Microwave-Assisted Synthesis of Symmetrical 1,4-Disubstituted Bis-1H-1,2,3-triazoles Using Copper N-Heterocyclic Carbene Catalysts
Abstract
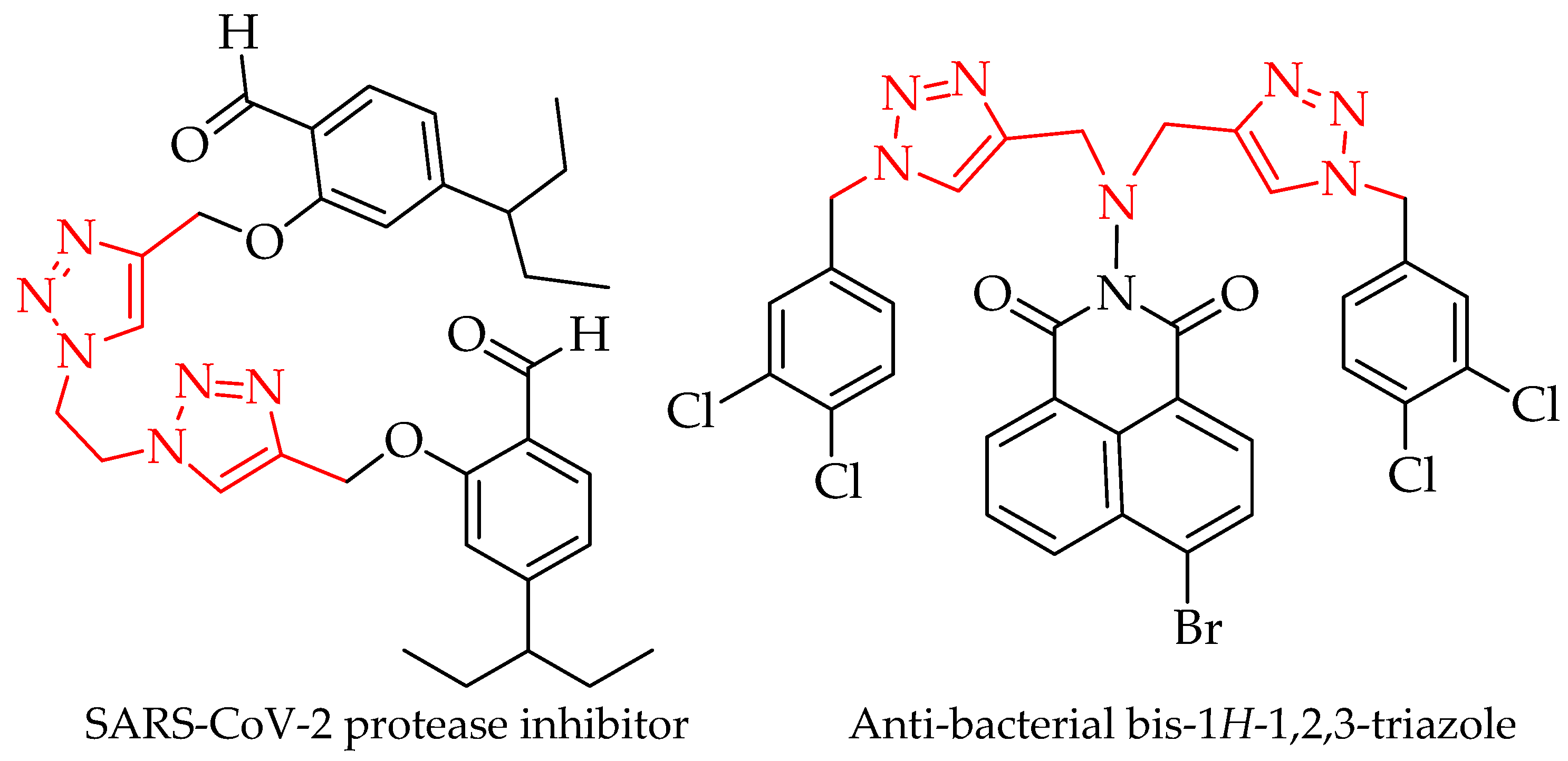
1. Introduction
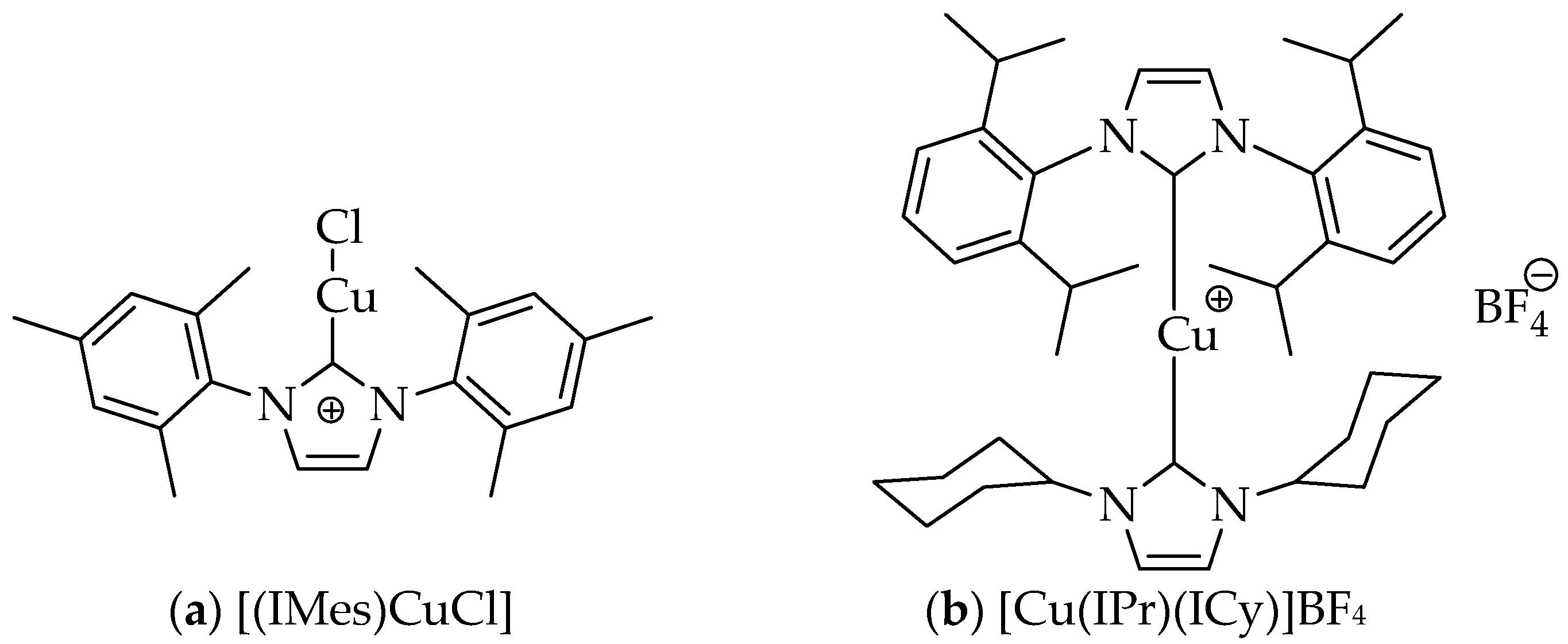
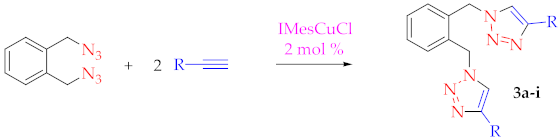
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Analysis Techniques
3.2. Synthetic Procedures and Data
3.2.1. Synthesis of α,α-Diazido-o-xylene
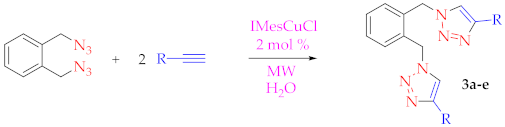
3.2.2. Procedure for the [(IMes)CuCl]-Catalyzed Cycloaddition Reaction in Water or 1:1 tert-Butyl Alcohol/Water (with or without MW Heating)
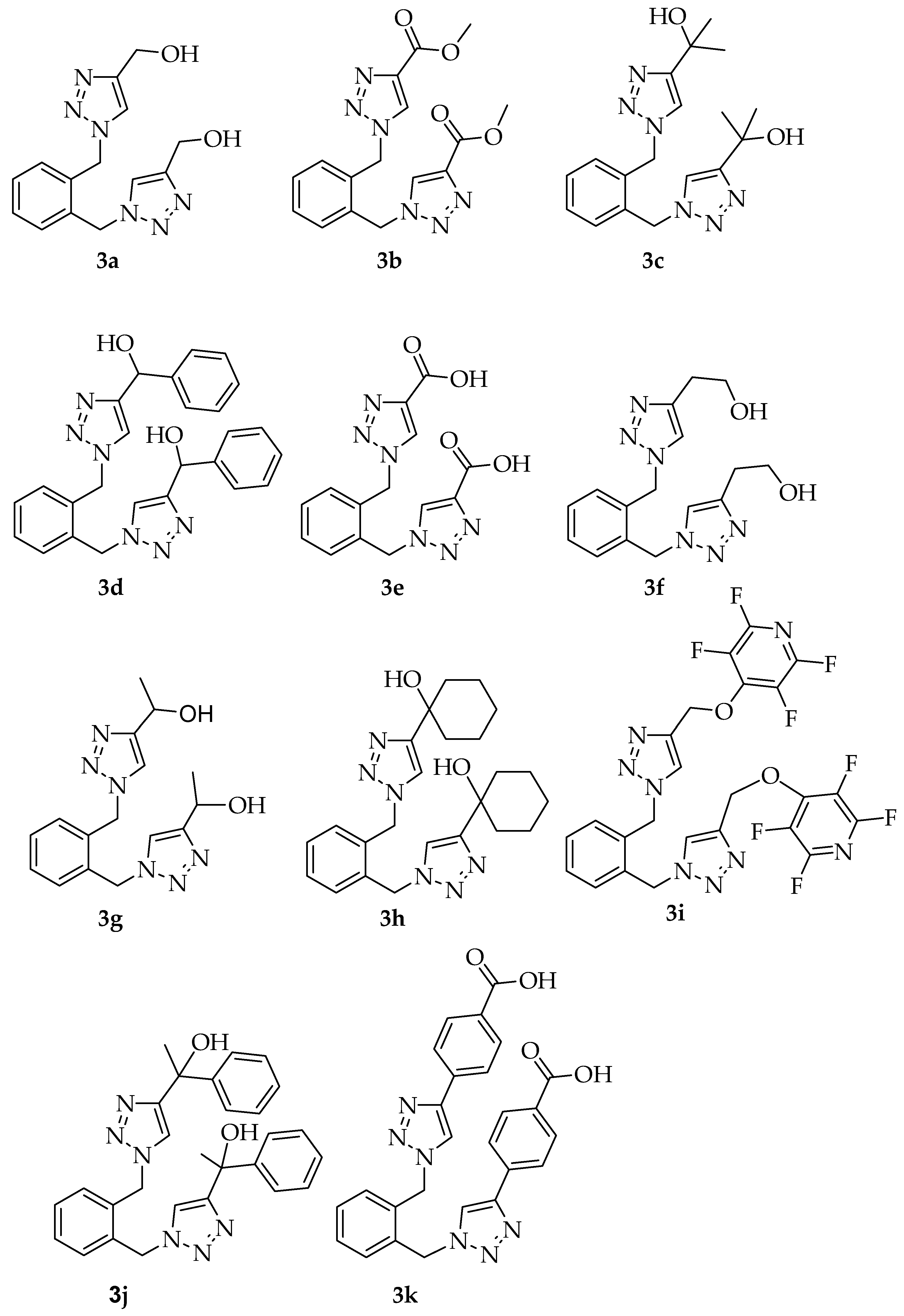
3.2.3. Spectral and Analytical Data for 3a–3k
3.2.4. Procedure for the [(IMes)CuCl]-Catalyzed Cycloaddition Reaction without Solvent (Note: This Procedure Should Not Be Scaled Up Due to the Buildup of Heat during the Reaction)
3.2.5. Spectral and Analytical Data for 4a–4c
3.2.6. Procedure for the Preparation of Compound 5, 1,1′-(o-phenylenedimethylene)bis [4,5-methyl-1-ol-1,2,3-triazole]
3.2.7. Procedure for the Ultrasonic Probe Assisted Synthesis of 1,1′-[(1,2-(Phenylene)bis(methylene)]bis-1H-1,2,3-triazole-4,4′-carboxylic Acid 3e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Z.-J.; Wang, D.; Xu, Z.; Xu, L.-W. Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry. Beilstein J. Org. Chem. 2015, 11, 2557–2576. [Google Scholar] [CrossRef]
- Dawood, K.M.; Abdel-Wahab, B.F.; Raslan, M.A. Synthesis and applications of bi- and bis-triazole systems. ARKIVOC Online J. Org. Chem. 2018, 2018, 179–215. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Zhang, H.; Tanimoto, T.; Morimoto, Y.; Nishiyama, K. Acid-mediated synthesis of fully substituted 1,2,3-triazoles: Multicomponent coupling reactions, mechanistic study, synthesis of serine hydrolase inhibitor and its derivatives. Tetrahedron 2014, 70, 9828–9835. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and reaction mechanisms: Selected examples from the experience of forty years. Pure Appl. Chem. 1989, 61, 613–628. [Google Scholar] [CrossRef]
- Sutton, D.A.; Popik, V.V. Sequential photochemistry of dibenzo[a,e]dicyclopropa[c,g][8]annulene-1,6-dione: Selective formation of didehydrodibenzo[a,e][8]annulenes with ultrafast SPAAC reactivity. J. Org. Chem. 2016, 81, 8850–8857. [Google Scholar] [CrossRef]
- Shamim, A.; Souza, F.B.; Trossini, G.H.G.; Gatti, F.M.; Stefani, H.A. Synthesis of C-glycosyl-bis-1,2,3-triazole derivatives from 3,4,6-tri-O-acetyl-D-glucal. Mol. Divers. 2015, 19, 423–434. [Google Scholar] [CrossRef]
- Aher, N.G.; Pore, V.S.; Mishra, N.N.; Kumar, A.; Shukla, P.K.; Sharma, A.; Bhat, M.K. Synthesis and antifungal activity of 1, 2, 3-triazole containing fluconazole analogues. Bioorg. Med. Chem. Lett. 2009, 19, 759–763. [Google Scholar] [CrossRef]
- Singh, G.; Mohit, P.; Suman, D.; Sushma, P.; Saini, A.; Kaur, A. Design of new bistriazolyl structure for identification of inhibitory activity on COVID-19 main protease by molecular docking approach. J. Mol. Struct. 2022, 1250, 131858. [Google Scholar] [CrossRef]
- Lv, J.S.; Peng, X.M.; Kishore, B.; Zhou, C.H. 1,2,3-Triazole-derived naphthalimides as a novel type of potential antimicrobial agents: Synthesis, antimicrobial activity, interaction with calf thymus DNA and human serum albumin. Bioorg. Med. Chem. Lett. 2014, 24, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Chaidam, S.; Saehlim, N.; Athipornchai, A.; Sirion, U.; Saeeng, R. Synthesis and biological evaluation of 1,6-bis-triazole-2,3,4-tri-O-benzyl-α-D-glucopyranosides as a novel α-glucosidase inhibitor in the treatment of Type 2 diabetes. Bioorg. Med. Chem. Lett. 2021, 50, 128331. [Google Scholar] [CrossRef] [PubMed]
- Elamari, H.; Slimi, R.; Chabot, G.G.; Quentin, L.; Scherman, D.; Girard, C. Synthesis and in vitro evaluation of potential anticancer activity of mono- and bis-1,2,3-triazole derivatives of bis-alkynes. Eur. J. Med. Chem. 2013, 60, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Krim, J.; Taourirte, M.; Engels, J.W. Synthesis of 1, 4-disubstituted mono and bistriazolocarboacyclonucleoside analogues of 9-(4-hydroxybutyl) guanine by Cu (I)-catalyzed click azide-alkyne cycloaddition. Molecules 2011, 17, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Munoz, M.; Perez-Balderas, F.; Morales-Sanfrutos, J.; Hernandez-Mateo, F.; Isac-Garcia, J.; Santoyo-Gonzalez, F. Click multivalent heterogeneous neoglycoconjugates-modular synthesis and evaluation of their binding affinities. Eur. J. Org. Chem. 2009, 2009, 2454–2473. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Chen, Z.-A.; Shen, Q.-K.; Quan, Z.-S. Application of triazoles in the structural modification of natural products. J. Enzym. Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef]
- Németh-Rieder, A.; Keglevich, P.; Hunyadi, A.; Latif, A.D.; Zupkó, I.; Hazai, L. Synthesis and in vitro anticancer evaluation of flavone—1,2,3-triazole hybrids. Molecules 2023, 28, 626. [Google Scholar] [CrossRef]
- Saleh, M.M.; Abuarqoub, D.A.; Hammad, A.M.; Hossan, M.S.; Ahmed, N.; Aslam, N.; Naser, A.Y.; Moody, C.J.; Laughton, C.A.; Bradshaw, T.D. In vitro anticancer properties of novel bistriazoles. Curr. Issues Mol. Biol. 2023, 45, 175–196. [Google Scholar] [CrossRef]
- Vermal, N.K.; Mondal, D.; Bera1, S. Pharmacological and cellular significance of triazole-surrogated compounds. Curr. Org. Chem. 2019, 23, 2305–2572. [Google Scholar] [CrossRef]
- Mondal, D.; Kundu, S.; Elramadi, E.; Valiyev, I.; Schmittel, M. Self-healing of a copper(I) [2]rotaxane shuttle monitored by fluorescence. Org. Lett. 2023, 25, 933–937. [Google Scholar] [CrossRef]
- Malkova, K.; Bubyrev, A.; Krivovicheva, V.; Dar’in, D.; Bunev, A.; Krasavin, M. A novel bistriazole scaffold accessed via two tandem [3 + 2] cycloaddition events including an uncatalyzed room temperature azide-alkyne click reaction. Beilstein J. Org. Chem. 2022, 18, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, S.; Dam, B.; Bhushan, M.; Singh, L.R.; Pal, A.K. CuO-NiO bimetallic nanoparticles supported on graphitic carbon nitride with enhanced catalytic performance for the synthesis of 1,2,3-triazoles, bis-1,2,3-triazoles, and tetrazoles in parts per million level. Appl. Organomet. Chem. 2022, 36, e6524. [Google Scholar] [CrossRef]
- Hsueh, F.-C.; Tsai, C.-Y.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. N-Heterocyclic carbene copper rotaxanes mediate sequential click ligations with all reagents premixed. Angew. Chem. Int. Ed. 2020, 59, 11278–11282. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J., III; Dias, V.R.; Calabrese, J.C.; Davidson, F. Homoleptic carbene-silver(I) and carbene-copper(I) complexes. Organometallics 1993, 12, 3405–3409. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic carbene complexes of copper, nickel, and cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Boreux, A.; Marion, N.; Gagosz, F. NHC-Copper, -silver and -gold complexes in catalysis. RSC Catal. Ser. 2017, 27, 421–455. [Google Scholar]
- Díez-González, S.; Correa, A.; Cavallo, L.; Nolan, S.P. (NHC)copper(I)-catalyzed [3 + 2] cycloaddition of azides and mono- or disubstituted alkynes. Chem. Eur. J. 2006, 12, 7558–7564. [Google Scholar] [CrossRef]
- Nakamura, T.; Terashima, T.; Ogata, K.I.; Fukuzawa, S. Copper(I) 1,2,3-triazol-5-ylidene complexes as efficient catalysts for click reactions of azides with alkynes. Org. Lett. 2011, 13, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Lazreg, F.; Slawin, A.M.Z.; Cazin, C.S.J. Heteroleptic bis(N-heterocyclic carbene)copper(I) complexes: Highly efficient systems for the [3+2] cycloaddition of azides and alkynes. Organometallics 2012, 31, 7969–7975. [Google Scholar] [CrossRef]
- Berg, R.; Straub, J.; Schreiner, E.; Mader, S.; Rominger, F.; Straub, B.F. Highly active dinuclear copper catalysts for homogeneous azide-alkyne cycloadditions. Adv. Synth. Catal. 2012, 354, 3445–3450. [Google Scholar] [CrossRef]
- Touj, N.; Chakchouk-Mtibaa, A.; Mansour, L.; Harrath, A.H.; Al-Tamimi, J.H.; Ozdemir, I.; Mellouli, L.; Yasar, S.; Hamdi, N. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) under mild condition in water: Synthesis, catalytic application and biological activities. J. Organomet. Chem. 2017, 853, 49–63. [Google Scholar] [CrossRef]
- Wan, L.; Cai, C. Multicomponent synthesis of 1,2,3-triazoles in water catalyzed by silica-immobilized NHC-Cu(I). Catal. Lett. 2012, 142, 1134–1140. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Pavlov, A.O.; Ogorodnov, Y.V.; Spiridonova, D.V.; Sorokoumov, V.N.; Balova, I.A. N-Propargylation and copper(I)-catalyzed azide-alkyne cycloaddition as a convenient strategy for directed post-synthetic modification of 4-oxo-1,4-dihydrocinnoline derivatives. Chem. Heterocycl. Compd. 2020, 56, 915–922. [Google Scholar] [CrossRef]
- Jing, L.; Hongwei, L.; Fanyu, M.; Liuqing, Y.; Yanpeng, S.; Yumin, Z.; Qiang, G. Microwave-assisted synthesis of new 1,2,3-triazoles bearing an isoxazole ring by the azide-alkyne cycloaddition click chemistry. Chem. Res. Chin. Univ. 2018, 34, 197–202. [Google Scholar] [CrossRef]
- Schotten, C.; Manson, J.; Chamberlain, T.W.; Bourne, R.A.; Nguyen, B.N.; Kapur, N.; Willans, C.E. Development of a multistep, electrochemical flow platform for automated catalyst screening. Catal. Sci. Technol. 2022, 12, 4266–4272. [Google Scholar] [CrossRef]
- Szadkowska, A.; Pawlowski, R.; Zaorska, E.; Staszko, S.; Trzybinski, D.; Wozniak, K. NHC copper complexes functionalized with sulfoxide and sulfone moieties. Appl. Organomet. Chem. 2019, 33, e4983. [Google Scholar] [CrossRef]
- Trujillo, M.; Hull-Crew, C.; Outlaw, A.; Stewart, K.; Taylor, L.; George, L.; Duensing, A.; Tracey, B.; Schoffstall, A. Green methodologies for copper(I)-catalyzed azide-alkyne cycloadditions: A comparative study. Molecules 2019, 24, 973. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S.; Escudero-Adan, E.C.; Benet-Buchholz, J.; Stevens, E.D.; Slawin, A.M.Z.; Nolan, S.P. [(NHC)CuX] complexes: Synthesis, characterization and catalytic activities in reduction reactions and Click Chemistry. On the advantage of using well-defined catalytic systems. J. Chem. Soc. Dalton Trans. 2010, 39, 7595–7606. [Google Scholar] [CrossRef]
- Yamada, Y.M.; Sarkar, S.M.; Uozumi, Y. Amphiphilic Self-Assembled Polymeric Copper Catalyst to Parts per Million Levels: Click Chemistry. J. Am. Chem. Soc. 2012, 134, 9285–9290. [Google Scholar] [CrossRef] [PubMed]
- Khajehzadeha, M.; Moghadamb, M.; Jamehbozorgic, S. Synthesis and characterization of a new poly(N–heterocyclic carbene Cucomplex) immobilized on nano–silica, (CuII–NHCs)n@nSiO2, and its application as an efficient and reusable catalyst in the synthesis of benzimidazoles, benzothiazoles, 1,2,3–triazoles, bis–triazoles andsonogashira–hagihara reactions. Inorg. Chim. Acta 2019, 485, 173–189. [Google Scholar] [CrossRef]
- Pawar, A.; Gajare, S.; Jagdale, A.; Patil, S.; Chandane, W.; Rashinkar, G.; Patil, S. Supported NHC-Benzimi@Cu Complex a Magnetically Separable and Reusable Catalyst for the Multicomponent and Click Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Huisgen 1,3-Dipolar Cycloaddition. Catal. Lett. 2022, 152, 1854–1868. [Google Scholar] [CrossRef]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, Y.-J.; Shih, T.-Y.; Chen, Y.-H.; Lai, Y.-C.; Chiang, M.Y.; Senadi, G.C.; Chen, H.-Y.; Chen, H.-Y. Mechanistic study in click reactions by using (N-heterocyclic carbene)copper(I) complexes: Anionic effects. Organometallics 2019, 38, 223–230. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, P.; Sun, Z. CuI/CuBr2-catalyzed decarboxylative/A3 reaction of propiolic acids for the facile synthesis of 1,4-diheterocycle-2-butynes. Tetrahedron Lett. 2015, 56, 5676–5680. [Google Scholar] [CrossRef]
- Sreedhar, B.; Reddy, P.S. Sonochemical Synthesis of 1,4-Disubstituted 1,2,3-Triazoles in Aqueous Medium. Synth. Commun. 2007, 37, 805–812. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.L.; Qu, L.; Wang, J.; Yuan, J.; Chen, S.; Li, X. An Efficient Ultrasound-assisted Method for the Synthesis of 1,4-Disubstituted Triazoles. Z. Naturforsch. 2011, 66, 77–82. [Google Scholar] [CrossRef]
- Shinde, K.S.; Philipp, M.; Fuhrmann, D.; Binder, W.H. A Mechanochemically Active Metal-Organic Framework (MOF) Based on Cu-Bis-NHC-Linkers: Synthesis and Mechano-Catalytic Activation. Macromol. Chem. Phys. 2023, 224, 2200207. [Google Scholar] [CrossRef]
- Cravotto, G.; Gaudino, E.C.; Cintas, P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 2013, 42, 7521–7534. [Google Scholar] [CrossRef]
- Alotaibi, S.H. Tretinoin (2,4-difluoro-phenyl) triazole activates proapoptotic protein expression and targets NRP2 protein to inhibit esophageal carcinoma cell growth. Environ. Toxicol. 2024, 39, 942–951. [Google Scholar] [CrossRef]
- Peddapayata, P.R.; Jakkula, S.K.; Ega, J.K. Ultrasonically assisted synthesis of chromen-2-one derivatives coupled 1,2,3-triazoles via 1,3-polariton cycloaddition. AIP Conf. Proc. 2024, 2971, 050001. [Google Scholar] [CrossRef]
- Seo, C.; Cheong, Y.-J.; Yoon, W.; Kim, J.; Shin, J.; Yun, H.; Kim, S.-J.; Jang, H.-Y. Mononuclear Copper Complexes with Tridentate Tris(N-heterocyclic carbene): Synthesis and Catalysis of Alkyne–Azide Cycloaddition. Organometallics 2021, 40, 16–22. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Harmon, R.E. Reaction of acetylenedicarboxaldehyde bis(diethyl acetal) with bis(azidomethyl)benzene. J. Chem. Eng. Data 1986, 31, 379–380. [Google Scholar] [CrossRef]
- Pavel, S.; Hopf, H.; Jones, P.G.; Lupu, V.V.; Birsa, L.M. Synthesis and structural characterization of some 1,2-bis[(1H-1,2,3-triazol-1-yl)methyl]benzene derivatives. Rev. Chim. 2016, 67, 683–686. [Google Scholar]
- Nagao, Y.; Takasu, A. Click polyester: Synthesis of polyesters containing triazole units in the main chain via safe and rapid “click” chemistry and their properties. J. Polym. Sci. A Polym. Chem. 2010, 48, 4207–4218. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Wang, L.; Xiao, J.; Tao, M. Copper(II)-Schiff base complex-functionalized polyacrylonitrile fiber as a green efficient heterogeneous catalyst for one-pot multicomponent syntheses of 1,2,3-triazoles and propargylamines. Adv. Synth. Catal. 2018, 360, 1673–1684. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Sankararaman, S. Palladium complexes with abnormal N-heterocyclic carbene ligands derived from 1,2,3-triazolium ions and their application in Suzuki coupling. Tetrahedron Lett. 2009, 50, 5834–5837. [Google Scholar] [CrossRef]
- Keshavarz, M.; Badri, R. A facile and one pot synthesis of 1,4-disubstituted-1H-1,2,3-triazoles from terminal alkynes and phenacyl azides prepared from styrenes by CAN oxidant and sodium azide. Mol. Divers. 2011, 15, 957–962. [Google Scholar] [CrossRef]




 | |||||
|---|---|---|---|---|---|
| Entry | Compound | R Substituent | 60 °C for 15 min | 80 °C for 15 min | 100 °C for 10 min |
| 1 | 3a | -CH2OH | 65% a | 93% a | 49% a |
| 2 | 3b | -COOCH3 | 70% a | 88% a | 75% a |
| 3 | 3c | -C(CH3)2OH | 56% a | 59% a | 59% a |
| 4 | 3d | -CH(Ph)OH | 80% b | 87% b | 72% b |
| 5 | 3e | -COOH | 77% a,c | 37% a,c | 39% a,c |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Compound | R Substituent | No Solvent, Time, Yield | H2O at rt Time, Yield | H2O at 80 °C Time, Yield | H2O in MW 80 °C Time, Yield |
| 1 | 3a | -CH2OH | 4 h, 29% a | 24 h, 80% d | 1 h, 59% d | 0.25 h, 93% d |
| 2 | 3b | -COOCH3 | 2 h, 64% | 20 h, 78% d | 1 h, 76% d | 0.25 h, 88% d |
| 3 | 3c | -C(CH3)2OH | 3 h, 67% a | 20 h, 56% d | 1 h, 68% d | 0.25 h, 59% c |
| 4 | 3d | -CH(Ph)OH | 3 h, 73% c | 12 h, 67% c | 1 h, 70% c | 0.2 h, 87% c,f |
| 5 | 3e | -COOH | 1 h, 41% a | 22 h, 73% d,e | 1 h, 82% d,e | 0.25 h, 77% d,e,g |
| 6 | 3f | -CH2CH2OH | 1 h, 35% a | 24 h, 35% d | 4 h, 46% d | 0.25 h, 70% d |
| 7 | 3g | -CH(CH3)OH | 1 h, 19% a | 42 h, 99% d | 2 h, 72% d | 0.25 h, 98% d |
| 8 | 3h | -C(CH2)5OH | b | 20 h, 80% d | 3 h, 72% d | 0.25 h, 83% d |
| 9 | 3i | -CH2O(C5NF4) | 3 h, 76% c | 43 h, 61% c | 2 h, 70% c | 0.25 h, 49% c |
| 10 | 3j | -C(Ph)(CH3)OH | b | 12 h, 84% c | -- | 0.2 h, 75% c |
| 11 | 3k | -PhCOOH | b | 22 h, 94% d,e | -- | 0.2 h, 87% d,e,f |
| Entry | Method | Time (h) | Temp (°C) | Solvent | Yield |
|---|---|---|---|---|---|
| 1 | Room temperature | 1 | rt | none | 41% |
| 2 | Room temperature | 22 | rt | t-BuOH/H2O (1:1) | 73% |
| 3 | Conventional heating | 1 | 80 | H2O | 82% |
| 4 | MW heating | 0.25 | 100 | H2O | 39% |
| 5 | MW heating | 0.25 | 80 | H2O | 37% |
| 6 | MW heating | 0.25 | 60 | H2O | 77% |
| 7 | Sonication | 0.25 | rt | t-BuOH/H2O (1:1) | 81% b |
| 8 | Sonication | 0.50 | rt | t-BuOH/H2O (1:1) | 53% c |
| Entry | Compound | Heating | N1 Substituent | Time (h) | Yield b |
|---|---|---|---|---|---|
| 1 | 4a | MW | -CH2Ph | 0.25 | 78% a |
| 2 | 4b | MW | -CH2COPh | 0.25 | 76% |
| 3 | 4c | MW | -(C5NF4) | 0.25 | 90% a |
| 4 | 4a | Conventional | -CH2Ph | 0.5 | 49% a |
| 5 | 4b | Conventional | -CH2COPh | 0.5 | 50% a |
| 6 | 4c | Conventional | -(C5NF4) | 3 h | 58% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, L.T.; Barnett, E.; Hexom, M.; Ruiz, A.; Schoffstall, A. Microwave-Assisted Synthesis of Symmetrical 1,4-Disubstituted Bis-1H-1,2,3-triazoles Using Copper N-Heterocyclic Carbene Catalysts. Catalysts 2024, 14, 702. https://doi.org/10.3390/catal14100702
Mitchell LT, Barnett E, Hexom M, Ruiz A, Schoffstall A. Microwave-Assisted Synthesis of Symmetrical 1,4-Disubstituted Bis-1H-1,2,3-triazoles Using Copper N-Heterocyclic Carbene Catalysts. Catalysts. 2024; 14(10):702. https://doi.org/10.3390/catal14100702
Chicago/Turabian StyleMitchell, Loren Taylor, Erin Barnett, Max Hexom, Alexander Ruiz, and Allen Schoffstall. 2024. "Microwave-Assisted Synthesis of Symmetrical 1,4-Disubstituted Bis-1H-1,2,3-triazoles Using Copper N-Heterocyclic Carbene Catalysts" Catalysts 14, no. 10: 702. https://doi.org/10.3390/catal14100702
APA StyleMitchell, L. T., Barnett, E., Hexom, M., Ruiz, A., & Schoffstall, A. (2024). Microwave-Assisted Synthesis of Symmetrical 1,4-Disubstituted Bis-1H-1,2,3-triazoles Using Copper N-Heterocyclic Carbene Catalysts. Catalysts, 14(10), 702. https://doi.org/10.3390/catal14100702






