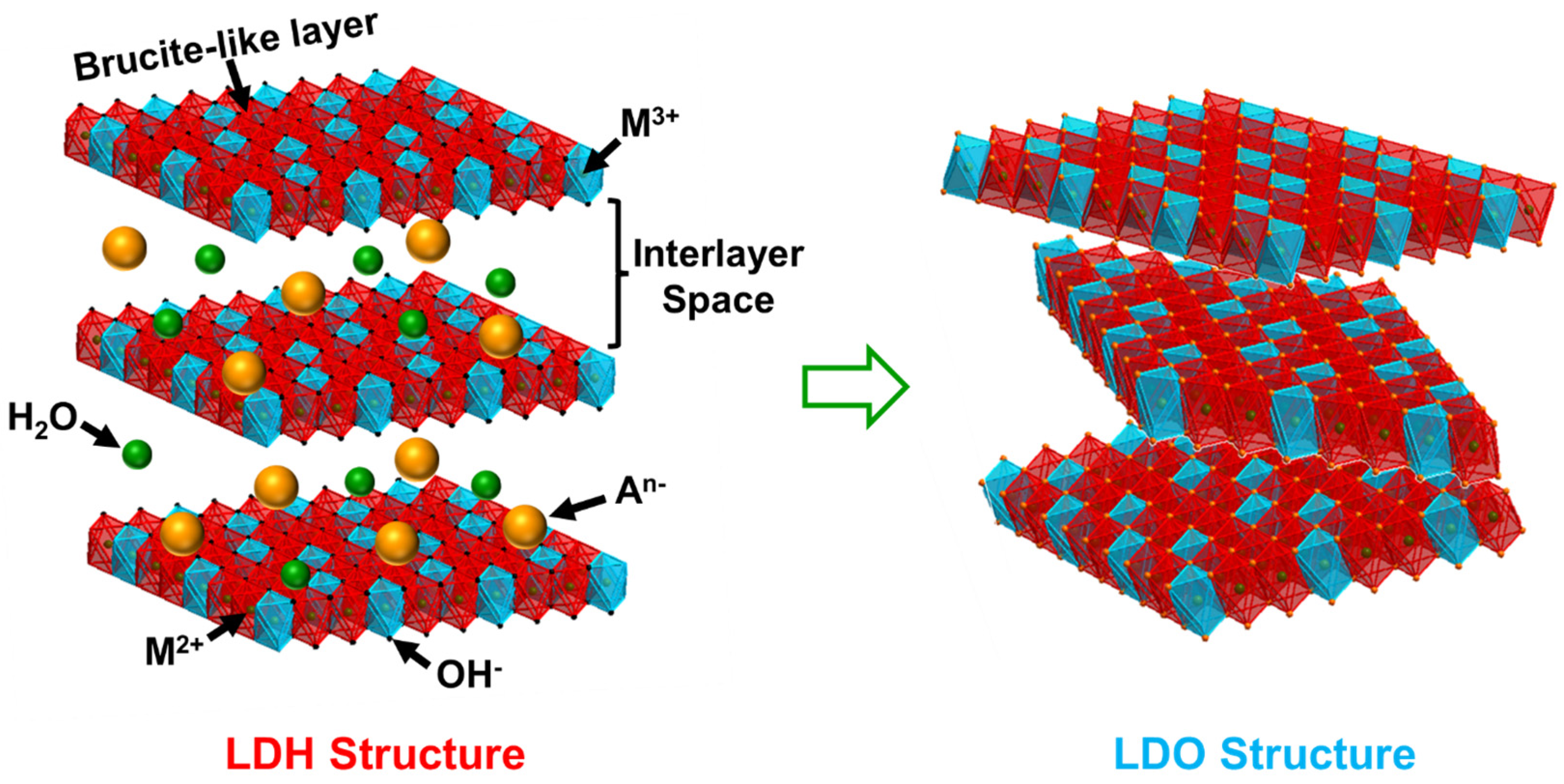
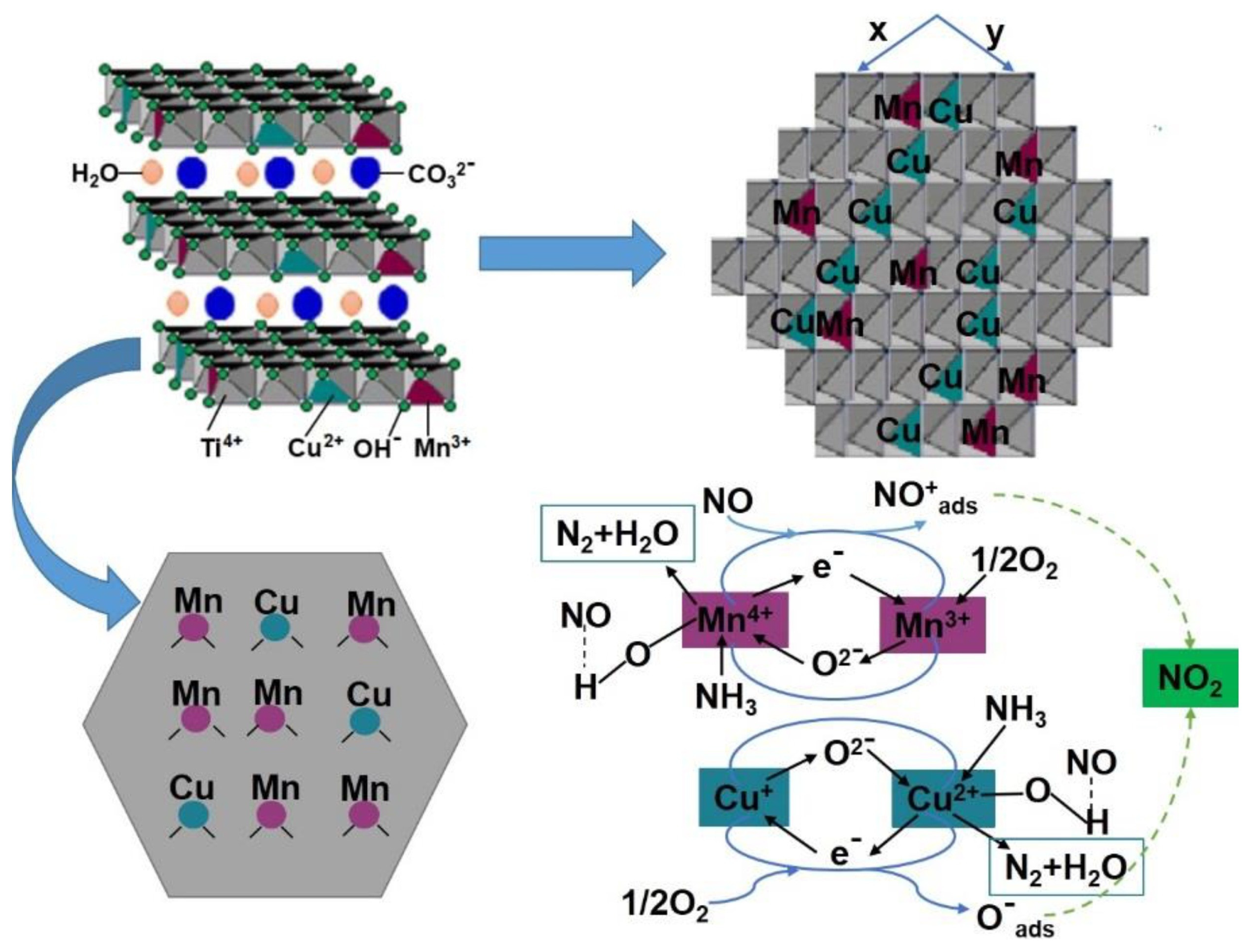
2.1. LDH Properties and Preparation
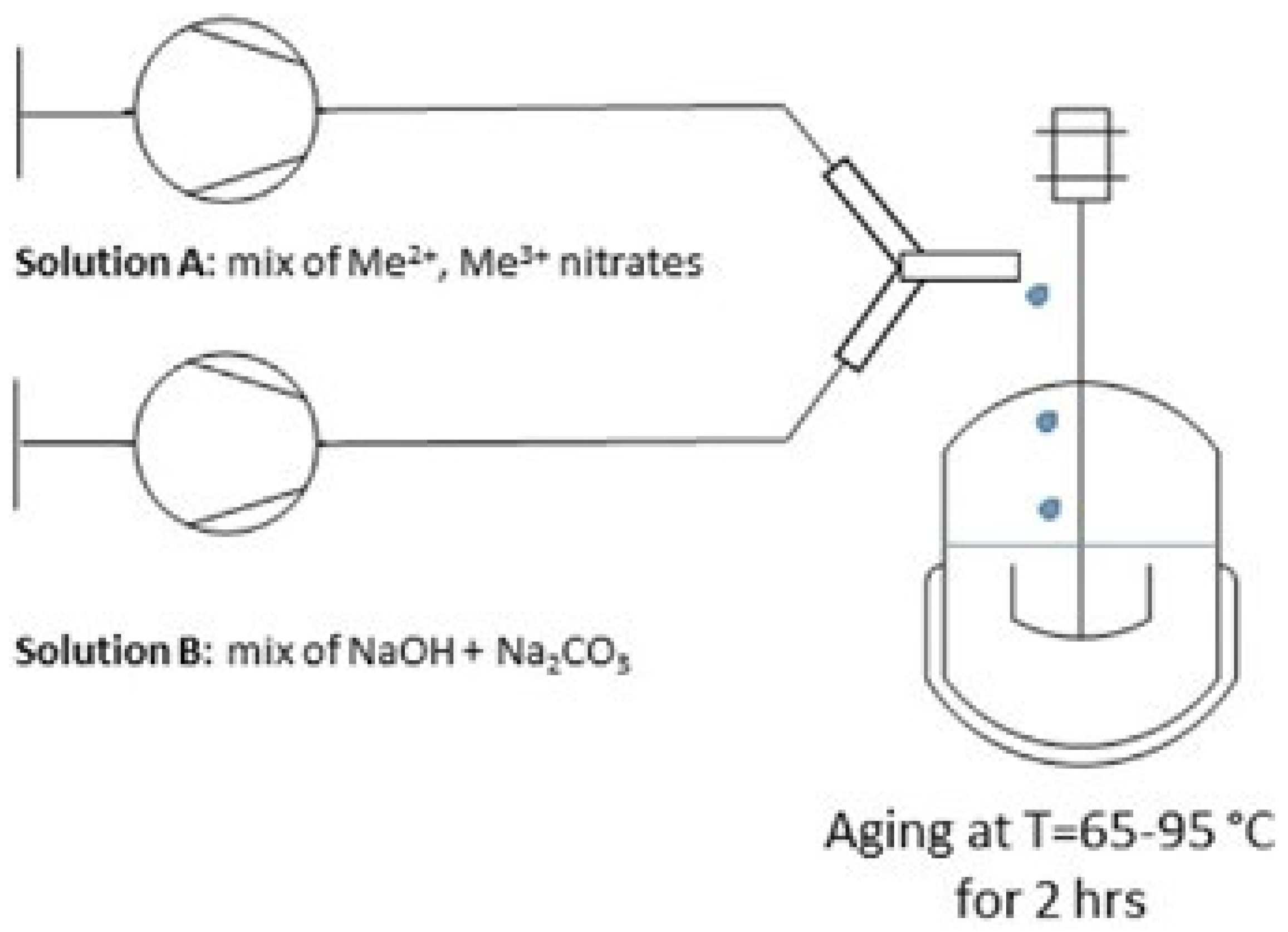
2.1.1. Coprecipitation
2.1.2. Urea Hydrothermal Method
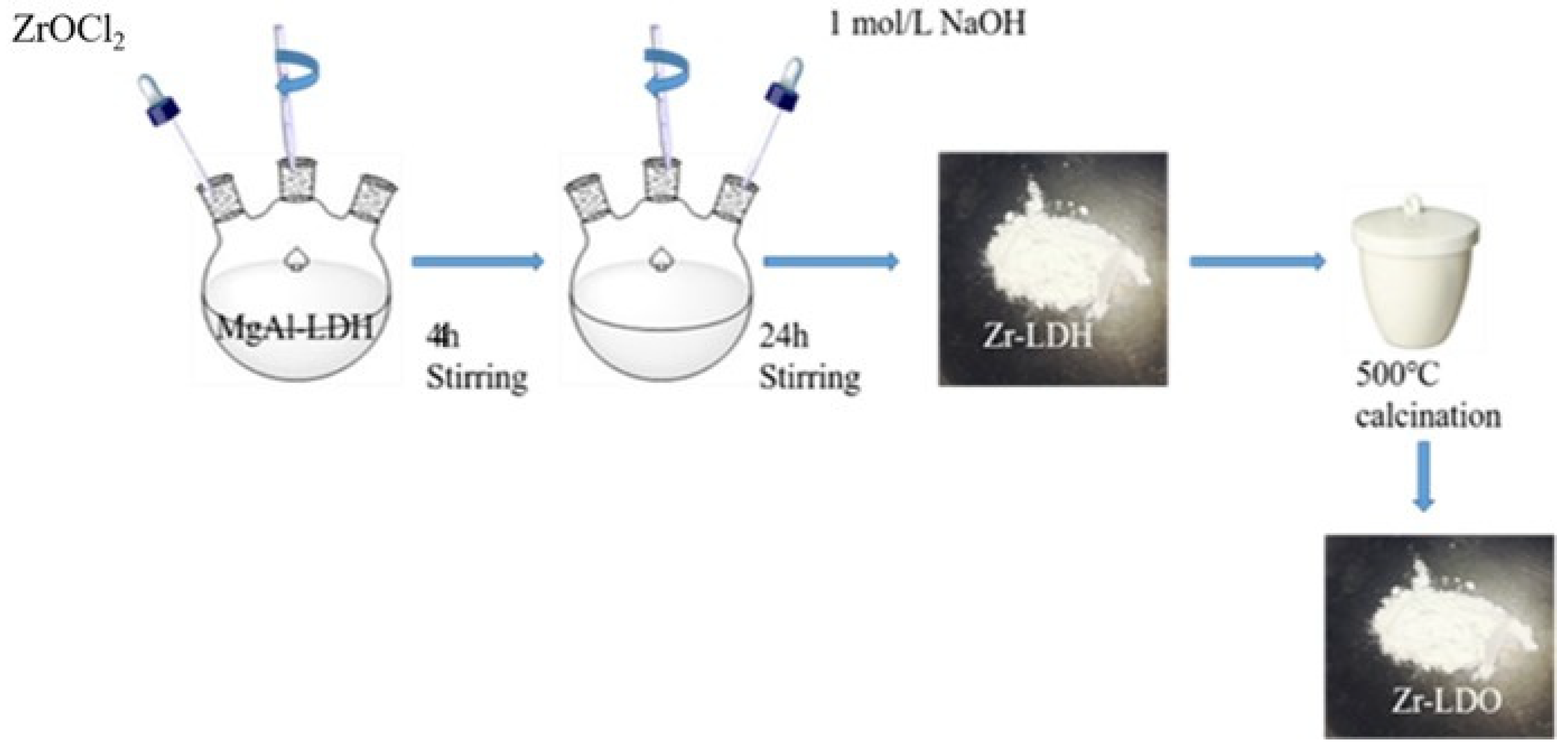
2.1.3. Ion Exchange Method
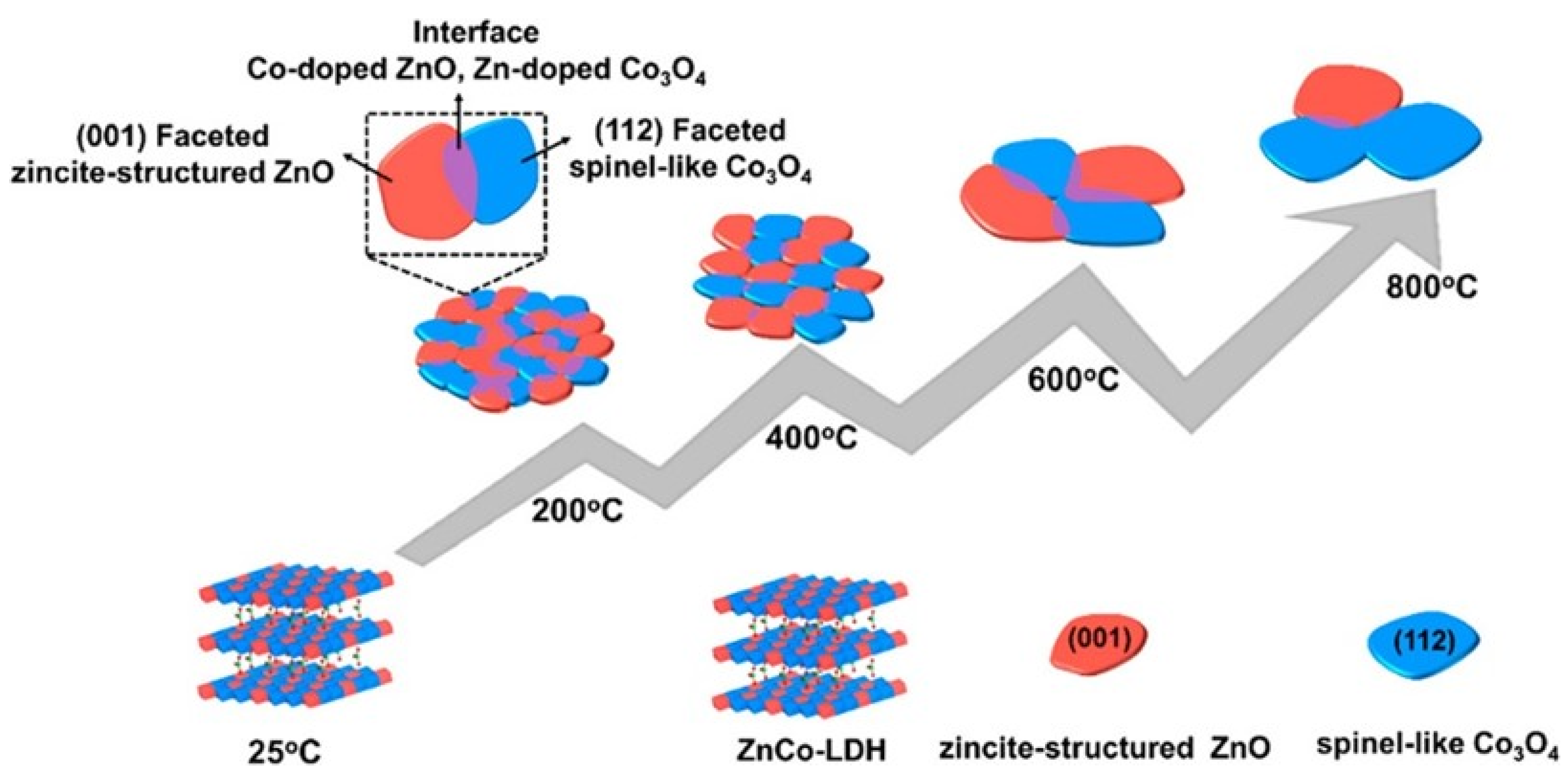
2.2. The Transition from LDH to LDO
3. LDO NH3-SCR Catalysts
3.1. The Influence of Preparation Methods and Structure on Catalytic Activity
3.2. The Influence of Morphology and Support on Catalytic Activity
3.3. Common LDO Catalysts Used in NH3-SCR Reactions
3.3.1. Mn-Based LDO Catalysts
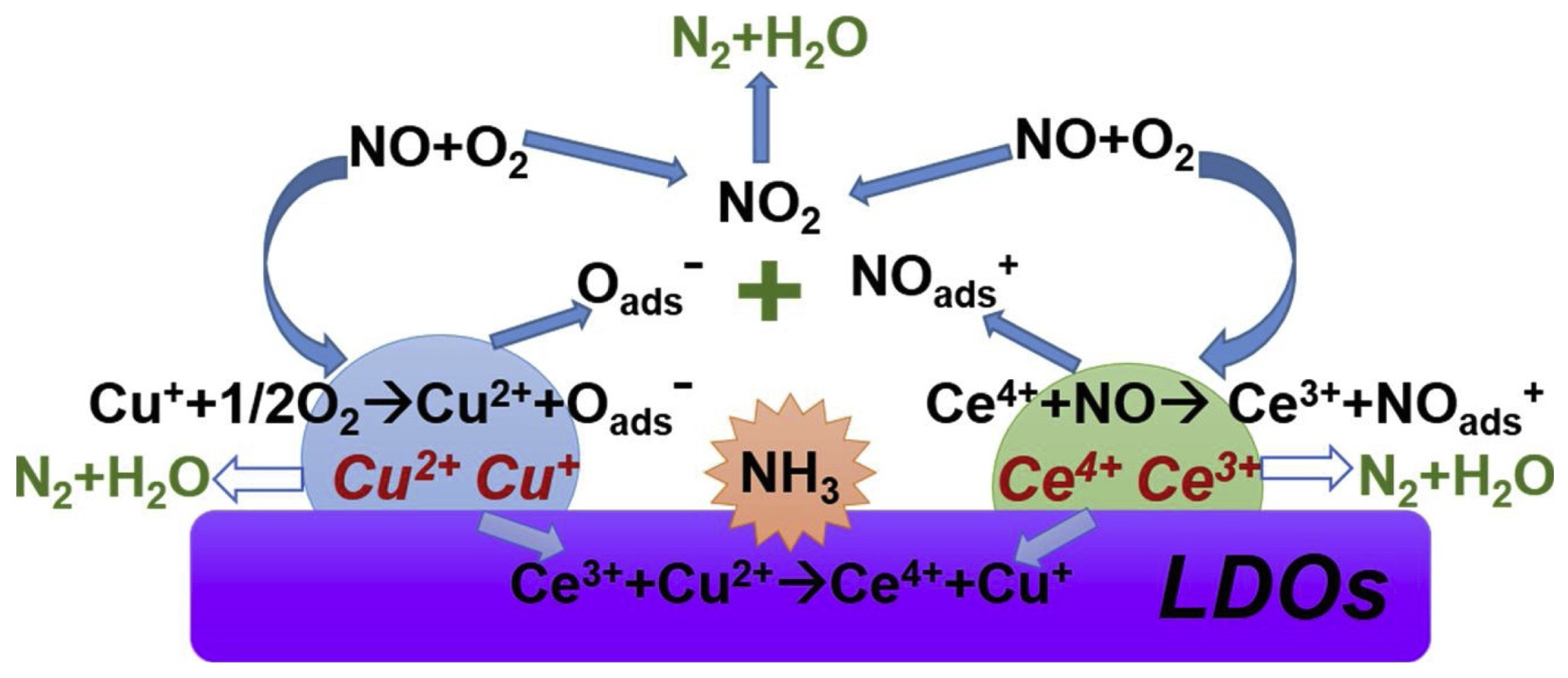
3.3.2. Cu-Based LDO Catalysts
3.3.3. Fe-Based LDO Catalysts
3.4. The NH3-SCR Process and Mechanism of LDO Catalysts
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Zou, R.; Wang, X. Toward an Atomic-Level Understanding of the Catalytic Mechanism of Selective Catalytic Reduction of NOx with NH3. ACS Catal. 2022, 12, 14347–14375. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, J.; Wang, J.; Chen, Y. Performances of CuSO4/TiO2 catalysts in selective catalytic reduction of NOx by NH3. Chin. J. Catal. 2016, 37, 281–287. [Google Scholar] [CrossRef]
- Lei, Z.; Wei, K.; Yang, J.; Zhang, L.; Lu, X.; Fang, B. Ultrasonication-Assisted Preparation of a Mn-Based Blast Furnace Slag Catalyst: Effects on the Low-Temperature Selective Catalytic Reduction Denitration Process. ACS Omega 2021, 6, 23059–23066. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Sun, Y.; Wang, X.; Li, H.; Tian, J.; Hu, W.; Liu, L. Comparative study on the toxic elements in spent selective catalytic reduction (SCR) catalyst from different industries: Occurrence, leaching behavior, and detoxification strategy. Fuel 2024, 366, 131298. [Google Scholar] [CrossRef]
- Kamasamudram, K.; Currier, N.W.; Chen, X.; Yezerets, A. Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol. Catal. Today 2010, 151, 212–222. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zhou, X.Y.; Zhou, J.L.; Hu, Z.; Cai, Q.; Lu, Q. A comprehensive review of the heavy metal issues regarding commercial vanadium-titanium-based SCR catalyst. Sci. Total Environ. 2023, 857, 159712. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Tan, W.; Ma, K.; Zou, W.; Tang, C.; Dong, L. Enhanced low-temperature NH3-SCR performance of CeTiO catalyst via surface Mo modification. Chin. J. Catal. 2020, 41, 364–373. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Lee, H.; Lee, S.G.; Cho, S.J.; Kim, D.H. Effects of microporous TiO2 support on the catalytic and structural properties of V2O5/microporous TiO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 210, 421–431. [Google Scholar] [CrossRef]
- Shamim, M.; Dana, K. Thermal decomposition of layered double hydroxides: Kinetic modeling and validation. Thermochim. Acta 2016, 632, 64–71. [Google Scholar] [CrossRef]
- Venugopal, B.R.; Shivakumara, C.; Rajamathi, M. A composite of layered double hydroxides obtained through random costacking of layers from Mg–Al and Co–Al LDHs by delamination–restacking: Thermal decomposition and reconstruction behavior. Solid State Sci. 2007, 9, 287–294. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Das, K.K.; Mansingh, S.; Sultana, S.; Parida, K. Recent progress in first row transition metal Layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis. Coord. Chem. Rev. 2022, 469, 214666. [Google Scholar] [CrossRef]
- Kasneryk, V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L. LDH has been grown: What is next? Overview on methods of post-treatment of LDH conversion coatings. Appl. Clay Sci. 2023, 232, 106774. [Google Scholar] [CrossRef]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, A.; Wang, Z.; Wang, M.; Wu, Q.; Shen, Y.; Zhu, Q.; Fu, Y.; Wen, M. Two-dimensional hetero-nanostructured electrocatalyst of Ni/NiFe-layered double oxide for highly efficient hydrogen evolution reaction in alkaline medium. Chem. Eng. J. 2021, 426, 131827. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, A.; Kumar, S.; Thakur, A.; Thakur, R.; Bhatia, S.K.; Sharma, A.K. Multifaceted potential applicability of hydrotalcite-type anionic clays from green chemistry to environmental sustainability. Chemosphere 2022, 306, 135464. [Google Scholar] [CrossRef]
- Cao, J.; Feng, Z.; Liang, H.; Lu, X.; Wang, W. Oriented self-assembly of anisotropic layered double hydroxides (LDHs) with 2D-on-3D hierarchical structure. Chem. Eng. J. 2023, 472, 144872. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Yang, N.; Ma, J.; Shi, J.; Yang, X.; Lu, J. Manipulate the nano-structure of layered double hydroxides via calcination for enhancing immobilization of anionic dyes on collagen fibers. J. Colloid Interface Sci. 2022, 610, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Yu, Y. Chloride binding by calcined layered double hydroxides and alumina-rich cementitious materials in mortar mixed with seawater and sea sand. Constr. Build. Mater. 2021, 293, 123493. [Google Scholar] [CrossRef]
- Bian, Q.; Zhang, M.; Liu, Y.; Liu, L.; Li, Y.; Wang, C.; He, G.; Liu, Y. Layered Double Hydroxide-Assisted Fabrication of Prussian Blue Membranes for Precise Molecular Sieving. Angew. Chem. Int. Ed. 2021, 61, e202113662. [Google Scholar] [CrossRef]
- Santos, R.M.M.; Tronto, J.; Briois, V.; Santilli, C.V. Thermal decomposition and recovery properties of ZnAl–CO3 layered double hydroxide for anionic dye adsorption: Insight into the aggregative nucleation and growth mechanism of the LDH memory effect. J. Mater. Chem. A 2017, 5, 9998–10009. [Google Scholar] [CrossRef]
- Lu, X.; Xue, H.; Gong, H.; Bai, M.; Tang, D.; Ma, R.; Sasaki, T. 2D Layered Double Hydroxide Nanosheets and Their Derivatives Toward Efficient Oxygen Evolution Reaction. Nano Micro Lett. 2020, 12, 1–32. [Google Scholar] [CrossRef]
- Gao, X.; Jia, Z.; Wang, B.; Wu, X.; Sun, T.; Liu, X.; Chi, Q.; Wu, G. Synthesis of NiCo-LDH/MXene hybrids with abundant heterojunction surfaces as a lightweight electromagnetic wave absorber. Chem. Eng. J. 2021, 419, 130019. [Google Scholar] [CrossRef]
- Pelalak, R.; Hassani, A.; Heidari, Z.; Zhou, M. State-of-the-art recent applications of layered double hydroxides (LDHs) material in Fenton-based oxidation processes for water and wastewater treatment. Chem. Eng. J. 2023, 474, 145511. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Yu, J.; Jiao, F. Layered double hydroxides materials for photo(electro-) catalytic applications. Chem. Eng. J. 2020, 397, 125407. [Google Scholar] [CrossRef]
- Motandi, M.K.; Zhang, Z.; Inkoua, S.; Yan, L. Application of zirconium modified layered double hydroxide and calcination product for adsorptive removal of phosphate from aqueous solution. Environ. Prog. Sustain. Energy 2021, 41, e13744. [Google Scholar] [CrossRef]
- Almojil, S.F.; Othman, M.A. Screening Different Divalent and Trivalent Metals Containing Binary and Ternary Layered Double Hydroxides for Optimum Phosphate Uptake. Sci. Rep. 2019, 9, 15511. [Google Scholar] [CrossRef]
- Yan, L.-G.; Yang, K.; Shan, R.-R.; Yan, T.; Wei, J.; Yu, S.-J.; Yu, H.-Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Pan, G.; Lundehøj, L.; Nielsen, U.G.; Shi, Y.; Hansen, H.C.B. Phosphate capture by ultrathin MgAl layered double hydroxide nanoparticles. Appl. Clay Sci. 2019, 177, 82–90. [Google Scholar] [CrossRef]
- Rahman, S.; Navarathna, C.M.; Krishna Das, N.; Alchouron, J.; Reneau, P.; Stokes, S.; Thirumalai, R.V.K.G.; Perez, F.; Barbary Hassan, E.; Mohan, D.; et al. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar. J. Colloid Interface Sci. 2021, 597, 182–195. [Google Scholar] [CrossRef]
- Li, D.; Chen, M.; Jiang, Y. Adsorptive removal of phosphate by ZnAlLa ternary layered double hydroxides: Synthesis conditions, adsorption performance, mechanism and reusability. Sep. Purif. Technol. 2025, 354, 128668. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials 2020, 10, 1361. [Google Scholar] [CrossRef]
- Wang, G.; Liao, Y.; Zhang, S.; Xu, X.; Lv, G.; Xu, C.; Cai, J.; Yang, Z.; Cheng, Z. Biodegradable chelator GLDA intercalated magnesium/aluminum layered double hydroxides for efficient phosphate capture and removal from wastewater. Environ. Technol. Innov. 2024, 35, 103701. [Google Scholar] [CrossRef]
- Tao, L.; Wang, J.; Qin, Q.; Chu, B.; Gao, P.; Qiu, J.; Li, Q.; Du, X.; Dong, L.; Li, B. Simple anion-modified layered double oxides use for controlling Cu valence states for low-temperature CO-SCR. Surf. Interfaces 2024, 44, 103654. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Costa, F.R.; Vyalikh, A.; Leuteritz, A.; Scheler, U.; Jehnichen, D.; Wagenknecht, U.; Häussler, L.; Heinrich, G. One-Step Synthesis of Organic LDH and Its Comparison with Regeneration and Anion Exchange Method. Chem. Mater. 2009, 21, 4490–4497. [Google Scholar] [CrossRef]
- Janani, F.Z.; Taoufik, N.; Khiar, H.; Boumya, W.; Elhalil, A.; Sadiq, M.; Puga, A.V.; Barka, N. Nanostructured layered double hydroxides based photocatalysts: Insight on synthesis methods, application in water decontamination/splitting and antibacterial activity. Surf. Interfaces 2021, 25, 101263. [Google Scholar] [CrossRef]
- Rouahna, N.; Barkat, D.; Ouakouak, A.; Srasra, E. Synthesis and characterization of Mg-Al layered double hydroxide intercalated with D2EHPA: Application for copper ions removal from aqueous solution. J. Environ. Chem. Eng. 2018, 6, 1226–1232. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Liu, X.; Wu, X.; Du, Y. Fabrication of carbon doped Cu-based oxides as superior NH3-SCR catalysts via employing sodium dodecyl sulfonate intercalating CuMgAl-LDH. J. Catal. 2022, 407, 265–280. [Google Scholar] [CrossRef]
- Aisawa, S.; Kudo, H.; Hoshi, T.; Takahashi, S.; Hirahara, H.; Umetsu, Y.; Narita, E. Intercalation behavior of amino acids into Zn–Al-layered double hydroxide by calcination–rehydration reaction. J. Solid State Chem. 2004, 177, 3987–3994. [Google Scholar] [CrossRef]
- Wang, J.; Fan, G.; Wang, H.; Li, F. Synthesis, Characterization, and Catalytic Performance of Highly Dispersed Supported Nickel Catalysts from Ni–Al Layered Double Hydroxides. Ind. Eng. Chem. Res. 2011, 50, 13717–13726. [Google Scholar] [CrossRef]
- Benito, P.; Labajos, F.M.; Rocha, J.; Rives, V. Influence of microwave radiation on the textural properties of layered double hydroxides. Microporous Mesoporous Mater. 2006, 94, 148–158. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.J.; Gao, W.; Li, B.; Wang, Q.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X.; O’Hare, D. Synthesis and antimicrobial activity of ZnTi-layered double hydroxide nanosheets. J. Mater. Chem. B 2013, 1, 5988–5994. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2017, 28, 1703868. [Google Scholar] [CrossRef]
- Yaseneva, P.; An, N.; Finn, M.; Tiedemann, N.; Jose, N.; Voutchkova-Kostal, A.; Lapkin, A. Continuous synthesis of doped layered double hydroxides in a meso-scale flow reactor. Chem. Eng. J. 2019, 360, 190–199. [Google Scholar] [CrossRef]
- Zadaviciute, S.; Baltakys, K.; Bankauskaite, A. The effect of microwave and hydrothermal treatments on the properties of hydrotalcite. J. Therm. Anal. Calorim. 2016, 127, 189–196. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Clissold, M.E.; Williams, P.A.; Kloprogge, J.T. Thermal decomposition of the natural hydrotalcites carrboydite and hydrohonessite. Thermochim. Acta 2003, 407, 1–9. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, H.; Kim, S.; Han, J.W.; Lee, K.B. Structural changes of hydrotalcite-based Co-containing mixed oxides with calcination temperature and their effects on NOx adsorption: A combined experimental and DFT study. Chem. Eng. J. 2022, 437, 135209. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Tan, L.; Zhao, Y.; Duan, H.; Song, Y.-F. Fine Tuning the Heterostructured Interfaces by Topological Transformation of Layered Double Hydroxide Nanosheets. Ind. Eng. Chem. Res. 2018, 57, 10411–10420. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Du, Y.; Wang, R.; Guo, X.; Hou, B. NOx Removal Performance Optimization of NiMnTi Mixed Oxide Catalysts by Tuning the Redox Capability. ChemCatChem 2019, 11, 1993–2003. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Du, Y.; Zou, C.; Meng, H.; Xie, X. Performance enhancement of NH3-SCR via employing hydrotalcite-like precursor to induce the decoration of NiO by TiO2 phase. Mol. Catal. 2019, 467, 150–160. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Zhou, X.; Yao, Y.; Wang, D.; Dan, J.; Dai, B.; Wang, Q.; Yu, F. Overwhelming low ammonia escape and low temperature denitration efficiency via MnO -decorated two-dimensional MgAl layered double oxides. Chin. J. Chem. Eng. 2020, 28, 1925–1934. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Yu, F.; Shi, Y.; Cao, P.; Dan, J.; Chen, K.; Lv, Y.; Guo, X.; Dai, B. Enhanced Oxygen Vacancies in a Two-Dimensional MnAl-Layered Double Oxide Prepared via Flash Nanoprecipitation Offers High Selective Catalytic Reduction of NOx with NH3. Nanomaterials 2018, 8, 620. [Google Scholar] [CrossRef]
- Du, Y.; Liu, J.; Li, X.; Liu, L.; Wu, X. SCR performance enhancement of NiMnTi mixed oxides catalysts by regulating assembling methods of LDHs-Based precursor. Appl. Organomet. Chem. 2020, 34, e5510. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, J.; Song, K.; Zeng, J.; Zhou, X.; Guo, X.; Lin, K.; Zhang, C.; Xie, C.; Shi, J.-W. Insight into the reasons for enhanced NH3-SCR activity and SO2 tolerance of Mn-Co layered oxides. Sep. Purif. Technol. 2024, 336, 126285. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Horky, O.; Slang, S.; Dubnova, L.; Gajewska, M.; Chmielarz, L.; Capek, L. Hollow @CuMgAl double layered hydrotalcites and mixed oxides with tunable textural and structural properties, and thus enhanced NH3-NOx-SCR activity. Nanoscale Adv. 2023, 5, 3063–3074. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Maitarad, P.; Shi, L.; Gao, R.; Zhang, J.; Cao, W. In situ synthesis of 3D flower-like NiMnFe mixed oxides as monolith catalysts for selective catalytic reduction of NO with NH3. Chem. Commun. 2012, 48, 10645–10647. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, X.; Liu, J.; Du, R.; Wu, X. DeNOx performance enhancement of Cu-based oxides via employing a TiO2 phase to modify LDH precursors. RSC Adv. 2022, 12, 10142–10153. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, H.; Du, Y.; Liu, J.; Hou, B.; Xie, X. Fabrication of Highly Dispersed Cu-Based Oxides as Desirable NH3-SCR Catalysts via Employing CNTs To Decorate the CuAl-Layered Double Hydroxides. ACS Appl. Mater. Interfaces 2019, 11, 32917–32927. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Du, Y.; Liu, X.; Ci, C.; Wu, X.; Xie, X. Tunable preparation of highly dispersed Nix Mn-LDO catalysts derived from Nix Mn-LDHs precursors and application in low-temperature NH3-SCR reactions. RSC Adv. 2019, 9, 24377–24385. [Google Scholar] [CrossRef]
- Yan, Q.; Nie, Y.; Yang, R.; Cui, Y.; O’Hare, D.; Wang, Q. Highly dispersed CuyAlOx mixed oxides as superior low-temperature alkali metal and SO2 resistant NH3 -SCR catalysts. Appl. Catal. A 2017, 538, 37–50. [Google Scholar] [CrossRef]
- Nie, Y.; Yan, Q.; Chen, S.; O’Hare, D.; Wang, Q. CuTi LDH derived NH3-SCR catalysts with highly dispersed CuO active phase and improved SO2 resistance. Catal. Commun. 2017, 97, 47–50. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, J.; Gui, R.; Chen, Z.; Li, Y.; Zhu, T.; Wang, Q.; Xin, Y. Mechanistic Insight into the Promotion of the Low-Temperature NH3–SCR Activity over NiMnFeOx LDO Catalysts: A Combined Experimental and DFT Study. Environ. Sci. Technol. 2023, 57, 20708–20717. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Guo, X.; Wang, R.; Hou, B.; Wu, X. Synthesis of a Novel NiMnTi Mixed Metal Oxides from LDH Precursor and Its Catalytic Application for Selective Catalytic Reduction of NOx with NH3. Catal. Lett. 2018, 149, 456–464. [Google Scholar] [CrossRef]
- Chen, S.; Vasiliades, M.A.; Yan, Q.; Yang, G.; Du, X.; Zhang, C.; Li, Y.; Zhu, T.; Wang, Q.; Efstathiou, A.M. Remarkable N2-selectivity enhancement of practical NH3-SCR over Co0.5Mn1Fe0.25Al0.75Ox-LDO: The role of Co investigated by transient kinetic and DFT mechanistic studies. Appl. Catal. B 2020, 277, 119186. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Wang, Q. Design of practical Ce/CoMnAl-LDO catalyst for low-temperature NH3-SCR. Catal. Commun. 2020, 142, 106037. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Liu, L.; Du, Y.; Wu, X. Superior CuMgFe mixed oxide catalysts engineered by tuning the redox cycle for enhancing NOx removal performance. J. Environ. Chem. Eng. 2022, 10, 108824. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5O mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance. Appl. Catal. B 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kustrowski, P.; Rafalska-Lasocha, A.; Majda, D.; Dziembaj, R. Catalytic activity of Co–Mg–Al, Cu–Mg–Al and Cu–Co–Mg–Al mixed oxides derived from hydrotalcites in SCR of NO with ammonia. Appl. Catal. B Environ. 2002, 35, 195–210. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; O’Hare, D.; Wang, Q. Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J. Colloid Interface Sci. 2018, 526, 63–74. [Google Scholar] [CrossRef]
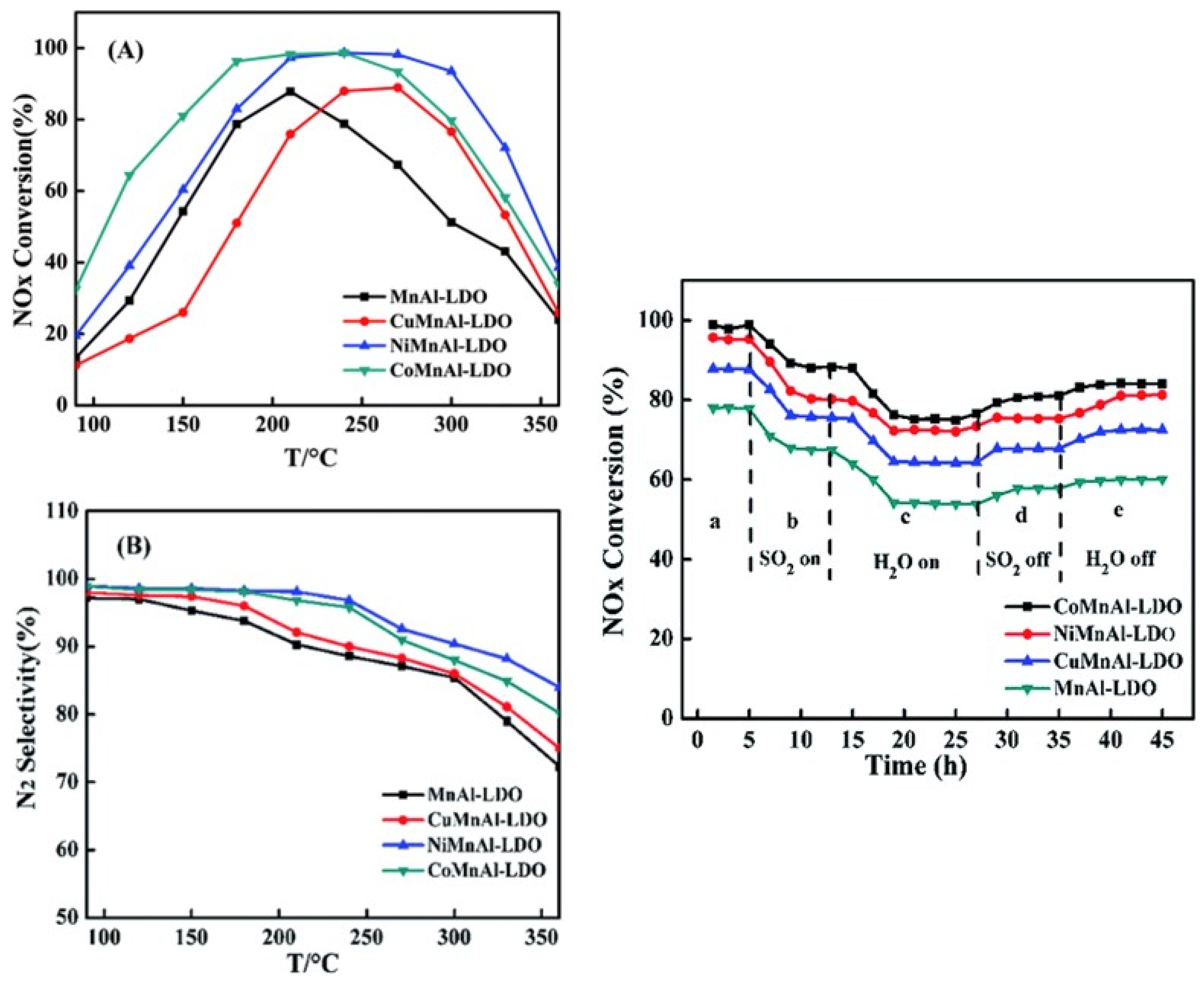
- Du, Y.; Liu, L.; Feng, Y.; Yang, B.; Wu, X. Enhancement of NH(3)-SCR performance of LDH-based MMnAl (M = Cu, Ni, Co) oxide catalyst: Influence of dopant M. RSC Adv. 2019, 9, 39699–39708. [Google Scholar] [CrossRef]
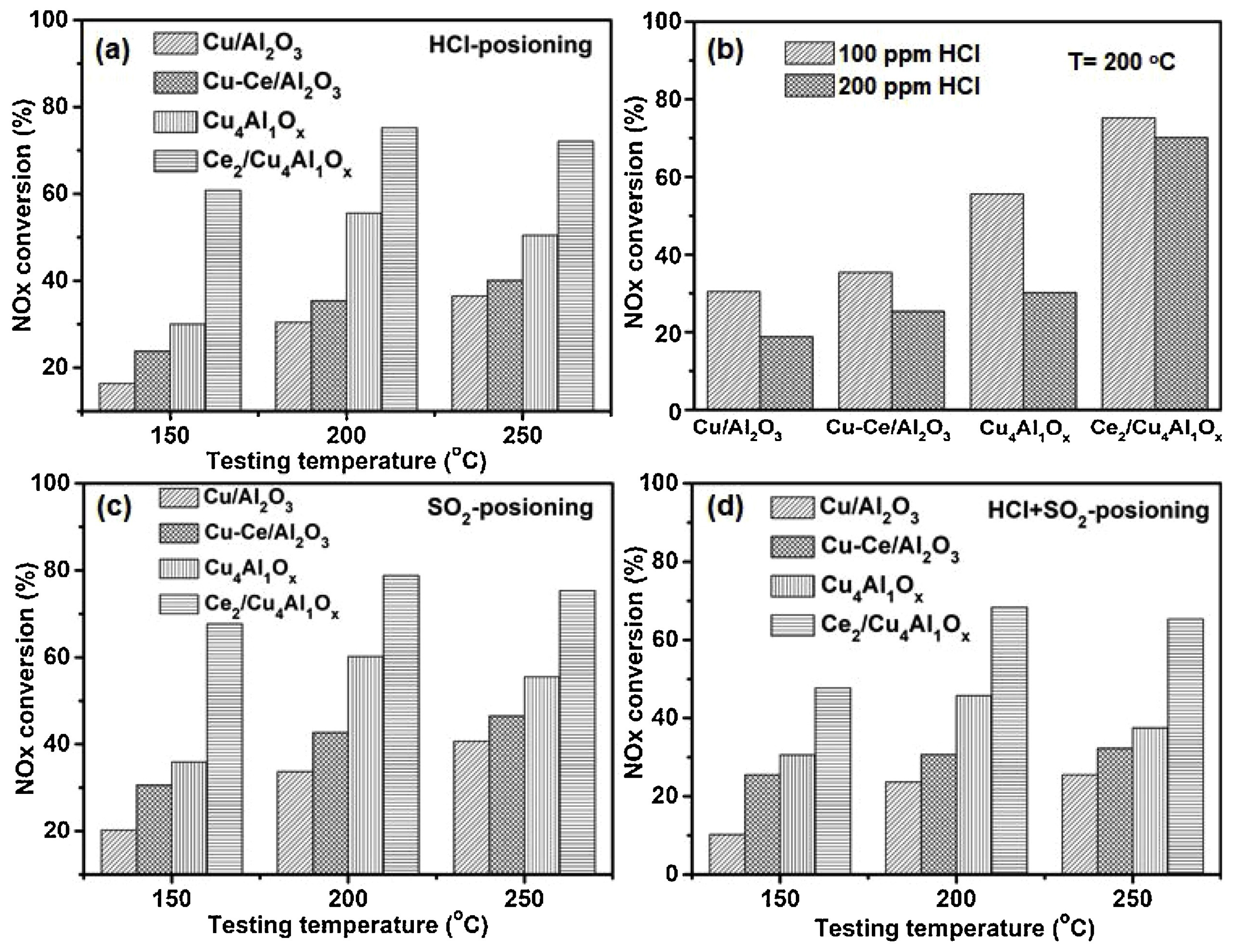
- Yan, Q.; Gao, Y.; Li, Y.; Vasiliades, M.A.; Chen, S.; Zhang, C.; Gui, R.; Wang, Q.; Zhu, T.; Efstathiou, A.M. Promotional effect of Ce doping in Cu4Al1Ox—LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies. Appl. Catal. B 2019, 255, 117749. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, F.; Sun, R.; Tian, J.; Wang, Q.; Dai, B.; Dan, J.; Pfeiffer, H. Two-dimensional MnFeCo layered double oxide as catalyst for enhanced selective catalytic reduction of NOx with NH3 at low temperature (25–150 °C). Appl. Catal. A 2020, 592, 117432. [Google Scholar] [CrossRef]
- Wu, X.; Meng, H.; Du, Y.; Liu, J.; Hou, B.; Xie, X. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: Enhanced activity and synergistic mechanism. J. Catal. 2020, 384, 72–87. [Google Scholar] [CrossRef]
- Feng, X.; Zeng, J.; Zhu, J.; Song, K.; Zhou, X.; Guo, X.; Xie, C.; Shi, J.-W. Gd-modified Mn-Co oxides derived from layered double hydroxides for improved catalytic activity and H2O/SO2 tolerance in NH3-SCR of NOx reaction. J. Colloid Interface Sci. 2024, 659, 1063–1071. [Google Scholar] [CrossRef]
- Yoon, W.; Kim, Y.; Kim, G.J.; Kim, J.-R.; Lee, S.; Han, H.; Park, G.H.; Chae, H.-J.; Kim, W.B. Boosting low temperature De-NOx performance and SO2 resistance over Ce-doped two dimensional Mn-Cr layered double oxide catalyst. Chem. Eng. J. 2022, 434, 134676. [Google Scholar] [CrossRef]
- Chen, S.; Yan, Q.; Zhang, C.; Wang, Q. A novel highly active and sulfur resistant catalyst from Mn-Fe-Al layered double hydroxide for low temperature NH3-SCR. Catal. Today 2019, 327, 81–89. [Google Scholar] [CrossRef]
- Carja, G.; Delahay, G. Mesoporous mixed oxides derived from pillared oxovanadates layered double hydroxides as new catalysts for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2004, 47, 59–66. [Google Scholar] [CrossRef]
- Zhang, Y.-s.; Li, C.; Yu, C.; Tran, T.; Guo, F.; Yang, Y.; Yu, J.; Xu, G. Synthesis, characterization and activity evaluation of Cu-based catalysts derived from layered double hydroxides (LDHs) for DeNO reaction. Chem. Eng. J. 2017, 330, 1082–1090. [Google Scholar] [CrossRef]
- Yoon, W.; Lee, S.; Maeng, J.; Jeon, N.; Yun, Y.; Kim, Y.; Chae, H.-J.; Kim, W.B. Improving catalytic performance with sulfur resistance over Co-Cr layered double oxide for low temperature NH3-SCR. Chem. Eng. J. 2023, 452, 139561. [Google Scholar] [CrossRef]
- Hou, Q.; Liu, Y.; Hou, Y.; Han, X.; Huang, Z. Tunable Highly Dispersed Nia-xMnxAlOy Catalysts Derived from Layered Double Oxide for Low Temperature NOx Removal. Catal. Lett. 2024, 154, 4389–4402. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Du, Y.; Li, X.; Meng, H.; Xie, X. NOx removal by selective catalytic reduction with ammonia over hydrotalcite-derived NiTi mixed oxide. New J. Chem. 2019, 43, 2640–2648. [Google Scholar] [CrossRef]
- Du, Y.; Wu, X.; Liu, L.; Li, X.; Liu, L.; Wu, X. Low-Temperature NH3 Selective Catalytic Reduction Performance Enhancement of Fe-Based Oxides by Employing Carbon Nanotubes to Decorate the MgFe-LDH. ChemistrySelect 2023, 8, e202203767. [Google Scholar] [CrossRef]
- Basąg, S.; Kocoł, K.; Piwowarska, Z.; Rutkowska, M.; Baran, R.; Chmielarz, L. Activating effect of cerium in hydrotalcite derived Cu–Mg–Al catalysts for selective ammonia oxidation and the selective reduction of NO with ammonia. React. Kinet. Mech. Catal. 2017, 121, 225–240. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Qiu, L.; Gao, Y.; O’Hare, D.; Wang, Q. The synthesis of CuyMnzAl1−zOx mixed oxide as a low-temperature NH3-SCR catalyst with enhanced catalytic performance. Dalton Trans. 2018, 47, 2992–3004. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Z.; Li, Y.; Liu, G.; Zhang, H.; Wang, H.; Xia, Y.; Zhan, S. Relationship between structure and performance of a novel highly dispersed MnOx on Co-Al layered double oxide for low temperature NH3-SCR. Appl. Catal. B 2019, 258, 117983. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Du, Y.; Liu, X.; Zou, C.; Li, Z. Enhancing DeNOx performance of CoMnAl mixed metal oxides in low-temperature NH3-SCR by optimizing layered double hydroxides (LDHs) precursor template. Appl. Surf. Sci. 2019, 467, 802–810. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Jin, W.; Liu, Y. Mn mixed oxide catalysts supported on Sn-doped CoAl-LDO for low-temperature NH3-SCR. Catal. Sci. Technol. 2023, 13, 3147–3157. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Chen, L.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Jansson, J.; Skoglundh, M.; Grönbeck, H. A Complete Multisite Reaction Mechanism for Low-Temperature NH3-SCR over Cu-CHA. ACS Catal. 2020, 10, 5646–5656. [Google Scholar] [CrossRef]
- Wang, X.; Du, X.; Yang, G.; Xue, J.; Chen, Y.; Zhang, L. Chemisorption of NO2 on V-Based SCR Catalysts: A Fundamental Study toward the Mechanism of “Fast SCR” Reaction. J. Phys. Chem. C 2019, 123, 20451–20458. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, J.; Zheng, R.; Guo, L.; Yuan, J.; Zhang, S.; Gu, M. Insight into the reaction mechanism over PMoA for low temperature NH3-SCR: A combined In-situ DRIFTs and DFT transition state calculations. J. Hazard. Mater. 2021, 412, 125258. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Z.; Luan, X.; Zhai, Y.; Zhang, J.; Wang, L.; Wang, Z. Optimizing the catalytic performance of MnCo2O4 spinel catalysts for the NH3-SCR: Structure-activity relationships and reaction mechanism. Appl. Surf. Sci. 2024, 674, 160915. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, N.; Huang, Z.; Zhao, G.; Chu, F.; Yang, R.; Tang, X.; Wang, G.; Gao, F.; Huang, X. Recent advances in low-temperature NH3-SCR of NOx over Ce-based catalysts: Performance optimizations, reaction mechanisms and anti-poisoning countermeasures. Chem. Eng. J. 2023, 476, 146889. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B 2018, 222, 76–87. [Google Scholar] [CrossRef]







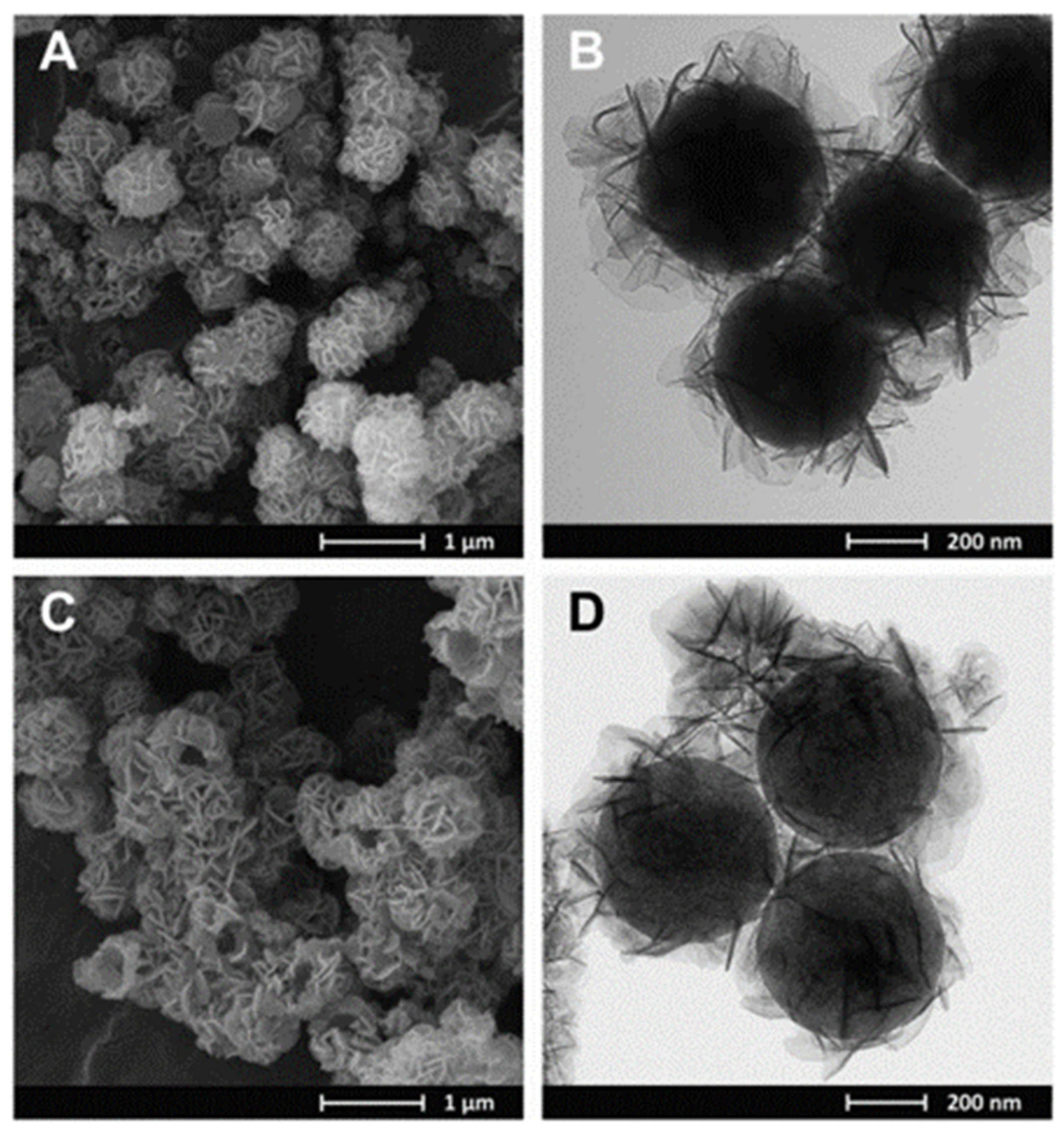




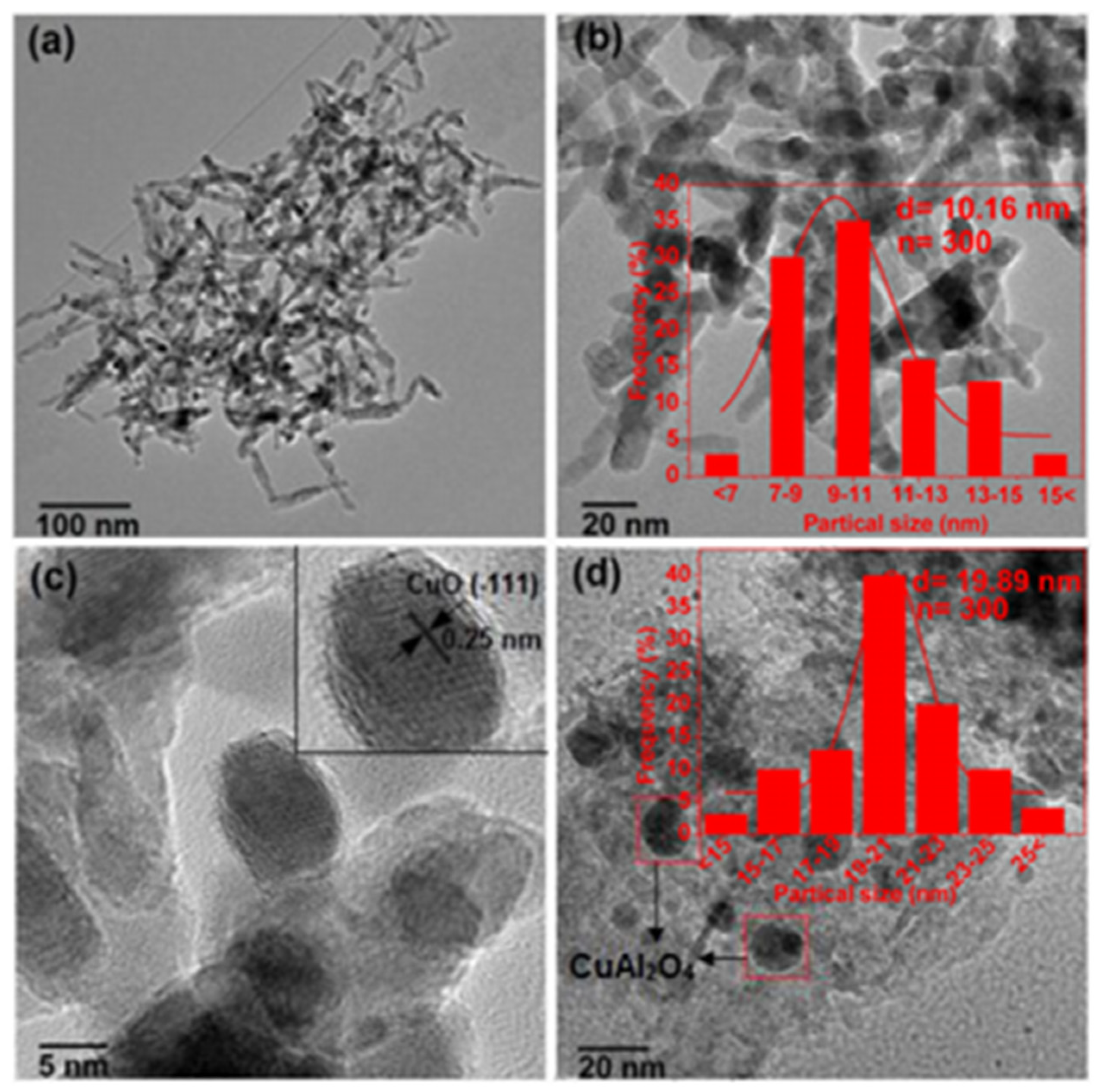
| Materials | Preparation Method | Size (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Phosphate Sorption Quantity (mg/g) | Refs. |
|---|---|---|---|---|---|---|---|
| Fe3O4@Zn–Al–LDH | Co-precipitation | 200 | 133 | 0.59 | 17.6 | 36.9 | [31] |
| Fe3O4@Mg–Al–LDH | Co-precipitation | 200 | 71.9 | 0.15 | 7.81 | 31.7 | [31] |
| Fe3O4@Ni–Al–LDH | Co-precipitation | 200 | 50.9 | 0.34 | 28.4 | 26.5 | [31] |
| AC/MgAl LDH | Co-precipitation | —— | 584.124 | 584.124 | —— | 337.2 | [32] |
| LDHns-U80 | Co-precipitation | 30 | 97 | 0.28 | 13 | 98 ± 11 | [33] |
| Fe/Mg-LDH-BC | Co-precipitation | —— | 267.3 | 0.0879 | —— | 117.2 | [34] |
| ZnAlLa-LDH | Co-precipitation | 200 | 28.631 | 0.138 | 20.316 | 105.42 | [35] |
| SBAC100MgFe | Co-precipitation | 30 | 169 | 0.21 | 1.82 | 104 | [36] |
| GLDA@MgAl–LDH | Co-precipitation | 50 | 60.15 | 0.13 | 1.76 | 44.17 | [37] |
| Catalysts | Preparation Method of LDH | Calcination | Morphology of LDO | Size (nm) | Structure Parameters | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | Time (h) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | |||||
| MnCo-LDO | Urea Hydrothermal | 400 | 4 | Layered structure | 4000 | 142.89 | 0.17 | 5.01 | [59] |
| Ni5Mn-LDO | Co-precipitation | 400 | 5 | Spherical | 13.23 | 70 | 0.17 | 9.35 | [64] |
| MnAl-LDO (FNP) | Flash Nanoprecipitation | 550 | 6 | Particles | 114.9 | 121 | 0.22 | 7.13 | [57] |
| Cu4AlOx | Co-precipitation | 600 | 5 | Flower-like | 10.16 | 108.4 | 0.92 | 20.6 | [65] |
| Cu1Ti1O | Homogeneous Precipitation | 400 | 5 | Particles | 10 | 150.5 | 0.377 | 5.02 | [66] |
| NiMnTi-LDO | Urea Homogeneous Precipitation | 400 | 5 | Flower-like | 60 | 210 | 0.32 | 5 | [58] |
| Ni2Mn2Ti1-LDO | Urea Homogeneous Precipitation | 400 | 5 | Flower-like | 7.157 | 210 | 0.32 | 5 | [54] |
| Ni0.5Mn0.5Fe0.5Ox | Co-precipitation | 400 | 5 | Flower-like | 50 | —— | —— | —— | [67] |
| NiMnTi-LDO | Urea Homogeneous Precipitation | 400 | 5 | Flower-like | 4.54 | 244 | 0.23 | 3.5 | [68] |
| Co0.5Mn1Fe0.25Al0.75Ox-LDO | Co-precipitation | 400 | 5 | Layered structure | —— | 316 | 1.5 | —— | [69] |
| Ce0.5/Co1Mn0.5Al0.5Ox-LDO | Co-precipitation | 500 | 5 | Flower-like | 200 | 210.1 | 0.77 | 7.29 | [70] |
| Cu0.5Mg2.5Fe1-LDO | Co-precipitation | 500 | 5 | Sheet | 10 | 102 | 0.34 | 10.4 | [71] |
| Cu1Mn0.5Ti0.5Ox | Co-precipitation | 400 | 5 | Sheet | 200 | 102.1 | 0.339 | 6.63 | [72] |
| Cu-Mg-Al | Co-precipitation | 600 | 16 | —— | —— | —— | —— | —— | [73] |
| Cu0.5Mg1.5Mn0.5Al0.5Ox | Co-precipitation | 400 | 5 | Flower-like | 230 | 228.7 | 1.68 | 14.69 | [74] |
| CuAl-LDO/CNTs(I) | In Situ Assembly | 500 | 5 | Stacked gauze-like | —— | —— | —— | —— | [63] |
| CoMnAl-LDO | Co-precipitation | 500 | 5 | Layered structure | 250 | 140.9 | —— | —— | [75] |
| CuMgAl-SDSO-LDO | Co-precipitation | 500/400 | 5/4 | Sheet | 300 | 85 | 0.2 | 7 | [42] |
| Ce2/Cu4Al1Ox-LDO | Co-precipitation | 400 | 5 | Nanosheet | 90 | 130.8 | 0.86 | 13.14 | [76] |
| NiTi-LDO | Urea Homogeneous Precipitation | 400 | 5 | Flake morphology | 5 | 224.6 | —— | 2.9 | [55] |
| Mn/MgAl-LDO (FP-CP) | FP-CP | 400 | 2 | Stacked nanosheet | 8 | 103.6 | 0.23 | 8.97 | [56] |
| MnFeCo-LDO | Co-precipitation | 500 | 4 | Layered structure | 200–300 | 92 | —— | —— | [77] |
| CuAl LDH/CNTs-2 | In Situ Assembly | 500 | 5 | —— | —— | 138 | —— | 6.2 | [78] |
| MnCoGd0.2 | Urea Hydrothermal | 400 | 4 | Nanoplates | —— | 181.1 | 0.18 | 5.88 | [79] |
| Ce0.2-Mn2Cr1Ox-LDO | Co-precipitation | 400 | 5 | Flower-like | 100 | 173.8 | 0.476 | 10.7 | [80] |
| Mn1Fe0.25Al0.75Ox | AMOST | 400 | 5 | Flower-like | 40 | 304.3 | 1.719 | 11.3 | [81] |
| CuVOLDHc | Co-precipitation | 470 | 5 | Flower-like | 80 | 72 | 0.344 | —— | [82] |
| (Cu4Zny)2Al-MMO | Co-precipitation | 450 | 4 | Sheet | 2000 | 58 | 0.15 | 10.4 | [83] |
| Co2Cr1Ox-LDO | Co-precipitation | 400 | 5 | Flower-like | 500 | 124.8 | 0.401 | 12.9 | [84] |
| Ni4-xMnxAlOy | Hydrothermal | 400 | 4 | Nanosheet | —— | 178.2 | 0.4 | 8.9 | [85] |
| @CuMgAl_0.5-MO | Template | 450 | 4 | Core–shell particles | 800 | 258 | 0.66 | —— | [60] |
| Ni4Ti1-400 | Urea Homogeneous Precipitation | 400 | 5 | Nanosheet | 5.6 | 224.6 | —— | 2.9 | [86] |
| Mg3Fe1-LDO/10CNTs | Co-precipitation | 500 | 5 | Thin sheets | —— | 112 | 0.62 | 15.4 | [87] |
| Cu5Mg62Al33-Ce3.0% | Co-precipitation | 600 | 12 | —— | —— | 242 | —— | —— | [88] |
| Cu2Mn0.5Al0.5Ox | Co-precipitation | 400 | 5 | Flower-like | —— | 136.4 | 1.029 | 15.09 | [89] |
| NiMnFe-IWM-C | In Situ Hydrothermal | 500 | 5 | 3D flower-like | 3000 | —— | —— | —— | [61] |
| Mn(0.25)/CoAl-LDO | Urea Homogeneous Precipitation | 500 | 4 | Nanosheet | 2000 | 155.1 | 0.31 | 3.48 | [90] |
| MnO2/CoAl-LDO | Ion-exchange/Redox Reaction | 500 | 5 | Particles | 9 | 156.8 | —— | —— | [91] |
| CuAl-LDO/TiO2NTs | In Situ Assembly | 500 | 5 | Nanosheet | —— | 80.5 | 0.67 | 28.7 | [62] |
| Catalysts | Reaction Conditions | Stability | Refs. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO (ppm) | NH3 (ppm) | O2 (%) | GHSV (h−1) | T (°C) | NOx Conversion | N2 Selectivity | H2O (%) | SO2 (ppm) | T (°C) | Activity (%) | ||
| MnCo-LDO | 500 | 500 | 5 | 36,000 | 60~270 | >90% | ~100% | 5 | 100 | 120 | 91 | [59] |
| Ni5Mn-LDO | 600 | 600 | 5 | 45,000 | 180~360 | >90% | >90% | 10 | 100 | 240 | 80 | [64] |
| MnAl-LDO (FNP) | 500 | 500 | 5 | 60,000 | 150~250 | 95% | >90% | —— | —— | —— | —— | [57] |
| Cu4AlOx | 500 | 500 | 5 | 80,000 | 200 | 91.1% | —— | 5 | 50 | 200 | 67.6 | [65] |
| Cu1Ti1O | 500 | 500 | 5 | 80,000 | 200 | 88.9% | —— | —— | 50 | 200 | 76.4 | [66] |
| NiMnTi-LDO | 600 | 600 | 5 | 45,000 | 150~360 | >90% | >95% | —— | —— | —— | —— | [58] |
| Ni2Mn2Ti1-LDO | 600 | 600 | 5 | 45,000 | 150~360 | >90% | >95% | 10 | 100 | 210 | 94 | [54] |
| Ni0.5Mn0.5Fe0.5Ox | 500 | 500 | 5 | 60,000 | 250 | >94% | 91.6% | 5 | 100 | 200 | 70.4 | [67] |
| NiMnTi-LDO | 600 | 600 | 5 | 45,000 | 150~360 | >90% | >90% | 10 | 100 | 210 | 94 | [68] |
| Co0.5Mn1Fe0.25Al0.75Ox-LDO | 500 | 500 | 5 | 60,000 | 80~250 | >92% | >75% | 5 | 100 | 150 | >60 | [69] |
| Ce0.5/Co1Mn0.5Al0.5Ox-LDO | 500 | 500 | 5 | 80,000 | 100~250 | >95% | >80% | 5 | 100 | 150 | 92.4 | [70] |
| Cu0.5Mg2.5Fe1-LDO | 500 | 500 | 5 | 36,000 | 180~240 | >80% | >85% | 8 | 100 | 240 | 80 | [71] |
| Cu1Mn0.5Ti0.5Ox | 500 | 500 | 5 | 80,000 | 200 | 90% | 99.4% | 5 | 100 | 200 | 64.6 | [72] |
| Cu-Mg-Al | 2500 | 2500 | 2.5 | 7000 | 200~250 | 80~95% | 93~97% | —— | —— | —— | —— | [73] |
| Cu0.5Mg1.5Mn0.5Al0.5Ox | 500 | 500 | 5 | 60,000 | 100~250 | 87.2~96.6% | >90% | 5 | 100 | 150 | 68.2 | [74] |
| CuAl-LDO/CNTs(I) | 600 | 600 | 5 | 45,000 | 180~300 | >80% | >90% | 10 | 100 | 240 | 83.3 | [63] |
| CoMnAl-LDO | 600 | 600 | 5 | 45,000 | 150~300 | >80% | >88% | 10 | 100 | 240 | 75 | [75] |
| CuMgAl-SDSO-LDO | 600 | 600 | 5 | 45,000 | 210 | 90% | 75% | 10 | 100 | 210 | 75/56 | [42] |
| Ce2/Cu4Al1Ox-LDO | 500 | 500 | 5 | 80,000 | 200 | 95.3% | 99% | 5 | 100 | 200 | 78.8 | [76] |
| NiTi-LDO | 600 | 600 | 5 | 45,000 | 240~360 | >90% | >95% | 10 | 100 | 240 | 90 | [55] |
| Mn/MgAl-LDO (FP-CP) | 500 | 500 | 5 | 60,000 | 25~150 | 76~100% | >90% | 5 | 100 | 100 | 65 | [56] |
| MnFeCo-LDO | 500 | 500 | 5 | 30,600 | 50~400 | >86% | >50% | 5 | 100 | 120 | 92 | [77] |
| CuAl LDH/CNTs-2 | 600 | 600 | 5 | 45,000 | 180~305 | >90% | —— | —— | —— | —— | —— | [78] |
| MnCoGd0.2 | 500 | 500 | 5 | 50,000 | 90~210 | 100% | ~100% | 5 | 100 | 240 | 92 | [79] |
| Ce0.2-Mn2Cr1Ox-LDO | 500 | 500 | 5 | 90,000 | 200 | 100% | >90% | 5 | 100 | 240 | 43.3 | [80] |
| Mn1Fe0.25Al0.75Ox | 500 | 500 | 5 | 60,000 | 150 | 97.6% | —— | —— | 100 | 150 | 76.6 | [81] |
| CuVOLDHc | 2000 | 2000 | 3 | 414,000 | 200~500 | 70~80% | >98% | —— | —— | —— | —— | [82] |
| (CuxZny)2Al-MMO | 600 | 480 | 5 | 30,000 | 240 | >80% | —— | —— | —— | —— | —— | [83] |
| Co2Cr1Ox-LDO | 500 | 500 | 5 | 90,000 | 150 | 100% | >90% | 5 | 100 | 200 | 43.3 | [84] |
| Ni4-xMnxAlOy | 500 | 500 | 6.5 | 45,000 | 120~210 | >90% | >50% | 5 | 1000 | 210 | <20 | [85] |
| @CuMgAl_0.5-MO | 2500 | 2500 | 2.5 | 24,000 | 275~300 | 89% | >99.7% | —— | —— | —— | —— | [60] |
| Ni4Ti1-400 | 600 | 600 | 5 | 45,000 | 240~360 | >90% | ~95% | 10 | 100 | 240 | 85 | [86] |
| Mg3Fe1-LDO/10CNTs | 500 | 500 | 5 | 36,000 | 240 | 90% | >80% | —— | 500 | 240 | ~80 | [87] |
| Cu5Mg62Al33-Ce3.0% | 2500 | 2500 | 2.5 | 12,000 | 275 | 96% | >90% | —— | —— | —— | —— | [88] |
| Cu2Mn0.5Al0.5Ox | 500 | 500 | 5 | 80,000 | 150 | 91.2% | —— | 5 | 100 | 150 | 62.18 | [89] |
| NiMnFe-IWM-C | 500 | 500 | 3 | 20,000 | 250~370 | >80% | ~100% | —— | 200 | 350 | 92 | [61] |
| Mn(0.25)/CoAl-LDO | 500 | 500 | 5 | 40,000 | 150~300 | >95% | >70% | 10 | 100 | 200 | 76.5 | [90] |
| MnO2/CoAl-LDO | 600 | 600 | 5 | 45,000 | 90~270 | >90% | >95% | 10 | 100 | 240 | 80 | [91] |
| CuAl-LDO/TiO2NTs | 600 | 600 | 5 | 45,000 | 210~330 | >80% | >90% | —— | 100 | 240 | 80 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Wang, X.; Zhang, J.; Wang, L.; Song, X.; Huo, P.; Liu, X. A Short Review of Layered Double Oxide-Based Catalysts for NH3-SCR: Synthesis and NOx Removal. Catalysts 2024, 14, 755. https://doi.org/10.3390/catal14110755
Sun T, Wang X, Zhang J, Wang L, Song X, Huo P, Liu X. A Short Review of Layered Double Oxide-Based Catalysts for NH3-SCR: Synthesis and NOx Removal. Catalysts. 2024; 14(11):755. https://doi.org/10.3390/catal14110755
Chicago/Turabian StyleSun, Tao, Xin Wang, Jinshan Zhang, Lan Wang, Xianghai Song, Pengwei Huo, and Xin Liu. 2024. "A Short Review of Layered Double Oxide-Based Catalysts for NH3-SCR: Synthesis and NOx Removal" Catalysts 14, no. 11: 755. https://doi.org/10.3390/catal14110755
APA StyleSun, T., Wang, X., Zhang, J., Wang, L., Song, X., Huo, P., & Liu, X. (2024). A Short Review of Layered Double Oxide-Based Catalysts for NH3-SCR: Synthesis and NOx Removal. Catalysts, 14(11), 755. https://doi.org/10.3390/catal14110755