Carbon Materials with Different Dimensions Supported Pt Catalysts for Selective Hydrogenation of 3,4-Dichloronitrobenzene to 3,4-Dichloroaniline
Abstract
1. Introduction
2. Results and Discussion
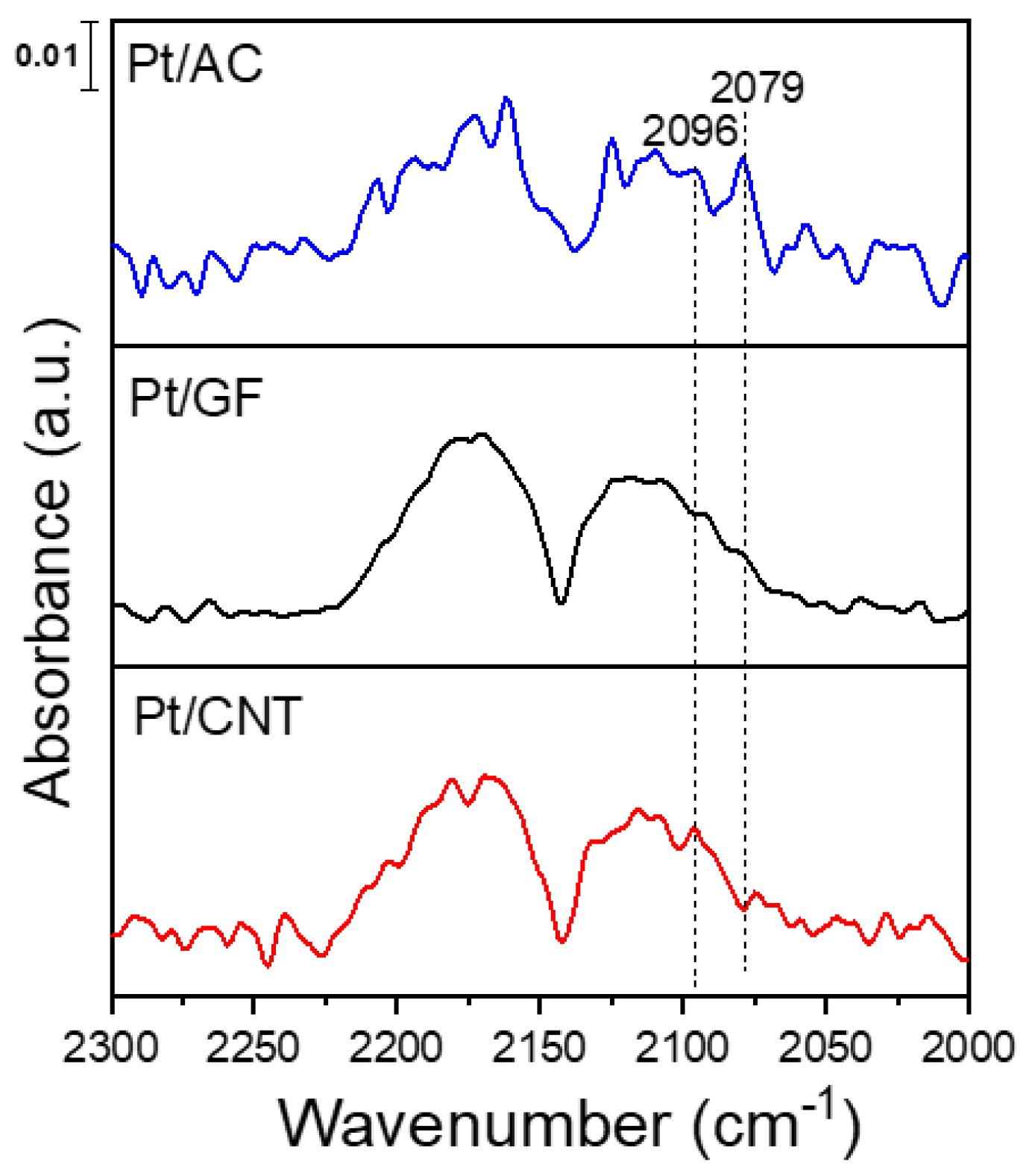
2.1. Characterization of the Catalysts
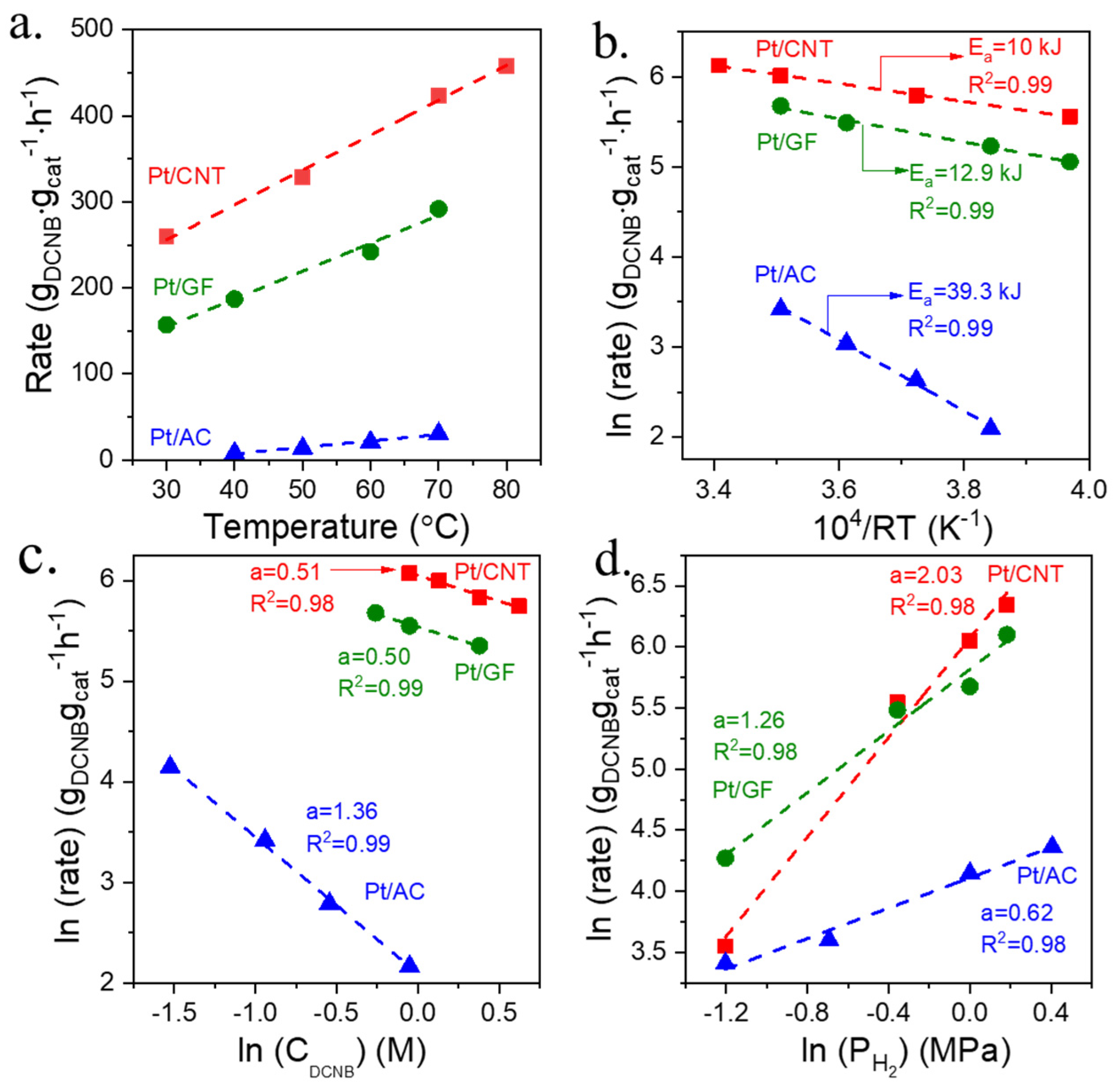
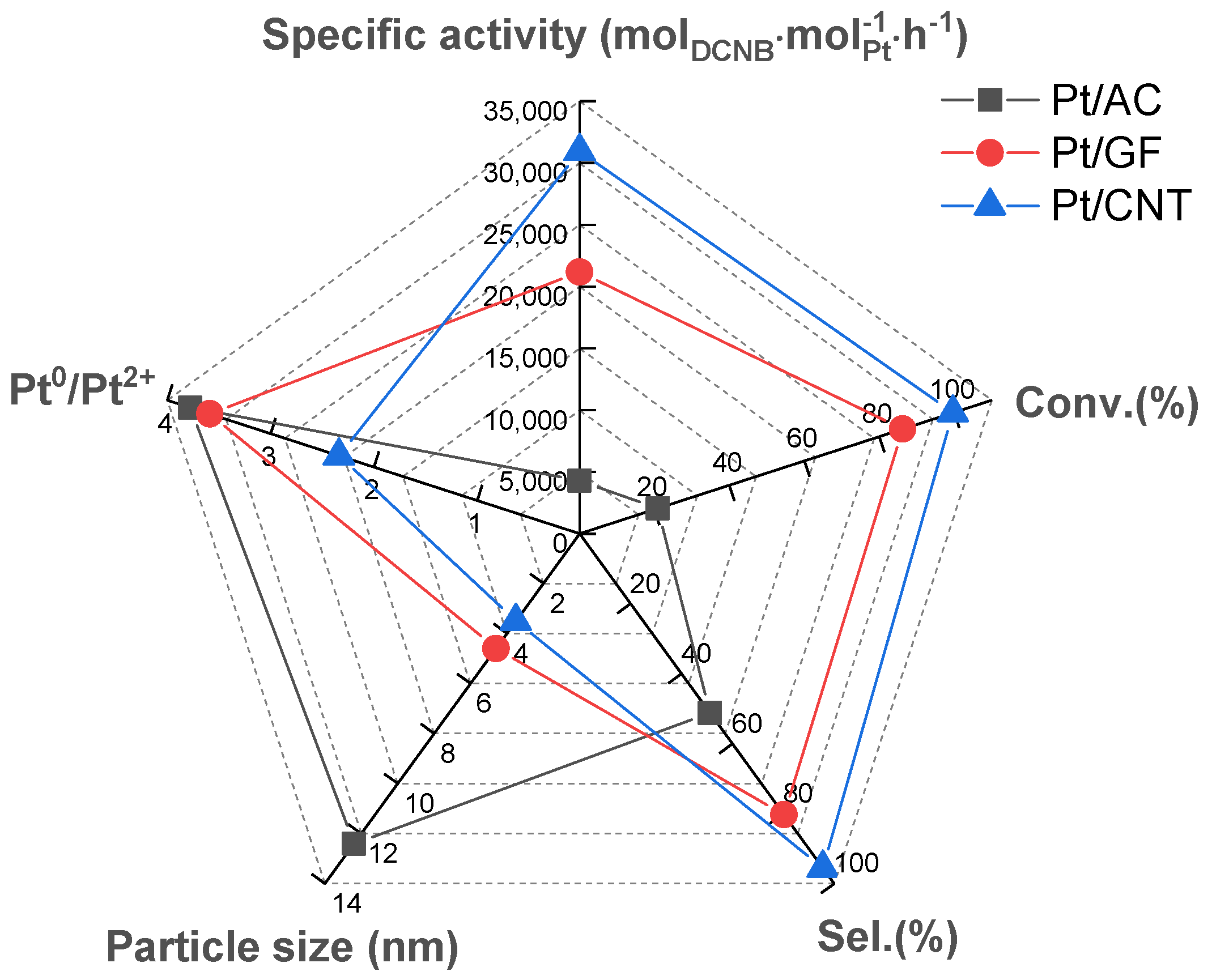
2.2. Catalytic Performances of Different Carbon Materials Supported Pt Catalysts
2.3. Dynamic Tests of Different Pt Catalysts for Selective Hydrogenation of 3,4-DCNB
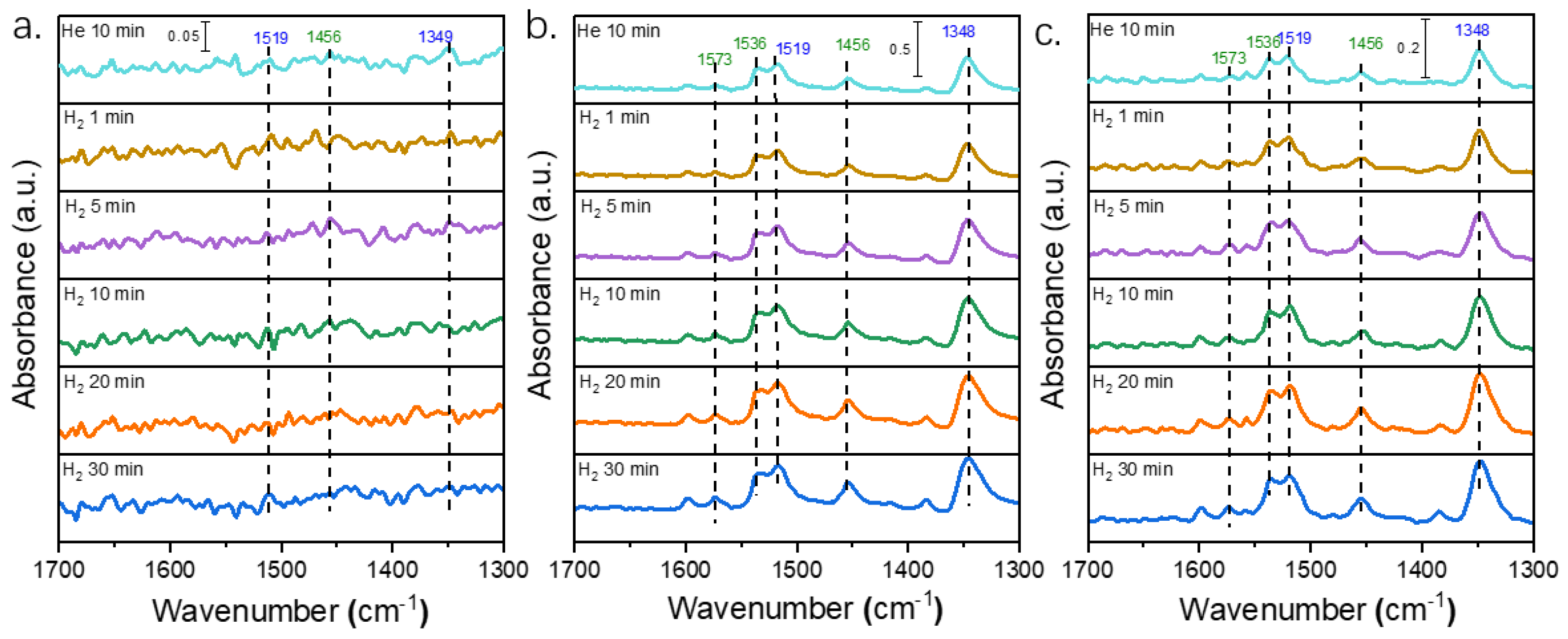
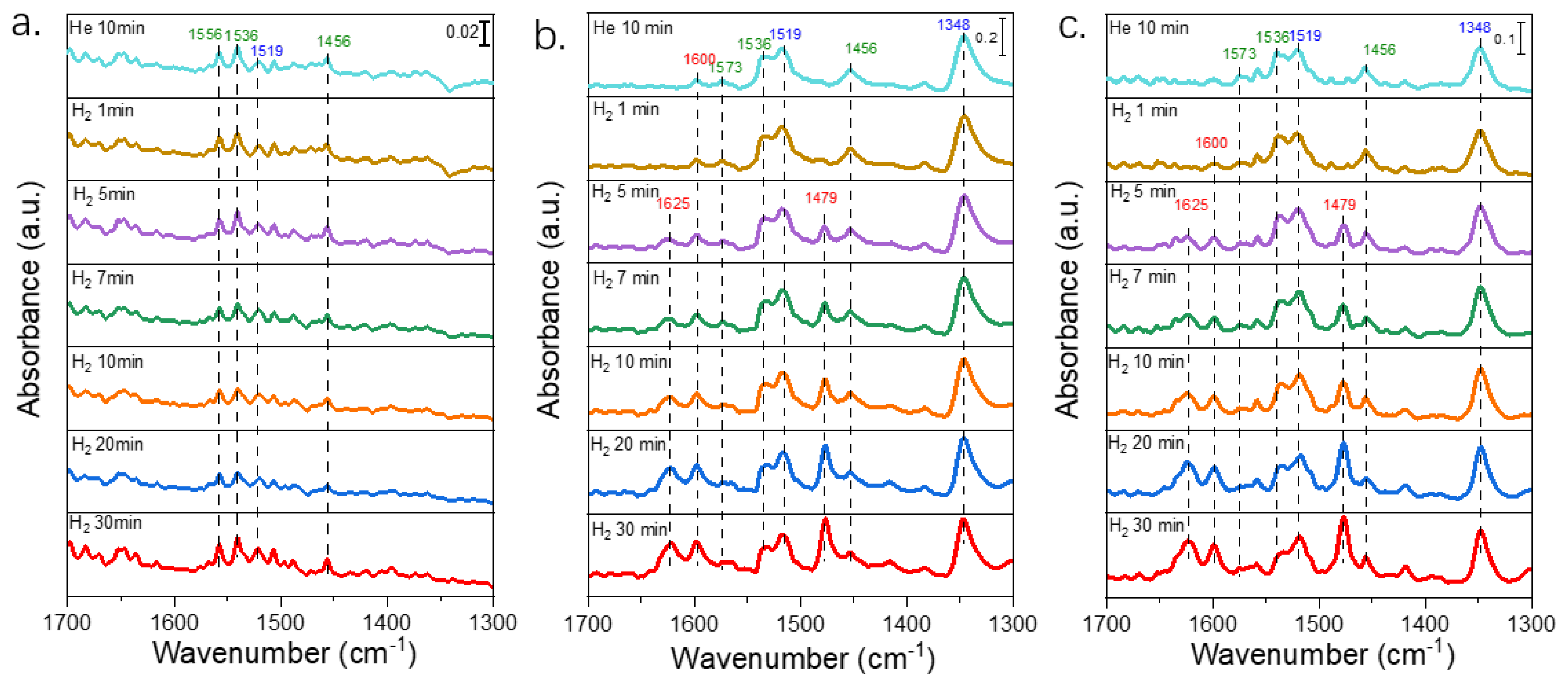
2.4. In Situ DRIFT Spectra of Different Pt Catalysts for Selective Hydrogenation of 3,4-DCNB
2.5. Influence on Catalytic Performances
3. Materials and Methods
3.1. Material
3.2. Preparation of Different Carbon Materials Supported Pt Catalysts
3.3. Catalytic Test
3.4. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, G. Nitro compounds, Aromatic. Ullmann’s Encycl. Ind. Chem. 2000, 4, 301–349. [Google Scholar]
- Das, A.; Mondal, S.; Hansda, K.M.; Adak, M.K.; Dhak, D. A critical review on the role of carbon supports of metal catalysts for selective catalytic hydrogenation of chloronitrobenzenes. Appl. Catal. A 2023, 649, 118955. [Google Scholar] [CrossRef]
- Blaser, H.U.; Steiner, H.; Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem 2009, 1, 210–221. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Macino, M.; Barnes, A.J.; Althahban, S.M.; Qu, R.; Gibson, E.K.; Morgan, D.J.; Freakley, S.J.; Dimitratos, N.; Kiely, C.J.; Gao, X.; et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat. Catal. 2019, 2, 873–881. [Google Scholar] [CrossRef]
- Gao, R.; Pan, L.; Li, Z.; Zhang, X.; Wang, L.; Zou, J.-J. Cobalt nanoparticles encapsulated in nitrogen-doped carbon for room-temperature selective hydrogenation of nitroarenes. Chin. J. Catal. 2018, 39, 664–672. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.Q.; Ye, S.; Liu, Y.M.; He, H.Y.; Cao, Y. Gold-catalyzed direct hydrogenative coupling of nitroarenes to synthesize aromatic azo compounds. Angew. Chem. Int. Ed. 2014, 126, 7754–7758. [Google Scholar] [CrossRef]
- Wu, Q.; Su, W.; Huang, R.; Shen, H.; Qiao, M.; Qin, R.; Zheng, N. Full selectivity control over the catalytic hydrogenation of nitroaromatics into six products. Angew. Chem. Int. Ed. 2024, 136, e202408731. [Google Scholar] [CrossRef]
- Dorokhov, V.G.; Dorokhova, G.F.; Savchenko, V.I. Liquid-phase catalytic hydrogenation of 3,4-dichloronitrobenzene over Pt/C catalyst under gradient free flow conditions in the presence of pyridine. Russ. Chem. Bull. Int. Ed. 2016, 65, 2040–2045. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Lu, C.; Zhang, Q.; Li, X. Highly selective hydrogenation of 3,4-dichloronitrobenzene over Pd/C catalysts without inhibitors. Catal. Today 2011, 173, 62–67. [Google Scholar] [CrossRef]
- Lu, C.S.; Lu, J.H.; Ma, L.; Zhang, Q.F.; Li, X.N. Effect of solvent polarity properties on the selectivity and activity for 3,4-dichloronitrobenzene hydrogenation over Pd/C catalyst. Adv. Mat. Res. 2011, 396–398, 2379–2383. [Google Scholar] [CrossRef]
- Yan, M.; Wu, T.; Chen, L.; Yu, Y.; Liu, B.; Wang, Y.; Chen, W.; Liu, Y.; Lian, C.; Li, Y. Effect of protective agents upon the catalytic property of platinum nanocrystals. ChemCatChem 2018, 10, 2433–2441. [Google Scholar] [CrossRef]
- Guo, M.; Guan, X.; Meng, Q.; Gao, M.L.; Li, Q.; Jiang, H.L. Tailoring catalysis of encapsulated platinum nanoparticles by pore wall engineering of covalent organic frameworks. Angew. Chem. Int. Ed. 2024, 136, e202410097. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J. Synergistic catalysis by a hybrid nanostructure Pt catalyst for high-efficiency selective hydrogenation of nitroarenes. J. Catal. 2021, 395, 445–456. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Mao, S.; Lu, B.; Chen, Y.; Zhang, X.; Chen, Z.; Wang, Y. Decoupling the electronic and geometric effects of Pt catalysts in selective hydrogenation reaction. Nat. Commun. 2022, 13, 3561. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-Q.; Hu, Z.-Y.; Li, W.-W.; Luo, S.-P.; Xu, Z.-Y. Hydrogenation in ionic liquids: An alternative methodology toward highly selective catalysis of halonitrobenzenes to corresponding haloanilines. J. Mol. Catal. A Chem. 2005, 235, 137–142. [Google Scholar] [CrossRef]
- Lv, Y.P.; Yu, F.; Wang, Z.P.; Liu, H.W.; Wang, L.Y.; Song, J.; Li, Y.; Huang, G.Q.; Cui, J. Regulating pore structures of carbon supports toward efficient selective hydrogenation of o-chloronitrobenzene on Pt nanoparticles. New J. Chem. 2023, 47, 11577–11583. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Y.; Lu, C.; Maity, B.; Chen, Y.; Ueno, T.; Liu, Z.; Lu, D. Apo-ferritin-caged Pt nanoparticles for selective hydrogenation of p-chloronitrobenzene. ACS Appl. Nano Mater. 2023, 6, 5835–5843. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Jin, B.; Liang, X.; Wang, Q.; Zhao, Z.; Li, Q. Pt–carbon interaction-determined reaction pathway and selectivity for hydrogenation of 5-hydroxymethylfurfural over carbon supported Pt catalysts. Catal. Sci. Technol. 2021, 11, 1298–1310. [Google Scholar] [CrossRef]
- Yu, L.; Li, D.; Xu, Z.; Zheng, S. Polyaniline coated Pt/CNT as highly stable and active catalyst for catalytic hydrogenation reduction of Cr(VI). Chemosphere 2023, 310, 136685. [Google Scholar] [CrossRef]
- Xia, L.; Li, D.; Long, J.; Huang, F.; Yang, L.; Guo, Y.; Jia, Z.; Xiao, J.; Liu, H. N-doped graphene confined Pt nanoparticles for efficient semi-hydrogenation of phenylacetylene. Carbon 2019, 145, 47–52. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, B.; Lin, Y.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced chemoselective hydrogenation through tuning the interaction between Pt nanoparticles and carbon supports: Insights from identical location transmission electron microscopy and X-ray photoelectron spectroscopy. ACS Catal. 2016, 6, 7844–7854. [Google Scholar] [CrossRef]
- Du, Y.; Meng, X.; Ma, Y.; Qi, J.; Xu, G.; Zou, H.; Qiu, J. Dimensionality engineering toward carbon materials for electrochemical CO2 reduction: Progress and prospect. Adv. Funct. Mater. 2024, 2408013. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Z. Porous carbon materials with different dimensions and their applications in supercapacitors. J. Phys. D Appl. Phys. 2024, 57, 433001. [Google Scholar] [CrossRef]
- Swamy, S.S. Stability of single-wall carbon nanotubes under hydrothermal conditions. J. Mater. Res. 2002, 17, 734–737. [Google Scholar] [CrossRef]
- Luo, J.; Yao, C.; Ma, D.; Chen, Y.; Tian, M.; Xie, H.; Chen, R.; Wu, J.; Zhen, Y.; Pan, L.; et al. Mechanism-guided design of sulfur-modified platinum catalysts for selective hydrogenation of nitrobenzene. Appl. Catal. A 2023, 660, 119198. [Google Scholar] [CrossRef]
- Hasa, B.; Martino, E.; Vakros, J.; Trakakis, G.; Galiotis, C.; Katsaounis, A. Effect of carbon support on the electrocatalytic properties of Pt−Ru catalysts. ChemElectroChem 2019, 6, 4970–4979. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Loh, K.P. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Tuo, Y.; Jiang, H.; Duan, X.; Li, P. Microwave-assisted hydrogen releasing from liquid organic hydride over Pt/CNT catalyst: Effects of oxidation treatment of CNTs. Catal. Today 2016, 276, 121–127. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Wu, X.; Shi, Y.; Nie, N.; Zhao, H.; Miao, H.; Chen, X.; Li, S.; Lai, J.; et al. Noble metal (Pt, Rh, Pd, Ir) doped Ru/CNT ultra-small alloy for acidic hydrogen evolution at high current density. Small 2021, 18, 2104559. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Gan, T.; Qi, H.; Zheng, B.; Chu, X.; Yu, G.; Yan, W.; Zou, Y.; Zhang, W.; Liu, G. Synergism of Pt nanoparticles and iron oxide support for chemoselective hydrogenation of nitroarenes under mild conditions. Chin. J. Catal. 2019, 40, 214–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Wang, F.; Zhao, X. Hybrid nanostructure catalyst with low loading of Pt for the high-efficiency catalytic hydrogenation of chloronitrobenzene. Langmuir 2022, 38, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Goetz, M.; Fuess, H. Synthesis and characterization of carbon-supported Pt-Ru-WOx catalysts by spectroscopic and diffraction methods. J. Appl. Electrochem. 2001, 31, 793–798. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, W.; Fan, G. Pt nanoparticles on Ti3C2Tx-based MXenes as efficient catalysts for the selective hydrogenation of nitroaromatic compounds to amines. Dalton Trans. 2020, 49, 14914–14920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; et al. Tuning defects in oxides at room temperature by lithium reduction. Nat. Commun. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.Y.; Zhang, L.; Wang, A.; Li, L.; Pan, X.; Miao, S.; Haruta, M.; Wei, H.; Wang, H.; et al. ZnAl-hydrotalcite-supported Au25 nanoclusters as precatalysts for chemoselective hydrogenation of 3-nitrostyrene. Angew. Chem. Int. Ed. 2017, 56, 2709–2713. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Bai, J.Q.; Miao, Z.; Tan, Y.; Zhan, N.; Liu, H.; Ma, M.; Cai, M.; Cheng, Q.; et al. Efficient Co/NSPC catalyst for selective hydrogenation of halonitrobenzenes and mechanistic insight. Catal. Sci. Technol. 2024, 14, 1167–1180. [Google Scholar] [CrossRef]
- Li, X.; Tan, Y.; Liu, Z.; Su, J.; Xiao, Y.; Qiao, B.; Ding, Y. NiOx-promoted Cu-based catalysts supported on AlSBA-15 for chemoselective hydrogenation of nitroarenes. J. Catal. 2022, 416, 332–343. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Słomkiewicz, P.M.; Szczepanik, B.; Garnuszek, M. Determination of adsorption isotherms of aniline and 4-chloroaniline on halloysite adsorbent by inverse liquid chromatography. Appl. Clay Sci. 2015, 114, 221–228. [Google Scholar] [CrossRef]
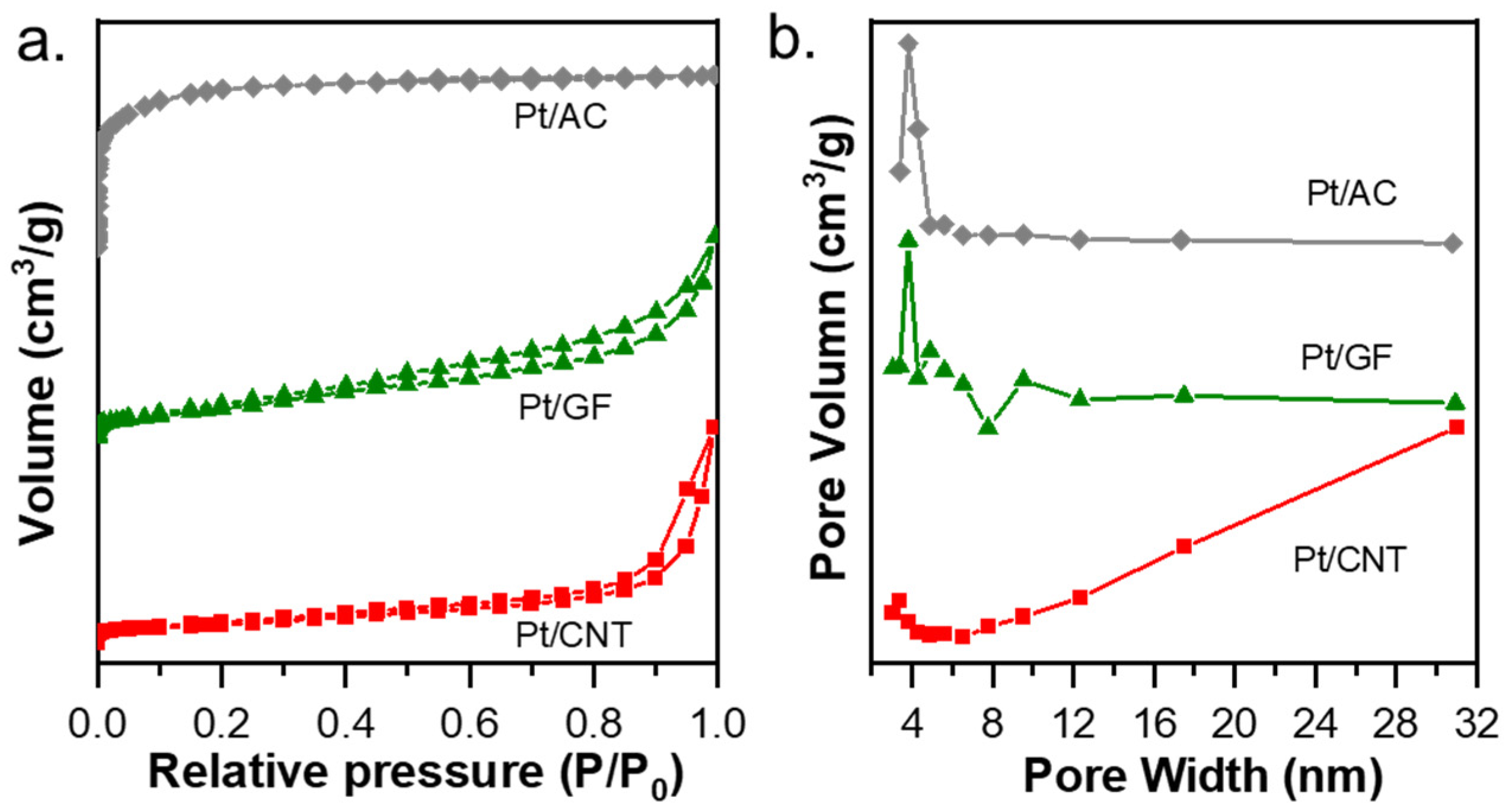
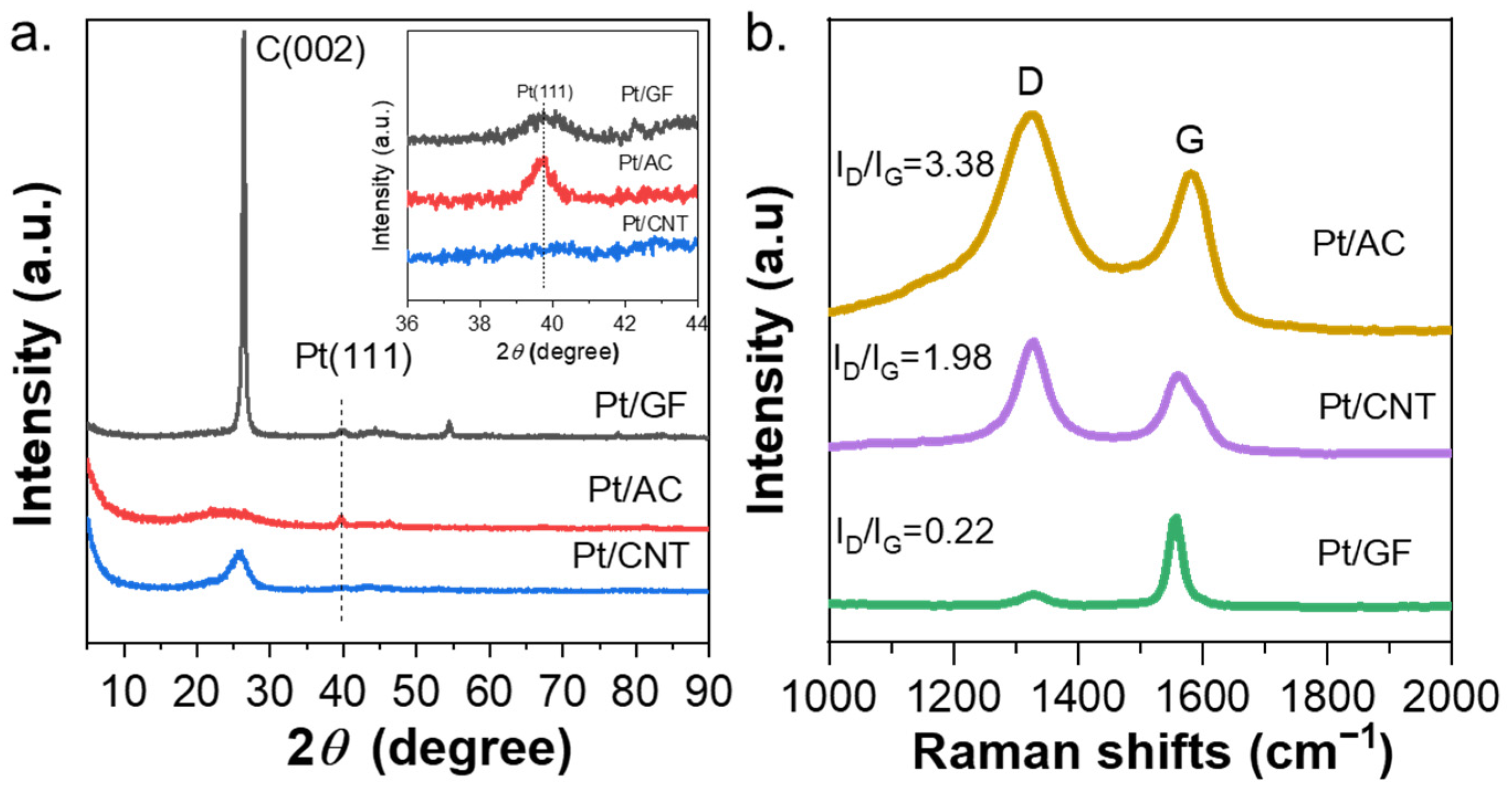
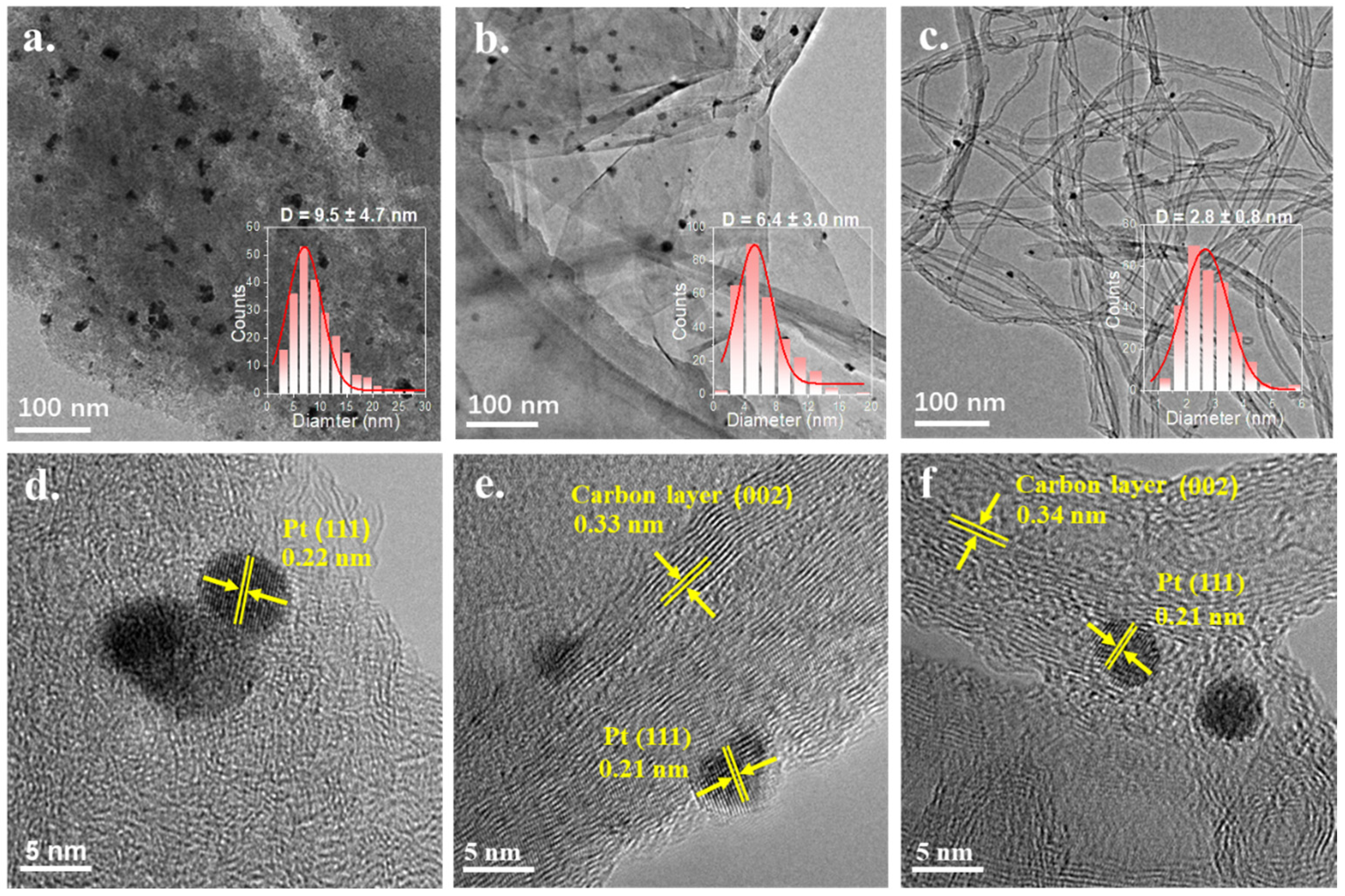

| Entry | Catalysts | Loading of Pt (%) a | SBET (m2·g−1) b | Vtotal (cm3·g−1) b | Dpore (nm) b | Particle size (nm) c | Particle Size (nm) d |
|---|---|---|---|---|---|---|---|
| 1 | Pt/AC | 0.7 | 1339.0 (Smicro: 1202) | 0.7 (Vmicro: 0.6) | 2.1 | 9.5 ± 4.7 | 12.4 |
| 2 | Pt/GF | 1.3 | 56.0 | 0.1 | 10.4 | 6.4 ± 3.0 | 4.6 |
| 3 | Pt/CNT | 1.3 | 315.3 | 1.6 | 20.0 | 2.8 ± 0.8 | 3.5 |
| Entry | Catalysts | C (002) | Pt (111) | ||||
|---|---|---|---|---|---|---|---|
| Position (°) | FWHM | Crystal Size (nm) | Position (°) | FWHM | Crystal Size (nm) | ||
| 1 | Pt/AC | 24.05 | 8.53 | 1.1 | 39.68 | 0.76 | 12.4 |
| 2 | Pt/GF | 26.39 | 0.51 | 19.0 | 39.86 | 1.90 | 4.6 |
| 3 | Pt/CNT | 25.71 | 2.71 | 3.1 | 39.63 | 2.48 | 3.5 |
| Catalyst | Pt 4f | O 1s | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt2+ (B.E.) | Pt2+ (%) | Pt0 (B.E.) | Pt0 (%) | Oγ (B.E.) | Oγ (%) | Oβ (B.E.) | Oβ (%) | Oα (B.E.) | Oα (%) | |
| Pt/AC | 72.2 | 20.9 | 71.2 | 79.1 | 532.9 | 54.6 | 531.8 | 30.7 | 530.5 | 14.7 |
| Pt/GF | 72.2 | 21.8 | 71.3 | 78.2 | 532.9 | 36.3 | 531.8 | 50.0 | 530.6 | 13.7 |
| Pt/CNT | 72.3 | 29.9 | 71.4 | 70.1 | 533.0 | 56.3 | 531.9 | 28.8 | 530.6 | 14.9 |
| Entry | Catalysts | T (°C) | t (min) | Conv. (%) | Sel. (%) | Reaction Rates (mol∙mol−1∙h−1) c | |||
|---|---|---|---|---|---|---|---|---|---|
| DCAN | NSB | AB | AOB | ||||||
| 1 | Pt/AC | 50 | 10 | 3.4 | 62.2 | 23.6 | 0 | 14.2 | |
| 2 | Pt/AC | 50 | 60 | 20.9 | 51.2 | 17.9 | 1.6 | 29.3 | |
| 3 | Pt/AC | 60 | 20 | 8.8 | 63.8 | 18.7 | 0.9 | 16.6 | |
| 4 | Pt/AC | 70 | 20 | 12.9 | 55.0 | 23.3 | 1.0 | 20.7 | 4312 |
| 5 | Pt/GF | 50 | 10 | 48.8 | 68.4 | 8.9 | 0.4 | 22.3 | |
| 6 | Pt/GF | 50 | 60 | 86.2 | 80.5 | 5.2 | 0.4 | 13.9 | |
| 7 | Pt/GF | 60 | 20 | 71.6 | 80.5 | 5.2 | 0.4 | 13.9 | |
| 8 | Pt/GF a | 70 | 5 | 15.4 | 71.4 | 10.0 | 0.2 | 18.4 | 21,176 |
| 9 | Pt/GF | 70 | 20 | 75.9 | 82.5 | 5.7 | 0.3 | 11.5 | |
| 10 | Pt/CNT b | 30 | 60 | 95.9 | 96.9 | 1.3 | 1.2 | 0.6 | |
| 11 | Pt/CNT | 50 | 10 | 59.1 | 77.8 | 6.8 | 2.9 | 12.5 | |
| 12 | Pt/CNT | 50 | 60 | 99.7 | 95.5 | 0.5 | 4.0 | 0 | |
| 13 | Pt/CNT | 60 | 20 | 90.4 | 90 | 3 | 3.7 | 3.3 | |
| 14 | Pt/CNT a | 70 | 5 | 21.5 | 72.9 | 9.5 | 0.5 | 17.9 | 30,960 |
| 15 | Pt/CNT | 70 | 20 | 92.9 | 91.6 | 3.0 | 2.3 | 3.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, N.; Xiao, Y.; Chen, X.; Tan, Y.; Ding, Y. Carbon Materials with Different Dimensions Supported Pt Catalysts for Selective Hydrogenation of 3,4-Dichloronitrobenzene to 3,4-Dichloroaniline. Catalysts 2024, 14, 724. https://doi.org/10.3390/catal14100724
Zhan N, Xiao Y, Chen X, Tan Y, Ding Y. Carbon Materials with Different Dimensions Supported Pt Catalysts for Selective Hydrogenation of 3,4-Dichloronitrobenzene to 3,4-Dichloroaniline. Catalysts. 2024; 14(10):724. https://doi.org/10.3390/catal14100724
Chicago/Turabian StyleZhan, Nannan, Yan Xiao, Xingkun Chen, Yuan Tan, and Yunjie Ding. 2024. "Carbon Materials with Different Dimensions Supported Pt Catalysts for Selective Hydrogenation of 3,4-Dichloronitrobenzene to 3,4-Dichloroaniline" Catalysts 14, no. 10: 724. https://doi.org/10.3390/catal14100724
APA StyleZhan, N., Xiao, Y., Chen, X., Tan, Y., & Ding, Y. (2024). Carbon Materials with Different Dimensions Supported Pt Catalysts for Selective Hydrogenation of 3,4-Dichloronitrobenzene to 3,4-Dichloroaniline. Catalysts, 14(10), 724. https://doi.org/10.3390/catal14100724









