Abstract
With increasing environmental awareness, the issue of atmospheric pollution has gained significant attention. Specifically, three types of atmospheric pollutants, namely, nitrogen oxides, volatile organic compounds, and carbon monoxide, have become the focus of widespread concern. In addressing these pollutants, mesoporous zeolites have emerged as promising materials due to their large specific surface area, which enables effective dispersion of active sites, and their large pore volume, which facilitates efficient diffusion. This article provides a comprehensive overview of the preparation methods of mesoporous zeolites and their applications in removing nitrogen oxides, volatile organic compounds, and carbon monoxide. It also highlights the challenges and limitations faced by the application of mesoporous zeolites in pollutant removal and emphasizes their potential as efficient catalysts.
1. Introduction
Human activities and natural processes generate various pollutants that can be released into the atmosphere and become part of the atmospheric circulation. These pollutants are eliminated from the atmosphere through chemical reactions, biological activities, physical sedimentation, and other ways. When the rate of pollutant removal is lower than the rate of production, these substances will accumulate in the atmosphere, leading to the formation of atmospheric pollution once a certain concentration is reached [1,2,3,4]. Atmospheric pollution has adverse effects on the ecological environment and poses significant risks to human health. Therefore, it is of great significance to eliminate and reduce atmospheric pollution [5,6]. Currently, the main pollutants contributing to atmospheric pollution are nitrogen oxides (NOx, including NO, NO2, and N2O), volatile organic compounds (VOCs), and carbon monoxide (CO).
NOx sources can be categorized into stationary sources (such as industrial boilers and coal-fired boilers) and mobile sources (vehicle emissions, etc.). High concentrations of nitrogen oxides in the air can lead to the formation of acid rain and photochemical smog, as well as damage to the central nervous system [7,8,9]. Various technologies are employed for NOx removal, including selective catalytic reduction (SCR), selective non-catalytic reduction (SNCR), non-thermal plasma (NTP), exhaust gas recirculation (EGR), and wet flue gas denitration (WFGD) [10,11,12,13]. Among them, SCR is a widely used technology in denitration. Another type of pollutant is volatile organic compounds (VOCs), which include many kinds of organic atmosphere substances, and they can stay in the atmosphere for up to 60 days. VOCs originated from various exhaust gases, mainly painting, printing, fuel storage, petroleum refineries, and motor vehicles. When the concentration of VOCs in the air is high, it will cause photochemical smog and destroy the ozone layer [14,15]. Several methods for removing VOCs have been developed, among which catalytic oxidation is widely used due to its certain advantages. It occurs at moderate temperatures (165–440 °C), which allow lower operating costs; in addition, gas flows with low oxygen content are required, and the formation of NOx in the combustion process is decreased [16,17,18]. Currently, catalysts used for VOC oxidation are usually metal particles or metal oxides-supported catalysts [19,20]. Finally, carbon monoxide (CO), which usually comes from the inadequate combustion of carbon-containing substances, is a colorless, odorless, and neurotoxic gas. The CO in the air can cause tissue hypoxia, leading to acute or chronic poisoning and even death [21]. The main method for eliminating carbon monoxide is catalytic oxidation. Among all the supported metal catalysts, zeolite-supported metal catalysts display excellent thermal/hydrothermal stability, and the ultrasmall and stable metal species can be dispersed [22]. In addition, the catalytic conversion of CO under mild conditions is also an interesting subject [23,24].
Zeolites possess significant potential for the treatment of atmospheric pollutants due to their remarkable adsorption capacity, high stability, and selective catalytic capability. Their three-dimensional structure is composed of interconnected tetrahedral networks of [SiO4]4− and [AlO4]5− linked by oxygen atoms [25,26,27]. The pore size of zeolites is less than 2 nm [28,29]. According to the definition provided by the International Union of Pure and Applied Chemistry (IUPAC), inorganic porous materials with pore sizes smaller than 2 nm are referred to as microporous materials, while those with pore sizes ranging from 2 nm to 50 nm are known as mesoporous materials, and materials with pore sizes exceeding 50 nm are categorized as macroporous materials [30,31]. The scaling up of zeolite-based catalysts is challenged mainly by the high cost required for its synthesis, and there have been efforts to reduce costs for zeolite preparation and enable high-value applications, such as using bagasse fly ash [32], palm oil mill fly ash [33], coal fly ash [34], and so on [35]. Despite possessing strong acidic sites, high thermal and hydrothermal stability, as well as unique “shape selective catalysis” properties, zeolites face limitations in applications within macromolecular reaction systems due to the narrow channels and high diffusion resistance [36]. Introducing a mesoporous structure into zeolites is considered an effective strategy for overcoming this constraint.
Mesoporous zeolites exhibit a large specific surface area and versatile pore structures, facilitating the diffusion and transportation of substances, thus enabling their utilization in macromolecular reactions [37]. They can be classified into disordered mesoporous zeolites and ordered mesoporous zeolites based on the level of mesoporous organization. Among these, ordered mesoporous zeolites such as the MCM series (Mobil Composition of Matter), SBA-n series (Santa Barbara USA), and MSU series (Michigan State University) have been extensively investigated due to their well-defined and interconnected mesopores [38,39]. Currently, the preparation methods for mesoporous zeolites have reached maturity, and their applications in catalysis have been widely explored. In catalytic conversions involving three types of atmospheric pollutants, mesoporous zeolites are typically loaded with specific metal particles to enhance their catalytic performance. For example, the addition of copper ions can effectively improve the catalytic conversion ability of NOx at low temperatures. In these catalytic reactions, mesoporous zeolites enhance catalytic performance by providing a large specific surface area that promotes the dispersion of metal particles.
This article introduces the preparation and application of mesoporous zeolites. Among them, the article provides a detailed summary of the catalytic ability of mesoporous zeolites in three main types of atmospheric pollutants (NOx, VOCs, and CO). The aim of this article is to elucidate the potential of mesoporous molecular sieves in environmental protection through investigation and research and to provide a theoretical basis for such research.
2. Synthesis of Mesoporous Zeolites
Since Barrer’s pioneering synthesis of artificial zeolites in the 1940s, extensive research has been conducted on zeolite synthesis. From the mid-1950s to the early 1980s, zeolites experienced a pinnacle in scientific investigation, practical application, and industrial advancement. Presently, the Structure Commission of the International Zeolite Association (IZA-SC) has cataloged 255 distinct zeolite framework structures [40]. In 1992, Mobil company reported the groundbreaking synthesis of mesoporous zeolites, sparking widespread interest in their design, preparation, and application. As of now, researchers have developed a variety of synthesis methods for mesoporous zeolites, which can be mainly divided into two categories: the post-synthesis method (also known as top-down) and the in situ method (also known as bottom-up) [41,42,43,44]. The post-synthesis method involves the removal of silicon or aluminum atoms from the synthesized zeolite to introduce a mesoporous structure. Conversely, the in situ method necessitates the utilization of templates to generate mesopores during the zeolite synthesis process.
2.1. Post-Synthesis Method (Top-Down)
The post-synthesis method requires a chemical treatment process, which can be divided into dealumination [45] or desilication [46,47] according to different methods. During this process, a fraction of the zeolite framework may collapse to form mesopores [48,49]. Typically, aluminum-rich zeolites are more conducive to dealumination, and the distribution of Si and Al atoms within the zeolite also influences the arrangement of mesopores generated through desilication or dealumination. That is to say, when Al atoms are randomly dispersed within the zeolite, the resulting mesopores tend to be disordered. Conversely, if the Al atoms are uniformly distributed, ordered mesopores are typically obtained [50,51,52].
2.1.1. Dealumination
Dealumination is a process used to remove aluminum from zeolite structures, resulting in the formation of mesopores and modification of the zeolite properties. Acid leaching is a commonly employed method for dealumination, and different types of acids can be used, including inorganic acids such as nitric acid and hydrochloric acid, as well as organic acids like oxalic acid and citric acid. The efficiency of dealumination can vary depending on the type of acid used and the specific zeolite involved. When weak acids with low concentrations of free H+ ions are used, they have a limited effect on removing amorphous silicon and aluminum from the surface and pore walls of zeolites. As a result, the decrease in crystallinity, expansion of pore volume, and increase in specific surface area are not significant. However, as the acidity of the leaching solution increases, the dealumination effect becomes more pronounced, leading to greater pore enlargement. Babic et al. conducted in-depth research on the influence of acidity and different commercial zeolites with varying framework topology on mesoporous structure formation. They treated three types of commercial zeolites (CHA: Chabazite, the structure is made of a double 6-ring (D6R) as the basic building, and its large CHA cavity is accessed by six eight-ring windows measuring around 0.38 nm × 0.38 nm [53]; MFI: (Mobil Five)-type zeolite, which has two types of decametric ring pore channels: a straight pore channel with a pore size of 5.6 × 5.3 Å and a sinusoidal pore channel with a pore size of 5.5 × 5.1 Å [54]; LTL: Linde type L zeolite, composed of composite building units of D6R, CAN and LTL [55]) with chromic acid (H2CrO4) solutions at concentrations ranging from 0.1 to 10 w/%. Among the samples, only LTL treated with 10 w/% chromic acid successfully formed mesoporous structures, while the others did not. This suggests that the formation of mesopores through dealumination is specific to certain types of zeolites [56]. Another study by Wei et al. explored the effects of organic and inorganic acids on mesoporous Y zeolite synthesis. They successfully synthesized mesoporous Y zeolite by treating commercial Y zeolite with citric acid (C6H8O7) and ammonium tetrafluoroborate (NH4BF4) as agents (Figure 1). The treated Y zeolite exhibited a higher degree of interconnected mesopores compared to the commercial Y zeolite. This is attributed to the penetration and corrosion of the zeolite crystal by the complexes formed by C6H8O7 and NH4BF4. The scale-up test also displayed a significant number of connected mesopores, indicating the suitability of this catalyst for industrial applications [57].
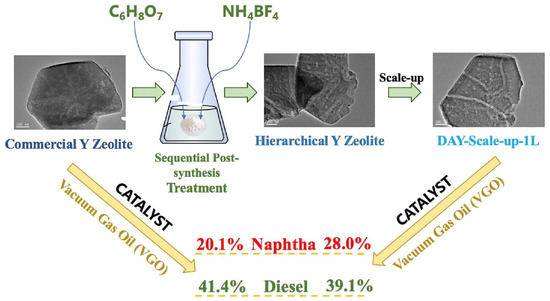
Figure 1.
The synthesis route of mesoporous Y zeolite [57]. Copyright 2023, Elsevier B.V.
2.1.2. Desilication
Alkaline desiliconization is the process of alkaline treatment over the synthesized zeolite within the range of 50–80 °C. High-silicon zeolites with a Si/Al ratio greater than 20 usually have better desiliconization effects [58]. The alkaline treatment desiliconization process is usually accompanied by a small amount of aluminum leaching, but the aluminum content on the outer surface of zeolite usually leads to uneven silicon dissolution during alkaline treatment [59]. Currently, alkaline media used in alkali treatment can be divided into strong bases (NaOH, LiOH, KOH, and CsOH) and weak bases (NaHCO3, Na2CO3, and NH3·H2O). Treating with different alkaline substances will lead to different results [60]. It was reported that zeolites treated with alkaline have a lower Si/Al ratio and an increased ion-exchange capacity [61]. Nguyen et al. prepared mesoporous ferrierite zeolite (FER) by using NaOH solution as a desilication agent. The results showed that mesoporous structures in the range of 5–40 nm can be formed on the surface of the FER zeolite. Alkaline desilication can effectively introduce interconnecting mesoporous pores. But it also has the disadvantages of easy destruction of zeolite skeleton and difficult adjustment of secondary porosity [62]. The mesopore size obtained from the NaOH solution is relatively large, and the pore channel is severely damaged. To avoid this problem, Zhang et al. prepared mesoporous β zeolite via protective desilication using quaternary ammonium hydroxide (TAAOH) and explored the mechanism of protective desilication of TAAOH. The protection of TAA+ coexists with the desilication function of OH− and the two act in different stages. As shown in Figure 2, TAA+ occupies the entrances of micropores of β zeolite and displays a protection effect. Then, OH− extracts the Si atom in dominance, thereby steadily forming abundant mesopores. Meanwhile, if β zeolite is treated with NaOH first, the added TAA+ cannot protect the β zeolite. Moreover, OH− concentration is also an important factor in the formation of mesopores in β zeolite. The mesoporosity of β zeolite can be tuned by adjusting the length of the TAAOH alkyl chain [63].
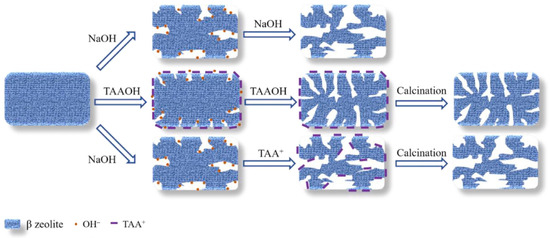
Figure 2.
Schematic diagram of protective desilication process on β zeolite by TAAOH and NaOH [63]. Copyright 2021, Elsevier B.V.
Based on the above discussion, the essence of dealumination and desilication is to sacrifice the parts of zeolite crystals for generating mesopores, which will reduce the crystallinity of zeolites and change the original Si/Al ratio and the micropore connectivity, resulting in uncontrollable secondary pores. It is also worth noting that dealumination is usually only applicable to aluminum-rich zeolites, which greatly narrows the application range of forming mesopores through dealumination [64].
2.2. In Situ Method (Bottom-Up)
The in situ method, also known as the bottom-up method, mainly includes the hard template method and the soft template method, which obtain materials with mesoporous structure by adding template agents in the process of zeolite synthesis [65,66]. In general, the template method is one of the ideal ways to synthesize mesoporous zeolites.
2.2.1. Hard Template Method
The hard template method refers to the addition of a hard template during the synthesis of mesoporous zeolites. Although a variety of hard template agents have been developed, the most commonly used material is still carbon. Cho et al. successfully prepared ordered mesoporous MFI zeolites by using mesoporous carbon as a hard template. The mesopores are highly ordered and have a diameter of approximately 10 nm. Moreover, the pore size of the template and skeleton stiffness are also important factors affecting the mesopores during synthesis [67]. Abdulridha et al. synthesized mesoporous Y zeolite by using multi-walled carbon nanotubes (CNTs) and nanocrystalline cellulose (NCC) as hard templates, respectively. The micropore and mesopore size distributions of Y zeolites in Figure 3a,b show that all the zeolites prepared with different templates have mesoporous structures. And the nitrogen adsorption–desorption curves also proved that the parent Y zeolite showed a type I curve, while the zeolites prepared by the hard template method showed a typical type IV curve with hysteresis loop, indicating that the mesoporous structure on the Y zeolite was successfully introduced by using the hard template method [68]. In addition, White et al. prepared mesoporous ZSM-5 zeolite by using the nitrogen-doped carbonaceous monolithic (NDC) as a hard template (Figure 3c). The NDC template was composed of sugar and egg protein precursors. The mesopore diameters of the prepared Meso-ZSM-5 were located at the range of 12–16 nm [69].
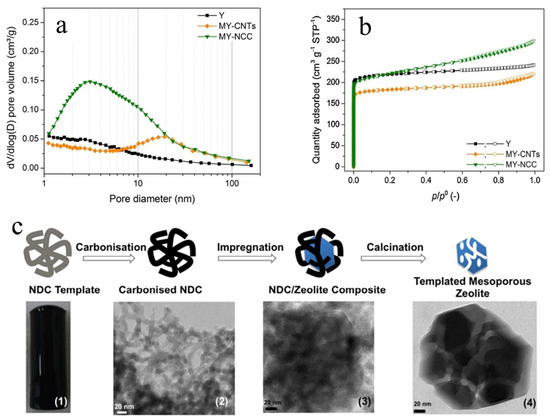
Figure 3.
Micropore and mesopore size distributions of MY zeolites using the Horváth–Kawazoe (HK) method (a) and by the Barrett–Joyner–Halenda (BJH) method (b) [68]. Copyright 2021, Elsevier B.V.; (c) The synthesis route of mesoporous zeolites (e.g., ZSM-5) (3) and (4) based on (1) and (2) biomass-derived NDC monolithic templates [69]. Copyright 2014, ACS Publications.
2.2.2. Soft Template Method
The soft template method can be traced back to 1992, when Mobil’s Kresge reported that the M41S series mesoporous materials were synthesized using surfactants as templates [70,71]. At present, the commonly used soft templates include silyliertes polymer [72], hydrophilic cationic polymer [73], cationic organosilane [74], and surfactant [75]. The synthesis method involves the interaction between structure-directing agents and templates within the synthetic gel, leading to the self-assembly and formation of mesopores. Hence, the selection of appropriate structure-directing agents is crucial for the successful synthesis of mesoporous zeolite. Miyake et al. prepared a uniform mesoporous MFI zeolite using poly vinyl alcohol with surface activity (PVA) as a secondary template agent and explored the optimum PVA contents in the starting mixture. MFI zeolite crystals with uniform mesopores (15 nm) can be prepared in the medium PVA content while low PVA content (1.5 wt%) or high PVA content (4.5 wt%) are not. Meanwhile, the diameter of mesopores is approximately consistent with the end-to-end distance of PVA molecules (10.8–14.4 nm), indicating that one PVA molecule is incorporated in one mesopore [76]. Zhao et al. prepared hierarchical ZSM-5 zeolite by using organic functionalized nano silica and organic silane mesoporous structure directing agents as raw materials through hydrothermal synthesis. The results showed that the crystallinity of MFI zeolite decreases with the increase of silicone content. The nanocrystalline size and surface topography of ZSM-5 are related to organic functionalized silica and organic additives, and the mesoporous volume and pore size of the catalyst are related to the degree of silanization of silica [41]. Guo et al. successfully prepared mesoporous silicalite-1 with the mesoporous size range of 5–20 nm using polyacrylic acid (PAA) as a template. The preparation process of mesoporous silicalite-1 is shown in Figure 4a; the positively charged alkylammonium moiety has a strong interaction with the negatively charged PAA, whereas the reaction between the hydrolysable methoxysilyl moiety and growing crystal domains is strong through covalent bonds with silica sources. Therefore, PAA chains introduced into zeolite can form mesopores. As shown in Figure 4b–e, the mesopores in the zeolite crystal are abundant and uniformly distributed in the range of 5–20 nm [77].
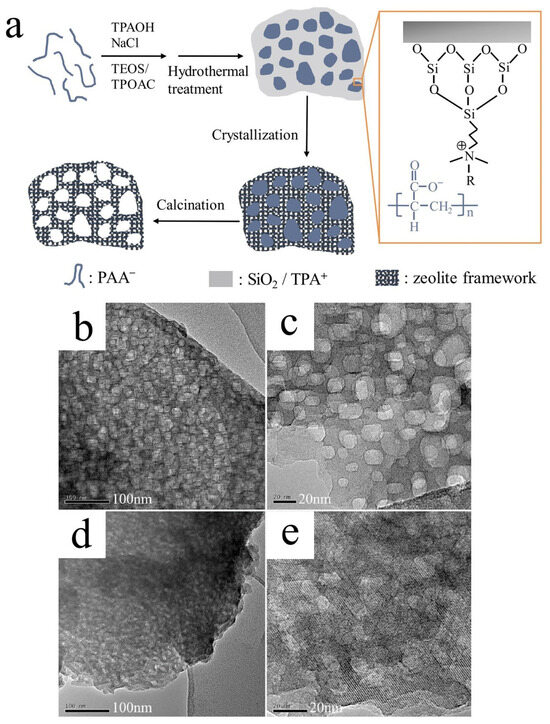
Figure 4.
(a) Formation mechanism of mesoporous Silicalite-1; TEM images of mesoporous silicalite-1 synthesized with different salts: (b,c) KCl and (d,e) NaNO3 [77]. Copyright 2020, Elsevier B.V.
In summary, the hard template method can strictly control the size and morphology of nanomaterials without considering the interaction between template agents and inorganic species nor controlling the hydrolysis and condensation of inorganic silicate species. However, the rigid framework exhibits a relatively simplistic structure [78,79]. The soft template method can flexibly adjust the pore size of mesopores, but compared to the hard template, the stability of the soft template structure is weaker.
3. Application of Mesoporous Zeolites for Catalytic Elimination of Air Pollutants
3.1. Removing NOx
Nitrogen oxides (NOx) mainly contain nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O). Among them, the content of NO accounts for over 90% of NOx emissions, and NO2 accounts for 0.5–10% [80]. The nitrogen oxides in the atmosphere pose significant hazards to the environment and human health. They can form a haze, acid rain, and photochemical smog and cause bronchitis, skin aging, and lung cancer. The main sources of NOx vary in different countries. For example, mobile sources account for 57.5% of NOx emissions in the US, while stationary sources account for 71% of NOx emissions in China [81]. To solve NOx emissions, many regulations and policies have been implemented by governments worldwide. Mainly, three ways can be used in the removal of NOx: source control, process control, and end-of-pipe control. Among them, selective catalytic reduction (SCR), which is end-of-pipe control, was widely used in the industry for removing NOx due to its high denitration efficiency, safe operation, and no secondary pollution [82,83,84,85]. One important application in industry is the marine SCR system for ocean-going vessels. For this system, its operating costs are mainly affected by operating time, NOx inlet concentration, and emission limits [86]. The preparation and application of catalysts are crucial for SCR. The catalysts used in SCR usually require high activity and selectivity toward N2 and high stability in the presence of SO2 and H2O. The traditional metal catalysts used in NH3-SCR (such as V2O5, manganese, and cerium) show some shortcomings, such as ammonia slip and unstable SO2 poisoning in low temperature. Meanwhile, the zeolite catalysts containing Cu can be used in low-temperature window operation without ammonia slip and are more stable in the presence of SO2. Further, zeolite catalysts containing Fe are more suitable for high-temperature window operation [87,88,89].
At present, Cu-SSZ-13 zeolite catalysts with small pore chabazite (CHA) structures have been widely studied for NH3-SCR reaction due to their ability to resist hydrocarbon poisoning and hydrothermal deactivation [90,91,92]. However, the small pore structure can restrict the transport of reactants to the active site, resulting in a decrease in reaction efficiency. Currently, one of the effective solutions for resolving diffusion limitations is to introduce mesopores into the zeolite framework [93,94]. Oord et al. introduced mesopores with a diameter of 2–10 nm into the zeolite using alkaline treatment (NaOH). The results showed that the catalytic activity of Cu-based zeolite catalyst with mesoporous structures was higher than that without mesoporous structures. Specifically, the conversion of NO over catalysts with mesoporous structures is 100% in the temperature range of 250–450 °C, and the presence of mesopores improves its performance at low temperatures (i.e., at 150 °C and 250 °C) by two times. It is worth noting that the formation of a mesopore is accompanied by a loss in the microporous surface area. Therefore, when the concentration of alkalinity is high, the crystallinity of the zeolite will be decreased. In addition, Oord et al. also found that mesopores are mainly formed outside the SSZ-13 zeolite and gradually corrode towards the interior of the SSZ-13 zeolite when increasing the amount of NaOH [95]. To deeply enhance activity, hydrothermal stability and propene poisoning resistance of zeolite should also be studied. Zhang et al. prepared mesoporous SSZ-13 and core–shell meso-SSZ-13@MAS (MAS: mesoporous aluminosilicate materials) via alkaline desilication and self-assembled methods (Figure 5a). The introduction of a mesoporous structure makes it easier to obtain more active Cu sites and effectively reduces the pore diffusion restriction. Meanwhile, the mesoporous zeolite catalyst shows higher SCR activity than that of traditional Cu-SSZ-13 over the whole temperature range (Figure 5b), which can be explained as follows: Cu ion exchange only occurs in the outer parts of traditional Cu-SSZ-13, and a part of the Cu ion could migrate and aggregate into CuO species on the external surface of SSZ-13. After the introduction of mesoporous structures, more ion-exchange sites in the internal parts of the SSZ-13 crystal can be exposed, and more active Cu sites can be obtained. The diffusion path length inside the SSZ-13 zeolite becomes shorter, and the diffusion resistance can be reduced remarkably [96]. Liang et al. prepared Cu-SSZ-13-Meso by using carbon black as a hard template (Figure 5c). In the NH3-SCR reaction, Cu-SSZ-13-Meso shows excellent low-temperature activity, and the existence of mesopores can effectively solve blockage of the micropores due to NH4NO3 or (NH4)2SO4 [97].
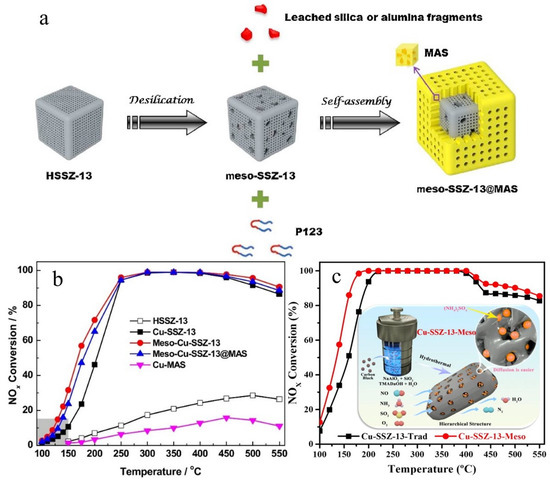
Figure 5.
(a) Schematic representation of the preparation of meso-SSZ-13@MAS composite; (b) NOx conversion in the NH3-SCR reaction over HSSZ-13, Cu-SSZ-13, meso-Cu-SSZ-13, meso-Cu-SSZ-13@MAS, and Cu-MAS catalysts [96]. Copyright 2016, Elsevier B.V.; (c) Schematic representation of the preparation of Cu-SSZ-13-Meso and NOx conversion over Cu-SSZ-13-Meso and Cu-SSZ-13-Trad [97]. Copyright 2021, Elsevier B.V.
In addition, ZSM-5 is also one of the early catalysts used for denitration research. Vennestrøm et al. synthesized mesoporous H-ZSM-5 by using sodium hydroxide, guanidinium hydroxide, and guanidinium carbonate, finding that all base-treated samples lead to longer catalyst lifetimes in the methanol-to-gasoline reaction. Compared to conventional zeolites, mesoporous zeolites are able to accommodate a higher amount of dispersed Fe species, which act as active species. The prepared sample (FeZ(0.3)NaOH(1.0)) possesses the highest degree of mesoporosity and shows the highest catalytic activity [98]. Similarly, Rutkowska et al. investigated the influence of mesopores on the catalytic performance of ZSM-5 commercial zeolite. The ZSM-5 zeolite catalyst with mesoporous structure was obtained by adjusting different ratios of NaOH and TPAOH, as well as different operating times. It was found that the enhancement of catalytic performance is closely related to the generation of mesopores [99]. Peng et al. prepared a Cu-ZSM-5 catalyst with a micro-mesoporous structure using a one-pot hydrothermal synthesis method and applied it to NH3-SCR. Compared with traditional Cu-ZSM-5 catalysts, the mesoporous Cu-ZSM-5 catalysts showed better catalytic performance, higher anti-SO2 toxicity activity, and higher hydrothermal stability in the range of 150–350 °C, which were attributed to the fact that a mesoporous structure can provide a larger specific surface area, as well as improve the mass transfer of reactants and NO-adsorption capacity [100].
3.2. Removing of VOCs
VOCs are recognized as a type of harmful substance released into the air. The World Health Organization (WHO) has a clear definition of VOCs, which refer to various organic compounds with boiling points between 50 °C and 260 °C, mainly including aliphatic hydrocarbons, halogenated hydrocarbons, aromatic hydrocarbons, alcohols, aldehydes, esters, ethers, ketones, carboxylic acids, amines, and sulfur-containing organic compounds [101]. Some VOCs can pass through the troposphere to the stratosphere through photolysis, physical adsorption, and other processes and react with atmospheric ozone, resulting in the destruction of the ozone layer. Under high temperatures or ultraviolet irradiation, the active free radicals from VOC cracking can participate in a series of chemical reactions and trigger a chain reaction, leading to the occurrence of ozone, photochemical smog, the greenhouse effect, and other phenomena. Among them, benzene can have a photochemical reaction with NOx under the action of light. When benzene coexists with NOx and SOx, the dry fallout becomes photochemical smoke, and the wet fallout turns into acid rain, causing great damage to the environment [102,103,104]. Moreover, VOCs can also harm human health: they can damage the eyeball, nasal cavity, and throat; cause memory and visual impairment; and even pose a risk of cancer. Meanwhile, VOCs can indirectly harm the human body as a viral carrier, which promotes the formation of secondary organic aerosol (SOA) and provides a carrier for bacteria and viruses [105,106,107,108]. At present, the removal of VOCs can be divided into two methods: the physical method and the chemical method. The common physical methods are absorption, condensation, and membrane separation. Physical methods, which usually enrich and separate VOCs by changing the physical conditions (such as pressure and temperature), are suitable for high concentrations (>5000 mg/m3) of VOCs. Chemical methods, mainly including the incineration method, biological method, and catalytic oxidation method, can convert VOCs into CO2, non-toxic or less-toxic inorganic substances, which are suitable for the treatment of medium- and low-concentration (<1000 mg/m3) VOCs.
Toluene is a type of VOCs. The treatment of toluene has attracted extensive attention because of its toxicity and damage to the environment. Kim et al. found that the adsorption properties of zeolite for toluene and methylethylketone (MEK) were closely related to the pore structure. The researchers removed toluene and MEK via the adsorption of mordenite and X- or Y-type FAU zeolite at 25 °C and investigated the relationship between the adsorption and desorption behavior of selected volatile organic compounds and the physicochemical properties of zeolite (acidity, Si/Al ratio, crystal structure, pore structure, specific surface area, and pore volume). The results showed that the pore volume has an important effect on the reaction. Among the selected zeolites, the commercial Y-zeolite shows the largest adsorption capacity due to its large mesoporous volume [109]. Lv et al. synthesized a Pd/HMAS-1 catalyst (HMAS: hollow mesoporous aluminosilicate sphere) by loading Pd onto a hollow mesoporous silica-aluminum sphere for toluene combustion. The result indicated that small-sized Pd nanoparticles existed in the ordered mesoporous pores of HMAS. The presence of mesopores provided a large specific surface area and improved the mass transfer efficiency, which made the catalyst exhibit excellent toluene oxidation performance at 150 °C. Moreover, Pd/HMAS-1 has excellent stability and can maintain 100% conversion efficiency at 200 °C for 20 h [110]. Li et al. investigated the adsorption and desorption properties of different industrial adsorbents (A zeolites, ZSM-5 zeolites, Y zeolites, X zeolites, and MCM-41 mesoporous silica) on VOCs before and after alkali modification. The p-xylene adsorption capacity of mesoporous USY zeolite after alkaline modification is improved due to the increase in mass transfer. These zeolites can also be applied to other volatile organic compounds, including benzene, chlorobenzene, and butyl acetate [111]. In addition, Khawaja et al. synthesized mesoporous NaY zeolite-supported palladium (Pd) or platinum (Pt) via three steps of dealuminization, desilication, and alkaline treatment. The prepared catalysts were applied in the oxidation of ethanol and toluene. According to the characterization and test results, the introduction of mesopores enabled the partial presence of catalytically active Pt0 species, and the temperature for oxidation of 90% toluene was 130 °C over meso-Pt/NaY, which is lower than that (151 °C) of Pt/NaY [112]. Meanwhile, Zhao et al. synthesized ZSM-5 with controllable intercrystalline and intracrystalline mesopores using the hydrothermal method and studied the catalytic performance of catalysts in toluene oxidation. Mesoporous ZSM-5 materials have excellent cycling properties due to their low spatial confinement and high diffusion rate for organic molecules, especially macromolecules [41]. In addition, Beta zeolites can also be used in the catalytic oxidation of toluene; for example, Chen et al. prepared mesoporous Beta zeolite (Beta-H) for the catalytic combustion of toluene. It was found that the Pt particle sizes are smaller and the Pt0/Pt2+ ratio is higher on mesoporous Pt-R/Beta-H than those on the traditional zeolite, which means that the mesoporous structures are favorable for the catalytically active Pt0 species loaded. As shown in Figure 6a,b, the catalytic activity and selectivity for toluene combustion of Beta-H zeolite was higher than that of Beta zeolite, which was related to the higher amount of Pt0 [113].
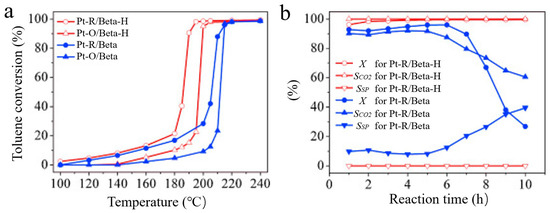
Figure 6.
(a) Catalytic combustion of toluene over Pt-R/Beta-H, Pt-O/Beta-H, Pt-R/Beta, and Pt-O/Beta catalysts; (b) Dependences of activities on reaction time in the catalytic combustion of toluene over Pt-R/Beta-H and Pt-R/Beta catalysts [113]. Copyright 2013, Elsevier B.V.
In addition, researchers have also carried out research on other types of pollutants in VOCs, such as light alkane, which is a typical volatile organic compound with strong carbon-hydrogen bonds and is not easily degraded into CO2 and H2O. At present, the most effective method for the removal of light alkanes is deep oxidation, but the small pore size of traditional zeolites limits the diffusion of molecules and the accessibility of metal active sites. Therefore, zeolite application in the oxidation of light alkanes is limited. In order to improve the mass transfer efficiency and the accessibility of the active site, the introduction of a mesopore is important. Peng et al. prepared Pd@IM-S-1 material with abundant mesopores. Compared with the commonly supported catalyst (Pd/S-1), the mesoporous Pd@IM-S-1 material showed excellent deep oxidation activity of methane and propane [114]. Similarly, Park et al. synthesized a mesoporous MFI zeolite-supported Pt3Y catalyst by using diammonium-type bromide surfactant [C18H37–N(CH3)2–C6H12–N(CH3)2–C4H9Br2]) and sodium-free silica source. The results indicated that more active sites existed in mesopores, which contributed to high catalytic durability, high propane conversion, and high propylene selectivity [115].
3.3. Removal of CO
Carbon monoxide (CO) is a common toxic gas that poses a threat to the environment and human health. The natural concentration of CO in air is approximately 0.2 parts per million (ppm), which is harmless to humans. But when the concentration of carbon monoxide is higher, it combines with hemoglobin present in blood cells and converts carboxyhemoglobin (CoHb), then reduces the oxygen-carrying capacity of the human body [116]. Carbon monoxide poisoning can cause headaches, fatigue, seizures, coma, nausea, vomiting, and even death [21]. Carbon monoxide is also an atmospheric pollutant, which is one of the direct factors contributing to global warming. Meanwhile, it can react with hydroxyl (OH) radicals present in the environment, thereby reducing their abundance [117,118]. In addition, the emissions of carbon monoxide are very extensive, mainly divided into natural sources and human sources. Human sources, including residential, industrial, and transportation, are the main factors causing air pollution [119,120]. Therefore, effective treatment and conversion of carbon monoxide is crucial [121]. Catalytic oxidation is considered to be the best solution to remove CO; many types of catalysts for CO oxidation have been studied, such as catalysts loaded with noble metal particles and non-noble metal particles [122]. The complete conversion temperature of catalysts loaded with non-noble metal particles for CO conversion is usually higher than 150 °C, and their catalytic activity significantly decreases under harsh conditions, which limits their application. Compared to the catalysts loaded with non-noble metal particles, the catalysts loaded with noble metal particles exhibit relatively high catalytic activity and stability for CO oxidation, but the price is relatively high for industrial applications. Among them, catalysts loaded with palladium are commonly studied, but they still face the challenge of water resistance and sulfur resistance [123].
To improve the water resistance of catalysts containing Pt, Chen’s group successfully prepared mesoporous zeolite (Pt/Fe-mBeta) catalysts with different iron contents using a one-pot hydrothermal method and ion-exchange method, active species Pt and Fe were highly dispersed on mesoporous zeolites. Among all the samples, the optimized sample Pt/Fe(3)-mBeta exhibits the best CO oxidation catalytic activity, and the complete oxidation temperature can be reduced to 90 °C, which is due to the introduction of mesopores increasing the specific surface area of the catalyst and providing abundant active sites, and the larger mesoporous pore volume is conducive to adsorption and diffusion of reaction gas. In addition, the possible catalytic reaction mechanisms under different conditions with/without the presence of H2O for CO oxidation on mesoporous zeolite (Pt/Fe-mBeta) have also been proposed. In the presence of CO and O2, Fe3+ species can obtain electrons from Pt0; then, CO and O2 participate in the reaction and finally produce CO2. Another reaction route happened when Pt2+ species obtained electrons from Fe2+; after a similar activation process, CO2 can be produced. When H2O is introduced, it can be activated into O– and/or OH groups and then participate in the reaction (Figure 7a) [123]. Moreover, in order to improve sulfur resistance, Chen’s group also designed and synthesized mesoporous zeolites Pt/m-Beta@TiO2-x with a core–shell structure via a facile photochemical reduction route. The obtained Pt/m-Beta@TiO2 catalysts possess rich oxygen vacancy defects, which are important in catalysis due to their unique properties and remarkable redox performance. Meanwhile, mesoporous zeolites provide a large specific surface area and abundant active sites, which provide a site for the high dispersion of Pt and show excellent catalytic activity in CO oxidation. Among them, the best sample, Pt/m-Beta@TiO2-3, showed higher CO catalytic oxidation activity and sulfur resistance. The complete conversion of CO of Pt/m-Beta@TiO2-3 can be reduced to 90 °C (Figure 7b), and the temperature for CO complete conversion is as low as 105 °C in the presence of both H2O and SO2 (Figure 7c). Furthermore, after three cycles, Pt/m-Beta@TiO2-3 still exhibits high CO catalytic activity, which demonstrates its high stability [124].
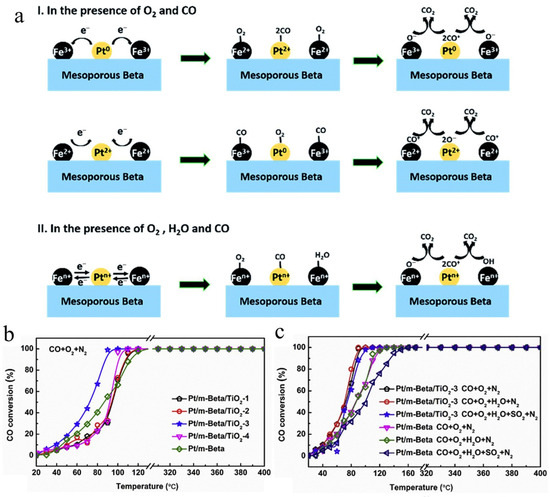
Figure 7.
(a) The possible catalytic pathways of CO oxidation over the catalyst Pt/Fe(3)-mBeta under different conditions [123]. Copyright 2019, pubs.rsc.org; (b) CO conversion vs. T profile of different catalyst samples in dry reaction atmosphere; (c) The effect of H2O and SO2 on the catalytic activity (CO conversion vs. T profile) of the Pt/m-Beta@TiO2-3 and Pt/m-Beta samples [124]. Copyright 2013, Elsevier B.V.
In addition, carbon monoxide can also be used to produce various organic derivatives through methanation for the synthesis of various organic products. Triwahyono’s group has conducted research on the use of mesoporous ZSM-5 zeolites for carbon monoxide methanation. They prepared mesoporous ZSM-5 (mZSM5) via a dual-templating method, and the prepared catalyst possesses both acidic and basic sites, which are beneficial for improving catalytic ability (Figure 8a). The catalytic performance of the CO methanation reaction is greatly influenced by both porosity and basicity. Among all the samples, the optimal mZSM5-0.5D exhibited the highest CO methanation rate (0.0226 μmol/m2 s) at 723 K [125]. Furthermore, the core–shell structure mesoporous ZSM-5 (FmZSM-5) was prepared to enhance the CO conversion rate (0.0708 μmol-CO/m2 s) and the CH4 formation rate (0.0488 μmol-CH4/m2 s) at 723 K (Figure 8b). Similarly, Teh et al. also illustrated that the presence of mesopores provides a large specific surface area and pore volume, as well as sufficient base active sites [126]. In addition, mesoporous ZSM-5 containing metal particles was also explored. The excellent catalytic performance of nickel-promoted mesoporous ZSM5 (Ni/mZSM5) is attributed to the presence of mesopores and basicity, which lead to the synergistic effect of Ni metal active sites and mZSM5 carriers. Two possible CO methanation mechanisms are proposed. The first mechanism is the main pathway, in which adrobed CO could react with H2 to form CH4 and H2O (Figure 8c) [127].
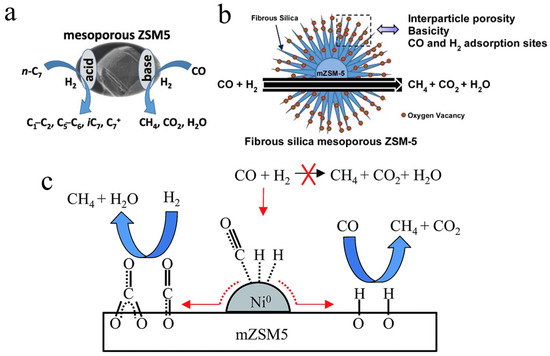
Figure 8.
(a) Methanation of CO on mesoporous ZSM5 [125]. Copyright 2015, Elsevier B.V.; (b) Methanation of CO on core–shell structure mesoporous ZSM-5 [126]. Copyright 2016, Elsevier B.V.; (c) Reaction mechanism of CO methanation over Ni/mZSM5 [127]. Copyright 2015, pubs.rsc.org.
4. Conclusions
The ecological environment is closely related to human life. However, with the progress of human production and technological civilization, the ecological environment has been destroyed, and both human life and health have been greatly threatened. How to solve air pollution effectively is an important subject. Mesoporous zeolite catalysts have attracted much attention because of their advantages, such as large specific surface area, easy adjustment of pore size, and strong acidic site. At present, the preparation methods of mesoporous zeolites have been widely studied. The preparation methods of mesoporous zeolites, including the post-treatment method (dealumination and desilication) and in situ method (the hard template method and soft template method), are described in detail in this paper. Moreover, the applications of mesoporous zeolite in nitrogen oxides, VOCs, and CO are also illustrated. The presence of mesopores in zeolites provides a large specific surface area and pore volume, making them better for dispersing active species. The presence of mesopores also facilitates the diffusion of reactants and increases adsorption capacity. Thus, the mesopores in zeolites significantly improved the catalytic and adsorption ability when compared with traditional zeolites.
Although mesoporous zeolites have been widely studied in environmental purification, some problems still limit the industrial application. With the development of science and technology, research methods and technical means are constantly updated, and the development of more low-cost, high-stability, and high-activity catalysts will contribute to shortening the distance between basic research and practical application. By changing the raw materials used for synthesizing zeolites, the cost of synthesizing zeolites can be effectively reduced. In short, mesoporous zeolites with excellent performance can be used in the treatment of air pollutants, which is of great significance for the governance of the ecological environment and the protection of human health.
Author Contributions
Literature search, C.M.; article structure design, L.W. and X.Y.; manuscript writing, C.Z. and S.Z.; graphics production, D.Y.; creating diagrams, S.G.; manuscript revision, X.Y. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (22372107, 22072095, U1908204), the National Key R&D Program of China (2022YFB3506200, 2022YFB3504100), the Excellent Youth Science Foundation of Liaoning Province (2022-YQ-20), the Shenyang Science and Technology Planning Project (22-322-3-28), the Liaoning Xingliao talented youth Top talent program (XLYC2203007), and the University Joint Education Project for China-Central and Eastern European Countries (2021097).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., III; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA A Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhang, X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. USA 2018, 115, 9193–9197. [Google Scholar] [CrossRef] [PubMed]
- Almetwally, A.A.; Bin-Jumah, M.; Allam, A.A. Ambient air pollution and its influence on human health and welfare: An overview. Environ. Sci. Pollut. Res. 2020, 27, 24815–24830. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Grossale, A.; Nova, I.; Tronconi, E. Study of a Fe-zeolite-based system as NH3-SCR catalyst for diesel exhaust aftertreatment. Catal. Today 2008, 136, 18–27. [Google Scholar] [CrossRef]
- Tronconi, E.; Nova, I.; Ciardelli, C.; Chatterjee, D.; Bandl-Konrad, B.; Burkhardt, T. Modelling of an SCR catalytic converter for diesel exhaust after treatment: Dynamic effects at low temperature. Catal. Today 2005, 105, 529–536. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Cao, C.; Wang, S.; Lv, J.; Zheng, W.; Tan, D. The development of diesel oxidation catalysts and the effect of sulfur dioxide on catalysts of metal-based diesel oxidation catalysts: A review. Fuel Process. Technol. 2022, 233, 107317. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C—Catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, H.; Zhu, J.; Tian, G.; Huang, J.; Zhang, F. An experimental study of heterogeneous NO reduction by biomass reburning. Fuel Process. Technol. 2015, 132, 111–117. [Google Scholar] [CrossRef]
- Tang, Q.; Denison, M.; Adams, B.; Brown, D. Towards comprehensive computational fluid dynamics modeling of pyrolysis furnaces with next generation low-NOx burners using finite-rate chemistry. Proc. Combust. Inst. 2009, 32, 2649–2657. [Google Scholar] [CrossRef]
- Ni, P.; Wang, X.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Carabineiro, S.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.; Órfão, J.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. The total oxidation of propane over supported Cu and Ce oxides: A comparison of single and binary metal oxides. J. Catal. 2010, 272, 109–120. [Google Scholar] [CrossRef]
- Carrillo, A.; Carriazo, J. Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Wang, C.; Bai, H. A comparative investigation on the low-temperature catalytic oxidation of acetone over porous aluminosilicate-supported cerium oxides. Chem. Eng. J. 2015, 264, 835–844. [Google Scholar] [CrossRef]
- Bastos, S.; Carabineiro, S.; Órfão, J.; Pereira, M.; Delgado, J.; Figueiredo, J. Total oxidation of ethyl acetate, ethanol and toluene catalyzed by exotemplated manganese and cerium oxides loaded with gold. Catal. Today 2012, 180, 148–154. [Google Scholar] [CrossRef]
- Sykes, O.T.; Walker, E. The neurotoxicology of carbon monoxide—Historical perspective and review. Cortex 2016, 74, 440–448. [Google Scholar] [CrossRef]
- Tian, X.; Shan, Y.; Zhang, J.; Yan, Z.; Sun, Y.; Ding, W.; Yu, Y. The study of Pt/zeolites for CO oxidation: Effects of skeleton structure and Si/Al ratio. Catal. Commun. 2023, 178, 106679. [Google Scholar] [CrossRef]
- Dey, S.; Sun, S.; Mehta, N.S. Carbon monoxide catalytic oxidation over various iron-based nanoparticles at ambient conditions: A Review. Carbon Capture Sci. Technol. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wu, X.; Ye, Q.; Xu, X.; Li, B.; Wang, F. Novel synthesis and shape-dependent catalytic performance of Cu-Mn oxides for CO oxidation. Appl. Surf. Sci. 2017, 403, 335–341. [Google Scholar] [CrossRef]
- Mgbemere, H.; Ekpe, I.; Lawal, G. Zeolite synthesis, characterization and application areas: A review. Int. J. Res. Environ. Sci. 2017, 6, 45–59. [Google Scholar]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef] [PubMed]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.; Karmakar, A.K.; Zhou, J.; Ahmed, M.B. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Yi, H.; Yu, Q.; Zhang, Y.; Wei, J.; Yuan, Y. Synthesis, characterization and application of Fe-zeolite: A review. Appl. Catal. A Gen. 2022, 630, 118467. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Xue, H.; Li, W.; Zhang, P. Characterization of shale pore system: A case study of Paleogene Xin’gouzui Formation in the Jianghan basin, China. Mar. Pet. Geol. 2017, 79, 321–334. [Google Scholar] [CrossRef]
- Guilera, J.; del Valle, J.; Alarcon, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Cunha, F.A.; Ruotolo, L.A.M. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J. Clean. Prod. 2019, 229, 956–963. [Google Scholar] [CrossRef]
- Kongnoo, A.; Tontisirin, S.; Worathanakul, P.; Phalakornkule, C. Surface characteristics and CO2 adsorption capacities of acid-activated zeolite 13X prepared from palm oil mill fly ash. Fuel 2017, 193, 385–394. [Google Scholar] [CrossRef]
- Pedrolo, D.R.S.; de Menezes Quines, L.K.; de Souza, G.; Marcilio, N.R. Synthesis of zeolites from Brazilian coal ash and its application in SO2 adsorption. J. Environ. Chem. Eng. 2017, 5, 4788–4794. [Google Scholar] [CrossRef]
- Gao, S.; Peng, H.; Song, B.; Zhang, J.; Wu, W.; Vaughan, J.; Zardo, P.; Vogrin, J.; Tulloch, S.; Zhu, Z. Synthesis of zeolites from low-cost feeds and its sustainable environmental applications. J. Environ. Chem. Eng. 2023, 11, 108995. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Kumar, D.; Schumacher, K.; von Hohenesche, C.d.F.; Grün, M.; Unger, K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterisation. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 109–116. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.orgdatabases (accessed on 5 August 2023).
- Zhao, C.; Hu, X.; Liu, C.; Chen, D.; Yun, J.; Jiang, X.; Wei, N.; Li, M.; Chen, Z. Hierarchical architectures of ZSM-5 with controllable mesoporous and their particular adsorption/desorption performance for VOCs. J. Environ. Chem. Eng. 2022, 10, 106868. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef]
- Ren, S.; Liu, G.; Wu, X.; Chen, X.; Wu, M.; Zeng, G.; Liu, Z.; Sun, Y. Enhanced MTO performance over acid treated hierarchical SAPO–34. Chin. J. Catal. 2017, 38, 123–130. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Desilication mechanism revisited: Highly mesoporous all-silica zeolites enabled through pore-directing agents. Chem.—A Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Pérez-Ramírez, J. Full compositional flexibility in the preparation of mesoporous MFI zeolites by desilication. J. Phys. Chem. C 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Janssen, A.; Koster, A.; De Jong, K. On the shape of the mesopores in zeolite Y: A three-dimensional transmission electron microscopy study combined with texture analysis. J. Phys. Chem. B 2002, 106, 11905–11909. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Oruji, S.; Khoshbin, R.; Karimzadeh, R. Preparation of hierarchical structure of Y zeolite with ultrasonic-assisted alkaline treatment method used in catalytic cracking of middle distillate cut: The effect of irradiation time. Fuel Process. Technol. 2018, 176, 283–295. [Google Scholar] [CrossRef]
- Sammoury, H.; Toufaily, J.; Cherry, K.; Hamieh, T.; Pouilloux, Y.; Pinard, L. Desilication of *BEA zeolites using different alkaline media: Impact on catalytic cracking of n-hexane. Microporous Mesoporous Mater. 2018, 267, 150–163. [Google Scholar] [CrossRef]
- Luo, X.-L.; Pei, F.; Wang, W.; Qian, H.-m.; Miao, K.-K.; Pan, Z.; Chen, Y.-S.; Feng, G.-D. Microwave synthesis of hierarchical porous materials with various structures by controllable desilication and recrystallization. Microporous Mesoporous Mater. 2018, 262, 148–153. [Google Scholar] [CrossRef]
- Yin, X.; Liu, N.; Han, M.; Xu, F.; Jia, Y.; Song, F.; Cui, H. Ultrasonic-pretreated hydrothermal synthesis of less dense zeolite CHA from the transformation of zeolite T. Ultrason. Sonochemistry 2023, 100, 106598. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Chen, G.; Zhu, H.; Liu, G.; Xie, Y.; Liu, G.; Jin, W. Stability of MFI zeolite-based membranes: Current status and perspectives. Sep. Purif. Technol. 2024, 330, 125603. [Google Scholar] [CrossRef]
- Tangale, N.P.; Niphadkar, P.S.; Joshi, P.N.; Dhepe, P.L. Hierarchical K/LTL zeolite as solid base for aqueous phase hydrogenation of xylose to xylitol. Microporous Mesoporous Mater. 2019, 278, 70–80. [Google Scholar] [CrossRef]
- Babić, V.; Koneti, S.; Moldovan, S.; Debost, M.; Gilson, J.-P.; Valtchev, V. Chromic acid dealumination of zeolites. Microporous Mesoporous Mater. 2022, 329, 111513. [Google Scholar] [CrossRef]
- Wei, L.-J.; Peng, P.; Qiao, K.; Cheng, G.-N.; Etim, U.J.; Yan, Z.-F. Hierarchical Y zeolite with accessible intracrystalline mesopores and enhanced hydrocracking performances via sequential multi-carboxyl acid and fluoroborate post-treatments. Fuel 2023, 354, 129289. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Groen, J.C.; Abelló, S.; Villaescusa, L.A.; Pérez-Ramírez, J. Mesoporous beta zeolite obtained by desilication. Microporous Mesoporous Mater. 2008, 114, 93–102. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite catalysts with tunable hierarchy factor by pore-growth moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Srivastava, R. Synthesis and applications of ordered and disordered mesoporous zeolites: Present and future prospective. Catal. Today 2018, 309, 172–188. [Google Scholar] [CrossRef]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qin, Y.; Jiang, H.; Wang, L. Protective desilication of β zeolite: A mechanism study and its application in ethanol-acetaldehyde to 1, 3-butadiene. Microporous Mesoporous Mater. 2021, 326, 111359. [Google Scholar] [CrossRef]
- Qin, Z.; Melinte, G.; Gilson, J.P.; Jaber, M.; Bozhilov, K.; Boullay, P.; Mintova, S.; Ersen, O.; Valtchev, V. The mosaic structure of zeolite crystals. Angew. Angew. Chem. Chem. 2016, 128, 15273–15276. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Malgras, V.; Tang, J.; Wang, J.; Kim, J.; Torad, N.L.; Dutta, S.; Ariga, K.; Hossain, M.S.A.; Yamauchi, Y.; Wu, K.C. Fabrication of nanoporous carbon materials with hard-and soft-templating approaches: A review. J. Nanosci. Nanotechnol. 2019, 19, 3673–3685. [Google Scholar] [CrossRef]
- Cho, H.S.; Ryoo, R. Synthesis of ordered mesoporous MFI zeolite using CMK carbon templates. Microporous Mesoporous Mater. 2012, 151, 107–112. [Google Scholar] [CrossRef]
- Abdulridha, S.; Jiao, Y.; Xu, S.; Zhang, R.; Ren, Z.; Garforth, A.A.; Fan, X. A comparative study on mesoporous Y zeolites prepared by hard-templating and post-synthetic treatment methods. Appl. Catal. A Gen. 2021, 612, 117986. [Google Scholar] [CrossRef]
- White, R.J.; Fischer, A.; Goebel, C.; Thomas, A. A sustainable template for mesoporous zeolite synthesis. J. Am. Chem. Soc. 2014, 136, 2715–2718. [Google Scholar] [CrossRef]
- Vartuli, J.; Roth, W.; Beck, J.; McCullen, S.; Kresge, C. The synthesis and properties of M41S and related mesoporous materials. In Synthesis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 97–119. [Google Scholar]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wang, H.; Pinnavaia, T.J. MFI zeolite with small and uniform intracrystal mesopores. Angew. Chem. 2006, 118, 7765–7768. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Yin, C.; Shan, Z.; Xiao, F.-S. Hierarchical mesoporous zeolites with controllable mesoporosity templated from cationic polymers. Microporous Mesoporous Mater. 2010, 131, 58–67. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.-H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef]
- Gu, F.N.; Wei, F.; Yang, J.Y.; Lin, N.; Lin, W.G.; Wang, Y.; Zhu, J.H. New strategy to synthesis of hierarchical mesoporous zeolites. Chem. Mater. 2010, 22, 2442–2450. [Google Scholar] [CrossRef]
- Miyake, K.; Hirota, Y.; Uchida, Y.; Nishiyama, N. Synthesis of mesoporous MFI zeolite using PVA as a secondary template. J. Porous Mater. 2016, 23, 1395–1399. [Google Scholar] [CrossRef]
- Guo, D.; Shi, C.; Zhao, H.; Chen, R.; Chen, S.; Sun, P.; Chen, T. Polyacrylic acid as mesoscale template for synthesis of MFI zeolite with plentiful intracrystalline mesopores. Microporous Mesoporous Mater. 2020, 293, 109821. [Google Scholar] [CrossRef]
- Feng, G.; Wang, J.; Boronat, M.; Li, Y.; Su, J.-H.; Huang, J.; Ma, Y.; Yu, J. Radical-facilitated green synthesis of highly ordered mesoporous silica materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Boronat, M.; Ferri, P.; Xu, Z.; Liu, P.; Shen, B.; Wang, Z.; Yu, J. Organic-Free Synthesis of Zeolite Y with High Si/Al Ratios: Combined Strategy of In Situ Hydroxyl Radical Assistance and Post-Synthesis Treatment. Angew. Chem. 2020, 132, 17378–17381. [Google Scholar] [CrossRef]
- Muhammad Farhan, S.; Pan, W.; Zhijian, C.; JianJun, Y. Innovative catalysts for the selective catalytic reduction of NOx with H2: A systematic review. Fuel 2024, 355, 129364. [Google Scholar] [CrossRef]
- Zhang, J.J.; Samet, J.M. Chinese haze versus Western smog: Lessons learned. J. Thorac. Dis. 2015, 7, 3. [Google Scholar] [PubMed]
- Zhang, P.; Yu, W.; Gao, B.; Cui, S.; Li, J.; Qi, L. Denitration performance and mechanism of Mn-Ce supported alkali-modified fly ash catalysts for NH3-SCR. Fuel 2024, 357, 129878. [Google Scholar] [CrossRef]
- Liu, B.; Peng, D.; Chiang, P.-C.; Chu, C. Performance evaluation of NOx absorption by different denitration absorbents in wet flue gas denitration. J. Taiwan Inst. Chem. Eng. 2023, 145, 104840. [Google Scholar] [CrossRef]
- Wei, Y.; Li, D.; Qiao, J.; Guo, X. Recovery of spent SCR denitration catalyst: A review and recent advances. J. Environ. Chem. Eng. 2023, 11, 110104. [Google Scholar] [CrossRef]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, H.; Li, T.; Zhu, Y.; Zhou, S.; Feng, Y.; Zhou, W. Relation analysis on emission control and economic cost of SCR system for marine diesels. Sci. Total Environ. 2021, 788, 147856. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Z.; Zhao, N.; Shi, M.; Sun, Y.; Nan, Z.; Wang, L. One-pot synthesis of CNT-SAPO-34 composite supported copper and cerium catalysts with excellent surface resistance to SO2 and H2O in NH3-SCR. J. Rare Earths 2023, 41, 1344–1352. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Sunil Kumar, M.; Alphin, M.S.; Manigandan, S.; Vignesh, S.; Vigneshwaran, S.; Subash, T. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 2023, 344, 128125. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, S.; Xu, S.; Xu, S.; Pei, M.; Yao, P.; Xu, H.; Dan, Y.; Chen, Y. Comprehensive effect of tuning Cu/SAPO-34 crystals using PEG on the enhanced hydrothermal stability for NH3-SCR. Catal. Sci. Technol. 2021, 11, 7640–7651. [Google Scholar] [CrossRef]
- Wang, C.; Yan, W.; Wang, Z.; Chen, Z.; Wang, J.; Wang, J.; Wang, J.; Shen, M.; Kang, X. The role of alkali metal ions on hydrothermal stability of Cu/SSZ-NH3-SCR catalysts. Catal. Today 2020, 355, 482–492. [Google Scholar] [CrossRef]
- Olsson, L.; Wijayanti, K.; Leistner, K.; Kumar, A.; Joshi, S.Y.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. A multi-site kinetic model for NH3-SCR over Cu/SSZ-13. Appl. Catal. B Environ. 2015, 174, 212–224. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T. Catalysis in a cage: Condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Oord, R.; Ten Have, I.; Arends, J.; Hendriks, F.; Schmidt, J.; Lezcano-Gonzalez, I.; Weckhuysen, B. Enhanced activity of desilicated Cu-SSZ-13 for the selective catalytic reduction of NOx and its comparison with steamed Cu-SSZ-13. Catal. Sci. Technol. 2017, 7, 3851–3862. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Liang, J.; Tao, J.; Mi, Y.; Liu, W.; Wang, Z.; Li, Z.; Wu, D.; Wu, P.; Peng, H. Unraveling the boosting low-temperature performance of ordered mesoporous Cu-SSZ-13 catalyst for NOx reduction. Chem. Eng. J. 2021, 409, 128238. [Google Scholar] [CrossRef]
- Vennestrøm, P.N.R.; Grill, M.; Kustova, M.; Egeblad, K.; Lundegaard, L.F.; Joensen, F.; Christensen, C.H.; Beato, P. Hierarchical ZSM-5 prepared by guanidinium base treatment: Understanding microstructural characteristics and impact on MTG and NH3-SCR catalytic reactions. Catal. Today 2011, 168, 71–79. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basąg, S.; Kowalczyk, A.; Piwowarska, Z.; Duda, M.; Tarach, K.; Góra-Marek, K.; Michalik, M.; Díaz, U. Catalytic performance of commercial Cu-ZSM-5 zeolite modified by desilication in NH3-SCR and NH3-SCO processes. Microporous Mesoporous Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Peng, H.; Mi, Y.; Liang, J.; Liu, W.; Wang, X.; Song, G.; Wu, P.; Liu, F. One-pot synthesis of layered mesoporous ZSM-5 plus Cu ion-exchange: Enhanced NH3-SCR performance on Cu-ZSM-5 with hierarchical pore structures. J. Hazard. Mater. 2020, 385, 121593. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, J.; Yu, G.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. Hydrophobic design of adsorbent for VOC removal in humid environment and quick regeneration by microwave. Microporous Mesoporous Mater. 2020, 294, 109869. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, F.; Sun, L.; Yu, X.; Zhao, J.; Hu, Y.; Wang, Y. Characterization of VOC emissions from composite wood furniture: Parameter determination and simplified model. Build. Environ. 2019, 161, 106237. [Google Scholar] [CrossRef]
- Zheng, C.; Shen, J.; Zhang, Y.; Huang, W.; Zhu, X.; Wu, X.; Chen, L.; Gao, X.; Cen, K. Quantitative assessment of industrial VOC emissions in China: Historical trend, spatial distribution, uncertainties, and projection. Atmos. Environ. 2017, 150, 116–125. [Google Scholar] [CrossRef]
- Zhang, T.; Li, G.; Yu, Y.; Ji, Y.; An, T. Atmospheric diffusion profiles and health risks of typical VOC: Numerical modelling study. J. Clean. Prod. 2020, 275, 122982. [Google Scholar] [CrossRef]
- Dai, H.; Jing, S.; Wang, H.; Ma, Y.; Li, L.; Song, W.; Kan, H. VOC characteristics and inhalation health risks in newly renovated residences in Shanghai, China. Sci. Total Environ. 2017, 577, 73–83. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.; Yan, Y.; Chen, N.; Yao, L.; Liu, X.; Wu, F.; Cheng, Y.; Niu, Z.; Zheng, S. Compositions, sources and health risks of ambient volatile organic compounds (VOCs) at a petrochemical industrial park along the Yangtze River. Sci. Total Environ. 2020, 703, 135505. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, L.; Liu, X.; Zhang, Y.; Li, C.; Zhang, Y.; Zhang, C.; Qu, Y.; An, J.; Ma, D. Characteristics, secondary transformation, and health risk assessment of ambient volatile organic compounds (VOCs) in urban Beijing, China. Atmos. Pollut. Res. 2021, 12, 33–46. [Google Scholar] [CrossRef]
- Kim, K.-J.; Ahn, H.-G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef]
- Lv, M.; Song, S.; Verma, P.; Wen, M. Hollow mesoporous aluminosilicate spheres imbedded with Pd nanoparticles for high performance toluene combustion. Catal. Today 2023, 410, 135–142. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Guo, Y.; Zhu, T.; Xu, W. Adsorption and desorption characteristics of hydrophobic hierarchical zeolites for the removal of volatile organic compounds. Chem. Eng. J. 2021, 411, 128558. [Google Scholar] [CrossRef]
- El Khawaja, R.; Sonar, S.; Barakat, T.; Heymans, N.; Su, B.-L.; Löfberg, A.; Lamonier, J.-F.; Giraudon, J.-M.; De Weireld, G.; Poupin, C. VOCs catalytic removal over hierarchical porous zeolite NaY supporting Pt or Pd nanoparticles. Catal. Today 2022, 405, 212–220. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.-S. Enhanced performance in catalytic combustion of toluene over mesoporous Beta zeolite-supported platinum catalyst. Appl. Catal. B Environ. 2013, 140, 199–205. [Google Scholar] [CrossRef]
- Peng, H.; Dong, T.; Yang, S.; Chen, H.; Yang, Z.; Liu, W.; He, C.; Wu, P.; Tian, J.; Peng, Y. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nat. Commun. 2022, 13, 295. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Kim, J.-C.; Choi, M.; Park, J.Y.; Ryoo, R. Sodium-free synthesis of mesoporous zeolite to support Pt-Y alloy nanoparticles exhibiting high catalytic performance in propane dehydrogenation. J. Catal. 2021, 404, 760–770. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Low-temperature complete oxidation of CO over various manganese oxide catalysts. Atmos. Pollut. Res. 2018, 9, 755–763. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Zhou, R. Catalytic performance of manganese doped CuO–CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Fuel 2016, 163, 56–64. [Google Scholar] [CrossRef]
- Qian, K.; Qian, Z.; Hua, Q.; Jiang, Z.; Huang, W. Structure–activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl. Surf. Sci. 2013, 273, 357–363. [Google Scholar] [CrossRef]
- Hoshyar, N.; Irankhah, A.I.; Jafari, M. Copper catalysts supported on CeMnO2 for CO oxidation in hydrogen-rich gas streams. Iran. J. Chem. Eng. (IJChE) 2015, 12, 3–14. [Google Scholar]
- Dey, S.; Dhal, G.C.; Prasad, R.; Mohan, D. Effect of nitrate metal (Ce, Cu, Mn and Co) precursors for the total oxidation of carbon monoxide. Resour.-Effic. Technol. 2017, 3, 293–302. [Google Scholar]
- Roderique, J.D.; Josef, C.S.; Feldman, M.J.; Spiess, B.D. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology 2015, 334, 45–58. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. A review of synthesis, structure and applications in hopcalite catalysts for carbon monoxide oxidation. Aerosol Sci. Eng. 2019, 3, 97–131. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, X.; Zhao, W.; Peng, C.; Wu, H.; Chen, H. Pt/Fe co-loaded mesoporous zeolite beta for CO oxidation with high catalytic activity and water resistance. RSC Adv. 2019, 9, 28089–28094. [Google Scholar] [CrossRef]
- Yan, D.; Li, Q.; Zhang, H.; Zhou, X.; Chen, H. A highly dispersed mesoporous zeolite@ TiO2-supported Pt for enhanced sulfur-resistance catalytic CO oxidation. Catal. Commun. 2020, 142, 106042. [Google Scholar] [CrossRef]
- Teh, L.P.; Triwahyono, S.; Jalil, A.A.; Mukti, R.R.; Aziz, M.A.A.; Shishido, T. Mesoporous ZSM5 having both intrinsic acidic and basic sites for cracking and methanation. Chem. Eng. J. 2015, 270, 196–204. [Google Scholar] [CrossRef]
- Teh, L.; Triwahyono, S.; Jalil, A.; Firmansyah, M.; Mamat, C.; Majid, Z. Fibrous silica mesoporous ZSM-5 for carbon monoxide methanation. Appl. Catal. A Gen. 2016, 523, 200–208. [Google Scholar] [CrossRef]
- Teh, L.; Triwahyono, S.; Jalil, A.; Mamat, C.; Sidik, S.; Fatah, N.; Mukti, R.; Shishido, T. Nickel-promoted mesoporous ZSM5 for carbon monoxide methanation. RSC Adv. 2015, 5, 64651–64660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).