Phase Transformation of Zr-Modified LaNiO3 Perovskite Materials: Effect of CO2 Reforming of Methane to Syngas
Abstract
1. Introduction
2. Results and Discussion
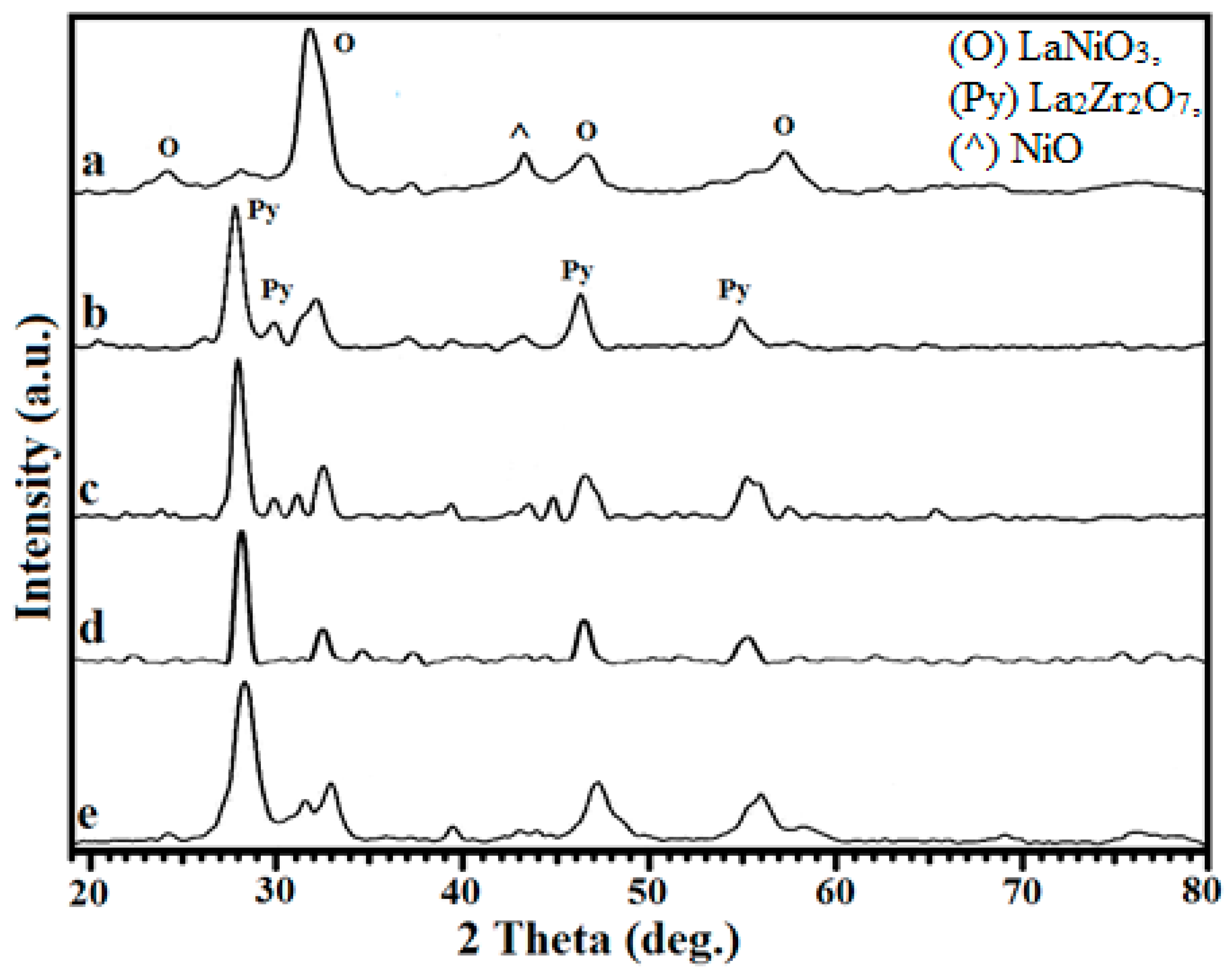
2.1. X-ray Diffraction Studies
2.2. Specific Surface Area Measurements
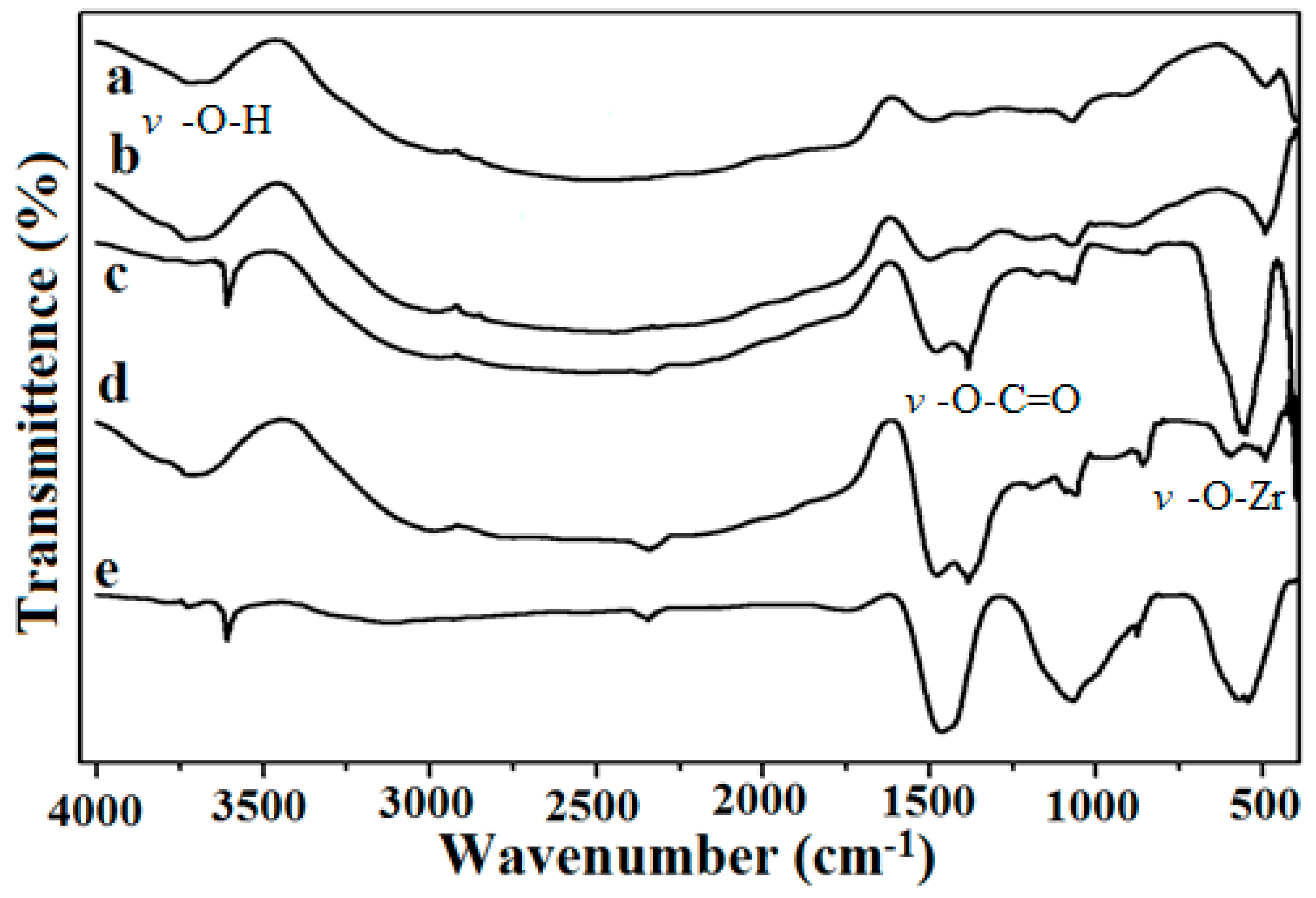
2.3. Fourier Transform Infrared Spectroscopy Studies
2.4. Temperature-Programmed Reduction Studies
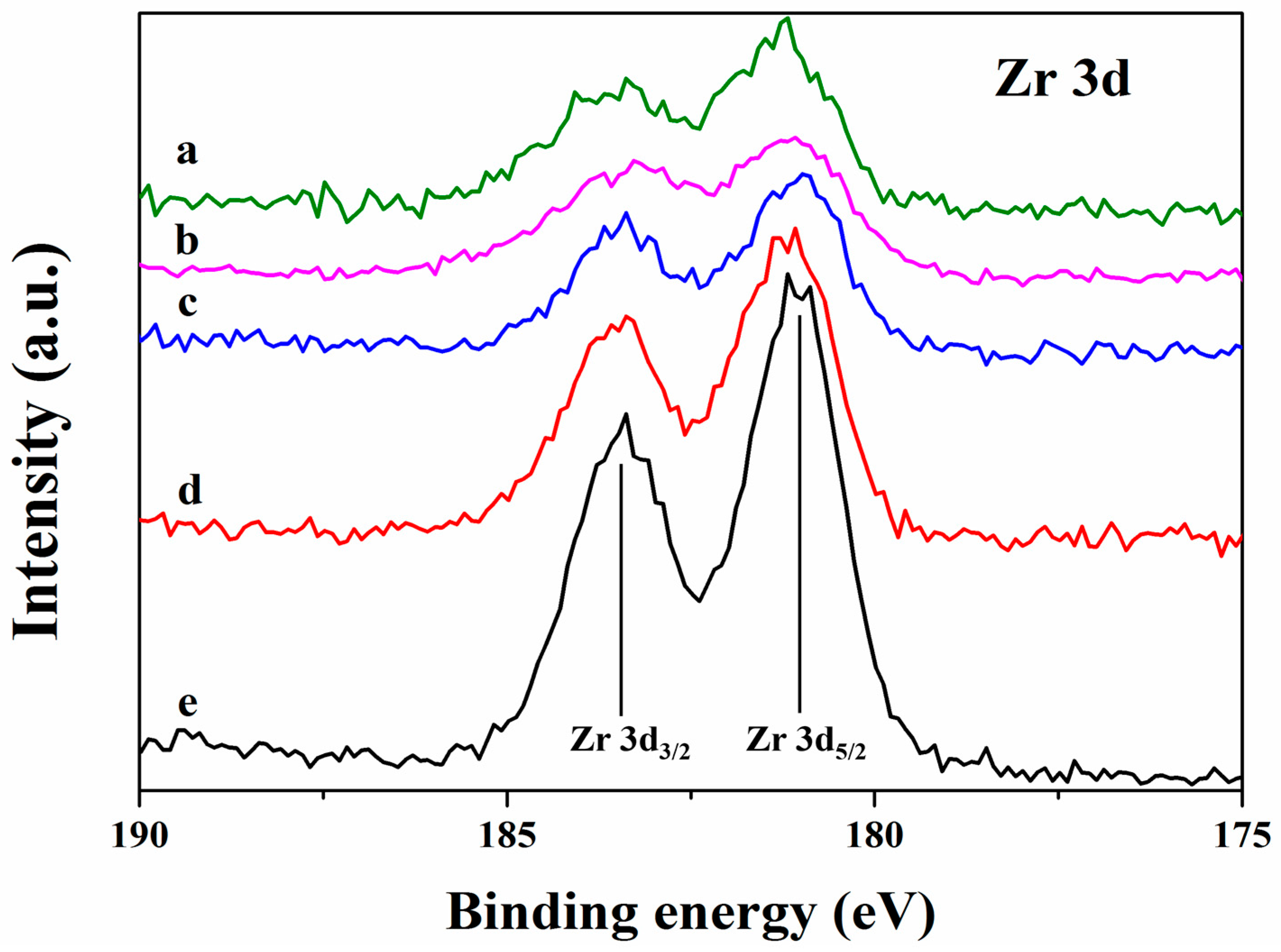
2.5. X-ray Photoelectric Spectroscopy Studies
2.6. Catalytic Activity
2.7. Carbon Analysis of the Used Catalysts
3. Experimental
3.1. Synthesis of Catalysts by Sol–Gel Method
3.2. Characterization Studies
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, Z.; Bukur, D.B.; Goodman, D.W. Fischer–Tropsch synthesis on a model Co/SiO2 catalyst. J. Catal. 2009, 268, 196–200. [Google Scholar] [CrossRef]
- Fidalgo, B.; Menéndez, J.A. Syngas production by CO2 reforming of CH4 under microwave heating-Challenges and opportunity. In Syngas: Production, Applications and Environmental Impact; Indarto., A., Palguandi., J., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 121–149. ISBN 978-1-62100-870-5. [Google Scholar]
- Mallikarjun, G.; Sagar, T.V.; Swapna, S.; Raju, N.; Chandrashekar, P.; Lingaiah, N. Hydrogen rich syngas production by bi-reforming of methane with CO2 over Ni supported on CeO2-SrO mixed oxide catalysts. Catal. Today 2020, 356, 597–603. [Google Scholar] [CrossRef]
- Gardner, T.H.; Spivey, J.J.; Campos, A.; Hissam, J.C.; Kugler, E.L.; Roy, A.D. Catalytic partial oxidation of CH4 over Ni-substituted barium hexaaluminate catalysts. Catal. Today 2010, 157, 166–169. [Google Scholar] [CrossRef]
- Sagar, T.V.; Padmakar, D.; Lingaiah, N.; Reddy, I.A.K.; Sai Prasad, P.S. Syngas production by CO₂ reforming of methane on LaNixAl1−xO₃ perovskite catalysts: Influence of method of preparation. J. Chem. Sci. 2017, 129, 1787–1794. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, V.C.; Štangar, U.L.; Mušič, B.; Mishra, I.M.; Meng, Y. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts. Catal. Rev. 2021, 63, 363–421. [Google Scholar] [CrossRef]
- Nuvula, S.; Sagar, T.V.; Valluri, D.K.; Prasad, P.S. Selective substitution of Ni by Ti in LaNiO3 perovskites: A parameter governing the oxy-carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2018, 43, 4136–4142. [Google Scholar] [CrossRef]
- Haynes, D.J.; Campos, A.; Berry, D.A.; Shekhawat, D.; Roy, A.; Spivey, J.J. Catalytic partial oxidation of a diesel surrogate fuel using an Ru-substituted pyrochlore. Catal. Today 2010, 155, 84–91. [Google Scholar] [CrossRef]
- Haynes, D.J.; Berry, D.A.; Shekhawat, D.; Spivey, J.J. Catalytic partial oxidation of n-tetradecane using Rh and Sr substituted pyrochlores: Effects of sulfur. Catal. Today 2009, 145, 121–126. [Google Scholar] [CrossRef]
- Yan, L.; Hongjian, L.; Lipeng, Q.; Zhijin, W.; Linhai, W.; Yun-Quan, L. Improving anti-coking and sulfur-resisting performance of Rh-based lanthanum zirconate pyrochlore catalysts for diesel reforming through partial substitution with alkali earth metals. J. Anal. Appl. Pyrolysis 2023, 169, 105865. [Google Scholar]
- Ma, Y.; Ma, Y.; Zhao, Z.; Ma, S.; Meng, Q.; Hu, X.; Buckley, C.E.; Dong, D. Fibrous La2Zr2O7 pyrochlore-supported Ni nanocatalysts for methane reforming. J. Phys. Chem. Solids 2020, 147, 109643. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Garcilaso, V.; Watson, D.J.; Centeno, M.A.; Odriozola, J.A.; Reina, T.R. Flexible syngas production using a La2Zr2-xNixO7-δ pyrochlore-double perovskite catalyst: Towards a direct route for gas phase CO2 recycling. Catal. Today 2020, 357, 583–589. [Google Scholar] [CrossRef]
- Kumar, N.; Kanitkar, S.; Wang, Z.; Haynes, D.; Shekhawat, D.; Spivey, J.J. Dry reforming of methane with isotopic gas mixture over Ni-based pyrochlore catalyst. Int. J. Hydrogen Energy 2019, 44, 4167–4176. [Google Scholar] [CrossRef]
- Kumar, N.; Roy, A.; Wang, Z.; L’Abbate, E.M.; Haynes, D.; Shekhawat, D.; Spivey, J.J. Bi-reforming of methane on Ni-based pyrochlore catalyst. Appl. Catal. A Gen. 2016, 517, 211–216. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Foord, J.A.; Green ML, H.; Grey, C.P. Selective oxidation of methane to synthesis gas using transition metal catalysts. Nature 1990, 344, 319–321. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Jones, R.H.; Natarajan, S.; Thomas, J.M.; Waller, D.; Clark, S.M. An in situ, energy-dispersive X-ray diffraction study of natural gas conversion by carbon dioxide reforming. J. Phys. Chem. 1993, 97, 3355–3358. [Google Scholar] [CrossRef]
- Bespalko, N.; Roger, A.C.; Bussi, J. Comparative study of NiLaZr and CoLaZr catalysts for hydrogen production by ethanol steam reforming: Effect of CO2 injection to the gas reactants. Evidence of Rh role as a promoter. Appl. Catal. A Gen. 2011, 407, 204–210. [Google Scholar] [CrossRef]
- Bussi, J.; Musso, M.; Veiga, S.; Bespalko, N.; Faccio, R.; Roger, A.C. Ethanol steam reforming over NiLaZr and NiCuLaZr mixed metal oxide catalysts. Catal. Today 2013, 213, 42–49. [Google Scholar] [CrossRef]
- Gaur, S.; Haynes, D.J.; Spivey, J.J. Rh, Ni, and Ca substituted pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2011, 403, 142–151. [Google Scholar] [CrossRef]
- Sagar, T.V.; Sreelatha, N.; Hanmant, G.; Surendar, M.; Lingaiah, N.; Rama Rao, K.S.; Reddy, I.K.A.; Prasad, P.S. Influence of method of preparation on the activity of La–Ni–Ce mixed oxide catalysts for dry reforming of methane. Rsc Adv. 2014, 4, 50226–50232. [Google Scholar] [CrossRef]
- Sagar, T.V.; Sreelatha, N.; Hanmant, G.; Upendar, K.; Lingaiah, N.; Rama Rao, K.S.; Reddy, I.K.A.; Prasad, P.S. Methane reforming with carbon dioxide over La-Nix-Ce1−x mixed oxide catalysts. Indian J. Chem.-Sect. A 2014, 53A, 478–483. [Google Scholar]
- Chen, H.S.; Kumar, R.V.; Glowacki, B.A. Chemical solution deposited lanthanum zirconium oxide thin films: Synthesis and chemistry. Mater. Chem. Phys. 2010, 122, 305–310. [Google Scholar] [CrossRef]
- Koteswara Rao, K.; Banu, T.; Vithal, M.; Swamy GY, S.K.; Kumar, K.R. Preparation and characterization of bulk and nano particles of La2Zr2O7 and Nd2Zr2O7 by sol–gel method. Mater. Lett. 2002, 54, 205–210. [Google Scholar] [CrossRef]
- Tong, Y.; Zhu, J.; Lu, L.; Wang, X.; Yang, X.J. Preparation and characterization of Ln2Zr2O7 (Ln = La and Nd) nanocrystals and their photocatalytic properties. J. Alloys Compd. 2008, 465, 280–284. [Google Scholar] [CrossRef]
- Bussi, J.; Bespalko, N.; Veiga, S.; Amaya, A.; Faccio, R.; Abello, M.C. The preparation and properties of Ni–La–Zr catalysts for the steam reforming of ethanol. Catal. Commun. 2008, 10, 33–38. [Google Scholar] [CrossRef]
- Lima, S.M.; Assaf, J.M.; Pena, M.A.; Fierro, J.L.G. Structural features of La1− xCexNiO3 mixed oxides and performance for the dry reforming of methane. Appl. Catal. A Gen. 2006, 311, 94–104. [Google Scholar] [CrossRef]
- Xu, Z.; He, L.; Zhao, Y.; Mu, R.; He, S.; Cao, X. Composition, structure evolution and cyclic oxidation behavior of La2(Zr0.7Ce0.3)2O7 EB-PVD TBCs. J. Alloys Compd. 2010, 491, 729–736. [Google Scholar] [CrossRef]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. Oxidation states at alkali-metal-doped Ni (110)–O surfaces. Phys. Chem. Chem. Phys. 2001, 3, 274–281. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, F.; Yang, Y.; Chen, P.; Yang, D.; Duan, Y.; Wang, X. Thin-film barrier performance of zirconium oxide using the low-temperature atomic layer deposition method. ACS Appl. Mater. Interfaces 2014, 6, 3799–3804. [Google Scholar] [CrossRef]
- Sagar, T.V.; Zavašnik, J.; Finšgar, M.; Novak Tušar, N.; Pintar, A. Evaluation of Au/ZrO2 Catalysts Prepared via Postsynthesis Methods in CO2 Hydrogenation to Methanol. Catalysts 2022, 12, 218. [Google Scholar] [CrossRef]
- Kumar, P.; Shah, A.K.; Lee, J.H.; Park, Y.H.; Štangar, U.L. Selective hydrogenolysis of glycerol over bifunctional copper–magnesium-supported catalysts for propanediol synthesis. Ind. Eng. Chem. Res. 2020, 59, 6506–6516. [Google Scholar] [CrossRef]
- Guan, D.; Ryu, G.; Hu, Z.; Zhou, J.; Dong, C.L.; Huang, Y.C.; Zhang, K.; Zhong, Y.; Komarek, A.C.; Zhu, M.; et al. Utilizing ion leaching effects for achieving high oxygen-evolving performance on hybrid nanocomposite with self-optimized behaviors. Nat. Commun. 2020, 11, 3376. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Mateos-Pedrero, C.; Soria, M.A.; Guerrero-Ruiz, A.; Rodemerck, U.; Rodríguez-Ramos, I. Transient studies of low-temperature dry reforming of methane over Ni-CaO/ZrO2-La2O3. Appl. Catal. B Environ. 2013, 129, 450–459. [Google Scholar] [CrossRef]



| S. No. | Catalysts | Specific Surface Area (m2/g) |
|---|---|---|
| 1 | LaNi0.2Zr0.8O3 | 14 |
| 2 | LaNi0.3Zr0.7O3 | 12 |
| 3 | LaNi0.4Zr0.6O3 | 7 |
| 4 | LaNi0.6Zr0.4O3 | 3 |
| 5 | LaNi0.8Zr0.2O3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagar, T.V.; Lingaiah, N.; Sai Prasad, P.S.; Tušar, N.N.; Štangar, U.L. Phase Transformation of Zr-Modified LaNiO3 Perovskite Materials: Effect of CO2 Reforming of Methane to Syngas. Catalysts 2024, 14, 91. https://doi.org/10.3390/catal14010091
Sagar TV, Lingaiah N, Sai Prasad PS, Tušar NN, Štangar UL. Phase Transformation of Zr-Modified LaNiO3 Perovskite Materials: Effect of CO2 Reforming of Methane to Syngas. Catalysts. 2024; 14(1):91. https://doi.org/10.3390/catal14010091
Chicago/Turabian StyleSagar, Tatiparthi Vikram, Nakka Lingaiah, Potharaju S. Sai Prasad, Nataša Novak Tušar, and Urška Lavrenčič Štangar. 2024. "Phase Transformation of Zr-Modified LaNiO3 Perovskite Materials: Effect of CO2 Reforming of Methane to Syngas" Catalysts 14, no. 1: 91. https://doi.org/10.3390/catal14010091
APA StyleSagar, T. V., Lingaiah, N., Sai Prasad, P. S., Tušar, N. N., & Štangar, U. L. (2024). Phase Transformation of Zr-Modified LaNiO3 Perovskite Materials: Effect of CO2 Reforming of Methane to Syngas. Catalysts, 14(1), 91. https://doi.org/10.3390/catal14010091









